Abstract
Understanding the pathophysiology of tuberculosis, and the bio-distribution of pathogen-associated molecules in the host is essential for the development of efficient methods of intervention. One of the key virulence factors in the pathology of tuberculosis infection is Lipoarabinomannan (LAM). Previously, we have demonstrated the reliable detection of LAM in urine from tuberculosis patients in a sandwich immunoassay format. We have also applied an ultra-sensitive detection strategy developed for amphiphilic biomarkers, membrane insertion, to the detection of LAM with a limit of detection of 10 fM. Herein, we evaluate the application of membrane insertion to the detection of LAM in patient serum, and demonstrate that the circulating concentrations of ‘monomeric’ LAM in serum are very low, despite significantly higher concentrations in the urine. Using spiked samples, we demonstrate that this discrepancy is due to the association of LAM with high-density lipoprotein (HDL) nanodiscs in human serum. Indeed, pull-down of HDL nanodiscs from human serum allows for the recovery of HDL-associated LAM. These studies suggest that LAM is likely associated with carrier molecules such as HDL in the blood of patients infected with tuberculosis. This phenomenon may not be limited to LAM in that many pathogen-associated molecular patterns like LAM are amphiphilic in nature and may also be associated with host lipid carriers. Such interactions are likely to affect host-pathogen interactions, pathogen bio-distribution and clearance in the host, and must be thoroughly understood for the effective design of vaccines and diagnostics.
Keywords: Lipoarabinomannan (LAM), amphiphiles, diagnostics, pathogen-associated molecular patterns, high-density lipoprotein, apolipoprotein A1
1. Introduction
One of the most well studied virulence factors associated with Mycobacterium tuberculosis infection is lipoarabinomannan (LAM). LAM is an abundant lipoglycan component of the Mycobacterial cell envelope and has been demonstrated to be critical to mycobacterial growth and viability in the host. This virulence factor also represents a pathogen-associated molecular pattern (PAMP) and triggers toll-like receptor-mediated responses in an infected host.1 LAM inactivates macrophages, among other functions, allowing replication of mycobacteria.2 Both metabolically active and inactive mycobacteria are known to shed cell-wall constituents such as LAM in the host and this biomarker has been detected in serum, urine, cerebrospinal fluid and sputum from infected patients.3-7 Hence, LAM has been thoroughly investigated as a biomarker for early diagnosis of tuberculosis, and several groups, including our own, have shown the presence of this biomarker in patient urine.
It has been suggested that the measurement of serum LAM might serve as an effective indicator of bacterial load in active tuberculosis. More recently, Wood et al have shown that urinary LAM expression correlates with host immune factors, and frequently indicates involvement of TB in the renal tract in patients with advanced HIV infection. This study demonstrates that urinary LAM can be used as an indicator of prognosis, and responsiveness to treatment.9 However, in contrast to studies demonstrating detection of LAM in urine, investigations as to the presence of this biomarker in serum are scarce.10 In fact, with the exception of agglutination studies, there are currently no studies that effectively measure LAM concentration in patient serum. We speculated that one of the reasons for this discrepancy is the need for ultra-sensitive detection to effectively pulldown the small concentrations of the biomarker in serum. The current study explores the circulating serum concentrations of LAM in a small cohort of samples (whose urinary LAM concentrations have previously been reported by our group) using an ultra-sensitive detection strategy (membrane insertion, limit of detection 10 fM) on a waveguide-based optical biosensor.11 Even with such a sensitive strategy, we were barely able to detect monomeric LAM in a small subset of patients with very high urinary burden. This observation, combined with the knowledge of the amphiphilic biochemistry of LAM, led us to speculate that serum LAM associates with carrier molecules such as, but not limited to, HDL. In this study we report the detection of HDL-associated LAM using a capture strategy that employs apolipoprotein A1 pull down on a waveguide-based optical biosensor platform and found a dramatic increase in recovery of serum LAM.
Understanding the interaction of pathogens with the host is critical to the development of effective strategies for prevention, diagnosis and treatment. In the case of bacteria, early host recognition of infection is achieved by toll-like receptors located on the cell membrane. These receptors recognize PAMPs, many of which are also virulence factors or endotoxins, critical to disease manifestation, very early in infection. Many bacterial PAMPs (e.g.; LAM, lipopolysaccharide from enteric bacteria, lipoteichoic acid of gram-positive bacteria) are amphiphilic in nature, encompassing both hydrophilic and lipophilic component, and are also likely associated with carrier molecules in the host. Understanding the interaction of bacterial PAMPs with host carrier molecules may thus have far reaching implications in elucidating the exact events in innate immune recognition, and thereby designing more effective strategies for prevention, diagnosis and treatment of infection.
2. Materials and methods
2.1. Materials
The waveguide-based sensor was developed at the Los Alamos National laboratory.12 Silicon oxynitride (SiONx) planar optical waveguides, described elsewhere, were fabricated at nGimat Ltd (Atlanta).13
LAM (14-19 Kda) from Mycobacterium tuberculosis H37Rv culture and, the goat anti-rabbit polyclonal antibody and monoclonal antibodies for the antigen were procured by a materials contract from the Colorado State University Materials Consortium (via BEI Resources). EZ-link Sulfo-NHS-LC-LC-Biotin and streptavidin were from Pierce. Alexa Fluor 647 (AF647) labeling kit was from Invitrogen. 1,2-Dioleoyl-sn-glycero-3-phosphocholine (DOPC) and 1,2-Dioleoyl-snglycero-3-phosphoethanolamine-N-(cap biotinyl) (cap-biotin-PE) were from Avanti Polar Lipids, Inc. Human serum, human lipoprotein deficient serum and human HDL were purchased from Biomedical Technologies Inc. Anti-apolipoprotein A1 antibody (anti-ApoA1)and biotinylated Anti-apolipoprotein A1 antibody were purchased from AbCam. Miniature G-25 sephadex columns were acquired from Harvard Apparatus and bovine serum was purchased from Hyclone Laboratories. All other assay reagents were from Sigma-Aldrich or Pierce Biologicals, unless otherwise specified.
2.2. Human Samples
The South Korean Ministry of Health and Welfare and NIAID co-sponsored a Natural History study conducted at the National Masan Tuberculosis Hospital (NMTH) to investigate factors involved in the development of multidrug resistant tuberculosis (MDR-TB) and the rate of relapse in Korean subjects undergoing treatment for tuberculosis for the first time or those that were being retreated (http://clinicaltrials.gov_NCT00341601). Initial blood and periodic sputum samples were collected from all subjects during treatment and subjects who had taken 7 days or less of anti-tubercular therapy were asked to provide a urine sample for analysis. Age and gender matched control subjects were recruited from the surrounding province and given a physical exam, chest X-ray, and sputum exam to exclude active TB. The blood of control subjects was also tested with the QuantiFERON-T Gold assay (QFT-G; Cellestis Limited, Carnegie, Victoria, Australia) to determine their exposure status to M. tb. The NMTH and NIAID institutional review boards approved the study protocol and all subjects provided written informed consent for the protocol. Serum samples were aliquoted, snap frozen in liquid nitrogen and shipped to Los Alamos for experimental evaluation.
2.3. Preparation of Antibodies
The anti-LAM rabbit polyclonal antibody was labeled with AF647 using a fluorescence labeling kit from Invitrogen, as per manufacturer’s instructions. Labeled antibody was separated from unlabeled by gel filtration and tested for activity using an immunoblot. Concentration and the degree of labeling were measured by UV-Vis spectroscopy.14 Anti-LAM AF647 labeled rabbit polyclonal antibody (20 nM) was used as reporter antibody in both the insertion and sandwich assays. For sandwich immunoassay 40 nM biotinylated anti-Apo AI (used as purchased) was used as a capture antibody.
2.4 Cleaning and Functionalization of Waveguides
Measurements were performed on a waveguide-based biosensor platform. Waveguides and glass coverslips were cleaned by sonication in chloroform and ethanol, and water (8 min each), followed by exposure to UV-light and ozone (40 min) and functionalized with either unilamellar bilayers (lipid bilayers) or self assembled monolayers (SAM), as described elsewhere. For lipid bilayers, DOPC vesicles were prepared by sonication, with or without 0.1% cap-biotinyl, and fused onto the cleaned waveguides, and allowed to stabilize for 12 hrs. at room temperature (RT).
For functionalization using SAMs15, clean waveguides were placed in a vacuum desiccator with ~50 μl of 1-(3-aminopropyl)-1-methyldiethoxy silane (APMDES) for 2 h. They were then baked in a hot air oven for 30 min, cooled in a desiccator, rinsed with ethanol, dried, and analyzed by contact angle measurements. Polyethylene glycol (PEG) reagents containing a methoxy group (99% of the total PEG volume) or an Fmoc-protected amine (1% of the PEG volume) at the ends were coupled to the amine surfaces of APMDES (overnight) using standard peptide coupling conditions. The protecting groups were removed by incubation in a piperidine/ N-methylpyrrolidinone (NMP) solution for 30 min. Using commercially available N-hydroxysuccinimidobiotin, biotin was attached to the resulting free-amine. The slides were rinsed with NMP, acetone, and ethanol and dried.
Functionalized waveguides were mounted on a flow cell holder and blocked for 30 min with PBS containing 2% bovine serum albumin (BSA) as a blocking agent. After addition of each reagent, the flow cell was washed with PBS (~1.5 ml, 60X flow cell volume), unless otherwise specified.
2.5. Membrane Insertion for Detection of LAM in Patient Serum
The essential components of a membrane insertion assay for LAM have been described before. In all experiments, the waveguide-associated background, coupling efficiency (typically 40-50%, incident power 440 μW) and the non-specific (NS) interactions were determined. For NS measurements, the signal associated with control human serum (1:10 dilution, 15 min, RT), followed by the reporter antibody (anti-LAM-AF647, 100 nM, 5 min, RT) was measured. Subsequently, LAM (spiked in human serum) was added to the flow cell (15 min, RT), followed by the reporter antibody (100 nM, 5 min, RT) and the interaction measured on the spectrometer interface. NS signal was subtracted from this measurement to yield the ‘specific signal’ for LAM. A standard curve was established using the specific signal measured when LAM (at varying concentrations) was spiked in serum immediately before testing (t = 0). For the time course, the samples were tested immediately (0) or at different time points (4, 7 hrs.).
To determine the concentration of monomeric LAM in patient serum, samples were thawed, diluted 4X and immediately added to a functionalized lipid bilayer. The specific signal measured was extrapolated against the standard curve for LAM by membrane insertion to determine the concentration of the biomarker in the sample. All experiments were repeated 2-3X for valid
2.6. Apolipoprotein A1 Sandwich Immunoassays
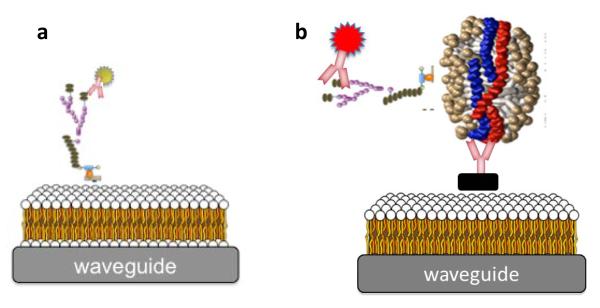
To evaluate whether HDL nanodiscs were likely carrier molecules for serum LAM, we designed a sandwich immunoassay using anti-ApoAI (recognizes coat-protein Apolipoprotein A1 on HDL nanodiscs) as a capture antibody, and AF647 labeled polyclonal rabbit Anti-LAM as a reporter antibody. Fig 1b depicts the sandwich immunoassay (HDL capture assay). LAM was spiked in control human serum and evaluated at 0, 6 and 16-24 h by both membrane insertion and HDL capture assay.
Figure 1.
Schematic representation (not to scale) of the membrane insertion approach for the detection of LAM (a) and the sandwich immunoassay for evaluating association of LAM with HDL (b) on the waveguide-based optical biosensor.
For the HDL capture assay, SAM functionalized waveguides with 0.1% surface biotin were used. Streptavidin (10 nM, 10 min RT) was added, followed by 100 nM of biotinylated anti-ApoA1 antibody (10 min, RT). NS interactions were determined by the addition by the reporter antibody (anti-LAM-AF647, 20 nM). Specific detection was measured by the addition of LAM in human serum (1 h, RT), followed by the reporter antibody (15 min, RT). The fluorescence signal associated with the binding of the biomarker to the reporter was measured.
To evaluate the reliability of these results, insertion with delipidated LAM was evaluated. Also, time course experiments with intact LAM were performed in lipid free serum (with no HDL). The absence of HDL nanodiscs was confirmed in commercial delipidated serum by evaluating binding of anti-ApoA1 antibody by immunoblot analysis.
3. Results
3.1. Membrane Insertion for Detection of LAM in Patient Serum
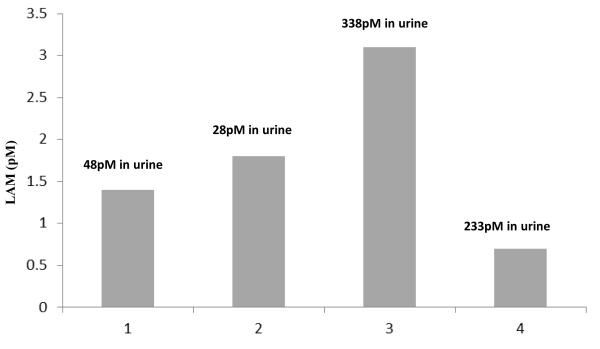
We evaluated monomeric LAM in sera from 8 tuberculosis patients by membrane insertion, and extrapolated the concentrations against a standard curve generated using the purified antigen (19 KDa, H37Rv, mannose-capped LAM). All eight patients were previously shown to have measurable concentrations of LAM in urine. Detectable concentrations of LAM were found only in four of these patients (1,2 3, 4). Fig 2 shows the extrapolated serum concentrations of monomeric LAM in these patients as measured by membrane insertion (Fig. 1a) in four patients to be 1.3 pM, 1.8 pM, 3.1 pM and 0.8 pM. The concentration of urinary LAM from the same patients (previously published data) was 48, 28, 338 and 233 pM respectively8, at least an order of magnitude greater than that observed in serum.
Figure 2.
Detection of circulating concentrations of monomeric LAM in serum from patients with tuberculosis. Only individuals with positive serum concentrations (4/8) are shown. The measured concentrations of urinary LAM from the same patients are indicated in the text boxes.
3.2. Time Dependent Decrease in Serum LAM (spiked samples)
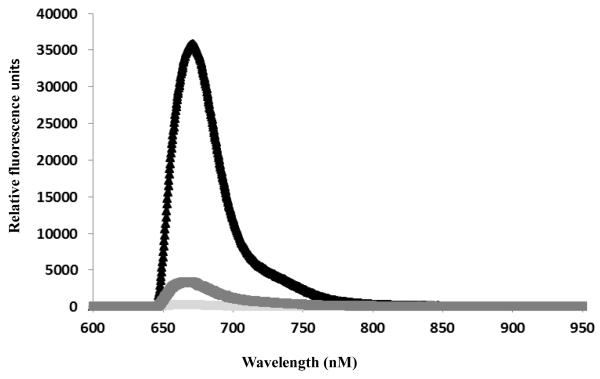
Fig 3 demonstrates the specific signal associated with 5 μM of LAM in human serum, as measured by membrane insertion, at 0, 6 and 24 h. after spiking. We attempted this experiment with lower (100 pM, 1nM) concentrations of LAM as well (data not shown) but free LAM was undetectable within 4 hours, and therefore, we repeated the experiment with 5 mM LAM, which is shown here. The specific signal associated with 5 μM LAM at 0 hrs. was 35778 RFU. At 6 hrs., the signal decreases to 3388 RFU, a tenth of the original measurement, whereas the NS measurement in serum (< 500 RFU) remains unchanged. LAM spiked in serum is undetectable after overnight incubation (> 16 hrs. below NS signal).
Figure 3.
Decrease in the specific signal for LAM over time in spiked serum by membrane insertion assay. Black triangles and black grey line indicate positive signal for detection of the antigen in the serum of LAM spiked human serum incubated for 0 and 6 h. LAM was undetectable after overnight (>16 hrs.) incubation. Light gray line indicated the measure of non-specific binding of the reporter antibody with the control serum.
Table 1 summarizes the signal over noise measured with 100 pM LAM in delipidated serum, as well as the lack of membrane insertion in 100 pM of delipidated LAM. The specific signal associated with the biomarker remains unchanged at 0, 6 and 24 hrs. of incubation in lipoprotein-free serum. Also, insertion was not detected with even 100 pM of delipidated LAM.
Table 1.
Lack of association of LAM with delipidated (HDL-free) serum. Spiking 100 pM of LAM into delipidated serum does not result in any decrease in signal/background, measured by membrane insertion after 12 hrs. As shown, a student T-test demonstrates the absence of significant differences in measurements (waveguide background, NS binding and specific signal) between 0 and 12 hrs.
| Measurement | 0hrs | 12hrs | Student T-test | ||
|---|---|---|---|---|---|
|
| |||||
| Wg Background | 40 | 30 | 35 | 35 | 1 |
| NS Singal | 260 | 280 | 280 | 290 | 0.3 |
| Specific Signal | 465 | 430 | 400 | 560 | 0.7 |
3.2.Pull-down and Detection of LAM associated with Serum HDL
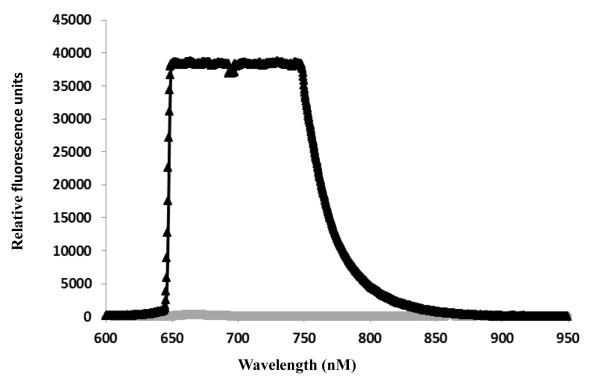
Fig 4 demonstrates the pull-down of HDL particles in serum using anti-Apo-A1 as the capture antibody (Fig. 1b). This approach detects LAM associated with HDL by using the anti-LAM antibody as the fluorescence reporter. When this sandwich assay strategy was evaluated on LAM spiked into human serum and incubated for 6 hrs. a saturating specific signal was observed (Fig 4) demonstrating the recovery of lost LAM using this approach. The NS binding associated with this experiment is 800 RFU.
Figure 4.
Detection of LAM ‘lost’ in serum following 6 hrs. of incubation (Fig. 3) by apo-A1 capture strategy to pull down HDL particles on the waveguide surface. The saturating signal measured with 5 mM LAM clearly demonstrates the association of LAM with HDL particles in serum. The gray line indicates NS binding signal associated with control serum (with HDL, without spiked LAM).
4. Discussion
Several investigators have shown that LAM is critical to virulence in M. tuberculosis infection, and that direct detection of this biomarker might be an efficient strategy for accurate diagnosis of the disease. We, and others, have demonstrated the detection of this biomarker in human urine, and its potential application to diagnosis of active infection. However, measurement of serum LAM has proven to be elusive. In 1992, Sada et al demonstrated the presence of LAM in serum using a co-agglutination test that required extensive sample preparation, which had 88% sensitivity in patients with sputum-positive tuberculosis.3 The sensitivity was markedly lower in sputum smear negative and HIV-positive individuals. The reason for the inability to effectively detect serum LAM was, at the time, unclear.
In this manuscript, we attempted to use a sensitive detection strategy (membrane insertion, limit of detection for LAM is 10 fM) to detect free LAM in serum. We found that monomeric, free LAM is barely detectable in serum from patients who had otherwise high concentrations of urinary LAM. Since immunoreactive LAM was detected in urine, chemical modification of serum LAM seemed unlikely. Therefore, we hypothesized that LAM, and other amphiphilic biomarkers, were associated with host carrier molecules in serum thereby reducing the concentration of monomeric form, making their direct detection difficult. One possible candidate for such a carrier molecule is human HDL. This hypothesis is supported by two previous studies. Glatman-Freedman et al evaluated the bio-distribution of LAM in mice and demonstrated that the biomarker was cleared from serum in as little as three hours, and is localized in the liver.17 Indeed, the association of amphiphilic PAMPs with human HDL has been previously suggested for lipopolysaccharide, a molecule with significant similarity to LAM in its chemical properties.18 Levine et al have shown data to support a simple leaflet insertion model for binding and neutralization of lipopolysaccharide by phospholipids on the surface of HDL.19 Pajkrt et al investigated the effect of reconstituted HDL on LPS responsiveness in humans in a double-blinded, randomized, placebo-controlled, crossover study. They concluded that reconstituted HDL might inhibit the toxic effects of LPS in humans by binding and neutralizing the antigen, and also reducing CD24 expression to monocytes.20
Based on the above studies, we evaluated the role of HDL in the binding of LAM, and if this association could be responsible for the poor efficacy for serum diagnostic assays for LAM. Because of the limited availability of patient sera, we performed these experiments on spiked human serum to first validate the hypothesis. Fig 3 shows the rapid decrease in signal associated with free monomeric LAM detection by membrane insertion as a function of time, likely because of the association of the amphiphile with carrier molecules like HDL. This theory is validated by the observation that this decrease in signal is not observed when delipidated LAM is used (Table 1). HDL is but one of the five major groups of lipoproteins in serum, the other four being chylomicrons, low-density lipoproteins, intermediate density lipoproteins and very low density lipoproteins, but it is the primary lipoprotein that is involved in the clearance of lipids from the vascular system. Any role of other lipoproteins in binding or transportation of LAM remains to be investigated, and is not addressed in this study. To investigate the role of HDL in the uptake of amphiphilic LAM, we designed a capture assay (Fig 1b) where HDL nanoparticles in serum were captured on a SAM functionalized waveguide (preventing membrane insertion) using an antibody directed against the coat protein of the nanodisc, ApoA1. Successful detection of LAM associated with HDL using this capture strategy (Fig. 4) demonstrates the association of HDL with LAM in serum. In control experiments, we showed the lack of specific signal (above NS background) in serum that was not spiked with LAM, as well the lack of signal when serum was spiked with delipidated LAM incapable of associating with HDL nanoparticles, as well as the lack of decrease in signal associated with LAM detection in delipidated (HDL-free) serum.
These experiments clearly demonstrate that amphiphilic biomarkers such as LAM associate with lipoprotein carriers like HDL in serum, and are likely available in free monomeric form at reduced concentrations in blood. This might be one of the reasons for the poor success associated with the direct detection of these molecules in serum. Further, association of PAMPs with carrier molecules may affect innate immune recognition by host toll-like receptors, a critical factor in understanding host-pathophysiology. It is significant to note that PAMPs associated with many bacterial pathogens are amphiphilic in nature, and this feature might be key to pattern recognition receptors, and their association with HDL might be a common characteristic of infection and immunity. This observation can be exploited to achieve universal strategies for bacterial biomarker discovery and detection. The combination of membrane insertion assays for free LAM and sandwich assays for LAM associated with HDL nanoparticles offers a useful approach to help determine the distribution of this important biomarker in patient samples. The same approach could also be useful for other amphiphilic biomarkers, including bacterial PAMPs. In future experiments we hope to evaluate bio-distribution of LAM in serum and urine in longitudinal samples by using three different techniques: sandwich immunoassays, membrane insertion and HDL capture, to effectively compute the relative concentrations of the biomarker and determine its form (as a monomer or associated with lipidic molecules or particles). Quantitative measurement of monomeric and trapped LAM can perhaps provide us with not just bio-distribution data for a significant biomarker, but be developed as an indicator of bacterial load.
Acknowledgements
We thank Mr. K. W. Grace for help in waveguide instrumentation and Mr. A. S. Anderson for SAM chemistry and technical help. We thank the Colorado State University (BEI Resources, operated by the NIAID) for purified LAM and antibodies used in this study. The work was supported by a Department of Energy and Los Alamos National Laboratory LDRD Directed Research Award to Drs. B.T. Korber and B.I. Swanson, and (in part) by the Intramural Research Program of the NIAID, NIH.
References
- 1.Kaur D, Guerin ME, Skovierova H, Brennan PJ, Jackson M. Chapter 2: Biogenesis of the cell wall and other glycoconjugates of Mycobacterium tuberculosis. Advances in applied microbiology. 2009;69:23–78. doi: 10.1016/S0065-2164(09)69002-X. doi: 10.1016/S0065-2164(09)69002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mishra AK, Driessen NN, Appelmelk BJ, Besra GS. Lipoarabinomannan and related glycoconjugates: structure, biogenesis and role in Mycobacterium tuberculosis physiology and host-pathogen interaction. FEMS microbiology reviews. 2011;35:1126–1157. doi: 10.1111/j.1574-6976.2011.00276.x. doi: 10.1111/j.1574-6976.2011.00276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sada E, Aguilar D, Torres M, Herrera T. Detection of lipoarabinomannan as a diagnostic test for tuberculosis. Journal of clinical microbiology. 1992;30:2415–2418. doi: 10.1128/jcm.30.9.2415-2418.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reither K, Saathoff E, Jung J, Minja LT, Kroidl I, Saad E, Huggett JF, Ntinginya EN, Maganga L, Maboko L, Hoelscher M. Low sensitivity of a urine LAM-ELISA in the diagnosis of pulmonary tuberculosis. BMC infectious diseases. 2009;9:141. doi: 10.1186/1471-2334-9-141. doi: 10.1186/1471-2334-9-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Achkar JM, Lawn SD, Moosa MY, Wright CA, Kasprowicz VO. Adjunctive tests for diagnosis of tuberculosis: serology, ELISPOT for site-specific lymphocytes, urinary lipoarabinomannan, string test, and fine needle aspiration. The Journal of infectious diseases. 2011;204(Suppl 4):S1130–1141. doi: 10.1093/infdis/jir450. doi: 10.1093/infdis/jir450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dheda K, Davids V, Lenders L, Roberts T, Meldau R, Ling D, Brunet L, van Zyl Smit R, Peter J, Green C, Badri M, Sechi L, Sharma S, Hoelscher M, Dawson R, Whitelaw A, Blackburn J, Pai M, Zumla A. Clinical utility of a commercial LAM-ELISA assay for TB diagnosis in HIV-infected patients using urine and sputum samples. PloS one. 2010;5:e9848. doi: 10.1371/journal.pone.0009848. doi: 10.1371/journal.pone.0009848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel VB, Singh R, Connolly C, Kasprowicz V, Zumla A, Ndungu T, Dheda K. Comparison of a clinical prediction rule and a LAM antigen-detection assay for the rapid diagnosis of TBM in a high HIV prevalence setting. PloS one. 2010;5:e15664. doi: 10.1371/journal.pone.0015664. doi: 10.1371/journal.pone.0015664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mukundan H, Kumar S, Price DN, Ray SM, Lee YJ, Min S, Eum S, Kubicek-Sutherland J, Resnick JM, Grace WK, Anderson AS, Hwang SH, Cho SN, Via LE, Barry C, 3rd, Sakamuri R, Swanson BI. Rapid detection of Mycobacterium tuberculosis biomarkers in a sandwich immunoassay format using a waveguide-based optical biosensor. Tuberculosis. 2012;92:407–416. doi: 10.1016/j.tube.2012.05.009. doi: 10.1016/j.tube.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wood R, Racow K, Bekker LG, Middelkoop K, Vogt M, Kreiswirth BN, Lawn SD. Lipoarabinomannan in urine during tuberculosis treatment: association with host and pathogen factors and mycobacteriuria. BMC infectious diseases. 2012;12:47. doi: 10.1186/1471-2334-12-47. doi: 10.1186/1471-2334-12-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peter JG, Theron G, Muchinga TE, Govender U, Dheda K. The Diagnostic Accuracy of Urine-Based Xpert MTB/RIF in HIV-Infected Hospitalized Patients Who Are Smear-Negative or Sputum Scarce. PloS one. 2012;7:e39966. doi: 10.1371/journal.pone.0039966. doi: 10.1371/journal.pone.0039966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mukundan H, Price DN, Goertz M, Parthasarathi R, Montano GA, Kumar S, Scholfield MR, Anderson AS, Gnanakaran S, Iyer S, Schmidt J, Swanson BI. Understanding the interaction of Lipoarabinomannan with membrane mimetic architectures. Tuberculosis. 2012;92:38–47. doi: 10.1016/j.tube.2011.09.006. doi: 10.1016/j.tube.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Martinez JS, Grace WK, Grace KM, Hartman N, Swanson BI. Pathogen detection using single mode planar optical waveguides. J Mater Chem. 2005;15:4639–4647. doi: 10.1039/B502329G. [Google Scholar]
- 13.Mukundan H, Anderson AS, Grace WK, Grace KM, Hartman N, Martinez JS, Swanson BI. Waveguide-based Sensors for pathogen detection. Sensors. 2009;9:5783–5809. doi: 10.3390/s90705783. doi: 10.3390/s90705783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mukundan H, Holt A, Shively JE, Martinez JS, Grace K, Grace WK, Swanson BI. Planar optical waveguides for the quantitative detection of tumor markers. Sensors & Actuators B. 2009;138:453–460. [Google Scholar]
- 15.Anderson AS, Dattelbaum AM, Montano GA, Price DN, Schmidt JG, Martinez JS, Grace WK, Grace KM, Swanson BI. Functional PEG-modified thin films for biological detection. Langmuir : the ACS journal of surfaces and colloids. 2008;24:2240–2247. doi: 10.1021/la7033438. doi: 10.1021/la7033438. [DOI] [PubMed] [Google Scholar]
- 16.Peter J, Green C, Hoelscher M, Mwaba P, Zumla A, Dheda K. Urine for the diagnosis of tuberculosis: current approaches, clinical applicability, and new developments. Current opinion in pulmonary medicine. 2010;16:262–270. doi: 10.1097/MCP.0b013e328337f23a. doi: 10.1097/MCP.0b013e328337f23a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glatman-Freedman A, Mednick AJ, Lendvai N, Casadevall A. Clearance and organ distribution of Mycobacterium tuberculosis lipoarabinomannan (LAM) in the presence and absence of LAM-binding immunoglobulin M. Infection and immunity. 2000;68:335–341. doi: 10.1128/iai.68.1.335-341.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Amersfoort ES, Van Berkel TJ, Kuiper J. Receptors, mediators, and mechanisms involved in bacterial sepsis and septic shock. Clinical microbiology reviews. 2003;16:379–414. doi: 10.1128/CMR.16.3.379-414.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levine DM, Parker TS, Donnelly TM, Walsh A, Rubin AL. In vivo protection against endotoxin by plasma high density lipoprotein. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:12040–12044. doi: 10.1073/pnas.90.24.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pajkrt D, Doran JE, Koster F, Lerch PG, Arnet B, van der Poll T, ten Cate JW, van Deventer SJ. Antiinflammatory effects of reconstituted high-density lipoprotein during human endotoxemia. The Journal of experimental medicine. 1996;184:1601–1608. doi: 10.1084/jem.184.5.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]