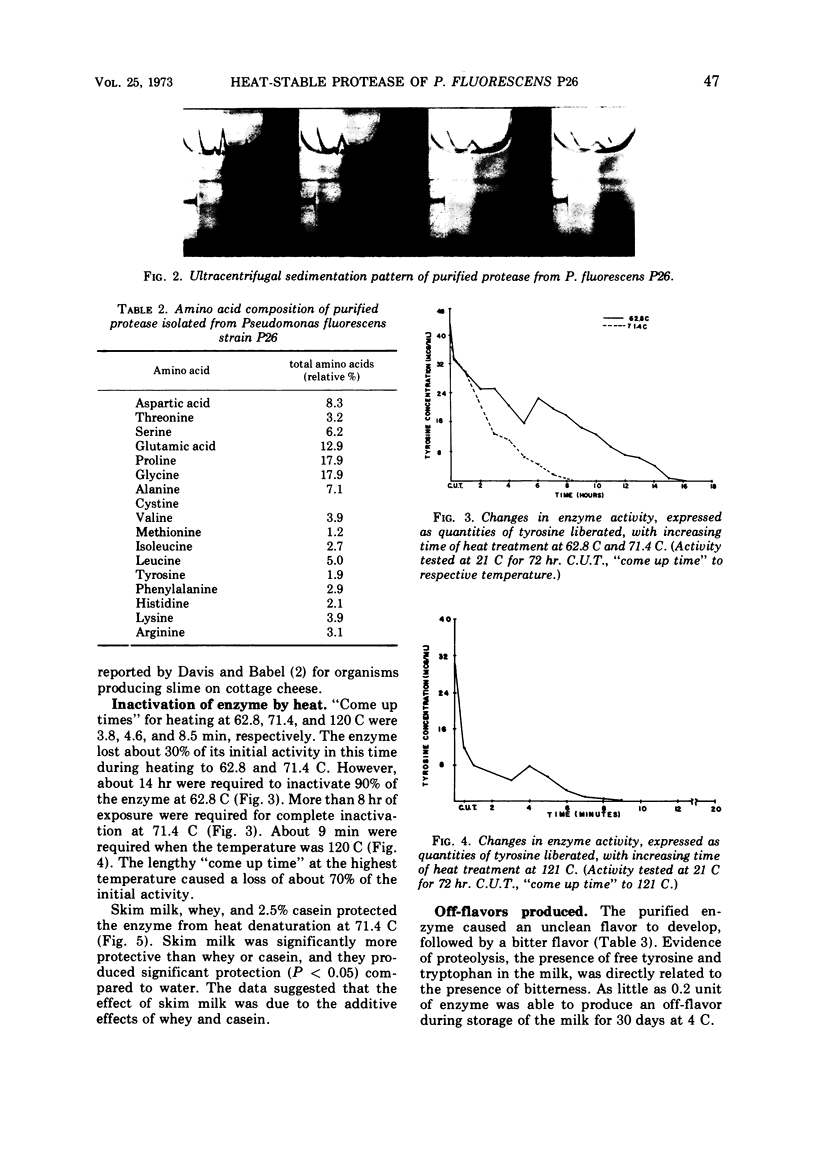
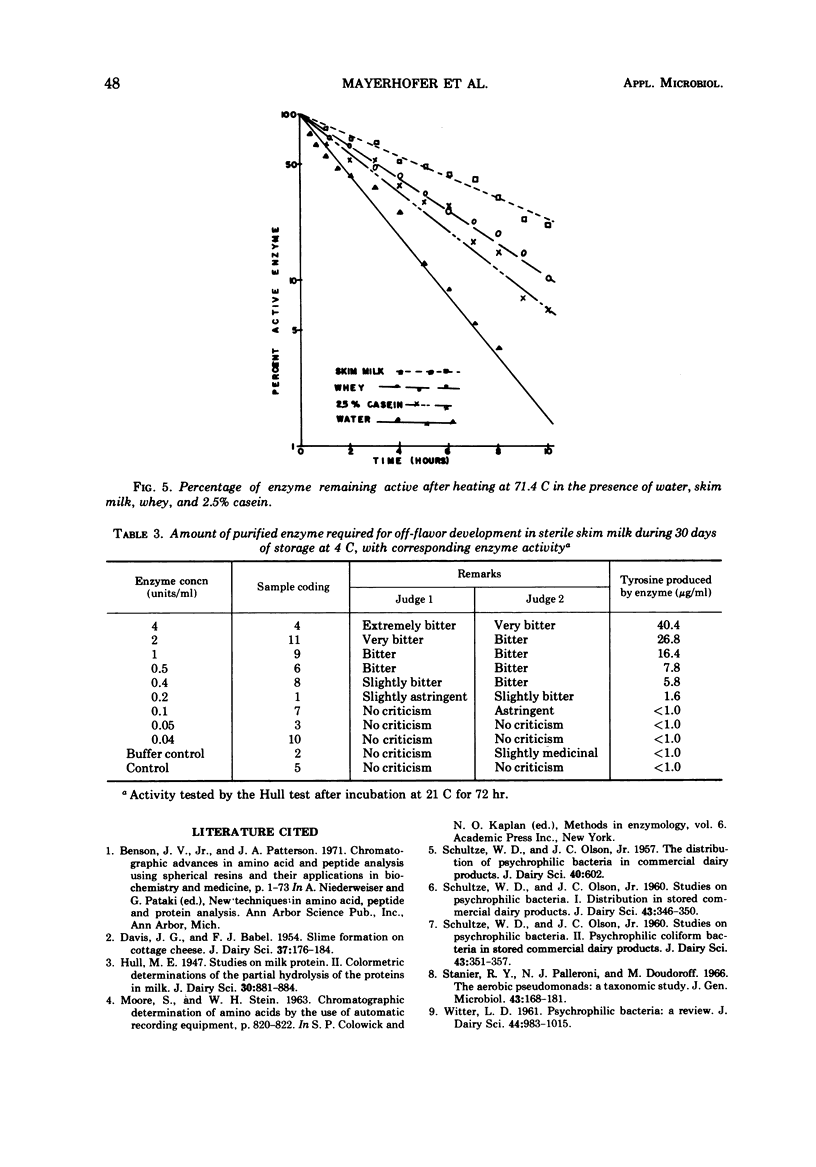
Abstract
A heat-stable, extracellular proteolytic enzyme was isolated from Pseudomonas fluorescens P26. Brain heart infusion broth (Fisher Scientific Co.), pH 7.5, and incubation at 21 C provided the optimal conditions for bacterial growth for enzyme production. The organism had a D value of 2.6 min at 62.8 C (145 F). The enzyme, however, was quite heat-stable, requiring 15 hr at 62.8 C, 8 hr at 71.4 C, and 9 min at 121 C for complete inactivation. Milk, whey, and casein each had a protective effect on the enzyme against heat inactivation. Purification was accomplished by growth of organisms in broth, centrifugation, sterilization by filtration, ammonium sulfate precipitation (55% saturation), dialysis (against six changes of water), and protein separation by passage through a Sephadex G-100 column. Ultracentrifugation revealed a single band with a sedimentation coefficient of 1.51 which suggested a molecular weight of approximately 23,000. As little as 0.2 unit of the purified enzyme caused detectable flavor defects during 30 days of storage at 4 C, and the Hull test for liberation of tyrosine compared favorably in sensitivity with the sensory method in milk.
Full text
PDF