Abstract
Identification of immune correlates of protection for viral vaccines is complicated by multiple factors, but there is general consensus on the importance of antibodies that neutralize viral attachment to susceptible cells. Development of new viral vaccines has mostly followed this neutralizing antibody paradigm, but as a recent clinical trial of human cytomegalovirus (HCMV) vaccination demonstrated, this singular approach can yield limited protective efficacy. Since HCMV devotes >50% of its coding capacity to proteins that modulate host immunity, it is hypothesized that expansion of vaccine targets to include this part of the viral proteome will disrupt viral natural history. HCMV and rhesus cytomegalovirus (RhCMV) each encode an ortholog to the cellular interleukin-10 (cIL-10) cytokine: cmvIL-10 and rhcmvIL10, respectively. Despite extensive sequence divergence from their host's cIL-10, each viral IL-10 retains nearly identical functionality to cIL-10. Uninfected rhesus macaques were immunized with engineered, nonfunctional rhcmvIL-10 variants, which were constructed by site-directed mutagenesis to abolish binding to the cIL-10 receptor. Vaccinees developed antibodies that neutralized rhcmvIL-10 function with no cross-neutralization of cIL-10. Following subcutaneous RhCMV challenge, the vaccinees exhibited both reduced RhCMV replication locally at the inoculation site and systemically and significantly reduced RhCMV shedding in bodily fluids compared to controls. Attenuation of RhCMV infection by rhcmvIL-10 vaccination argues that neutralization of viral immunomodulation may be a new vaccine paradigm for HCMV by expanding potential vaccine targets.
INTRODUCTION
There is ample precedent from studies of licensed viral and microbial vaccines that vaccine-mediated stimulation of pathogen-specific B cell immunity is critical for protection from infection and/or disease (1). Many new vaccine designs are predicated on the induction of antibodies that neutralize viral attachment to susceptible cells. As phase 2 clinical trials on human cytomegalovirus (HCMV) vaccination have shown, however, this approach only partially protects against challenge virus infection. In one study, seronegative women who had given birth within the previous year were vaccinated against HCMV glycoprotein B (gB) (2), the predominant virion target for antibodies that neutralize infection of fibroblasts. While significant protection against primary HCMV infection was observed in the vaccine group, the effect was limited in magnitude (50%) and duration. In the second placebo-controlled, randomized study, liver and kidney transplant candidates were similarly immunized with gB and prospectively evaluated for parameters of HCMV infection posttransplantation (3). Vaccine-mediated increases in gB-specific antibody titers were inversely correlated with the duration of HCMV viremia posttransplantation. Similar to the trial involving seronegative women, however, vaccination against gB alone in transplant recipients did not completely protect those at risk for HCMV infection. The absence of greater protection in both studies offers compelling justification that HCMV vaccine optimization is required. Potential improvements on initial clinical trials include improved epitope targeting, increased durability of vaccine-mediated immune responses, and/or expansion of viral antigens as vaccine candidates. For the latter, vaccination against HCMV proteins that modulate host immunity is an untested strategy. This may be especially relevant for viruses, such as HCMV, that establish a persistent infection, since early manipulation of the immune microenvironment may be an obligatory prerequisite for persistence. For HCMV, accumulating evidence indicates that particular HCMV immunomodulating proteins may be vulnerable to vaccine-mediated inhibition.
Exploitation of cellular interleukin-10 (cIL-10) or IL-10 receptor (IL-10R) signaling is a common theme in the natural histories of diverse pathogenic and nonpathogenic microbes that establish lifelong persistence, including viruses, pathogenic and commensal bacteria, protozoa, helminths, and fungi (4–7). This unifying linkage via cIL-10 suggests the potential for development of novel interventions to prevent and/or disrupt persistent infections. Indeed, treatment of mice infected with either murine cytomegalovirus (MCMV) or lymphocytic choriomeningitis virus with a monoclonal antibody that blocks cIL-10 or IL-10R engagement enhances immune system-mediated clearance of both persistent viruses (8, 9). Unlike MCMV, which stimulates cIL-10 expression (10, 11), HCMV and rhesus cytomegalovirus (RhCMV) each encode a cIL-10 ortholog (cmvIL-10 for HCMV and rhcmvIL-10 for RhCMV). cmvIL-10 retains only 27% amino acid identity to cIL-10, yet it binds with higher affinity to IL-10R than cIL-10 and retains nearly identical functionality to that of cIL-10 (12–14). cmvIL10 downregulates proinflammatory cytokine production and professional antigen-presenting cell functions in activated lymphoid cells in vitro (15–17), suggestive of cmvIL-10-mediated modulation of both innate and adaptive immunity in an infected host. In support of this, rhesus macaques inoculated with an RhCMV variant lacking rhcmvIL-10 function exhibit increased inflammatory responses and greater RhCMV-specific antibody and T cell responses than animals inoculated with the parental virus expressing rhcmvIL-10 (18). A mechanistic basis for these observations is suggested by the fact that rhcmvIL-10 suppresses the production of IL-12 in lipopolysaccharide (LPS)-activated peripheral blood mononuclear cells (PBMC) (19, 20). Together, these studies highlight HCMV modulation of host immunity and lead to the hypothesis that strategies that prevent HCMV-mediated activation of IL-10R signaling during the earliest stage of primary infection should significantly alter the virus-host relationship to augment host antiviral immunity. To this end, biologically inert forms of rhcmvIL-10 (rhcmvIL-10M1 and rhcmvIL-10M2), which were previously demonstrated to be incapable of both binding to IL-10R and suppressing activation of lymphoid cells (20), were evaluated as vaccine antigens in RhCMV-uninfected rhesus macaques.
MATERIALS AND METHODS
Animals.
Healthy, genetically outbred rhesus macaques from the California National Primate Research Center, confirmed to be RhCMV uninfected, were used for these studies. Their ages ranged from 1 to 2 years at the time of RhCMV inoculation. The University of California, Davis (UC Davis), is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC; Animal Assurance no. A3433-01), a private, nonprofit group that promotes the humane treatment of animals in science through voluntary accreditation. UC Davis is one of more than 640 research institutions and other organizations that have earned AAALAC accreditation, demonstrating its commitment to responsible animal care and use. In addition, the CNPRC receives unannounced inspections by the U.S. Department of Agriculture, as required by the Animal Welfare Act, and inspections by the Food and Drug Administration. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals (21) and in accordance with the recommendations of The Use of Nonhuman Primates in Research—the Weatherall Report (22). The Institutional Animal Care and Use Committee of UC Davis approved in advance all animal use protocols. Animals were anesthetized with ketamine during all procedures (blood draws, saliva and urine collection, and vaccinations). Care was taken to ensure that the animals were adequately sedated under all conditions, as assessed by the veterinarian and/or animal care staff.
DNA or protein immunization and RhCMV inoculation of rhesus macaques.
The construction of two nonfunctional forms of rhcmvIL-10, termed rhcmvIL-10M1 and rhcmvIL-10M2, has been described previously (20). The M1 and M2 variants of rhcmvIL-10 each contain two site-directed amino acid changes that prevent the recombinant proteins from (i) binding IL-10R and (ii) suppressing proinflammatory cytokine production by LPS-activated PBMC. Four RhCMV-uninfected animals were immunized with rhcmvIL-10M1 and -M2 plasmids at week 0, as previously described (23), and subsequently boosted with rhcmvIL-10M1/M2 protein at weeks 6, 12, and 26, as described previously (19, 20). In brief, DNA recombinant expression constructs, pND/rhcmvIL-10M1 and pND/rhcmvIL-10M2 (20), were purified using an endotoxin-free plasmid purification kit (Qiagen), and the DNA concentration was determined spectrophotometrically. DNA was then diluted in phosphate-buffered saline (PBS) buffer at 1 mg/ml and stored at −80°C. rhcmvIL-10M1 and rhcmvIL-10M2 proteins, expressed from a pMT expression vector system in Drosophila melanogaster S2 cells, were purified by nickel affinity purification protocol as previously described (20). Four RhCMV-negative rhesus macaques were immunized with a combination of rhcmvIL-10M1 and rhcmvIL-10M2 using a heterologous DNA prime-protein boost immunization strategy (23). Animals were immunized first with injection of rhcmvIL-10M1/M2 plasmid DNA (150 μg intramuscularly [i.m.], 50 μg intradermally [i.d.]) at week 0 and subsequently given three intramuscular boosts of rhcmvIL-10M1/M2 protein (50 μg each of M1 and M2) at weeks 6, 12, and 26. The proteins were adjuvanted in Montanide ISA 720 as described previously (23). The four immunized macaques and four mock-immunized RhCMV-uninfected controls were then challenged subcutaneously with the epitheliotropic RhCMV strain UCD59 at 1,000 PFU (24).
Sample collection and processing.
Oral swabs, urine, and blood were collected from anesthetized animals and processed according to our published procedures (24, 25). Saliva was collected via oral swabs of the buccal pouch and subsequently mixed with 2 ml of PBS. This represented an approximate 1:10 dilution of saliva. Urine was collected via cystocentesis. All samples were stored at −80°C until use.
Quantitative real-time PCR.
Real-time PCR quantification of RhCMV DNA in plasma, oral swabs, and urine was done according to previously published protocols (26).
ELISA.
Enzyme-linked immunosorbent assays (ELISAs) for rhcmvIL-10 binding, RhCMV binding antibodies, and RhCMV neutralizing (fibroblast) antibodies (NAb) were performed as described previously (19, 20, 23, 27). The avidity of rhcmvIL-10-specific antibodies was quantified, as described previously (19).
Neutralization of rhcmvIL-10 function in vitro.
Neutralizing antibodies against rhcmvIL-10 function were characterized by a bioassay measuring the ability of rhesus plasma to neutralize wild-type rhcmvIL-10-mediated suppression of IL-12 production in LPS-stimulated PBMC (19, 20).
Microscopy.
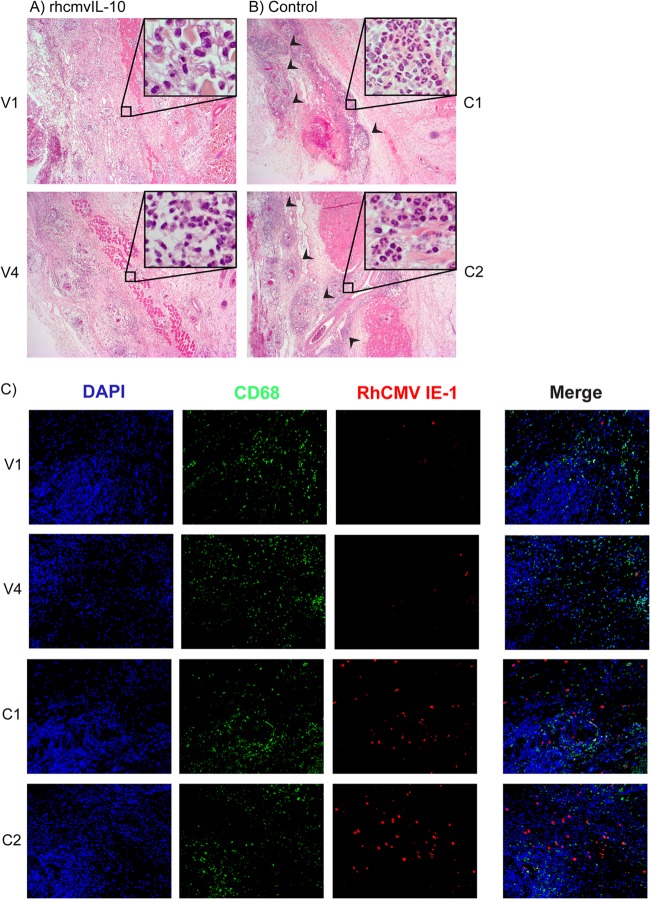
Skin biopsy specimens were analyzed for nuclear content (DAPI [4′,6-diamidino-2-phenylindole]), the CD68 macrophage marker, and RhCMV immediate early 1 (IE-1) protein (18). Skin biopsy specimens were fixed in paraformaldehyde, paraffin embedded, serially sectioned, and processed for hematoxylin and eosin (H&E) staining and immunofluorescence labeling. All sections were deparaffinized with 100% xylene (3 washes, 5 min each) and washed 3 times in 100% ethanol (EtOH). Endogenous peroxidase activity was inactivated by immersing sections in 3% H2O2 in methanol (MeOH) for 20 min followed by 2 additional 100% EtOH washes and one wash each in 95% and 70% EtOH. Sections were then washed for 10 min in deionized H2O, treated with Antigen Decloaker solution (Biocare Medical) for 4 h at 97°C, and gradually brought down to room temperature (RT). After the samples were washed in PBS (2 times for 5 min each), they were blocked in Dako universal blocker (Invitrogen) for 30 min. Sections were then stained with monoclonal antibodies cross-reactive to monkey CD68 (KP1) (Thermo Scientific) and rabbit anti-RhCMV IE-1 polyclonal antibodies (27). Sections were washed and then fluorescently stained with DyLight 488 and DyLight 595 (Vector Laboratories) and subsequently mounted with Prolong Gold antifade reagent with DAPI (Invitrogen). Images were taken using fluorescent light and a single-pass filter (Omega Optical) with a digital camera (Axicam, Carl Zeiss, Germany) operated by AxioVision software. Images were processed with Adobe Photoshop (Adobe systems).
Statistical analysis.
All statistical analysis was performed using Prism 4 (GraphPad Software, Inc.).
RESULTS
Induction of rhcmvIL-10-specific antibody responses.
Using a DNA prime-protein boost immunization strategy (23), four RhCMV-uninfected macaques were primed with plasmid vectors for rhcmvIL-10M1 and rhcmvIL-10M2 and boosted three times with insect cell-expressed rhcmvIL-10M1 and -M2 proteins (20). Vaccinees developed rhcmvIL-10-specific binding antibodies and antibodies that neutralized wild-type (WT) rhcmvIL-10 function (19) (Fig. 1A and B). rhcmvIL-10 binding antibodies were detected in all animals after the first protein boost, whereas antibodies that neutralized rhcmvIL-10 function (NAb) required two protein boosts for detectable NAbs in all 4 vaccinees. The final immunization stimulated peak responses that exceeded those observed after the second protein boost and were comparable to those observed in macaques naturally infected with RhCMV (19) (data not shown). Previous studies demonstrated that NAb against WT rhcmvIL-10 function, induced by RhCMV infection, do not cross-neutralize rhesus cIL-10 (19, 20). To determine if rhcmvIL-10 M1/M2 immunization of naive animals stimulated de novo antibodies that inhibited cIL-10, plasma samples from the vaccinees were evaluated for neutralization of cIL-10 in an LPS-activated PBMC bioassay. The basis of this assay was that both WT rhcmvIL-10 and cIL-10 suppress IL-12 production in PBMC activated by exposure to LPS. Whereas all postvaccination plasma samples neutralized rhcmvIL-10-mediated inhibition of IL-12 production (24 to 96%), there was no detectable neutralization of cIL-10 function (Fig. 1C). The absence of detectable cross-reactivity to cIL-10 in rhcmvIL-10-vaccinated animals was not unexpected. There is exceedingly low protein identity between the two IL-10 orthologs (25% amino acid identity) (14), and no evidence of an anti-cIL-10-like autoimmunity has been described in RhCMV-infected macaques, in which the seroreactivity to rhcmvIL-10 is 100% (19).
Fig 1.
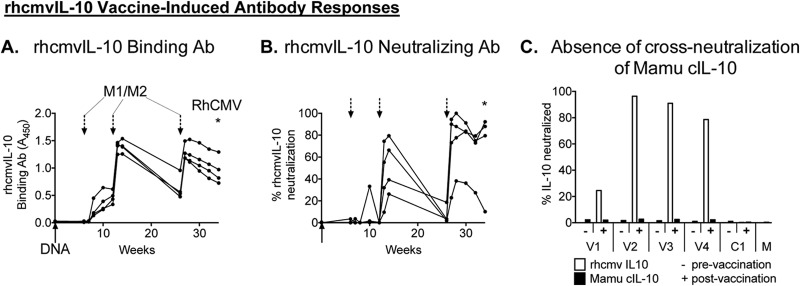
rhcmvIL-10 vaccine-induced antibody responses. Four RhCMV-uninfected juvenile macaques were immunized over the course of 26 weeks with a mixture of two different nonfunctional forms of rhcmvIL-10, rhcmvIL-10M1 and -M2, by a DNA prime (solid arrow) and 3 protein boosts (dashed arrows). All animals were challenged with RhCMV at 34 weeks (asterisk). (A) rhcmvIL-10 binding antibodies (Ab). (B) rhcmvIL-10 neutralizing antibodies. (C) Plasma samples from the four vaccinees (V1 to V4) and one control (C1) were evaluated before (−) and after (+) immunization for neutralization of rhcmvIL-10 (white) and cIL-10 (black) functions.Mamu, Macaca mulatta.
Vaccine-induced protection against local RhCMV replication.
The protective efficacy of rhcmvIL-10M1/M2 vaccination was assessed by subcutaneous challenge of the vaccinees and four unvaccinated controls with 103 PFU of an epitheliotropic strain of RhCMV (UCD59) (24). To determine whether rhcmvIL-10 vaccination modified acute RhCMV infection, biopsy specimens of the inoculation site, obtained 1 week postchallenge (pc), were characterized for the nature of the host cellular infiltrate and the extent of local RhCMV infection. Whereas all animals in both groups were noted for a vascularly oriented edema, the two groups were distinguished by the severity of inflammation. Biopsy specimens from the control animals were noted for marked vascularly oriented inflammation and edema in the subcutis, panniculus carnosis, and underlying connective tissue with focally intense areas of neutrophil infiltration and macrophages that had engulfed degenerate neutrophils (Fig. 2B [only two animals shown]). Inflammatory changes did not extend into the dermis. The inflammation was centered in and around venous blood vessels (and, perhaps, lymphatics), with margination of neutrophils and hypertrophy of lining endothelium. In contrast, biopsy specimens of the vaccinated animals exhibited less inflammation, ranging from mild, often multifocal, inflammation in three of the four vaccinees to a more prominent inflammation in one vaccinee (Fig. 2A [only two animals shown]). Another notable distinction between both groups was that the intensity of neutrophilic inflammation in the epicenter of the inflammatory lesions was markedly reduced in the vaccine group compared to the controls. The infiltrate in the vaccinees was predominantly mononuclear. Cytomegalic cells, characteristic of productive RhCMV infection, were observed in all animals. To assess whether there was local control of RhCMV replication at the site of inoculation in the vaccinated animals, immunohistochemical staining was performed to detect infected cells expressing RhCMV immediate early 1 (IE-1) protein. In foci with comparable macrophage infiltration, there were markedly fewer cells expressing RhCMV IE-1 protein in the vaccinees than the controls (Fig. 2C). Taken together, the analyses of the inoculation site biopsy specimens were consistent with the interpretation that prior vaccination against rhcmvIL-10 alone elicited qualitative and quantitative changes in host immune responses to acute infection coincident with reduced viral replication locally.
Fig 2.
RhCMV replication and host inflammation 7 days post-RhCMV challenge (at inoculation site). Skin biopsy specimens were taken at sites of infection 1 week postinoculation. Serial sections were stained with H&E (4×; 40× for inset). Representative images for vaccinees V1 and V4 (A) and controls C1 and C2 (B) are shown. Vaccinees showed a diffused decrease in the inflammatory infiltrate, with a specific decrease in polymorphonuclear cells. Arrows indicate focal infiltrates in control animals. (C) Skin biopsy specimens were stained for RhCMV IE-1 (red), the CD68 macrophage marker (green), and nuclei (DAPI) (20×). Vaccinees had fewer infected cells, observed by both the presence of cytomegalic cells, as seen in the H&E stain (A and B), and IE-1-positive cells, as seen in the RhCMV IE-1-stained cells (C).
Vaccine-induced protection against systemic RhCMV replication.
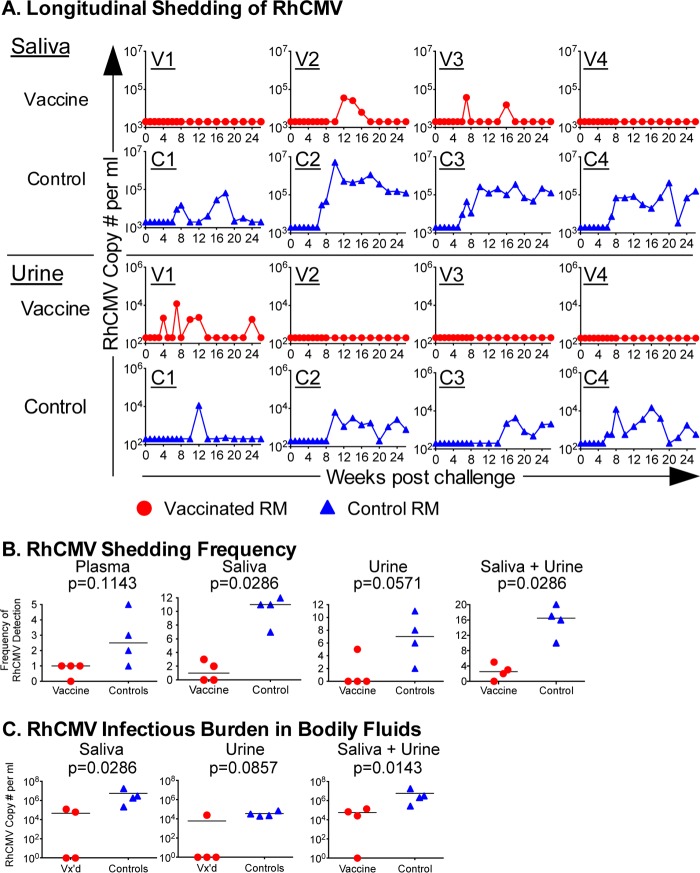
Primary infection of rhesus macaques with wild-type RhCMV naturally circulating in breeding cohorts of rhesus macaques or epitheliotropic strains of RhCMV, including UCD59, results in long-term excretion of RhCMV in saliva and urine in the vast majority of infected animals (24, 25, 28). To assess if rhcmvIL-10 vaccination altered the long-term pattern of RhCMV infection, RhCMV genomes were prospectively quantified in saliva and urine of both treatment groups. Whereas RhCMV shedding generally persisted in the saliva and urine of unvaccinated controls once shedding began, RhCMV DNA was notably absent in the saliva and urine in 2 and 3 of the vaccinees, respectively, throughout 26 weeks pc (Fig. 3A). The individual frequencies of RhCMV DNA detection in saliva and urine and the combined frequency of RhCMV detection in both fluids were significantly lower than the frequencies of RhCMV detected in the control animals (Fig. 3B). The frequency of RhCMV detection was also reduced in plasma, although statistical significance was not reached (Fig. 3B). Three vaccinees had detectable RhCMV DNA in one bodily fluid only, and the fourth did not have detectable RhCMV DNA in either compartment. In contrast, all controls shed virus in both fluids. Commensurate with a reduction in the frequencies of RhCMV shedding, the cumulative infectious burden of RhCMV in bodily fluids (determined as an area under the curve [AUC]) of the vaccinees was significantly reduced in both saliva and urine individually and in both fluids combined (median, 4.7 × 104 RhCMV copies), compared to that of the controls (median, 2.6 × 106 RhCMV copies) (Fig. 3C). In sum, rhcmvIL-10 vaccination elicited prominent reductions in both the frequency and magnitude of RhCMV shedding postchallenge.
Fig 3.
RhCMV shedding in saliva and urine postchallenge. Saliva and urine samples were prospectively quantified for RhCMV genome copy numbers by quantitative PCR (qPCR) analysis. (A) Individual longitudinal qPCR analyses of RhCMV viral loads in saliva and urine of vaccinees (V1 to V4; red circles) and controls (C1 to C4; blue triangles). Quantities are shown as copies/ml, with limits of detection set at 2,000 and 200 copies/ml for saliva and urine, respectively. (B) Frequency of detection of RhCMV in plasma, saliva, urine, and combined saliva and urine. The horizontal line represents the median for each group. (C) Cumulative infectious burden of RhCMV (area under the curve) in saliva, urine, and combined saliva and urine. Vx'd, vaccinated. A two-tailed Mann-Whitney t test was performed to assess statistical significance.
Post-RhCMV challenge antibody responses.
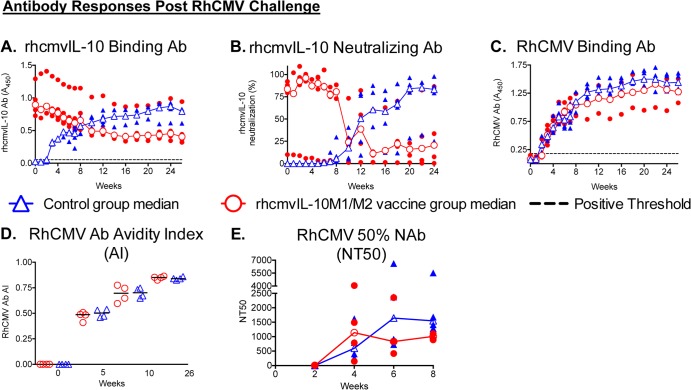
Decreased RhCMV shedding and restricted RhCMV replication at the inoculation site suggested that univalent immunization against rhcmvIL-10 elicited both local and distal protective immunity. Postchallenge immune responses were consistent with a model in which the magnitude of long-term RhCMV shedding inversely correlated with the presence of rhcmvIL-10-specific immunity at the time of and soon after RhCMV challenge. Every vaccinee had rhcmvIL-10 binding (Fig. 4A) and rhcmvIL-10 NAb (Fig. 4B) in plasma at the time of challenge and throughout the first 10 weeks of challenge, in addition to rhcmvIL-10-specific CD4+ T cell responses at 0 or 4 weeks pc (data not shown). Notably, all four vaccinees exhibited increased rhcmvIL-10 binding and/or neutralizing antibodies within 2 weeks post-RhCMV challenge. In the controls, rhcmvIL-10 binding antibodies steadily increased from 3 to 20 weeks postchallenge to levels comparable to those in macaques naturally exposed to RhCMV (Fig. 1D) (19). However, rhcmvIL-10 NAb were undetectable in the controls until ∼8 weeks pc (Fig. 4B), which was generally contemporaneous with the initial detection of RhCMV DNA in urine and saliva of the controls (Fig. 3A). The peak binding and neutralizing levels of the controls were comparable in the vaccinees, although the kinetics of the antibody responses were reversed. As opposed to the controls, median rhcmvIL-10 binding and neutralizing antibody titers in the vaccinees greatly declined over the same time frame to levels below those of the controls, particularly for rhcmvIL-10 NAb.
Fig 4.
Antibody responses post-RhCMV challenge for vaccinees (red circles) and controls (blue triangles). Open circles and triangles, median responses for vaccine and control groups, respectively; solid circles and triangles, individual animal responses. Note that because the symbols for the median value for each group are superimposed onto the symbols representing the individual values for each group, some of the individual values may be obscured. (A) rhcmvIL-10 binding antibodies. (B) rhcmvIL-10 neutralizing antibodies. (C) RhCMV binding antibodies. (D) RhCMV binding antibody avidity index (AI). (E) Fifty percent neutralizing antibody titers (NT50) on fibroblasts. (Only the first 8 weeks postchallenge were analyzed.)
Despite significant differences between both groups in RhCMV infectious burdens (Fig. 3) and rhcmvIL-10-specific antibody responses (Fig. 4A and B), no differences were noted in total RhCMV antibody responses postchallenge (Fig. 4C to E). RhCMV binding antibodies were first detected 2 to 4 weeks pc in all animals of both groups, and plateau titers were mostly reached by 16 to 20 weeks pc (Fig. 4C). While the median responses were 1.5- to 2-fold higher for the control animals, the differences were not statistically different. Similarly, increases in RhCMV antibody avidity (Fig. 4D) and the kinetics of antibodies that neutralized infection of fibroblasts (Fig. 4E through 8 weeks pc) were essentially equivalent between the two groups.
DISCUSSION
The goal of this study was to broaden the current paradigm for vaccine candidates to prevent primary HCMV infection in susceptible individuals (i.e., gB, pp65, and IE-1) (29) by demonstrating in the rhesus macaque model that vaccine-mediated targeting of a single RhCMV-encoded immunomodulatory protein elicits biologically relevant reductions in RhCMV replication. The rationale for this approach was that modulation of host immune responses is central to successful reiteration of CMV natural history in immunocompetent hosts (30), as well as the life cycles of a wide variety of mammalian pathogens. Although such a vaccine approach has not been reported for any species of CMV, there is ample precedent to now warrant evaluation of CMV immunomodulatory proteins as vaccine candidates, particularly the HCMV IL-10 ortholog (cmvIL-10). As previous reviews have noted, activation of IL-10R-mediated signaling is a phenotype of many microbial infections following either activation of cIL-10 expression by incompletely defined microbial components or expression of virus-encoded cIL-10 orthologs (vIL-10) (4–7). Importantly, engagement of IL-10R by either the cIL-10 or vIL-10 ligand appears to be a virulence factor for pathogenic infections or a requisite step for commensal bacterial infections (5). Multiple approaches have been employed to demonstrate that abrogation of cIL-10 induction and signaling attenuates infection and/or disease of bacteria (31–39), fungi (40), and viruses (9, 18, 41–43). Based on the premise that microbial manipulation of IL-10 signaling confers greater replication fitness in the infected host, targeted disruption of microbial exploitation of IL-10 signaling offers a clinically relevant option for preventing primary infection or reducing viral sequelae.
There are relatively few studies addressing vaccine targeting of microbial antigens that activate IL-10R signaling, and a range of outcomes have been observed following vaccination and challenge. Together, the results of these studies offer a road map for vaccination against cmvIL-10. Vaccination against the CyaA virulence toxin of Bordetella pertussis, which activates cIL-10 expression via Toll-like receptor 4 (TLR4) (44), significantly reduces pathogenic outcomes and bacterial loads following intranasal challenge of vaccinated mice (45). This, however, is the singular example in which vaccination against the microbial activator of IL-10R signaling induces protective efficacy (i.e., vaccine-induced reductions in infection and/or disease). In contrast, vaccination of mice with either the YopM type III secretion system protein of Yersinia pestis or the PrpA virulence factor of Brucella abortus does not induce any protective efficacy against bacterial challenge (46, 47). Since, in the case of YopM, purified protein can enter into the cell by “autonomous translocation” (48), vaccination with an unmodified form of the protein may preclude the efficient induction of antibodies that neutralize the function of the protein during primary challenge. Similarly, vaccination of mice with a recombinant form of the SSP4 immunomodulatory glycoprotein of Trypanosoma cruzi increases both parasite loads in the blood and lethality following challenge with T. cruzi trypomastigotes, whereas vaccination with an SSP4 cDNA expression plasmid reduces parasitemia and mortality (49). While the mechanism of increased pathogenesis following challenge of mice immunized with the functional protein form of SSP4 remains to be determined, it may be related to the fact that SSP4 protein immunization alone stimulates the production of immunoregulatory IL-10+ and/or gamma interferon-positive (IFN-γ+) CD4+ T cells (50). A salient implication of the immunization studies with YopM, PrpA, and SSP4 is that immunization with fully functional protein may not elicit protective immune responses due to inappropriate sequestration of antigen and/or antigen-induced skewing of host immune responses prior to challenge. Accordingly, the structural biology of cmvIL-10 or IL-10R engagement (12) was used to engineer minimal mutations into rhcmvIL-10 to ablate both binding to IL-10R and the immunosuppressive functionality of the parental form of rhcmvIL-10, while retaining the capability to stimulate memory antibody responses following vaccination of RhCMV-immune rhesus macaques (20).
This study extends our previous study by demonstrating that the combined rhcmvIL-10M1/M2 regimen is highly immunogenic in naive monkeys, stimulating de novo rhcmvIL-10-specific antibodies that bind and neutralize wild-type rhcmvIL-10. The titers of rhcmvIL-10-neutralizing antibodies are biologically relevant, since the range of vaccine-induced neutralizing titers is within the normative range of RhCMV-infected animals (19). This study also confirms previous observations that antibodies to rhcmvIL-10 do not neutralize cIL-10 following (i) RhCMV infection of naive animals (19), (ii) DNA or protein booster immunizations in RhCMV-immune animals (20), and (iii) priming and booster immunizations in RhCMV-uninfected animals (this study). The targeting of cmvIL-10 represents targeting of a virus-encoded cytokine especially amenable to vaccine-mediated neutralization because of its extensive genetic drift from human cIL-10 (14, 30). In contrast, the viral IL-10 ortholog encoded by Epstein-Barr virus (EBV) (ebvIL-10) retains 90% identity to human cIL-10 (7), and monoclonal and polyclonal antibodies specific to cIL-10 can both bind and neutralize ebvIL-10 (51). Antibodies to ebvIL-10 are elevated in clinically apparent EBV infections, including chronic infectious mononucleosis (CIM), nasopharyngeal carcinoma, and EBV-associated lymphoproliferative disease, compared to those in EBV-infected but asymptomatic hosts and EBV-uninfected individuals (52). In CIM patients, however, increased antibody titers to ebvIL-10 are observed in conjunction with elevated binding antibodies to cIL-10, suggestive of CIM-related induction of cross-reactive antibodies to cIL-10. The absence of cross-neutralization of rhcmvIL-10-specific antibodies to rhesus cIL-10 is not unexpected given the extensive sequence divergence between the two orthologs, highlighted by only a single instance of three contiguous identical amino acids and seven instances of two contiguous amino acids dispersed throughout the alignment of rhcmvIL-10 and rhesus cIL-10 (GenBank accession no. AAF59907 and NP_001038192, respectively). A previous study of ours did not demonstrate any clear protective effect conferred by DNA immunization with an rhcmvIL-10 expression plasmid (53). While the basis for an apparent absence of protection with rhcmvIL-10 vaccination is not known, two factors may have impacted the results. DNA immunization at the amounts of DNA used in the previous study has not proven to induce robust neutralizing responses, such as those directed to RhCMV gB. Furthermore, the expression plasmid for rhcmvIL-10 was constructed using the fully functional sequence of rhcmvIL-10. It may be that, similar to what has been observed with bacterial activators of cIL-10, immunization with a functional rhcmvIL-10 may have precluded generation of a protective immune response.
Considering that rhcmvIL-10 is a secreted protein not involved in attachment or entry of RhCMV to susceptible cells and is not a virion protein (18, 54, 64), it is quite remarkable that immunization against this single RhCMV immunomodulatory protein markedly enhanced both local and systemic control of challenge RhCMV replication within the infected host. Alterations in the magnitude and type of inflammatory cells at the site of inoculation together with reductions in the frequency and magnitude of shedding in saliva and urine are indicative of acute and long-term restriction of persistent high-level excretion in bodily fluids (24, 28). While the mechanisms by which rhcmvIL-10 vaccination restricted RhCMV challenge were not defined in this study, it is certain that they are distinct from those operative for neutralization of gB- and UL128 pentamer-mediated entry into fibroblasts and epithelial/endothelial cells (25, 55–58). Since RhCMV antibody responses were indistinguishable between groups, the data indicate that the salient immune correlate for reduced shedding in the vaccinees was the presence of rhcmvIL-10 NAb at the time of and/or soon after challenge. The evidence presented here, along with other studies on cmvIL-10 and rhcmvIL-10, lead to the following model invoking a temporal race between dissemination of virus to sites of persistence and generation of protective adaptive immune responses. Expression of rhcmvIL-10 in the control animals suppresses innate effector cells at the primary site of infection to enable dissemination to sites of persistent shedding in the salivary glands and genitourinary tract prior to development of protective adaptive responses (15, 17–19, 59–61). In contrast, vaccine-mediated neutralization of rhcmvIL-10 facilitates innate effector functions to greatly restrict robust dissemination of progeny virions until development of protective adaptive responses blocks further dissemination. Consistent with this interpretation was a reduced frequency of RhCMV detection in the plasma of the vaccinated animals compared to the controls. There are precedents for this scenario in which the quality and magnitude of the innate immune response are determinants for the quality and durability of adaptive immune responses (62).
Primary MCMV infection stimulates expression of cIL-10, which disrupts dendritic cell-natural killer cell interactions, leading in turn to poor priming of MCMV-specific CD4 T cell responses (63). The authors of this study concluded that “early induction of IL-10 during MCMV infection critically regulates the strength of the innate-adaptive immune cell cross talk” in ways that affected the long-term virus-host relationship. While the induction of cIL-10 lessened host-induced immunopathology (by minimizing expression of tumor necrosis factor alpha [TNF-α]), alteration of innate-adaptive cross talk by cIL-10 also enabled MCMV persistence within an immune host. The results of this MCMV study provide a mechanistic basis for the results observed in rhesus macaques vaccinated with nonfunctional rhcmvIL-10 and challenged with RhCMV. They emphasize the importance of early virus-mediated disruption of innate responses and the importance of vaccine targeting of those viral mediators of altered innate immunity. Since no differences were noted in overall anti-RhCMV antibody responses between the two treatment groups, our model postulates improved innate protective efficacy in the absence of rhcmvIL-10-mediated suppression. However, it should be noted that there was no clear correlation in our study between the magnitude of reductions in RhCMV excretion and the magnitudes of the vaccine-induced rhcmvIL-10 neutralizing antibody responses. Three of the four vaccinees (V1 to V3) were RhCMV DNA positive in one bodily fluid (saliva or urine) but not the other, and one (V4) was not positive in either fluid or plasma as well. Whereas the rhcmvIL-10 neutralizing responses were comparable for three of the vaccinees (V2 to V4), the neutralizing response for V1 was considerably lower at both the time of challenge and postchallenge. Future studies need to address whether there are interanimal differences in rhcmvIL-10-neutralizing epitopes and, if so, whether targeted expansion of critical epitopes is required to provide broader protective efficacy. Moreover, the limited detection of RhCMV DNA in plasma postchallenge indicates that rhcmvIL-10 should not be a singular vaccine component. Undoubtedly, the composition of an HCMV vaccine will be a multicomponent cocktail of viral proteins that include humoral and cellular epitopes representing different classes of the viral proteome. Ongoing studies are directed at determining whether the combination of RhCMV gB and rhcmvIL-10 proteins elicits greater protection against challenge than either protein alone. Since HCMV immunomodulators are critical for HCMV natural history, inclusion of additional HCMV proteins that attenuate host immunity should be explored for vaccine efficacy.
In sum, we have presented a novel change in the paradigm of HCMV vaccine design by neutralizing a central RhCMV immunomodulatory protein, rhcmvIL-10, resulting in enhanced innate immunity that restricts local viral replication until the development of protective adaptive immune responses. Successful alteration of infection and shedding patterns by rhcmvIL-10 vaccination argues that blocking of immunomodulation may be a useful approach to vaccine or therapeutic strategies for HCMV by augmenting the protective role of innate immunity to hold challenge virus in check until the development of protective antiviral adaptive responses.
ACKNOWLEDGMENTS
We thank William J. Britt and Don J. Diamond for critical reading of the manuscript prior to submission.
This work was supported by NIH grants R01 AI49342 to P.A.B. and M.R.W. and RO1 AI047300 and AI047300-S1 to M.R.W., the Margaret Deterding Infectious Disease Research Support Fund to P.A.B., and P51 OD011107 to the California National Primate Research Center.
Footnotes
Published ahead of print 14 August 2013
REFERENCES
- 1.Plotkin SA. 2010. Correlates of protection induced by vaccination. Clin. Vaccine Immunol. 17:1055–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pass RF, Zhang C, Evans A, Simpson T, Andrews W, Huang ML, Corey L, Hill J, Davis E, Flanigan C, Cloud G. 2009. Vaccine prevention of maternal cytomegalovirus infection. N. Engl. J. Med. 360:1191–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griffiths PD, Stanton A, McCarrell E, Smith C, Osman M, Harber M, Davenport A, Jones G, Wheeler DC, O'Beirne J, Thorburn D, Patch D, Atkinson CE, Pichon S, Sweny P, Lanzman M, Woodford E, Rothwell E, Old N, Kinyanjui R, Haque T, Atabani S, Luck S, Prideaux S, Milne RS, Emery VC, Burroughs AK. 2011. Cytomegalovirus glycoprotein-B vaccine with MF59 adjuvant in transplant recipients: a phase 2 randomised placebo-controlled trial. Lancet 377:1256–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duell BL, Tan CK, Carey AJ, Wu F, Cripps AW, Ulett GC. 2012. Recent insights into microbial triggers of interleukin-10 production in the host and the impact on infectious disease pathogenesis. FEMS Immunol. Med. Microbiol. 64:295–313 [DOI] [PubMed] [Google Scholar]
- 5.Eberhardt MK, Barry PA. Pathogen manipulation of cIL-10 signaling pathways: opportunities for vaccine development? Curr. Top. Microbiol. Immunol., in press [DOI] [PubMed] [Google Scholar]
- 6.Redpath S, Ghazal P, Gascoigne NR. 2001. Hijacking and exploitation of IL-10 by intracellular pathogens. Trends Microbiol. 9:86–92 [DOI] [PubMed] [Google Scholar]
- 7.Slobedman B, Barry PA, Spencer JV, Avdic S, Abendroth A. 2009. Virus-encoded homologs of cellular interleukin-10 and their control of host immune function. J. Virol. 83:9618–9629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brooks DG, Lee AM, Elsaesser H, McGavern DB, Oldstone MB. 2008. IL-10 blockade facilitates DNA vaccine-induced T cell responses and enhances clearance of persistent virus infection. J. Exp. Med. 205:533–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Humphreys IR, de Trez C, Kinkade A, Benedict CA, Croft M, Ware CF. 2007. Cytomegalovirus exploits IL-10-mediated immune regulation in the salivary glands. J. Exp. Med. 204:1217–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell AE, Cavanaugh VJ, Slater JS. 2008. The salivary glands as a privileged site of cytomegalovirus immune evasion and persistence. Med. Microbiol. Immunol. 197:205–213 [DOI] [PubMed] [Google Scholar]
- 11.Cavanaugh VJ, Deng Y, Birkenbach MP, Slater JS, Campbell AE. 2003. Vigorous innate and virus-specific cytotoxic T-lymphocyte responses to murine cytomegalovirus in the submaxillary salivary gland. J. Virol. 77:1703–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones BC, Logsdon NJ, Josephson K, Cook J, Barry PA, Walter MR. 2002. Crystal structure of human cytomegalovirus IL-10 bound to soluble human IL-10R1. Proc. Natl. Acad. Sci. U. S. A. 99:9404–9409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kotenko SV, Saccani S, Izotova LS, Mirochnitchenko OV, Pestka S. 2000. Human cytomegalovirus harbors its own unique IL-10 homolog (cmvIL-10). Proc. Natl. Acad. Sci. U. S. A. 97:1695–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lockridge KM, Zhou SS, Kravitz RH, Johnson JL, Sawai ET, Blewett EL, Barry PA. 2000. Primate cytomegaloviruses encode and express an IL-10-like protein. Virology 268:272–280 [DOI] [PubMed] [Google Scholar]
- 15.Chang WL, Baumgarth N, Yu D, Barry PA. 2004. Human cytomegalovirus-encoded interleukin-10 homolog inhibits maturation of dendritic cells and alters their functionality. J. Virol. 78:8720–8731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raftery MJ, Wieland D, Gronewald S, Kraus AA, Giese T, Schonrich G. 2004. Shaping phenotype, function, and survival of dendritic cells by cytomegalovirus-encoded IL-10. J. Immunol. 173:3383–3391 [DOI] [PubMed] [Google Scholar]
- 17.Spencer JV, Lockridge KM, Barry PA, Lin G, Tsang M, Penfold ME, Schall TJ. 2002. Potent immunosuppressive activities of cytomegalovirus-encoded interleukin-10. J. Virol. 76:1285–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang W, Barry P. 2010. Attenuation of innate immunity by cytomegalovirus IL-10 establishes a long-term deficit of adaptive antiviral immunity. Proc. Natl. Acad. Sci. U. S. A. 107:22647–22652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eberhardt MK, Chang WL, Logsdon NJ, Yue Y, Walter MR, Barry PA. 2012. Host immune responses to a viral immune modulating protein: immunogenicity of viral interleukin-10 in rhesus cytomegalovirus-infected rhesus macaques. PLoS One 7:e37931. 10.1371/journal.pone.0037931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Logsdon NJ, Eberhardt MK, Allen CE, Barry PA, Walter MR. 2011. Design and analysis of rhesus cytomegalovirus IL-10 mutants as a model for novel vaccines against human cytomegalovirus. PLoS One 6:e28127. 10.1371/journal.pone.0028127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Research Council 2011. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC [Google Scholar]
- 22.Medical Research Council 12 December 2006, posting date The use of non-human primates in research—the Weatherall report. Medical Research Council, London, United Kingdom: www.mrc.ac.uk [Google Scholar]
- 23.Abel K, Strelow L, Yue Y, Eberhardt MK, Schmidt KA, Barry PA. 2008. A heterologous DNA prime/protein boost immunization strategy for rhesus cytomegalovirus. Vaccine 26:6013–6025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oxford KL, Strelow L, Yue Y, Chang WL, Schmidt KA, Diamond DJ, Barry PA. 2011. Open reading frames carried on UL/b′ are implicated in shedding and horizontal transmission of rhesus cytomegalovirus in rhesus monkeys. J. Virol. 85:5105–5114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wussow F, Yue Y, Martinez J, Deere JD, Longmate J, Herrmann A, Barry PA, Diamond DJ. 2013. A vaccine based on the rhesus cytomegalovirus UL128 complex induces broadly neutralizing antibodies in rhesus macaques. J. Virol. 87:1322–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sequar G, Britt WJ, Lakeman FD, Lockridge KM, Tarara RP, Canfield DR, Zhou SS, Gardner MB, Barry PA. 2002. Experimental coinfection of rhesus macaques with rhesus cytomegalovirus and simian immunodeficiency virus: pathogenesis. J. Virol. 76:7661–7671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lockridge KM, Sequar G, Zhou SS, Yue Y, Mandell CM, Barry PA. 1999. Pathogenesis of experimental rhesus cytomegalovirus infection. J. Virol. 73:9576–9583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huff JL, Eberle R, Capitanio J, Zhou S-S, Barry PA. 2003. Differential detection of B virus and rhesus cytomegalovirus in rhesus macaques. J. Gen. Virol. 84:83–92 [DOI] [PubMed] [Google Scholar]
- 29.Griffiths P, Plotkin S, Mocarski E, Pass R, Schleiss M, Krause P, Bialek S. 2013. Desirability and feasibility of a vaccine against cytomegalovirus. Vaccine 31(Suppl 2):B197–B203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Früh K, Malouli D, Oxford K, Barry P. 2013. Non-human-primate models of cytomegalovirus infection, prevention, and therapy. In Reddehase M. (ed), Cytomegaloviruses: from molecular pathogenesis to therapy, vol II, p 463–496 Caister Academic Press/Horizon, Norfolk, United Kingdom. [Google Scholar]
- 31.Cyktor JC, Carruthers B, Beamer GL, Turner J. 2013. Clonal expansions of CD8+ T cells with IL-10 secreting capacity occur during chronic Mycobacterium tuberculosis infection. PLoS One 8:e58612. 10.1371/journal.pone.0058612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horikawa M, Weimer ET, DiLillo DJ, Venturi GM, Spolski R, Leonard WJ, Heise MT, Tedder TF. 2013. Regulatory B cell (B10 cell) expansion during Listeria infection governs innate and cellular immune responses in mice. J. Immunol. 190:1158–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahajan S, Dkhar HK, Chandra V, Dave S, Nanduri R, Janmeja AK, Agrewala JN, Gupta P. 2012. Mycobacterium tuberculosis modulates macrophage lipid-sensing nuclear receptors PPARgamma and TR4 for survival. J. Immunol. 188:5593–5603 [DOI] [PubMed] [Google Scholar]
- 34.Marks E, Tam MA, Lycke NY. 2010. The female lower genital tract is a privileged compartment with IL-10 producing dendritic cells and poor Th1 immunity following Chlamydia trachomatis infection. PLoS Pathog. 6:e1001179. 10.1371/journal.ppat.1001179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McPhee JB, Mena P, Bliska JB. 2010. Delineation of regions of the Yersinia YopM protein required for interaction with the RSK1 and PRK2 host kinases and their requirement for interleukin-10 production and virulence. Infect. Immun. 78:3529–3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McPhee JB, Mena P, Zhang Y, Bliska JB. 2012. Interleukin-10 induction is an important virulence function of the Yersinia pseudotuberculosis type III effector YopM. Infect. Immun. 80:2519–2527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meghari S, Bechah Y, Capo C, Lepidi H, Raoult D, Murray PJ, Mege JL. 2008. Persistent Coxiella burnetii infection in mice overexpressing IL-10: an efficient model for chronic Q fever pathogenesis. PLoS Pathog. 4:e23. 10.1371/journal.ppat.0040023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mukherjee S, Mukherjee B, Mukhopadhyay R, Naskar K, Sundar S, Dujardin JC, Das AK, Roy S. 2012. Imipramine is an orally active drug against both antimony sensitive and resistant Leishmania donovani clinical isolates in experimental infection. PLoS Negl. Trop. Dis. 6:e1987. 10.1371/journal.pntd.0001987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sing A, Rost D, Tvardovskaia N, Roggenkamp A, Wiedemann A, Kirschning CJ, Aepfelbacher M, Heesemann J. 2002. Yersinia V-antigen exploits Toll-like receptor 2 and CD14 for interleukin 10-mediated immunosuppression. J. Exp. Med. 196:1017–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Felonato M, Pina A, de Araujo EF, Loures FV, Bazan SB, Feriotti C, Calich VL. 2012. Anti-CD25 treatment depletes Treg cells and decreases disease severity in susceptible and resistant mice infected with Paracoccidioides brasiliensis. PLoS One 7:e51071. 10.1371/journal.pone.0051071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB. 2006. Interleukin-10 determines viral clearance or persistence in vivo. Nat. Med. 12:1301–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ejrnaes M, Filippi CM, Martinic MM, Ling EM, Togher LM, Crotty S, von Herrath MG. 2006. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J. Exp. Med. 203:2461–2472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fleming SB, Anderson IE, Thomson J, Deane DL, McInnes CJ, McCaughan CA, Mercer AA, Haig DM. 2007. Infection with recombinant orf viruses demonstrates that the viral interleukin-10 is a virulence factor. J. Gen. Virol. 88:1922–1927 [DOI] [PubMed] [Google Scholar]
- 44.Ross PJ, Lavelle EC, Mills KH, Boyd AP. 2004. Adenylate cyclase toxin from Bordetella pertussis synergizes with lipopolysaccharide to promote innate interleukin-10 production and enhances the induction of Th2 and regulatory T cells. Infect. Immun. 72:1568–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hormozi K, Parton R, Coote J. 1999. Adjuvant and protective properties of native and recombinant Bordetella pertussis adenylate cyclase toxin preparations in mice. FEMS Immunol. Med. Microbiol. 23:273–282 [DOI] [PubMed] [Google Scholar]
- 46.Lowry JE, Isaak DD, Leonhardt JA, Vernati G, Pate JC, Andrews GP. 2011. Vaccination with Brucella abortus recombinant in vivo-induced antigens reduces bacterial load and promotes clearance in a mouse model for infection. PLoS One 6:e17425. 10.1371/journal.pone.0017425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nemeth J, Straley SC. 1997. Effect of Yersinia pestis YopM on experimental plague. Infect. Immun. 65:924–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruter C, Buss C, Scharnert J, Heusipp G, Schmidt MA. 2010. A newly identified bacterial cell-penetrating peptide that reduces the transcription of pro-inflammatory cytokines. J. Cell Sci. 123:2190–2198 [DOI] [PubMed] [Google Scholar]
- 49.Arce-Fonseca M, Ramos-Ligonio A, Lopez-Monteon A, Salgado-Jimenez B, Talamas-Rohana P, Rosales-Encina JL. 2011. A DNA vaccine encoding for TcSSP4 induces protection against acute and chronic infection in experimental Chagas disease. Int. J. Biol. Sci. 7:1230–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Flores-Garcia Y, Rosales-Encina JL, Satoskar AR, Talamas-Rohana P. 2011. IL-10-IFN-gamma double producers CD4+ T cells are induced by immunization with an amastigote stage specific derived recombinant protein of Trypanosoma cruzi. Int. J. Biol. Sci. 7:1093–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brodeur ND, Spencer JV. 2010. Antibodies to human IL-10 neutralize ebvIL-10-mediated cytokine suppression but have no effect on cmvIL-10 activity. Virus Res. 153:265–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tanner JE, Diaz-Mitoma F, Rooney CM, Alfieri C. 1997. Anti-interleukin-10 antibodies in patients with chronic active Epstein-Barr virus infection. J. Infect. Dis. 176:1454–1461 [DOI] [PubMed] [Google Scholar]
- 53.Yue Y, Kaur A, Eberhardt MK, Kassis N, Zhou SS, Tarantal AF, Barry PA. 2007. Immunogenicity and protective efficacy of DNA vaccines expressing rhesus cytomegalovirus glycoprotein B, phosphoprotein 65-2, and viral interleukin-10 in rhesus macaques. J. Virol. 81:1095–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Avdic S, Cao JZ, Cheung AK, Abendroth A, Slobedman B. 2011. Viral interleukin-10 expressed by human cytomegalovirus during the latent phase of infection modulates latently infected myeloid cell differentiation. J. Virol. 85:7465–7471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hahn G, Revello MG, Patrone M, Percivalle E, Campanini G, Sarasini A, Wagner M, Gallina A, Milanesi G, Koszinowski U, Baldanti F, Gerna G. 2004. Human cytomegalovirus UL131-128 genes are indispensable for virus growth in endothelial cells and virus transfer to leukocytes. J. Virol. 78:10023–10033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Isaacson MK, Compton T. 2009. Human cytomegalovirus glycoprotein B is required for virus entry and cell-to-cell spread but not for virion attachment, assembly, or egress. J. Virol. 83:3891–3903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ryckman BJ, Rainish BL, Chase MC, Borton JA, Nelson JA, Jarvis MA, Johnson DC. 2008. Characterization of the human cytomegalovirus gH/gL/UL128-131 complex that mediates entry into epithelial and endothelial cells. J. Virol. 82:60–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yue Y, Zhou SS, Barry PA. 2003. Antibody responses to rhesus cytomegalovirus glycoprotein B in naturally infected rhesus macaques. J. Gen. Virol. 84:3371–3379 [DOI] [PubMed] [Google Scholar]
- 59.Chang WL, Barry PA, Szubin R, Wang D, Baumgarth N. 2009. Human cytomegalovirus suppresses type I interferon secretion by plasmacytoid dendritic cells through its interleukin 10 homolog. Virology 390:330–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chang WL, Baumgarth N, Eberhardt MK, Lee CY, Baron CA, Gregg JP, Barry PA. 2007. Exposure of myeloid dendritic cells to exogenous or endogenous IL-10 during maturation determines their longevity. J. Immunol. 178:7794–7804 [DOI] [PubMed] [Google Scholar]
- 61.Spencer JV, Cadaoas J, Castillo PR, Saini V, Slobedman B. 2008. Stimulation of B lymphocytes by cmvIL-10 but not LAcmvIL-10. Virology 374:164–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Castellino F, Galli G, Del Giudice G, Rappuoli R. 2009. Generating memory with vaccination. Eur. J. Immunol. 39:2100–2105 [DOI] [PubMed] [Google Scholar]
- 63.Mandaric S, Walton SM, Rulicke T, Richter K, Girard-Madoux MJ, Clausen BE, Zurunic A, Kamanaka M, Flavell RA, Jonjic S, Oxenius A. 2012. IL-10 suppression of NK/DC crosstalk leads to poor priming of MCMV-specific CD4 T cells and prolonged MCMV persistence. PLoS Pathog. 8:e1002846. 10.1371/journal.ppat.1002846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Malouli D, Nakayasu ES, Viswanathan K, Camp DG, II, Chang WLW, Barry PA, Smith RD, Früh K. 2012. Reevaluation of the coding potential and proteomic analysis of the BAC-derived rhesus cytomegalovirus strain 68-1. J. Virol. 86:8959–8973 [DOI] [PMC free article] [PubMed] [Google Scholar]