Abstract
Wolbachia blocks dengue virus replication in Drosophila melanogaster as well as in Aedes aegypti. Using the Drosophila model and mutations in the Toll and Imd pathways, we showed that neither pathway is required for expression of the dengue virus-blocking phenotype in the Drosophila host. This provides additional evidence that the mechanistic basis of Wolbachia-mediated dengue virus blocking in insects is more complex than simple priming of the host insect innate immune system.
TEXT
The common intracellular bacterium Wolbachia pipientis (1) is maternally inherited through the eggs of its insect hosts. It is able to successfully invade host populations through a range of reproductive manipulations that either directly or indirectly favor its transmission between insect generations (2–8). In its natural host, it has recently been shown that the presence of Wolbachia can block the replication of RNA viruses (9–13). This effect is the basis for the recent development of Wolbachia as a biocontrol approach to block dengue virus (DENV) transmission by the mosquito Aedes aegypti (14, 15).
The main vector of DENV, the mosquito Aedes aegypti, is not naturally infected with Wolbachia. However, different strains of Wolbachia have recently been artificially introduced from Drosophila melanogaster (Wolbachia strains wMel and wMelPop) or Aedes albopictus (Wolbachia strain wAlbB) into A. aegypti and are stably maintained in laboratory and wild mosquito populations (8, 16–18). The expectation is that the negative impact that Wolbachia has on DENV replication in the insect will reduce virus transmission to humans and subsequent disease (18–20).
The mechanism(s) that underlies the ability of Wolbachia to affect the replication of DENV appears complex. Using the heterologous association of Wolbachia-infected A. aegypti, transcriptomic and biochemical studies have demonstrated that Wolbachia induces the production of reactive oxygen species (ROS); primes the innate immune system of the mosquito, especially the Toll signaling pathway; and induces the production of various antimicrobial effectors (19–23). In addition, the use of RNA interference (RNAi) depletion to partially knock down defensin and cecropin genes in Wolbachia-infected A. aegypti lowered resistance to DENV and suggested a role for the innate immune system in mediating virus resistance (22). In contrast, in their natural host D. melanogaster, the same Wolbachia strains do not induce overexpression of immune genes, including the Toll pathway and cecropin- and defensin-encoding genes, yet RNA virus interference, including DENV interference, occurs (23, 24). These results demonstrated that induction of the Toll pathway by Wolbachia is not the exclusive mechanism mediating resistance. However, gene expression studies are not sufficient to make a link between a phenotype and a genetic pathway. Since previous studies confirmed the ability of DENV to replicate, and of Wolbachia to block its replication in Drosophila (23), we took advantage of preexisting and well-characterized mutant fly strains lacking functional Toll or Imd pathways to determine a precise role for these pathways in the Wolbachia-mediated viral blocking phenotype.
Role of the Toll and Imd pathways in controlling Wolbachia wMelPop density and tissue tropism.
In order to characterize the role of the Toll and Imd pathways in the phenotype of Wolbachia-mediated viral blocking, we introduced the Wolbachia strain wMelPop into Drosophila lines carrying loss-of-function mutations in genes essential to the two pathways. For the Toll pathway, strong hypomorphic mutations of spätzle (spz) were tested (spz2 and spz4). For the Imd pathway, a null allele of relish (relE20) was used (25, 26). These particular alleles of spz and rel were used as they have been well documented to cause pathogen susceptibility due to reduced production of antimicrobial effector peptides such as cecropins and defensins (25, 26). Prior to introduction of wMelPop into the different mutant strains, we tested each for the presence of Wolbachia using Wolbachia-specific PCR, targeting the wsp gene that encodes the major surface protein of the bacterium (27). All strains were determined to be uninfected with the exception of the original spz4 strain, which was positive for Wolbachia infection. Further characterization by sequencing the wsp amplicon showed that it was identical to the wsp sequence of wMel, a Wolbachia strain known to commonly infect D. melanogaster (7). Since Wolbachia is only maternally transmitted, we could use simple crosses to establish wMelPop and wMel single-infected lines in different host genetic backgrounds. To generate wMelPop-infected balancer lines, D. melanogaster w1118wMelPop virgin females were crossed with virgin TM3/TM6B males (w1118 background). wMelPop-infected TM6B/+ virgin females were crossed with wMelPop-infected TM3/+ males to generate the wMelPop-infected TM3/TM6B balancer line. The Wolbachia-infected mutant lines were established by crossing wMelPop-infected TM3/TM6B virgin females with males of the Toll and Imd mutant lines. The spz2wMelPop, spz4wMelPop, spz4wMel, and relE20wMelPop flies were maintained as heterozygotes balanced and as homozygotes for each experiment. The tetracycline-cured lines were derived by the addition of tetracycline (0.3 mg/ml) to the larval diet for two generations and confirmed to be free of Wolbachia by PCR (28). The wMelPop-infected mutant lines and their uninfected counterparts were confirmed to be homozygous mutant for the different Toll and Imd pathway alleles by bacterial challenge using appropriate pathogens (data not shown; see also reference 29).
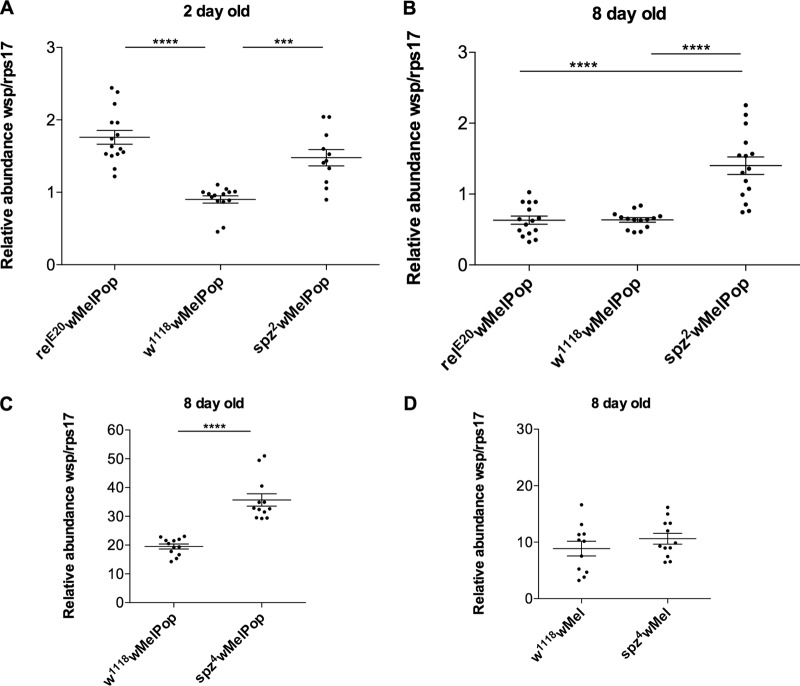
Previous studies suggest that the magnitude of virus blocking and Wolbachia density are positively correlated (30, 31). Moreover, the wMelPop strain of Wolbachia is known to overreplicate in host tissues, causing pathology and ultimately reducing the life span of its host (17, 32). Given these observations, we first examined the impact of both the Toll and Imd pathways on Wolbachia density and tissue tropism before testing DENV replication in wMelPop-infected mutant backgrounds. To assay Wolbachia density, 15 virgin females from each line were maintained in vials under controlled conditions, at 26°C with 60% relative humidity and a 12-h light/dark cycle. DNA was extracted from 9 to 15 individual females either 2 or 8 days posteclosion, using the ReliaPrep gDNA Tissue Miniprep system (Promega), according to the manufacturer's instructions. Wolbachia density was then determined by relative quantitative PCR (qPCR) by comparing the abundance of the single-copy Wolbachia surface protein gene (wsp) to that of the single-copy D. melanogaster rps17 gene as previously described (18). Data were analyzed using Mann-Whitney U tests (GraphPad Prism 5). Three independent experimental replicates were performed to confirm the results obtained with relE20 and spz2 mutants. Surprisingly, we found a significant increase in wMelPop density (approximately 2-fold) in both mutants (relE20 and spz2) in 2-day-old flies compared to the wild-type line w1118wMelPop (Fig. 1A). However, in 8-day-old flies only the Toll signaling pathway mutant spz2 exhibited a significant difference in wMelPop density for all three replicates (Fig. 1B). Similar results were obtained with another genetic background for loss of function of Toll pathway, using 2- and 8-day-old spz4 mutants (Fig. 1C). This suggests that Toll and Imd pathways both have a role in modulating wMelPop density.
Fig 1.
Wolbachia density in Drosophila lines deficient for Toll and Imd pathways. (A and B) wMelPop relative density in 2 (A)- and 8 (B)-day-old virgin females deficient for Imd (relE20wMelPop) and Toll (spz2wMelPop) pathways and the control line (w1118wMelPop). (C and D) wMelPop (C) and wMel (D) relative density in 8-day-old virgin females deficient for Toll pathway (allele spz4). n = 11 to 15; Mann-Whitney U test; ***, P < 0.001; ****, P < 0.0001.
We took advantage of having spz4 mutants infected with a different strain of Wolbachia to test whether the impact of the Toll signaling pathway on wMelPop density extended to other strains of Wolbachia. No significant difference in wMel density was observed between 8-day-old virgin females deficient for Toll pathway and the wild-type line w1118wMel (Fig. 1D). This suggests that only the overreplicative strain wMelPop is affected.
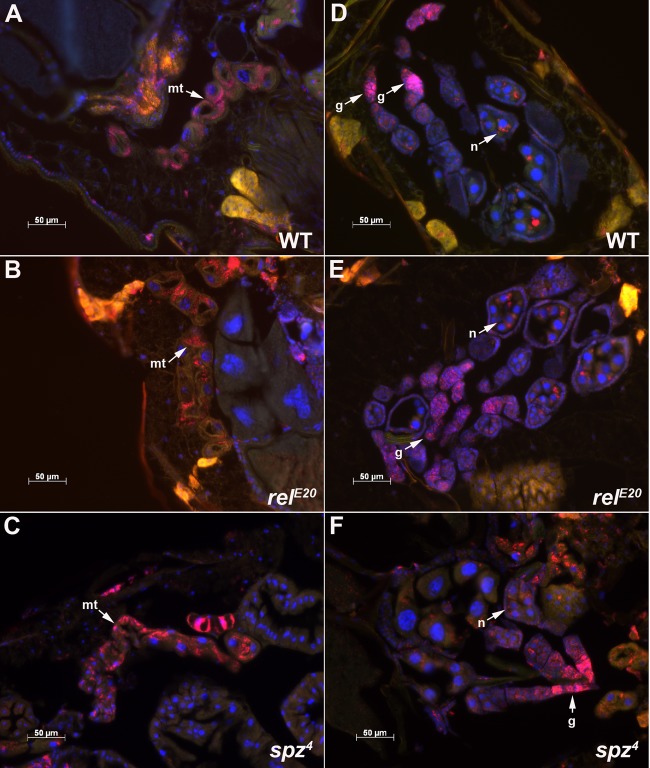
We then verified whether the observations made with wMelPop were linked with its tissue tropism in Drosophila strains deficient for Toll and Imd pathways. Fluorescence in situ hybridizations (FISH) to detect Wolbachia in tissues were made on paraffin sections of spz2wMelPop, spz4wMelPop, relE20wMelPop, and wild-type w1118wMelPop females, as described previously (19). No difference in Wolbachia localization was observed between the different lines in 8-day-old flies (Fig. 2).
Fig 2.
Wolbachia tissue tropism in Drosophila lines deficient for Toll and Imd pathways. Wolbachia is shown in red and DNA in blue. (A to C) Wolbachia localization in Malpighian tubules (mt) from w1118wMelPop (WT), relE20wMelPop, and spz4wMelPop flies. (D to F) Ovary sections, showing the localization of Wolbachia in the germarium (g) and in nurse cells (n) in developing oocytes.
Role of the Toll and Imd pathways in the Wolbachia-mediated DENV-blocking phenotype.
D. melanogaster has already been shown to be a good model for studying interactions between human viruses or parasites (including DENV) and innate immunity (33, 34). In a previous study, we artificially infected D. melanogaster with DENV, showing that the virus can replicate in Drosophila and, secondly, that Wolbachia interferes with this replication in flies (23). Using Drosophila as a model for DENV infection allows both utilization of the natural Wolbachia host and access to the genetic tools of this model species to gain a deeper understanding of the complexity of the Wolbachia-induced phenotype. After confirmation that the different Drosophila mutants used for this study retained Wolbachia infection with a cellular tropism similar to that for the wild type, we measured the impact of spätzle and relish loss of function on the DENV-Wolbachia interaction.
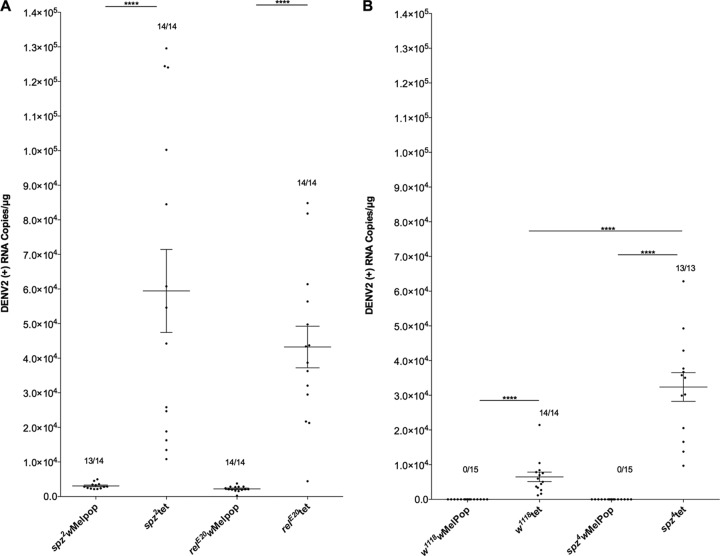
Two-day-old Drosophila females, lines spz2wMelPop and relE20wMelPop and their uninfected counterparts, spz2tet and relE20tet, were intrathoracically injected with 69 nl of DENV-2 suspension (strain ET300, 2.7 × 107 PFU/ml). Virus propagation and injection were performed as described previously (23, 35). Each experiment was repeated independently 2 to 3 times with 15 females injected per line. After injection, flies were maintained under identical controlled conditions; low density (15 females per vial), 26°C, 60% relative humidity, and 12-h light/dark cycle. Insects were collected 6 days postinjection and kept at −80°C until RNA extraction. Accumulation of genomic (+RNA) RNA strands was assessed by quantitative real-time PCR (qRT-PCR) using hydrolysis probes (TaqMan) specific to the 3′ untranscribed region (UTR) of the four serotypes of DENV (36). Only individuals with detectable levels of DENV infection were used to examine the effect of wMelPop on virus titer using Mann-Whitney U tests (GraphPad Prism 5). Regardless of the loss of function of Toll (spz2) and Imd (relE20) pathways, Wolbachia still dramatically reduced DENV replication in flies (Fig. 3A). The Toll pathway result was further confirmed with a second mutant allele, spz4 (Fig. 3B). The number of DENV copies is approximately 6 times higher in spz4tet than in w1118tet, in which the Toll pathway is intact (Fig. 3B). These results indicate that the Toll pathway, independently of Wolbachia infection status, influences DENV replication in Drosophila, as has been described previously in A. aegypti (37), reinforcing the relevance of this model to studying DENV-Wolbachia interactions. This study clearly demonstrates that both Toll and Imd pathways are not required for viral replication blocking by Wolbachia.
Fig 3.
Dengue virus blocking in Wolbachia-infected Drosophila lines deficient for Toll and Imd pathways. (A) DENV-2 was injected into 2-day-old female flies, spz2wMelPop and relE20wMelPop, and their tetracycline-treated uninfected counterparts, spz2tet and relE20tet. (B) DENV-2 was injected into 2-day-old females, spz4wMelPop and w1118wMelPop control flies, and their tetracycline-treated counterparts, spz4tet and w1118tet. Total DENV-2 in whole female Drosophila (n = 13 to 15) was measured 6 days postinjection by qRT-PCR using a TaqMan assay specific to DENV in 1 μg of total RNA. The fraction of flies that had detectable DENV-2 infections is shown above each set of data points. Mann-Whitney U test; ****, P < 0.0001.
Conclusion.
Utilizing Drosophila mutants for key regulatory genes of both the Toll and Imd pathways, we showed a clear interaction with infection density of the pathogenic Wolbachia strain wMelPop. Considering that this effect was not shared with the nonpathogenic wMel strain that grows to lower densities within the fly, it is possible that this effect is a response of the fly to the pathology and overreplication associated with this infecting Wolbachia strain. This is the first evidence to suggest an active role by the host insect in regulating Wolbachia densities. Our results show that the mutant genetic backgrounds do not negatively impact on Wolbachia densities in the host, by decreasing Wolbachia infection, which could confound interpretation of virus-blocking effects. Our results clearly demonstrate that functional Toll and Imd pathways are not required for the DENV interference phenotype to be expressed in wMelPop-infected flies. Other work done in Drosophila has shown that the antiviral small interfering RNA pathway is not involved either (38). It would be interesting to look at the impact of Wolbachia on dengue virus replication using Drosophila mutants for other immune pathways such as the JAK-STAT pathway (39).
This study provides further evidence that the mechanism of DENV blocking is likely to be more complicated than a simple priming of the insect innate immune system (23). Two recent studies support this notion: Wolbachia and Drosophila C virus compete for cholesterol, resulting in a delay in virus-induced mortality for Wolbachia-infected flies (40), and upregulation of an A. aegypti methyltransferase gene by Wolbachia contributes to dengue virus inhibition (41). These results are important in an applied context of utilizing Wolbachia infections for dengue virus control. If the mechanism of interference has a complex basis, then there is a reasonable expectation that the development of resistance by the virus may be slower than if a single interference mechanism is involved.
ACKNOWLEDGMENTS
We thank members of the O'Neill, McGraw, Warr, and Burke labs for helpful discussions and Tova Crossman and Lauren Forbes-Beadle for their help with bacterial infections. We also thank Bruno Lemaitre (Global Health Institute, EPFL, Lausanne, Switzerland), the Bloomington Drosophila Stock Centre, and the Australian Drosophila Biomedical Research Support Facility (OzDros) for supplying Drosophila strains.
This study was supported by a grant from the Foundation for the National Institutes of Health through the Grand Challenges in Global Health initiative of the Bill and Melinda Gates Foundation.
Footnotes
Published ahead of print 28 August 2013
REFERENCES
- 1.Werren JH, Baldo L, Clark ME. 2008. Wolbachia: master manipulators of invertebrate biology. Nat. Rev. Microbiol. 6:741–751 [DOI] [PubMed] [Google Scholar]
- 2.Hurst GDD, Jiggins FM, von der Schulenburg JHG, Bertrand D, West SA, Goriacheva II, Zakharov IA, Werren JH, Stouthamer R, Majerus MEN. 1999. Male-killing Wolbachia in two species of insect. Proc. Biol. Sci. 266:735–740 [Google Scholar]
- 3.Rousset F, Bouchon D, Pintureau B, Juchault P, Solignac M. 1992. Wolbachia endosymbionts responsible for various alterations of sexuality in arthropods. Proc. Biol. Sci. 250:91–98 [DOI] [PubMed] [Google Scholar]
- 4.Stouthamer R, Breeuwert JA, Luck RF, Werren JH. 1993. Molecular identification of microorganisms associated with parthenogenesis. Nature 361:66–68 [DOI] [PubMed] [Google Scholar]
- 5.O'Neill SL, Karr TL. 1990. Bidirectional incompatibility between conspecific populations of Drosophila simulans. Nature 348:178–180 [DOI] [PubMed] [Google Scholar]
- 6.Turelli M, Hoffmann AA. 1991. Rapid spread of an inherited incompatibility factor in California Drosophila. Nature 353:440–442 [DOI] [PubMed] [Google Scholar]
- 7.Riegler M, Sidhu M, Miller WJ, O'Neill SL. 2005. Evidence for a global Wolbachia replacement in Drosophila melanogaster. Curr. Biol. 15:1428–1433 [DOI] [PubMed] [Google Scholar]
- 8.Hoffmann A, Montgomery B, Popovici J, Iturbe-Ormaetxe I, Johnson P, Muzzi F, Greenfield M, Durkan M, Leong YS, Dong Y, Cook H, Axford J, Callahan AG, Kenny N, Omodei C, McGraw EA, Ryan PA, Ritchie SA, Turelli M, O'Neill SL. 2011. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 476:454–457 [DOI] [PubMed] [Google Scholar]
- 9.Hedges LM, Brownlie JC, O'Neill SL, Johnson KN. 2008. Wolbachia and virus protection in insects. Science 322:702. 10.1126/science.1162418 [DOI] [PubMed] [Google Scholar]
- 10.Teixeira L, Ferreira A, Ashburner M. 2008. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol. 6:e2. 10.1371/journal.pbio.1000002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osborne SE, San Leong Y, O'Neill SL, Johnson KN. 2009. Variation in antiviral protection mediated by different Wolbachia strains in Drosophila simulans. PLoS Pathog. 5:e1000656. 10.1371/journal.ppat.1000656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glaser RL, Meola MA. 2010. The native Wolbachia endosymbionts of Drosophila melanogaster and Culex quinquefasciatus increase host resistance to West Nile virus infection. PLoS One 5:e11977. 10.1371/journal.pone.0011977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mousson L, Zouache K, Arias-Goeta C, Raquin V, Mavingui P, Failloux AB. 2012. The native Wolbachia symbionts limit transmission of dengue virus in Aedes albopictus. PLoS Negl. Trop. Dis. 6:e1989. 10.1371/journal.pntd.0001989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iturbe-Ormaetxe I, Walker T, O'Neill SL. 2011. Wolbachia and the biological control of mosquito-borne disease. EMBO Rep. 12:508–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGraw EA, O'Neill SL. 2013. Beyond insecticides: new thinking on an ancient problem. Nat. Rev. Microbiol. 11:181–193 [DOI] [PubMed] [Google Scholar]
- 16.Xi ZY, Khoo CCH, Dobson SL. 2005. Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science 310:326–328 [DOI] [PubMed] [Google Scholar]
- 17.McMeniman CJ, Lane RV, Cass BN, Fong AWC, Sidhu M, Wang YF, O'Neill SL. 2009. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science 323:141–144 [DOI] [PubMed] [Google Scholar]
- 18.Walker T, Johnson PH, Moreira LA, Iturbe-Ormaetxe I, Frentiu FD, McMeniman CJ, Leong YS, Dong Y, Axford J, Kriesner P, Lloyd AL, Ritchie SA, O'Neill SL, Hoffmann AA. 2011. A non-virulent Wolbachia infection blocks dengue transmission and rapidly invades Aedes aegypti populations. Nature 476:450–453 [DOI] [PubMed] [Google Scholar]
- 19.Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu G, Pyke AT, Hedges LM, Rocha BC, Hall-Mendelin S, Day A, Riegler M, Hugo LE, Johnson KN, Kay BH, McGraw EA, van den Hurk AF, Ryan PA, O'Neill SL. 2009. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 139:1268–1278 [DOI] [PubMed] [Google Scholar]
- 20.Bian G, Xu Y, Lu P, Xie Y, Xi Z. 2010. The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathog. 6:e1000833. 10.1371/journal.ppat.1000833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kambris Z, Cook PE, Phuc HK, Sinkins SP. 2009. Immune activation by life-shortening Wolbachia and reduced filarial competence in mosquitoes. Science 326:134–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan X, Zhou G, Wu J, Bian G, Lu P, Raikhel AS, Xi Z. 2012. Wolbachia induces reactive oxygen species (ROS)-dependent activation of the Toll pathway to control dengue virus in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. U. S. A. 109:E23–E31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rancès E, Yixin HY, Woolfit M, McGraw EA, O'Neill SL. 2012. The relative importance of innate immune priming in Wolbachia-mediated dengue interference. PLoS Pathog. 8:e1002548. 10.1371/journal.ppat.1002548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bourtzis K, Pettigrew MM, O'Neill SL. 2000. Wolbachia neither induces nor suppresses transcripts encoding antimicrobial peptides. Insect Mol. Biol. 9:635–639 [DOI] [PubMed] [Google Scholar]
- 25.Lemaitre B, Nicolas E, Michaut L, Reichhart J-M, Hoffmann JA. 1996. The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86:973–983 [DOI] [PubMed] [Google Scholar]
- 26.Hedengren M, Dushay MS, Ando I, Ekengren S, Wihlborg M, Hultmark D. 1999. Relish, a central factor in the control of humoral but not cellular immunity in Drosophila. Mol. Cell 4:827–837 [DOI] [PubMed] [Google Scholar]
- 27.Braig HR, Zhou W, Dobson SL, O'Neill SL. 1998. Cloning and characterization of a gene encoding the major surface protein of the bacterial endosymbiont Wolbachia pipientis. J. Bacteriol. 180:2373–2378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoffmann AA, Turelli M, Simmons GM. 1986. Unidirectional incompatibility between populations of Drosophila simulans. Evolution 40:692–701 [DOI] [PubMed] [Google Scholar]
- 29.Romeo Y, Lemaitre B. 2008. Drosophila immunity: methods for monitoring the activity of Toll and Imd signaling pathways. Methods Mol. Biol. 415:379–394 [DOI] [PubMed] [Google Scholar]
- 30.Lu P, Bian G, Pan X, Xi Z. 2012. Wolbachia induces density-dependent inhibition to dengue virus in mosquito cells. PLoS Negl. Trop. Dis. 6:e1754. 10.1371/journal.pntd.0001754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Osborne SE, Iturbe-Ormaetxe I, Brownlie JC, O'Neill SL, Johnson KN. 2012. Antiviral protection and the importance of Wolbachia density and tissue tropism in Drosophila simulans. Appl. Environ. Microbiol. 78:6922–6929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Min KT, Benzer S. 1997. Wolbachia, normally a symbiont of Drosophila, can be virulent, causing degeneration and early death. Proc. Natl. Acad. Sci. U. S. A. 94:10792–10796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hughes TT, Allen AL, Bardin JE, Christian MN, Daimon K, Dozier KL, Hansen CL, Holcomb LM, Ahlander J. 2012. Drosophila as a genetic model for studying pathogenic human viruses. Virology 423:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cherry S, Silverman N. 2006. Host-pathogen interactions in Drosophila: new tricks from an old friend. Nat. Immunol. 7:911–917 [DOI] [PubMed] [Google Scholar]
- 35.Frentiu FD, Robinson J, Young PR, McGraw EA, O'Neill SL. 2010. Wolbachia-mediated resistance to dengue virus infection and death at the cellular level. PLoS One 5:e13398. 10.1371/journal.pone.0013398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warrilow D, Northill JA, Pyke A, Smith GA. 2002. Single rapid TaqMan fluorogenic probe based PCR assay that detects all four dengue serotypes. J. Med. Virol. 66:524–528 [DOI] [PubMed] [Google Scholar]
- 37.Xi Z, Ramirez JL, Dimopoulos G. 2008. The Aedes aegypti toll pathway controls dengue virus infection. PLoS Pathog. 4:e1000098. 10.1371/journal.ppat.1000098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hedges LM, Yamada R, O'Neill SL, Johnson KN. 2012. The small interfering RNA pathway is not essential for Wolbachia-mediated antiviral protection in Drosophila melanogaster. Appl. Environ. Microbiol. 8:6773–6776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Souza-Neto JA, Sim S, Dimopoulos G. 2009. An evolutionary conserved function of the JAK-STAT pathway in anti-dengue defense. Proc. Natl. Acad. Sci. U. S. A. 106:17841–17846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caragata EP, Rancès E, Hedges LM, Gofton AW, Johnson KN, O'Neill SL, McGraw EA. 2013. Dietary cholesterol modulates pathogen blocking by Wolbachia. PLoS Pathog. 9:e1003459. 10.1371/journal.ppat.1003459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang G, Hussain M, O'Neill SL, Asgari S. 2013. Wolbachia uses a host microRNA to regulate transcripts of a methyltransferase, contributing to dengue virus inhibition in Aedes aegypti. Proc. Natl. Acad. Sci. U. S. A. 110:10276–10281 [DOI] [PMC free article] [PubMed] [Google Scholar]