Abstract
Human noroviruses are a major cause of food-borne illness, accountable for 50% of all-etiologies outbreaks of acute gastroenteritis (in both developing and developed countries). There is no vaccine or antiviral drug for the prophylaxis or treatment of norovirus-induced gastroenteritis. We recently reported the inhibitory effect of 2′-C-methylcytidine (2CMC), a hepatitis C virus polymerase inhibitor, on the in vitro replication of murine norovirus (MNV). Here we evaluated the inhibitory effect of 2CMC on in vitro human norovirus replication through a Norwalk virus replicon model and in a mouse model by using AG129 mice orally infected with MNV. Survival, weight, and fecal consistency were monitored, and viral loads in stool samples and organs were quantified. Intestines were examined histologically. 2CMC reduced Norwalk virus replicon replication in a dose-dependent manner and was able to clear cells of the replicon. Treatment of MNV-infected AG129 mice with 2CMC (i) prevented norovirus-induced diarrhea; (ii) markedly delayed the appearance of viral RNA and reduced viral RNA titers in the intestine, mesenteric lymph nodes, spleen, lungs, and stool; (iii) completely prevented virus-induced mortality; and (iv) resulted in protective immunity against a rechallenge. We demonstrate for the first time that a small-molecule inhibitor of norovirus replication protects from virus-induced disease and mortality in a relevant animal model. These findings pave the way for the development of potent and safe antivirals as prophylaxis and therapy of norovirus infection.
INTRODUCTION
Human noroviruses are the leading cause of food-borne disease and are responsible for ∼50% of the acute gastroenteritis outbreaks that occur worldwide. Diarrheal diseases are associated with high mortality rates in the developing world, where noroviruses are annually responsible for 200,000 deaths of children under 5 years of age (1). They affect people of all ages, and their importance in children begins to surpass that of rotavirus since vaccination against the latter pathogen becomes more and more widespread (2, 3). Among young children, the elderly, or the immunocompromised, norovirus disease may be severe and prolonged, eventually leading to hospitalization (71,000/year in the United States) and/or evolving into chronic gastroenteritis (4).
The burden of norovirus disease is best understood by adding up the unique features of this enteric pathogen: (i) its highly infectious nature (18 virus particles are sufficient for infection); (5); (ii) shedding in stool for 20 to 40 days, on average (10 times the mean duration of illness), also in asymptomatic individuals; (6, 7); (iii) long persistence in the environment (resistance to disinfectants, heating, and freezing), up to 2 weeks on surfaces; (8); and (iv) its many routes of transmission, including contaminated food and water (at the source or by food handlers), fomites, recreational waters, or aerosolized particles from vomitus or stool. Outbreaks of norovirus gastroenteritis thus occur in semiclosed environments such as long-term care facilities (>50%), schools, restaurants, hospitals, and cruise ships and can scale up to hundreds of sick individuals. Prevention and control of such episodes depend on the use of disinfectants and hand sanitizers to contain virus spreading and electrolyte replenishment for dehydrated individuals. There are no vaccines or antiviral drugs available. Potent and safe antiviral strategies for the treatment and prophylaxis of norovirus infections may help to reduce the severity of disease in infected patients and reduce the chance of transmission and might be used prophylactically to prevent infection during an outbreak.
Since human noroviruses are not cultivatable in vitro, the Norwalk virus (a human genogroup I.1 [GI.1] norovirus) replicon-bearing cell line and the surrogate murine norovirus (MNV) (a GV norovirus) are the available models that can be used to discover antivirals against norovirus (9, 10). It was very recently reported that intraperitoneal infection of BALB/c Rag-yc-deficient mice with human norovirus (GII.4 strain) results in subclinical infection of the mice with the virus (11). The Norwalk virus replicon model was constructed by the insertion of a neomycin phosphotransferase gene into a plasmid containing the Norwalk virus genome, which was then transfected into mammalian cell lines (9). The neomycin gene acts as a selection marker and stops translation. Hence, only the nonstructural proteins (NS1 to NS7) are translated. These transfected cell lines are capable of constitutively replicating the viral RNA genome and expressing the replicative enzymes and other nonstructural proteins of the Norwalk virus (9). MNV replicates in mice, is transmitted by the fecal-oral route after being shed in feces, and produces pathophysiological events comparable to those of norovirus infection in humans (10, 12). Immunocompetent mice show a persistent asymptomatic infection when infected with the majority of MNV strains. However, MNV-1 results in an acute and lethal infection in mice lacking components of innate immunity, such as signal transducer and activator of transcription 1, the recombination-activating gene, or alpha/beta interferon (IFN-α/β) and IFN-γ receptors (13–15). For this study, we used the MNV-1.CW3 strain to infect IFN-α/β and IFN-γ receptor-deficient AG129 mice. These AG129 mice have, among others, been used as a small-animal model for studies of vaccine against the dengue virus (16).
We reported recently that the hepatitis C virus (HCV) nucleoside polymerase inhibitor 2′-C-methylcytidine (2CMC) inhibits the in vitro replication of MNV (17). We report here that 2CMC also inhibits the replication of the Norwalk virus replicon and is able to cure cells of the replicon. We further demonstrated that 2CMC protects AG129 mice against MNV-induced disease and death. To the best of our knowledge, this study constitutes the first evidence that a small-molecule inhibitor of norovirus replication protects (in a relevant animal model) against virus-induced diarrhea and death.
MATERIALS AND METHODS
Cells, viruses, and compound.
2CMC was synthesized as previously described (18). For in vitro assays, a stock solution of 2CMC was prepared in dimethyl sulfoxide. For in vivo assays 2CMC was dissolved in sterile saline. Strain MNV-1.CW3 (kindly provided by Herbert Virgin, Washington University, St. Louis, MO) was propagated in RAW 264.7 cells (ATCC, Barcelona, Spain) grown in Dulbecco's modified Eagle's medium (DMEM; Life Technologies, Ghent, Belgium) supplemented with 10% fetal bovine serum (FBS), 2 mM l-glutamine, 20 mM HEPES, 0.075 g/liter sodium bicarbonate, 1 mM sodium pyruvate, 100 U penicillin/ml, and 100 μg/ml streptomycin at 37°C in a humidified atmosphere of 5% CO2. Virus stocks were generated from their sixth passage in cell culture, and viral titers were determined by endpoint titration. HG23 cells (Norwalk virus replicon-bearing Huh-7 cell origin) (9) were grown in DMEM supplemented with 10% FBS, 2 mM l-glutamine, 20 mM HEPES, 0.075 g/liter sodium bicarbonate, 100 U penicillin/ml, 100 μg/ml streptomycin, and 1.25 mg/ml Geneticin (G418; Life Technologies) at 37°C in a humidified atmosphere of 5% CO2.
Antiviral assay.
The in vitro antiviral activity of 2CMC was evaluated by quantification of intracellular Norwalk virus replicon RNA and the β-actin mRNA reference (housekeeping) gene (by quantitative reverse transcription-PCR [qRT-PCR]).
HG23 cells (5,000/well) were seeded into the wells of 96-well plates in complete DMEM without G418. After 24 h of incubation, serial dilutions of compounds were added. Cells were further incubated for 72 h, after which cell culture supernatant was removed and monolayers were washed with phosphate-buffered saline (PBS). Cell monolayers were collected for RNA load quantification by qRT-PCR. To determine relative levels of Norwalk virus replicon RNA, β-actin was used as a normalizer and ratios were calculated by the Pfaffl method (19). The Norwalk virus replicon/β-actin ratio was calculated as follows: Ratio = ENorwalkΔCT, Norwalk (CC − TC)/Eβ-actinΔCT, β-actin (CC − TC), where ENorwalk and Eβ-actin represent the amplification efficiencies (E = 10−1/slope) of the Norwalk virus replicon and β-actin qRT-PCRs, respectively; ΔCT, Norwalk virus (CC − TC) is the CT (threshold cycle) of untreated control cells (CC) minus the CT of cells treated with a compound concentration (TC) obtained with Norwalk virus replicon primers and probe; and ΔCT, β-actin (CC − TC) is the CT of untreated control cells (CC) minus the CT of cells treated with a compound concentration (TC) obtained with β-actin primers and probe. Efficiency values (ENorwalk and Eβ-actin) were determined for each qRT-PCR. The 50% effective concentration (EC50) and EC90 were defined as the compound concentrations that resulted in 50 and 90% reductions of the relative Norwalk virus replicon RNA levels.
Clearance rebound assay.
HG23 cells were seeded at 2.5 × 105/25-cm2 T flask with complete DMEM and 1.25 mg/ml G418. After 24 h of incubation, the cell culture medium was replaced with complete DMEM without G418 containing either one fixed concentration of 2CMC or no antiviral compound. Selected concentrations of 2CMC (6.25 to 25 μg/ml) were up to 5 times the EC50 but were not cytostatic. When 90% confluence was reached, cultures were trypsinized and (i) 2.5 × 105 cells were seeded into a new 25-cm2 T flask in complete DMEM with the same concentration of compound and (ii) 1.5 × 105 cells from each flask were washed with PBS and collected for quantification of the Norwalk virus replicon and β-actin RNA contents. Cells were passaged a total of six consecutive times in the presence or absence of 2CMC and in the absence of G418 (clearance phase). For Norwalk virus replicon/β-actin ratio calculations, each passage of 2CMC-treated cells was compared to untreated control cells with the same passage number. Every 2 passages (after passages 2, 4, and 6), cells from each flask were passaged three consecutive times in the presence of 1.25 mg/ml G418 and in the absence of the compound (rebound phase). Also here 2.5 × 105 cells were seeded into new 25-cm2 T flasks and 1.5 × 105 cells from each flask were washed with PBS and collected for quantification of Norwalk virus replicon and β-actin RNA contents by qRT-PCR.
Assessment of antiviral activity in an MNV-infected mouse model.
AG129 mice (129/Sv mice deficient in both alpha/beta interferon [IFN-α/β] and IFN-γ receptors) were bred and housed at the Rega Institute under specific-pathogen-free conditions. All experiments were performed under the guidelines and with the authorization of the Ethical Committee of the KU Leuven (P101/2012).
For all experiments, age- and sex-matched mice 6 to 8 weeks of age were infected by oral gavage with 3 × 106 50% cell culture infectious dose (CCID50) of MNV (total volume of 200 μl). Treatment with 2CMC was initiated 1 h before infection with a dose of 100 mg/kg/day divided into two daily treatments (2 × 50 mg/kg) for 7 consecutive days by the subcutaneous route (n = 15). Saline was administered to untreated control mice on the same schedule as 2CMC (n = 12). Treated and untreated mice were kept separate in independently ventilated cages for all of the experiments. Bedding was replaced once a week. Starting at day 0 postinfection (p.i.), mice were weighed daily and stool samples were collected from each animal and scored for consistency (0, normal feces; 1, mixed stool samples containing both solid and pasty feces; 2, pasty feces; 3, semiliquid feces; 4, liquid feces). When animals were severely ill, they were humanely euthanized with pentobarbital (Nembutal). Blood was collected by cardiac puncture. Following extensive transcardial perfusion with PBS, ∼100 to 150 mg of organ tissue (small intestine, proximal colon, mesenteric lymph nodes, spleen, and lung) was either fixed in 4% formaldehyde for later histological examination or collected in 2-ml tubes containing 2.8 mm zirconium oxide beads (Precellys/Bertin Technologies) for quantification of viral RNA by qRT-PCR. To these, RLT lysis buffer (Qiagen, The Netherlands) was added and tissue homogenates were prepared with an automated homogenizer (Precellys24; Bertin Technologies). Homogenates were cleared by centrifugation, and total RNA was extracted from the supernatant. For histological examination, organ tissue was embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E).
RNA isolation and qRT-PCR.
To extract RNA from mouse organs and stool samples, as well as from HG23 cells, the RNeasy minikit (Qiagen) was used according to the manufacturer's protocol. For detection of Norwalk virus replicon RNA, forward (5′-CCG GCT ACC TGC CCA TTC-3′) and reverse (5′-CCA GAT CAT CCT GAT CGA CAA G-3′) primers and a probe (5′–6-carboxyfluorescein–ACA TCG CAT CGA GCG AGC ACG TAC–6-carboxytetramethylrhodamine–3′) for the neomycin gene were used as previously described (20). For detection of β-actin mRNA, forward (5′-GGC ATC CAC GAA ACT ACC TT-3′) and reverse (5′-AGC ACT GTG TTG GCG TAC AG-3′) primers and a probe (5′–6-hexachlorofluorescein–ATC ATG AAG TGT GAC GTG GAC ATC CG–Black Hole Quencher 1–3′) were used as previously described (21). For MNV qRT-PCR, the primers and probe were used as described before (22).
One-step qRT-PCR was performed with a 25-μl reaction mixture containing 12.5 μl of One-Step Reverse Transcriptase qPCR Master Mix Plus Low ROX (Eurogentec), 0.125 μl of an RT-PCR enzyme mixture, 5 μl of template RNA, and 300 nM Norwalk virus replicon primers and probe, 300 nM β-actin primers and 200 nM probe, or 900 nM MNV primers and 200 nM MNV probe. Cycling conditions were RT at 48°C for 30 min, initial denaturation at 95°C for 10 min, and 40 cycles of denaturation at 95°C for 15 s, annealing, and extension at 60°C for 1 min (ABI 7500 Fast Real-Time PCR System; Applied Biosystems). For absolute MNV quantification, standard curves were generated by using 10-fold dilutions of a known concentration of MNV template DNA.
Statistical analysis.
All data were analyzed with Prism 5 software (Graph-Pad Software, San Diego, CA). The D'Agostino and Pearson omnibus normality test was performed. In all graphs, *** indicates a P value of <0.001, ** indicates a P value of <0.01, * indicates a P value of <0.05, and NS indicates no statistically significant difference (P > 0.05). P values were determined with an unpaired t test (with n ≥ 8 and a Gaussian distribution) or a nonparametric Mann-Whitney test (with n < 8 or a non-Gaussian distribution). The one-sample t test was used to compare means to the limit of detection (LOD). All error bars depict the standard errors of the means (SEM). Grubbs' test was used to detect significant outliers (P < 0.01).
RESULTS
2CMC inhibits the in vitro replication of human norovirus.
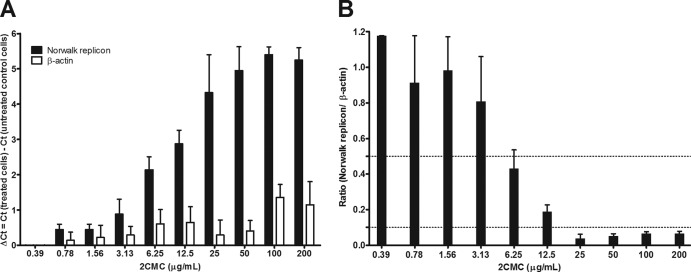
The inhibitory effect of 2CMC on human norovirus replication was evaluated by using the Norwalk virus replicon system. The reduction of Norwalk virus RNA in the presence of 2CMC was quantified by qRT-PCR and normalized to β-actin mRNA levels. 2CMC reduced levels of Norwalk virus RNA in a dose-dependent manner with an EC50 of 4.7 ± 1.0 μg/ml (18 ± 4 μM) and an EC90 of 22 ± 5 μg/ml (84 ± 19 μM) (Fig. 1B). The effect of 2CMC on the host cell was monitored by quantifying the effect of the compound on the housekeeping gene for β-actin. At 2CMC concentrations of up to 100 μg/ml, a ΔCT of <1 was observed with β-actin (Fig. 1A). Hence, 2CMC inhibits the Norwalk virus replicon at concentrations without significant cytotoxicity.
Fig 1.
Antiviral activity of 2CMC against the Norwalk virus replicon. HG23 cells were exposed to serial concentrations of 2CMC for a period of 72 h, after which cell monolayers were collected for quantification of RNA loads by qRT-PCR. Intracellular RNA loads are represented by the variation in Norwalk virus replicon and β-actin CT values (A) and by relative genome quantification of the Norwalk virus replicon versus β-actin (B). Dotted lines represent 50 and 90% reductions of the relative Norwalk virus replicon RNA levels. Results are mean values ± SEM of five independent experiments.
2CMC clears cells of the Norwalk virus replicon.
To evaluate whether 2CMC is able to completely eliminate the norovirus replicon from host cells, clearance and rebound experiments were performed. For the clearance phase, cells were treated for six consecutive passages with 2CMC in the absence of G418 selective pressure. In cells treated with 25 μg/ml 2CMC (5 times the EC50), Norwalk virus replicon levels were reduced by 5 log10 after four passages (at 6.25 and 12.5 μg/ml, 2 log10 was the maximum observed reduction) (Fig. 2A). Additionally, in untreated control cells, the variation in the quantity of Norwalk virus RNA (and β-actin mRNA) among the six different passages was <2 CT units. This further attests that the Norwalk virus replicon levels are relatively stable during passaging of the replicon-containing cultures. For the rebound phase, 2CMC was omitted from the culture medium but cells were again cultured under the selective pressure of G418. When cultures are successfully cleared of the replicon, they lose the ability to grow in the presence of the selection marker G418. When the drug does not clear the cells of the replicon, the cultures survive and proliferate in the presence of G418. Cultures that had been treated for 4 or 6 passages with 25 μg/ml 2CMC were no longer able to proliferate in the presence of G418 (Fig. 2). Hence, 2CMC at 25 μg/ml (but not at lower concentrations) is able to clear cells of the replicon following four consecutive passages of antiviral pressure.
Fig 2.
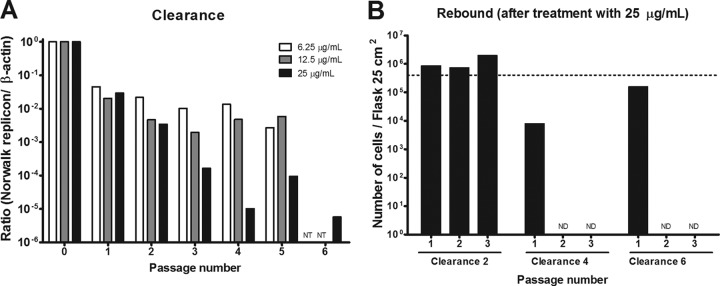
Effect of 2CMC on Norwalk virus replicon clearance from HG23 cells. (A) In the clearance phase, cells were treated for six consecutive passages with 2CMC (at concentrations of 6.25 to 25 μg/ml) in the absence of G418 selective pressure and the ratio of viral RNA to beta-actin RNA was determined (NT, not tested). (B) In the rebound phase (for cells treated with 25 μg/ml 2CMC), 2CMC was omitted from the culture medium but cells were again cultured under the selective pressure of G418 (1.25 mg/ml). The graph shows the number of cells/25-cm2 T flask for three consecutive rebound passages with cells from each clearance passage, namely, passages 2, 4, and 6 (ND, not detected). The dotted line represents the number of cells that was set as the starting point to start a new culture. Results are representative of two independent experiments.
2CMC protects AG129 mice against MNV-induced diarrhea and death.
AG129 mice were infected orally with MNV 1 h after a first dose (50 mg/kg) of 2CMC or vehicle was given. Treatment was continued twice daily for 7 consecutive days. The first signs of disease in the infected, vehicle-treated group appeared at 3 days p.i.; mice became lethargic and had a hunched posture, ruffled fur, and watery squinted eyes. The severity of disease increased rapidly, culminating in a mean time to death or euthanasia of 3.5 + 0.5 days p.i. (Fig. 3). Macroscopic observation of the abdominal cavities of untreated mice revealed gastric bloating (data not shown). All of the animals in the 2CMC-treated group survived the infection (Fig. 3). Mice exhibited somewhat lethargic behavior and had moderately ruffled fur on days 5 and 6 p.i. but appeared healthy and active on other days.
Fig 3.
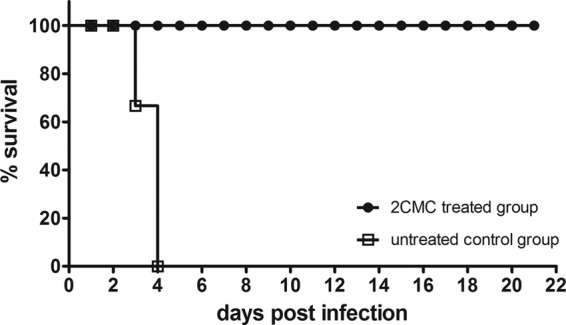
Survival curves of 2CMC-treated (n = 15) and untreated (n = 12) MNV-infected AG129 mice. Treatment with 2CMC (100 mg/kg/day) was initiated 1 h before infection. The compound was administered twice daily by the subcutaneous route for 7 consecutive days. The graph depicts Kaplan-Meier survival curves as percent survival versus time postinfection.
Between days 2 and 4 p.i., untreated mice lost 14.5% ± 0.6% of their body weight while the weight of 2CMC-treated mice decreased 7.2% ± 1% (Fig. 4). The 2CMC-treated animals continued to lose weight up to 6 days p.i., with a maximum weight loss of 13.9% + 1.5%. As of day 7 p.i., these mice started to regain body weight and recovered all of the lost weight by 11 days p.i. (Fig. 4). Untreated mice became diarrheic at day 3 p.i., with a stool score that increased to 4 (liquid stool) at 4 days p.i. (Fig. 5). The fecal consistency score of 2CMC-treated mice was markedly and significantly lower than that of vehicle-treated animals, and a majority of the stool samples of the treated mice had a more or less normal consistency and appearance during the entire duration of the experiment (Fig. 5). Treatment with 2CMC was stopped at 7 days p.i. Mice were then kept for 21 days (following the cessation of treatment with 2CMC) and remained in good health during the entire period of time.
Fig 4.
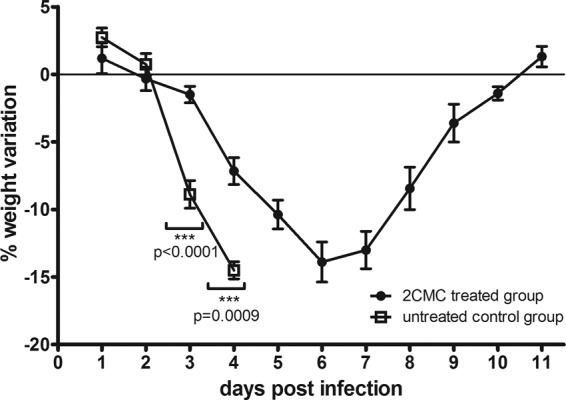
Weight variation of MNV-infected AG129 mice treated with 2CMC (n = 15) or left untreated (n = 12). The graph shows percent body weight variation (compared to day 0 p.i.) versus time postinfection and mean values ± SEM. Values obtained with 2CMC-treated and untreated groups on the same days postinfection were compared.
Fig 5.
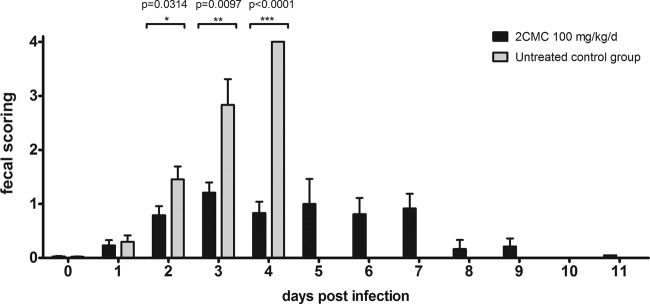
Fecal consistency of MNV-infected AG129 mice treated with 2CMC or left untreated. Scores for consistency of stool (0, normal feces; 1, mixed stool samples containing both solid and pasty feces; 2, pasty feces; 3, semiliquid feces; 4, liquid feces) versus time postinfection. Data are expressed as mean values ± SEM. In each group, 6 to 15 individual stool samples were collected each day, with the exceptions of the untreated group at 4 days p.i. and the 2CMC-treated group at 11 days p.i., when 3 stool samples were collected. Values obtained with the 2CMC-treated and untreated groups on the same days postinfection were compared.
2CMC-treated mice shed virus in feces later than untreated animals.
Stool samples collected from animals throughout these experiments were used for quantification of viral RNA loads by qRT-PCR. Samples were collected individually from each animal between 0 and 11 days p.i. (unless the animal died) (Fig. 6). Stool samples collected at day 0 had no detectable levels of viral RNA (in both vehicle- and 2CMC-treated animals). Viral RNA was detected 1 day after infection in the untreated and 2CMC-treated groups. This may possibly reflect the inoculum that had passed the gastrointestinal tract.
Fig 6.
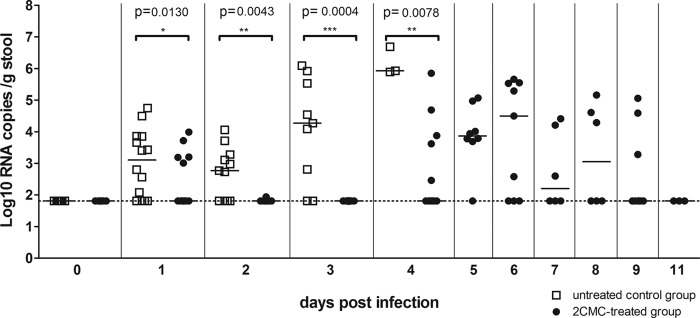
Viral RNA loads in stool samples of 2CMC-treated or untreated MNV-infected AG129 mice. Data are presented as log10 RNA copy numbers per gram of stool versus time postinfection for untreated or 2CMC-treated animals. In each group, RNA was quantified in 6 to 15 individual stool samples per day, with the exceptions of the untreated group at 4 days p.i. and the 2CMC-treated group at 11 days p.i., when 3 stool samples were collected. The dotted line represents the LOD. Values obtained with the 2CMC-treated and untreated groups on the same days postinfection were compared.
Whereas viral RNA remained undetectable in stool samples of 2CMC-treated mice at 2 and 3 days p.i., levels of viral RNA in stool samples of untreated mice increased to 5.5 ± 0.3 log10 copies/g stool at day 3 (Fig. 6). At 4 days p.i., the viral loads in stool samples from untreated mice were as high as 6.2 ± 0.3 log10 copies/g stool. From 4 days p.i. onward, an increase in viral RNA titers in the stool samples of 2CMC-treated mice (to a maximum of 4.7 ± 0.6 log10 copies/g stool at 6 days p.i.) was observed. This maximum viral load at 6 days p.i. coincided with (i) the slightly lethargic behavior of the treated animals and (ii) the maximum weight loss (Fig. 4). Viral RNA levels in the stool of these mice at 6 days p.i. proved still significantly lower than those in the stool samples of untreated mice at 4 days p.i. (P = 0.0238). After this peak at 6 days p.i., viral RNA loads decreased to 2.8 ± 0.6 log10 copies/g stool at 9 days p.i. (P = 0.1763 compared to the LOD) and were undetectable at day 11.
2CMC treatment decreases viral replication in the intestine and prevents tissue damage.
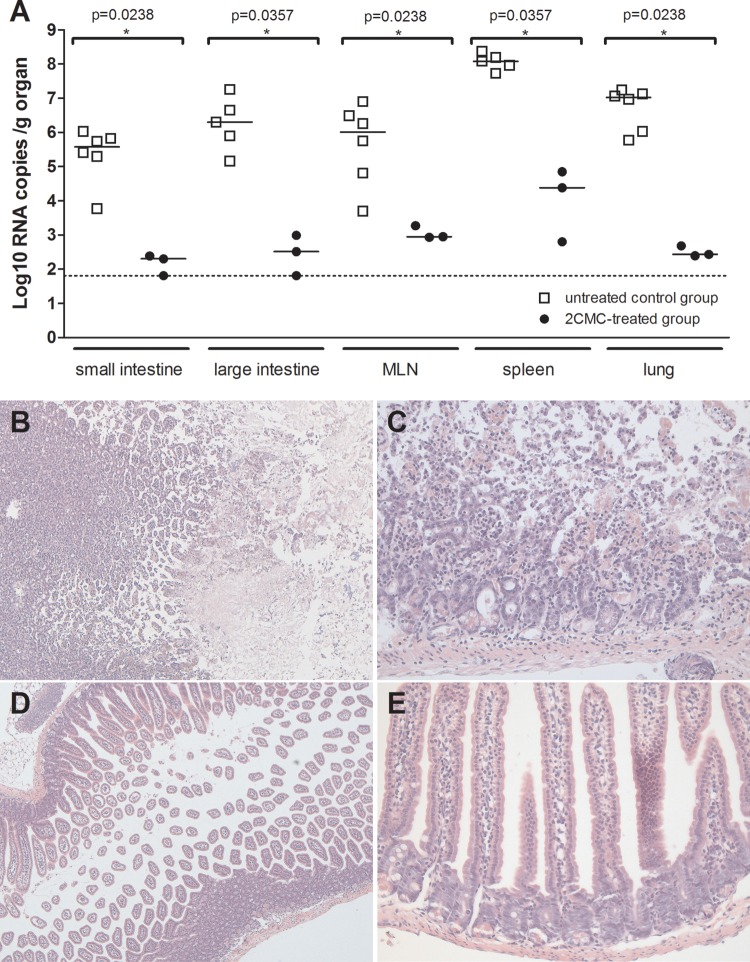
At 3 days p.i., three 2CMC-treated and six vehicle-treated infected mice were sacrificed; their small intestines, proximal colons, mesenteric lymph nodes, spleens, and lungs were collected for quantification of viral RNA by qRT-PCR. The viral RNA levels in the organs of the 2CMC-treated mice were significantly (3 to 4 log10) lower than those in the corresponding organs of the vehicle-treated group (Fig. 7A). Histological examination of the small intestine revealed multifocal necrosis of the intestinal mucosa, associated with a mild lymphocytic inflammation in the vehicle-treated mice (Fig. 7B and C). The intestinal mucosa of 2CMC-treated mice appeared totally normal (Fig. 7D and E).
Fig 7.
(A) Viral RNA loads in organs of 2CMC-treated or untreated MNV-infected AG129 mice. Data are presented as log10 RNA copy numbers per gram of organ at 3 days p.i. for the small intestines, large intestines, mesenteric lymph nodes, spleens, and lungs of untreated or 2CMC-treated animals. The dotted line represents the LOD. An asterisk indicates that a P value of <0.05 was determined with the Mann-Whitney test, and medians obtained with 2CMC-treated and untreated groups at 3 days p.i. were compared. (B to E) Histopathology of the small intestines of 2CMC-treated and untreated MNV-infected AG129 mice. Images show H&E staining of the small intestines of representative untreated AG129 mice (B and C) and 2CMC-treated mice (D and E) at ×50 (B and D) and ×200 (C and E) magnifications.
Norovirus-infected mice treated with 2CMC develop protective immunity.
Two weeks after the cessation of treatment with 2CMC (i.e., 3 weeks after infection), six mice were rechallenged with MNV. During the next 7 days, animals were weighed daily and stool samples were collected. All of the animals appeared healthy during the entire week following the rechallenge, whereas a control group (n = 3) developed disease and had to be euthanized at 3 days p.i. The variation in the weight of mice previously treated with 2CMC mice was, on average, <2% (not statistically significant). In stool samples from these drug-treated mice, MNV RNA remained undetectable during the entire observation period.
DISCUSSION
We reported recently that the HCV nucleoside polymerase inhibitor 2CMC inhibits the in vitro replication of MNV (a surrogate for human norovirus), with EC50s of ∼2 μM (17). We now report anti-norovirus activity of 2CMC in the Norwalk virus replicon model, the single available human norovirus cell culture model. This nucleoside analogue reduced Norwalk virus replicon replication in a dose-dependent manner with an EC50 of ∼5 μg/ml (∼20 μM). Another group recently also reported the in vitro anti-norovirus activity of 2CMC; they reported 10-fold lower EC50s, which may be related to differences in either the methodology used or the purity of the compound used (the activity of our batch of compound resulted in an inhibition of the replication of an unrelated virus, i.e., the HCV, comparable to the inhibition reported by other groups). At a concentration close to the EC90, 2CMC was able to clear cells of their Norwalk virus replicon following four consecutive passages of antiviral pressure. Our attempts to select for resistance to 2CMC in Norwalk virus replicon-carrying cells were unsuccessful (data not shown), which is in line with our failure to select 2CMC-resistant MNV (17). Also against HCV, the barrier to resistance of this nucleoside analogue is high (23).
We next studied whether 2CMC is able to protect against norovirus-induced morbidity/mortality in a murine infection model. To this end, we established a model of norovirus infection and disease in IFN-α/β and IFN-γ receptor-deficient AG129 mice following oral infection with MNV-1.CW3. Innate-immunity-deficient mice have been shown to develop severe illness following MNV infection, with significant mortality in particular when infected with the MNV-1.CW3 strain (13, 15). MNV-infected AG129 mice became visibly ill 3 days after oral infection, with >10% body weight loss and diarrhea. Changes in stool consistency were detected from 2 days p.i. onward and progressed to liquid stool at 4 days p.i., with intense virus shedding and clear signs (following histological examination of the intestines) of acute mucosal necrosis. The severity of illness progressed rapidly, resulting in 100% mortality 4 days after infection (n = 12).
The great diversity of norovirus strains and the genetic drift associated with the more clinically relevant GII.4 strains will require broad-spectrum norovirus inhibitors. Valopicitabine, the 3′-valyl ester oral prodrug of 2CMC, was shown to markedly reduce viral loads in chronically HCV-infected patients (24, 25); however, its development was halted because of adverse effects. 2CMC, through its 5′-triphosphate metabolite, targets the active site of the viral RNA-dependent RNA polymerases (18, 26). Since we demonstrated in vitro inhibitory activity against two genogroups of the Norovirus genus, it may be expected that 2CMC (as well as other 2′-C methyl nucleoside analogues) is effective against the more prevalent GII.4 noroviruses. 2CMC-treated, MNV-infected AG129 mice presented no symptoms and lost less weight up to 4 days after infection, at which time untreated mice had died or been euthanized. 2CMC-treated animals did not become diarrheic, and no macroscopic or histological signs of disease were observed in their intestines. Treated animals developed a mild disease on days 5 and 6 p.i. and lost significant body weight but shed less virus than untreated animals did. They finally recovered fully, and no rebound was observed following the cessation of treatment at day 7 p.i. It remains to be studied why a viral load increase in treated animals is apparent on day 4 p.i. Since mice may eat their feces, reinfection could be the cause of this delayed viremia. Importantly, the viral RNA levels in the stool samples of treated mice proved to be significantly lower than those in the stool samples of untreated mice. Whether the viruses shed in the stool of 2CMC-treated animals are infectious virus particles was not assessed in this study.
Since AG129 mice lack key elements of innate immunity, acquired immunity is expected to result in the recovery of 2CMC-treated animals from MNV infection. MNV-specific IgG antibodies have been described as potentially neutralizing and have been shown to play a part in the immune response of MNV-infected mice, limiting MNV replication in the intestine and spleen (27, 28). We hypothesize that 2CMC limits the replication of MNV in the gut for a sufficiently long period of time to allow the development of neutralizing antibodies that will (together with a T-cell response) go on to clear the infection. Such protective immunity should also protect against a rechallenge with MNV. Two weeks after the cessation of treatment with 2CMC, mice were therefore rechallenged with MNV. All of the animals remained healthy during the week following the rechallenge, without diarrhea, viral shedding in stool, or significant weight loss. This observation is in line with previous studies with mice (27, 28). Also in human volunteers who were challenged with a GII human norovirus, a Th1 cellular immune response leading to B-cell differentiation and IgG production was suggested to be associated with protection from infection following a challenge (29).
It will be relevant to study the immune response (such as cytokine response, kinetics of antibody production, duration of immunity) of these MNV-infected and 2CMC-treated mice.
The use of antiviral drugs to treat symptomatic norovirus infections might be somewhat challenging, given the fact that the onset of symptoms in humans is just 12 to 48 h after infection and that the mean duration of illness is <3 days. However, when given soon after the start of the first symptoms, a potent drug may be able to reduce the severity of the illness and, importantly, shedding of infectious virus (thus reducing the chance of transmission). An anti-norovirus drug would also be very useful for prophylaxis during a norovirus outbreak in communal settings such as nursing homes and hospitals, where large outbreaks of norovirus often occur (and that may lead to hundreds of affected individuals). A potent and safe drug may contain the spread of this highly infectious virus in such settings.
It will also be relevant to assess the impact of treatment with an antiviral compound (such as 2CMC) on persistent norovirus infections, in particular given the increasingly recognized role of noroviruses as agents of chronic gastroenteritis in immunocompromised patients. To that end, a norovirus model will need to be used that employs MNV strains that cause a persistent infection.
In conclusion, we have demonstrated, to the best of our knowledge, for the first time that an antiviral molecule protects against norovirus-induced diarrhea and death in a relevant animal model. This model and reference compound can be employed in future efforts to develop highly potent, broad-spectrum, and safe anti-norovirus drugs.
ACKNOWLEDGMENTS
This work was funded by EU FP7 project SILVER (260644) and KU Leuven GOA grant GOA/10/014. J. Rocha-Pereira is supported by a Ph.D. grant (SFRH/BD/48156/2008) from the Fundação para a Ciência e Tecnologia.
We acknowledge Herbert W. Virgin (Washington University, St. Louis, MO) for the MNV and Kyeong-Ok Chang (Kansas State University, Manhattan, KS) for the Norwalk virus replicon-bearing cells. We thank Carolien De Keyser and Tom Bellon for excellent technical assistance and Dominque Brabants for fine editorial help.
Footnotes
Published ahead of print 28 August 2013
REFERENCES
- 1.Patel MM, Widdowson MA, Glass RI, Akazawa K, Vinje J, Parashar UD. 2008. Systematic literature review of role of noroviruses in sporadic gastroenteritis. Emerg. Infect. Dis. 14:1224–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hemming M, Rasanen S, Huhti L, Paloniemi M, Salminen M, Vesikari T. 2013. Major reduction of rotavirus, but not norovirus, gastroenteritis in children seen in hospital after the introduction of RotaTeq vaccine into the National Immunization Programme in Finland. Eur. J. Pediatr. 172:739–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Payne DC, Vinje J, Szilagyi PG, Edwards KM, Staat MA, Weinberg GA, Hall CB, Chappell J, Bernstein DI, Curns AT, Wikswo M, Shirley SH, Hall AJ, Lopman B, Parashar UD. 2013. Norovirus and medically attended gastroenteritis in U.S. children. N. Engl. J. Med. 368:1121–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bok K, Green KY. 2012. Norovirus gastroenteritis in immunocompromised patients. N. Engl. J. Med. 367:2126–2132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teunis PF, Moe CL, Liu P, Miller SE, Lindesmith L, Baric RS, Le Pendu J, Calderon RL. 2008. Norwalk virus: how infectious is it? J. Med. Virol. 80:1468–1476 [DOI] [PubMed] [Google Scholar]
- 6.Atmar RL, Opekun AR, Gilger MA, Estes MK, Crawford SE, Neill FH, Graham DY. 2008. Norwalk virus shedding after experimental human infection. Emerg. Infect. Dis. 14:1553–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siebenga JJ, Beersma MF, Vennema H, van Biezen P, Hartwig NJ, Koopmans M. 2008. High prevalence of prolonged norovirus shedding and illness among hospitalized patients: a model for in vivo molecular evolution. J. Infect. Dis. 198:994–1001 [DOI] [PubMed] [Google Scholar]
- 8.Lopman B, Gastanaduy P, Park GW, Hall AJ, Parashar UD, Vinje J. 2012. Environmental transmission of norovirus gastroenteritis. Curr. Opin. Virol. 2:96–102 [DOI] [PubMed] [Google Scholar]
- 9.Chang KO, Sosnovtsev SV, Belliot G, King AD, Green KY. 2006. Stable expression of a Norwalk virus RNA replicon in a human hepatoma cell line. Virology 353:463–473 [DOI] [PubMed] [Google Scholar]
- 10.Karst SM, Wobus CE, Lay M, Davidson J, Virgin HW., IV 2003. STAT1-dependent innate immunity to a Norwalk-like virus. Science 299:1575–1578 [DOI] [PubMed] [Google Scholar]
- 11.Taube S, Kolawole AO, Hohne M, Wilkinson JE, Handley SA, Perry JW, Thackray LB, Akkina R, Wobus CE. 2013. A mouse model for human norovirus. mBio 4:e00450–00413. 10.1128/mBio.00450-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wobus CE, Karst SM, Thackray LB, Chang KO, Sosnovtsev SV, Belliot G, Krug A, Mackenzie JM, Green KY, Virgin HW. 2004. Replication of Norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLoS Biol. 2:e432. 10.1371/journal.pbio.0020432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kahan SM, Liu G, Reinhard MK, Hsu CC, Livingston RS, Karst SM. 2011. Comparative murine norovirus studies reveal a lack of correlation between intestinal virus titers and enteric pathology. Virology 421:202–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mumphrey SM, Changotra H, Moore TN, Heimann-Nichols ER, Wobus CE, Reilly MJ, Moghadamfalahi M, Shukla D, Karst SM. 2007. Murine norovirus 1 infection is associated with histopathological changes in immunocompetent hosts, but clinical disease is prevented by STAT1-dependent interferon responses. J. Virol. 81:3251–3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strong DW, Thackray LB, Smith TJ, Virgin HW. 2012. Protruding domain of capsid protein is necessary and sufficient to determine murine norovirus replication and pathogenesis in vivo. J. Virol. 86:2950–2958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson AJ, Roehrig JT. 1999. New mouse model for dengue virus vaccine testing. J. Virol. 73:783–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rocha-Pereira J, Jochmans D, Dallmeier K, Leyssen P, Cunha R, Costa I, Nascimento MS, Neyts J. 2012. Inhibition of norovirus replication by the nucleoside analogue 2′-C-methylcytidine. Biochem. Biophys. Res. Commun. 427:796–800 [DOI] [PubMed] [Google Scholar]
- 18.Pierra C, Amador A, Benzaria S, Cretton-Scott E, D'Amours M, Mao J, Mathieu S, Moussa A, Bridges EG, Standring DN, Sommadossi JP, Storer R, Gosselin G. 2006. Synthesis and pharmacokinetics of valopicitabine (NM283), an efficient prodrug of the potent anti-HCV agent 2′-C-methylcytidine. J. Med. Chem. 49:6614–6620 [DOI] [PubMed] [Google Scholar]
- 19.Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45. 10.1093/nar/29.9.e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paeshuyse J, Kaul A, De Clercq E, Rosenwirth B, Dumont JM, Scalfaro P, Bartenschlager R, Neyts J. 2006. The non-immunosuppressive cyclosporin DEBIO-025 is a potent inhibitor of hepatitis C virus replication in vitro. Hepatology 43:761–770 [DOI] [PubMed] [Google Scholar]
- 21.Spann KM, Tran KC, Chi B, Rabin RL, Collins PL. 2004. Suppression of the induction of alpha, beta, and lambda interferons by the NS1 and NS2 proteins of human respiratory syncytial virus in human epithelial cells and macrophages. J. Virol. 78:4363–4369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rocha-Pereira J, Jochmans D, Dallmeier K, Leyssen P, Nascimento MS, Neyts J. 2012. Favipiravir (T-705) inhibits in vitro norovirus replication. Biochem. Biophys. Res. Commun. 424:777–780 [DOI] [PubMed] [Google Scholar]
- 23.Delang L, Vliegen I, Froeyen M, Neyts J. 2011. Comparative study of the genetic barriers and pathways towards resistance of selective inhibitors of hepatitis C virus replication. Antimicrob. Agents Chemother. 55:4103–4113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Afdhal N, Brien O C, Godofsky E, Rodriguez-Torres M, Pappas SC, Pockros P, Lawitz E, Bzowej N, Rustgi V, Sulkowski M, Sherman K, Jacobson I, Chao G, Knox S, Pietropaolo K, Brown N. 2006. Valopicitabine (NM283), alone or with peginterferon, compared to peg interferon/ribavirin (pegifn/RBV) retreatment in hepatitis patients with prior nonresponse to PEGIFN/RBV: week 24 results. J. Hepatol. 44(Suppl 2):S1916356583 [Google Scholar]
- 25.Afdhal N, O'Brien C, Godofsky E, Rodriguez-Torres M, Pappas SC, Lawitz E, Pockros P, Sulkowski M, Jacobson I, Chao G, Knox S, Pietropaolo K, Brown NA. 2007. Valopicitabine (nm283), alone or with peg-interferon, compared to peg interferon/ribavirin (pegifn/rbv) retreatment in patients with HCV-1 infection and prior non-response to pegifn/rbv: one-year results. J. Hepatol. 46(Suppl 1):S5 [Google Scholar]
- 26.Carroll SS, Tomassini JE, Bosserman M, Getty K, Stahlhut MW, Eldrup AB, Bhat B, Hall D, Simcoe AL, LaFemina R, Rutkowski CA, Wolanski B, Yang Z, Migliaccio G, De Francesco R, Kuo LC, MacCoss M, Olsen DB. 2003. Inhibition of hepatitis C virus RNA replication by 2′-modified nucleoside analogs. J. Biol. Chem. 278:11979–11984 [DOI] [PubMed] [Google Scholar]
- 27.Chachu KA, LoBue AD, Strong DW, Baric RS, Virgin HW. 2008. Immune mechanisms responsible for vaccination against and clearance of mucosal and lymphatic norovirus infection. PLoS Pathog. 4:e1000236. 10.1371/journal.ppat.1000236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chachu KA, Strong DW, LoBue AD, Wobus CE, Baric RS, Virgin HW., IV 2008. Antibody is critical for the clearance of murine norovirus infection. J. Virol. 82:6610–6617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindesmith L, Moe C, Lependu J, Frelinger JA, Treanor J, Baric RS. 2005. Cellular and humoral immunity following Snow Mountain virus challenge. J. Virol. 79:2900–2909 [DOI] [PMC free article] [PubMed] [Google Scholar]