Abstract
In this study, we demonstrate that killer cell lectin-like receptor subfamily G member 1 (KLRG1), a transmembrane protein preferentially expressed on T cells, is highly expressed on CD56+ NK cells, which are significantly reduced in their numbers and functions in the peripheral blood of patients with chronic hepatitis C virus (HCV) infection compared to subjects without infection. KLRG1 expression is also upregulated on healthy NK cells exposed to Huh-7 hepatocytes infected with HCV in vitro. Importantly, the expression levels of KLRG1 are inversely associated with the capacity of NK cells to proliferate and to produce gamma interferon (IFN-γ) but positively associated with apoptosis of NK cells in response to inflammatory cytokine stimulation. KLRG1+ NK cells, including CD56bright and CD56dim subsets, exhibit impaired cell activation and IFN-γ production but increased apoptosis compared to KLRG1− NK cells, particularly in HCV-infected individuals. Importantly, blockade of KLRG1 signaling significantly recovered the impaired IFN-γ production by NK cells from HCV-infected subjects. Blockade of KLRG1 also enhanced the impaired phosphorylation of Akt (Ser473) in NK cells from HCV-infected subjects. Taken together, these results indicate that KLRG1 negatively regulates NK cell numbers and functions via the Akt pathway, thus providing a novel marker and therapeutic target for HCV infection.
INTRODUCTION
Hepatitis C virus (HCV) infection is a global health problem, affecting approximately 200 million people worldwide (1). The most remarkable feature of this blood-borne virus is its ability to evade host innate and adaptive immunity, resulting in over 80% of infected individuals developing a chronic infection. The current treatment is not optimal, and no vaccine is available, in part due to our incomplete understanding of HCV-host interactions that lead to viral persistence (2). While a high rate of genetic variability in the HCV E1/E2 envelope proteins (quasispecies) may facilitate viral escape and persistence in the face of antiviral immunity, HCV immune responses appear to be too weak to resolve infection in most humans or to protect against reinfection in chimpanzees (3, 4). It is now clear that HCV-mediated impairment of cellular immune responses is a major mechanism by which persistent infection is established. Efforts to define clinical correlates of HCV persistence have focused primarily on CD4+ and CD8+ T cell responses (5); the role of innate immunity, in particular natural killer (NK) cells, in determining the outcome of HCV infection has recently received intense scrutiny (6, 7).
NK cells comprise the first line of host defense against invading pathogens. They generally become activated in an early phase of viral infection and play an essential role in eliminating HCV-infected cells directly by cytolytic killing and indirectly by secreting cytokines (8). They also interact with professional dendritic cells (DC) and virus-specific T cells in implementing antiviral immunity (9). Not surprisingly, HCV has evolved multiple strategies to counter the host's NK cell response (6). Compromised NK cell functions have been reported in chronically HCV-infected individuals, whereas normalization of depressed NK activity after antiviral therapy is associated with a low frequency of relapse and improved sustained virologic responses (SVR) (10–15). Notably, aberrant NK activities may also contribute toward liver injury (8). Therefore, further studies are required to understand how NK cells are fine-tuned during host defense and in liver injury during HCV infection.
Human NK lymphocytes have been functionally divided into several subpopulations based on the relative expression of CD3, CD16, and CD56 markers (16–18). The CD56bright and CD56dim NK cell subsets differ in their maturation status, proliferative capacity, cytotoxic activity, and cytokine production (16–18). The majority (90%) of peripheral blood and spleen NK cells are CD56dim and are considered to be the mature effectors, with enhanced natural and major histocompatibility complex (MHC)-unrestricted lymphokine-activated killing (LAK) ability, whereas a minority of the NK cells are CD56bright, which function as an important source of immune-regulatory cytokines (16–18). Shifts of these subsets have been reported in patients with acute to chronic HCV infection and following antiviral therapy (10–15). The antiviral activity of NK cells is regulated through surface NK receptors, including the inhibitory killer immunoglobulin-like receptors (KIRs) and the leukocyte immunoglobulin-like receptors (LIRs) in humans and the Ly49 molecules in mice. Natural cytotoxicity receptors NKp30, NKp44, NKp46, and C-type lectin-like receptors CD94/NKG2, comprising inhibitory (NKG2A) or activating (NKG2C/NKG2D) isoforms, represent additional NK cell receptors (16–18). Classical and nonclassical MHC molecules serve as ligands for these NK receptors. It is noteworthy that inheritance of particular KIR genes or their polymorphisms involved in the control of NK activity predispose to chronic infection or treatment resistance (19–23). Expression levels of these inhibitory and stimulatory receptors on NK cells and their ligands on hepatocytes, cholangiocytes, stellate cells, and Kupffer cells are significantly altered during HCV infection and contribute to the pathogenesis and progression of liver disease (24, 25).
Killer cell lectin-like receptor subfamily G member 1 (KLRG1) is a transmembrane protein with immunoreceptor tyrosine-based inhibitory motifs (ITIMs) in its cytoplasmic domain and a C-type lectin-like domain in the extracellular region (26). KLRG1 was first identified on the rat mucosal-type mast cell line RBL-2H3, as antibody-mediated ligation of KLRG1 inhibits release of inflammatory mediators from these cells; thus, the protein was originally named mast cell function-associated antigen (MAFA) (27). In humans and mice, KLRG1 is found preferentially expressed on subsets of NK and T cells, but not on monocytes or granulocytes, and it binds three members (E, N, and R) of the cadherin family to regulate lymphocyte activities (28–30). After birth, KLRG1 expression in T cells from peripheral blood decrease rapidly, to reappear in effector/memory T cells in adults (31). Of note, monoclonal antibody (MAb)-mediated cross-linking of KLRG1 inhibits both cytokine production and NK cell-mediated cytotoxicity (32). Interestingly, pathogenic infections increase the percentage of KLRG1-expressing lymphocytes (32–41). More specifically, ongoing antigen triggering in chronic viral infection increases the abundant expression of KLRG1 by virus-specific CD8 T cells infected with human immunodeficiency virus (HIV), cytomegalovirus (CMV), or Epstein-Barr virus (EBV) with impaired proliferative capacity, whereas influenza virus, a typical acute viral infection, elicits a considerably lower frequency of KLRG1+ cells within influenza virus-specific T cells (39). Despite these insights into KLRG1 functions, very little information is currently available about the expression of KLRG1 on NK cells and its role in NK subset function during chronic infection.
In this study, we examined KLRG1 expression on NK cells in individuals with chronic HCV infection and assessed the effects of KLRG1 on NK cell subset (CD56bright and CD56dim) functions and the Akt signaling pathway. We found that KLRG1 expression is upregulated and inversely associated with a reduced NK cell number, impaired NK cell activation and proliferation, suppressed gamma interferon (IFN-γ) production, and defective Akt (Ser473) phosphorylation but positively correlated with apoptosis of NK cells in patients with chronic HCV infection. We also found that antiviral therapy with interferon/ribavirin (IFN/RBV) corrects the NK cell subset discrepancy and KLRG1 expression levels, and blockade of KLRG1 signaling significantly improves NK cell IFN-γ production and Akt phosphorylation. These results suggest that KLRG1 negatively regulates NK cell frequency and function in HCV infection.
MATERIALS AND METHODS
Subjects.
The study subjects comprised three populations: 32 subjects with chronic HCV infection prior to antiviral treatment, 10 subjects who had received IFN/RBV therapy with SVR, and 10 healthy subjects (HS). The study protocol was approved by an institutional review board at East Tennessee State University and the James H. Quillen VA Medical Center (ETSU/VA IRB, Johnson City, TN). HCV genotype (70% type 1, 30% type 2 or 3) and viral load (ranging from 12,300 to 500,000 IU/ml) were determined by Lexington VAMC, and all subjects were virologically and serologically positive for HCV. Healthy subjects were negative for HBV, HCV, and HIV infections. Of the study subjects, 85% were male. The mean age of HCV-infected individuals was comparable to that among HS (P > 0.05).
Cell isolation and culture.
Human peripheral blood mononuclear cells (PBMCs) were isolated from the peripheral blood of study subjects by Ficoll-density centrifugation with lympho-H (Atlanta Biological, Lawrenceville, GA). Where indicated, CD56+ NK cells were further purified from PBMCs by magnetic beads conjugated to anti-CD56 antibody; CD3− CD56+ NKs were also purified from PBMCs by negative selection according to the manufacturer's instructions (purity, >95%; Miltenyi Biotec Inc., Auburn, CA). The cells were cultured as previously described (42).
Flow cytometry.
Procedures for detection of cell surface markers and intracellular cytokine staining were performed essentially as described previously (42, 43). Briefly, PBMCs (0.2 × 106 per well in a 96-well plate) were stimulated with 10 ng/ml recombinant human interleukin-12 (rhIL-12; eBioscience, San Diego, CA) for 18 h, followed by 1 μg/ml Brefeldin A (BioLegend, San Diego, CA) 4 h prior to harvesting the cells, thus forbidding cytokine secretion. Cell surface markers were stained with specific conjugated antibodies that included phycoerythrin (PE)-CD3 and peridinin chlorophyll protein (PerCP)-CD56 (eBioscience, San Diego, CA), PE-Annexin V (BD Biosciences), allophycocyanin (APC)-CD69 (eBioscience), CD107a (Miltenyi Biotec Inc., Auburn, CA), Alexa Fluor 488-KLRG1 (H. Pircher), and Alexa Fluor 488–E-cadherin (R&D Systems Inc., Minneapolis, MN) (31). For staining of intracellular IFN-γ (Miltenyi Biotec Inc., Auburn CA) and granzyme B (eBioscience), the cells were fixed and permeabilized by adding Cytofix/Cytoperm (BD Pharmingen). Cells were washed three times and fixed in 100 μl CellFix (BD Pharmingen) per well. The intracellular cytokine staining was carried out using an Inside Stain kit (Miltenyi Biotec) per the manufacturer's instructions. Isotype-matched control antibodies (eBioscience) and fluorescence minus-one (FMO) controls were used to determine background levels of staining and to adjust multicolor compensation as a gating strategy. The cells was sorted on a FACSCalibur flow cytometer or Accuri C6 flow cytometer (BD, Franklin Lakes, NJ) and analyzed by using CellQuest or FlowJo software (Tree Star, Inc., Ashland, OR).
Proliferation assays.
PBMCs were labeled with carboxyfluorescein succinimidyl ester (CFSE; 2.5 μM; Invitrogen) for 10 min at 37°C per the manufacturer's instructions, washed with complete medium, and cultured (5 × 104 cells/well) in a 96-well plate in the presence rhIL-12 (10 ng/ml; eBioscience) and rhIL-2 (50 U/ml; R&D Systems). After culture for 6 days, the cells were immunostained with PE-CD3, PerCP-CD56, and Alexa Fluor 488-KLRG1 and analyzed with a FACSCalibur flow cytometer (BD).
Blocking assay.
Purified NK cells from HCV-infected patients were incubated with anti-human KLRG1 (3 μg/ml; obtained from Hanspeter Pircher), anti-human E-cadherin (5 μg/ml; EMD Millipore Corporation, Billerica, MA), or isotype control IgG for 54 h, followed by stimulation with rhIL-12 (10 ng/ml; eBioscience) and rhIL-2 (50 U/ml; eBioscience) for an additional 18 h, and then subjected to flow cytometric analysis for intracellular IFN-γ and pAkt expression as described above.
Phosphocytometry.
Purified NK cells were incubated with anti-human KLRG1 (3 μg/ml; from H. Pircher) or isotype control IgG in 96-well plate with complete RPMI 1640 medium containing rhIL-12 (10 ng/ml) and rhIL-2 (50 U/ml) (eBioscience) for 72 h, after which the cells were pulsed with rhIL-15 (100 ng/ml; eBioscience) for 1 h. The NK cells were fixed, permeabilized, and sequentially incubated with pAkt (ser473) antibody (D9E; Cell Signaling, Boston, MA) or rabbit isotype control IgG (DA1E; Cell Signaling, Boston, MA) for 1 h at room temperature. The cells were analyzed on a FACSCalibur flow cytometer (BD, Franklin Lakes, NJ) by using FlowJo software (Tree Star, Inc., Ashland, OR).
Coculture of healthy PBMCs with HCV-transfected or untransfected Huh-7 hepatocytes.
Transfection of Huh-7 hepatocytes (kindly provided by T. J. Liang, Liver Section, NIH NIDDK) with HCV JFH-1 strain (kindly provided by T. Wakita) was carried out as described previously (42, 43). Prior to the coculture experiment, HCV-transfected or untransfected Huh-7 hepatocytes were serum starved for 18 h, then activated with rhIFN-γ (0.1 μg/ml; R&D Systems) for 48 h. Activated hepatocytes were removed from plates with 0.05% trypsin–EDTA and then plated at 5 × 105 cells/well in a 12-well plate. PBMCs or negatively purified NKs were then added to the adherent hepatocytes in RPMI 1640 medium and cocultured for an additional 48 h, and the expression levels of KLRG-1, CD69, CD107a, IFN-γ, and granzyme B in CD56+ NK cells were analyzed by flow cytometry.
Statistical analysis.
Study results were summarized for each group, and results are expressed as the means ± standard deviations (SD). Comparisons between two groups were performed by multiple comparisons testing with the least significant difference or Turkey's procedure, depending on an analysis of variance (ANOVA) F test (Prism software, version 4; GraphPad Software) and a nonparametric Mann-Whitney U test. A pairwise t test was used to compare the significance of changes in KLRG1 blockage experiments. Correlations between KLRG1 expression on NK cells and IFN-γ or Annexin V expression were analyzed by using a Pearson correlation program. P values of <0.05, <0.01, and <0.001 were considered significant or very significant.
RESULTS
NK cells are significantly reduced, with a discrepancy of CD3− CD56bright and CD3− CD56dim subsets in the peripheral blood of patients with chronic HCV infection.
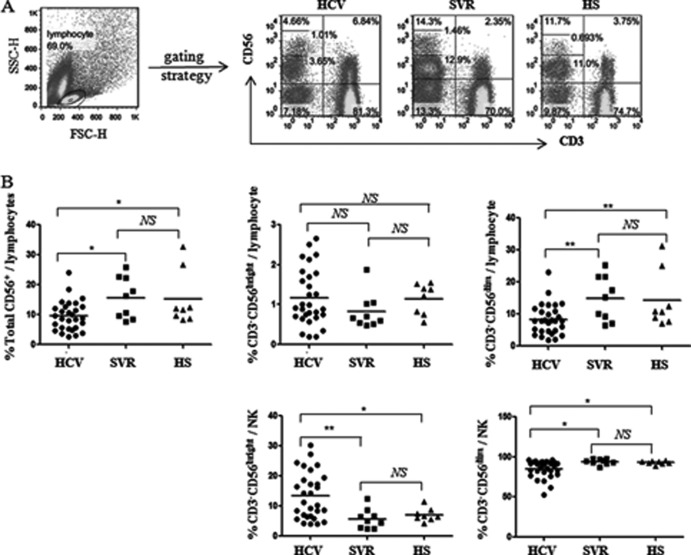
Human NK cells are divided into two subsets, CD56bright and CD56dim, according to their expression levels of CD56. About 90% of peripheral blood NK cells belong to the CD56dim subset, which is mainly cytolytic, while the remaining 10% of NK cells are in the CD56bright subset and primarily produce cytokines (16–18). To characterize the effects of HCV infection on NK cells and their subsets, we compared the percentages of total CD56+ NK cells, and the percentages of CD56bright or CD56dim cells within NK cells, in the gated lymphocytes or NK populations in PBMCs from 29 HCV patients, 9 SVR individuals, and 8 HS. As shown in the representative dot plots and summary data in Fig. 1A and B, CD3− CD56+ NK lymphocyte frequencies in patients with chronic HCV infection were significantly reduced compared with those with SVR and HS, while there was no difference between the SVR and HS groups. Interestingly, CD56bright NK subsets were expanded and CD56dim NK subsets contracted in chronic HCV patients compared to SVR and HS; this finding held true with regard to the percentages of cell subsets based on whole lymphocytes as well as the NK populations, although there was no significant difference in the CD56bright NK subset in the whole lymphocyte populations in HCV-infected versus uninfected subjects (Fig. 1B). These results suggest that HCV infection reduces the total NK cell numbers and shifts the NK subsets, whereas successful antiviral therapy recovers this HCV-induced NK cell discrepancy.
Fig 1.
NK cells are reduced with shifting distribution of CD3− CD56dim and CD3− CD56bright subsets in patients with chronic HCV infection. PBMCs from 29 HCV patients, 9 SVR individuals, and 8 HS were analyzed by flow cytometry for the frequencies of different subsets of NK cells. The cells were immunostained with PE-CD3 and PerCP-CD56; the gating strategies are described in Materials and Methods. (A) Representative dot plots showing the gating strategy for CD3− CD56+ NK cells and CD3− CD56bright and CD3− CD56dim subsets in patients with chronic HCV infection, individuals following treatment with SVR, and HS. Percentage of cell frequency in the gated area is shown in the dot plots. (B) Summary data showing the percentages of CD3− CD56+ NK cells, CD3− CD56bright and CD3− CD56dim subsets in the lymphocyte populations (upper panel) and in the NK cell populations (lower panel). Each dot represents one individual, and horizontal bars represent mean values. *, P < 0.05; **, P < 0.01; NS, no significance.
KLRG1 expression is upregulated on human NK cells upon HCV infection.
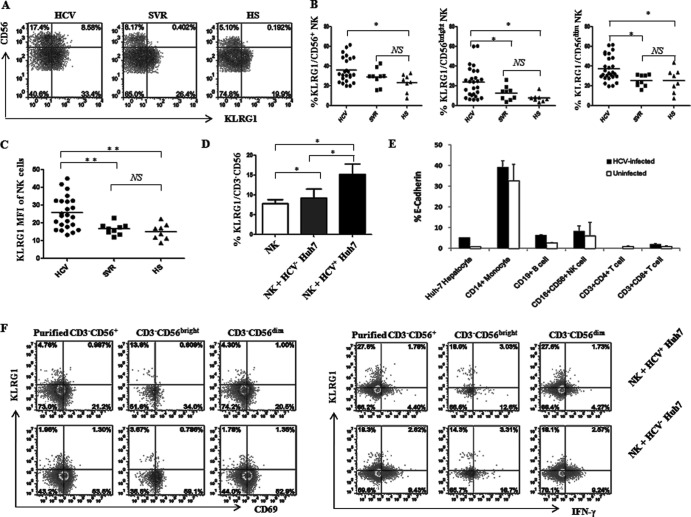
KLRG1 is preferentially expressed on subsets of NK cells and antigen-experienced T lymphocytes, as it binds to cadherins and exhibits inhibitory effects on lymphocyte activities (27–29). To determine the effects of KLRG1 on human NK subsets during HCV infection, we examined KLRG1 expression levels on gated NK cells in PBMCs from 24 chronically HCV-infected patients, 9 SVR individuals, and 8 HS. The representative dot plots and summary data for the percentages of KLRG1+ cells on CD56+, CD56bright, and CD56dim NK cells are shown in Fig. 2A and B. Of note, KLRG1 expression in chronically HCV-infected individuals was significantly elevated, not only on CD3− CD56+ NK cells but also on CD3− CD56bright and CD3− CD56dim NK subsets compared to SVR subjects and HS. In addition to the percentages of positive cell frequencies, the mean fluorescence intensity (MFI) of KLRG1 expression on NK cells in HCV-infected patients was also significantly higher than for SVR subjects and HS (Fig. 2C). However, there was no significant difference for KLRG1 expression levels on NK cell subsets between SVR and HS (Fig. 2B and C). These results demonstrate that HCV infection upregulates KLRG1 expression, and IFN/RBV therapy in individuals with SVR normalizes the NK cell number as well as KLRG1 expression levels on these cells.
Fig 2.
KLRG1 is upregulated on NK cells by HCV infection. (A) PBMCs from 24 HCV patients, 9 SVR individuals, and 8 HS were analyzed by flow cytometry for expression of KLRG1 on different subsets of NK cells. Representative dot plots showing the gating strategy for KLRG1 expression on total CD3− CD56+ NK cells and CD3− CD56bright and CD3− CD56dim subsets in patients with chronic HCV infection, individuals after treatment with SVR, and HS. Percent cell frequency in the gated area is shown in the dot plots. (B) Summary data showing the percentages of KLRG1 expression in total CD3− CD56+ NK cells and CD3− CD56bright and CD3− CD56dim subsets. Each dot represents one individual, and the horizontal bars represent mean values. *, P < 0.05; NS, no significance. (C) MFI of KLRG1 expression on CD3− CD56+ NK cells. **, P < 0.01; NS, no significance. (D) Upregulation of KLRG1 expression on NK cells incubated with or without HCV-transfected (HCV+) or untransfected (HCV−) Huh-7 hepatocytes. Briefly, healthy PBMCs were cocultured with HCV-transfected or untransfected Huh-7 cells, and KLRG1 expression on CD3− CD56+ NK cells was analyzed by flow cytometry as described in Materials and Methods. Summary data (means ± SD) from three independent experiments are shown. *, P < 0.05; NS, no significance. (E) Expression of the KLRG1 ligand E-cadherin on HCV-infected and uninfected Huh-7 hepatocytes as well as on different populations of PBMCs from 3 HCV-infected and 3 uninfected subjects. The percentage of cells expressing E-cadherin was calculated as the percentage in the sample minus that in the isotype control. (F) Representative dot plots of CD69 expression (left panel) and IFN-γ production (right panel) by KLRG1+ or KLRG1− NKs, including total purified CD3− CD56+ NKs as well as CD56bright and CD56dim subsets, cocultured with HCV+ or HCV− Huh-7 hepatocytes. The percent cell frequency in the gated area is shown in the dot plots.
The above-observed KLRG1 expression might be a result, rather than a cause, of NK cell dysregulation during HCV infection. Additionally, the driving force for KLRG1 upregulation during HCV infection remains to be determined. To further elucidate the role of HCV in regulation of KLRG1 expression, we employed a newly established cell culture system by transfecting Huh-7 hepatocytes with the HCV JFH-1 strain in vitro to mimic the in vivo setting of early HCV infection (42, 43). To this end, PBMCs or purified NKs from HS were cocultured with HCV-transfected or untransfected Huh-7 hepatocytes for 48 h, followed by flow cytometric analysis for KLRG1 expression and NK functions. Consistent with the data observed in natural HCV infection, HCV+ Huh-7 cells significantly enhanced KLRG1 expression on cocultured CD3− CD56+ cells (Fig. 2D).
In addition to the KLRG1 receptor, we also examined the expression of the KLRG1 ligand E-cadherin on HCV-transfected or untransfected hepatocytes as well as different populations of PBMCs from HCV-infected and uninfected individuals. As shown in Fig. 2E, E-cadherin was detected at higher levels in HCV-transfected Huh-7 than HCV-untransfected Huh-7 cells. Additionally, E-cadherin was expressed at high levels on CD14+ monocytes, at low levels on CD19+ B cells and CD56+ NKs, but not on CD4+ or CD8+ T lymphocytes. Interestingly, HCV infection seems to upregulate KLRG1 expression on these cells. These accessory cells may provide a ligand for interaction with KLRG1 that is expressed on T cells and NKs but not on monocytes or granulocytes (data not shown).
Since KLRG1 has been recognized as an inhibitory receptor and senescence marker for T cells (31–41), it may also function as a regulator to control NK cell function. To exclude the possible secondary effects from accessory cells in bulk PBMCs, we repeated the coculture assay with purified NK cells. While these NK cells cocultured with HCV-transfected hepatocytes expressed higher levels of KLRG1, they expressed less CD69 and IFN-γ, primarily by KLRG1− NKs, than those cocultured with HCV-untransfected hepatocytes (Fig. 2F), suggesting that HCV may induce KLRG1 expression, as well as its ligand's expression, to inhibit NK cell activation and function.
Increased KLRG1 expression is associated with decreased IFN-γ production by NKs in HCV infection.
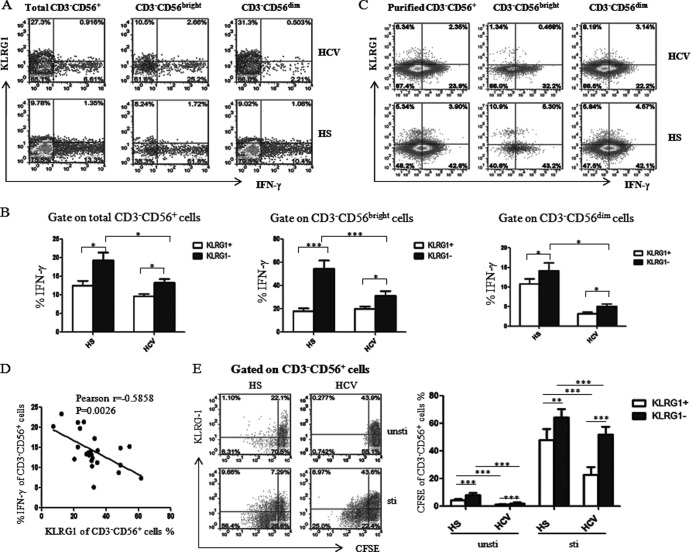
NK and T cells produce IFN-γ, a critical cytokine for clearance of infectious pathogens (44). Previous reports have implicated the increased KLRG1 expression on T cells in impairing their capacity for proliferation and IFN-γ production (33, 45). To determine the role of KLRG1 in regulation of NK cell function, here we examined the relationship between KLRG1 expression and IFN-γ production in NK cells and its subsets in individuals with HCV and HS. As shown in Fig. 3A, an impaired production of IFN-γ was observed in CD3− CD56+ NK cells, including CD3− CD56bright and CD3− CD56dim subpopulations in PBMCs, derived from chronically HCV-infected patients compared with those from HS, and this was very significant in the CD56bright populations, the major subsets for cytokine secretion, suggesting that HCV inhibits IFN-γ production by NK cells (Fig. 3B). Notably, the majority of IFN-γ was primarily produced by KLRG1− NK cells, and this was also observed in CD56bright and CD56dim subpopulations, both in HS and HCV-infected individuals (Fig. 3B). To determine whether isolated NKs behave in the same manner as in bulk PBMCs and whether accessory cells are required for their behavior, we examined the relationship between KLRG1 expression and IFN-γ production by using purified NKs from HCV-infected and uninfected subjects in response to cytokine stimulation. As shown in Fig. 3C with isolated NKs, similar to the gated NKs in bulk PBMCs (Fig. 3A), NKs from HCV-infected individuals produced less IFN-γ than those of HS, and the majority of IFN-γ was expressed by KLRG1− NK cells, whereas KLRG1+ NKs produced little IFN-γ, if any, in both populations. Notably, the KLRG1 expression level on purified NK cells was lower than those in bulk PBMCs, which we believe was likely due to secondary effects from accessory cells (such as monocytes or T or B lymphocytes, either directly or indirectly by cytokine secretion) in response to ex vivo stimulation. Moreover, KLRG1 expression on CD3− CD56+ NK cells was inversely correlated with the IFN-γ production by these cells (Fig. 3D), indicating that KLRG1 may negatively control IFN-γ production by NK cells.
Fig 3.
KLRG1 expression is inversely associated with the low levels of IFN-γ production by NK cells in HCV infection. (A) PBMCs from chronically HCV-infected patients and HS were stimulated with rhIL-12 (10 ng/ml) for 18 h and incubated for the last 4 h with brefeldin A (10 μg/ml) to halt cytokine secretion. The cells were immunostained with PE-CD3, PerCP-CD56, APC–IFN-γ, and Alexa Fluor 488-KLRG1, and the IFN-γ production by KLRG1+ and KLRG1− NK cells was analyzed by flow cytometry. Representative dot plots for IFN-γ production by KLRG1+ and KLRG1− NK cells, including total CD3− CD56+, CD3− CD56bright, and CD3− CD56dim subsets from an HCV-infected subject and HS. (B) The average percentage of IFN-γ production by the KLRG1+ versus KLRG1− fraction of total CD3− CD56+ NK cells, or in the CD3− CD56bright and CD3− CD56dim subsets. Results are expressed as means ± SD of the percentages of IFN-γ production by NK cells from 24 HCV-infected patients versus 8 HS. *, P < 0.05; ***, P < 0.001. (C) Representative dot plots for KLRG1 expression and IFN-γ production by negatively purified NKs, including total CD3− CD56+ and CD3− CD56bright or CD3− CD56dim subsets from an HCV-infected subject and HS. The percentage of cells in the gated area is shown in the dot plots. (D) Relationship between KLRG1 expression and IFN-γ production by CD3− CD56+ NK cells from 24 HCV-infected patients based on Pearson correlation analysis (r = −0.585; **, P < 0.01). E) KLRG1+ and KLRG1− NK cell proliferation levels were assessed in a CFSE assay. PBMCs were labeled with CFSE and cultured with or without rhIL-12 and rhIL-2 for 6 days. The cells were immunostained with PE-CD3, PerCP-CD56, and Alexa Fluor 488-KLRG1 and analyzed by flow cytometry as described in Materials and Methods. Representative dot plots (left panel) and summary data (right panel; mean ± SD) of CFSE dilution in the KLRG1+ and KLRG1− NK cells from 8 HCV patients versus 8 HS, with and without ex vivo stimulation, are shown. ***, P < 0.001.
We also assayed the proliferative capability of NK cells from HCV-infected patients versus those from HS in a CFSE dilution assay with or without stimulation. As shown in Fig. 3E, the proliferation of NK cells with increased KLRG1 expression from chronically HCV-infected patients was much lower than in HS. Moreover, in both HCV-infected individuals and HS, the proliferative capacity of KLRG1+ NK cells was much lower than that of KLRG1− NK cells. These results clearly illustrated that HCV infection upregulates KLRG1 expression that is linked to a low capacity for proliferation and cytokine production by NK cells, suggesting that KLRG1 is a negative regulator or biomarker for NK cells.
KLRG1 expression is associated with apoptosis of NK cells in HCV infection.
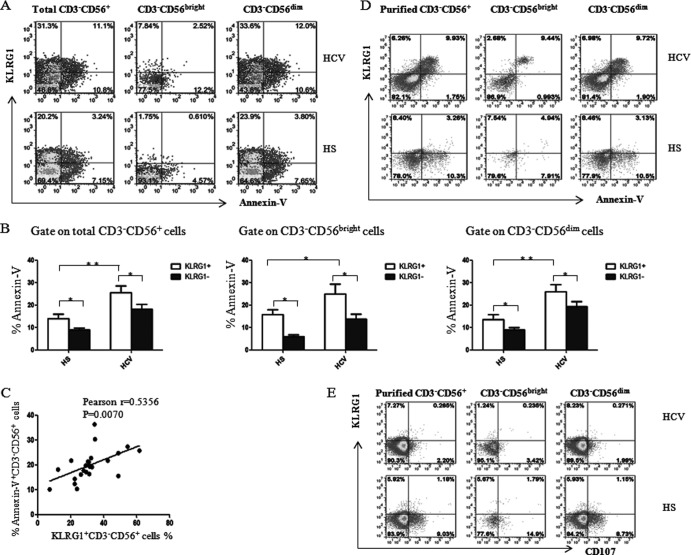
KLRG1+ NK cells have been reported to exhibit properties of end-stage or senescent cells during the contraction phase of immune responses, including decreased proliferation, increased apoptosis, and reduced effector function in mice (32, 46). To further determine whether the reduced NK cell number and impaired NK cell function was due to apoptosis of NK cells during HCV infection, we analyzed Annexin V expression on total CD3− CD56+ NK cells, as well as CD3− CD56bright and CD3− CD56dim NK subsets of PBMCs, in response to inflammatory cytokine stimulation. Indeed, CD56+ NK cells, including CD56bright and CD56dim subsets, derived from HCV-infected patients were more susceptible to apoptosis than were cells from HS following cell stimulation ex vivo (Fig. 4A and B). Intriguingly, KLRG1+ NK cells and their subsets were found to be more susceptible to apoptosis in response to ex vivo stimulation than were KLRG1− NK cells. Furthermore, KLRG1 expression on NK cells was positively correlated with the apoptosis of NK cells from HCV-infected patients (Fig. 4C). Again, we examined the relationship between KLRG1 and Annexin V expression on purified NK cells in response to ex vivo stimulation. As shown in Fig. 4D, HCV patient NKs exhibited more apoptotic cells, which were mainly KLRG1+ NKs, than did those from HS. In contrast, NKs derived from HCV-infected subjects expressed less of the degranulation marker CD107 (Fig. 4E) and cytolytic enzyme granzyme B (data not shown), which are primarily produced by KLRG1− NKs, than did NKs from HS. These findings revealed that the apoptotic and cytolytic activities of NK cells clearly correspond to higher surface expression of KLRG1 on NK cells, implying that KLRG1 might also be a hallmark for infection-mediated NK cell senescence or premature cell aging.
Fig 4.
KLRG1 expression is positively associated with apoptosis of NK cells following HCV infection. (A) PBMCs from HCV-infected patients and HS were stimulated with 10 ng/ml rhIL-12 for 18 h, followed by 1 μg/ml brefeldin A 4 h prior to harvest of the cells (to block cytokine secretion). PBMCs were immunostained and then analyzed for Annexin V expression on total CD3− CD56+ and CD3− CD56bright and CD3− CD56dim NK cells by flow cytometry. Representative dot plots for Annexin V expression on KLRG1+ and KLRG1− NK cells, including CD3− CD56+ and the CD3− CD56bright and CD3− CD56dim subsets from an HCV-infected patient and HS are shown. (B) Average percentage of Annexin V expression from the KLRG1+ versus KLRG1− cell fraction of total CD3− CD56+ NK cells and the CD3− CD56bright and CD3− CD56dim subsets. Results are expressed as means ± SD of the percentages of Annexin V expression by NK cells from 24 HCV-infected patients versus 8 HS. *, P < 0.05; **, P < 0.01. (C) Relationship between KLRG1 expression and Annexin V expression on CD3− CD56+ NK cells from 24 HCV-infected patients, based on Pearson correlation analysis. r = 0.535; **, P < 0.01. (D) Representative dot plots for KLRG1 and Annexin V expressions on negatively selected NK cells, including CD3− CD56+ and the CD3− CD56bright and CD3− CD56dim subsets, from an HCV-infected subject and HS. (E) Representative dot plots for KLRG1 and CD107a expression levels on negatively selected NK cells, including CD3− CD56+ and the CD3− CD56bright and CD3− CD56dim subsets, from an HCV-infected subject and HS. The percentage of cells in the gated area is shown in the dot plots.
KLRG1 blockade recovers the impaired IFN-γ production by NK cells in chronic HCV infection.
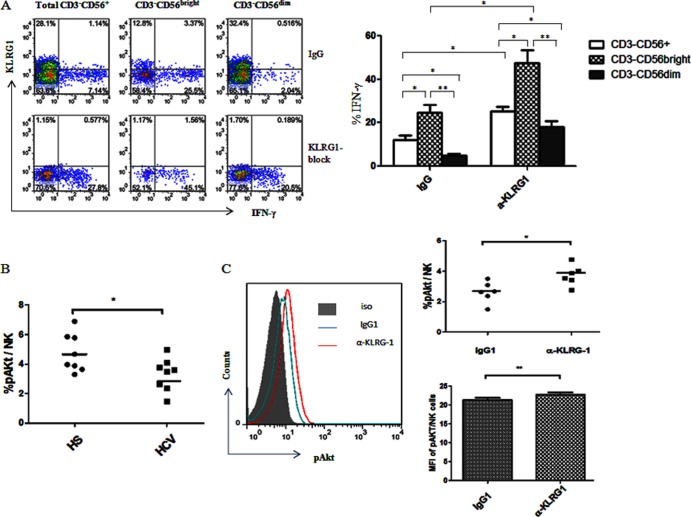
Given the fact that high levels of KLRG1 expression are inversely associated with the impaired capability of NK cells to produce IFN-γ in chronic HCV infection, we next sought to determine whether blockade of KLRG1 signaling improved IFN-γ production by NK cells from HCV-infected patients. It has been reported that MAb-mediated cross-linking of KLRG1 inhibits mouse NK functions (32); therefore, to further elucidate the role of KLRG1 in human NK function and exclude the possibility of secondary effects on NK cells from DC and T cells, we investigated IFN-γ production by activated NK cells with or without blockade of KLRG1 signaling. To this end, purified CD56+ NK cells from 6 chronically HCV-infected patients were incubated with anti-KLRG1 or control IgG antibodies for 54 h, followed by rhIL-12 and rhIL-2 stimulation for an additional 18 h. The representative flow cytometric dot plots and summary data of intracellular IFN-γ production by total CD3− CD56+ NK cells, as well as CD3− CD56bright and CD3− CD56dim subsets, are shown in Fig. 5. Notably, blockade of KLRG1 signaling in NK cells from chronically HCV-infected individuals significantly improved IFN-γ production by CD3− CD56+, CD3− CD56bright, and CD3− CD56dim NK cell populations compared with the cells treated with isotype IgG control, indicating that blockade of KLRG1 reverses the functional defects associated with overexpression of this receptor. The question remains as to whether this is due to removal of a negative signal generated by KLRG1-ligand interactions or a positive signal generated by antibody interaction with this receptor. Our preliminary data revealed that NK cells treated with anti-KLRG1 alone without rhIL-12/rhIL-2 stimulation failed to secrete IFN-γ, suggesting that the blocking antibody inhibited negative signaling by KLRG1 rather than directly activating NK cells, and its effect requires additional positive signals generated by cytokine stimulation to drive cell proliferation. These findings further supported the notion that KLRG1 negatively controls IFN-γ production by NK cells during HCV infection.
Fig 5.
KLRG1 blockade recovers the impaired IFN-γ production through the Akt pathway by NK cells in chronic HCV infection. (A) Purified NK cells from HCV-infected patients were incubated with anti-human KLRG1 or isotype IgG for 54 h, followed by stimulation with rhIL-12 and rhIL-2 for 18 h, and then subjected to flow cytometric analysis of intracellular IFN-γ expression levels as described Materials and Methods. With the blockade by KLRG1 antibody, IFN-γ production was upregulated. Representative dot plots measuring IFN-γ production by total CD3− CD56+ and CD3− CD56bright and CD3− CD56dim NK cell subsets with the KLRG1 blocking antibody or control IgG are shown on the left, and the average percentages of IFN-γ production by different subsets of NK cells are shown on the right. Results are expressed as means ± SD from 8 independent experiments. *, P < 0.05; **P, < 0.01. (B) The frequency of pAkt expression by purified NKs from 8 HCV-infected and 8 uninfected subjects following ex vivo stimulation as described in Materials and Methods. *, P < 0.05. (C) KLRG1 blockade restores the phosphorylation of Akt in NK cells from HCV-infected patients. Purified CD56+ NK cells from HCV-infected patients and HS were incubated with anti-human KLRG1 or isotype control IgG in the presence of rhIL-12 and rhIL-2 for 72 h, then costimulated with rhIL-15 for 1 h, followed by intracellular staining of phosphorylated Akt, and then analyzed by flow cytometry as described in Materials and Methods. The representative flow histogram overlap against isotype control staining (gray-filled area) for pAkt expression in NK cells with IgG (blue line) versus anti-KLRG1 (red line) treatment is shown on the left, and summary data of the percentage and mean fluorescence intensity of pAkt expression in NK cells from 6 HS versus 6 HCV-infected patients, treated with the IgG control versus KLRG1 blocking antibody is shown on the right. *, P < 0.05; **, P < 0.01.
The ligands of KLRG1 are E-, N-, and R-cadherins. We have examined E-cadherin expression on different populations in PBMCs and hepatocytes. Interestingly, HCV infection seems to upregulate E-cadherin expression on some of these cells (Fig. 2E). We also attempted to block KLRG1 signaling with anti-E-cadherin antibodies, but only a minimal rescuing effect on IFN-γ production was observed in PBMCs stimulated with rhIL-12/rhIL-2 in the presence of anti-E-cadherin versus the IgG control (data not shown). Because of multiple other KLRG1 ligands existing in the system, we would expect less efficiency in blocking KLRG1 ligands than in blocking the KLRG1 receptor itself.
KLRG1 blockade reinvigorates NK cells by correcting the impaired Akt (Ser473) phosphorylation in HCV infection.
Lymphocyte activation is initiated by Akt phosphorylation at Ser473 (45). To determine the underlying mechanism for correction of NK cell function via blockade of KLRG1 signaling, we further investigated the phosphorylation of Akt in NK cell activation with cytokine stimulation following KLRG1 blockade by flow cytometric analysis. As shown in Fig. 5B, Akt phosphorylation was impaired in NK cells derived from 8 individuals with chronic HCV infection compared to those from HS following ex vivo stimulation, and blockade of KLRG1 signaling in purified CD56+ NK cells significantly enhanced Akt (Ser473) phosphorylation compared to the cells treated with control IgG. These results were reproducible in independent experiments using purified NK cells from multiple HCV-infected individuals (n = 6), in terms of both the percentage of pAkt-positive cell numbers and the MFI of pAkt expression levels in NK cells, indicating an inhibitory role for KLRG1 in regulation of human NK cell functions through the Akt pathway.
DISCUSSION
Chronic HCV infection is a worldwide infectious disease, leading to chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma (1). Although the pathogenesis of HCV infection remains only partly elucidated, it has become evident that, in addition to viral mutational escape, the dysregulation of innate to adaptive immune responses plays a major role in viral persistence and disease progression. The mechanisms involved in the HCV chronicity include dysfunction of monocyte and dendritic cells, impairment or depletion of NK cells, suppression of CD4+ and CD8+ effector T cells, and accumulation of Th17 cells and Foxp3+ regulatory T cells (2–10, 42–43, 47–49). Since NK cells comprise the first line of host defense against invading pathogens, reduction of NK cell frequency and cytokine secretion may contribute significantly to the impaired cellular immune response and virus persistence in HCV infection. Despite intensive research on the regulation of NK cell frequency, phenotype, and function during HCV infection (10–15, 47), the exact mechanisms for contraction of NK cell subsets with impaired NK cell function in the setting of HCV infection are not completely understood.
KLRG1 is an inhibitory receptor preferentially expressed by NK and antigen-experienced T cells in mice and humans (28–41). Recent studies have indicated that repetitive antigen stimulation leads to an increase of KLRG1 expression by virus-specific CD8+ T cells in chronic viral infections, such as infections with HIV, CMV, and EBV, but not in resolved infection, such as influenza virus infection (39). Chronic viral infections yield virus-specific, CD127− KLRG1+ T cells with an impaired proliferative capacity, whereas resolved infections are characterized by the presence of CD127+ KLRG1− CD8+ memory T cells with a potent proliferative ability (40, 41). Therefore, KLRG1 is also regarded as an immune senescence marker reflecting infection-mediated cell aging (35). While the majority of studies have focused on KLRG1 expression and inhibitory effects on CD4+ and CD8+ T lymphocytes in pathogenic infections (32–41), information regarding the expression of KLRG1 and its role in NK cells during chronic viral infections is limited.
In this study, we analyzed the expression of KLRG1 and its role in NK cell number and function in the peripheral blood of individuals with chronic HCV infection. We found that (i) there is a significant difference in the NK cell frequency between patients with chronic HCV infection and individuals after antiviral treatment with SVR or HS without infection. Although the frequency of CD3− CD56bright NK cells in HCV patients was higher than observed in those with SVR and HS (perhaps reflecting the NK cell differentiation and maturation status in the setting of chronic HCV infection), the frequency of CD3− CD56dim NK cells, the major NK cell subset, in HCV patients was remarkably lower than in those with SVR and HS, leading to a total CD56+ NK population contraction in chronic HCV infection. However, there was no significant difference in the total NK cell frequency, or in the CD3− CD56bright and CD3− CD56dim NK subsets, between SVR and HS, indicating that successful antiviral treatment can correct the frequency and phenotype of NK cells in HCV infection. (ii) KLRG1 expression levels on CD3− CD56+ NK cells, including CD3− CD56bright and CD3− CD56dim subsets, were significantly increased in chronically HCV-infected subjects compared to SVR subjects or HS. This was recapitulated in vitro, as we showed that KLRG1 expression was upregulated on NK cells cocultured with hepatocytes expressing HCV. Notably, KLRG1 was preferentially expressed on the less-responsive subset of CD56+, CD56bright, and CD56dim NK cells with respect to IFN-γ production. Moreover, the level of KLRG1 expression was inversely correlated with the ability to produce IFN-γ in CD3− CD56+ cells, and KLRG1+ NK cells produced less IFN-γ than KLRG1− subsets, suggesting that KLRG1+ NK cells are dysfunctional or senescent. This is consistent with the observation that production of IFN-γ during CMV infection in C57BL/6 mice is primarily by KLRG1− NK cells (32). Intriguingly, KLRG1+ NK cells, including the total CD3− CD56+ NK population, as well as CD3− CD56bright and CD3− CD56dim subsets, were found to be more sensitive, whereas KLRG1− NKs were resistant, to inflammatory cytokine-mediated cell apoptosis, which was more prominently observed in HCV-infected subjects versus HS. (iii) The elevated KLRG1 expression on NK cells and subsets was corrected by antiviral therapy with IFN/RBV in individuals with SVR. Furthermore, blockade of KLRG1 signaling significantly recovered the impaired IFN-γ production by NK cells from HCV-infected individuals. Additionally, blockade of KLRG1 also enhanced the impaired phosphorylation of Akt (Ser473) in NK cells from chronically HCV-infected patients. Taken together, these results indicate that HCV-induced expression of KLRG1 negatively controls NK cell number and function via the Akt pathway during chronic viral infection.
The CD56dim population is the major subset of NK cells that exhibit cytolytic activity and can destroy virally infected cells, malignant cells, and play regulatory roles in autoimmunity. In this regard, we believe that KLRG1 primarily mediates the loss of CD56dim NK cells, which may abolish the protective role of NK cells against virally infected hepatocytes. Indeed, KLRG1 is highly expressed on the CD56dim subset from HCV-infected patients, leading to very significant cellular apoptosis (P < 0.01) compared to NK cells from HS in response to the inflammatory cytokine stimulation. On the other hand, the CD56bright population is a minor subset of NK cells that primarily produces cytokines to indirectly combat virally infected cells. In this context, KLRG1 is also found to be upregulated in the CD56bright subset from HCV-infected patients, leading to very significant impairment of this subset in its ability to produce IFN-γ (P < 0.001) compared to HS. These results are in agreement with our previous observations that another negative signaling molecule, T cell immunoglobulin and mucin domain protein 3 (Tim-3), is upregulated on NK cells to dampen their functions, with inhibited activation (CD69), proliferation (Ki69), degranulation (CD107a), and killing activity (granzyme B) following HCV infection (data not shown). This in turn interplays with monocytes and T cells (including Th17 and Foxp3+ Tregs) in immune dysregulation that contribute to viral persistence (42–43, 48–49). This also supports the notion that HCV may utilize KLRG1—an intrinsic mercenary of cell feedback regulation—for the purpose of virus persistence and thus facilitate chronic infection.
In conclusion, our results indicate that KLRG1 plays a pivotal role in control of NK cell number and function during HCV infection. HCV appears to induce KLRG1 expression on NK cells to disrupt NK cell function, and blocking KLRG1 signaling may reverse HCV-induced NK cell dysfunction. It is not clear, however, how KLRG1 is upregulated by HCV infection at this point. Additionally, KLRG1 is an inhibitory receptor, but only when it binds to its ligands (E-, N-, or R-cadherin) to transmit an inhibitory signal (28–30). The mechanism of how anti-KLRG1 MAb treatment improves NK cell function is also unclear. We suspect that either KLRG1 ligands are also expressed on NK cells and the anti-KLRG1 MAb blocks the binding sites of cadherins, or antibody ligation of KLRG1 itself delivers a positive signaling and reverses the unresponsiveness of NK cells from virally infected individuals. Since E-cadherin is upregulated on HCV-infected hepatocytes, antigen-presenting cells, and NKs, we believe that interference of the interaction of NK KLRG1 with its ligands on accessory cells, rather than a direct activation of NKs, is likely the mechanism for NK functional recovery after anti-KLRG1 treatment. Nevertheless, given the unique functions of NK cells as the initial defense against intruding pathogens, identification of factors such as KLRG1 that control NK differentiation, maturation, homeostasis, and activation status is critical to understanding innate immune regulation and to improving immunotherapy of chronic viral infection.
ACKNOWLEDGMENTS
This work was supported by an NIH NIDDK grant to Z.Q.Y. and J.P.M. (R01DK093526) and an NIAID grant to J.P.M. and Z.Q.Y. (1R15AI103828-01). L. Shi is a visiting scholar who is partially supported by the Guanghua Foundation of Xian Jiaotong University, China. Y. Q. Cheng is a visiting scholar who is partially supported by Beijing 302 Hospital, Beijing, China. R. S. Ying, a visiting scholar, holds a grant for viral hepatitis research from Guangzhou Municipal Health Bureau, China.
We greatly appreciate support from T. Wakita, Department of Virology II, NIH, Japan, for transferring the HCV JFH-1 strain through an MTA and T. J. Liang, Liver Section, NIH NIDDK, for sending the Huh-7 cells. We also acknowledge Hanspeter Pircher, Department of Immunology, University of Freiburg, Freiburg, Germany, for generously providing KLRG1 antibodies.
This publication is the result of work supported with resources and the use of facilities of the James H. Quillen Veterans Affairs Medical Center. The contents in this publication do not represent the views of the Department of Veterans Affairs or the U.S. Government.
Footnotes
Published ahead of print 21 August 2013
REFERENCES
- 1.Shepard CW, Finelli L, Alter MJ. 2005. Global epidemiology of hepatitis C virus infection. Lancet Infect. Dis. 5:558–567 [DOI] [PubMed] [Google Scholar]
- 2.Liang TJ, Rehermann B, Seeff LB, Hoofnagle JH. 2000. Pathogenesis, natural history, treatment, and prevention of hepatitis C. Ann. Intern. Med. 132:296–305 [DOI] [PubMed] [Google Scholar]
- 3.Farci P, Alter HJ, Govindarajan S, Wang DC, Engle R, Desai SM, Miller RH, Ogata N. 1992. Lack of protective immunity against reinfection with hepatitis C virus. Science 258:135–140 [DOI] [PubMed] [Google Scholar]
- 4.Major ME, Dahari H, Mihalik K, Puig M, Rice CM. 2004. Hepatitis C virus kinetics and host responses associated with disease and outcome of infection in chimpanzees. Hepatology 39:1709–1720 [DOI] [PubMed] [Google Scholar]
- 5.Walker CM. 2010. Adaptive immunity to the hepatitis C virus. Adv. Virus Res. 78:43–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golden-Mason L, Rosen HR. 2006. Natural killer cells: primary target for hepatitis C virus immune evasion strategies? Liver Transpl. 12:363–372 [DOI] [PubMed] [Google Scholar]
- 7.Mondelli MU, Varchetta S, Oliviero B. 2010. Natural killer cells in viral hepatitis: facts and controversies. Eur. J. Clin. Invest. 40:851–863 [DOI] [PubMed] [Google Scholar]
- 8.Ahmad A, Alvarez F. 2004. Role of NK and NKT cells in the immunopathogenesis of HCV-induced hepatitis. J. Leukoc. Biol. 76:743–759 [DOI] [PubMed] [Google Scholar]
- 9.Kanto T, Hayashi N. 2007. Innate immunity in hepatitis C virus infection: interplay among dendritic cells, natural killer cells and natural killer T cells. Hepatol Res. 37:S319–S326 [DOI] [PubMed] [Google Scholar]
- 10.Meier UC, Owen RE, Taylor E, Worth A, Naoumov N, Willberg C, Tang K, Newton P, Williams I, Klenerman P, Borrow P. 2005. Shared alteration in NK cell frequency, phenotype, and function in chronic human immunodeficiency virus and hepatitis C virus infections. J. Virol. 79:12365–12374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nattermann J, Feldmann G, Ahlenstiel G, Langhan B, Spengler U. 2006. Surface expression and cytolytic function of natural killer cell receptors is altered in chronic hepatitis C. Gut 55:869–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golden-Mason L, O'Farrelly C, Rosen HR. 2007. Phenotypic and functional changes of cytotoxic CD56+ natural T cells determine outcome of acute hepatitis C virus infection. J. Virol. 81:9292–9298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golden-Mason L, Cox AL, Randall JA, Cheng L, Rosen HR. 2010. Increased natural killer cell cytotoxicity and NKp30 expression protects against hepatitis C virus infection in high-risk individuals and inhibits replication in Vitro. Hepatology 52:1581–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okumura A, Ishikawa T, Maeno T, Sato K, Ayada M, Hotta N, Yamauchi T, Fukuzawa Y, Kakumu S. 2005. Change in natural killer T cells subsets during therapy in type C hepatitis and hepatocellular carcinoma. Hepatol. Res. 32:213–217 [DOI] [PubMed] [Google Scholar]
- 15.Stegmann KA, Bjokstrom NK, Veber H, Ciesek S, Riese P, Wiegand J, Hadem J, Suneetha PV, Jaroszewicz J, Wang C, Schlaphoff V, Fytili P, Cornberg M, Manns MP, Geffers R, Pietschmann T, Guzman CA, Lunggren HG, Wedemeyer H. 2010. Interferon-alpha-induced TRAIL on natural killer cells is associated with control of hepatitis C virus infection. Gastroenterology 138:1885–1897 [DOI] [PubMed] [Google Scholar]
- 16.Cooper MA, Fehniger TA, Caligiuri MA. 2001. The biology of human natural killer-cell subsets. Trends Immunol. 22:633–640 [DOI] [PubMed] [Google Scholar]
- 17.Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC. 2001. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu. Rev. Immunol. 19:197–223 [DOI] [PubMed] [Google Scholar]
- 18.Lanier CA. 2005. NK cell recognition. Annu. Rev. Immunol. 23:225–274 [DOI] [PubMed] [Google Scholar]
- 19.Brown MG, Dokun AO, Heusel JW, Smith HR, Beckman DL, Blattenberger EA, Dubbelde CE, Stone LR, Scalzo AA, Yokoyama WM. 2001. Vital involvement of a natural killer cell activation receptor in resistance to viral infection. Science 292:934–937 [DOI] [PubMed] [Google Scholar]
- 20.Khakoo SI, Thio CL, Martin MP, Brooks CR, Gao X, Astemborski J, Cheng J, Goedert JJ, Vlahov D, Hilgartner M, Cox S, Little AM, Alexander GJ, Cramp ME, O'Brien SJ, Rosenberg WM, Thomas DL, Carrington M. 2004. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science 305:872–874 [DOI] [PubMed] [Google Scholar]
- 21.Zúñiga J, Romero V, Azocar J, Terreros D, Vargas-Rojas MI, Torres-García D, Jiménez-Alvarez L, Vargas-Alarcón G, Granados-Montiel J, Husain Z, Chung RT, Alper CA, Yunis EJ. 2009. Protective KIR-HLA interactions for HCV infection in intravenous drug users. Mol. Immunol. 46:2723–2727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knapp S, Warshow U, Hegazy D, Brackenbury L, Guha IN, Fowell A, Little AM, Alexander GJ, Rosenberg WM, Cramp ME, Khakoo SI. 2010. Consistent beneficial effects of killer cell immunoglobulin-like receptor 2DL3 and group 1 human leukocyte antigen-C following exposure to hepatitis C virus. Hepatology 51:1168–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carneiro VL, Lemaire DC, Bendicho MT, Souza SL, Cavalcante LN, Angelo AL, Freire SM, Mendes CM, Santana N, Lyra LG, Lyra AC. 2010. Natural killer cell receptor and HLA-C gene polymorphisms among patients with hepatitis C: a comparison between sustained virological responders and non-responders. Liver Int. 30:567–573 [DOI] [PubMed] [Google Scholar]
- 24.Long EO. 2008. Negative signaling by inhibitory receptors: the NK cell paradigm. Immunol. Rev. 224:70–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao B. 2010. NKG2D, its ligands, and liver disease: good or bad? Hepatology 51:8–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butcher S, Arney KL, Cook GP. 1998. MAFA-L, an ITIM-containing receptor encoded by the human NK cell gene complex and expressed by basophils and NK cells. Eur. J. Immunol. 28:3755–3762 [DOI] [PubMed] [Google Scholar]
- 27.Abramson J, Xu R, Pecht I. 2002. An unusual inhibitory receptor: the mast cell function-associated antigen (MAFA). Mol. Immunol. 38:1307–1313 [DOI] [PubMed] [Google Scholar]
- 28.Ito M, Maruyama T, Saito N, Koganei S, Yamamoto K, Matsumoto N. 2006. Killer cell lectin-like receptor G1 binds three members of the classical cadherin family to inhibit NK cell cytotoxicity. J. Exp. Med. 203:289–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grundemann C, Bauer M, Schweier O, von Oppen N, Lassing U, Saudan P, Becker KF, Karp K, Hanke T, Bachmann MF, Pircher H. 2006. Cutting edge: identification of E-cadherin as a ligand for the murine killer cell lectin-like receptor G1. J. Immunol. 176:1311–1315 [DOI] [PubMed] [Google Scholar]
- 30.Tessmer MS, Fugere C, Stevenaert F, Naidenko OV, Chong HJ, Leclercq G, Brossay L. 2007. KLRG1 binds cadherins and preferentially associates with SHIP-1. Int. Immunol. 19:391–400 [DOI] [PubMed] [Google Scholar]
- 31.Marcolino I, Przybylski GK, Koschella M, Schmidt CA, Voehringer D, Schlesier M, Pircher H. 2004. Frequent expression of the natural killer cell receptor KLRG1 in human cord blood T cells: correlation with replicative history. Eur. J. Immunol. 34:2672–2680 [DOI] [PubMed] [Google Scholar]
- 32.Robbins SH, Nguyen KB, Takahashi N, Mikayama T, Biron CA, Brossay L. 2002. Cutting edge: inhibitory functions of the killer cell lectin-like receptor G1 molecule during the activation of mouse NK cells. J. Immunol. 168:2585–2589 [DOI] [PubMed] [Google Scholar]
- 33.Voehringer D, Koschella M, Pircher H. 2002. Lack of proliferative capacity of human effector and memory T cells expressing killer cell lectinlike receptor G1 (KLRG1). Blood 100:3698–3702 [DOI] [PubMed] [Google Scholar]
- 34.Ibegbu CC, Xu YX, Harris W, Maggio D, Miller JD, Kourtis AP. 2005. Expression of killer cell lectin-like receptor G1 on antigen-specific human CD8+ T lymphocytes during active, latent, and resolved infection and its relation with CD57. J. Immunol. 174:6088–6094 [DOI] [PubMed] [Google Scholar]
- 35.Ouyang Q, Wagner WM, Voehringer D, Wikby A, Klatt T, Walter S, Müller CA, Pircher H, Pawelec G. 2003. Age-associated accumulation of CMV-specific CD8+ T cells expressing the inhibitory killer cell lectin-like receptor G1 (KLRG1). Exp. Gerontol. 38:911–920 [DOI] [PubMed] [Google Scholar]
- 36.Voehringer D, Blaser C, Brawand P, Raulet DH, Hanke T, Pircher H. 2001. Viral infections induce abundant numbers of senescent CD8 T cells. J. Immunol. 167:4838–4843 [DOI] [PubMed] [Google Scholar]
- 37.McMahon CW, Zajac AJ, Jamieson AM. 2002. Viral and bacterial infections induce expression of multiple NK cell receptors in responding CD8+ T cells. J. Immunol. 169:1444–1452 [DOI] [PubMed] [Google Scholar]
- 38.Wilson DC, Matthews S, Yap GS. 2008. IL-12 signaling drives CD8+ T cell IFN-gamma production and differentiation of KLRG1 effector subpopulations during Toxoplasma gondii infection. J. Immunol. 180:5935–5945 [DOI] [PubMed] [Google Scholar]
- 39.Thimme R, Appay V, Koschella M, Panther E, Roth E, Hislop AD, Rickinson AB, Rowland-Jones SL, Blum HE, Pircher H. 2005. Increased expression of the NK cell receptor KLRG1 by virus-specific CD8 T cells during persistent antigen stimulation. J. Virol. 79:12112–12116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bengsch B, Spangenberg HC, Kersting N, Neumann-Haefelin C, Panther E, Fvon Weizsacker Blum HE, Pircher H, Thimme R. 2007. Analysis of CD127 and KLRG1 expression on hepatitis C virus-specific CD8+ T cells reveals the existence of different memory T-cell subsets in the peripheral blood and liver. J. Virol. 81:945–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bengsch B, Seigel B, Ruhl M, Timm J, Kuntz M, Blum HE, Pircher H, Thumme R. 2010. Coexpression of PD-1, 2B4, CD160 and KLRG1 on exhausted HCV-specific CD8+ T cells is linked to antigen recognition and T cell differentiation. PLoS Pathog. 6(6):e1000947. 10.1371/journal.ppat.1000947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Ma CJ, Wang JM, Ji XJ, Wu XY, Jia ZS, Moorman JP, Yao ZQ. 2011. Tim-3 negatively regulates IL-12 expression by monocytes in HCV infection. PLoS One 6(5):e19664. 10.1371/journal.pone.0019664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y, Ma CJ, Ni L, Zhang CL, Wu XY, Kumaraguru U, Li CF, Moorman JP, Yao ZQ. 2011. Cross-talk between programmed death-1 and suppressor of cytokine signaling-1 in inhibition of IL-12 production by monocytes/macrophages in hepatitis C virus infection. J. Immunol. 186:3093–3103 [DOI] [PubMed] [Google Scholar]
- 44.Trotta R, Chen L, Ciarlariello D, Josyula S, Mao C, Costinean S, Yu L, Butchar JP, Tridandapani S, Croce CM, Caligiuri MA. 2012. miR-155 regulates IFN-γ production in natural killer cells. Blood 119:3478–3485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Henson SM, Franzese O, Macaulay R, Libri V, Azevedo RI, Kiani-Alikhan S, Plunkett FJ, Masters JE, Jackson S, Griffiths SJ, Pircher HP, Soares MV, Akbar AN. 2009. KLRG1 signaling induces defective Akt (Ser473) phosphorylation and proliferative dysfunction of highly differentiated CD8+ T cells. Blood 113:6619–6628 [DOI] [PubMed] [Google Scholar]
- 46.Robbins SH, Tessmer MS, Mikayama T, Brossay L. 2004. Expansion and contraction of the NK cell compartment in response to murine cytomegalovirus infection. J. Immunol. 173:259–266 [DOI] [PubMed] [Google Scholar]
- 47.Dessouki O, Kamiya Y, Nagahama H, Tanaka M, Suzu S, Sasaki Y, Okada S. 2010. Chronic hepatitis C viral infection reduces NK cell frequency and suppresses cytokine secretion: reversion by anti-viral treatment. Biochem. Biophys. Res. Commun. 393:331–337 [DOI] [PubMed] [Google Scholar]
- 48.Wang JM, Shi L, Ma CJ, Ji XJ, Ying RS, Wu XY, Wang KS, Li GY, Moorman JP, Yao ZQ. 2013. Differential regulation of IL-12/IL-23 by Tim-3 drives TH17 cell development during HCV infection. J. Virol. 87:4372–4383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moorman JP, Wang JM, Zhang Y, Ji XJ, Ma CJ, Wu XY, Jia ZS, Wang KS, Yao ZQ. 2012. Tim-3 controls regulatory and effector T cell balance during HCV Infection. J. Immunol. 189:755–766 [DOI] [PMC free article] [PubMed] [Google Scholar]