Abstract
Inhibition of host-directed gene expression by the matrix (M) protein of vesicular stomatitis virus (VSV) effectively blocks host antiviral responses, promotes virus replication, and disables the host cell. However, dendritic cells (DC) have the capacity to resist these effects and remain functional during VSV infection. Here, the mechanisms of DC resistance to M protein and their subsequent maturation were addressed. Flt3L-derived murine bone marrow dendritic cells (FDC), which phenotypically resemble resident splenic DC, continued to synthesize cellular proteins and matured during single-cycle (high-multiplicity) and multicycle (low-multiplicity) infection with VSV. Granulocyte-macrophage colony-stimulating factor (GM-CSF)-derived myeloid DC (GDC), which are susceptible to M protein effects, were nevertheless capable of maturing, but the response was delayed and occurred only during multicycle infection. FDC resistance was manifested early and was type I interferon (IFN) receptor (IFNAR) and MyD88 independent, but sustained resistance required IFNAR. MyD88-dependent signaling contributed to FDC maturation during single-cycle infection but was dispensable during multicycle infection. Similar to FDC, splenic DC were capable of maturing in vivo during the first 24 h of infection with VSV, and neither Toll-like receptor 7 (TLR7) nor MyD88 was required. We conclude that FDC resistance to M protein is controlled by an intrinsic, MyD88-independent mechanism that operates early in infection and is augmented later in infection by type I IFN. In contrast, while GDC are not intrinsically resistant, they can acquire resistance during multicycle infection. In vivo, splenic DC resist the inhibitory effects of VSV, and as in multicycle FDC infection, MyD88-independent signaling events control their maturation.
INTRODUCTION
Suppression and evasion of antiviral immune responses are strategies that viruses use to promote their replication in the host organism. Vesicular stomatitis virus (VSV), a prototypic negative-strand RNA virus, utilizes the dual function viral matrix (M) protein to suppress the host response. M protein is a structural protein, but it also suppresses host antiviral responses by inhibiting host-directed gene expression. M protein induces global inhibition of host gene expression at the levels of transcription, nuclear-cytoplasmic RNA transport, and translation (reviewed in reference 1). This activity of M protein effectively inhibits the synthesis of most cellular proteins, including type I interferon (IFN) and other antiviral gene products (2, 3), thus promoting virus replication. M protein mutations that inactivate its ability to suppress host responses without affecting virus assembly or replication (4) attenuate VSV pathogenicity in vivo (5, 6).
Immunocompetent animals mount an effective immune response against VSV (7–10), giving rise to the prediction that some innate immune cell types are relatively resistant to the suppressive effects of M protein. We and others have shown that dendritic cells (DC) derived from murine bone marrow in the presence of Flt3L continue to synthesize cellular proteins, produce type I IFN, and retain function during infection with VSV (11, 12). DC are a phenotypically and functionally heterogeneous group of innate immune cells that are indispensable for the activation of an adaptive immune response. DC utilize surface and intracellular pattern recognition receptors to detect the presence of pathogens. The triggering of pattern recognition receptors, such as Toll-like receptors (TLR), induces DC maturation, a complex gene expression program that promotes the activation of antigen-specific naive T cells and polarizes the adaptive immune response toward the activation of effector cells that are appropriate for elimination of the pathogen (13–15).
In light of the critical role of DC in activating a neutralizing antiviral response, the goal of the experiments presented here was to address the mechanisms by which DC manage to resist the inhibitory effects of M protein and remain functional for the activation of an immune response. For these studies, we utilized two well-characterized primary murine DC culture systems (16–18) that model four of the major DC subtypes (19). The cultivation of DC from murine bone marrow in the presence of Flt3L (referred to here as FDC) gives rise to DC that resemble the three resident splenic DC subtypes, i.e., myeloid (CD11c+ CD11b+) and nonmyeloid (CD11c+ CD8+) conventional DC and plasmacytoid (CD11c+ B220+) DC. DC cultured in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF) (referred to here as GDC) resemble monocyte-derived myeloid DC (CD11c+ CD11b+) that migrate from blood and bone marrow into inflammatory sites. We have previously demonstrated that both FDC and GDC become infected to a similar extent with VSV, but FDC are relatively resistant to VSV-induced inhibition of host gene expression compared to GDC. Consequently, FDC mature in response to high- and low-multiplicity infection with wild-type (wt) VSV over the course of 24 h (11). In contrast, over the same time course, GDC fail to mature but rather succumb to wt-VSV infection (20).
In innate immune cells, the binding of viral proteins and nucleic acids to pattern recognition receptors drives the expression of antiviral and proinflammatory genes. The products of these genes not only orchestrate the activation of an adaptive immune response but also inhibit virus replication (21). An important component of this response is driven by the maturation of plasmacytoid DC (pDC), which leads to the production of high levels of type I IFN (22, 23). The maturation of murine pDC in response to VSV depends on Toll-like receptor 7 (TLR7) (24), which is coupled to intracellular signaling pathways through the adapter protein MyD88 (25). Similarly, our previous data indicate that MyD88 and TLR7, as well as type I IFN receptor (IFNAR), contribute to the maturation of FDC in response to wt VSV (11). Thus, we hypothesized that TLR7 and/or MyD88 signaling would control DC resistance to the inhibitory effects of VSV M protein. However, the data presented here indicate that MyD88-independent events largely control resistance and the maturation of FDC and splenic DC in response to wt-VSV infection. In addition, we found that GDC, which are not intrinsically resistant to the effects of M protein, are nevertheless capable of maturing by an acquired resistance mechanism that is only apparent when the cells encounter virus at low multiplicity, as would occur during infection in vivo.
MATERIALS AND METHODS
Mice and viruses.
C57BL/6 mice were purchased from Frederick Cancer Research Facility/Charles River, Frederick, MD. MyD88−/− mice (26) were obtained from D. Golenbock (University of Massachusetts) with the permission of S. Akira. TLR7−/− mice (24) were obtained from R. Flavell (Yale University). P14 transgenic mice, which produce T cells with receptors specific for lymphocytic choriomeningitis virus (LCMV) H-2Db-restricted peptide, gp33-41 (27), were a kind gift from J. Grayson, Wake Forest School of Medicine. Mice were maintained and bred in the Wake Forest School of Medicine vivarium according to approved IACUC protocols. The study has complied with all relevant federal guidelines and institutional policies related to animal care and use. Stocks of recombinant wild-type VSV and recombinant M protein mutant M51R VSV (a mutant with Arg substituted for Met at position 51 of the M protein) were prepared and their titers were determined by infection of BHK cells as described previously (20).
Antibodies and reagents.
Phycoerythrin (PE) or allophycocyanin (APC)-labeled hamster and rat antibodies against murine DC surface markers were purchased from BD Biosciences (San Jose, CA) and included CD11c (clone HL3), CD40 (clone 3/23), CD80 (clone 16-10A1), CD86 (clone GL1), CD8α (clone 53-6.7), CD11b (clone M1/70), and CD45R/B220 (clone RA3-6B2). Species- and isotype-matched control IgGs were from the same source. Monoclonal anti-VSV M protein (23H12) and N protein (10G4) antibodies have been described previously (28). Monoclonal anti-β-actin antibody was from Sigma. The synthetic TLR7 agonist loxoribine was obtained from InvivoGen (San Diego, CA).
VSV infection in vivo.
Eight- to 20-week-old C57BL/6, MyD88−/−, or TLR7−/− mice (both on C57BL/6 background) were inoculated intravenously with 1 × 108 PFU of wt or M51R VSV. Twenty-four hours later, the mice were sacrificed and spleens were harvested. Single-cell suspensions of red blood cell-depleted splenocytes were stained with fluorescent antibodies to detect total DC (CD11c+ cells) and DC of the 3 resident subtypes (CD8+, CD11b+, and B220+ cells). Maturation after in vivo infection was assessed as described below for DC infected in culture.
Preparation of murine bone marrow-derived DC.
Bone marrow-derived DC were prepared according to standard protocols (16, 18). Briefly, bone marrow flushed from femurs and tibias was depleted of red blood cells and seeded in 24-well (GDC) or 6-well (FDC) dishes in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), glutamine, and 2-mercaptoethanol (DC medium). For GDC, cells were seeded at 5 × 105/ml in DC medium containing 10 ng/ml recombinant murine GM-CSF (produced in baculovirus, a gift from S. Mizel, Wake Forest School of Medicine). Cells were fed at days 2, 4, and 5 and harvested on day 6. For FDC, cells were seeded at 1.5 × 106 to 2.0 × 106/ml in DC medium containing 150 ng/ml recombinant human Flt3L (Invitrogen), fed on day 3, and harvested on day 8 or 9. For both culture systems, the nonadherent and loosely adherent cells were collected for experiments, leaving behind firmly adherent macrophages (16, 29).
VSV infection of cultured DC and measurement of maturation.
DC were seeded in DC medium without growth factor at 5 × 105/well in 48-well dishes. VSV was added at multiplicities of 10, 1.0, and 0.1 PFU/cell, and the cells were incubated at 37°C with 5% CO2 for 24 or 48 h. Maturation was measured by staining the cells for the T cell costimulatory molecule CD86, using a fluorescently labeled antibody. For staining of intracellular VSV M and N proteins, infected cells were fixed and permeabilized (catalog number 554714 Cytofix/Cytoperm; BD) and then incubated with monoclonal anti-M protein (23H12) or anti-N protein (10G4) antibody, followed by rabbit anti-mouse IgG conjugated to AF568 (Invitrogen). Fluorescence data were acquired using a BD FACSCalibur flow cytometer (BD Biosciences, San Jose, CA) and analyzed using FlowJo software (Tree Star, Inc., Ashland, OR). For all experiments, live cells were analyzed by gating based on forward and side scatter properties. Fluorescence-positive/-negative gates were set based on nonspecific staining of cells with fluorescently labeled isotype-matched control IgGs.
Immunoblotting.
DC were infected with VSV at a multiplicity of infection (MOI) of 10, and cell lysates were prepared at 4, 8, and 12 h postinfection. Briefly, cells were washed and incubated for 10 min on ice in lysis buffer (0.15 M NaCl, 1% sodium deoxycholate, 1% Triton X-100, 10 mM Tris, pH 7.4) containing protease inhibitors (protease inhibitor cocktail set 1; Calbiochem). Cleared lysates were analyzed for protein content (Bio-Rad), and equivalent amounts of protein per sample were subject to SDS-PAGE, followed by blotting to polyvinylidene difluoride (PVDF) membrane. Membranes were blocked and probed with monoclonal anti-VSV M protein antibody 23H12 (1:5,000), followed by goat anti-mouse IgG–horseradish peroxidase. For detection, membranes were incubated with chemiluminescent substrate (SuperSignal West Dura; Thermo Scientific) and subjected to autoradiography. Membranes were stripped and reprobed with monoclonal anti-β-actin antibody (Sigma). The integrated pixel intensities of M protein bands were quantified using ImageQuant software, and the values were normalized using the pixel densities of actin bands as the reference.
[35S]methionine radiolabeling of infected cells.
To measure the rate of protein synthesis during virus infection, GDC and FDC (2 × 106 cells/well in 6-well dishes) were infected with wt VSV at an MOI of 10 in DC medium. At the indicated times, the cells were washed once and incubated for 10 min in methionine-free medium containing 3% dialyzed FBS and then pulse labeled for 15 min with [35S]methionine (100 μCi/ml) in a total volume of 0.25 ml of the same medium. Cells were then washed several times with phosphate-buffered saline (PBS), and lysates were prepared according to the immunoblotting protocol. Equivalent amounts of protein from each lysate were loaded to 10% SDS polyacrylamide gels, subjected to electrophoresis, and analyzed by phosphorescence imaging as described previously (30). Host protein synthesis was quantitated using ImageQuant software by measuring pixel intensities in regions of the gel that were devoid of viral protein bands. The results are expressed as the percentage of host protein synthesis in virus-infected samples compared to that in mock-infected samples.
T cell proliferation and activation assay.
To determine if virus-infected FDC were capable of presenting antigen, wt FDC were infected with VSV (MOI of 10) to induce maturation. Twenty-four hours after infection, H-2Db-restricted LCMV gp33 peptide (KAVYNFATC) and naive CD8+ T cells from P14 transgenic mice (27) were added for an additional 72 h. T cells were purified from the spleens of P14 mice by negative selection using a CD8+ T cell isolation kit II (order number 130-095-236; Miltenyi Biotec) and labeled with carboxyfluorescein succinimidyl ester (CFSE) (catalog number C1157; Invitrogen) according to established methods (31). The DC/T cell ratio was 1:5. After 72 h, the cells were restimulated with 1 μg/ml gp33 peptide for 5 h. T cell proliferation was evaluated by measuring the dilution of the CFSE label (31), and T cell activation was measured by intracellular cytokine staining for gamma interferon (IFN-γ). The data were acquired using a BD FACSCanto 2 flow cytometer and analyzed with FlowJo software.
Statistical analysis.
All results are reported as the means ± standard deviations (SD) from at least 3 independent experiments; P values were calculated using the unpaired Student's t test.
RESULTS
Flt3L DC phenotype and infection with VSV.
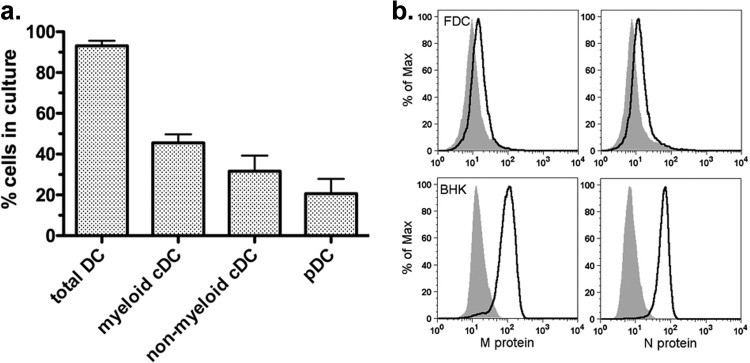
Murine bone marrow cells cultured in the presence of Flt3L produce three major subsets of DC that are similar to DC resident in the spleen: myeloid conventional DC (cDC), nonmyeloid cDC, and plasmacytoid DC (pDC) (17). The cells derived from the cultures used in the current study were analyzed for surface markers to confirm the presence of the 3 subtypes. As shown in Fig. 1a, immunofluorescence and flow cytometry analysis indicated that the cultures were comprised of the three major DC subsets in proportions similar to those reported in the literature for this culture method (17, 29). To confirm that VSV infects FDC, they were stained for intracellular VSV M and N proteins 12 h after infection at a multiplicity of 10 (Fig. 1b). As shown by the solid-line graphs in Fig. 1b, FDC expressed viral antigens but at low levels compared to their expression in the permissive cell type BHK, in which high levels of viral proteins were detected. These results are in agreement with our previous studies, in which the viral titers produced by both pDC and cDC purified from FDC were 3 orders of magnitude lower than the titers produced by fibroblasts and epithelial cells (11, 30). In addition, these results are in agreement with a published report demonstrating that DC derived from Flt3L cultures become infected with VSV but produce modest levels of viral proteins (12).
Fig 1.
Phenotype of Flt3L DC (FDC) and infection with wt VSV. (a) FDC cultured as described in Materials and Methods were stained for cell surface markers to determine DC subtype composition. Total DC were defined as CD11c+ cells, myeloid cDC were defined as CD11c+ CD11bhigh, pDC were defined as CD11c+ B220+ CD11blow/−, and nonmyeloid cDC were defined as CD11c+ CD11blow/− B220−. Results are expressed as percentage of each cell type in the culture (mean ± SD; n = 7). (b) Graphs depicting mock-infected FDC (gray shaded areas) and wt-VSV-infected FDC (solid lines). At 12 h, the cells were stained for intracellular VSV M and N proteins by 2-step immunofluorescence. Results for BHK cells infected and stained in the same manner are depicted as a positive control. Data for one of 2 independent experiments with similar results are shown.
Type I IFN feedback through IFNAR is not required for FDC resistance to M protein at early times but promotes resistance at late times postinfection.
FDC are resistant to inhibition of cellular protein synthesis and produce type I IFN in response to VSV. In contrast, GDC are susceptible to VSV-induced inhibition and produce little to no IFN (11, 20). Type I IFN is critical for the host response to VSV (10), and feedback amplification of the response via the type I IFN receptor (IFNAR) is necessary for CD86 production by FDC infected with wt VSV (11). This raised the question of the role of type I IFN feedback amplification in the resistance of FDC to inhibition of host gene expression by wt VSV. To address this question, FDC were prepared from IFNAR−/− mice. Phenotyping by surface marker staining indicated that IFNAR−/− FDC were comprised of 64% ± 1.5% (mean ± SD) myeloid DC, 6% ± 1% pDC, and 30% ± 2% nonmyeloid DC (n = 3). Therefore, compared to wt FDC, the IFNAR−/− bone marrow cultures yielded significantly more myeloid cDC and fewer pDC than the wt (P < 0.01 compared to wt FDC for both DC subtypes).
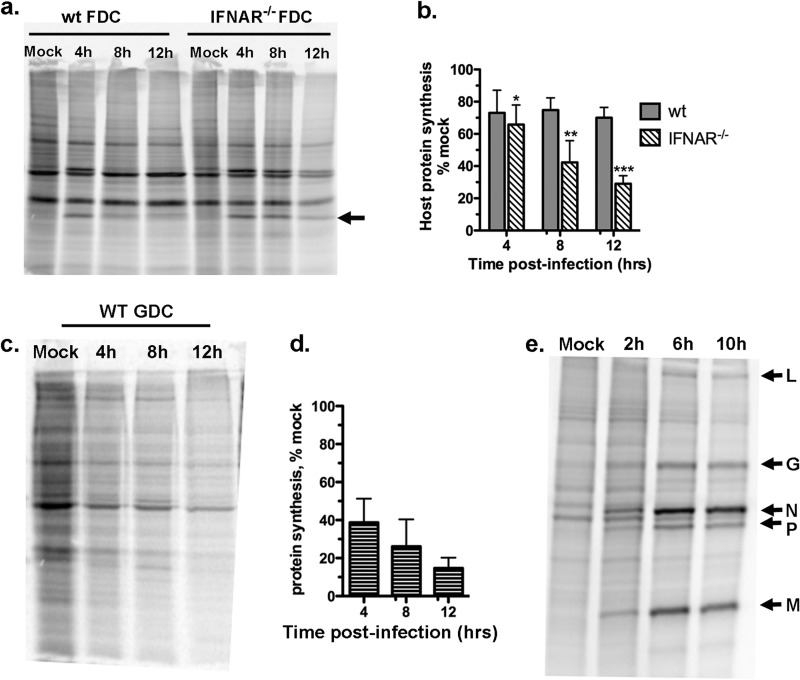
Wild-type and IFNAR−/− FDC were infected with wt VSV at an MOI of 10 (single-cycle infection). The cells were pulse labeled with [35S]methionine at various times during infection, and the rate of protein synthesis was analyzed by SDS-PAGE and phosphorimaging. For comparison, GDC, which were shown previously to be susceptible to protein synthesis inhibition by VSV (20), were infected and labeled under the same conditions. Figure 2a depicts a representative gel in which equivalent amounts of protein lysates from mock- and virus-infected FDC were loaded per lane. In Fig. 2b, the integrated pixel intensity measurements of host protein bands from three separate experiments are plotted as the percentages of protein synthesis in mock-infected cells. As shown in Fig. 2a and b, wt FDC were resistant to the inhibitory effects of M protein, as indicated by a consistent rate of protein synthesis across the population of cells over the 12-h infection period (gray bars). This is in agreement with our previously published data, in which all three DC subtypes present in FDC cultures are shown to have the capacity to resist protein synthesis inhibition by M protein and mature in response to infection with VSV (11). There was no difference between the rate of host protein synthesis in wt and IFNAR−/− DC at 4 h postinfection (P = 0.47). However, by 8 h and 12 h postinfection, the rate of host protein synthesis in IFNAR-deficient FDC was reduced relative to the rate in wt FDC (P = 0.006 and 0.001, respectively). In comparison, in GDC, VSV inhibited protein synthesis starting at 4 h postinfection, and the rate of synthesis continued to decrease throughout the 12-h time course (Fig. 2c and d). Together, these experiments indicate that type I IFN feedback amplification through IFNAR is not required for the early resistance of FDC to the effects of wt VSV but that it contributes to resistance at late times postinfection.
Fig 2.
Type I IFN receptor controls late but not early resistance of FDC to M protein-mediated inhibition of host gene protein synthesis. The rates of host protein synthesis were measured in wt and IFNAR−/− FDC after infection with wt VSV. Wild-type and IFNAR−/− FDC were infected with wt VSV (MOI of 10) and pulse labeled with [35S]methionine at the indicated times postinfection, and the rates of host protein synthesis were evaluated by gel electrophoresis, phosphorimaging, and densitometry. (a) Representative gel containing lysates from wt and IFNAR−/− FDC. Arrow indicates M protein. (b) Cumulative results expressed as percentages of synthesis in the mock-infected control. Data represent means ± SD from at least 3 independent experiments per group. P values for IFNAR−/− FDC compared to wt FDC are as follows: *, P = 0.467; **, P = 0.006; ***, P = 0.001. (c) Protein synthesis in wt GDC. (d) Cumulative results from multiple experiments. Host protein synthesis is expressed as percentage of the synthesis in the mock-infected control. (e) Protein synthesis in L929 cells; viral proteins are indicated with arrows.
Both GDC and FDC become productively infected with VSV (11). However, although M protein was detected in some experiments (Fig. 2a, arrow), the synthesis of viral proteins (L, G, N, P, and M) was not reproducibly detected above the background of host protein synthesis by this analysis (Fig. 2 a and c) (11). This is in contrast to the results for L929 murine fibroblasts (Fig. 2e) and other nonimmune cell types (32, 33), in which viral proteins were prominent during the latter stages of infection. These results suggest that DC are less permissive for VSV than nonimmune cells, in that they provide a less-favorable environment for the production of viral proteins.
M protein levels are similar in FDC and GDC.
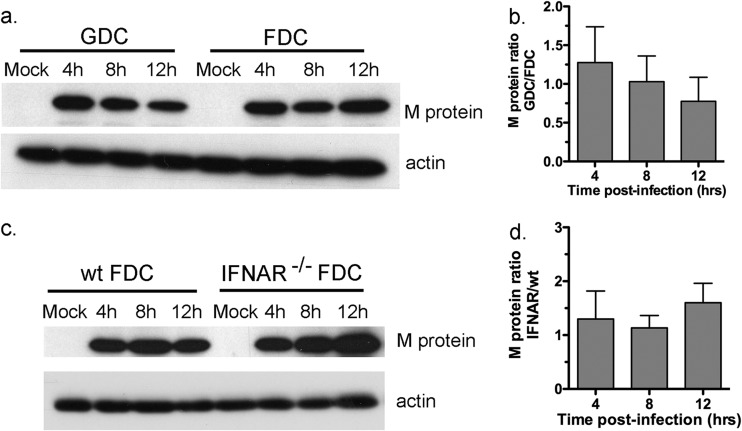
The results in Fig. 2 indicate that FDC are able to resist the effects of M protein early after infection with high-dose VSV (MOI of 10) and that early resistance does not depend on IFNAR. A possible explanation for the resistance of FDC relative to that of GDC is that M protein does not accumulate as early or as effectively in FDC as in GDC. Such an effect could be due to a lag in M protein production or a higher turnover rate in FDC. To address this possibility, FDC and GDC were infected with wt VSV at an MOI of 10, and M protein levels were measured at 4, 8, and 12 h postinfection by Western blot analysis (Fig. 3a). The results are expressed as the ratio of pixel intensities (GDC/FDC, both normalized to actin), in which 1.0 represents equivalency (Fig. 3b). As shown in Fig. 3, the ratio of M protein levels in GDC and FDC at all 3 time points was approximately 1.0, with experimental variation of less than 2-fold. Therefore, M protein accumulated to similar levels in the two DC types, further substantiating previous data demonstrating that FDC and GDC become infected with wt VSV to a similar extent (11) and yet respond quite differently to the infection. The same analysis indicated that M protein levels did not vary by more than 2-fold over the course of infection in IFNAR−/− and wt FDC (Fig. 3c and d). Collectively, these results indicate that differential accumulation of M protein is not likely to explain the resistance or susceptibility of DC subtypes to the inhibitory effects of wt VSV.
Fig 3.
VSV M protein accumulates to similar levels in FDC and GDC. FDC and GDC were infected with wt VSV at an MOI of 10. (a) Lysates were prepared at the indicated time points postinfection and subjected to immunoblotting using monoclonal anti-VSV M protein antibody. Blots were stripped, and actin was probed as an internal reference to normalize pixel intensities at each time point. (b) Results are expressed as the ratios of M protein integrated pixel intensities from infected GDC and infected FDC. (c) Lysates were prepared and processed from infected wt and IFNAR−/− FDC as described for panel a. (d) M protein levels expressed as ratios of the levels in IFNAR−/− and wt cells. For immunoblots, 1 of 3 experiments with similar results is shown, and for bar graphs, the means ± SD from 3 independent experiments are shown.
Resistance of FDC to inhibitory effects of M protein is independent of MyD88.
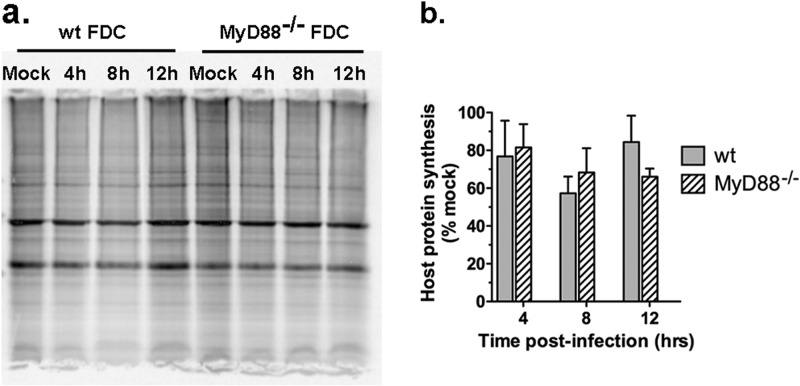
TLR7/MyD88-dependent signaling regulates the production of type I IFN by pDC in response to VSV (24). Given the role of IFN at later times postinfection, the role of MyD88 in the resistance of FDC to M protein was addressed. FDC were prepared from MyD88−/− mice and determined to have a composition of DC subtypes similar to that in wt FDC (45% ± 8% myeloid cDC, 39% ± 5% nonmyeloid cDC, and 17% ± 4% pDC; n = 3). Wild-type and MyD88−/− FDC were infected with VSV at an MOI of 10 and analyzed by metabolic labeling. As shown in Fig. 4a and b, there was no difference in the rate of host protein synthesis in MyD88−/− FDC compared to the rate in wt FDC over the course of a 12-h infection (P > 0.1, all time points), indicating that the resistance of FDC to the inhibitory effects of wt VSV is MyD88 independent.
Fig 4.
MyD88 does not control resistance of FDC to inhibition of host-directed protein synthesis by wt VSV. Wild-type and MyD88−/− FDC were infected with wt VSV (MOI of 10) and analyzed as described in the legend to Fig. 2. (a) Representative gel containing lysates from wt and MyD88−/− FDC. (b) Cumulative results from multiple experiments. Host protein synthesis is expressed as percentages of synthesis in the mock-infected control. Data represent means ± SD from 4 experiments per group. P > 0.10 for wt FDC versus MyD88−/− FDC at all time points.
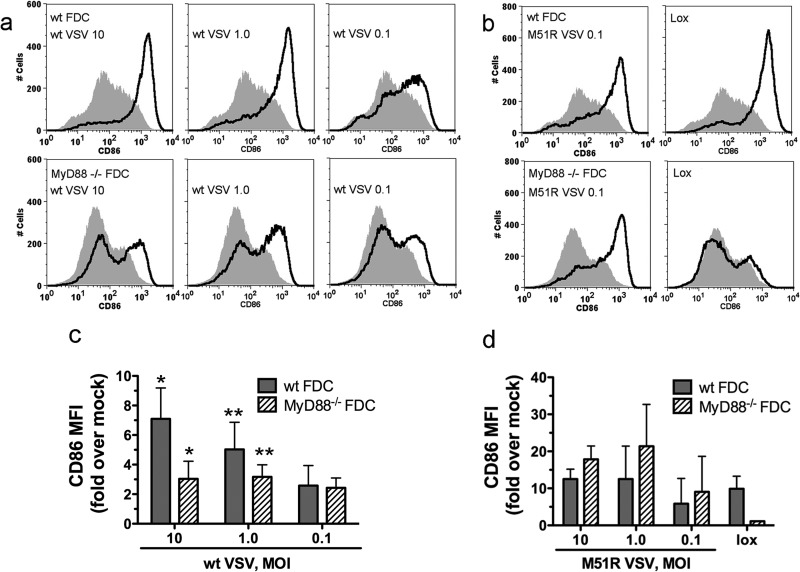
The maturation of FDC in response to wt VSV is partially dependent on MyD88.
The data in Fig. 4 indicate that MyD88 is not required for DC resistance to the effects of M protein. We next addressed the role of MyD88 in DC maturation, the functional outcome of resistance, by measuring the levels of CD86 on the surface of infected cells. Wild-type or MyD88−/− FDC were infected with VSV at multiplicities of 10, 1.0, and 0.1 PFU/cell. An MOI of 10 results in a single-cycle infection, and an MOI of 0.1 results in multicycle infection. In the latter case, only a small percentage of cells are infected initially, and additional cells become infected as a result of viral spread, as for the dynamics of infection in vivo. Figure 5a and b depict representative graphs of CD86 expression, and Fig. 5c and d show the cumulative results of multiple experiments expressed as fold increases in the CD86 mean fluorescence intensity (MFI) over the CD86 MFI in mock-infected cells. At an MOI of 10, wt VSV induced robust CD86 expression in wt FDC by 24 h postinfection (Fig. 5a and c), consistent with their capacity to resist M protein-mediated inhibition of host gene expression (Fig. 2). In MyD88−/− cells, CD86 expression was reduced relative to the level in wt cells (P = 0.012). The biphasic expression curve indicated that a subpopulation of cells utilized a MyD88-dependent pathway to mature during single-cycle infection (Fig. 5a). In contrast, the response at an MOI of 0.1 was similar in the presence and absence of MyD88 signaling (Fig. 5a and c). At the intermediate MOI of 1.0, CD86 expression was reduced in MyD88−/− FDC compared to its expression in wt FDC, but the difference did not reach statistical significance (Fig. 5a and c) (P = 0.1). M51R VSV is severely defective for inhibition of host-directed gene expression, due to a single amino acid substitution in the M protein (2). The maturation of FDC in response to M51R VSV was robust and was somewhat greater than that in response to wt VSV (Fig. 5c and d; note the different scales). As we have reported previously (11), in contrast to the maturation induced by wt VSV, the maturation induced by M51R VSV was independent of MyD88 regardless of the MOI (Fig. 5b and d). As a further control, there was no response to the synthetic TLR7 agonist loxoribine (lox) in the absence of MyD88 (Fig. 5b and d). Collectively, these results demonstrate that a subpopulation of cells in FDC cultures is capable of engaging a MyD88-dependent signaling pathway to increase the expression of CD86 when the viral load is high. However, when the initial viral load is low, FDC are capable of increasing their expression of CD86 using a MyD88-independent pathway.
Fig 5.
FDC maturation in response to wt VSV is partially dependent on MyD88. FDC prepared from wt or MyD88−/− mice were infected with wt or M51R VSV at the indicated MOI. Controls were mock treated or treated with 1 mM loxoribine (lox). Maturation of the total DC population (>90% CD11c+) gated on live cells was evaluated by flow cytometry after staining for CD86. (a) Representative histograms from wt-VSV-infected cells. Top, wt FDC; bottom, MyD88−/− FDC. (b) Representative histograms from M51R VSV-infected or lox-treated cells. Top, wt FDC; bottom, MyD88−/− DC. (c and d) Cumulative data from multiple experiments showing fold increases in CD86 MFI for wt-VSV-infected cells, M51R VSV-infected cells, or lox-treated cells. Mock-infected FDC did not significantly increase CD86 expression over the course of the 24-h culture period relative to levels expressed at start of culture (data not shown).
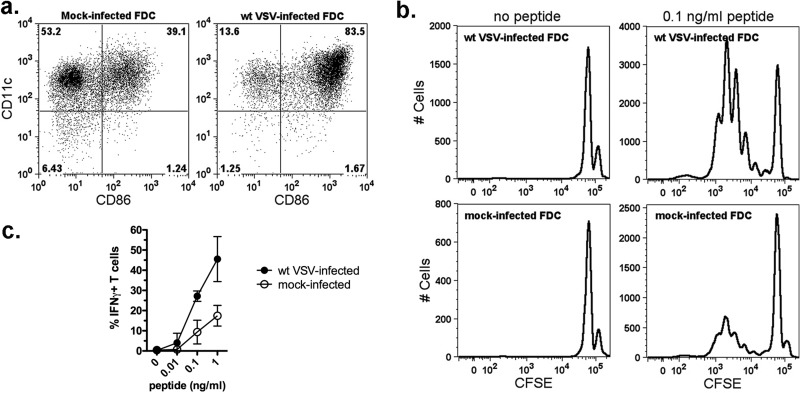
FDC infected with wt VSV are competent to induce T cell proliferation and activation.
To measure the functional effects of maturation induced in wt-VSV-infected FDC, the cells were tested for their ability to induce the proliferation and activation of naive CD8+ T cells. For these experiments, CD8+ T cells were isolated from the spleens of P14 transgenic mice, labeled with CFSE, and cultured with wt-VSV-infected or mock-infected FDC in the presence or absence of LCMV gp33-41 peptide. After 72 h, proliferation and activation were measured by flow cytometry analysis of CFSE dilution and IFN-γ expression, respectively. As shown previously for GDC (20), mock-infected FDC contained a subpopulation of CD86+ cells that induced a background level of T cell activation, but the extent of CD86 expression was dramatically increased following VSV infection (Fig. 6a). VSV-matured DC induced T cell proliferation, as indicated by the abundant presence of weakly fluorescent T cells in the cultures that received peptide (Fig. 6b; note the shift to left), whereas mock-infected DC were much less effective at inducing antigen-specific T cell proliferation (Fig. 6b, compare histograms for cultures with peptide). Furthermore, VSV-matured FDC were capable of activating T cells, as indicated by the production of IFN-γ (Fig. 6c). Similar to the proliferation results, VSV-infected FDC activated significantly more T cells to produce IFN-γ than did mock-infected cells. Collectively, these results indicate that FDC remain functional for T cell activation during wt-VSV infection and further support the conclusion that they are resistant to the inhibitory effects of VSV on host gene expression.
Fig 6.
VSV-infected FDC are capable of inducing CD8+ T cell proliferation and activation. (a) FDC used as antigen-presenting cells. The cells were cultured for 24 h without virus (mock infected) or with wt VSV (MOI of 10), and maturation was measured by surface staining for CD11c and CD86. The number in each quadrant of the dot plots indicates the percentage of positive cells in the gate. (b) Mock- or virus-infected FDC were cultured for an additional 72 h with LCMV H-2Db-restricted gp33 peptide (KAVYNFATC) and CFSE-labeled CD8+ T cells from P14 transgenic mice. T cell proliferation and activation were measured by flow cytometry. Histograms depict proliferation of T cells cultured with wt-VSV-infected FDC or mock-infected FDC ± gp33 peptide as indicated. The data are from 1 of 3 experiments with similar results. (c) CD8+ T cell activation in response to gp33 peptide presented by mock-infected or VSV-infected FDC. Results are expressed as percentages of IFN-γ+ T cells (mean ± SD; n = 3 VSV infected, n = 2 mock infected).
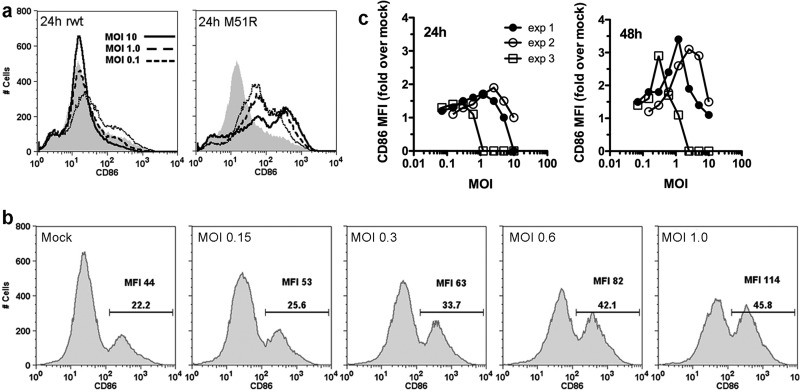
M protein-sensitive GDC are capable of maturing but only during multicycle infection.
Further evidence that DC utilize TLR7-independent signaling pathway(s) to mature when they encounter VSV at low multiplicity was obtained in experiments with GDC, which do not express TLR7 at levels sufficient to respond to synthetic TLR7 agonists (20, 34). Cellular protein synthesis is inhibited in GDC infected with wt VSV (Fig. 2), and consequently, the cells fail to mature during 24 h of infection with wt virus but mature robustly in response to M51R VSV (Fig. 7a) (20). However, we found that GDC were capable of maturing over the course of a 48-h infection period when infected with wt VSV at low multiplicity, as measured by the percentages of cells expressing CD86 and by CD86 MFI (Fig. 7b). The results of three independent experiments, shown in Fig. 7c, demonstrate enhanced CD86 expression in GDC cultured for 48 h compared to the CD86 expression in GDC cultured for 24 h. Although the MOI at which the CD86 response peaked varied in individual experiments, VSV in the dose range of 0.1 to 2.5 PFU/cell induced maturation (Fig. 7c). These results indicate that GDC are capable of maturing by a TLR7-independent mechanism that is delayed and is only acquired upon encounter with wt VSV during low-multiplicity infection.
Fig 7.
GDC acquire the ability to mature under conditions of multicycle infection and extended culture period. (a) GDC were infected with wt or M51R VSV at the indicated MOI, and CD86 expression was measured at 24 h postinfection by flow cytometry. Shaded areas show data for mock-infected cells. (b) Representative graphs depicting CD86 fluorescence 48 h after infection of GDC with wt VSV at the indicated MOI; the percentage of CD86+ cells (gated on rat IgG isotype control stain) and MFI are indicated in each panel. (c) GDC were infected with wt VSV at the indicated MOI, and CD86 expression was measured at 24 h and 48 h postinfection. Results from 3 independent experiments, expressed as fold increases in CD86 MFI over values in mock-infected cells, are shown.
Together, our data indicate that the fate and functional outcome of DC infection with wt VSV depends on both the DC subtype and the MOI. In GDC, a high MOI leads to cell death (20), whereas FDC under the same conditions mature and become competent to present antigen to naive T cells. At low multiplicities of infection, similar to infection in vivo, both FDC and GDC are capable of maturing in response to wt VSV by a mechanism that does not require signaling through TLR7 or MyD88.
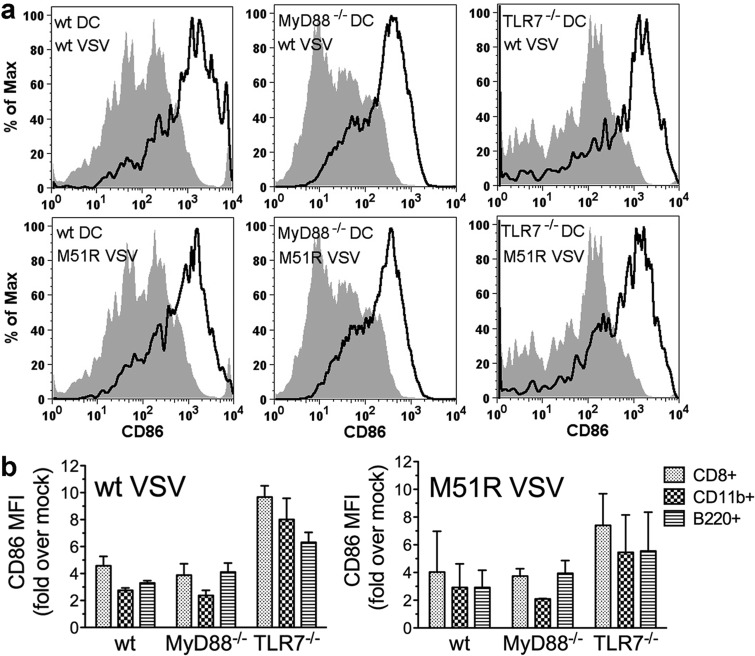
VSV induces DC maturation in the absence of TLR7 or MyD88 in vivo.
The results reported above demonstrate that MyD88 is not required for FDC resistance to M protein and their subsequent maturation in vitro. We next tested the role of MyD88 and TLR7 in the in vivo DC response using mice with specific deletions of each gene. Wild-type, MyD88−/−, and TLR7−/− mice were infected with either wt VSV or M51R VSV. At 24 h postinfection, splenocytes were isolated and the levels of T cell costimulatory molecules were measured by flow cytometry to evaluate DC maturation. Total splenic DC were analyzed for the expression of CD40, CD80, and CD86 by gating on CD11c+ cells (Fig. 8a). As reported previously for FDC (11), the most-dramatic increases in the expression of costimulatory molecules on splenic DC were in the levels of CD86. The representative histograms of CD86 expression shown in Fig. 8a indicate that wt DC matured in response to wt VSV. Furthermore, the responses were similar in magnitude in MyD88−/− and TLR7−/− DC (Fig. 8a, top). In addition, the extent of DC maturation in response to wt VSV was similar to that in response to M51R VSV (Fig. 8a, bottom).
Fig 8.
Murine splenic DC maturation after in vivo infection with VSV is MyD88 and TLR7 independent. Wild-type, MyD88−/−, or TLR7−/− mice were infected intravenously with wt VSV or M51R VSV. At 24 h postinfection, splenocytes were harvested and splenic DC maturation was measured by immunofluorescence staining for CD11c and T cell costimulatory molecules followed by flow cytometry. (a) Representative graphs of live splenocytes gated on CD11c to identify total DC and analyzed for CD86 expression to identify DC that had matured. Top, wt VSV; bottom, M51R VSV; shaded, mock infected; solid line, virus infected. (b) Cumulative data from multiple experiments expressed as fold increases in CD86 geometric MFI over values from mock-infected mice. Live splenocytes were gated on CD11c and DC subset marker CD8 (CD8+, nonmyeloid DC), CD11b (myeloid DC), or B220 (pDC). Results represent means ± SD; n = 3 MyD88−/− or n = 6 wt or TLR7−/− mice per group.
Specific DC subtypes were analyzed by gating on CD11c and markers that distinguish the three resident splenic DC types, myeloid DC (CD11b+), plasmacytoid DC (B220+), and nonmyeloid, CD8+ DC (CD11b− CD8+). The results from multiple experiments, expressed as the fold change in induction of CD86 expression over that in mock-infected mice, are shown in Fig. 8b. DC of all three subtypes from all three genotypes of mice increased their surface expression of CD86 to a similar extent in response to wt VSV. In each case, the cells responded equally well to infection with wt and M51R VSV. Similar results were obtained when the expression levels of CD40 and CD80 were analyzed (data not shown). Therefore, resident splenic DC of all three subtypes matured in response to wt VSV, and there was no evidence of the inhibitory activity of M protein. Furthermore, DC maturation as measured by T cell costimulatory molecule production did not require signaling through TLR7 or MyD88. Collectively, these results indicate that, like FDC, splenic DC are capable of resisting the inhibitory effects of wt VSV in vivo and that they respond to infection using MyD88-independent signaling pathways. Since we have not had success in detecting viral gene expression in splenic DC populations (data not shown), it is likely that most of the responding cells had acquired resistance indirectly by interacting with a small number of initially infected cells.
DISCUSSION
VSV utilizes M protein to suppress host-directed gene expression. This activity of M protein is highly effective in fibroblasts, epithelial cells, and neurons, which are the target cells for VSV infection in mouse models (1). The inhibitory activity of M protein is apparent within the first several hours of infection with wt VSV (32, 33). In susceptible cells, the suppression of host protein synthesis promotes robust virus replication and culminates in apoptotic cell death (30, 35). However, DC derived from bone marrow in the presence of Flt3L (FDC) are relatively resistant to the effects of M protein (11). Here, we demonstrate that FDC resistance reflects both intrinsic mechanisms that operate early after infection and acquired mechanisms that operate later in infection. The latter are mediated through the production of type I IFN and, likely, other factors that act in an autocrine fashion in a single-cycle infection and in a paracrine fashion in multicycle infection (Fig. 2). M protein-susceptible GDC are also able to mature by an acquired-resistance mechanism but only during multicycle infection. Importantly, murine splenic DC, the in vivo counterparts of FDC, survive and mature 24 h after intravenous inoculation with VSV. This demonstrates in vivo resistance to the inhibitory effects of wt VSV that allows DC to retain functions that are critical for the initiation of adaptive immunity. Notably, we found that the DC response to wt VSV was of similar magnitude to the response to M protein mutant M51R VSV, which does not effectively inhibit host gene expression. Therefore, our study is the first to show that DC of the types found in the spleen are minimally affected in vivo by the inhibitory activities of VSV M protein on host gene expression that are readily apparent in nonimmune cells (32, 33). Similar resistance mechanisms may be present in some subtypes of macrophages, which, along with DC, are among the earliest responders to virus infection (36). That DC remain functional during the early stages of in vivo infection with VSV is consistent with the well-documented ability of immunocompetent hosts to mount an immune response that effectively neutralizes VSV (7–10, 36).
We hypothesized that FDC resistance to inhibition by wt VSV would involve signaling through TLR7 or, perhaps, TLR13, both of which are MyD88-dependent sensors of VSV (24, 37). However, there was no difference in the ability of wt and MyD88−/− FDC to synthesize cellular proteins following infection with wt VSV. Therefore, the resistance of FDC to the inhibitory effects of M protein is independent of TLR7, TLR13, and other known TLRs that depend on MyD88. Although this study does not address the role in resistance of TLR3, a MyD88-independent TLR (38), the available evidence indicates that TLR3 has minimal involvement in the host response to VSV (7, 39).
FDC resistance to wt VSV required IFNAR, but only late in infection (≥8 h), a time point at which IFN feedback amplification of antiviral gene expression would be expected to be fully engaged (40). Early in infection (4 h), the rate of cellular protein synthesis in IFNAR−/− cells was comparable to that in wt cells. Thus, the ability of FDC to resist the inhibitory effects of wt VSV early in infection provides a window of opportunity for the cells to produce type I IFN and initiate a maturation response. FDC, which are resistant, go on to mature (11), while GDC, which are susceptible, do not mature during single-cycle infection (20). We predicted that the absence of TLR7 and/or MyD88 in vivo would decrease DC maturation in the spleen. Our previous study demonstrated that MyD88-dependent signaling contributes to FDC maturation in response to wt VSV (11). TLR7 is necessary for type I IFN production by plasmacytoid DC (pDC) in response to VSV infection ex vivo (24), and pDC are major IFN-producing cells during VSV infection in vivo (41). Since type I IFN promotes the maturation of conventional (nonplasmacytoid) DC (40, 42), decreased IFN production by pDC in VSV-infected TLR7−/− and MyD88−/− mice could have a negative impact on DC maturation in the spleen. However, the data presented here demonstrate that in vivo maturation of splenic DC did not depend on TLR7 or MyD88 (Fig. 8). Similarly, when cultured FDC encountered VSV under multicycle infection conditions, as would be expected to occur in vivo, maturation did not depend on MyD88. These results strongly suggest that signals mediated by sensors other than TLR control early splenic DC responses to wt VSV.
Consistent with these findings, others have reported that type I IFN production and restriction of peripheral virus replication after intravenous infection with wt VSV did not depend on MyD88 (7). Nevertheless, MyD88 is critical for preventing death by viral spread to the central nervous system, due in part to the MyD88-dependent recruitment of monocytic cells that restrict VSV replication to the periphery (7). In our study, MyD88 contributed to the ability of FDC to increase CD86 expression when the cells encountered high viral loads (an MOI of 10, at which every cell is infected initially). In MyD88-deficient FDC, the resulting biphasic distribution of CD86 expression (Fig. 5) suggests that a subset of cells in FDC cultures utilized MyD88 signaling to mature in response to high doses of virus. Similarly, there may be cells in the periphery in which the involvement of TLR7 and MyD88 in in vivo responses to VSV is necessary to prevent viral invasion of the central nervous system. However, in the spleen, all 3 major subsets of splenic DC, including pDC (CD11c+ B220+ cells), myeloid DC (CD11c+ CD11b+), and nonmyeloid CD8α DC (CD11c+ CD11b−), increased their expression of CD86, CD40, and CD80 by 24 h after infection with VSV regardless of the presence or absence of MyD88. Therefore, during viral infection in vivo, when splenic cells would be expected to encounter VSV at low infectious doses, MyD88-independent signaling pathways promoted DC maturation.
These experiments also revealed that GDC, which are susceptible to the inhibitory effects of M protein, can acquire resistance during wt-VSV infection. Neither high nor low infectious doses of VSV induced maturation of GDC over the course of a 24-h infection period (Fig. 7) (20). However, during multicycle infection, a subpopulation of GDC was capable of maturing over the course of a 48-h time period. Maturation under these circumstances likely reflects priming of uninfected cells by type I IFN, other antiviral cytokines, and/or dead cell material released into the local milieu by cells that became infected initially. Priming mediated by type I IFN endows cells (of all types) that have yet to become infected with the ability to perpetuate and amplify the systemic antiviral response when they eventually confront virus (40). Priming by-products of infected cells may additionally confer upon uninfected GDC the capacity to mature. This was proposed as a mechanism by which myeloid DC could contribute to anti-VSV immune responses despite an overall cytolytic effect in single-cycle infections (43). Similar mechanisms have been demonstrated for human monocyte-derived DC, which acquired the ability to control Newcastle disease virus (NDV) infection and an enhanced capacity to mature in response to products released from NDV-infected DC (44). Likewise, pretreatment of human GDC with type I IFN confers the ability to mature in response to infection with influenza virus, which otherwise suppresses DC responses (45). We are currently addressing the role of type I IFN and apoptotic material in the maturation of GDC after low-dose virus infection and extended culture. It is possible that the initiation of maturation in response to the ingestion of infected-cell material (as opposed to live virus particles) is delayed, explaining why maturation was evident at 48 h but not 24 h after multicycle infection with VSV. In any case, the ability of GDC to mature in response to wt VSV under multicycle infection conditions may not be mediated by type I IFN alone, because priming of GDC with a broad dose range of type I IFN prior to infection did not confer upon these cells the capacity to mature (data not shown).
M protein-mediated suppression of host-directed gene expression and similar mechanisms used by other viruses to suppress the antiviral response are barriers that the host must overcome in order to generate a virus-neutralizing immune response. DC function must be preserved during infection in order for the host to initiate an adaptive immune response. This study indicates that wt-VSV-infected FDC retain their ability to undergo the complex gene expression program that leads to their maturation and confers the ability to induce T cell proliferation and activation. The data further indicate that FDC have both intrinsic and acquired mechanisms of resistance to the inhibitory effects of VSV M protein. With regard to DC resistance in vivo, the current study does not address which splenocyte subtypes are directly infected by VSV after inoculation by the intravenous route. Therefore, it is not possible make conclusions about which cell types are responsible for in vivo DC resistance. Because viral infection in vivo proceeds via multiple cycles of infection, splenic DC resistance to the effects of M protein (Fig. 8) is very likely acquired by signals received from locally infected cells. However, our in vitro experiments indicate that FDC continue to synthesize protein at a normal rate after high-multiplicity infection with VSV, suggesting that splenic DC may also have an intrinsic resistance to M protein. The only known cellular target for VSV M protein that has been identified is the protein Rae1, which is involved in the M protein-induced inhibition of host transcription and mRNA transport (33, 46). We found no difference between FDC and GDC in the overall cellular levels of Rae1 (M. M. Westcott and D. S. Lyles, unpublished data), leaving open the question of the mechanism of intrinsic resistance. Resistance in FDC may involve proteins akin to HIV restriction factors, such as SAMHD1 and APOBEC3, that limit the ability of HIV to complete its life cycle in macrophages and other cell types (47). Identification of the pathways that control M protein resistance in DC should broaden our understanding of how the mammalian host has adapted to cope with viral pathogens that use immune-suppressive tactics to promote their replication.
ACKNOWLEDGMENTS
We thank Jason Grayson, Department of Microbiology and Immunology, Wake Forest School of Medicine, for P14 transgenic mice.
This study was supported by NIH grants R01 AI032983 and P01 AI082325.
Footnotes
Published ahead of print 28 August 2013
REFERENCES
- 1.Gerlier D, Lyles DS. 2011. Interplay between innate immunity and negative-strand RNA viruses: towards a rational model. Microbiol. Mol. Biol. Rev. 75:468–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed M, McKenzie MO, Puckett S, Hojnacki M, Poliquin L, Lyles DS. 2003. Ability of the matrix protein of vesicular stomatitis virus to suppress beta interferon gene expression is genetically correlated with the inhibition of host RNA and protein synthesis. J. Virol. 77:4646–4657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faul EJ, Lyles DS, Schnell MJ. 2009. Interferon response and viral evasion by members of the family Rhabdoviridae. Viruses 1:832–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black BL, Rhodes RB, McKenzie M, Lyles DS. 1993. The role of vesicular stomatitis virus matrix protein in inhibition of host-directed gene expression is genetically separable from its function in virus assembly. J. Virol. 67:4814–4821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed M, Marino TR, Puckett S, Kock ND, Lyles DS. 2008. Immune response in the absence of neurovirulence in mice infected with M protein mutant vesicular stomatitis virus. J. Virol. 82:9273–9277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stojdl DF, Lichty BD, ten Oever BR, Paterson JM, Power AT, Knowles S, Marius R, Reynard J, Poliquin L, Atkins H, Brown EG, Durbin RK, Durbin JE, Hiscott J, Bell JC. 2003. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell 4:263–275 [DOI] [PubMed] [Google Scholar]
- 7.Lang KS, Navarini AA, Recher M, Lang PA, Heikenwalder M, Stecher B, Bergthaler A, Odermatt B, Akira S, Honda K, Hengartner H, Zinkernagel RM. 2007. MyD88 protects from lethal encephalitis during infection with vesicular stomatitis virus. Eur. J. Immunol. 37:2434–2440 [DOI] [PubMed] [Google Scholar]
- 8.Zhou S, Kurt-Jones EA, Fitzgerald KA, Wang JP, Cerny AM, Chan M, Finberg RW. 2007. Role of MyD88 in route-dependent susceptibility to vesicular stomatitis virus infection. J. Immunol. 178:5173–5181 [DOI] [PubMed] [Google Scholar]
- 9.Hangartner L, Zinkernagel RM, Hengartner H. 2006. Antiviral antibody responses: the two extremes of a wide spectrum. Nat. Rev. Immunol. 6:231–243 [DOI] [PubMed] [Google Scholar]
- 10.Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. 1994. Functional role of type I and type II interferons in antiviral defense. Science 264:1918–1921 [DOI] [PubMed] [Google Scholar]
- 11.Ahmed M, Mitchell LM, Puckett S, Brzoza-Lewis KL, Lyles DS, Hiltbold EM. 2009. Vesicular stomatitis virus M protein mutant stimulates maturation of Toll-like receptor 7 (TLR7)-positive dendritic cells through TLR-dependent and -independent mechanisms. J. Virol. 83:2962–2975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waibler Z, Detje CN, Bell JC, Kalinke U. 2007. Matrix protein mediated shutdown of host cell metabolism limits vesicular stomatitis virus-induced interferon-alpha responses to plasmacytoid dendritic cells. Immunobiology 212:887–894 [DOI] [PubMed] [Google Scholar]
- 13.Kapsenberg ML. 2003. Dendritic-cell control of pathogen-driven T-cell polarization. Nat. Rev. Immunol. 3:984–993 [DOI] [PubMed] [Google Scholar]
- 14.Steinman RM. 1991. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 9:271–296 [DOI] [PubMed] [Google Scholar]
- 15.Heath WR, Belz GT, Behrens GM, Smith CM, Forehan SP, Parish IA, Davey GM, Wilson NS, Carbone FR, Villadangos JA. 2004. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol. Rev. 199:9–26 [DOI] [PubMed] [Google Scholar]
- 16.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. 1992. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 176:1693–1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naik SH, Proietto AI, Wilson NS, Dakic A, Schnorrer P, Fuchsberger M, Lahoud MH, O'Keeffe M, Shao QX, Chen WF, Villadangos JA, Shortman K, Wu L. 2005. Cutting edge: generation of splenic CD8+ and CD8- dendritic cell equivalents in Fms-like tyrosine kinase 3 ligand bone marrow cultures. J. Immunol. 174:6592–6597 [DOI] [PubMed] [Google Scholar]
- 18.Gilliet M, Boonstra A, Paturel C, Antonenko S, Xu XL, Trinchieri G, O'Garra A, Liu YJ. 2002. The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 195:953–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merad M, Sathe P, Helft J, Miller J, Mortha A. 2013. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu. Rev. Immunol. 31:563–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmed M, Brzoza KL, Hiltbold EM. 2006. Matrix protein mutant of vesicular stomatitis virus stimulates maturation of myeloid dendritic cells. J. Virol. 80:2194–2205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takeuchi O, Akira S. 2009. Innate immunity to virus infection. Immunol. Rev. 227:75–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colonna M, Trinchieri G, Liu YJ. 2004. Plasmacytoid dendritic cells in immunity. Nat. Immunol. 5:1219–1226 [DOI] [PubMed] [Google Scholar]
- 23.Gilliet M, Cao W, Liu YJ. 2008. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat. Rev. Immunol. 8:594–606 [DOI] [PubMed] [Google Scholar]
- 24.Lund JM, Alexopoulou L, Sato A, Karow M, Adams NC, Gale NW, Iwasaki A, Flavell RA. 2004. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. U. S. A. 101:5598–5603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takeda K, Akira S. 2005. Toll-like receptors in innate immunity. Int. Immunol. 17:1–14 [DOI] [PubMed] [Google Scholar]
- 26.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9:143–150 [DOI] [PubMed] [Google Scholar]
- 27.Brandle D, Brduscha-Riem K, Hayday AC, Owen MJ, Hengartner H, Pircher H. 1995. T cell development and repertoire of mice expressing a single T cell receptor alpha chain. Eur. J. Immunol. 25:2650–2655 [DOI] [PubMed] [Google Scholar]
- 28.Lyles DS, Puddington L, McCreedy BJ., Jr 1988. Vesicular stomatitis virus M protein in the nuclei of infected cells. J. Virol. 62:4387–4392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naik SH, O'Keeffe M, Proietto A, Shortman HH, Wu L. 2010. CD8+, CD8-, and plasmacytoid dendritic cell generation in vitro using flt3 ligand. Methods Mol. Biol. 595:167–176 [DOI] [PubMed] [Google Scholar]
- 30.Kopecky SA, Willingham MC, Lyles DS. 2001. Matrix protein and another viral component contribute to induction of apoptosis in cells infected with vesicular stomatitis virus. J. Virol. 75:12169–12181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lyons AB, Hasbold J, Hodgkin PD. 2001. Flow cytometric analysis of cell division history using dilution of carboxyfluorescein diacetate succinimidyl ester, a stably integrated fluorescent probe. Methods Cell Biol. 63:375–398 [DOI] [PubMed] [Google Scholar]
- 32.Connor JH, Lyles DS. 2005. Inhibition of host and viral translation during vesicular stomatitis virus infection. eIF2 is responsible for the inhibition of viral but not host translation. J. Biol. Chem. 280:13512–13519 [DOI] [PubMed] [Google Scholar]
- 33.Rajani KR, Pettit Kneller EL, McKenzie MO, Horita DA, Chou JW, Lyles DS. 2012. Complexes of vesicular stomatitis virus matrix protein with host Rae1 and Nup98 involved in inhibition of host transcription. PLoS Pathog. 8:e1002929. 10.1371/journal.ppat.1002929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scheu S, Dresing P, Locksley RM. 2008. Visualization of IFNbeta production by plasmacytoid versus conventional dendritic cells under specific stimulation conditions in vivo. Proc. Natl. Acad. Sci. U. S. A. 105:20416–20421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koyama AH. 1995. Induction of apoptotic DNA fragmentation by the infection of vesicular stomatitis virus. Virus Res. 37:285–290 [DOI] [PubMed] [Google Scholar]
- 36.Oehen S, Odermatt B, Karrer U, Hengartner H, Zinkernagel R, Lopez-Macias C. 2002. Marginal zone macrophages and immune responses against viruses. J. Immunol. 169:1453–1458 [DOI] [PubMed] [Google Scholar]
- 37.Shi Z, Cai Z, Sanchez A, Zhang T, Wen S, Wang J, Yang J, Fu S, Zhang D. 2011. A novel Toll-like receptor that recognizes vesicular stomatitis virus. J. Biol. Chem. 286:4517–4524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blasius AL, Beutler B. 2010. Intracellular Toll-like receptors. Immunity 32:305–315 [DOI] [PubMed] [Google Scholar]
- 39.Edelmann KH, Richardson-Burns S, Alexopoulou L, Tyler KL, Flavell RA, Oldstone MB. 2004. Does Toll-like receptor 3 play a biological role in virus infections? Virology 322:231–238 [DOI] [PubMed] [Google Scholar]
- 40.Stetson DB, Medzhitov R. 2006. Type I interferons in host defense. Immunity 25:373–381 [DOI] [PubMed] [Google Scholar]
- 41.Swiecki M, Gilfillan S, Vermi W, Wang Y, Colonna M. 2010. Plasmacytoid dendritic cell ablation impacts early interferon responses and antiviral NK and CD8(+) T cell accrual. Immunity 33:955–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fitzgerald-Bocarsly P, Feng D. 2007. The role of type I interferon production by dendritic cells in host defense. Biochimie 89:843–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ludewig B, Maloy KJ, Lopez-Macias C, Odermatt B, Hengartner H, Zinkernagel RM. 2000. Induction of optimal anti-viral neutralizing B cell responses by dendritic cells requires transport and release of virus particles in secondary lymphoid organs. Eur. J. Immunol. 30:185–196 [DOI] [PubMed] [Google Scholar]
- 44.Borderia AV, Hartmann BM, Fernandez-Sesma A, Moran TM, Sealfon SC. 2008. Antiviral-activated dendritic cells: a paracrine-induced response state. J. Immunol. 181:6872–6881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Phipps-Yonas H, Seto J, Sealfon SC, Moran TM, Fernandez-Sesma A. 2008. Interferon-beta pretreatment of conventional and plasmacytoid human dendritic cells enhances their activation by influenza virus. PLoS Pathog. 4:e1000193. 10.1371/journal.ppat.1000193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Faria PA, Chakraborty P, Levay A, Barber GN, Ezelle HJ, Enninga J, Arana C, van Deursen J, Fontoura BM. 2005. VSV disrupts the Rae1/mrnp41 mRNA nuclear export pathway. Mol. Cell 17:93–102 [DOI] [PubMed] [Google Scholar]
- 47.Harris RS, Hultquist JF, Evans DT. 2012. The restriction factors of human immunodeficiency virus. J. Biol. Chem. 287:40875–40883 [DOI] [PMC free article] [PubMed] [Google Scholar]