Abstract
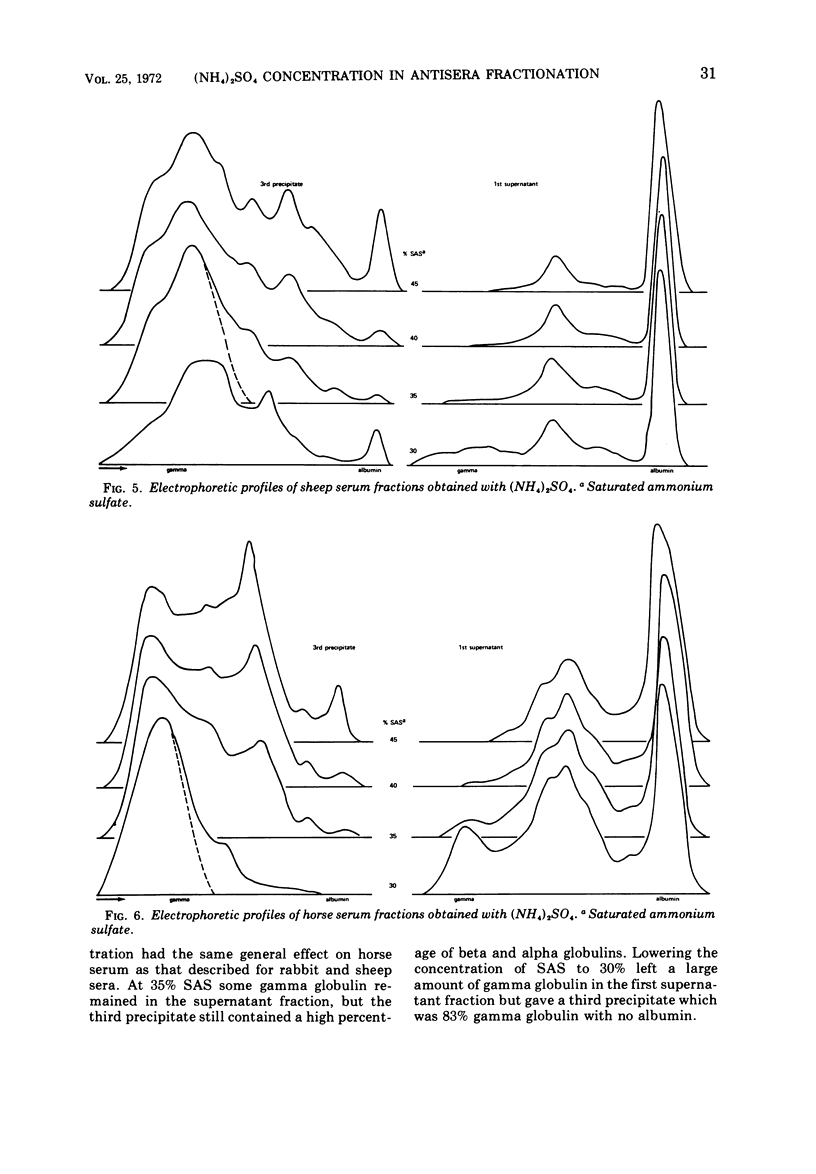
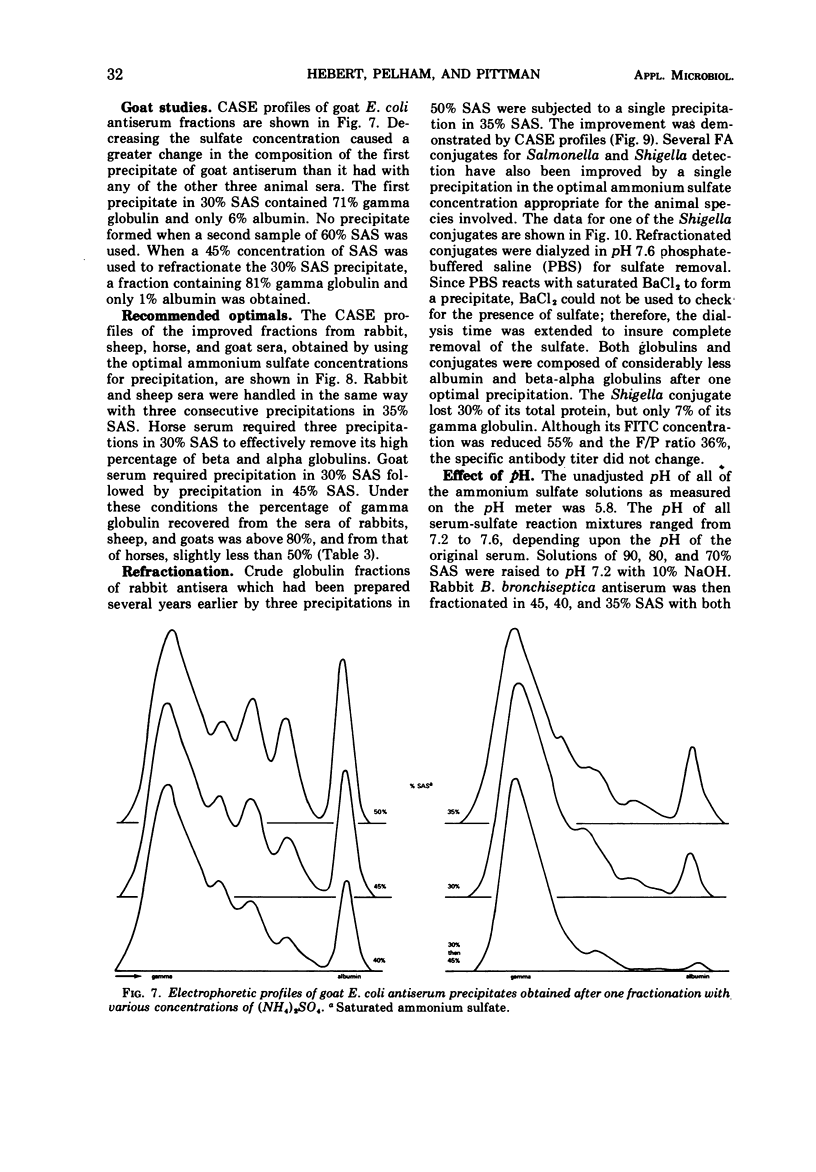
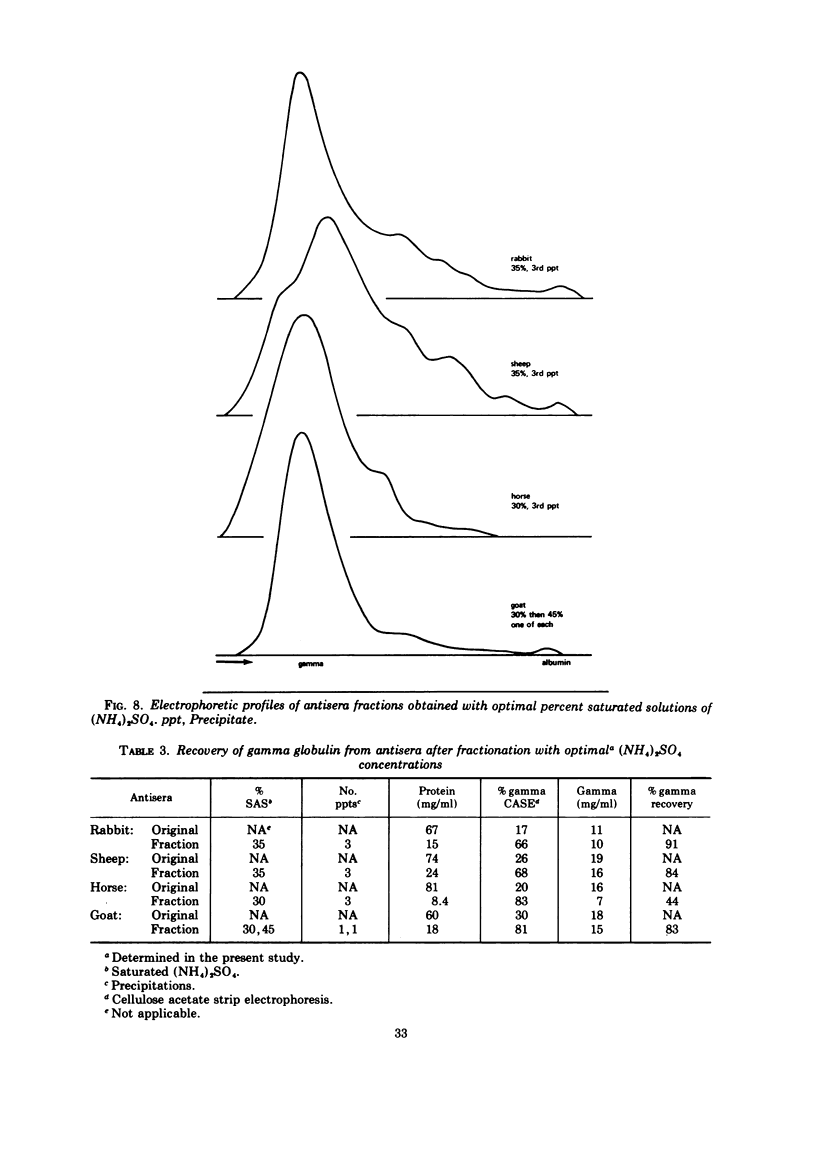
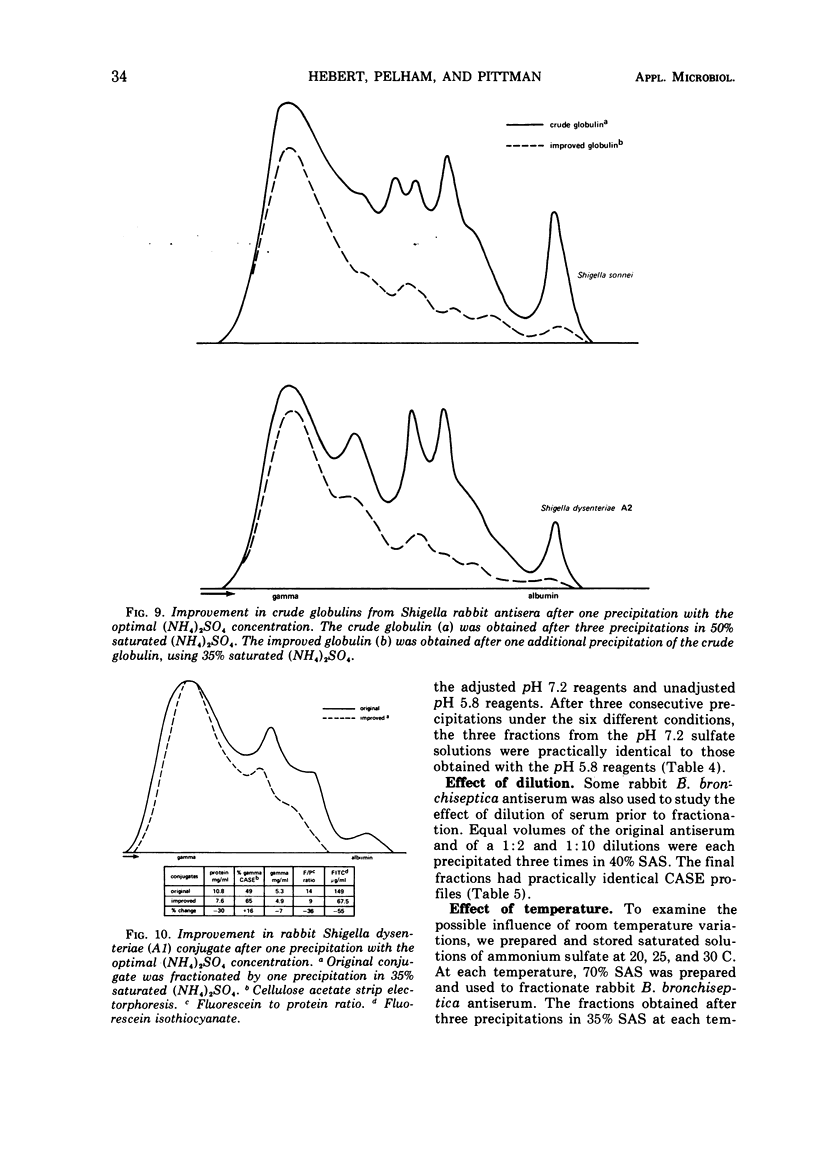
Various ammonium sulfate concentrations and reaction conditions were employed in the fractionation of sera from rabbits, sheep, horses, and goats. Precipitates and supernatant fluids were analyzed by electrophoresis to study the effects of the controlled variables. At room temperature, the third precipitate in 35% saturated (NH4)2SO4 was the best fraction from both rabbit and sheep sera; 80 to 90% of the gamma globulins were recovered. The second and third precipitates of horse sera proteins in 30% saturated (NH4)2SO4 were both satisfactory, but only 44% of the gamma globulin was recovered after three precipitations. Goat sera yielded a very satisfactory fraction; 80% of the gamma globulin was recovered after two precipitations—the first in 30% and the second in 45% saturated (NH4)2SO4. The composition of these fractions was not influenced by the pH of the sulfate solutions (pH 5.8 and 7.2), by a range of normal room temperatures (20 to 30 C), or by diluting the sera before fractionation. Crude globulins and fluorescein isothiocyanate-labeled globulins were successfully refractionated by one precipitation in the optimal sulfate concentration for the appropriate animal species. The refractionated products contained considerably less beta and alpha globulins than did the original crude fractions and little or no albumin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- HEBERT G. A., PITTMAN B. FACTORS AFFECTING REMOVAL OF (NH4)2SO4 FROM SALT FRACTIONATED SERUM GLOBULINS EMPLOYING A SPECTROPHOTMETRIC PROCEDURE FOR DETERMINATION OF SULFATE. Health Lab Sci. 1965 Jan;2:48–53. [PubMed] [Google Scholar]
- Hebert G. A., Pittman B., Cherry W. B. Factors affecting the degree of nonspecific staining given by fluorescein isothiocyanate labelled globulins. J Immunol. 1967 Jun;98(6):1204–1212. [PubMed] [Google Scholar]
- Hebert G. A., Pittman B., Cherry W. B. The definition and application of evaluation techniques as a guide for the improvement of fluorescent antibody reagents. Ann N Y Acad Sci. 1971 Jun 21;177:54–69. doi: 10.1111/j.1749-6632.1971.tb35033.x. [DOI] [PubMed] [Google Scholar]
- KAUFMAN L., CHERRY W. B. Technical factors affecting the preparation of fluorescent antibody reagents. J Immunol. 1961 Jul;87:72–79. [PubMed] [Google Scholar]
- MCKINNEY R. M., SPILLANE J. T., PEARCE G. W. FACTORS AFFECTING THE RATE OF REACTION OF FLUORESCEIN ISOTHIOCYANATE WITH SERUM PROTEINS. J Immunol. 1964 Aug;93:232–242. [PubMed] [Google Scholar]
- MCKINNEY R. M., SPILLANE J. T., PEARCE G. W. FLUORESCEIN DIACETATE AS A REFERENCE COLOR STANDARD IN FLUORESCENT ANTIBODY STUDIES. Anal Biochem. 1964 Dec;9:474–476. doi: 10.1016/0003-2697(64)90208-8. [DOI] [PubMed] [Google Scholar]
- THOMASON B. M., COWART G. S., CHERRY W. B. CURRENT STATUS OF IMMUNOFLUORESCENCE TECHNIQUES FOR RAPID DETECTION OF SHIGELLAE IN FECAL SPECIMENS. Appl Microbiol. 1965 Jul;13:605–613. doi: 10.1128/am.13.4.605-613.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason B. M., Wells J. G. Preparation and testing of polyvalent conjugates for fluorescent-antibody detection of salmonellae. Appl Microbiol. 1971 Nov;22(5):876–884. doi: 10.1128/am.22.5.876-884.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]


