Abstract
HCMV UL76 is a member of a conserved Herpesviridae protein family (Herpes_UL24) that is involved in viral production, latency, and reactivation. UL76 presents as globular aggresomes in the nuclei of transiently transfected cells. Bioinformatic analyses predict that UL76 has a propensity for aggregation and targets cellular proteins implicated in protein folding and ubiquitin-proteasome systems (UPS). Furthermore, fluorescence recovery after photobleaching experiments suggests that UL76 reduces protein mobility in the aggresome, which indicates that UL76 elicits the aggregation of misfolded proteins. Moreover, in the absence of other viral proteins, UL76 interacts with S5a, which is a major receptor of polyubiquitinated proteins for UPS proteolysis via its conserved region and the von Willebrand factor type A (VWA) domain of S5a. We demonstrate that UL76 sequesters polyubiquitinated proteins and S5a to nuclear aggresomes in biological proximity. After knockdown of endogenous S5a by RNA interference techniques, the UL76 level was only minimally affected in transiently expressing cells. However, a significant reduction in the number of cells containing UL76 nuclear aggresomes was observed, which suggests that S5a may play a key role in aggresome formation. Moreover, we show that UL76 interacts with S5a in the late phase of viral infection and that knockdown of S5a hinders the development of both the replication compartment and the aggresome. In this study, we demonstrate that UL76 induces a novel nuclear aggresome, likely by subverting S5a of the UPS. Given that UL76 belongs to a conserved family, this underlying mechanism may be shared by all members of the Herpesviridae.
INTRODUCTION
Human cytomegalovirus (HCMV) infection is one of the most prevalent infections (global seroprevalence, 60 to 90%) in the human population (1). Recent reports indicate that HCMV is able to superinfect hosts despite long-term viral latency, and for most healthy individuals, the infection remains asymptomatic (2). However, severe neurological defects associated with HCMV can occur with congenital infection. The affected neonates develop mental retardation, microcephaly, seizures, and sensorineural hearing impairment. In immunocompromised patients, the reactivation of latent HCMV can result in systemic opportunistic infections, including pneumonitis, hepatitis, retinitis, and gastrointestinal disease. Growing evidence in recent years has linked HCMV infection to malignant tumors, vascular diseases, and mental disorders (3–5).
The ubiquitin (Ub)-proteasome system (UPS) is a major intracellular, nonlysosomal proteolytic system that is involved in many important cellular pathways, including protein quality control, protein homeostasis, DNA repair, cell cycle progression, pathogen infection, transcriptional regulation, cellular differentiation, and immune modulation (reviewed more thoroughly in references 6 and 7). The target substrates are attached to a branched polyubiquitinated chain that is ubiquitinated mainly at the Lys48 linkage through the activation of E1 (ubiquitin-activating enzyme), in addition to conjugation with E2 (ubiquitin-conjugated enzyme) and E3 (ubiquitin ligase), prior to being recognized, bound, and degraded by the 26S proteasome. The main body of the 26S proteasome is composed of two substructures: a 19S regulatory particle responsible for the recognition and translocation of ubiquitinated substrates and a 20S catalytic particle composed of proteolytic enzymes. In the integral 26S proteasome components, the subunits S5a (PSMD4; Rpn10) and Rpn13 of the regulatory particle are the two major receptors responsible for direct binding of polyubiquitinated substrates following translocation into the catalytic particle for degradation (8–10).
Aberrant or misfolded proteins often cause adverse cell stresses and therefore need to be recognized immediately and removed efficiently (11). In the cytoplasm, misfolded proteins are degraded by two different processes: the UPS and autophagy involved in lysosomal proteolysis. Because of the lack of autophagy, the clearing of misfolded proteins residing in the nucleus appears to be conducted entirely by the UPS (12–14). Failure to remove misfolded proteins elicits protein aggregation or deposition as insoluble aggresomes, which are associated with severe neurological diseases, including Creutzfeldt-Jakob disease.
Recent studies have explicitly revealed that the UPS is robustly involved with and manipulated by viruses during every phase of the viral life cycle: entry, uncoating, replication, egress, and immune evasion (15–17). In addition, growing evidence indicates that HCMV extensively modulates UPS proteins and associated proteolytic functions for the presumable purpose of subverting the homeostasis of cellular proteins to create suitable microenvironments that better suit the virus at various stages of its intracellular life cycle. In the initial stage of an HCMV lytic infection, virus-infected cells maintain a state of G1/S transition, which is a hallmark of HCMV-infected cells. To arrest cell cycle progression, HCMV alters several proteins in the UPS. During these initial alterations, the multifunctional E3 ligase anaphase-promoting complex/cyclosome (APC/C) is inactivated, which contributes to the degradation of APC4 and APC5 via the UPS and consequently results in the promotion of entry into G1 (18, 19). Following viral entry, the host cells elicit intrinsic defense mechanisms characterized by repressive effects in promyelocytic leukemia (PML) bodies (also called PML oncogenic domains or nuclear domain 10), which inhibit the expression of HCMV immediate-early (IE) gene promoters. HCMV counteracts these repressive cellular effects by promoting proteasome-dependent degradation of the cellular repressors Daxx and SP100 in PML bodies (20). Evidence supports the idea that the HCMV-associated major tegument protein UL82, when delivered into cells during viral infection, elicits the degradation of RB and Daxx proteins via a novel ubiquitin-independent and proteasome-dependent mode (21). As a result, the activation of the major immediate-early (MIE) gene (UL122-123) transcription and the acceleration of cell cycle progression from G1 to S phase can occur. Moreover, during HCMV infection, UPS proteins relocate to adjacent replication compartments, which presumably implicates the proteins in the viral replication process (22). This speculation has yet to be confirmed, however. Together, these data present a scenario where the homeostasis of protein pools is manipulated after viral infection.
UL76 is a Herpes_UL24 family (PF01646) member in HCMV that has been shown to display multiple functions by several independent teams (23). We have reported that UL76 represses the expression of immediate-early-, early-, and late-gene promoters to inhibit viral production (24, 25). Plausibly, as a correlated regulatory mode, UL76 is able to regulate gene expression at the posttranslational level (26). Moreover, UL76 is detected as one of the viral latency-associated transcripts in CD34+ hematopoietic cells latently infected with HCMV (27). Specifically, UL76 transcripts are present in CD34+ CD38− cells, which comprise a subpopulation that allows long-term maintenance of the latent HCMV genome and supports viral reactivation from latency (28). However, contradictory results were obtained for the experiment of Cheung et al. showing that UL76 transcripts are not detected in latent infected myeloid progenitor cells (29). In cells presenting long-term expression of UL76, we observed the emergence of a supernumerary centrosome, micronuclei, chromosomal misalignments, lagging, and bridging in the mitotic phase (30). Another group found that UL76 strongly reduces the number of PML bodies by an unknown mechanism (31).
To elucidate the underlying mechanism governing the multiple functions of UL76, we conducted a CytoTrap yeast two-hybrid assay to gain insight into the cellular pathways targeted by UL76. In this system, UL76 and human protein interactions are anchored beneath the cytoplasmic membrane of yeast to avoid the repressive effect of UL76 on gene expression (25, 32). Given that HCMV pathology can present as severe neurological defects in fetuses, we screened a human fetal brain cDNA library using UL76 as a bait protein. By employing bioinformatic analyses, we determined that prey candidates were mainly implicated in the UPS and protein-folding pathways. In this study, we hypothesized that UL76 modulates the UPS to produce the accumulation of polyubiquitinated proteins by two interrelated modes: the aggregation propensity of UL76 and the interaction of UL76 and S5a, which is a receptor for polyubiquitinated proteins in the UPS. This is the first report to examine the specific targeting of human S5a in the UPS by HCMV UL76. We present multiple lines of evidence to support the idea that the UL76-induced nuclear aggresome accumulates misfolded UL76 and polyubiquitinated substrates for UPS-dependent degradation. S5a is critically involved in the development of the UL76-associated aggresome, and we demonstrate that the knockdown of cellular S5a dramatically reduces the number of cells containing UL76-induced nuclear aggresomes. Collectively, these results indicate that UL76 utilizes a novel mechanism for aggresome formation that may be implicated in the pathogenic effects of UL76. Overall, this mechanism may be a general regulatory mode for the conserved Herpes_UL24 family members in Herpesviridae.
MATERIALS AND METHODS
Cells and viruses.
Human embryonic lung (HEL 299) cells and HCMV AD169 (VR-538) were purchased from the American Type Culture Collection (Manassas, VA). The HEL cells and HCMV AD169 were propagated as described previously (24). Human embryonic kidney large-T antigen-transformed (HEK293T) cells were cultured in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum. All cells were kept in an incubator supplemented with 5% CO2 at 37°C.
Plasmids.
The plasmid pEF-UL76, which expresses UL76 in eukaryotic cells, was described previously (30). The UL76 deletion constructs containing the N- and C-terminal regions were all derived using PCR amplification (the primers are listed in Table S1 in the supplemental material). The amplified DNA fragments, encoding amino acids 1 to 190 and 187 to 325, were designed to contain the restriction endonuclease sites BamHI at the 5′ end and EcoRI at the 3′ end. The vector pEF1/Myc-His C (Invitrogen) DNA was doubly digested with BamHI and EcoRI. The restriction enzyme-digested vector DNA was then ligated to the UL76 fragment produced by PCR. The resulting plasmids were designated pEF-UL76(1-190) and pEF-UL76(187-325), respectively. Each UL76 deletion construct was fused with a c-Myc sequence at the C-terminal end. The plasmid pEGFP-UL76 has been described previously (25). To subclone the UL76 inserts into the pEGFP-C3 vector, the vectors pEF-UL76(1-190) and pEF-UL76(187-325) were digested with BamHI and EcoRI, respectively. After ligation and transformation, the resulting constructs were designated pEGFP-UL76(1-190) and pEGFP-UL76(187-325), respectively.
Plasmid pcDNA3-HA-S5a, encoding the full-length S5a, was kindly provided by Yael Gus (Hebrew University, Israel) (33). Deletion constructs containing the von Willebrand factor type A (VWA) domain (amino acids 1 to 191), VWA to ubiquitin-interacting motif 1 (UIM1) (amino acids 1 to 253), and UIM1 to UIM2 (amino acids 196 to 377) were PCR amplified from the template pcDNA3-HA-S5a (the primers are listed in Table S1 in the supplemental material). The PCR-produced DNA fragments and vector pcDNA3 were all digested with compatible restriction enzymes that recognized sites created using PCR and then religated. The resulting constructs were designated pcDNA3-HA-S5a(1-199), pcDNA3-HA-S5a(1-253), and pcDNA3-HA-S5a(196-377), respectively. To construct a fusion protein of S5a in frame with the fluorescent protein DsRed1, S5a was excised from pcDNA3-HA-S5a by digestion with EcoRI and SalI. In parallel, the vector pDsRed1 (Clontech) was digested using the same set of restriction enzymes. Both the S5a insert and the vector were religated. The resulting construct was designated pDsRed-S5a.
The plasmid pCGN-HA-Ub, containing ubiquitin, was kindly provided by Jeang Kuan-Teh (National Institute of Allergy and Infectious Diseases). To construct ubiquitin tagged with the FLAG epitope, both pcDNA3-FLAG and pCGN-HA-Ub were doubly digested with EcoRI and EcoRV to produce the modified vector and the ubiquitin insert, which were religated. The resulting plasmid was designated pcDNA3-FLAG-Ub. The DsRed-ubiquitin fusion protein was constructed. First, the ubiquitin insert was obtained from pCGN-HA-Ub by digestion with EcoRI and ApaI. The vector pDsRed1 was digested using the same set of restriction enzymes. The enzyme-modified DNA from both the insert and vector were religated. The resulting construct was designated pDsRed-Ub.
FRAP analysis.
To monitor live images of fusion proteins, cells were transfected with pEGFP-UL76. After 48 h of transfection, images were acquired using a laser scanning confocal microscope (Olympus FV1000). The region of interest (ROI) in the cells was UV bleached for 1 s, and the fluorescence intensities of the cells were monitored before and after the photobleaching for a duration of 105 s. The fluorescence intensities of the designated regions were subtracted from the lowest level observed after UV bleaching. The values were then normalized based on the intensity difference before and after UV bleaching. The fluorescence recovery after photobleaching (FRAP) assay was conducted using the diffuse measurement package of FlowView software (Olympus Corporation) (34).
Bioinformatic tools.
The networks involving UL76-interacting proteins obtained from yeast two-hybrid screening were generated using Ingenuity Pathway Analysis (IPA) (Ingenuity System). Bioinformatics-based computational analyses were employed to predict the protein conformation. The programs used for prediction were as follows: for protein nuclear localization, WoLF PSORT (http://wolfpsort.org/) (35), and for aggregation propensity, AGGRESCAN (http://bioinf.uab.es/aggrescan/) (36, 37) and TANGO (http://tango.crg.es/about.jsp) (38).
Antibodies.
A polyclonal antibody (Ab) against UL76 was raised in this study via immunization of mice using oligopeptide amino acids 244 to 267 (CRAHGPGAQTVSASGAQGSGSQGAD). Polyclonal rabbit anti-UL112 was described previously (39). Monoclonal mouse anti-myc tag (clone 9B11), mouse anti-hemagglutinin (HA) tag (clone 6E2), anti-ubiquitin (clone P4D1), polyclonal rabbit anti-K48 linkage-specific polyubiquitin, and monoclonal rabbit anti-S5a (D17E4) antibodies were obtained from Cell Signaling. Monoclonal mouse anti-α-tubulin (clone B-5-1-2) was obtained from Sigma-Aldrich. Mouse anti-HA tag (clone HA-7) conjugated to agarose and rabbit anti-Myc tag conjugated to agarose were obtained from Sigma-Aldrich. Monoclonal mouse anti-K63 linkage-specific polyubiquitin antibody (HWA4C4) was obtained from eBioscience. Mouse anti-HA (clone 3F10)-peroxidase and mouse anti-c-Myc (9E10)-peroxidase were obtained from Roche. Polyclonal rabbit antibody to proteasome subunit S5a was purchased from Enzo or Cell Signaling. The secondary anti-mouse IgG-horseradish peroxidase (HRP) and anti-rabbit IgG-HRP antibodies were purchased from GE Healthcare. Alexa Fluor 488 anti-mouse IgG and Alexa Fluor 594 anti-rabbit IgG antibodies were purchased from Molecular Probes.
DNA transfection and immunoblot (IB) analysis.
To assay transient gene expression via immunoblotting, 3 μg of DNA was transfected into 3 × 106 HEL cells using a microporator kit (Invitrogen) according to the manufacturer's recommended protocol. For gene expression in HEK293T cells, the transfection was mediated by Lipofectamine Plus and Lipofectamine (Invitrogen). In the live illumination experiments, the transfected pEGFP-UL76, pDsRed-S5a, or pDsRed-Ub was expressed for 48 h in the cells. In the proteasome inhibitory assay, after 3 h of DNA transfection, the cells were exposed to MG132 (10 μM; CalBiochem) or clasto-lactacystin β-lactone (10 μM; CalBiochem) for an additional 21 h as indicated in the text. Forty-eight hours after transfection, the transfected cells were harvested and lysed in RIPA buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 1% NP-40, 0.05% sodium deoxycholate, and 0.01% SDS) containing complete protease inhibitor cocktail (Roche). The soluble proteins were saved and quantified using a Bio-Rad Bradford protein assay kit (Bio-Rad). Forty micrograms of protein from each sample was used for resolution by 12% SDS-PAGE and then transferred to an Immobilon membrane (Millipore, Merck) in Towbin transfer buffer (48 mM Tris, 39 mM glycine [pH 9.2]). The membranes were blocked in Tris-buffered saline (TBS) (50 mM Tris, 150 mM NaCl [pH 7.5]) containing 1% skim milk for 1 h. The antibodies were diluted 1:3,000 in SignalBoost Immunoreaction Enhancer (Merck) and incubated with the membranes for 1 h. After addition of the secondary antibody, chemiluminescent signals were generated using an Immobilon Immunoblotting Detection Reagent (Merck), and the signals were recorded on HyBlot CL autoradiography film (Denville Scientific, Inc.). A BioSpectrum Imaging System (UVP) was used for densitometry quantification.
Quantitative RT-PCR.
S5a mRNA expression was determined using real-time (RT) PCR. RNA samples were extracted using an RNeasy Mini Kit (Qiagen; 74106) according to the manufacturer's instructions. RNA samples were reverse transcribed for 120 min at 37°C using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems; 4368814) according to the standard protocol of the supplier. The primers used for S5a gene expression are listed in Table S1 in the supplemental material. Each sample was tested in triplicate, with 10 ng per 20-μl reaction volume and forward and reverse primers at a concentration of 200 nM. Quantitative PCR was performed using the following conditions: 2 min at 50°C, 10 min at 95°C, 40 cycles of 15 s at 95°C, and 1 min at 60°C using 2× Fast SYBR green PCR Master Mix (Applied Biosystems). The assays were run in an Applied Biosystems Prism 7000 Sequence Detection system.
Immunofluorescent cell staining.
The detailed steps for immunofluorescent cell staining have been described previously (24). In brief, HEL cells were seeded onto a coverslip (10 × 104 cells per well) in six-well culture plates 2 days before DNA transfection or HCMV infection at a multiplicity of infection (MOI) of three PFU/cell. At the time points described in the text, the cells were fixed in 1% paraformaldehyde in phosphate-buffered saline (PBS) for 10 min at room temperature and then permeabilized with 0.1% NP-40 in PBS on ice for 30 min and stained with antibody at 37°C for 30 min in a humid chamber. After extensive washing in PBS, the cells were immersed in a solution containing 1 μg/ml DAPI (4′,6-diamidino-2-phenylindole) and secondary antibodies, i.e., anti-mouse IgG conjugated to Alexa Fluor 488 and/or anti-rabbit IgG conjugated to Alexa Fluor 594 (1:1,000 dilution), for 30 min at 37°C. After extensive washing in PBS, the coverslips were air dried and preserved in Prolong Gold antifade reagent (Molecular Probes). Confocal images were acquired using a laser scanning confocal microscope (Olympus FV1000). Images were obtained by sequential excitation at 559 nm (Alexa Fluor 594), 488 nm (Alexa Fluor 488), and 405 nm (DAPI) and collection of emissions at 618 nm, 517 nm, and 461 nm, respectively. Adobe Photoshop (version 9.0) software was used to compile images.
FRET analysis.
Live images of cells were acquired with a laser scanning confocal microscope (Olympus FluoView 1000) equipped with an UPLSAPO 100×/1.4-numerical-aperture oil objective at 25°C. For the experiment, 20 × 104 HEK293T cells were seeded in glass bottom dishes and transfected with pEGFP-UL76, pDsRed-S5a, or pDsRed-Ub or cotransfected with two plasmids. Images were acquired by sequential excitation at 488 nm and 559 nm using argon and diode lasers, respectively. Emission was recorded between 505 and 540 nm for enhanced green fluorescent protein (EGFP) and between 575 and 675 nm for DsRed. The Föster distance between EGFP and DsRed was set at 4.73 ± 0.09 nm (40). Fluorescence resonance energy transfer (FRET) images were acquired, and the data were analyzed using the FluoView package with the sensitized emission method to calculate the transfer efficiency and Föster distance between the two illuminating proteins EGFP-UL76 and DsRed-S5a (41), whereas the Föster distance between Alexa Fluor 488 and 594 was set at 6.00 nm in the FRET assay using HCMV-infected HEL cells stained with immunofluorescent antibodies (Molecular Probes).
Immunoprecipitation assay.
HEK293T cells were transfected with eukaryotic expression plasmids for 48 h, and then the cell extracts were prepared by lysing the cells in RIPA buffer. For each immunoprecipitation (IP) assay, 400 μg of cell extracts was mixed with 15 μl of anti-HA (for immunoprecipitation of S5a) or anti-c-Myc (for immunoprecipitation of UL76) antibody-conjugated agarose (Sigma-Aldrich) at 4°C for 16 h. Protein-Ab-conjugated agarose was precipitated by centrifugation at 300 × g for 10 min. The precipitated agarose was washed with RIPA buffer. The washing process was repeated four times in total. Subsequently, the agarose was resuspended in 15 μl of loading buffer that was subjected to PAGE and immunoblotting analyses. To conduct coimmunoprecipitation assays in virus-infected HEL cells, the ImmunoCruz IP/WB system (Santa Cruz Biotechnology) was used to prepare cell lysates harvested at 96 h post-HCMV infection. Cell lysates (2 g) were cleared with preclearing matrix by incubation at 4°C for 2 h. In addition, rabbit monoclonal antibody for S5a (1:300) was incubated with IP matrix at 4°C for 2 h. Then, the precleared lysates were mixed with S5a antibody conjugated with IP matrix, and the mixtures were incubated with rotation at 4°C for 16 h. Subsequently, the mixtures were washed four times with RIPA buffer, and the protein complexes with S5a were analyzed by immunoblot analysis using UL76 antibody and secondary anti-mouse antibody recognizing intact IgG molecules.
RNA interference (RNAi).
To knock down the expression of S5a, a lentivirus-based approach was utilized. S5a short hairpin RNA (shRNA) plasmids expressing shRNA I (TRCN0000003939), shRNA II (TRCN0000003940), and a control plasmid (pLKO_TRC025) were provided by the National RNAi Core Facility. Pseudoviruses were prepared by cotransfection with the packaging vectors pCMV-ΔR8.91, pMD.G, and S5a shRNA I or shRNA II. Pseudoviruses were harvested from the medium 60 h after transfection. To knock down endogenous S5a, HEL or HEK293T cells were transduced with pseudovirus at an MOI of 3 relative infectious units/ml in the presence of Polybrene. After 24 h of transduction, cells were selected in medium containing 2 μg/ml puromycin and then further cultured for an additional 3 days.
TissueFaxs analysis.
Quantitative analysis of the aggresome (UL76) and replication compartment (UL112) in cells were performed with the TissueFaxs system (TissueGnostics, Austria). Whole-field slides were automatically scanned by a Zeiss AxioImager Z2 microscope. TissueQuest software was used for quantitation of immunofluorescent staining. To analyze cells expressing the UL76 aggresome, TissueQuest analyzed the UL76 fluorescence as the range of intensity, which counted cells emitting a peak fluorescence intensity. Replication compartments of cells were calculated as the sum intensity of UL112 fluorescence.
RESULTS
Determinant region for UL76 aggregation.
Previous publications have documented that HCMV UL76 in the absence of other viral proteins is present as globular aggresomes in the nuclei of transfected cells (25, 31) (see Fig. 2A and elsewhere in this study). When investigating the distribution of UL76 during the HCMV infectious cycle, we observed that UL76, which is a virus-associated tegument protein, localizes exclusively in the nucleus in an aggresome phenotype in the late phase, i.e., 72 to 96 h postinfection (Fig. 1A). Based on multiple protein sequence alignments of the Herpes_UL24 family, UL76, as well as other family members, was found to contain five conserved amino acid blocks at the N terminus and a variable sequence at the C terminus. The amino acids of the blocks are as follows: block I, 19 to 35; block II, 67 to 82; block III, 97 to 106; block IV, 123 to 135; and block V, 151 to 162 (Fig. 1B). The functions of the conserved regions of UL76 remain uncharacterized. Therefore, the UL76 N-terminal sequence (amino acids 1 to 190) represents a conserved region, and the C-terminal sequence (amino acids 187 to 325) represents a nonconserved variable region. We analyzed the characteristics of the UL76 protein by employing two computational analyses: AGGRESCAN and TANGO (Fig. 1C). AGGRESCAN predicted nine peaks within UL76 above the threshold, suggesting that these regions are involved in aggregation. Five beta aggregation peaks were consistently predicted by TANGO, and their positions coincide with the peaks obtained from AGGRESCAN (Fig. 1B). Considering the amino acid positions within UL76, we found that eight out of the nine AGGRESCAN peaks and four out of the five TANGO peaks were positioned in the N-terminal region within the conserved domains of the Herpes_UL24 family.
Fig 2.
UL76 conserved region implicated in protein aggregation. (A) Assessment of the aggresome determinant region within UL76. HEK293T cells transiently expressed control EGFP and the fluorescent fusion proteins EGFP-UL76 wt, EGFP-UL76(1-190), and EGFP-UL76(187-325). (B and C) The cells were counterstained with DAPI. FRAP analyses were performed by selecting the ROIs in HEK293T cells transfected with full-length pEGFP-UL76 wt (B) and pEGFP-UL76(1-190) (C). The zero time point was defined as the time when the intensity reached the lowest point after photobleaching, and then single-scan images were obtained at consecutive time points. To assess EGFP-UL76 protein mobility within the aggresome, the ROI (the region between the solid and dotted lines) for photobleaching was defined as the region of the total nucleoplasm excluding the aggresome region. To assess EGFP-UL76 protein mobility in the nucleoplasm, the ROI for photobleaching is the region enclosed with white dots. The normalized intensities are shown to the right and were determined for each time point as the average of four cells. Representative images are shown.
Fig 1.
UL76 protein elicits aggregation and is expressed as an aggresome. (A) UL76 protein localized at nuclear aggresomes of HEL cells 96 h postinfection with HCMV. DIC, differential interference contrast. (B) Schematic depiction of UL76 aggregation hot spots predicted by AGGRESCAN and TANGO. The conserved blocks of the Herpes_UL24 family in Herpesviridae are shown as gray boxes. (C) AGGRESCAN and TANGO predict UL76 aggregation determinant regions. Regions above the hot-spot threshold of AGGRESCAN are considered aggregation prone. As calculated by TANGO, the beta aggregation scores were plotted against the UL76 sequence.
To verify this prediction, we constructed two UL76 deletion mutations: pEGFP-UL76(1-190) and pEGFP-UL76(187-325). Following transient expression in HEK293T cells (Fig. 2A), cells transfected with pEGFP-UL76(1-190) expressed fluorescent nuclear aggresomes, as observed for the full-length pEGFP-UL76 wild type (wt), whereas in the cells transfected with pEGFP-UL76(187-325), the fluorescence intensities were diffusely distributed throughout most of the cell. These images validated the computational prediction (Fig. 2A) that the conserved N-terminal region is the determinant region for protein aggregation.
Nuclear aggresomes are the hallmarks of nuclear misfolded proteins and are found in many neurodegenerative diseases (42). In principle, the protein sequence is a key determinant in the transition from the soluble functional conformation to insoluble misfolded forms of aggregation (43). To assess protein mobility, we conducted a FRAP experiment that revealed the differential mobility of EGFP-UL76 wt in the nucleoplasm and in UL76-induced nuclear aggresomes. The ROI is depicted in the photobleached area. EGFP-UL76 wt is a highly nuclear-bound protein, so we photobleached the entire nucleoplasm area except for the nuclear aggresome, and the fluorescence intensity of the bleached area was monitored (Fig. 2B, row 1). The photobleaching moment was set as zero seconds, and the intensity at this point was set as a relative zero. The recovered intensities after photobleaching were normalized by the differences before and after bleaching at zero seconds. The fluorescence intensities of the images were recorded in a time series, and the normalized intensities are depicted (Fig. 2B, right). After 90 s of photobleaching, the intensities of the bleached area recovered to only 40% of the initial baseline value, which indicates that soluble EGFP-UL76 wt was moving out of the aggresome, whereas the immobile or insoluble form of EGFP-UL76 wt was retained.
EGFP-UL76 wt was retained in the aggresome, which suggests that EGFP-UL76 wt was predominantly insoluble in the aggresome. We observed a different scenario for EGFP-UL76 wt distribution in the nucleoplasm (Fig. 2B, row 2). The ROI is depicted in the photobleached area. The nucleoplasm was partially photobleached, and the fluorescence intensities were recorded in a time series. Normalized intensities are shown (Fig. 2B, right). The fluorescence intensity of EGFP-UL76 wt in the bleached area almost fully recovered after 30 s of photobleaching, which suggests that the EGFP-UL76 wt at the nucleoplasm was soluble and diffused freely to the bleached region.
To determine whether the aggregation-prone region also determines UL76 protein solubility, we conducted FRAP analyses using pEGFP-UL76(1-190). Consistent with the results of the full-length UL76 experiment, most of the EGFP-UL76(1-190) in the aggresomes was insoluble (Fig. 2C, row 1), and the fluorescence intensity of the nucleoplasm partially recovered (approximately 20%), with most of the aggregated proteins largely unaffected (Fig. 2C, right), whereas the intensity of EGFP-UL76(1-190) in the nucleoplasm was almost recovered within 20 s after photobleaching (Fig. 2C, row 2).
Cellular targets of UL76.
To gain insight into the mechanism governing the multifunctionality of UL76, we performed a genome-wide cDNA library screening using a yeast two-hybrid system. Based upon the common presentation of severe neural defects following HCMV congenital infection, we chose a fetal brain cDNA library as prey for the assay. Because UL76 acts as a transcriptional repressor, a conventional yeast two-hybrid system, in which the protein-protein interactions are based on transcriptional activation, may not be suitable for evaluating UL76. Therefore, we employed a CytoTrap yeast two-hybrid system for screening. The basis of the system is the fact that the human Sos protein (hSos; guanyl nucleotide exchange factor) is able to complement the temperature-sensitive mutant of yeast cdc25, which is homologous to hSos (25°C). The recruitment of cellular hSos to the plasma membrane allows the activation of the yeast Ras signaling pathway and, consequently, the growth of the cdc25H yeast strain at the nonpermissive temperature of 37°C (32, 44).
For the initial effort, pSos-UL76 expressing a full-length UL76 protein was used for screening (see Fig. S1 in the supplemental material). However, we did not recover any prey clones in this experiment. We reasoned that in the CytoTrap system, the protein interaction occurs in the membrane, and UL76 is a strong nuclear-bound protein that contains six putative nuclear localization signals (NLSs) (three NLSs in amino acids 20 to 40 and the other three in amino acids 191 to 207, 285 to 291, and 310 to 316, as predicted by WoLF PSORT software) that may hinder protein recruitment to the plasma membrane (see Fig. S1). We then constructed an NLS deletion clone, pSos-UL76ΔNLS (see Fig. S1). As a positive control, pSos MAFB encodes a transcriptional factor belonging to the bZIP family that is able to form homodimers or heterodimers with other transcriptional factors (45). Our results were consistent with previous reports in which cdc25H mutants cotransformed with pMyr MAFB and pSos MAFB were able to grow at the nonpermissive temperature of 37°C (see Fig. S1, row 1), whereas the negative controls indicated that MAFB did not interact with lamin C or collagenase IV, and the two outcomes were also as expected (see Fig. S1, rows 2 and 3). Transformation with pMyr SB, which expressed a Sos-binding domain with a myristoylation signal, served as an additional positive control to verify the cdc25H yeast context (see Fig. S1, row 4). In this experiment, the pSos UL76ΔNLS bait construct passed the control tests, as the cotransformed yeast colonies expressing pMyr Lamin and pMyr SB (see Fig. S1, rows 5 and 6, respectively) demonstrated lack of growth and growth, respectively, at 37°C. After all of the control experiments, 7.5 million yeast colonies were screened. We obtained 207 clones capable of complementing cdc25H yeast. Nucleic acid sequencing analysis verified the identities of these clones, which comprised 68 unique genes. Computational analyses were performed on these UL76 candidate interacting proteins.
Predictions were made using IPA, which suggested that UL76 targets two interrelated networks involved in the process of protein aggregation: protein folding and proteasome-mediated proteolysis (see Fig. S2 in the supplemental material). S5a (PSMD4), which is a universal E3 ligase that plays a major role in accepting polyubiquitinated substrates for proteasome degradation, was selected for further investigation (46). The originally selected clone pMyr 1311, which encodes a truncated S5a (amino acids 52 to 377) (see Fig. S2, row 8), and the clone pMyr S5a, which encodes full-length S5a (see Fig. S2, row 7), were able to complement the growth defect of cdc25H cultured on synthetic galactose minimal medium without uracil and leucine [SD/Gal(-UL)] plates at 37°C.
Interacting domains between UL76 and S5a.
To investigate whether S5a and UL76 form a complex in cells, we expressed plasmids encoding UL76 and S5a sequences in a transient-cotransfection cell culture system. In addition, DNA fragments corresponding to the wild type and deletion mutants of UL76 and S5a, as determined by the identified protein domains, were also cloned into the eukaryotic vectors pEF1/Myc-His C and pcDNA3-HA and tagged with the Myc and HA epitopes, respectively (Fig. 3A). Therefore, in this experiment, the UL76 and S5a constructs were detected using Myc and HA antibodies, respectively. HEK293T cells were transfected with the eukaryotic constructs pEF-UL76 and pcDNA3-S5a. After 48 h of transfection, the cells were harvested, and the protein lysates were prepared for immunoblotting analysis. All of the cell lysates expressed the plasmid-encoded proteins, as expected (Fig. 3B, bottom). In this experiment, UL76 was detected in a protein complex immunoprecipitated by anti-HA-conjugated agarose targeting S5a (Fig. 3B, top). Consistent with this result, in a protein complex precipitated by anti-Myc-conjugated agarose targeting UL76, S5a was detected (Fig. 3B, middle). Our results indicate that UL76 and S5a were in a protein complex. Further investigation was performed to identify the interacting domains.
Fig 3.
UL76 and S5a are in a complex in vivo and interact via conserved domains. (A) Schematic diagrams of the functional domains in full-length (wt) UL76 and S5a, as well as their deletion constructs. The conserved blocks of the Herpes_UL24 family in Herpesviridae are shown as gray boxes. The S5a VWA domain is depicted as a hatched box and UIM motifs as dark-gray boxes. The constructs pEF-UL76, pEF-UL76(1-190), and pEF-UL76(187-325) expressed UL76 wt, UL76(1-190), and UL76(187-325), respectively. The constructs pcDNA3-HA-S5a, pcDNA3-HA-S5a(1-253), pcDNA3-HA-S5a(1-191), and pcDNA3-HA-S5a(196-377) expressed S5a wt, S5a(1-253), S5a(1-191), and S5a(196-377) proteins, respectively. The UL76 and S5a constructs were tagged with Myc and HA epitopes, respectively. As a positive control, 1/100 of the input lysate was used. The lysates from the transfected samples were first analyzed by immunoblotting to confirm their expression. In each group of transfected cells, a plus sign indicates the presence of the designated constructs, whereas a minus sign indicates the absence of the constructs. The numbers on the left of the blots indicate the molecular mass in kDa. (B) UL76 was immunoprecipitated with S5a (HA) antibody and vice versa. HEK293T cells were cotransfected with plasmids expressing wild-type UL76 and/or S5a. (C) The VWA domain of S5a interacts with UL76. S5a deletion mutants coexpressed wild-type full-length UL76. (D) The N-terminal conserved region of UL76 interacts with S5a. UL76 deletion mutants coexpressed wild-type full-length S5a.
The plasmid vectors pEF-UL76(1-190) and pEF-UL76(187-325) were constructed (Fig. 3A). S5a (amino acids 1 to 377) contains an N-terminal consensus domain consisting of a VWA domain (amino acids 2 to 188) and two C-terminal ubiquitin-interacting motifs (UIM1, amino acids 210 to 227, and UIM2, amino acids 282 to 301) (Fig. 3A). The UIM domains contribute to the binding of the polyubiquitinated substrates, and the VWA domain contributes to the binding of the proteasome complex and the importation of substrates to the 20S subunit for proteolytic degradation (9, 47, 48). Accordingly, we constructed deletion mutant plasmids, i.e., pDNA3-S5a(1-191), pDNA3-S5a(1-253), and pDNA3-S5a(196-377), that express the VWA domain, the VWA and UIM1 domains, and the UIM1 and UIM2 domains, respectively.
As shown in Fig. 3C, wild-type UL76 was cotransfected with each of the S5a deletion mutants. Lysate controls indicated that every transfected plasmid expressed its corresponding protein. Subsequent immunoblotting assays revealed that two types of S5a (amino acids 1 to 253 and amino acids 1 to 191) coprecipitated with UL76 upon precipitation with Myc or HA antibody-conjugated agarose, whereas S5a(196-377) was not detected. These results indicate that UL76 interacts with S5a via the VWA domain. Conversely, we also investigated the region of UL76 that interacts with S5a. Using full-length UL76 (UL76 wt) as a positive control, we demonstrated that the conserved domain of UL76 (amino acids 1 to 190), but not the variable domain of UL76 (amino acids 187 to 325), coprecipitated along with S5a (Fig. 3D). In summary, these results indicate that the N-terminal conserved region of UL76 interacts with the VWA domain of S5a and is the aggregation determinant region.
UL76 promotes the accumulation of polyubiquitinated proteins.
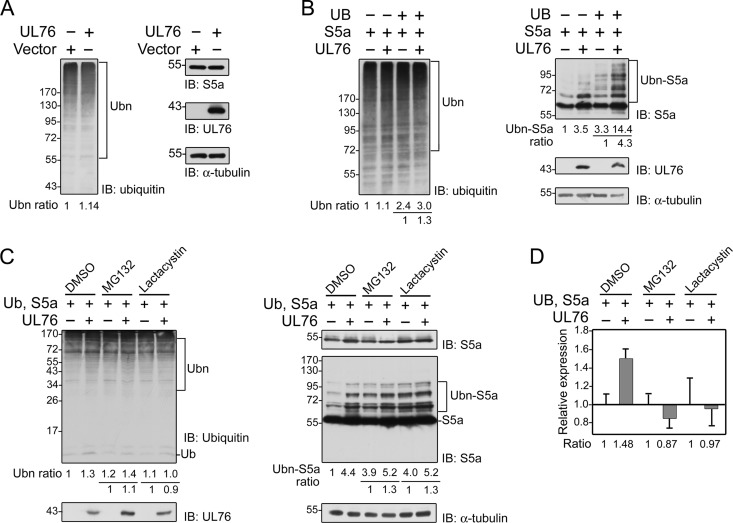
In light of the major role S5a plays as a receptor for polyubiquitinated proteins that are subjected to proteasome-dependent degradation, we investigated whether UL76 affects the expression of total endogenous ubiquitin-conjugated proteins and S5a. With equal loading of control α-tubulin, UL76 transiently expressed in HEK293T cells produced a slight increase in the level of endogenous ubiquitin-conjugated proteins but almost no effect on S5a (Fig. 4A). S5a is known to be extremely unstable and to be degraded and ubiquitinated by almost all types of E3 ligases (46). To detect the effect of UL76 on polyubiquitinated proteins and S5a, S5a and ubiquitin were highly expressed in HEK293T cells. The expression of exogenous ubiquitin-conjugated proteins was moderately enhanced by UL76 (Fig. 4B, left). When the combinations with and without the addition of UL76 were compared, the levels of polyubiquitinated S5a were enhanced by more than 3- to 4-fold. The level of monoubiquitinated S5a was particularly enhanced (Fig. 4B, right).
Fig 4.
UL76 promotes the accumulation of polyubiquitinated proteins at the posttranslational level. (A) The level of endogenous ubiquitinated proteins was enhanced in the presence of UL76. HEK293T cells were transfected with the cloning vector pEF1/Myc-His C or with pEF-UL76. Cell lysates were harvested after 48 h of expression. Endogenous ubiquitin-conjugated proteins and S5a were assessed using anti-ubiquitin and anti-S5a antibodies, respectively. UL76 was detected by a Myc antibody that recognizes the Myc-tagged epitope at the N terminus. The intensities of the indicated polyubiquitinated region (Ubn, marked by brackets) were quantified using a densitometer. The intensity ratios were normalized by the control value. (B) UL76 increased the expression of ubiquitin-conjugated proteins and ubiquitinated S5a in HEK293T cells. Ubiquitin, S5a, and UL76 were produced by transfection with pcDNA3-FLAG-Ub, pcDNA3-HA-S5a, and pEF-UL76, respectively, and detected by antibodies against the FLAG, HA, and Myc epitopes, respectively. (C) UL76 synergistically enhances the accumulation of polyubiquitinated proteins in the presence of proteasome inhibitors. The effects of UL76 with the additional proteasome inhibitors MG132 and clasto-lactacystin β-lactone (Lactacystin) were compared. Dimethyl sulfoxide (DMSO) was used as the control solvent. The expression of exogenous ubiquitinated proteins, S5a, and polyubiquitinated S5a was assessed by immunoblotting analysis. The data were obtained from the average of two representative images. The molecular mass markers are shown on the left in kDa. An equal level of α-tubulin was used as an internal loading control in each experiment. (D) The transcriptional levels of total S5a transcripts in the transfected cells were validated by quantitative PCR. The data are the averages of three repeat experiments. The error bars indicate standard deviations.
Previous studies suggested that inhibition of proteasome-dependent protein degradation leads to the accumulation of undegraded polyubiquitinated proteins that make up globular nuclear- or nucleolar-like aggresomes (49). UL76 produced similar results. To compare the effects of UL76 and proteasome inhibitors, combinational constructs containing a control cloning vector or UL76 were transfected into HEK293T cells, which were then supplemented with MG132 or clasto-lactacystin β-lactone. The levels of cellular polyubiquitinated proteins (Fig. 4C, left) and polyubiquitinated S5a (Fig. 4C, right) were quantified. UL76, the proteasome inhibitor MG132, and, to a lesser extent, clasto-lactacystin β-lactone all increased the levels of polyubiquitinated proteins (Fig. 4C). Even more profound, these three factors increased the level of polyubiquitinated S5a by 4.4-, 3.9-, and 4.0-fold, respectively (Fig. 4C). UL76 synergistically enhanced the effects of MG132 and clasto-lactacystin β-lactone on polyubiquitinated S5a to produce a 1.3-fold increase. In contrast, the combined effects of UL76 and MG132 on the overall level of polyubiquitinated proteins were mild compared to that of MG132 alone (1.1-fold), and no enhancement was detected with the combined treatment of UL76 and clasto-lactacystin β-lactone (1.0-fold). These results suggest that UL76 specifically enhances the accumulation of polyubiquitinated S5a.
Previously, we demonstrated that UL76 is able to modulate the HCMV immediate-early promoter (MIEP) in both the activation and repression modes (25), and we took into account that the eukaryotic expression vector pcDNA3 uses the HCMV IE promoter to drive the expression of exogenous ubiquitin and S5a in these experiments. Among proteasome inhibitors, MG132 is reported to either repress or activate the transcription of MIEP, depending on the specific cell type and cell line (50, 51). To resolve the possibility that the enhancement mediated by UL76 may be caused by the activation of transcription, we assessed the transcriptional levels of S5a in treated cell samples (Fig. 4D). The total cellular RNA was purified, reverse transcribed, and subjected to quantitative RT-PCR amplification using a pair of S5a-specific primers. As expected, UL76 activated the expression of S5a (1.48-fold increase). Moreover, we confirmed previous reports that the two proteasome inhibitors enhance transcription of MIEP by 1.97- and 1.92-fold (data not shown). Nevertheless, the effect of UL76 plus treatment with MG132 reduced S5a transcript expression in comparison with MG132 applied alone (Fig. 4D). Despite the relative transcription repression, the level of polyubiquitinated S5a increased. Consistent outcomes were obtained, and S5a-related transcripts were moderately decreased in cells transfected with UL76 and also subjected to treatment with clasto-lactacystin β-lactone. Polyubiquitinated S5a was consistently shown to be upregulated by 1.3-fold. These results suggest that UL76 stimulates the accumulation of polyubiquitinated S5a independently of transcriptional activation, possibly at a posttranslational step.
UL76 induces accumulation of polyubiquitinated proteins in aggresomes and localizes in proximity to the polyubiquitinated proteins.
Normally, proteasome-dependent ubiquitinated proteins have very short half-lives and high turnover rates in cells. Nevertheless, ubiquitin-conjugated proteins are frequently detected in intranuclear inclusions in neurodegenerative diseases (42). Even though UL76 only slightly increased the level of ubiquitin-conjugated proteins, we continued to explore whether UL76 affects the localization of endogenous ubiquitin-conjugated proteins and whether the ubiquitins are polymerized in a branched linkage. It is known that all seven lysine residues in ubiquitin can be conjugated to proteins, mostly in monoubiquitinated forms. The extended polyubiquitinated chains that predominantly occur in lysine-48-linked Ub are associated with proteolytic function, whereas proteins with a lysine-63 linkage are mainly involved in nonproteolytic pathways, such as protein aggregation (52, 53). Antibodies that recognize specific ubiquitin lysine-48 (Fig. 5A, row 1) or lysine-63 (row 2) linkages were used for immunofluorescence staining of cells expressing EGFP-UL76. Both types of endogenous lysine-linked polyubiquitinated proteins always colocalized with EGFP-UL76 within the nuclear aggresomes. In addition, polyubiquitinated proteins were observed in scattered fine foci without EGFP-UL76 expression (row 1, arrows). These results suggest that UL76 induces redistribution of polyubiquitinated proteins into the aggresome, possibly without selective preference.
Fig 5.
UL76 induces the sequestration of ubiquitin-conjugated proteins in the nuclear aggresome, and UL76 and ubiquitin-conjugated proteins are colocalized in biological proximity. (A) The polyubiquitinated proteins are in either the Lys48- or Lys63-linked chain within the UL76-induced nuclear aggresome. HEK293T cells were transfected with pEGFP-UL76. After 48 h of expression, the cells were fixed and stained by antibodies specific for endogenous ubiquitin Lys48-(Ub-K48) or Lys63-(Ub-K63) linkage (red). The nuclei were stained with DAPI. Immunofluorescence and DIC images were acquired using confocal microscopy. The arrows indicate a cell not expressing EGFP-UL76. (B) Live fluorescence images of HEK293T cells coexpressing EGFP-UL76 and DsRed-Ub. After 48 h of transfection, the fluorescence intensities of EGFP-UL76 and DsRed-Ub along the ROI were measured and are depicted on the right. FRET analysis was used to calculate the efficiency of fluorescence energy transfer and relative Föster distances between EGFP-UL76 and DsRed-Ub. (C) Values corresponding to the ROI.
We evaluated whether ubiquitin-conjugated proteins and UL76 localize in proximity by constructing the plasmid pDsRed-Ub, in which the ubiquitin was expressed in frame with the fluorescent protein DsRed1 to produce the fusion protein DsRed-Ub. Both pEGFP-UL76 and pDsRed-Ub were cotransfected into living HEK293T cells for measurement of the proximity of UL76 and ubiquitin. In this experiment, we again observed few visible red-fluorescent cells among the cells transiently expressing pDsRed-Ub alone (Fig. 5B, row 1) or in the absence of EGFP-UL76, which suggests that the proteins were unstable. We consistently observed that DsRed-Ub was superimposed on EGFP-UL76 in the nuclear aggresome (row 2). FRET analysis was employed to calculate the fluorescence energy transfer efficiency and to measure the relative Föster distance between EGFP-UL76 and DsRed-Ub. At 2.5 to 6 μm, the intensities of both proteins reached a peak (Fig. 5C, top). Moreover, FRET analyses revealed that DsRed-Ub and EGFP-UL76 displayed their highest efficiencies for fluorescence energy transfer, i.e., approximately 0.25 to 0.3, within the nuclear aggresomes (Fig. 5C, middle). In addition, the proteins were separated by 5.5 nm, which is considered to be close enough to allow plausible biological interaction between two proteins (Fig. 5C, bottom). In addition, these proteins were also found to closely colocalize within the nucleoplasm, but the overall distribution was much more scattered.
Normally, S5a has a high turnover rate and a short protein half-life in cells (46). We could hardly detect immunofluorescence signals of endogenous S5a in cells. To visualize the dynamic status of S5a and UL76 in cells, we used two constructs expressing the fluorescent fusion proteins DsRed-S5a and EGFP-UL76. For this experiment, two cell lines were transiently transfected. HEL cells and HEK293T cells are permissive and nonpermissive for the HCMV productive cycle, respectively. As expected, when transfected with pDsRed-S5a without the coexpression of EGFP-UL76, DsRed-S5a fluorescence was diffusely distributed in the cytosol of both HEL and HEK293T cells (Fig. 6A, row 1). EGFP-UL76 intensity was concentrated in nuclear globular aggresomes (row 2). When DsRed-S5a and EGFP-UL76 were coexpressed in both cells, the DsRed-S5a fluorescence was relocalized to the nucleus (row 3). Moreover, DsRed-S5a foci were superimposed or juxtaposed with the EGFP-UL76-associated globular aggresomes. At a high level of expression in HEK23T cells in the absence of UL76, the red DsRed-S5a fluorescence was too scattered and dim to produce any sharp images. However, in the presence of UL76, DsRed-S5a was distributed diffusely in the cytoplasm and also heavily concentrated in nuclear aggresomes, where it was visibly superimposed on EGFP-UL76 in both fixed (Fig. 6A) and live (Fig. 6B) HEK293T cells. Through measurement of the fluorescence intensity of EGFP-UL76 and DsRed-S5a along the ROI (Fig. 6B), we demonstrated through both visualization and quantitative analysis that DsRed-S5a was present at low levels in the cytoplasm (Fig. 6C, top). In contrast, the DsRed-S5a signal was dramatically amplified and colocalized with that of EGFP-UL76 in the nucleus, particularly along the 4.5- to 9-μm ROI. These results demonstrate that EGP-UL76 induces the accumulation and relocalization of DsRed-S5a. To determine whether these two proteins are actually close enough for biological reactions, we conducted FRET analysis, calculated the fluorescence energy transfer efficiency, and measured the relative Föster distance between EGFP-UL76 and DsRed-S5a. Our results demonstrate that the ratio of transfer efficiency exhibited the highest peak of approximately 0.25 (Fig. 6C, middle). Consistently, at the aggresome locations, the Föster distances between the proteins were determined to be approximately 5.5 nm, which falls well within the range that allows biochemical reactions between two proteins (Fig. 6C, bottom). Within the aggresome, both EGFP-UL76 and DsRed-S5a emitted the brightest fluorescence, which indicates the most effective energy transfer and the shortest distances between the proteins (Fig. 6B and C). Outside the aggresomes, a few spots were also detected to colocalize with distances of less than 7 nm, which suggests that protein-protein interactions between UL76 and S5a can also occur in the nucleoplasm. It is unlikely that any interaction occurs in the cytoplasm, as EGFP-UL76 was not detected in the cytoplasm, no trace of energy transfer was detected, and the cytoplasmic distances between EGFP-UL76 and DsRed-S5a were greater than 10 nm. All of these results suggest that UL76 affects the nuclear portion of S5a. Additionally, it is evident that the aggregation-prone region UL76(1-190) is responsible for the sequestration of S5a and Ub (Fig. 6D) in aggresomes. In the cells coexpressing EGFP-UL76(187-325) and DsRed-S5a or DsRed-Ub, only diffusive fluorescence was observed.
Fig 6.
UL76 induces the redistribution of S5a, and UL76 and S5a are colocalized in biological proximity. (A) Images of cells cotransfected with pEGFP and pDsRed-S5a (row 1) or pDsRed and pEGFP-UL76 (row 2) served as controls. The fluorescent fusion proteins EGFP-UL76 and DsRed-S5a were expressed in both HEL and HEK293T cells by transfection (row 3). At 48 h posttransfection, the cells were fixed, and images were acquired. The nuclei were counterstained with DAPI. (B) Live fluorescence and DIC images for HEK293T cells coexpressing EGFP-UL76 and DsRed-S5a. The fluorescence intensities of EGFP-UL76 and DsRed-S5a, along with the ROI, were measured and are depicted on the right. FRET analysis was used to calculate the efficiency of fluorescence energy transfer and relative Föster distances between EGFP-UL76 and DsRed-S5a. (C) Values corresponding to the ROI. (D) The N-terminal conserved region of UL76 shows the sequestration of ubiquitin and S5a. Live fluorescence images of HEK293T cells coexpressing EGFP-UL76(1-190) and DsRed-S5a, EGFP-UL76 and DsRed-Ub, EGFP-UL76(187-325) and DsRed-S5a, and EGFP-UL76(187-325) and DsRed-Ub are shown.
S5a mediates the accumulation of UL76-induced nuclear aggresomes.
There are two subunits of the 26S proteasome that act as receptors for the polyubiquitinated proteins: S5a and Rpn13 (8). S5a plays a major role in mediating the proteolysis of approximately one-fourth of cellular ubiquitinated proteins (54). We hypothesized that UL76-induced accumulation of undigested polyubiquitinated proteins is mediated by interaction with S5a. Therefore, we conducted an investigation using RNA interference techniques to reduce the production of endogenous S5a. Pseudoviruses harboring shRNA I and shRNA II that specifically targeted S5a sequences were prepared and transduced into two cell lines, HEL and HEK293T, respectively. After 3 days of expression in both cell types, immunoblotting analyses were performed to quantify the protein expression (Fig. 7A). In both cell types, S5a shRNA I treatment resulted in approximately 13% reduction of S5a protein (not statistically significant), whereas shRNA II caused significant 37% and 46% reductions at the time of pEGFP-UL76 transfection (Fig. 7A). Following transduction, pEGFP-UL76 was transfected into S5a-knockdown HEK293T and HEL cells. EGFP-UL76 protein was expressed for 48 h. Immunoblotting analyses performed for the transduced and transfected HEK293T and HEL cells showed that the production of EGFP-UL76 remained constant for the cells regardless of S5a reduction (Fig. 7A). In a concurrent experiment, the treated cells were fixed and counterstained with DAPI. The number of cells expressing EGFP-UL76 fluorescence was analyzed using a TissueFaxs system. The shRNA II knockdown of S5a reduced the number of cells expressing EGFP-UL76 aggresomes in both HEK293 and HEL cells (Fig. 7B). After S5a was knocked down using shRNA II pseudovirus, we consistently obtained a significant reduction in the range intensity (peak intensity) of fluorescent aggresomes in both cell types. In Fig. 7C, HEK293T cells exhibited 45%, whereas HEL cells exhibited 43% aggresome intensity following S5a shRNA II treatment. Overall, these results indicate that the knockdown of S5a by RNA interference reduced the EGFP-UL76 range intensity associated with aggresomes, even though total EGFP-UL76 protein levels were minimally affected.
Fig 7.
Knockdown of S5a reduces the number of aggresomes in EGFP-UL76-expressing cells. HEL and HEK293T cells were transduced with control (Ctr) and human S5a-specific shRNA I and shRNA II pseudoviruses. After transfection of EGFP-UL76 for 48 h, the cells were processed. (A) The protein production of S5a, EGFP-UL76, and tubulin was assessed by immunoblot analysis. (B) Representative images of control or S5a-suppressed cells transfected with EGFP-UL76. The total number of cells was determined by DAPI staining. Cells containing EGFP-UL76 nuclear aggresomes were counted by TissueFaxs. (C) The percentage of cells with EGFP-UL76 aggresomes was calculated and normalized against the value in cells transduced with the Ctr pseudovirus. The data are reported as the averages of three independent experiments. The error bars indicate standard deviations. Total cell counts were >7,000 for each treatment. **, 0.005 < P < 0.01; ***, P < 0.005.
S5a is closely complexed with UL76 during HCMV infection.
To investigate the relative distributions of UL76 and S5a during the HCMV infectious cycle, we used an immunoblotting analysis that indicated that the expression of UL76 and S5a increased over time, reaching peaks at 96 h postinfection (Fig. 8A). These findings validate the idea that UL76 is a late gene. To investigate whether UL76 and S5a also interact in HCMV-infected cells, we conducted a coimmunoprecipitation experiment by infecting HEL cells with HCMV and preparing protein lysates from mock- and HCMV-infected cells. Specific S5a antibody was applied to form protein complexes that were subjected to immunoblotting analysis using a UL76 antibody. As shown in Fig. 8B, a UL76 signal was detected in the lysate of cells harvested 96 h postinfection. This result indicates that UL76 and S5a complex together in the late phase of infection. Furthermore, the appearance of both proteins was tracked by immunofluorescent cell staining following visualization under a laser scanning confocal microscope. As shown in Fig. 8C, we hardly observed UL76 before 16 h of viral infection had passed. At 16 h postinfection, UL76 appeared at low intensity and was distributed as speckled foci. At 72 to 96 h after HCMV infection, UL76 appeared exclusively in the nucleus, predominantly in granular aggresomes. In mock-infected HEL cells, the fluorescence of S5a was observed as diffuse foci distributed in both the nucleus and cytoplasm. In the late phase of infection, S5a foci became more compact and localized to the vicinity of or within UL76 globular aggresomes in the nucleus. In Fig. 8C, the arrows show the putative colocalization of UL76 and S5a. In the subsequent FRET assay, we measured the energy transfer efficiency and Föster distance between UL76 and S5a. The ROI is depicted as a white line crossing the UL76 aggresome, nucleus, and cytoplasm (Fig. 8D). Along the ROI, the intensities of UL76 and S5a were measured. Within the aggresome, the UL76 and S5a signals were generally more condensed and stronger than the signals scattered in the nucleoplasm (Fig. 8E). The efficiency of energy transfer in this assay reached 0.66. Föster distances of UL76 and S5a were between 5.4 and 6.7 nm. We therefore conclude that the interaction of UL76 and S5a occurs in the aggresome, as well as the nucleus, in the late stage of HCMV infection. In addition, we measured the mean intensities of UL76 and S5a for two aggresomes and 11 randomly selected ROIs in the nucleus. We found that the mean intensities of UL76 versus S5a are fitted to a linear regression curve (r2 = 0.9586; P < 0.0001), suggesting concurrent increases in both UL76 and S5a intensities in the infected cells (Fig. 8F). This suggests the possibility that UL76 sequesters S5a in HCMV-infected cells.
Fig 8.
UL76 and S5a interactions in the HCMV infectious cycle. (A) Immunoblot analyses of the HCMV UL76 and S5a proteins during the HCMV replication cycle. HEL cells were infected at an MOI of 3 PFU/cell. Cell lysates harvested at 8, 16, 24, 48, 72, and 96 h postinfection were resolved by SDS-PAGE. The immunoblots were incubated with polyclonal mouse anti-UL76, polyclonal rabbit anti-S5a, and monoclonal mouse anti-tubulin antibodies. The sizes of the molecular mass markers are shown on the left in kDa. (B) UL76 and S5a are in a protein complex at 96 h postinfection. Lysates of mock-infected (M) and HCMV-infected (V) cells 96 h postinfection were harvested for coimmunoprecipitation. A rabbit monoclonal antibody for S5a was used to precipitate UL76. After the matrix was washed, the conjugated proteins were analyzed using UL76 antibody. As the control, 1/400 of the input lysate was used. (C) Distribution of UL76 and S5a in the late phase of infection. Immunofluorescent cell staining was performed on HCMV-infected HEL cells harvested during the viral productive cycle at 16, 72, and 96 h postinfection. The nuclei were counterstained with DAPI. The arrows indicate the putative UL76 and S5a colocalized spots. (D) UL76 and S5a were in close proximity in the late phase of infection, as shown by FRET analysis. Fluorescence intensities of UL76 and S5a were evaluated 96 h after infection with HCMV. The ROI was measured and is depicted on the right. FRET analysis was used to calculate the efficiency of fluorescence energy transfer and the relative Föster distance between UL76 and S5a. (E) Values corresponding to the ROI. (F) Mean intensities of S5a and UL76 staining in the nucleus of the cell in panel D. Data points were calculated from the two aggresomes and 11 randomly selected ROIs in the nucleus. The r2 value and P value of the linear regression line are shown.
Appearance of UL76 aggresome related to the replication compartment.
Members of the Herpes_UL24 family, including HCMV UL76, are associated with mature viral particles (24, 55). It is plausible that UL76 is linked to mature virus production. For this reason, we performed immunofluorescent cell staining to visualize the localization of the UL76 aggresome with respect to the replication compartment marked by UL112 protein, which is involved in the replication compartment by association with UL44 (DNA polymerase processivity factor) and the HCMV lytic origin of replication (56, 57). As shown in Fig. 9A, we observed that a distinguishable UL76 aggresome emerged at 72 h postinfection and was juxtaposed with a replication compartment within scattered UL76 foci. In addition, UL76 localized to a lesser extent around the replication compartment. We noticed that the UL76 aggresome emerges only with a fully developed replication compartment. In particular, the fluorescence intensity was highest along the periphery of the aggresome and replication compartment. In cells containing UL112 speckled foci that indicated an immature replication compartment, the UL76 aggresome was not observed (Fig. 9A, arrows). To obtain a precise quantification, the fluorescence intensities of UL76 and UL112 in infected cells were measured and statistically analyzed. In the representative plot (Fig. 9B), the UL76 intensity increased along with the increase in UL112 intensity over time. Few cells expressed only UL76, and no aggresomes or aberrant cellular masses were observed. Statistical analysis revealed that cells with UL76 aggresomes had a mature replication compartment (Fig. 9C), and the fraction of UL76+ UL112+ cells increased from 72 to 96 h postinfection.
Fig 9.
The UL76 aggresomes are adjacent to fully developed replication compartments. HEL cells were infected with HCMV at an MOI of 1 PFU/ml. At 72 and 96 h postinfection (hpi), the cells were fixed and stained by antibodies specific for UL76 (aggresome; green) and UL112 (replication compartment; red). The nuclei were counterstained with DAPI. (A) Confocal images of the XY plane were sequentially acquired in the Z axis. Images of the XZ and YZ planes were acquired from the ROI in the XY plane stacked by Z axis sequential images. The arrows indicate cells with an immature replication compartment. (B) Representative distribution of fluorescence intensities for UL76 versus UL112 from TissueFaxs analyses. The summed intensities of UL76 or UL112 in log scale within one cell are plotted. The total cell numbers and relative positions are marked by DAPI. (C) Quantification of cells expressing UL112 (replication compartment) and the UL76 aggresome according to TissueFaxs microscopy. (D) Knockdown of S5a reduces the development of the replication compartment and the UL76 aggresome in the late phase of infection. Pseudoviruses expressing control or S5a shRNA II were transduced into cells that were postinfected with HCMV for 24 or 48 h. At 96 h of infection, the cells were subjected to immunofluorescent cell staining and quantitative analyses. Fluorescence intensities above and below the cutoff were considered positive (+) and negative (−), respectively. The cutoff values were set automatically by the software. The cells were divided into four groups: UL112+ UL76+, UL112+ UL76−, UL112− UL76+, and UL112− UL76−. The percentiles were derived from three replicates, and at least 7,000 cells were analyzed for each slide.
We further investigated whether S5a is involved in the formation of UL76 aggresomes in HCMV-infected cells. Pseudovirus of S5a shRNA II was transduced in HEL cells at 24 and 48 h postinfection. Subsequent incubation continued to 96 h after infection; then, the cells were subjected to immunofluorescent cell staining of UL76 and UL112. The TissueFaxs system was employed for quantitation analysis of the fluorescence intensity and distribution profile. This experiment verified that knockdown of S5a reduced the fraction of UL76+ UL112+ cells in comparison with cells transduced with control pseudovirus (Fig. 9D). There was marginal decrease of cells sorted as UL76+ UL112+ when knockdown of S5a occurred at 24 h postinfection (P = 0.0992). Notably, when the knockdown of S5a started at 48 h postinfection, there was a significant reduction of UL76+ UL112+ (P = 0.006) cells compared to control transduction, suggesting the involvement of S5a in the formation of the mature replication compartment and aggresome in the late phase of infection.
DISCUSSION
In this study, we demonstrated two novel characteristics of the HCMV UL76 protein. (i) The UL76 aggresome is involved in UL76-mediated aggregation by interaction with S5a. (ii) The UL76 aggresome is associated with a mature replication compartment. Protein integrity requires folding into appropriate three-dimensional conformations to allow the protein to perform its distinctive biological function. Misfolded proteins have reduced motility and solubility and can produce adverse stresses in cells. The removal of misfolded nuclear proteins in mammalian cells has not been fully elucidated but may result from the combined processes of the UPS and translational machinery (see Fig. S2 in the supplemental material) (58). One determinant of the initiation of protein misfolding appears to be the protein sequence, for which the transition from hidden to exposed hydrophobicity is critical and has to be recognized by specific E3 ligases of UPS, such as San1 in yeast (12–14, 59). Although insoluble protein aggresomes are the hallmark of misfolded proteins, previous studies by Bennett and colleagues provide insight into the induction of dysfunctional protein folding. They demonstrated that protein misfolding impairs UPS proteolytic function before any aggregated proteins are deposited into visible foci (60). Therefore, the UPS threshold in the recognition of protein misfolding is a highly stringent control with regard to the global biological pathways that involve the UPS. Consistent results are obtained upon inhibition of the UPS by proteasome-specific inhibitors. In vitro, when cultured cells are treated with MG132 or lactacystin, insoluble aggresomes develop in the nucleus (49, 61).
Protein aggregation is determined by several factors, including amino acid composition, sequence, exposed hydropathicity, charge, and β-structure propensity (43). In computational analyses of the UL76 sequence, two separate programs (AGGRESCAN and TANGO) predicted that an aggregation-prone region of UL76 would be located in the N-terminal conserved blocks of the Herpesviridae Herpes_UL24 family (Fig. 1B). These results suggest that UL76 is aggregation prone (36, 38). In the HCMV infectious cycle (Fig. 1A, 8C and D, and 9A) and in the absence of other viral proteins (Fig. 2, 5, and 6), UL76 was predominantly observed in globular nuclear aggresomes. During the monitoring of live cells, we observed that UL76 proteins were soluble in the nucleoplasm and strongly insoluble in the aggresome (Fig. 2), which suggests that the transition into a misfolded insoluble conformation is possibly initiated in the nucleoplasm.
The interaction of UL76 with S5a of the UPS (see Fig. S1 in the supplemental material) was shown to be mediated by the conserved blocks of UL76 and the VWA domain of S5a (Fig. 3). Previous studies indicated that S5a plays a role as a hinge in the 19S and 20S proteasome complexes and presumably stabilizes the integral structure of the 26S proteasome (62). Additionally, an electron cryomicroscopy study mapped the location of S5a to the apical region of the 19S regulatory proteasome and indicated that the VWA domain is embedded within the other 19S proteasome subunit (63, 64). If the S5a VWA domain is deleted, the 19S proteasome still binds polyubiquitinated proteins but does not deliver them into the 20S proteasome. As a result, the cells accumulate polyubiquitinated proteins, which suggests the VWA domain is likely required for translocation of polyubiquitinated proteins into the 20S proteasome (48). These results support our finding that the interaction of UL76 with VWA likely blocks the further translocation of polyubiquitinated proteins for proteolytic processes. Our S5a depletion experiments demonstrated reduced aggresome formation when S5a was knocked down by RNA interference (Fig. 7 and 9). We propose that the interaction of UL76 and S5a in the VWA domain may interfere with the importation of polyubiquitinated proteins into the 20S proteasome. Recently, FAT10, a ubiquitin-like modifier, was shown to be conjugated to proteins that are accepted by S5a via binding to the VWA domain for UPS degradation (65). Proteins that undergo FAT10 conjugation include polyglutamine proteins in the cytoplasm and nucleus (66). Whether UL76 affects the degradation of FAT10-conjugated substrates remains to be investigated. In addition, our results indicate that S5a is critical in protein aggregation, as the knockdown of S5a reduced UL76 aggregation in cells (Fig. 7). Similar findings have been previously described in cells exposed to ionizing radiation and DNA damage, which activate the signaling pathways for the development of radiation-induced nuclear foci. Depletion of S5a reduces the formation of foci associated with BRCA1, RAD51, and FANCD2 (67). In reviewing the literature, we found that the depletion of S5a by RNA interference increases the level of polyubiquitinated, FAT10-conjugated proteins and the transcriptional levels of 26S proteasome subunits in various cell types (65, 68, 69). Therefore, the connection between an aggregation-prone protein phenotype and S5a may be decisive in the development of protein aggresomes. Intriguingly, our results suggest that S5a is involved, not only in the formation of aggresomes, but also in the development of the replication compartment (Fig. 9). Coincidentally, Tran et al. demonstrated that S5a is relocated to the replication compartment (22).
We noted the robust fluorescence intensities of UL76 in transfected HEK293T cells (Fig. 2, 5, and 6). In these cells, extensive interactive signals (energy transfer efficiency) and close proximity (distance) were recorded within aggresomes. However, only low levels of coimmunoprecipitated UL76 and S5a proteins were recovered in either transfected or virus-infected cells (Fig. 3 and 8B). The discrepancy between immunofluorescent staining and immunoprecipitation/immunoblotting results likely reflects the low solubility (or immobility) of UL76, which was not fully solubilized in the protein extracts used for coimmunoprecipitation (data not shown), within aggresomes (Fig. 2). The protein isoelectric point (pI) is considered an important factor affecting the efficiency of transferring proteins onto polyvinylidene difluoride (PVDF) membranes mediated by electrophoresis. UL76 and its family members (Herpes_UL24 and PF01646) are extraordinary for their highly basically charged amino acid sequences. The UL76 protein exhibits a theoretical pI value of 11.64, presumably resulting in low efficiency of protein transfer in immunoblot analyses.
Virus-induced protein aggresomes are found in the cytoplasm and the nuclei in many virus-infected cells (70). Various roles for virus-induced aggresomes have been proposed. The protein contexts of these aggresomes implicate them in viral infection. It is generally perceived that the virus uses protein aggresomes to fine-tune protein levels in the cellular microenvironment for different purposes. During viral infection, unusual virus-associated inclusion bodies or aggresomes are observed (31, 71). In the case of HCMV, UL76 and the other eight proteins encoded by HCMV (TRL5, TRL9, UL31, UL35, UL76, UL80a, US32, and US33) visibly aggregate in the nuclei of HEK293T cells (31). Among them, UL35, which presents as a nuclear aggregate distinct from that of UL76, is required for efficient HCMV replication (72). In contrast, overexpression of UL76 inhibits virus production and strongly represses gene expression (24). Protein analysis reveals that the UL35 nuclear body recruits the E3 ligases involved in DNA repair, a ubiquitin-specific protease USP7, and ubiquitinated proteins with Lys48 linkages (72), which potentially implicates them in the viral replication process. The nuclear body UL80a serves as a capsid assembly site where proteins encoded by UL80a interact with the major capsid protein UL86 (73). Herpes simplex virus 1 (HSV-1) modulates the UPS and molecular chaperones, which indicates that a nuclear body, VICE (virus-induced chaperone-enriched domain), which harbors abundant polyubiquitinated proteins, is associated with viral replication (74). HCMV may target proteolytic machinery as a strategy for efficient alteration of the protein microenvironment. Our results support the idea that UL76-induced aggresomes are a distinct type of nuclear aggresome emerging along with the viral replication compartment. Regarding the deletion of UL76 in the context of viruses, contradictory results have been reported for virus production. Recombinant HCMV with a UL76 mutation derived from either deletion or transposition exhibits dramatically decreased virus production (75, 76). However, other evidence demonstrates that UL76 is not an essential gene and that the recombinant virus with a UL76 deletion has a reductive effect on virus production at low MOI, but the effect is not obvious at high MOI (26).
Various reports indicate that the Herpes_UL24 family is engaged in a wide range of roles during herpesvirus infection. Proteins of the Herpes_UL24 family are distributed predominantly in the nucleus in globular aggresomes (HCMV, HSV-1, human herpesvirus 8 [HHV-8], and murine gammaherpesvirus 68 [MHV-68]) (31, 77, 78). In HSV-1, several nucleolar proteins have been associated with these nuclear aggresomes (31, 79). Approaching the late phase of infection, the dispersion of UL24-associated nuclear aggresomes is related to the N-terminal conserved PD-(D/E)XK motif, which is predicted to encode a potential endonuclease (80). In addition, an HSV-1 UL24-specific aggresome has been shown to be a separate viral nuclear substructure independent of the replication compartment (HSV-1) (81), which implicates the protein in a nonessential role in viral lytic replication in cell culture (HSV-1, HSV-2, MHV-68, varicella-zoster virus [VZV], and equine herpesvirus 1 [EHV-1]) (82–85). The aggresomes are capable of modulating protein expression at both the transcriptional and translational levels (HCMV) (25, 26). Notably, in vivo animal models provide several lines of evidence to support the idea that Herpes_UL24 family proteins (HSV-1, HSV-2, EHV-1, and HMV-68) may be pathogenic determinants for neurological and lung infections and may be critical in the efficient reactivation of latent virus from sensory ganglia (82–84, 86, 87). Moreover, the conserved N-terminal region (HSV-1) is required for pathogenic manifestation (86). Taking all these data together, we conclude that the N-terminal conserved region of the Herpes_UL24 family contributes to aggregation, the usurping of the UPS, potential endonuclease activity, and pathogenic effects.
In this study, we presented evidence that the interaction of UL76 and S5a modulates the proteolytic function of the UPS, which may be a common underlying mechanism explaining the diverse activities of the Herpes_UL24 family. Currently, we are investigating the relationship between the UL76 aggresome and the replication compartment.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by grants from the National Science Council (NSC), Taiwan, Republic of China (NSC99-2320-B-037-005-MY3 and NSC100-2320-B-037-005-MY3) and from the Kaohsiung Medical University (KMU-M098002) awarded to S.-K.W. The RNAi reagents were obtained from the National RNAi Core Facility, supported by the National Research Program for Genomic Medicine Grants from the NSC.
We thank the Center for Research Resources and Development of Kaohsiung Medical University for providing technical support for the laser scanning confocal microscope and TissueFaxs machines.
Footnotes
Published ahead of print 21 August 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.01568-13.
REFERENCES
- 1.Mocarski ES, Shenk T, Pass RF. 2007. Cytomegalovirus, p 2701–2772 In Knipe M, Howley PM. (ed). Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 2.Hansen SG, Powers CJ, Richards R, Ventura AB, Ford JC, Siess D, Axthelm MK, Nelson JA, Jarvis MA, Picker LJ, Fruh K. 2010. Evasion of CD8+ T cells is critical for superinfection by cytomegalovirus. Science 328:102–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michaelis M, Doerr HW, Cinatl J. 2009. The story of human cytomegalovirus and cancer: increasing evidence and open questions. Neoplasia 11:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caposio P, Orloff SL, Streblow DN. 2011. The role of cytomegalovirus in angiogenesis. Virus Res. 157:204–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carter CJ. 2009. Schizophrenia susceptibility genes directly implicated in the life cycles of pathogens: cytomegalovirus, influenza, herpes simplex, rubella, and Toxoplasma gondii. Schizophr. Bull. 35:1163–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weissman AM, Shabek N, Ciechanover A. 2011. The predator becomes the prey: regulating the ubiquitin system by ubiquitylation and degradation. Nat. Rev. Mol. Cell Biol. 12:605–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciechanover A. 2005. Proteolysis: from the lysosome to ubiquitin and the proteasome. Nat. Rev. Mol. Cell Biol. 6:79–87 [DOI] [PubMed] [Google Scholar]
- 8.Husnjak K, Elsasser S, Zhang N, Chen X, Randles L, Shi Y, Hofmann K, Walters KJ, Finley D, Dikic I. 2008. Proteasome subunit Rpn13 is a novel ubiquitin receptor. Nature 453:481–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verma R, Oania R, Graumann J, Deshaies RJ. 2004. Multiubiquitin chain receptors define a layer of substrate selectivity in the ubiquitin-proteasome system. Cell 118:99–110 [DOI] [PubMed] [Google Scholar]
- 10.Kang Y, Chen X, Lary JW, Cole JL, Walters KJ. 2007. Defining how ubiquitin receptors hHR23a and S5a bind polyubiquitin. J. Mol. Biol. 369:168–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gardner RG, Nelson ZW, Gottschling DE. 2005. Degradation-mediated protein quality control in the nucleus. Cell 120:803–815 [DOI] [PubMed] [Google Scholar]
- 12.Matsuo Y, Kishimoto H, Tanae K, Kitamura K, Katayama S, Kawamukai M. 2011. Nuclear protein quality is regulated by the ubiquitin-proteasome system through the activity of Ubc4 and San1 in fission yeast. J. Biol. Chem. 286:13775–13790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenbaum JC, Fredrickson EK, Oeser ML, Garrett-Engele CM, Locke MN, Richardson LA, Nelson ZW, Hetrick ED, Milac TI, Gottschling DE, Gardner RG. 2011. Disorder targets misorder in nuclear quality control degradation: a disordered ubiquitin ligase directly recognizes its misfolded substrates. Mol. Cell 41:93–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fredrickson EK, Rosenbaum JC, Locke MN, Milac TI, Gardner RG. 2011. Exposed hydrophobicity is a key determinant of nuclear quality control degradation. Mol. Biol. Cell 22:2384–2395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blanchette P, Branton PE. 2009. Manipulation of the ubiquitin-proteasome pathway by small DNA tumor viruses. Virology 384:317–323 [DOI] [PubMed] [Google Scholar]
- 16.Martin-Serrano J. 2007. The role of ubiquitin in retroviral egress. Traffic 8:1297–1303 [DOI] [PubMed] [Google Scholar]
- 17.Hewitt EW. 2003. The MHC class I antigen presentation pathway: strategies for viral immune evasion. Immunology 110:163–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiebusch L, Bach M, Uecker R, Hagemeier C. 2005. Human cytomegalovirus inactivates the G0/G1-APC/C ubiquitin ligase by Cdh1 dissociation. Cell Cycle. 4:1435–1439 [DOI] [PubMed] [Google Scholar]
- 19.Tran K, Mahr JA, Choi J, Teodoro JG, Green MR, Spector DH. 2008. Accumulation of substrates of the anaphase-promoting complex (APC) during human cytomegalovirus infection is associated with the phosphorylation of Cdh1 and the dissociation and relocalization of APC subunits. J. Virol. 82:529–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tavalai N, Stamminger T. 2011. Intrinsic cellular defense mechanisms targeting human cytomegalovirus. Virus Res. 157:128–133 [DOI] [PubMed] [Google Scholar]
- 21.Kalejta RF. 2008. Functions of human cytomegalovirus tegument proteins prior to immediate early gene expression. Curr. Top. Microbiol. Immunol. 325:101–115 [DOI] [PubMed] [Google Scholar]
- 22.Tran K, Mahr JA, Spector DH. 2010. Proteasome subunits relocalize during human cytomegalovirus infection, and proteasome activity is necessary for efficient viral gene transcription. J. Virol. 84:3079–3093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bateman A, Coin L, Durbin R, Finn RD, Hollich V, Griffiths-Jones S, Khanna A, Marshall M, Moxon S, Sonnhammer EL, Studholme DJ, Yeats C, Eddy SR. 2004. The Pfam protein families database. Nucleic Acids Res. 32:D138–D141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang SK, Duh CY, Wu CW. 2004. Human cytomegalovirus UL76 encodes a novel virion-associated protein that is able to inhibit viral replication. J. Virol. 78:9750–9762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang SK, Duh CY, Chang TT. 2000. Cloning and identification of regulatory gene UL76 of human cytomegalovirus. J. Gen. Virol. 81:2407–2416 [DOI] [PubMed] [Google Scholar]
- 26.Isomura H, Stinski MF, Murata T, Nakayama S, Chiba S, Akatsuka Y, Kanda T, Tsurumi T. 2010. The human cytomegalovirus UL76 gene regulates the level of expression of the UL77 gene. PLoS One 5:e11901. 10.1371/journal.pone.0011901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodrum FD, Jordan CT, High K, Shenk T. 2002. Human cytomegalovirus gene expression during infection of primary hematopoietic progenitor cells: a model for latency. Proc. Natl. Acad. Sci. U. S. A. 99:16255–16260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodrum F, Jordan CT, Terhune SS, High K, Shenk T. 2004. Differential outcomes of human cytomegalovirus infection in primitive hematopoietic cell subpopulations. Blood 104:687–695 [DOI] [PubMed] [Google Scholar]
- 29.Cheung AK, Abendroth A, Cunningham AL, Slobedman B. 2006. Viral gene expression during the establishment of human cytomegalovirus latent infection in myeloid progenitor cells. Blood 108:3691–3699 [DOI] [PubMed] [Google Scholar]
- 30.Siew VK, Duh CY, Wang SK. 2009. Human cytomegalovirus UL76 induces chromosome aberrations. J. Biomed. Sci. 16:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salsman J, Zimmerman N, Chen T, Domagala M, Frappier L. 2008. Genome-wide screen of three herpesviruses for protein subcellular localization and alteration of PML nuclear bodies. PLoS Pathog. 4:e1000100. 10.1371/journal.ppat.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aronheim A, Zandi E, Hennemann H, Elledge SJ, Karin M. 1997. Isolation of an AP-1 repressor by a novel method for detecting protein-protein interactions. Mol. Cell. Biol. 17:3094–3102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gus Y, Karni R, Levitzki A. 2007. Subunit S5a of the 26S proteasome is regulated by antiapoptotic signals. FEBS J. 274:2815–2831 [DOI] [PubMed] [Google Scholar]
- 34.Axelrod D, Koppel DE, Schlessinger J, Elson E, Webb WW. 1976. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys. J. 16:1055–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horton P, Park KJ, Obayashi T, Fujita N, Harada H, Adams-Collier CJ, Nakai K. 2007. WoLF PSORT: protein localization predictor. Nucleic Acids Res. 35:W585–W587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conchillo-Sole O, de Groot NS, Aviles FX, Vendrell J, Daura X, Ventura S. 2007. AGGRESCAN: a server for the prediction and evaluation of “hot spots” of aggregation in polypeptides. BMC Bioinformatics 8:65–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanchez de Groot N, Pallares I, Aviles FX, Vendrell J, Ventura S. 2005. Prediction of “hot spots” of aggregation in disease-linked polypeptides. BMC Struct. Biol. 5:18–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fernandez-Escamilla AM, Rousseau F, Schymkowitz J, Serrano L. 2004. Prediction of sequence-dependent and mutational effects on the aggregation of peptides and proteins. Nat. Biotechnol. 22:1302–1306 [DOI] [PubMed] [Google Scholar]
- 39.Wang SK, Hu CH, Lu MC, Duh CY, Liao PC, Tyan YC. 2009. Novel virus-associated proteins encoded by UL112-113 of human cytomegalovirus. J. Gen. Virol. 90:2840–2848 [DOI] [PubMed] [Google Scholar]
- 40.Patterson GH, Piston DW, Barisas BG. 2000. Föster distances between green fluorescent protein pairs. Anal. Biochem. 284:438–440 [DOI] [PubMed] [Google Scholar]
- 41.Elangovan M, Wallrabe H, Chen Y, Day RN, Barroso M, Periasamy A. 2003. Characterization of one- and two-photon excitation fluorescence resonance energy transfer microscopy. Methods 29:58–73 [DOI] [PubMed] [Google Scholar]
- 42.Woulfe JM. 2007. Abnormalities of the nucleus and nuclear inclusions in neurodegenerative disease: a work in progress. Neuropathol. Appl. Neurobiol. 33:2–42 [DOI] [PubMed] [Google Scholar]
- 43.Chiti F. 2006. Relative importance of hydrophobicity, net charge, and secondary structure propensities in protein aggregation, p 43–59, In Uversky VN, Fink AL. (ed), Protein misfolding, aggregation, and conformational diseases. Part A. Protein aggregation and conformational diseases. Protein reviews, vol 4 Springer, New York, NY [Google Scholar]
- 44.Aronheim A, Engelberg D, Li N, al-Alawi N, Schlessinger J, Karin M. 1994. Membrane targeting of the nucleotide exchange factor Sos is sufficient for activating the Ras signaling pathway. Cell 78:949–961 [DOI] [PubMed] [Google Scholar]
- 45.Kataoka K, Fujiwara KT, Noda M, Nishizawa M. 1994. MafB, a new Maf family transcription activator that can associate with Maf and Fos but not with Jun. Mol. Cell. Biol. 14:7581–7591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uchiki T, Kim HT, Zhai B, Gygi SP, Johnston JA, O'Bryan JP, Goldberg AL. 2009. The ubiquitin-interacting motif protein, S5a, is ubiquitinated by all types of ubiquitin ligases by a mechanism different from typical substrate recognition. J. Biol. Chem. 284:12622–12632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Q, Young P, Walters KJ. 2005. Structure of S5a bound to monoubiquitin provides a model for polyubiquitin recognition. J. Mol. Biol. 348:727–739 [DOI] [PubMed] [Google Scholar]
- 48.Chandra A, Chen L, Madura K. 2010. Synthetic lethality of rpn11-1 rpn10Delta is linked to altered proteasome assembly and activity. Curr. Genet. 56:543–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Latonen L, Moore HM, Bai B, Jaamaa S, Laiho M. 2011. Proteasome inhibitors induce nucleolar aggregation of proteasome target proteins and polyadenylated RNA by altering ubiquitin availability. Oncogene 30:790–805 [DOI] [PubMed] [Google Scholar]
- 50.Kaspari M, Tavalai N, Stamminger T, Zimmermann A, Schilf R, Bogner E. 2008. Proteasome inhibitor MG132 blocks viral DNA replication and assembly of human cytomegalovirus. FEBS Lett. 582:666–672 [DOI] [PubMed] [Google Scholar]
- 51.Sadanari H, Tanaka J, Li Z, Yamada R, Matsubara K, Murayama T. 2009. Proteasome inhibitor differentially regulates expression of the major immediate early genes of human cytomegalovirus in human central nervous system-derived cell lines. Virus Res. 142:68–77 [DOI] [PubMed] [Google Scholar]
- 52.Clague MJ, Urbe S. 2010. Ubiquitin: same molecule, different degradation pathways. Cell 143:682–685 [DOI] [PubMed] [Google Scholar]
- 53.Wong E, Bejarano E, Rakshit M, Lee K, Hanson HH, Zaarur N, Phillips GR, Sherman MY, Cuervo AM. 2012. Molecular determinants of selective clearance of protein inclusions by autophagy. Nat. Commun. 3:1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mayor T, Graumann J, Bryan J, Maccoss MJ, Deshaies RJ. 2007. Quantitative profiling of ubiquitylated proteins reveals proteasome substrates and the substrate repertoire influenced by the rpn10 receptor pathway. Mol. Cell. Proteomics 6:1885–1895 [DOI] [PubMed] [Google Scholar]
- 55.Bortz E, Whitelegge JP, Jia Q, Zhou ZH, Stewart JP, Wu TT, Sun R. 2003. Identification of proteins associated with murine gammaherpesvirus 68 virions. J. Virol. 77:13425–13432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim YE, Ahn JH. 2010. Role of the specific interaction of UL112-113 p84 with UL44 DNA polymerase processivity factor in promoting DNA replication of human cytomegalovirus. J. Virol. 84:8409–8421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kagele D, Rossetto CC, Tarrant MT, Pari GS. 2012. Analysis of the interactions of viral and cellular factors with human cytomegalovirus lytic origin of replication, oriLyt. Virology 424:106–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pegoraro G, Voss TC, Martin SE, Tuzmen P, Guha R, Misteli T. 2012. Identification of mammalian protein quality control factors by high-throughput cellular imaging. PLoS One 7:e31684. 10.1371/journal.pone.0031684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rosenbaum JC, Gardner RG. 2011. How a disordered ubiquitin ligase maintains order in nuclear protein homeostasis. Nucleus 2:264–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bennett EJ, Bence NF, Jayakumar R, Kopito RR. 2005. Global impairment of the ubiquitin-proteasome system by nuclear or cytoplasmic protein aggregates precedes inclusion body formation. Mol. Cell 17:351–365 [DOI] [PubMed] [Google Scholar]
- 61.Latonen L. 2011. Nucleolar aggresomes as counterparts of cytoplasmic aggresomes in proteotoxic stress. Proteasome inhibitors induce nuclear ribonucleoprotein inclusions that accumulate several key factors of neurodegenerative diseases and cancer. Bioessays 33:386–395 [DOI] [PubMed] [Google Scholar]
- 62.Riedinger C, Boehringer J, Trempe JF, Lowe ED, Brown NR, Gehring K, Noble ME, Gordon C, Endicott JA. 2010. Structure of Rpn10 and its interactions with polyubiquitin chains and the proteasome subunit Rpn12. J. Biol. Chem. 285:33992–34003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sakata E, Bohn S, Mihalache O, Kiss P, Beck F, Nagy I, Nickell S, Tanaka K, Saeki Y, Forster F, Baumeister W. 2012. Localization of the proteasomal ubiquitin receptors Rpn10 and Rpn13 by electron cryomicroscopy. Proc. Natl. Acad. Sci. U. S. A. 109:1479–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bohn S, Beck F, Sakata E, Walzthoeni T, Beck M, Aebersold R, Forster F, Baumeister W, Nickell S. 2010. Structure of the 26S proteasome from Schizosaccharomyces pombe at subnanometer resolution. Proc. Natl. Acad. Sci. U. S. A. 107:20992–20997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rani N, Aichem A, Schmidtke G, Kreft SG, Groettrup M. 2012. FAT10 and NUB1L bind to the VWA domain of Rpn10 and Rpn1 to enable proteasome-mediated proteolysis. Nat. Commun. 3:749–769 [DOI] [PubMed] [Google Scholar]
- 66.Nagashima Y, Kowa H, Tsuji S, Iwata A. 2011. FAT10 protein binds to polyglutamine proteins and modulates their solubility. J. Biol. Chem. 286:29594–29600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jacquemont C, Taniguchi T. 2007. Proteasome function is required for DNA damage response and fanconi anemia pathway activation. Cancer Res. 67:7395–7405 [DOI] [PubMed] [Google Scholar]
- 68.Lundgren J, Masson P, Mirzaei Z, Young P. 2005. Identification and characterization of a Drosophila proteasome regulatory network. Mol. Cell. Biol. 25:4662–4675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lundgren J, Masson P, Realini CA, Young P. 2003. Use of RNA interference and complementation to study the function of the Drosophila and human 26S proteasome subunit S13. Mol. Cell. Biol. 23:5320–5330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moshe A, Gorovits R. 2012. Virus-induced aggregates in infected cells. Viruses 4:2218–2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wileman T. 2007. Aggresomes and pericentriolar sites of virus assembly: cellular defense or viral design? Annu. Rev. Microbiol. 61:149–167 [DOI] [PubMed] [Google Scholar]
- 72.Salsman J, Jagannathan M, Paladino P, Chan PK, Dellaire G, Raught B, Frappier L. 2012. Proteomic profiling of the human cytomegalovirus UL35 gene products reveals a role for UL35 in the DNA repair response. J. Virol. 86:806–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Loveland AN, Nguyen NL, Brignole EJ, Gibson W. 2007. The amino-conserved domain of human cytomegalovirus UL80a proteins is required for key interactions during early stages of capsid formation and virus production. J. Virol. 81:620–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Livingston CM, Ifrim MF, Cowan AE, Weller SK. 2009. Virus-induced chaperone-enriched (VICE) domains function as nuclear protein quality control centers during HSV-1 infection. PLoS Pathog. 5:e1000619. 10.1371/journal.ppat.1000619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu D, Silva MC, Shenk T. 2003. Functional map of human cytomegalovirus AD169 defined by global mutational analysis. Proc. Natl. Acad. Sci. U. S. A. 100:12396–12401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dunn W, Chou C, Li H, Hai R, Patterson D, Stolc V, Zhu H, Liu F. 2003. Functional profiling of a human cytomegalovirus genome. Proc. Natl. Acad. Sci. U. S. A. 100:14223–14228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sander G, Konrad A, Thurau M, Wies E, Leubert R, Kremmer E, Dinkel H, Schulz T, Neipel F, Sturzl M. 2008. Intracellular localization map of human herpesvirus 8 proteins. J. Virol. 82:1908–1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pearson A, Coen DM. 2002. Identification, localization, and regulation of expression of the UL24 protein of herpes simplex virus type 1. J. Virol. 76:10821–10828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lymberopoulos MH, Pearson A. 2007. Involvement of UL24 in herpes-simplex-virus-1-induced dispersal of nucleolin. Virology 363:397–409 [DOI] [PubMed] [Google Scholar]
- 80.Knizewski L, Kinch L, Grishin NV, Rychlewski L, Ginalski K. 2006. Human herpesvirus 1 UL24 gene encodes a potential PD-(D/E)XK endonuclease. J. Virol. 80:2575–2577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lymberopoulos MH, Pearson A. 2010. Relocalization of upstream binding factor to viral replication compartments is UL24 independent and follows the onset of herpes simplex virus 1 DNA synthesis. J. Virol. 84:4810–4815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kasem S, Yu MH, Yamada S, Kodaira A, Matsumura T, Tsujimura K, Madbouly H, Yamaguchi T, Ohya K, Fukushi H. 2010. The ORF37 (UL24) is a neuropathogenicity determinant of equine herpesvirus 1 (EHV-1) in the mouse encephalitis model. Virology 400:259–270 [DOI] [PubMed] [Google Scholar]
- 83.Nascimento R, Costa H, Dias JD, Parkhouse RM. 2011. MHV-68 open reading frame 20 is a nonessential gene delaying lung viral clearance. Arch. Virol. 156:375–386 [DOI] [PubMed] [Google Scholar]
- 84.Blakeney S, Kowalski J, Tummolo D, DeStefano J, Cooper D, Guo M, Gangolli S, Long D, Zamb T, Natuk RJ, Visalli RJ. 2005. Herpes simplex virus type 2 UL24 gene is a virulence determinant in murine and guinea pig disease models. J. Virol. 79:10498–10506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ito H, Sommer MH, Zerboni L, Baiker A, Sato B, Liang R, Hay J, Ruyechan W, Arvin AM. 2005. Role of the varicella-zoster virus gene product encoded by open reading frame 35 in viral replication in vitro and in differentiated human skin and T cells in vivo. J. Virol. 79:4819–4827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Leiva-Torres GA, Rochette PA, Pearson A. 2010. Differential importance of highly conserved residues in UL24 for herpes simplex virus 1 replication in vivo and reactivation. J. Gen. Virol. 91:1109–1116 [DOI] [PubMed] [Google Scholar]
- 87.Jacobson JG, Chen SH, Cook WJ, Kramer MF, Coen DM. 1998. Importance of the herpes simplex virus UL24 gene for productive ganglionic infection in mice. Virology 242:161–169 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.