Abstract
Infection with Theiler's murine encephalomyelitis virus (TMEV) in the central nervous system (CNS) of susceptible mice results in an immune-mediated demyelinating disease which is considered a relevant viral model of human multiple sclerosis. We previously demonstrated that the expression of positive costimulatory molecules (CD40, CD80, and CD86) is higher on the microglia of TMEV-resistant C57BL/6 (B6) mice than the microglia of TMEV-susceptible SJL/J (SJL) mice. In this study, we analyzed the expression levels of the negative costimulatory molecules PD-1 and PDL-1 in the CNS of TMEV-infected SJL mice and B6 mice. Our results indicated that TMEV infection induces the expression of both PD-1 and PDL-1 on microglia and macrophages in the CNS but not in the periphery. The expression of PD-1 only on CNS-infiltrating macrophages and not on resident microglia was considerably higher (>4-fold) in TMEV-infected SJL mice than TMEV-infected B6 mice. We further demonstrated that interleukn-6 (IL-6) is necessary to induce the maximal expression of PDL-1 but not PD-1 after TMEV infection using IL-6-deficient mice and IL-6-transgenic mice in conjunction with recombinant IL-6. In addition, cells from type I interferon (IFN) receptor knockout mice failed to upregulate PD-1 and PDL-1 expression after TMEV infection in vitro, indicating that type I IFN signaling is associated with the upregulation. However, other IFN signaling may also participate in the upregulation. Taken together, these results strongly suggest that the expression of PD-1 and PDL-1 in the CNS is primarily upregulated following TMEV infection via type I IFN signaling and the maximal expression of PDL-1 additionally requires IL-6 signaling.
INTRODUCTION
Theiler's murine encephalomyelitis virus (TMEV) is a common enteric pathogen in mice. The virus contains a single-stranded RNA genome with positive polarity (1). Intracerebral infection with TMEV in susceptible mice induces a chronic progressive white matter-demyelinating disease that is clinically and histopathologically similar to human multiple sclerosis (MS) (2, 3). Susceptibility to the TMEV-induced demyelinating disease (TMEV-IDD) is genetically associated with the major histocompatibility complex (MHC) loci (4). The persistence of the virus in the central nervous system (CNS) of susceptible SJL/J (SJL) mice leads to extensive immune-mediated demyelination. Resistant C57BL/6 (B6) mice rapidly clear the virus and do not develop TMEV-IDD (5, 6). Thus, the immune responses to TMEV in these mouse strains have often been compared to delineate the differences in the immune responses (7–9).
The level and type of both CD4+ and CD8+ T cell responses to TMEV affect viral persistence and the susceptibility to TMEV-IDD (10). For example, TMEV capsid-specific CD4+ T cell responses protect against TMEV-IDD, but non-capsid-reactive (RNA polymerase-reactive) CD4+ T cell responses are pathogenic in susceptible SJL mice (9, 11). In addition, the level of virus-specific Th1 cells in the CNS of infected mice is significantly higher in resistant B6 mice than susceptible SJL mice, suggesting a protective role for Th1 cells (9). Th17 cells, in contrast to Th1 cells, promote viral persistence by enhancing the survival of TMEV-infected cells (12). Moreover, the immunization of SJL mice with UV-inactivated TMEV prior to viral infection, which promotes the Th1 response, confers protection, whereas immunization after viral infection, which promotes the Th17 response, exacerbates the development of TMEV-IDD (11, 13). These data suggest that distinct immune responses against TMEV involve the protection and/or pathogenesis of TMEV-IDD. To understand the underlying mechanisms of the differential immune responses between resistant B6 and susceptible SJL mice, the levels of the costimulatory molecules associated with T cell activation have been compared in these mouse strains after TMEV infection (7). The expression levels of the positive costimulatory molecules (CD80, CD86, and CD40) on microglia, the main antigen-presenting cells in the CNS, were significantly lower in TMEV-infected susceptible SJL mice than resistant B6 mice. Therefore, the lower level of expression of positive costimulatory molecules may have contributed to the insufficient T cell activation in susceptible mice, leading to a higher viral persistence and the increased pathogenesis of TMEV-IDD (7). However, the potential contribution of negatively functioning costimulatory molecules, such as programmed cell death protein 1 (PD-1; CD279) and its ligand PDL-1 (B7-H1; CD274) in the development of TMEV-IDD is unclear.
The binding of PD-1 with its ligands PDL-1 and PDL-2 (B7-DC; CD273) delivers inhibitory signals that downregulate T cell activation and upregulate tolerance (14). The PD-1/PDL-1 pathway leads to the inhibition of T cell receptor-mediated lymphocyte proliferation and cytokine secretion (15). PD-1/PDL-1 signaling appears to be involved in the maintenance of peripheral self-tolerance and the prevention of autoimmune disease (16, 17). In addition, PD-1 signaling inhibits antiviral immunity (18) and regulates the sensitivity of CD8+ T cells to apoptosis (19). Furthermore, an elevated level of PD-1 is expressed on the exhausted T cells, and inhibition of the PD-1/PDL-1 pathway restores the function of exhausted CD8+ T cells in chronic lymphocytic choriomeningitis virus (LCMV) infection (20). These results indicate that the PD-1/PDL-1 pathway plays a critical role in regulating both CD4+ and CD8+ T cell function, particularly in antiviral immunity. The expression of PD-1 is inducible in activated T, B, and myeloid cells (15, 21). In particular, type I interferon (IFN) promotes the expression of PD-1 on T cells (22). In contrast, PDL-1 is expressed broadly on hematopoietic and parenchymal cells (23). In addition, PDL-1 is constitutively expressed on T cells, B cells, macrophages (MPs), and dendritic cells (DCs). The expression is further upregulated on T cells, MPs, and DCs after stimulation with type I IFN, gamma IFN (IFN-γ), granulocyte-macrophage colony-stimulating factor (GM-CSF), or interleukn-4 (IL-4) (24, 25).
The potential inhibitory functions of PD-1 and PDL-1 against protective T cell responses leading to the development of TMEV-induced demyelinating disease have been detected when antibodies against either PDL-1 (26) or PD-1 (C.-S. Koh et al., unpublished observation) were administered to susceptible SJL mice. In this study, we investigated the expression levels of these molecules (PD-1 and PDL-1) that inhibit T cell stimulation in the CNS of TMEV-infected susceptible SJL mice and resistant B6 mice, as well as the viral infection-induced signals associated with the expression. We compared the expression of PD-1 and PDL-1 on T cells and potential antigen-presenting cells in the periphery and the CNS of susceptible SJL mice and resistant B6 mice with and without TMEV infection. Our results indicated that the expression of PD-1, but not PDL-1, is far greater on macrophages from virus-infected susceptible SJL mice than on those from resistant B6 mice. In addition, IL-6 was necessary to induce the maximal expression of PDL-1 after TMEV infection in the experiments using cells from IL-6-deficient mice and IL-6-transgenic (IL-6-Tg) mice in conjunction with recombinant IL-6 (rIL-6). Further experiments using cells from type I IFN receptor-deficient (IFN-IR knockout [KO]) mice indicated that type I IFNs are also involved in the upregulation of PD-1 and PDL-1. Therefore, IL-6 and type I IFN signaling appear to be associated with the maximal upregulation of PD-1 and PDL-1 expression following TMEV infection. Taken together, the innate immune responses following viral infection may play a pivotal role in the expression of inhibitory costimulatory molecules, contributing to the poor T cell responses that promote further viral persistence. However, the expression of PD-1 and PDL-1 in the CNS of TMEV-infected IFN-IR KO, Toll-like receptor 3 (TLR3) KO, and melanoma differentiation-associated gene 5 (MDA5) KO mice with high viral loads was much elevated, suggesting that signaling by other IFNs (e.g., IFN-γ) may also participate in the upregulation in the CNS.
MATERIALS AND METHODS
Mice.
SJL/J (SJL) and C57BL/6 (B6) mice were purchased from the Charles River Laboratories (Charles River, MA) through the National Cancer Institute (Frederick, MD). IL-6 KO mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Human IL-6-transgenic (IL-6-Tg) mice (27) were obtained from Jeff Kansas at the Department of Microbiology-Immunology, Northwestern University. Type I IFN receptor knockout (IFN-IR KO) mice were kindly provided by Michel Aguet (University of Zürich, Zürich, Switzerland) via Herbert Virgin (Washington University, St. Louis, MO). Female 129S2/SVPasCrl (129S2/SP) mice, a control for IFN-IR KO mice, were purchased from Charles River Laboratories. B6:129S-Tlr3tm1Flv/J mice (TLR3 KO), purchased from The Jackson Laboratory, were backcrossed to SJL mice for 6 generations to obtain TLR3 KO mice in the SJL background. MDA5 KO mice were obtained from Marco Colonna (Washington University School of Medicine, St. Louis, MO). Control 129X1/SvJ (129SvJ) mice were purchased from The Jackson Laboratory. All experimental procedures approved by the Animal Care and Use Committee of Northwestern University were used in this study.
Virus.
The BeAn strain of TMEV was propagated in BHK-21 cells grown in Dulbecco modified Eagle medium supplemented with 7.5% donor calf serum (Mediatech Inc.). The viral titer was determined via plaque assay on BHK cell monolayers. Approximately 30 μl (1 × 106 PFU) of TMEV was injected into the right hemisphere of 5- to 7-week-old mice anesthetized with isoflurane.
Isolation of CNS-infiltrating lymphocytes.
Mice were perfused through the left ventricle with 40 ml of sterile Hanks balanced salt solution. The excised brains and spinal cords were forced through wire mesh and incubated at 37°C for 45 min in 250 μg/ml of collagenase type 4 (Worthington Biochemical Corp., Newark, NJ). CNS-infiltrating lymphocytes were then enriched at the bottom 1/3 of a continuous 100% Percoll (GE) gradient after centrifugation for 30 min at 27,000 × g.
Flow cytometry.
Fc receptors of isolated CNS cells or splenocytes were blocked using 50 μl of 2.4 G2 hybridoma (ATCC) supernatant by incubation at 4°C for 30 min. The cells were stained with anti-CD11b (clone M1/70) and anti-CD45 (clone Ly-5) for residential microglial cells and infiltrated macrophages. Anti-CD8 (clone 53-6.7), anti-CD4 (clone GK1.5), anti-CD69 (clone H1.2F3), anti-PD-1 (clone J43), anti-PDL-1 (clone MIH5), and anti-CD11c (clone HL3) antibodies were also used to stain the cells. All antibodies were purchased from BD Pharmingen. The cells were analyzed using a Becton, Dickinson LSRII flow cytometer (BD) and FlowJo software (TreeStar).
Generation of BM DCs.
Bone marrow (BM) cells were harvested from the femurs and tibias of adult mice and cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum and 20 ng/ml murine recombinant GM-CSF (PeproTech) as described previously (28).
Reverse transcription-PCR and quantitative PCR.
Total RNA was isolated using the TRIzol reagent (Invitrogen) and reverse transcribed to cDNA using Moloney murine leukemia virus reverse transcriptase (Invitrogen). The cDNAs were amplified with specific primer sets using the SYBR green Supermix (Bio-Rad) and an iCycler apparatus (Bio-Rad). The sense and antisense primer sequences used are as follows: for TMEV (VP1), 5′-TGACTAAGCAGGACTATGCCTTCC-3′ and 5′-CAACGAGCCACATATGCGGATTAC-3′; for IL-17A, 5′-CTCCAGAAGGCCCTCAGACTAC-3′ and 5′-AGCTTTCCCTCCGCATTGACACAG-3′; for PD-1, 5′-CGGTTTCAAGGCATGGTCATTGTT-3′ and 5′-TCCTTCAGAGTGTCGTCCTTGCTT-3′; for PDL-1, 5′-GTCAACGCCACAGCGAATGATGTT-3′ and 5′-ACAACAGGATGGATCCCAGAAGCA-3′; for IL-6, 5′-AGTTGCCTTCTTGGGACTGA-3′ and 5′-TCCACGATTTCCCAGAGAAC-3′; for IFN-γ, 5′-ACTGGCAAAAGGATGGTGAC-3′ and 5′-TGAGCTCATTGAATGCTT GG-3′; and for GAPDH (glyceraldehyde-3-phosphate dehydrogenase), 5′-AACTTTGGCATTGTGGAAGGGCTC-3′ and 5′-TGCCTGCTTCACCACCTTCTTGAT-3′). GAPDH expression served as an internal reference for normalization. Real-time PCR was performed in triplicate.
Cytotoxicity assay.
Aliquots of splenocytes from naive B6 mice were pulsed with viral epitope (VP2 residues 121 to 130 [VP2121-130]) or irrelevant peptide (ovalbumin residues 323 to 339 [OVA323-339]) for 1 h and labeled with low (0.5 μM) and high (10 μM) concentrations of carboxyfluorescein succinimidyl ester (CFSE), respectively (12). The irrelevant peptide was used to generate nonspecific target cells manipulated similarly to the specific epitope-loaded target cells because the manipulated target cells could be differentially vulnerable to cytolysis. The mixture of these two populations (1 × 107 total cells per mouse) was intravenously injected into TMEV-infected B6 mice at 8 days postinfection. After 15 h, splenocytes were isolated from the recipients and the CFSE-stained populations were analyzed using flow cytometry.
Statistical analyses.
The statistical significance of the differences between experimental groups (two-tailed P value) was analyzed with the unpaired Student's t test using the InStat program (GraphPad). P values of <0.05 were considered significant.
RESULTS
Intrinsic expression of PD-1 and PDL-1 on CD11b+ cells is markedly greater in the spleen of susceptible SJL mice than the spleen of resistant B6 mice.
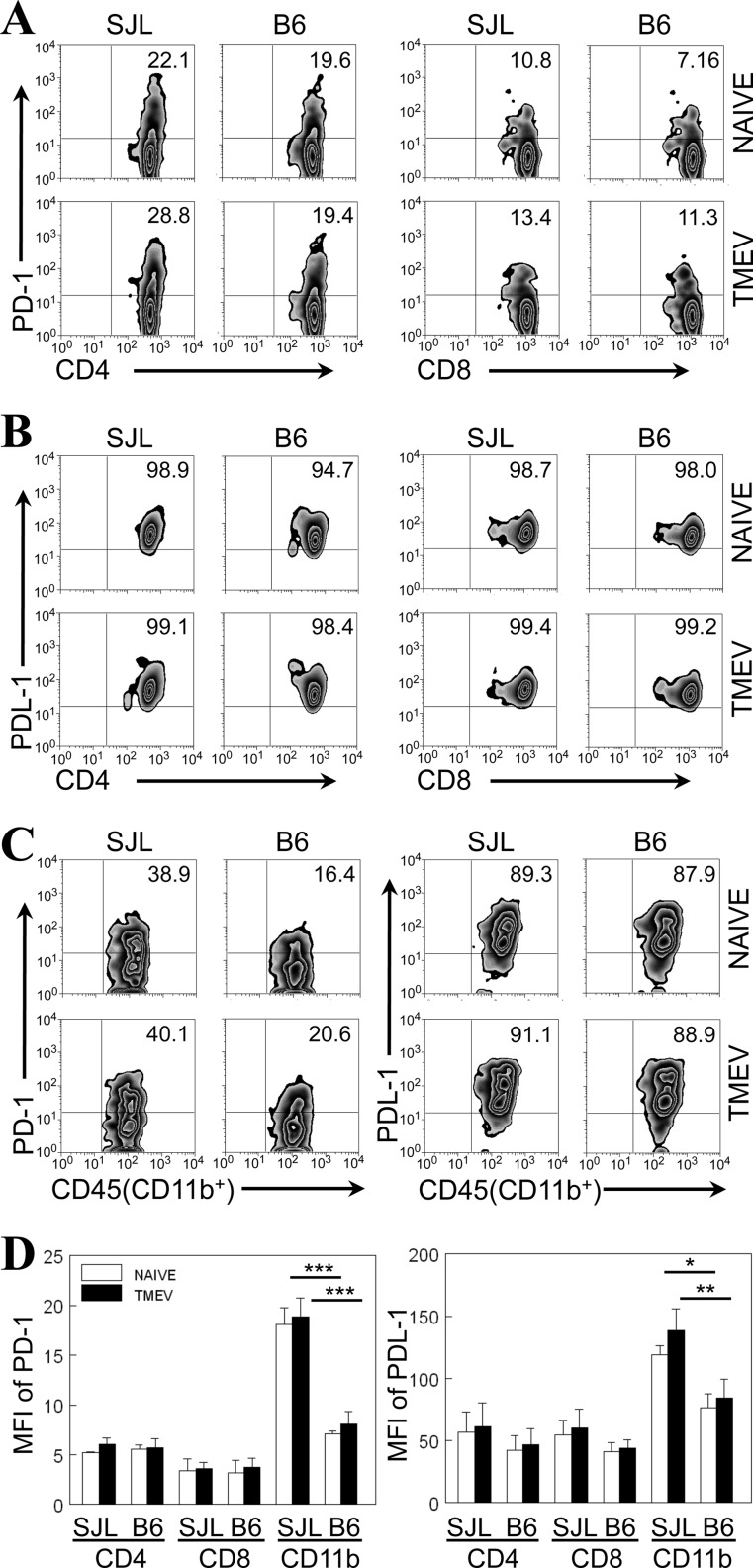
To understand the potential contribution of PD-1 and PDL-1 molecules, we first analyzed the expression levels in spleens from naive or TMEV-infected SJL and B6 mice (Fig. 1). The proportions of PD-1-expressing (PD-1+) splenic CD4+ and CD8+ T cells from naive SJL mice were similar to those from B6 mice (Fig. 1A and D). Interestingly, the proportions of PD-1+ splenic CD4+ and CD8+ T cells were not significantly changed at 8 days after virus infection. Unlike the relatively low proportions of PD-1+ T cells (<30%), the majority (>90%) of splenic CD4+ and CD8+ T cells from naive SJL and B6 mice expressed PDL-1 molecules and the proportion of PDL-1-expressing (PDL-1+) splenic T cells was not altered after viral infection (Fig. 1B). These results suggest that the expression levels of either PD-1 or PDL-1 on CD4+ and CD8+ T cells in the periphery are not significantly different between susceptible SJL mice and resistant B6 mice during TMEV infection.
Fig 1.
Expression of PD-1 and PDL-1 molecules on splenocytes of naive or TMEV-infected susceptible SJL mice and resistant B6 mice. (A) The proportion of PD-1-positive CD4+ and CD8+ T cells in the spleens of naive or TMEV-infected SJL mice and B6 mice was determined at 8 days postinfection using flow cytometry; (B) the proportion of PDL-1-positive CD4+ and CD8+ T cells in the spleens of naive or TMEV-infected SJL and B6 mice was determined at 8 days postinfection using flow cytometry; (C) the proportion of PD-1- and PDL-1-positive CD45+ CD11b+ cells in the spleens of naive or TMEV-infected SJL and B6 mice was determined at 8 days postinfection. The numbers in the fluorescence-activated cell sorter plots represent the percentages of total CD4+ or CD8+ T cells or CD45+ CD11b+ cells in the spleen. Data are representative of three experiments using three mice per group. The percentages of PD-1- and PDL-1-positive cells were determined on the basis of the staining of the cells with the corresponding isotype control antibodies (data not shown). (D) The mean fluorescence intensity (MFI) of PD-1 and PDL-1 expression on splenic cells was compared between naive and TMEV-infected SJL and B6 mice. The values given represent the means ± standard deviations of three independent experimental results. Statistically significant differences are indicated with asterisks (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
We further compared the expression levels of PD-1 and PDL-1 on splenic CD45+ CD11b+ cells in naive or TMEV-infected SJL and B6 mice because these cells include antigen-presenting macrophages and DCs (Fig. 1C). The proportion of PD-1-expressing CD45+ CD11b+ splenic cells in naive SJL mice was significantly higher than that in naive B6 mice (39.6% ± 1.2% versus 15.8% ± 3.9%) and remained similarly higher after TMEV infection (Fig. 1C). The difference in the proportion of PD-1+ splenic CD11b+ cells was consistent with the mean fluorescence intensity (MFI) of PD-1+ cells from three separate experiments (Fig. 1D). In contrast, the proportions of PDL-1+ splenic CD11b+ cells from naive SJL and B6 mice were very high (∼90%), and the proportions of these cells were not altered after virus infection (Fig. 1C). However, the MFI of PDL-1 in CD45+ CD11b+ splenic cells in naive SJL mice was higher than the MFI in naive B6 mice, and it remained similarly higher after TMEV infection (Fig. 1D). Taken together, these data suggest that the intrinsic expression levels of PD-1 and PDL-1 on splenic CD45+ CD11b+ cells are markedly higher in susceptible SJL mice than resistant B6 mice and the expression is not significantly altered after TMEV infection.
Expression of both PD-1 and PDL-1 on CD11b+ CNS cells is upregulated after TMEV infection.
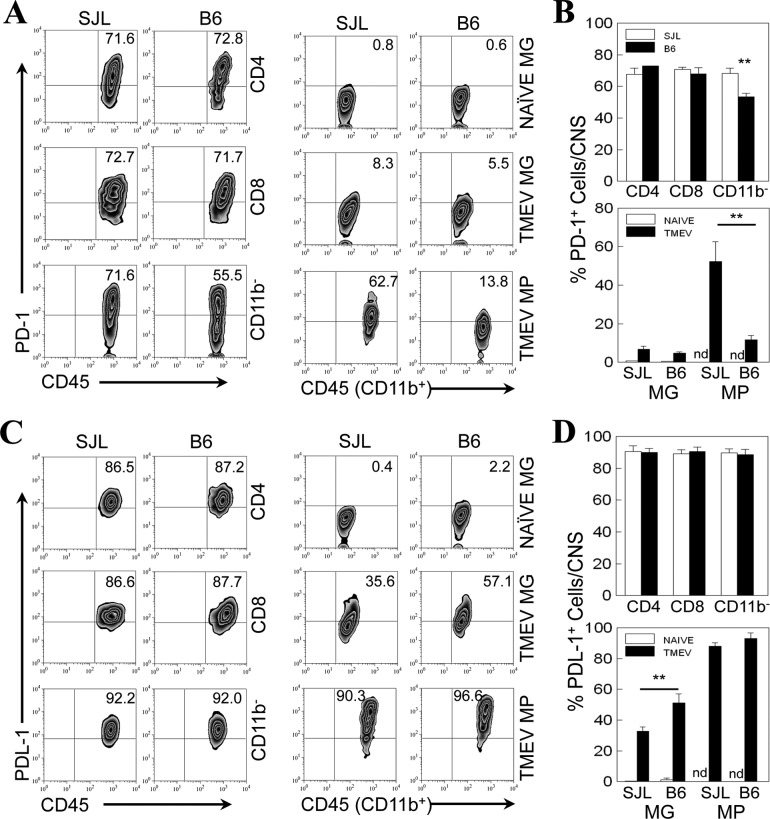
We have previously shown that the microglia of resistant B6 mice express higher levels of positive costimulatory molecules (CD40, CD80, and CD86) than the microglia of susceptible SJL mice (7). To understand the overall balance of the positive and negative costimulatory signals, we analyzed the expression levels of PD-1 and PDL-1 on CNS-infiltrating mononuclear cells in TMEV-infected SJL and B6 mice at 8 days postinfection (Fig. 2). The proportions of PD-1+ CD4+ and CD8+ T cells infiltrating the CNS of infected SJL and B6 mice (Fig. 2A) were drastically increased (to ∼70%) compared with the proportions (10 to 20%) infiltrating the spleen (Fig. 1). However, the proportions of PD-1+ CNS-infiltrating CD4+ and CD8+ T cells were very similar in infected SJL and B6 mice. The proportion of the PD-1+ CNS-infiltrating CD45hi CD11b− population containing non-macrophage-like lymphocytes, including CD4+ and CD8+ T cells and B cells, was consistently higher (P < 0.01) in susceptible SJL mice than in B6 mice (Fig. 2B). Microglia (CD45int CD11b+) from naive SJL and B6 did not express PD-1 molecules. However, after 8 days postinfection, PD-1 expression on microglia in the CNS similarly increased in both SJL and B6 mice. In contrast, the proportion of PD-1+ CNS-infiltrating macrophages (CD45hi CD11b+) in infected SJL mice was much higher (∼4.5-fold) than that in infected B6 mice (Fig. 2A and B). Similarly, the MFI of PD-1 on the CNS-infiltrating macrophages from infected SJL mice was also significantly higher than that of PD-1 on the CNS-infiltrating macrophages from B6 mice (not shown). These results suggest that the expression of PD-1 is higher on macrophages, as well as CNS-infiltrating non-T lymphocytes, which are potentially antigen-presenting cells in the CNS, in virus-infected SJL mice than on macrophages in virus-infected B6 mice.
Fig 2.
Expression of PD-1 and PDL-1 molecules on CNS cells of naive or TMEV-infected SJL mice and B6 mice. (A) The proportions of PD-1-positive CD4+ and CD8+ T cells, overall CD45+ CD11b− cells, microglia (MG; CD45int CD11b+) and macrophages (MPs; CD45hiCD11b+) in the CNS of naive or TMEV-infected SJL mice and B6 mice were determined using flow cytometry at 8 days postinfection. (C) The proportions of PDL-1-positive CD4+ and CD8+ T cells and overall CD45+ CD11b− microglia and MPs in the CNS of TMEV-infected SJL mice and B6 mice were determined using flow cytometry at 8 days postinfection. The percentages of PD-1- and PDL-1-positive cells were assessed after gating on the basis of negative staining with isotype control antibodies (data not shown). Data are representative of three separate experiments using three mice per group. (B and D) The mean percentages of PD-1-positive (B) and PDL-1-positive (D) CD4+ and CD8+ T cells, overall CD45+ CD11b− cells, microglia, and MPs were compared between naive and TMEV-infected SJL and B6 mice. The values given represent the means ± standard deviations of three independent experimental results. Statistically significant differences are indicated with asterisks (**, P < 0.01). nd, not detected.
The proportions of PDL-1+ CD4+ and CD8+ T cells (∼90%) in the CNS of infected SJL and B6 mice were similarly high (Fig. 2C). In addition, no significant differences in the proportions of PDL-1+ CD45+ CD11b− cells were observed between SJL and B6 mice. Similar to the expression of PD-1, low proportions of microglia (CD45int CD11b+) from naive SJL and B6 mice expressed PDL-1 molecules (Fig. 2C). However, the proportions of PDL-1+ microglia were markedly increased after 8 days postinfection in both SJL and B6 mice (Fig. 2C), but the proportion was significantly higher in B6 mice (Fig. 2D). The percentages of PDL-1+ CNS-infiltrating macrophages (CD45hi CD11b+) in infected SJL and B6 mice were similarly very high (>90%) (Fig. 2C). These results collectively suggest that PD-1 and PDL-1 molecules are not expressed on microglia in uninfected naive mice but that the expression is induced after TMEV infection. The expression of PD-1 on macrophages in the CNS of virus-infected SJL mice is much higher than that in the CNS of B6 mice. However, the expression of PDL-1 on microglia in infected SJL mice was lower than that in B6 mice. In contrast, the expression levels of these molecules on CNS-infiltrating CD4+ and CD8+ T cells in virus-infected SJL and B6 mice were similar.
IL-6-Tg mice exhibit upregulated expression of PD-1 and PDL-1 molecules in the CNS and poor cytolysis of viral epitope-bearing target cells.
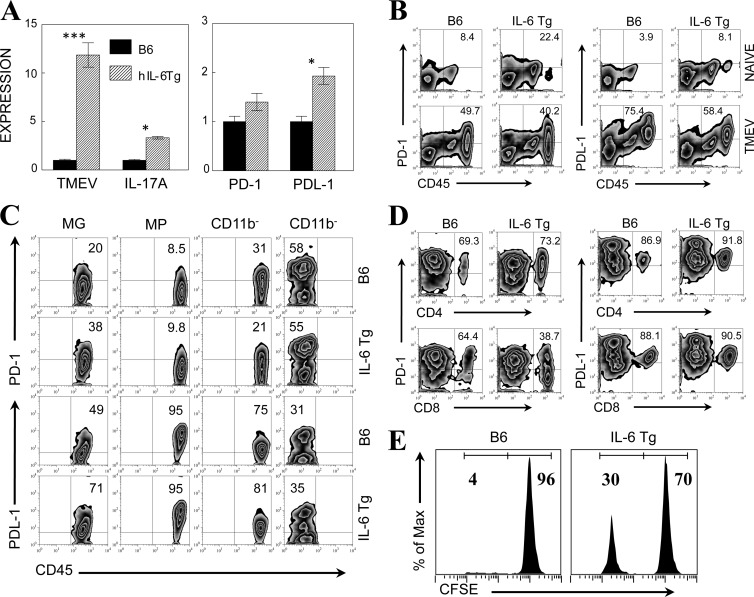
We have previously shown that resistant B6 mice treated with lipopolysaccharide (LPS) produce high levels of IL-6 and become susceptible to TMEV-induced demyelinating disease via an elevated level of pathogenic Th17 cells (12). Thus, we have examined the levels of IL-17A production and PD-1 and PDL-1 expression in B6 mice expressing intrinsically high levels of IL-6 by using IL-6-Tg B6 mice. As expected, IL-6-Tg B6 mice infected with TMEV displayed a higher viral load and a higher level of IL-17A production in the CNS at 8 days postinfection than the control B6 mice. In addition, the increased expression of PD-1 and PDL-1 was observed with quantitative PCR (Fig. 3A). To further determine whether excessive expression of IL-6 in IL-6-Tg mice affects the levels of PD-1 and PDL-1, we assessed the expression of these molecules in pooled CNS cells from naive and TMEV-infected B6 and IL-6-Tg mice (Fig. 3B). Interestingly, CNS cells from naive IL-6-Tg mice displayed a markedly increased number of CD45+ cells (∼3-fold) representing activated cells compared to naive B6 mice and >2-fold higher proportions of PD-1+ and PDL-1+ CD45+ cells than naive B6 mice. After TMEV infection, the proportions of PD-1+ and PDL-1+ cells were further increased in both B6 and IL-6-Tg mice. To further identify the cell type expressing PD-1 and PDL-1, the levels of PD-1 and PDL-1 in microglia (CD45int CD11b+), macrophages (CD45hi CD11b+), lymphocytes (CD45hi CD11b−), and other glial cells (CD45− CD11b−) in the CNS of infected mice were measured at 8 days postinfection (Fig. 3C). The proportions of PD-1+ and PDL-1+ microglia were higher in infected IL-6-Tg mice than in B6 mice. CNS-infiltrating macrophages presented only negligible increases of these molecules in IL-6-Tg mice. Glial cells (CD45− CD11b−) other than microglia expressed these molecules at similar levels in these mice. Further analysis of the T cell populations indicated that the proportion of PD-1+ CD4+ T cells was similar in IL-6-Tg and B6 mice, but the proportion of PD-1+ CD8+ T cells observed in IL-6-Tg mice was lower than that observed in B6 mice (43.7% ± 7.0% versus 69.5% ± 7.2%; P < 0.05) (Fig. 3D). Consistent with the CD45+ CD11b− populations in Fig. 3C, the proportions of PDL-1+ CD4+ and CD8+ T cells were very similar in B6 and IL-6-Tg mice. These results suggest that a high intrinsic IL-6 level in mice elevates the expression of PD-1 and PDL-1, particularly on microglia in naive mice, and further upregulates the expression following viral infection in the CNS.
Fig 3.
Elevated levels of PD-1 and PDL-1 expression on CNS-resident microglia of IL-6-Tg B6 mice. (A) The TMEV RNA and IL-17, PD-1, and PDL-1 mRNA levels in the CNS of TMEV-infected B6 mice and IL-6-Tg B6 mice were assessed at 8 days postinfection using quantitative PCR. The values given represent the means ± standard deviations of triplicates of the fold induction after normalization to GAPDH mRNA levels. Statistically significant differences are indicated with asterisks (*, P < 0.05; ***, P < 0.001). (B) The proportions of PD-1- and PDL-1-positive CD45+ CNS cells of naive and TMEV-infected B6 mice and IL-6-Tg mice were determined using flow cytometry. (C) The levels of PD-1 and PDL-1 expression on microglia (MG; CD45intCD11b+), macrophages (MPs; CD45highCD11b+), CD45+ CD11b−, and CD45− CD11b− cells were assessed using flow cytometry. CNS cells from B6 mice and IL-6-Tg mice were used after 8 days postinfection. (D) The levels of PD-1 and PDL-1 expression on CNS-infiltrating CD4+ and CD8+ T cells from TMEV-infected B6 mice and IL-6-Tg mice at 8 days postinfection are shown. (E) In vivo cytolysis of virus-specific versus control target cells was determined using peptide-loaded and CFSE-labeled target cells. Virus-specific VP2121-130 and control OVA323-339 peptide-loaded target cells were labeled with low and high concentrations of CSFE, respectively. The ratios between specific (VP2121-130) and nonspecific (OVA323-339) target cells are shown in the histograms. Data are representative of three experiments using three mice per group.
To further determine whether the elevated expression of PD-1 and PDL-1 in IL-6-Tg mice is associated with reduced cytolytic function, we compared the specific target cell lysis by CD8+ T cells in virus-infected B6 and IL-6-Tg mice after administration of epitope-loaded target cells (Fig. 3E). In B6 mice, the lysis of specific target cells was nearly complete (96%) compared with that for nonspecific target cells, whereas the lysis occurred partially (70%) in IL-6-Tg mice. Therefore, these results are consistent with the notion that excessive IL-6 inhibits target lysis through upregulation of PD-1 and PDL-1 molecules in the CNS. Because IL-6-Tg mice also display increased levels of IL-17 (Fig. 3A), which inhibits target cell lysis (12), IL-17 may also participate in the inhibition of cytolysis.
The presence of IL-6 is essential for the upregulation of PDL-1 in microglia following TMEV infection.
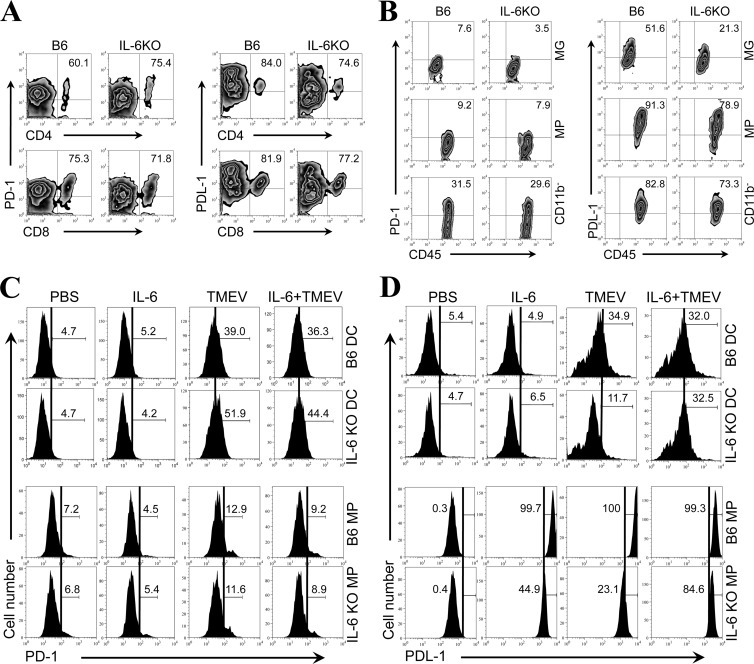
The above-presented results demonstrating the elevated expression of PD-1 and PDL-1 in IL-6-Tg mice (Fig. 3) suggest that the presence of IL-6 may be involved in the upregulation. To directly assess the involvement of IL-6 in the upregulation of these molecules following TMEV infection, the expression levels of PD-1 and PDL-1 were first compared between TMEV-infected control B6 mice and IL-6 KO mice lacking IL-6 production. The proportions of PD-1+ and PDL-1+ CD4+ and CD8+ T cells in the CNS of virus-infected IL-6 KO mice were only marginally different from those in the wild-type B6 mice, which were not deficient in IL-6 production (Fig. 4A). However, the proportions of PD-1+ and PDL-1+ microglia (CD45int CD11b+) in the CNS of virus-infected IL-6 KO mice were >2-fold lower than those in the CNS of the wild-type B6 mice (Fig. 4B). The levels of these molecules on other cell types, including macrophages (CD45hi CD11b+), of IL-6 KO mice were only slightly lower than those on cells of the control B6 mice. These results suggest that the presence of IL-6 contributes to the upregulation of PD-1 and PDL-1 molecules, particularly in microglia following TMEV infection.
Fig 4.
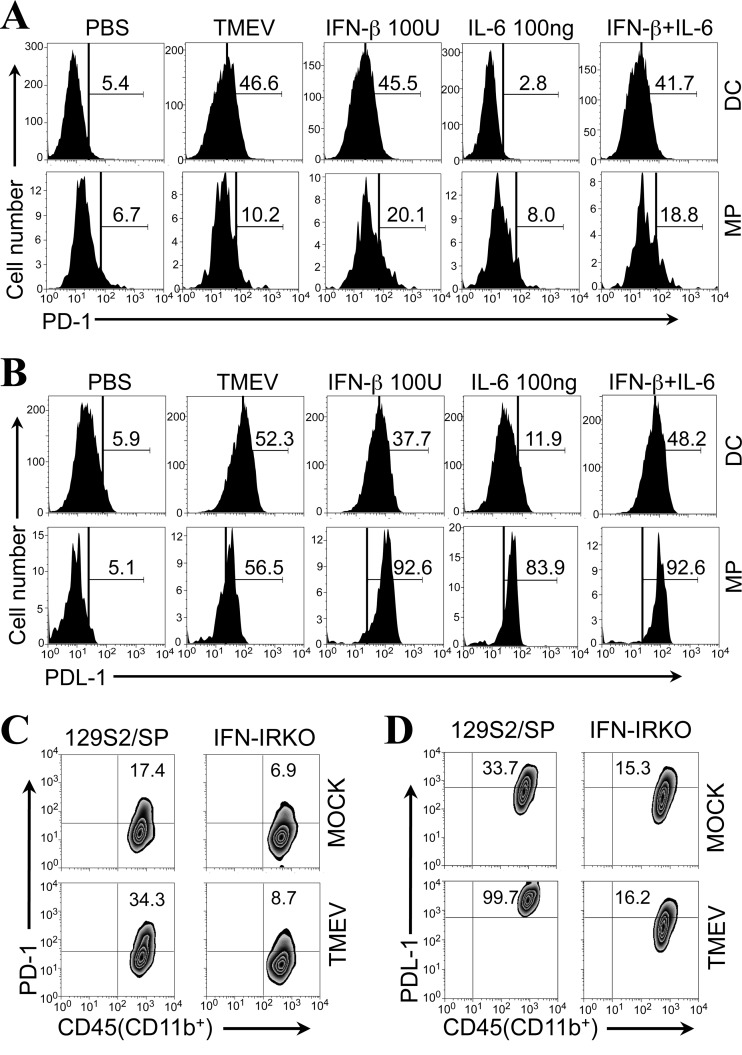
Lack of PDL-1 upregulation in the absence of IL-6 following in vitro TMEV infection. (A) Flow cytometry plots show the proportions of PD-1 and PDL-1 positive CD4+ and CD8+ cells in the CNS of TMEV-infected control B6 mice and IL-6 KO B6 mice at 8 days postinfection. (B) The proportions of PD-1 and PDL-1 positive microglia (MG; CD45int CD11b+), macrophages (MPs; CD45hi CD11b+), and CD45+ CD11b− cells in the CNS of virus-infected mice were compared at 8 days postinfection. Data are representative of three experiments using three mice per group. (C and D) Expression of PD-1 (C) and PDL-1 (D) on bone marrow-derived DCs and peritoneal MPs from B6 and IL-6 KO mice was assessed using flow cytometry after 24 h with or without TMEV infection (multiplicity of infection = 10) and with or without addition of 10 ng of rIL-6. The numbers in the histograms represent the percentages of PD-1+ or PDL-1+ cells in CD11c+ bone marrow cells cultured for 5 days, as described previously (28), or CD11b+ peritoneal macrophages. Data are representative of three experiments using three mice per group. PBS, phosphate-buffered saline.
It would be best to use microglial cultures to further investigate the role of IL-6 in the upregulated expression of PD-1 and PDL-1 molecules in vitro. However, it is not technically possible to maintain microglial cultures without external stimulation. Therefore, to further confirm the potential involvement of IL-6 in the upregulation of PD-1 (Fig. 4C) and PDL-1 expression (Fig. 4D) in conjunction with TMEV infection, we utilized cultures of bone marrow-derived DCs and peritoneal macrophages derived from B6 mice and IL-6 KO B6 mice. PD-1 expression was not affected by the presence or absence of IL-6 in either DC or macrophage cultures, although TMEV infection upregulated the expression in both DC and macrophage cultures (Fig. 4C). In contrast, the expression of PDL-1 was significantly upregulated on DCs and macrophages from B6 mice following TMEV infection (Fig. 4D). However, the levels of PDL-1 expressed on DCs and macrophages from IL-6 KO mice were lower than the levels for B6 mice, and this deficiency in the upregulation was fully restored after addition of rIL-6. These results indicate that IL-6 induction following TMEV infection is necessary for maximal upregulation. It is interesting to note that the addition of rIL-6 in the absence of viral infection did not affect the level of PDL-1 on DCs but significantly elevated the expression by macrophages, although the maximal upregulation appeared to require both IL-6 and TMEV infection. These results further suggest that the sensitivity to IL-6-dependent upregulation is different depending on the cell type. The level of rIL-6 required for PDL-1 upregulation in conjunction with TMEV infection was further assessed to exclude the possibility that the addition of a nonphysiological level of rIL-6 might have resulted in the upregulation. The level of rIL-6 necessary for the TMEV-infected IL-6 KO DCs appeared to be very low. Only 10 ng of rIL-6, which is well within the range of physiological production, was sufficient for maximal PDL-1 upregulation (data not shown). These data suggest that IL-6 is directly involved in the upregulation of PDL-1, unlike the upregulation of PD-1. Nevertheless, viral infection may also provide a signal(s) for the upregulation of PDL-1, in addition to IL-6, and these signals appear to collectively participate in PDL-1 upregulation.
IL-6 and/or type I IFN mediates the upregulation of PD-1 and PDL-1 expression on DCs and macrophages infected with TMEV.
It was previously shown that type I IFN treatment promotes PD-1 and PDL-1 expression on T cells and monocytes (29, 30). Thus, the upregulated expression of PD-1 and PDL-1 after TMEV infection could be mediated by infection-induced IL-6 and/or type I IFNs. To further confirm the role of IL-6 and type I IFN in the upregulation of PD-1 and PDL-1 expression, bone marrow-derived DCs and peritoneal macrophages derived from B6 mice were treated with IL-6 and/or type I IFN (Fig. 5A and B). The expression of PD-1 on both DCs and macrophages was upregulated after treatment with IFN-β without TMEV infection (Fig. 5A). The level of upregulation of PD-1 by IFN-β was similar to the level obtained after TMEV infection on DCs and exceeded the level obtained after TMEV infection on macrophages. However, PD-1 expression was not elevated on DCs and was elevated very little on macrophages after treatment with IL-6 alone, consistent with the results in Fig. 4C. In contrast, the proportions of PDL-1+ DCs and macrophages were significantly increased after treatment with IFN-β or IL-6 (Fig. 5B). However, the proportion of PDL-1+ DCs induced after treatment with IFN-β or IL-6 was lower than that induced after TMEV infection. When DCs were treated with IFN-β plus IL-6, the proportion of PDL-1+ DCs induced became similar to that induced after viral infection. Because the proportion of PDL-1+ DCs induced after treatment with IFN-β plus IL-6 was similar to the sum of the proportions of PDL-1+ DCs induced by IFN-β and IL-6 separately, the effect of these cytokines on PDL-1 expression is additive. On the other hand, the proportion of PDL-1+ macrophages far exceeded that induced after TMEV infection (93.3% ± 0.9% versus 61.6% ± 7.2%; P < 0.01). Similarly, the proportion of PDL-1+ macrophages induced after treatment with IL-6 alone was also greater than that induced by TMEV infection (80.9% ± 4.2% versus 61.6% ± 7.2%; P < 0.05). Thus, the induction of PDL-1 expression on macrophages appears to be much more sensitive to these cytokines. In addition, either IL-6 or IFN-β alone is sufficient to trigger the expression of PDL-1 on most macrophages, unlike the expression on DCs. These results further confirm that the innate cytokines IFN-β and IL-6 are associated with the maximal expression of PDL-1 and IFN-β is associated with the expression of PD-1 after TMEV infection. However, the sensitivity of the induction to the cytokines is cell type dependent.
Fig 5.
Expression of PD-1 and PDL-1 molecules on peritoneal macrophages from IFN-IR KO mice after in vitro TMEV infection. (A and B) Expression of PD-1 (A) and PDL-1 (B) on bone-marrow-derived DCs and peritoneal MPs from B6 mice was determined using flow cytometry after 24 h of TMEV infection (multiplicity of infection = 10) or with addition of 100 U IFN-β and/or 100 ng of rIL-6. The numbers in the histograms represent the percentages of PD-1+ or PDL-1+ cells in CD11c+ bone marrow-derived cells cultured for 5 days or CD11b+ peritoneal macrophages. (C and D) Expression levels of PD-1 (C) and PDL-1 (D) on peritoneal macrophages from control 129S2/SP mice and IFN-IR KO mice were determined after 24 h of in vitro TMEV infection (multiplicity of infection = 10) using flow cytometry. The numbers in the fluorescence-activated cell sorter plots represent the percentages of PD-1+ or PDL-1+ cells among the total peritoneal macrophages (CD45hi CD11b+). Data are representative of three separate experiments using two mice per group.
To confirm the effects of type I IFN signaling on the upregulated expression of PD-1 and PDL-1 following TMEV infection, peritoneal macrophages (CD45hi CD11b+) from type I IFN receptor-deficient (IFN-IR KO) mice and the control mice (129S2/SP) were infected with TMEV in vitro. The expression levels of PD-1 and PDL-1 on the peritoneal macrophages from control mice were elevated 2- to 3-fold after TMEV infection compared with the levels for uninfected cultures (Fig. 5C and D). In contrast, TMEV-infected macrophages from IFN-IR KO mice failed to upregulate PD-1 and PDL-1 expression from the level of the mock-infected macrophages. In addition, it was interesting to note that the intrinsic levels of both PD-1 and PDL-1 expressed on the macrophages from IFN-IR KO mice were 2-fold lower than those expressed on the macrophages from IFN-IR-sufficient control mice. Furthermore, IFN-IR KO macrophages infected with TMEV produced a similar level of IL-6 as control macrophages (not shown). Thus, the lower level of expression of PD-1 and PDL-1 on IFN-IR KO macrophages is not likely due to a lower level of IL-6 production. These results suggest that the expression of PD-1 and PDL-1 on macrophages following TMEV infection is dependent on the presence of type I IFN signaling. These results are consistent with previous reports on the upregulated expression of PD-1 and PDL-1 by type I IFNs (29, 30).
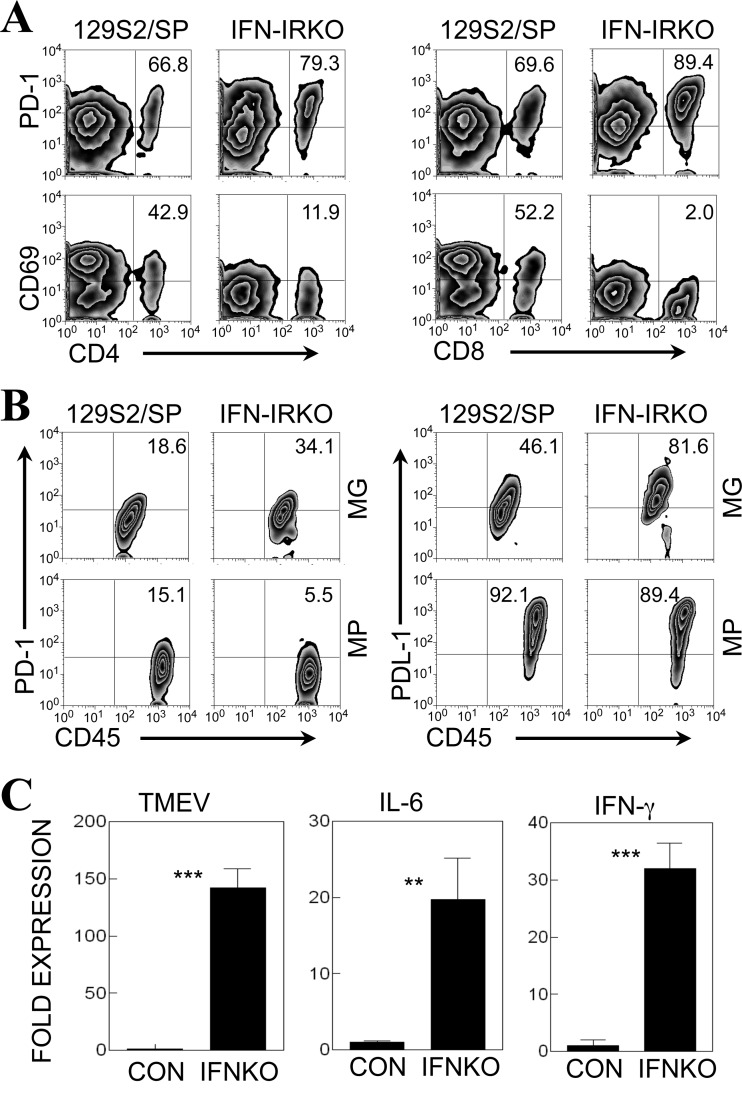
The proportions of PD-1+ CNS-infiltrating T cells and PDL-1+ microglia are significantly higher in IFN-IR KO mice after TMEV infection.
To further examine the effects of type I IFN signaling on the expression of PD-1 and PDL-1 on cells in the CNS of TMEV-infected mice, we compared the expression levels in TMEV-infected control and IFN-IR KO mice (Fig. 6). Surprisingly, our results displayed that the expression of PD-1 on both CD4+ and CD8+ T cells in the CNS of IFN-IR KO mice at 7 days postinfection was consistently higher than the expression observed on cells from control 129S2/SP mice (Fig. 6A). In contrast, the expression of CD69, an activation marker, on these T cells was severalfold lower than that on cells from virus-infected control (129S2/SP) mice, suggesting a deficiency in T cell activation (Fig. 6A). However, the proportion of PDL-1+ T cells was very high (∼90%) in both mouse strains (not shown), as shown in virus-infected B6 and IL-6-Tg mice (Fig. 3D). These results suggest that the elevated PD-1 expression is associated with reduced T cell activation, which is in agreement with previous reports (20).
Fig 6.
Expression of PD-1 and PDL-1 molecules in the CNS of TMEV-infected control and IFN-IR KO mice. (A) The proportions of PD-1- and CD69-positive CD4+ T cells and CD8+ T cells in the CNS of TMEV-infected control (129S2/SP) mice and IFN-IR KO mice were compared at 7 days postinfection by flow cytometry. The numbers in the fluorescence-activated cell sorter plots represent the percentages of total CD4 or CD8 cells in the CNS. (B) The proportions of PD-1- and PDL-1-positive microglia (MG; CD45int CD11b+), macrophages (MPs; CD45hi CD11b+), and CD45+ CD11b− cells representing infiltrating lymphocytes in the CNS of TMEV-infected 129S2/SP mice and IFN-IR KO mice were determined at 7 days postinfection using flow cytometry. (C) TMEV and cytokine mRNA expression levels in the CNS of TMEV-infected control (CON; 129S2/SP) mice and IFN-IR KO (IFNKO) mice at 7 days postinfection were analyzed by quantitative PCR. Data are expressed as the fold expression after normalization to the GAPDH mRNA levels. The values given are means ± standard deviations of triplicates. Statistically significant differences are indicated with asterisks (**, P < 0.01; ***, P < 0.001). Data are representative of three separate experiments using three mice per group.
We also compared the proportions of PD-1- and PDL-1-positive microglia (CD45int CD11b+) and infiltrating microphages (MPs; CD45hi CD11b+) in the CNS of IFN-IR KO and control mice at 7 days postinfection (Fig. 6B). The expression of PD-1 on these cell types was relatively low. However, the expression of PD-1 was ∼2-fold elevated on the microglia, but not on the macrophages, of infected IFN-IR KO mice compared with that on the cells of control mice. In addition to PD-1, the expression levels of PDL-1 on microglia, but not infiltrating macrophages, were also elevated (∼2-fold) in TMEV-infected IFN-IR KO mice compared with the expression levels on the cells of control mice. These results are similar to the expression pattern observed in IL-6-Tg mice, which displayed elevated viral loads in the CNS (Fig. 3A). We further examined the possibility that the elevated expression of PD-1 and PDL-1 in the CNS of virus-infected IFN-IR KO mice is mediated via alternate signaling pathways using IL-6 and/or IFN-γ (Fig. 6C). Consistent with this possibility, mRNA levels of both IL-6 and IFN-γ were significantly higher in the CNS of infected IFN-IR KO mice than in the CNS of wild-type control mice. Therefore, the upregulation of PD-1 expression on T cells and PDL-1 on microglia in IFN-IR KO mice with high viral loads (Fig. 6C) (31) may be achieved via signaling molecules other than type I IFNs, such as IL-6 and IFN-γ.
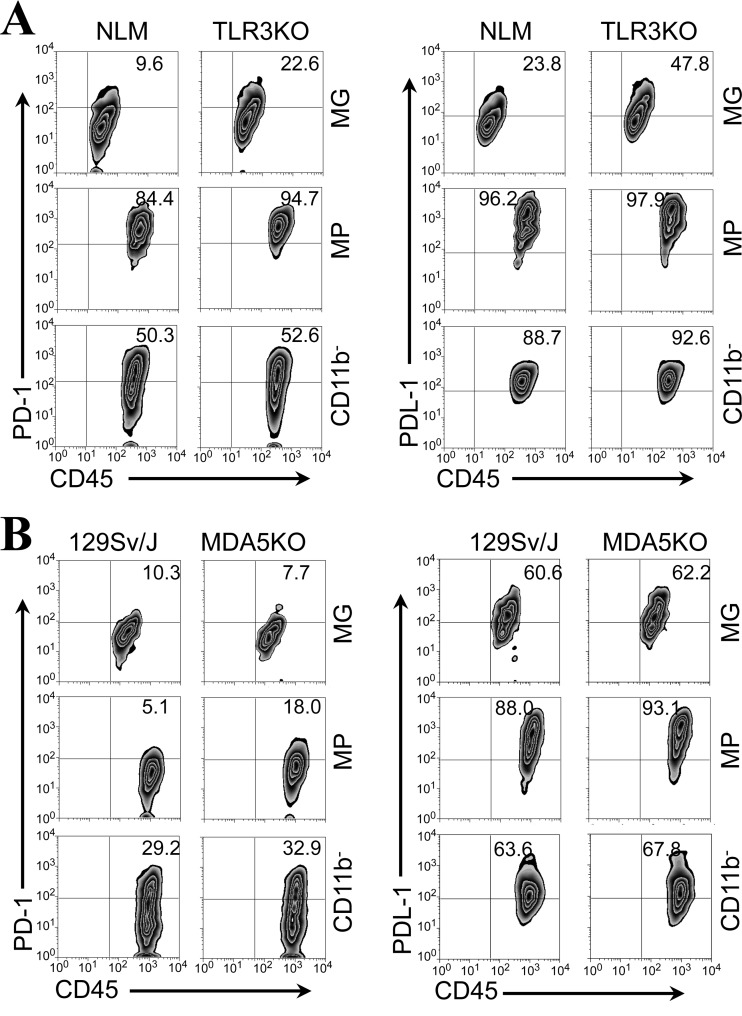
Expression of PD-1 and PDL-1 on CD11b+ cells is also higher in the CNS of TMEV-infected mice deficient in TLR3 or MDA5 signaling.
It was previously reported that innate immune responses to viral infection, such as the TLR response, upregulate the expression of PD-1 and PDL-1 molecules on various cell types (32, 33). In addition, TMEV infection induces strong TLR3 and melanoma differentiation-associated gene 5 (MDA5) responses, and these innate immune responses play important protective roles in controlling viral loads in the CNS and the development of demyelinating disease (34, 35). If a high viral load in the CNS affects the expression levels of PD-1 and PDL-1, we predicted that mice lacking these receptors for innate immunity to TMEV would display an elevated expression of PD-1 and PDL-1 molecules. To test this hypothesis, we used TMEV-infected TRL3 KO mice and MDA5 KO mice to assess the levels of PD-1 and PDL-1 in the CNS (Fig. 7). When we compared the expression of PD-1 on CD4+ and CD8+ T cells in the CNS from SJL and TLR3 KO SJL mice at 7 days postinfection, there was no significant difference (data not shown). However, the expression of both PD-1 and PDL-1 on microglia (CD45int CD11b+), but not macrophages (CD45hi CD11b+), in the CNS of TMEV-infected TLR3 KO SJL mice was approximately 2-fold higher than that in the CNS of infected SJL mice (Fig. 7A). The expression levels of PD-1 and PDL-1 molecules on the CNS-infiltrating macrophages reached the maximal levels in these mice. The CD45+ CD11b− population containing T cells and B cells also expressed similarly high levels in these mice. Therefore, high viral loads in the absence of TLR3-mediated signaling (34) appear to result in the differentially elevated expression of PD-1 and PDL-1 on the CNS-resident microglia but not on infiltrating cell populations, including similar macrophages, following TMEV infection.
Fig 7.
Expression of PD-1 and PDL-1 molecules in the CNS of TMEV-infected TLR3 KO mice and MDA5 KO mice. (A) The proportions of PD-1- and PDL-1-positive microglia (MG; CD45int CD11b+), macrophages (MPs; CD45hi CD11b+), and CD45+ CD11b− cells representing infiltrating lymphocytes in the CNS of TMEV-infected control SJL mice and TLR3 KO SJL mice (34) were determined using flow cytometry at 7 days postinfection. The numbers in the fluorescence-activated cell sorter plots represent the percentages of total microglial, MP, or CD45+ CD11b− cells in the CNS. Data are representative of three separate experiments using three mice per group. (B) The proportions of PD-1- and PDL-1-positive microglia (CD45int CD11b+), macrophages (CD45hi CD11b+), and CD45+ CD11b− cells representing infiltrating lymphocytes in the CNS of TMEV-infected control 129Sv/J mice and MDA5 KO mice (35) were determined at 7 days postinfection. The numbers in the fluorescence-activated cell sorter plots represent the percentages of total microglial, MP, or CD45+CD11b− cells in the CNS. Data are representative of three separate experiments using three mice per group.
MDA5 binds double-stranded RNA and also induces a variety of innate responses, including type I IFN responses (36, 37). MDA5 KO mice display an elevated viral load accompanied by a highly reduced type I IFN response, leading to the development of demyelinating disease after TMEV infection (35). To assess the potential association between MDA5-mediated signaling and the expression of PD-1 and PDL-1, we analyzed the expression of these molecules in the CNS of TMEV-infected control (129Sv/J) and MDA5 KO mice at 7 days postinfection (Fig. 7B). In contrast to TLR3 KO mice, the expression level of PD-1 on CNS-infiltrating macrophages, but not microglia, was higher (>3-fold) in MDA5 KO mice than control mice. The PDL-1 levels on the microglia and macrophages were similarly high in both control and MDA5 KO mice. These data indicate that MDA5-mediated signaling is not involved in the regulation of these molecules on microglia, unlike TLR3-mediated signaling. However, it appears that MDA5-mediated signaling may be involved in the downregulation of PD-1 on CNS-infiltrating macrophages.
DISCUSSION
It is well established that the binding of PD-1 on T cells to PDL-1 on antigen-presenting cells leads to the inhibition of T cell activation and function at the sites of inflammation (14, 15). For example, infection with rabies virus induces PDL-1 expression on infected neurons and PDL-1-deficient mice have demonstrated resistance to the viral infection, suggesting a counterprotective function of PDL-1 in the CNS (33). Similarly, infection with a neurotropic strain of JHMV (the JHM strain of mouse hepatitis virus) induces the expression of PD-1 on virus-specific CD8+ T cells and PDL-1 on oligodendroglia and microglia in the CNS, and PDL-1-deficient mice manage more efficient JHMV control in the CNS (38). In addition, it has been extensively shown that immunologically exhausted hosts with chronic viral infections display poorly functional CD8+ T cells or CD4+ T cells associated with PD-1/PDL-1 signaling (20, 39, 40).
We have previously shown that lower-level expression of costimulatory molecules on the antigen-presenting cells in the CNS of susceptible mice results in poor antiviral T cell responses, leading to a higher viral load and the pathogenesis of TMEV-IDD (7). In addition, blocking of PD-1/PDL-1 signaling in susceptible mice with antibodies to PDL-1 (26) or PD-1 (C.-S. Koh, unpublished results) results in the delayed development of the demyelinating disease. To further understand the mechanisms underlying the elevated expression of PD-1 and PDL-1 following TMEV infection, we compared the levels of these molecules expressed on splenocytes and CNS cells in naive and TMEV-infected susceptible SJL mice and resistant B6 mice. We also assessed the innate immune signals associated with the expression levels of these molecules. Interestingly, our data indicated that the levels of PD-1 and PDL-1 expressed on T cells (CD4+ and CD8+ cells) in the periphery are similar between naive and TMEV-infected mice, regardless of the susceptibility to TMEV (Fig. 1). However, the levels of PD-1 and PDL-1 expressed on macrophages (CD45hi CD11b+ cells) in the periphery of naive susceptible SJL mice were significantly higher than the levels expressed in naive resistant B6 mice, and the higher levels in SJL mice were maintained after TMEV infection (Fig. 1). Although the cells in the CNS of naive mice expressed neither PD-1 nor PDL-1, the proportions of PD-1+ microglia and macrophages in the CNS of TMEV-infected mice were elevated, particularly on the macrophages of susceptible SJL mice (Fig. 2). However, the proportions of PDL-1+ microglia and macrophages were markedly elevated in the CNS of both susceptible and resistant mice. It was interesting to note that the proportion of PDL-1+ microglia in the CNS of resistant B6 mice was higher than that in the CNS of susceptible SJL mice (Fig. 2). Thus, it is possible that the higher expression of PDL-1 on B6 microglia may dampen T cell-mediated CNS damage and consequent inflammatory responses. The expression levels of both PD-1 (>70%) and PDL-1 (>80%) on CNS-infiltrating T cells in TMEV-infected mice were virtually identical in susceptible SJL mice and resistant B6 mice (Fig. 2). These results strongly suggest that the differential expression of PD-1, but not PDL-1, on macrophages, the major potential antigen-presenting cells in the CNS, could exert a higher level of negative signaling against the development and function of locally expanding antiviral T cell responses. On the other hand, a higher level of PDL-1 expression on microglia may protect the integrity of the CNS in virus-infected resistant B6 mice. This notion is consistent with recent reports indicating that PD-1 expression on antigen-presenting cells negatively affects host protection against bacterial or viral infections, similar to the findings for PDL-1 expression (40, 41). Therefore, both PD-1/PDL-1 and PDL-1/PD-1 on antigen-presenting cells versus T cells, respectively, may exert negative signals on T cell responses and function.
The role of the highly expressed PD-1 on CNS-infiltrating T cells in the development of TMEV-induced demyelinating disease is unknown. Previously, it was shown that the expression of PD-1 is drastically upregulated on the exhausted T cells and the administration of anti-PDL-1 antibodies restores the CD8 function during chronic lymphocytic choriomeningitis virus (LCMV) infection (20). However, T cells expressing elevated PD-1 levels in HIV-1 infection do not appear to be functionally exhausted (42). Furthermore, we have repeatedly demonstrated that T cells in the CNS of TMEV-infected resistant B6 mice vigorously function for cytolysis and IFN-γ production (34), yet the majority (∼70%) of the T cells express PD-1, similar to functionally compromised T cells in the CNS of infected susceptible SJL mice (Fig. 2). These results strongly suggest that the differential expression of this negative signaling molecule on antigen-presenting cells plays a critical role in regulating the T cell responses and functions in the CNS during virally induced inflammation.
We have previously demonstrated that treatment of resistant B6 mice with LPS renders the mice susceptible to TMEV-induced demyelinating disease (5). In addition, we have demonstrated that administration of LPS induces the production of IL-6, which promotes the development of pathogenic Th17 responses (12). In addition, it was shown that DCs, microglia, and CNS-infiltrating macrophages from TMEV-infected SJL mice produce higher levels of IL-6 than such cells from infected B6 mice (7, 28). These previous results strongly suggested that the IL-6 induced following TMEV infection plays an important role in the pathogenesis of the demyelinating disease. It was reported that expression of PDL-1 on LPS-treated tolerogenic antigen-presenting cells is regulated in an IL-6-, IL-10-, and STAT-3-dependent manner (43). However, the signals that are critical for the upregulation of PDL-1 following TMEV infection are unknown. In this study, we observed that IL-6-Tg mice display increased IL-17, PD-1, and PDL-1 levels and CD8+ T cells exhibit compromised activity for the lysis of specific target cells after TMEV infection (Fig. 3). Therefore, these data suggest that IL-6 may inhibit specific lysis through the upregulation of PD-1 and PDL-1 molecules in the CNS after TMEV infection. Our results indicate that the expression level of PDL-1 on CD11b+ non-T cells in the CNS of TMEV-infected IL-6 KO mice was significantly lower and the expression level of PDL-1 in the CNS of TMEV-infected IL-6-Tg mice was higher than the levels for control B6 mice (Fig. 3 and 4). Moreover, DCs and macrophages derived from IL-6 KO mice failed to upregulate the expression of PDL-1 following TMEV infection. However, these DCs and macrophages were able to upregulate PDL-1 expression in the presence of IL-6 following viral infection (Fig. 4). These results strongly suggest that an IL-6-dependent signal is involved in PDL-1 expression in the CNS after TMEV infection. However, the expression of PDL-1 was not fully upregulated in the presence of IL-6 alone without TMEV infection, suggesting that IL-6 alone is not sufficient for the upregulation of PDL-1 expression, and TMEV infection may provide an additional signal(s), including type I IFNs, for the upregulation of PDL-1.
Previous studies indicated that type I IFN is the main inducer of PD-1 and PDL-1 expression. For example, IFN-α treatment induces PD-1 expression on T cells in vitro and in vivo (22). In addition, IFN-β treatment results in the upregulated expression of PDL-1 on monocytes and dendritic cells in vitro and in vivo in multiple sclerosis patients (29). It has also been reported that PDL-1 expression on neurons is dependent on TLR3-mediated signaling, inducing type I IFNs after rabies virus infection (33). Infection with TMEV induces robust upregulation of type I IFN production in vitro and in vivo (7, 28, 44). Therefore, type I IFNs produced after TMEV infection were reasonable candidates for the signal required for the expression of PD-1 and PDL-1. To examine this possibility, we assessed the effects of type I IFNs and IL-6 on the expression of PD-1 and PDL-1 by DCs and macrophages in vitro in the absence of TMEV infection (Fig. 5). The expression of PD-1 on DCs and macrophages was mainly derived in the presence of IFN-β but was poorly derived in the presence of IL-6, as shown in Fig. 4. In contrast, the expression level of PDL-1 on these cells after treatment with IFN-β or IL-6 approached or exceeded the expression level on TMEV-infected cells, depending on the cell types. These cytokines appear to cooperatively induce PDL-1 expression on DCs but to independently induce PDL-1 expression on macrophages, with the expression level on macrophages exceeding the level induced after TMEV infection. Thus, the sensitivity to the cytokines for the expression of PD-1 and PDL-1 is cell type dependent. As expected, the expression of PD-1 and PDL-1 on macrophages from IFN-IR KO mice was impaired following in vitro TMEV infection compared with that on macrophages from the control mice (Fig. 5). These results strongly suggest that type I IFNs play a major role in the upregulation of PD-1 and PDL-1 expression following TMEV infection and function cooperatively with IL-6 for PDL-1 expression (Fig. 4). Surprisingly, however, the PD-1 and PDL-1 expression levels on the microglia of TMEV-infected IFN-IR KO mice were further elevated compared with those in their counterpart control mice (Fig. 6B). Because viral loads and the levels of CNS-infiltrating T cells producing IFN-γ are significantly higher in IFN-IR KO mice (31), alternative signals, such as IFN-γ, may provide the upregulation of PD-1 and PDL-1 expression (45) and/or elevated IL-6 levels may compensate for the deficiency of upregulation. In fact, we have found that TMEV-infected IFN-IR KO mice produce higher levels of IL-6 and IFN-γ due to an elevated viral load in the CNS (Fig. 6C). In addition, IFN-IR KO macrophages produced levels of IL-6 similar to those produced by wild-type B6 macrophages after in vitro TMEV infection (not shown). These results indicate that type I IFN signaling is not directly associated with IL-6 production.
TMEV infection induces signals of innate immunity for the production of many cytokines, including IL-6 and type I IFNs, mainly via TLR3 and MDA5 (35, 46). If these cytokines were induced after TMEV infection via these innate immune receptors, mice lacking these receptors would fail to upregulate the expression of PD-1 and PDL-1 molecules. However, the expression level of PD-1 and PDL-1 on CD11b+ cells in the CNS of TLR3 KO or MDA5-KO mice after TMEV infection was even higher than that in receptor-sufficient control mice (Fig. 7). Moreover, there was no impairment in the expression of PD-1 and PDL-1 on macrophages from TLR3 KO mice after in vitro TMEV infection (not shown). Interestingly, type I IFN (IFN-α and IFN-β) and IL-6 levels are higher in TLR3 KO mice than in control mice due to the elevated viral load in the CNS (34). Thus, the elevated levels of type I IFNs caused by high viral loads in TLR3 KO mice may circumvent TLR3-dependent signaling for the induction of type I IFNs and IL-6 (46, 47). Therefore, it is most likely that the lack of one innate immune receptor may be compensated for by other innate immune receptors. Interestingly, the expression of PD-1 and PDL-1 on CNS-resident microglia but not infiltrating macrophages is differentially elevated in virus-infected TLR3 KO mice, and only PD-1 expression on infiltrating macrophages differentially increased in MDA5 KO mice (Fig. 7). In contrast to the elevated levels of type I IFNs in TMEV-infected TLR3 KO mice, the level of type I IFNs, particularly the induction of IFN-α during early viral infection (up to 7 to 8 days postinfection), was compromised in TMEV-infected MDA5 KO mice (35). Therefore, the signals involved in controlling the expression levels of these molecules are differentially provided by TLR3 and by MDA5. It has previously been reported that PDL-1 on microglia was upregulated in the presence of IFN-γ (45). Therefore, it is conceivable that different levels of T cell responses producing IFN-γ in these receptor-deficient mice may affect the expression of PD-1 and PDL-1 on microglia. The presence of high levels of CNS-infiltrating T cells producing IFN-γ in TLR3 KO and MDA5 KO mice following TMEV infection is consistent with this possibility (31, 34, 35).
Taken together, the results of this study strongly suggest that the level of PD-1 expressed on macrophages is potentially important for presenting antigens to T cells in the CNS of TMEV-infected mice and corresponds to the susceptibility to demyelinating disease. Therefore, the elevated expression of PD-1 by the potentially antigen-presenting cells in the CNS of susceptible mice appears to exert a powerful negative role in the protection from virus-induced demyelinating disease by dampening the T cell responses and functions. Our results further suggest that the IL-6 and type I IFNs generated after TMEV infection cooperatively upregulate the expression of PD-1 and PDL-1, although IFN-γ may also participate in upregulation. Because IL-6 critically contributes to the pathogenesis of the demyelinating disease after TMEV infection by elevating the generation of pathogenic Th17 responses (12) and the expression of negative costimulatory molecules PD-1 and PDL-1 on CNS antigen-presenting cells, as shown in this study, the IL-6 generated upon viral infection appears to play an important pathogenic role in chronic viral inflammatory responses, including the possibility that the upregulated expression of PDL-1 on virus-infected microglia hinders the cytolytic function of CD8+ T cells, permitting viral persistence. Therefore, inhibition of IL-6 production or function may be a therapeutic strategy to control the chronic immune-mediated demyelinating disease resulting from viral infection.
ACKNOWLEDGMENTS
This work was supported by United States Public Health Service grants (RO1 NS28752 and RO1 NS33008) and by a grant from the National Multiple Sclerosis Society (RG 4001-A6).
Footnotes
Published ahead of print 21 August 2013
REFERENCES
- 1.Theiler M. 1934. Spontaneous encephalomyelitis of mice—a new virus. Dis. Sci. 80:122. [DOI] [PubMed] [Google Scholar]
- 2.Dal Canto MC, Kim BS, Miller SD, Melvold RW. 1996. Theiler's murine encephalomyelitis virus (TMEV)-induced demyelination: a model for human multiple sclerosis. Methods 10:453–461 [DOI] [PubMed] [Google Scholar]
- 3.Kim BS, Lyman MA, Kang BS, Kang HK, Lee HG, Mohindru M, Palma JP. 2001. Pathogenesis of virus-induced immune-mediated demyelination. Immunol. Res. 24:121–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez M, Leibowitz J, David CS. 1986. Susceptibility to Theiler's virus-induced demyelination. Mapping of the gene within the H-2D region. J. Exp. Med. 163:620–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pullen LC, Park SH, Miller SD, Dal Canto MC, Kim BS. 1995. Treatment with bacterial LPS renders genetically resistant C57BL/6 mice susceptible to Theiler's virus-induced demyelinating disease. J. Immunol. 155:4497–4503 [PubMed] [Google Scholar]
- 6.Yamada M, Zurbriggen A, Fujinami RS. 1990. The relationship between viral RNA, myelin-specific mRNAs, and demyelination in central nervous system disease during Theiler's virus infection. Am. J. Pathol. 137:1467–1479 [PMC free article] [PubMed] [Google Scholar]
- 7.Jin YH, Mohindru M, Kang MH, Fuller AC, Kang B, Gallo D, Kim BS. 2007. Differential virus replication, cytokine production, and antigen-presenting function by microglia from susceptible and resistant mice infected with Theiler's virus. J. Virol. 81:11690–11702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lyman MA, Myoung J, Mohindru M, Kim BS. 2004. Quantitative, not qualitative, differences in CD8(+) T cell responses to Theiler's murine encephalomyelitis virus between resistant C57BL/6 and susceptible SJL/J. mice. Eur. J. Immunol. 34:2730–2739 [DOI] [PubMed] [Google Scholar]
- 9.Mohindru M, Kang B, Kim BS. 2006. Initial capsid-specific CD4(+) T cell responses protect against Theiler's murine encephalomyelitisvirus-induced demyelinating disease. Eur. J. Immunol. 36:2106–2115 [DOI] [PubMed] [Google Scholar]
- 10.Kim BS, Palna JP. 1999. Immune mechanisms of Theiler's virus-induced demyelination. Exp. Mol. Med. 31:115–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin YH, Kang B, Kim BS. 2009. Theiler's virus infection induces a predominant pathogenic CD4+ T cell response to RNA polymerase in susceptible SJL/J. mice. J. Virol. 83:10981–10992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hou W, Kang HS, Kim BS. 2009. Th17 cells enhance viral persistence and inhibit T cell cytotoxicity in a model of chronic virus infection. J. Exp. Med. 206:313–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crane MA, Yauch R, Dal Canto MC, Kim BS. 1993. Effect of immunization with Theiler's virus on the course of demyelinating disease. J. Neuroimmunol. 45:67–73 [DOI] [PubMed] [Google Scholar]
- 14.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. 2008. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 26:677–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. 2000. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 192:1027–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. 1999. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 11:141–151 [DOI] [PubMed] [Google Scholar]
- 17.Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, Honjo T. 2001. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 291:319–322 [DOI] [PubMed] [Google Scholar]
- 18.Iwai Y, Terawaki S, Ikegawa M, Okazaki T, Honjo T. 2003. PD-1 inhibits antiviral immunity at the effector phase in the liver. J. Exp. Med. 198:39–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC, Koup RA. 2006. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J. Exp. Med. 203:2281–2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439:682–687 [DOI] [PubMed] [Google Scholar]
- 21.Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T. 1996. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int. Immunol. 8:765–772 [DOI] [PubMed] [Google Scholar]
- 22.Terawaki S, Chikuma S, Shibayama S, Hayashi T, Yoshida T, Okazaki T, Honjo T. 2011. IFN-alpha directly promotes programmed cell death-1 transcription and limits the duration of T cell-mediated immunity. J. Immunol. 186:2772–2779 [DOI] [PubMed] [Google Scholar]
- 23.Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, Koulmanda M, Freeman GJ, Sayegh MH, Sharpe AH. 2006. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J. Exp. Med. 203:883–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishida M, Iwai Y, Tanaka Y, Okazaki T, Freeman GJ, Minato N, Honjo T. 2002. Differential expression of PD-L1 and PD-L2, ligands for an inhibitory receptor PD-1, in the cells of lymphohematopoietic tissues. Immunol. Lett. 84:57–62 [DOI] [PubMed] [Google Scholar]
- 25.Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, Shin T, Tsuchiya H, Pardoll DM, Okumura K, Azuma M, Yagita H. 2002. Expression of programmed death 1 ligands by murine T cells and APC. J. Immunol. 169:5538–5545 [DOI] [PubMed] [Google Scholar]
- 26.Duncan DS, Miller SD. 2011. CNS expression of B7-H1 regulates pro-inflammatory cytokine production and alters severity of Theiler's virus-induced demyelinating disease. PLoS One 6:e18548. 10.1371/journal.pone.0018548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suematsu S, Matsusaka T, Matsuda T, Ohno S, Miyazaki J, Yamamura K, Hirano T, Kishimoto T. 1992. Generation of plasmacytomas with the chromosomal translocation t(12;15) in interleukin 6 transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 89:232–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hou W, So EY, Kim BS. 2007. Role of dendritic cells in differential susceptibility to viral demyelinating disease. PLoS Pathog. 3:e124. 10.1371/journal.ppat.0030124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schreiner B, Mitsdoerffer M, Kieseier BC, Chen L, Hartung HP, Weller M, Wiendl H. 2004. Interferon-beta enhances monocyte and dendritic cell expression of B7-H1 (PD-L1), a strong inhibitor of autologous T-cell activation: relevance for the immune modulatory effect in multiple sclerosis. J. Neuroimmunol. 155:172–182 [DOI] [PubMed] [Google Scholar]
- 30.Wiesemann E, Deb M, Trebst C, Hemmer B, Stangel M, Windhagen A. 2008. Effects of interferon-beta on co-signaling molecules: upregulation of CD40, CD86 and PD-L2 on monocytes in relation to clinical response to interferon-beta treatment in patients with multiple sclerosis. Mult. Scler. 14:166–176 [DOI] [PubMed] [Google Scholar]
- 31.Jin YH, Hou W, Kim SJ, Fuller AC, Kang B, Goings G, Miller SD, Kim BS. 2010. Type I interferon signals control Theiler's virus infection site, cellular infiltration and T cell stimulation in the CNS. J. Neuroimmunol. 226:27–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Groschel S, Piggott KD, Vaglio A, Ma-Krupa W, Singh K, Goronzy JJ, Weyand CM. 2008. TLR-mediated induction of negative regulatory ligands on dendritic cells. J. Mol. Med. (Berl.) 86:443–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lafon M, Megret F, Meuth SG, Simon O, Velandia Romero ML, Lafage M, Chen L, Alexopoulou L, Flavell RA, Prehaud C, Wiendl H. 2008. Detrimental contribution of the immuno-inhibitor B7-H1 to rabies virus encephalitis. J. Immunol. 180:7506–7515 [DOI] [PubMed] [Google Scholar]
- 34.Jin YH, Kaneyama T, Kang MH, Kang HS, Koh CS, Kim BS. 2011. TLR3 signaling is either protective or pathogenic for the development of Theiler's virus-induced demyelinating disease depending on the time of viral infection. J. Neuroinflammation 8:178. 10.1186/1742-2094-8-178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jin YH, Kim SJ, So EY, Meng L, Colonna M, Kim BS. 2012. Melanoma differentiation-associated gene 5 is critical for protection against Theiler's virus-induced demyelinating disease. J. Virol. 86:1531–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang DC, Gopalkrishnan RV, Lin L, Randolph A, Valerie K, Pestka S, Fisher PB. 2004. Expression analysis and genomic characterization of human melanoma differentiation associated gene-5, mda-5: a novel type I interferon-responsive apoptosis-inducing gene. Oncogene 23:1789–1800 [DOI] [PubMed] [Google Scholar]
- 37.Kang DC, Gopalkrishnan RV, Wu Q, Jankowsky E, Pyle AM, Fisher PB. 2002. mda-5: an interferon-inducible putative RNA helicase with double-stranded RNA-dependent ATPase activity and melanoma growth-suppressive properties. Proc. Natl. Acad. Sci. U. S. A. 99:637–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phares TW, Ramakrishna C, Parra GI, Epstein A, Chen L, Atkinson R, Stohlman SA, Bergmann CC. 2009. Target-dependent B7-H1 regulation contributes to clearance of central nervous system infection and dampens morbidity. J. Immunol. 182:5430–5438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jeong HY, Lee YJ, Seo SK, Lee SW, Park SJ, Lee JN, Sohn HS, Yao S, Chen L, Choi I. 2008. Blocking of monocyte-associated B7-H1 (CD274) enhances HCV-specific T cell immunity in chronic hepatitis C infection. J. Leukoc. Biol. 83:755–764 [DOI] [PubMed] [Google Scholar]
- 40.Said EA, Dupuy FP, Trautmann L, Zhang Y, Shi Y, El-Far M, Hill BJ, Noto A, Ancuta P, Peretz Y, Fonseca SG, Van Grevenynghe J, Boulassel MR, Bruneau J, Shoukry NH, Routy JP, Douek DC, Haddad EK, Sekaly RP. 2010. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat. Med. 16:452–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yao S, Wang S, Zhu Y, Luo L, Zhu G, Flies S, Xu H, Ruff W, Broadwater M, Choi IH, Tamada K, Chen L. 2009. PD-1 on dendritic cells impedes innate immunity against bacterial infection. Blood 113:5811–5818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sadagopal S, Lorey SL, Barnett L, Sutherland D, Basham R, Erdem H, Kalams SA, Haas DW. 2010. Enhanced PD-1 expression by T cells in cerebrospinal fluid does not reflect functional exhaustion during chronic human immunodeficiency virus type 1 infection. J. Virol. 84:131–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolfle SJ, Strebovsky J, Bartz H, Sahr A, Arnold C, Kaiser C, Dalpke AH, Heeg K. 2011. PD-L1 expression on tolerogenic APCs is controlled by STAT-3. Eur. J. Immunol. 41:413–424 [DOI] [PubMed] [Google Scholar]
- 44.Palma JP, Kwon D, Clipstone NA, Kim BS. 2003. Infection with Theiler's murine encephalomyelitis virus directly induces proinflammatory cytokines in primary astrocytes via NF-kappaB activation: potential role for the initiation of demyelinating disease. J. Virol. 77:6322–6331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Magnus T, Schreiner B, Korn T, Jack C, Guo H, Antel J, Ifergan I, Chen L, Bischof F, Bar-Or A, Wiendl H. 2005. Microglial expression of the B7 family member B7 homolog 1 confers strong immune inhibition: implications for immune responses and autoimmunity in the CNS. J. Neurosci. 25:2537–2546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.So EY, Kang MH, Kim BS. 2006. Induction of chemokine and cytokine genes in astrocytes following infection with Theiler's murine encephalomyelitis virus is mediated by the Toll-like receptor 3. Glia 53:858–867 [DOI] [PubMed] [Google Scholar]
- 47.So EY, Kim BS. 2009. Theiler's virus infection induces TLR3-dependent upregulation of TLR2 critical for proinflammatory cytokine production. Glia 57:1216–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]