Abstract
MicroRNA-155 (miR-155) is expressed in many cancers. It also executes evolutionary conserved functions in normal B cell development. We show that the Kaposi's sarcoma-associated herpesvirus (KSHV) latency locus, which contains an ortholog of miR-155, miR-K12-11, complements B cell deficiencies in miR-155 knockout mice. Germinal center (GC) formation was rescued in spleen, lymph node, and Peyer's patches. Immunoglobulin levels were restored. This demonstrates that KSHV can complement the normal, physiological function of miR-155.
TEXT
Like other herpesviruses, Kaposi's sarcoma-associated herpesvirus (KSHV) employs two life cycles: lytic and latent. During viral latency, a small set of viral genes are expressed, including those encoding kaposin, viral Fas-associated death domain interleukin-1β-converting enzyme inhibitory protein (vFLIP), viral cyclin (vCYC), latency-associated nuclear antigen (LANA), and 12 pre-microRNAs (miRNAs), called miR-K12-1 to miR-K12-12, which give rise to up to 24 mature miRNAs (1, 2). These genes form the KSHV latency locus. They share a promoter and are constitutively expressed in all KSHV-infected cells (3–6). We recently showed that transgenic expression of the KSHV latency locus in mice augmented B cell response to thymus-dependent (TD) antigen to induce chronic marginal zone (MZ) expansion, plasma cell hyperplasia, hyperglobulinemia, and lymphoma (7).
KSHV miR-K12-11, which was part of the transgene, is an ortholog to miR-155 (8, 9). Human miR-155 is downregulated in KSHV-associated lymphomas, but the viral ortholog, miR-K12-11, is highly expressed (6, 9). miR-155 is transcribed from the non-protein-coding region of the B cell integration cluster (bic) gene (10, 11). It is expressed in Hodgkin's lymphoma, primary mediastinal B cell lymphoma, and diffuse large B cell lymphoma (10, 12, 13); miR-155 overexpression in mice led to B cell lymphoma (14). Ectopic expression of either miR-155 or miR-K12-11 in CD34 hematopoietic stem cells led to increased B cell expansion in vivo (15, 16). Conversely lack of miR-155 compromised B and T cell function, such as germinal center (GC) development (17, 18) and reduced B cell response to TD and T cell-independent antigens (19). All lymphotropic herpesviruses seem to rely on miR-155 functions. Marek's disease virus (MDV) miR-M4 also functions as an ortholog to miR-155 and is required for lymphomagenesis in chicken (20, 21). Epstein-Barr virus (EBV) induces miR-155 (22–25). This suggests that the herpesvirus miR-155 dependency coevolved and is conserved across viruses and across host species.
To examine if KSHV latency genes could overcome miR-155 deficiency in normal B cell function, the KSHV latency locus transgenic mice were crossed to miR-155 knockout (miR-155ko) mice (strain B6.Cg-Mirn155tm1.1Rsky/J) (18). Human and mouse miR-155 are highly similar beyond the seed region and show identical end-maturation patterns (Fig. 1A). The genotype of the miR-155 gene was ascertained by PCR (Fig. 1B). The absence of miR-155 in miR-155ko mice was confirmed by TaqMan assay (catalog no. 4427975; Life Technologies) (Fig. 1C and D).
Fig 1.
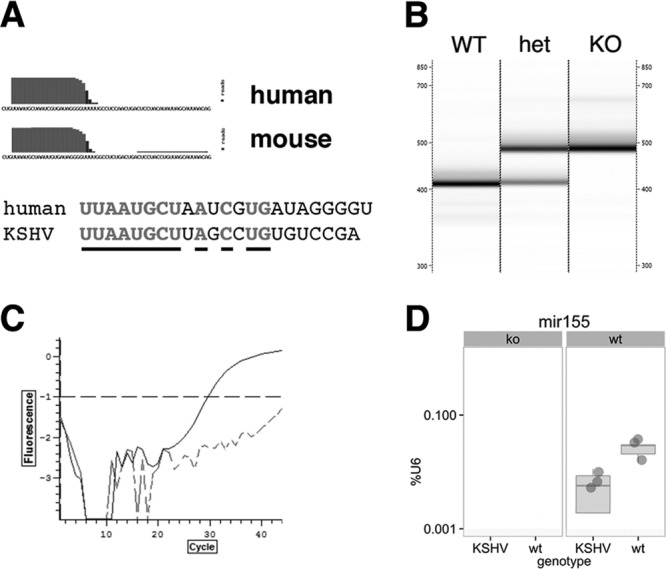
Generation of KSHV latency locus transgenic mice without miR-155. (A) Sequence of human and mouse miR-155. Overlaid is the deep sequencing coverage at each nucleotide position. This represents a new feature in mirBase version 19, which integrates sequence coverage from multiple, previously published RNAseq experiments. Underlined are identical residues. (B) Genotyping was done using qPCR, and PCR products were visualized via the LabChip system (Caliper). WT, KSHV latency locus transgenic mouse; het, KSHV latency locus transgene+/− miR-155+/− mouse; KO, miR-155−/− mouse. Shown is a representative of 3 independent experiments. (C) Expression of mature miR-155 was confirmed by qPCR-based TaqMan assay (catalog no. 4427975; Life Technologies). A dotted or solid line represents an miR-155−/− or an miR-155+/+ KSHV latency locus transgenic mouse. (D) Quantitative analysis of miR-155. The expression level is expressed as percentage of U6 in log10 scale on the vertical axis and genotype for KSHV transgene on the vertical axis. The miR-155 genotype is shown on top of each subpanel. Shown are individual data points in dark gray and box-whisker plots in gray. The line indicated median expression. The limit of detection was 0.001% of U6 levels.
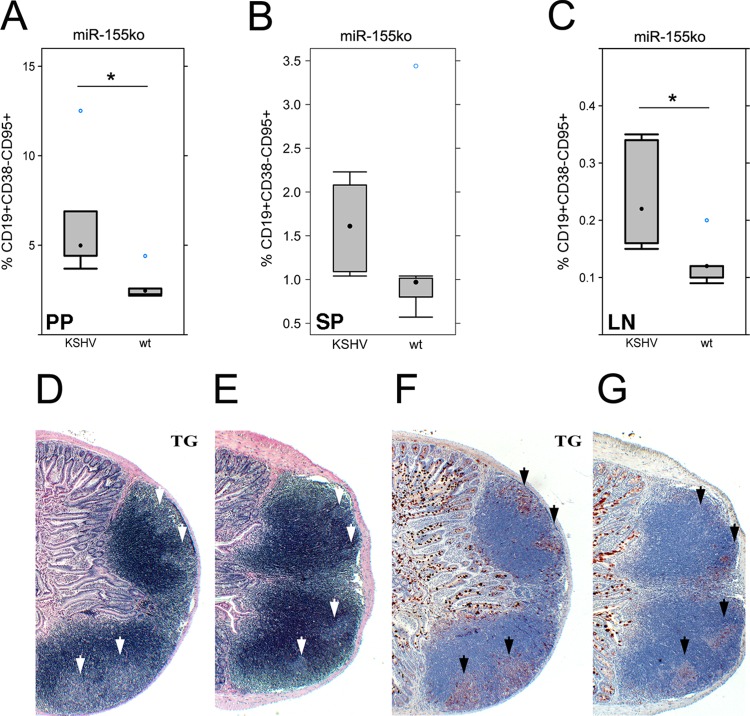
miR-155ko mice show reduced GC B cells in gut-associated lymphoid tissue (GALT), including in Peyer's patches (PP) and in mesenteric lymph nodes (mLN) but not in spleen (SP) (18). The GC B cells were analyzed using flow cytometry. The number of GC B cells (CD19+ CD38− CD95+) in PP and LN was restored to wild-type (wt) levels in KSHV latency locus transgenic mice × miR-155ko mice compared to miR-155ko mice not carrying the KSHV transgene (Fig. 2A and C). This phenotype was confirmed by histology (Fig. 2D and E). The GC area of PP in KSHV × miR-155ko mice was larger than that of KSHV-negative miR-155ko mice. Peanut agglutinin (PNA) is a well-validated marker for activated GC (26–28). PNA+ GC B cells were increased in the PP of KSHV × miR-155ko mice compared to in wt × miR-155ko mice (Fig. 2F and G). The fraction of GC B cells (CD19+ CD38− CD95+) in SP was increased in KSHV transgenic mice × miR-155ko animals in response to a single challenge with a T-dependent antigen, (4-hydroxy-3-nitrophenyl)acetyl-chicken γ-globulin (NP-CGG), compared to wt × miR-155ko mice (Fig. 2B).
Fig 2.
Rescue of miR-155 deficiency-induced phenotypes by the KSHV latency locus. Lymphocytes from KSHV latency locus transgenic mice on the miR-155ko background (n = 6) were subjected to flow cytometry analysis. miR-155ko mice (n = 7) were used as a control. The percentage of GC B cells (CD19+ CD38− CD95+) was plotted from PP (A), spleen (B), and mLN (C). *, P ≤ 0.05. (C) KSHV latency locus transgenic mice on the miR-155ko background (n = 6) and miR-155ko mice (n = 6) were immunized intraperitoneally (i.p.) with 200 μg NP-CGG (Biosearch Technologies) per mouse. Percentage of SP GC B cells (CD19+ CD38− CD95+) was determined on day 28 by flow cytometry. *, P ≤ 0.05. All flow cytometry data were acquired on CyAn (Beckman Coulter) and analyzed using FlowJo (version 7.6.5; Tree Star). Two-tailed Student's t tests were used for statistical analysis. General architecture of PP revealed by H&E staining was shown in the transgenic (D) and wild-type (E) mice on the miR-155ko background. White arrows indicate GC area. PNA+ GC B cells (black arrows) were shown in the transgenic (F) and wild-type (G) mice on the miR-155ko background. Formalin-fixed, paraffin-embedded tissue sections were stained with PNA (Vector Laboratories). Black arrows indicate PNA+ GC B cells. Magnification, ×40. TG means KSHV latency transgenic mice on the miR-155ko background.
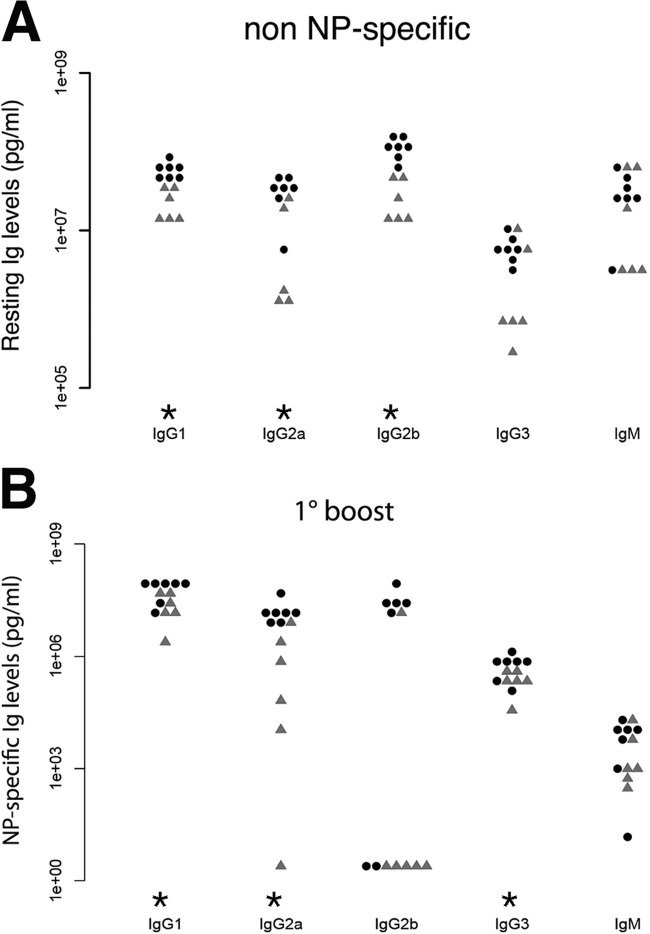
B cells from miR-155ko animals show a lower production of low- and high-affinity IgG1 antibodies (19) than that of wt animals. To examine if the KSHV latency locus could rescue this phenotype, the KSHV × miR-155ko animals were immunized with a TD antigen, (4-hydroxy-3-nitrophenyl)acetyl-keyhole limpet hemocyanin (NP-KLH), and boosted on days 21 and 42 to induce affinity maturation and class switching. Non-NP-specific immunoglobulin (Ig) levels in KSHV × miR-155ko mice were always higher than in miR-155ko mice, even in the absence of specific immunization (Fig. 3A), indicating that the KSHV-induced hyperglobulinemia (7) does not require miR-155. The KSHV × miR-155ko mice exhibited higher NP-specific IgG responses than KSHV-negative miR-155ko mice, indicating that the KSHV latency locus rescued miR-155 deficiency in antigen-dependent B cell differentiation (Fig. 3B). This represents the first in vivo evidence that viral latent genes can fulfill the normal function of miR-155 in normal B cell development.
Fig 3.
KSHV latency locus complements reduced immunoglobulin levels in miR-155ko mice. KSHV latency locus transgenic mice with the miR-155ko background (n = 7) and miR-155ko mice (n = 6) were immunized i.p. with 100 μg alum-precipitated NP-KLH and boosted on day 21. Serum was collected before immunization and on day 25. Levels of non-NP-specific (resting) and NP-specific Ig isotypes were determined using unlabeled anti-mouse Ig antibodies (Southern Biotech) or NP34-bovine serum albumin (Biosearch Technologies). Alkaline phosphatase-labeled antibodies and p-nitrophenyl phosphate were used for detection (all from Southern Biotech). (A) Levels of resting non-NP-specific immunoglobulins of the KSHV transgenic × miR-155ko mice (black dots, n = 7) and miR-155ko (gray triangles, n = 6) were plotted, respectively. (B) Levels of NP-specific immunoglobulins of the KSHV transgenic × miR-155ko mice (black dots, n = 7) and miR-155ko (gray triangles, n = 6) were analyzed 5 days after the first boost with NP-KLH and plotted. *, P ≤ 0.05.
There are some limitations to this experiment. One would not expect the single KSHV miRNA ortholog to complement all immunological defects associated with lack of miR-155 in vivo. For instance, miR-155 is also required for the function of T cells (17, 18). KSHV does not normally infect T cells (30, 31), and in this transgenic mouse the KSHV transgenes were not expressed in T cells (26, 29). Some miR-155 targets are nonoverlapping with miR-K12-11 and may require recognition sites beyond the seed sequence (1, 2, 9); others are conserved among mice, virus, and humans and so are the miR-155-dependent physiological consequences (9, 32). Both KSHV miR-K12-11 and miR-155 are present at low levels in mice, while the oncogenic phenotypes of miR-155 and of miR-K12-11 were most evident in ectopic expression experiments, which mimic elevated miR-155 and miR-K12-11 levels in lymphoma cells (9, 15, 16, 23). Our transgenic model (and the miR-155ko phenotype) reflects the preneoplastic, long-term latency of KSHV in normal B cells. Lastly, this transgenic mouse model expresses all KSHV miRNAs as well as latent viral proteins, which in conjunction complemented the miR-155 deficiency. Studies are under way to understand the individual contributions of each viral miRNA to the B cell phenotype.
Taken together, our data indicate that the KSHV latency locus restores B cell differentiation defects associated with the lack of miR-155 in normal, precancerous B cells and allow B cells to survive and expand in the absence of miR-155. Since miR-155 is required for GC development, one could speculate that by providing the miR-155 orthology (as well as additional functionalities), KSHV latently drives infected B cells into the GC reaction and beyond.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants CA109232 and CA019014 from the National Cancer Institute.
Footnotes
Published ahead of print 21 August 2013
REFERENCES
- 1.Gottwein E, Corcoran DL, Mukherjee N, Skalsky RL, Hafner M, Nusbaum JD, Shamulailatpam P, Love CL, Dave SS, Tuschl T, Ohler U, Cullen BR. 2011. Viral microRNA targetome of KSHV-infected primary effusion lymphoma cell lines. Cell Host Microbe 10:515–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haecker I, Gay LA, Yang Y, Hu J, Morse AM, McIntyre LM, Renne R. 2012. Ago HITS-CLIP expands understanding of Kaposi's sarcoma-associated herpesvirus miRNA function in primary effusion lymphomas. PLoS Pathog. 8:e1002884. 10.1371/journal.ppat.1002884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dittmer D, Lagunoff M, Renne R, Staskus K, Haase A, Ganem D. 1998. A cluster of latently expressed genes in Kaposi's sarcoma-associated herpesvirus. J. Virol. 72:8309–8315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fakhari FD, Dittmer DP. 2002. Charting latency transcripts in Kaposi's sarcoma-associated herpesvirus by whole-genome real-time quantitative PCR. J. Virol. 76:6213–6223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Hara AJ, Vahrson W, Dittmer DP. 2008. Gene alteration and precursor and mature microRNA transcription changes contribute to the miRNA signature of primary effusion lymphoma. Blood 111:2347–2353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Hara AJ, Wang L, Dezube BJ, Harrington WJ, Damania B, Dittmer DP. 2009. Tumor suppressor microRNAs are underrepresented in primary effusion lymphoma and Kaposi sarcoma. Blood 113:5938–5941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sin S-H, Dittmer DP. 2013. Viral latency locus augments B-cell response in vivo to induce chronic marginal zone enlargement, plasma cell hyperplasia, and lymphoma. Blood 121:2952–2963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gottwein E, Mukherjee N, Sachse C, Frenzel C, Majoros WH, Chi J-TA, Braich R, Manoharan M, Soutschek J, Ohler U, Cullen BR. 2007. A viral microRNA functions as an orthologue of cellular miR-155. Nature 450:1096–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skalsky RL, Samols MA, Plaisance KB, Boss IW, Riva A, Lopez MC, Baker HV, Renne R. 2007. Kaposi's sarcoma-associated herpesvirus encodes an ortholog of miR-155. J. Virol. 81:12836–12845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eis PS, Tam W, Sun L, Chadburn A, Li Z, Gomez MF, Lund E, Dahlberg JE. 2005. Accumulation of miR-155 and BIC RNA in human B cell lymphomas. Proc. Natl. Acad. Sci. U. S. A. 102:3627–3632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tam W. 2001. Identification and characterization of human BIC, a gene on chromosome 21 that encodes a noncoding RNA. Gene 274:157–167 [DOI] [PubMed] [Google Scholar]
- 12.Metzler M, Wilda M, Busch K, Viehmann S, Borkhardt A. 2004. High expression of precursor microRNA-155/BIC RNA in children with Burkitt lymphoma. Genes Chromosomes Cancer 39:167–169 [DOI] [PubMed] [Google Scholar]
- 13.van den Berg A, Kroesen B-J, Kooistra K, de Jong D, Briggs J, Blokzijl T, Jacobs S, Kluiver J, Diepstra A, Maggio E, Poppema S. 2003. High expression of B-cell receptor inducible gene BIC in all subtypes of Hodgkin lymphoma. Genes Chromosomes Cancer 37:20–28 [DOI] [PubMed] [Google Scholar]
- 14.Costinean S, Zanesi N, Pekarsky Y, Tili E, Volinia S, Heerema N, Croce CM. 2006. Pre-B cell proliferation and lymphoblastic leukemia/high-grade lymphoma in Eμ-miR155 transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 103:7024–7029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boss IW, Nadeau PE, Abbott JR, Yang Y, Mergia A, Renne R. 2011. A Kaposi's sarcoma-associated herpesvirus-encoded ortholog of microRNA miR-155 induces human splenic B-cell expansion in NOD/LtSz-scid IL2Rγnull mice. J. Virol. 85:9877–9886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dahlke C, Maul K, Christalla T, Walz N, Schult P, Stocking C, Grundhoff A. 2012. A microRNA encoded by Kaposi sarcoma-associated herpesvirus promotes B-cell expansion in vivo. PLoS One 7:e49435. 10.1371/journal.pone.0049435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez A, Vigorito E, Clare S, Warren MV, Couttet P, Soond DR, van Dongen S, Grocock RJ, Das PP, Miska EA, Vetrie D, Okkenhaug K, Enright AJ, Dougan G, Turner M, Bradley A. 2007. Requirement of bic/microRNA-155 for normal immune function. Science 316:608–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thai T-H, Calado DP, Casola S, Ansel KM, Xiao C, Xue Y, Murphy A, Frendewey D, Valenzuela D, Kutok JL, Schmidt-Supprian M, Rajewsky N, Yancopoulos G, Rao A, Rajewsky K. 2007. Regulation of the germinal center response by microRNA-155. Science 316:604–608 [DOI] [PubMed] [Google Scholar]
- 19.Vigorito E, Perks KL, Abreu-Goodger C, Bunting S, Xiang Z, Kohlhaas S, Das PP, Miska EA, Rodriguez A, Bradley A, Smith KGC, Rada C, Enright AJ, Toellner K-M, MacLennan ICM, Turner M. 2007. microRNA-155 regulates the generation of immunoglobulin class-switched plasma cells. Immunity 27:847–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao Y, Xu H, Yao Y, Smith LP, Kgosana L, Green J, Petherbridge L, Baigent SJ, Nair V. 2011. Critical role of the virus-encoded MicroRNA-155 ortholog in the induction of Marek's disease lymphomas. PLoS Pathog. 7:e1001305. 10.1371/journal.ppat.1001305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao Y, Yao Y, Xu H, Lambeth L, Smith LP, Kgosana L, Wang X, Nair V. 2009. A functional microRNA-155 ortholog encoded by the oncogenic Marek's disease virus. J. Virol 83:489–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gatto G, Rossi A, Rossi D, Kroening S, Bonatti S, Mallardo M. 2008. Epstein-Barr virus latent membrane protein 1 trans-activates miR-155 transcription through the NF-κB pathway. Nucleic Acids Res. 36:6608–6619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linnstaedt SD, Gottwein E, Skalsky RL, Luftig MA, Cullen BR. 2010. Virally induced cellular microRNA miR-155 plays a key role in B-cell immortalization by Epstein-Barr virus. J. Virol. 84:11670–11678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu F, Weidmer A, Liu C-G, Volinia S, Croce CM, Lieberman PM. 2008. Epstein-Barr virus-induced miR-155 attenuates NF-κB signaling and stabilizes latent virus persistence. J. Virol. 82:10436–10443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yin Q, McBride J, Fewell C, Lacey M, Wang X, Lin Z, Cameron J, Flemington EK. 2008. MicroRNA-155 is an Epstein-Barr virus-induced gene that modulates Epstein-Barr virus-regulated gene expression pathways. J. Virol. 82:5295–5306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fakhari FD, Jeong JH, Kanan Y, Dittmer DP. 2006. The latency-associated nuclear antigen of Kaposi sarcoma-associated herpesvirus induces B cell hyperplasia and lymphoma. J. Clin. Invest. 116:735–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato S, Steeber D, Tedder T. 1995. The CD19 signal transduction molecule is a response regulator of B-lymphocyte differentiation. Proc. Natl. Acad. Sci. U. S. A. 92:11558–11562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sin S-H, Fakhari FD, Dittmer DP. 2010. The viral latency-associated nuclear antigen augments the B-cell response to antigen in vivo. J. Virol. 84:10653–10660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeong JH, Hines-Boykin R, Ash JD, Dittmer DP. 2002. Tissue specificity of the Kaposi's sarcoma-associated herpesvirus latent nuclear antigen (LANA/orf73) promoter in transgenic mice. J. Virol. 76:11024–11032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dittmer D, Stoddart C, Renne R, Linquist-Stepps V, Moreno ME, Bare C, McCune JM, Ganem D. 1999. Experimental transmission of Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) to SCID-hu Thy/Liv mice. J. Exp. Med. 190:1857–1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hassman LM, Ellison TJ, Kedes DH. 2011. KSHV infects a subset of human tonsillar B cells, driving proliferation and plasmablast differentiation. J. Clin. Invest. 121:752–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qin Z, Freitas E, Sullivan R, Mohan S, Bacelieri R, Branch D, Romano M, Kearney P, Oates J, Plaisance K, Renne R, Kaleeba J, Parsons C. 2010. Upregulation of xCT by KSHV-encoded microRNAs facilitates KSHV dissemination and persistence in an environment of oxidative stress. PLoS Pathog. 6:e1000742. 10.1371/journal.ppat.1000742 [DOI] [PMC free article] [PubMed] [Google Scholar]