Abstract
A characteristic clinical feature of dengue virus infection is thrombocytopenia, though its underlying mechanism is not definitively determined. By adoptive transfer of human CD34+ fetal liver cells into immunodeficient mice, we have constructed humanized mice with significant levels of human platelets, monocytes/macrophages, and hepatocytes. Infection of these mice with both lab-adapted and clinical strains of dengue virus induces characteristic human hematological changes, including transient leukopenia and thrombocytopenia. We show that the specific depletion of human platelets is not mediated by antibodies in the periphery or reduced production of human thrombopoietin in the liver but reduction of human megakaryocytes and megakaryocyte progenitors in the bone marrow of the infected mice. These findings identify inhibition of platelet production in the bone marrow as a key mechanism underlying dengue-induced thrombocytopenia and suggest the utility of the improved humanized mouse model in studying dengue virus infection and pathogenesis in a human cell context.
INTRODUCTION
Dengue is an acute febrile illness caused by dengue virus (DENV), which is spread through mosquito vectors. Dengue manifests in a wide range of clinical symptoms and is usually accompanied by hematological changes, such as leukopenia and thrombocytopenia in mild cases and plasma leakage, hemorrhage, or organ impairment, such as liver damage, in severe cases (1). Even after decades of research, the cause of thrombocytopenia or platelet drop during dengue disease is still unclear.
Platelets are small (2 to 3 μm), anucleated cells which play a major role in homeostasis and coagulation. They are derived from megakaryocytes, which are large (30- to 100-μm), nucleated, polyploid cells. Megakaryocytes differentiate from hematopoietic stem cells in the bone marrow (BM) (2, 3) through progenitor cells known as megakaryocytic CFU (CFU-MK) that express CD34 and CD41 (4). Thrombopoietin (TPO) is the principal cytokine that drives the expansion and differentiation of the progenitors to megakaryocytes (3, 5) and is produced mainly by hepatocytes in the liver (6).
Two hypotheses have been proposed to explain thrombocytopenia during DENV infection: clearance of platelets from periphery and loss of platelet production in the BM. The peripheral mechanism is thought to involve antibody-mediated depletion where the antibody-opsonized DENV binds to platelets, which are then cleared by activated immune cells (7, 8). Another possibility is that antibodies to the viral nonstructural protein (NS1) cross-react with autoantigens expressed on platelets, which binds and tags them for clearance (9, 10). Alternatively, platelet production in the BM could be suppressed, although the evidence supporting this hypothesis is lacking due to difficulties in obtaining BM biopsy specimens from acute dengue patients. One report shows that the BM was hypocellular early during dengue disease but later became hypercellular, as if recovered from acute suppression (11). There is also evidence that DENV infection in an in vitro artificial BM reduced its ability to support hematopoiesis (12). Recently, evidence of direct infection in the BM was reported in a nonhuman primate model of DENV infection (13). Despite these significant efforts, the underlying mechanism of thrombocytopenia during dengue remains to be elucidated.
A major road block in the study and development of therapeutics for dengue is the lack of a robust small-animal model. The development of humanized mice (humice), which are immunodeficient mice stably reconstituted with human immune cells (14, 15), has made it possible to study DENV infection in a human cell context. Recently, DENV infection in NOD-scid Il2rg−/− (NSG) mice reconstituted with human immune cells has been reported with some clinical symptoms and immune responses (16–18). However, neither of these groups reported the presence of human platelets in their humice, precluding the induction and the study of thrombocytopenia and its underlying mechanisms.
Here, we have constructed humice by adoptive transfer of human CD34+ fetal liver cells into NSG mice that develop significant levels of human platelets, monocytes/macrophages, and hepatocytes. Infection of these mice with DENV serotype 2 (DENV2) virus recaptures some of the characteristic features of DENV infection in humans, including transient leukopenia and thrombocytopenia. In particular, we show that depletion of human platelets is not due to antibody-mediated clearance or a lack of human TPO but is instead due to specific inhibition of production of human megakaryocytes and their progenitor cells in the BM, thus elucidating the likely mechanism of thrombocytopenia during dengue disease.
MATERIALS AND METHODS
Construction of humanized mice.
Human fetal livers of 15 to 23 weeks of gestation were collected in accordance with the institutional ethical guidelines of the National University Hospital of Singapore and National University of Singapore (NUS). All women gave written informed consent for the donation of their fetal tissue for research. The human CD34+ cells were isolated from the fetal liver and purified using the CD34-positive selection kit (Stem Cell Technologies), and 2 × 105 cells were injected into sublethally irradiated NSG pups within 24 to 48 h of birth as previously described (19). All experiments involving mice were performed in compliance with the guidelines of the institutional committees at NUS and Massachusetts Institute of Technology.
Production of viruses and plaque assay.
DENV2 strains (NGC and 07K2861) were propagated in C6/36 mosquito cells in RPMI 1640 medium (GIBCO) with 10% fetal bovine serum (FBS) (Lonza) at 32°C. To concentrate the virus, 50 ml of the cell culture supernatant was loaded onto the VivaCell 100 centrifugal concentrator (Sartorius) and centrifuged at 2,000 × g for 10 min, and the supernatant remaining in the concentrator was aliquoted and stored at −80°C. For quantification of virus, BHK-21 cells were grown to a confluent monolayer in RPMI 1640 medium with 10% FBS (Lonza) and 1% penicillin-streptomycin-glutamine (GIBCO) in 24-well plates at 37°C. The virus was serially diluted in serum-free medium and then inoculated with the cells at 37°C with gentle shaking every 15 min for 1 h. Then it was replaced with RPMI 1640 medium containing 2% carboxymethyl cellulose and 2% FBS (Lonza; overlay medium) and kept at 37°C. After 5 days, the cells were fixed in 3% formalin in phosphate-buffered saline (PBS) for 1 h, washed, and stained with 0.1% crystal violet in 10% formalin solution for 1 h. Then the plates were washed with water and the plaques were counted. One PFU of the cell culture supernatant was observed to have close to 1,000 copies of viral RNA.
Infection of humanized mice.
Humanized mice were infected by injecting 1 × 107 PFU of the concentrated virus in 200 μl of RPMI 1640 medium through the tail vein. Control humanized mice reconstituted with the same batch of human CD34+ fetal liver cells were injected with 200 μl of plain RPMI 1640 medium. In some experiments, virus was heat inactivated (at 60°C for 1 h) and then injected into the humanized mice as the control. Blood, liver, spleen, lymph node (LN; axillary), and BM (femur) were collected from control and infected mice at different days postinfection (dpi) for flow cytometry assays, measuring viral RNA levels, amplification of infectious virus, alanine aminotransferase (ALT) and aspartate transaminase (AST) assays, platelet counts, and histology.
RNA extraction and quantitative RT-PCR.
Viral RNA was extracted from sera using a QIAamp viral RNA minikit (Qiagen) and from the liver, spleen, LN, and BM (femur) using the RNeasy minikit (Qiagen). Reverse transcription and amplification was done using the one-step quantitative reverse transcription-PCR (qRT-PCR)-based QuantiFast probe RT-PCR kit (Qiagen) with forward and reverse primers of the viral E gene, 5′ACACCACAGAGTTCCATCACAGA3′ and 5′CATCTCATTGAAGTCNAGGCC3′, respectively, along with the 6-carboxyfluorescein (FAM)-conjugated probe sequence CGATGGAATGCTCTC. The synthetic DNA containing the sequences containing both the primers and the probe (5′ACACCACAGAGTTCCATCACAGAAGCAGAACTAACAGGCTATGGCACTGTCACGATGGAATGCTCTCCGAGAACGGGCCTCGACTTCAATGAGATG-3′) was serially diluted to achieve the standard curve. qRT-PCR was performed using the CFX96 real-time system (Bio-Rad). The sensitivity of the qRT-PCR was around 100 copies of viral RNA.
To quantify the levels of hTPO transcript, total RNA was extracted from the liver of NSG mice, uninfected and infected humanized mice, and human fetal liver. The amount of RNA was quantified using NanoDrop 1000 (Thermo Scientific). One microgram of RNA was converted to cDNA using an iScript cDNA synthesis kit (Bio-Rad) and then amplified using the SsoFast Eva Low Rox kit (Bio-Rad) according to the manufacturer's instructions in the CFX96 real-time system (Bio-Rad). The forward and reverse primers used for hTPO were 5′AACTGCAAGGCTAACGCTGT3′ and 5′GACATGGGAGTCACGAAGCA3′, respectively, and for hGAPDH were 5′CGCCCCACTTGATTTTGGA3′ and 5′TTGCCATCAATGACCCCTTCA3′, respectively. The PCR products were confirmed by electrophoresis on a 2.5% agarose gel. The relative fold change in hTPO expression was calculated using the comparative cycle threshold (CT) method.
Amplification of infectious virus from sera in the C6/36 cell line.
Serum from infected mice was diluted in plain RPMI 1640 to a final volume of 1 ml and incubated with a monolayer of C6/36 mosquito cells grown in a T25 flask for 1 h at 37°C with gentle shaking every 15 min. The medium was replaced with 5 ml of fresh RPMI 1640 medium with 2% FBS, and the culture was kept in a 28°C incubator for 5 days. The supernatant was removed and centrifuged at 2,000 rpm for 10 min. A total of 100 μl of the supernatant was taken for plaque assay and RNA extraction, and the rest was loaded to a Vivaspin 20 (Sartorius). The supernatant was centrifuged at 5,000 × g for 5 to 7 min until only 1 ml of the medium remained. The concentrated supernatant was then incubated with a monolayer of C6/36 mosquito cells as described above. This was repeated for a total of three rounds to amplify infectious virus in the sera.
Western blotting.
C6/36 cells incubated with serum from infected mice (7 dpi) or viral cell culture supernatant were lysed with 2× Laemmli sample buffer containing bromophenol blue, and the protein concentration was determined by the Bio-Rad protein assay kit. A total of 4 μg of protein was separated on a 10% SDS-PAGE gel and transferred to a nitrocellulose membrane (Bio-Rad) using the wet-transfer technique. The membrane was blocked with 5% skim milk in PBS (blocking buffer) for 1 h at room temperature and incubated with rabbit anti-dengue NS1 primary antibody (GeneTex) (1:1,000 in blocking buffer) overnight at 4°C. The membranes were washed and subsequently incubated with horseradish peroxidase (HRP)-conjugated anti-rabbit secondary antibody (Dako) (1:3,000 in blocking buffer) for 1 h at room temperature. Detection was performed using the enhanced chemiluminescence (ECL) prime detection reagents (GE Healthcare).
Antibodies and flow cytometry analysis.
Antibodies specific for human CD34 (581), CD45 (HI30), CD14 (HCD14), CD19 (HIB19), CD3 (HIT3A/OKT3), CD4 (OKT4), CD8 (HIT8A), CD56 (HIT56), and CD41 (HIP8) and mouse CD45.1 (A20) and CD41 (MWReg30) were purchased as fluorescein isothiocyanate (FITC), phycoerythrin (PE), allophycocyanin (APC), or PE/Cy7 conjugates from BioLegend Inc. Blood was lysed of red blood cells (RBCs) by incubating with ACK lysis buffer (GIBCO) for 10 min. After being washed and filtered with 100-μm-pore-size nylon mesh (SEFAR), the single-cell suspension (SCS) was collected. Livers were grinded through a 100-μm-pore-size stainless steel mesh and centrifuged at 50 × g for 5 min to remove hepatocytes, and SCS was prepared by Percoll gradient centrifugation. Spleen and LN were gently mashed between the coarse surfaces of two glass slides in RPMI 1640 medium, filtered, and lysed of RBCs, and SCS was collected. Cells from the BM were gently flushed out, lysed of RBCs, and filtered, and SCS was prepared. Cells were suspended in 80 μl of flow cytometry (FC) buffer (PBS containing 0.5% bovine serum albumin [BSA] and 0.02% sodium azide) and stained with fluorophore-conjugated antibodies for 30 min on ice. The cells were then washed, resuspended in FC buffer containing DAPI (4′,6-diamidino-2-phenylindole), and analyzed using an LSRII flow cytometer (Becton Dickinson). A total of 100,000 to 1,000,000 events were collected, and the data were analyzed using the FlowJo software (FlowJo).
To count platelets, we mixed 10 μl of whole blood from mice with 600 μl FC buffer. Antibodies specific for human CD41 and mouse CD41 were added and incubated for 10 min. A total of 25 μl of CountBright absolute counting beads (Invitrogen) was added to each sample and analyzed until 1,000 beads were recorded per sample. The number of human or mouse platelets was calculated as follows: count of platelets/μl of whole blood = (target platelet events/beads event) × (bead count/10 μl). All FC analysis was done using an LSRII flow cytometer (Becton Dickinson).
ALT and AST measurement and ELISA.
ALT and AST in the sera from mice at 7 dpi were measured using a Cobas C111 analyzer (Roche) in the comparative medicine in-house veterinary diagnostic laboratory, NUS. Human albumin in sera was quantified using the human albumin enzyme-linked immunosorbent assay (ELISA) quantification kit (Bethyl Laboratories). The presence of human dengue-specific IgM and IgG was measured in sera using the dengue IgG capture ELISA kit (Panbio) and dengue IgM capture ELISA kit (Panbio).
Histology.
For human albumin staining, liver sections of 5-μm thickness were incubated with primary rabbit anti-human albumin (Abcam) at a 1:100 dilution at 4°C overnight. The endogenous peroxidases were inactivated by using 3% H2O2, and the slides were incubated with HRP polymer conjugate followed by 3,3′-diaminobenzidine (DAB) solution. They were then washed and mounted on a coverslip with Permount medium (Fisher Scientific) and scanned with the MIRAX MIDI system with bright-field illumination (Carl Zeiss). For hematoxylin and eosin (H&E) staining, the liver sections were stained with hematoxylin solution, washed, stained with aqueous eosin, and serially dehydrated in an increasing concentration of ethanol (from 70 to 100%). The slides were then mounted on a coverslip and scanned using a MIRAX MIDI system with bright-field illumination (Carl Zeiss). For immunofluorescence, liver sections were incubated with mouse anti-dengue E antibody (D1-4G2-4-15; ATCC) at a 1:10 dilution at 4°C overnight. The slides were washed and stained with the Alexa Fluor 488-conjugated donkey anti-mouse secondary antibody (Invitrogen) for 1 h at room temperature. After being washed, the slides were stained with SlowFade gold reagent with DAPI (Invitrogen) and covered with a glass coverslip. The slides were scanned and visualized with the MIRAX MIDI system with fluorescence illumination (Carl Zeiss). Images were analyzed using the MIRAX viewer software.
Statistical analysis.
Student's t test was used to determine statistical significance between various samples. P values of <0.05, 0.01, and 0.001 were considered to be 95%, 99%, and 99.9% significant, respectively. All error bars show standard errors of the means (SEM).
RESULTS
Generation of human platelets in humanized mice.
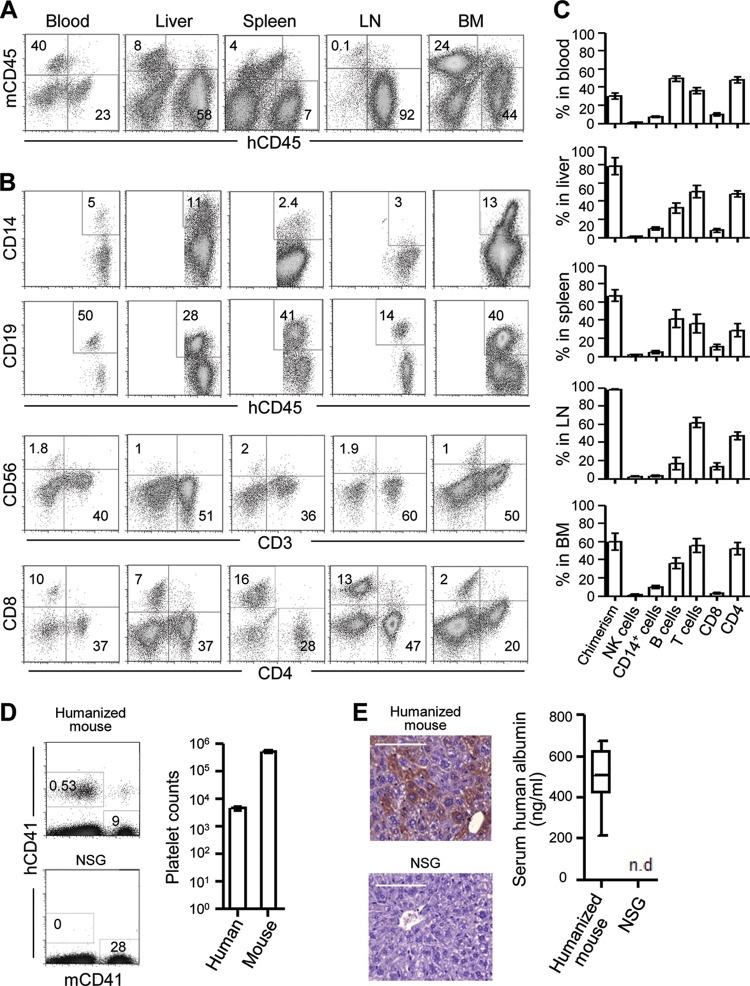
Humice were constructed by injecting sublethally irradiated newborn NSG pups with CD34+ cells from human fetal liver. Twelve to 15 weeks later, peripheral blood mononuclear cells (PBMCs) were analyzed for mouse CD45 (mCD45) and human CD45 (hCD45), and the reconstitution level of human leukocytes (chimerism) was calculated as follows: % hCD45+ cells/(% hCD45+ cells + % mCD45+ cells). Humice with 20% or more chimerism in the blood were used for subsequent experiments. Detailed analyses of humice showed reconstitution of human leukocytes in various organs, including blood, liver, spleen, LN, and BM (Fig. 1A). Among human leukocytes, CD19+ B cells and CD3+ T cells, including both CD4+ and CD8+ subsets, were most abundant, followed by CD14+ monocytes/macrophages and a low level of CD56+ NK cells (Fig. 1B and C), consistent with previous reports (14, 20, 21). Notably, human CD41+ platelets were consistently detected in the blood of the humice (Fig. 1D). In addition, there was the presence of human hepatocytes as indicated by positive staining of human albumin-expressing cells in the liver sections and human albumin in the serum (Fig. 1E) as reported previously (19). The presence of human monocytes/macrophages and platelets makes the humice suitable for DENV infection and for studying dengue-induced thrombocytopenia.
Fig 1.
Human cell reconstitution in humanized mice. (A to C) Single-cell suspensions were prepared from the indicated tissues of humice 12 to 15 weeks after reconstitution, stained for various combinations of cell surface markers, and analyzed by flow cytometry. (A) Representative hCD45 versus mCD45 staining profiles of blood, liver, spleen, LN, and BM gating on live cells (DAPI negative); (B) representative staining profiles for CD14 versus CD45, CD19 versus CD45, CD56 versus CD3, and CD8 versus CD4 gating on hCD45+ cells in various tissues; (C) average percentages of human CD56+ NK cells, CD14+ monocytes/macrophages, CD19+ B cells, CD3+ T cells, CD8+ T cells, and CD4+ T cells in various tissues. Human leukocyte chimerism is also shown for each tissue (n = 10 to 15). (D) Representative hCD41 versus mCD41 staining profile of whole blood of a humanized mouse and plain NSG mouse (left) and the average counts of human and mouse platelets per microliter of blood among 28 humice (right); (E) representative immunohistochemical staining of human albumin (brown) of a liver section of a humanized mouse and plain NSG mouse (left; scale bar, 100 μm) and average values of the human albumin in the serum of humice (n = 16) and plain NSG mice (n = 5) as measured by ELISA (right). n.d., not detectable. Data for panels A to D were generated from humice reconstituted with CD34+ cells from at least three different fetal liver samples. The numbers in the flow cytometry plots indicate percentages of cells in the gated regions. Error bars are SEM.
Systemic DENV infection in humanized mice.
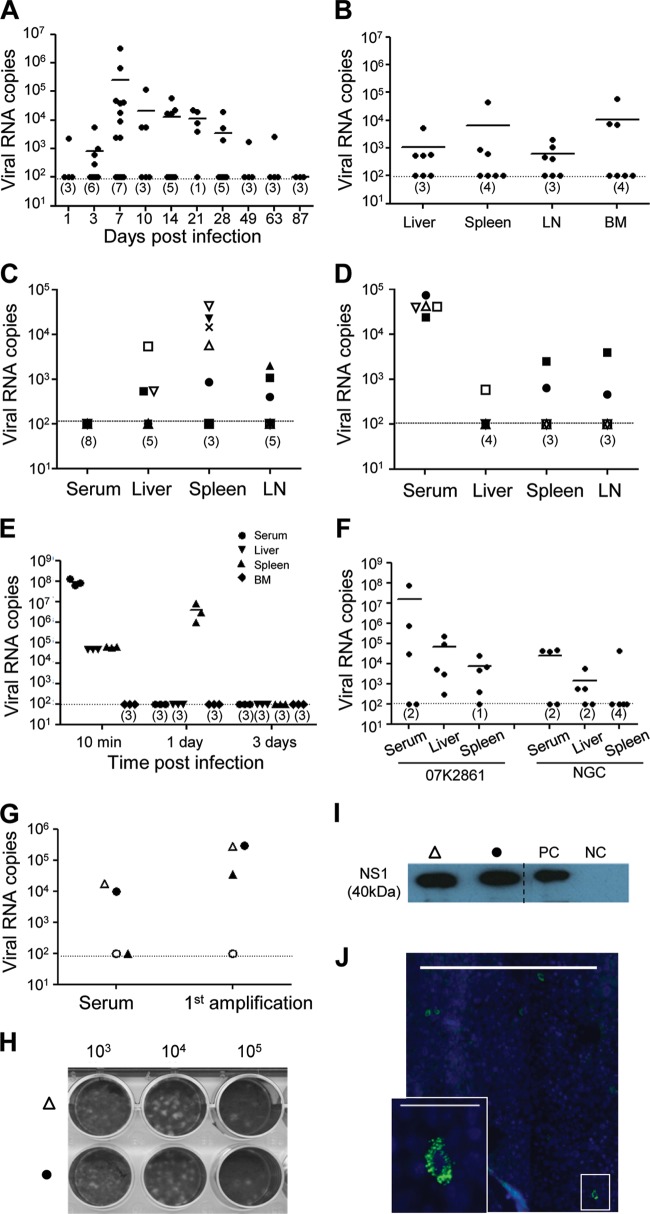
Humice were infected intravenously (i.v.) with 1 × 107 PFU of DENV2 NGC virus and analyzed on different days postinfection (dpi). Viremia in the serum was measured by qRT-PCR for the envelope E gene of the viral RNA. Viremia was detected in the infected mice from 3 dpi, peaked at 7 dpi, persisted until 28 dpi, and then was detected only in one mouse by 49 and 63 dpi (Fig. 2A). In a significant proportion of mice, the viremia level was below the detection limit of the qRT-PCR of 100 copies per reaction. Viral load was also measured by qRT-PCR in the selected tissues at 7 dpi, and significant levels were detected in the liver, spleen, LN, and BM of approximately 50% of the mice (Fig. 2B). Notably, some infected mice with viremia below the detection limit in the blood had detectable levels of DENV in the tissues and vice versa (Fig. 2C and D), suggesting that most of the mice were infected, although viremia was below the detection limit by qRT-PCR in the serum. As a control, NSG mice (nonhumanized) were injected with NGC virus (1 × 107 PFU), and viral levels in the serum, liver, spleen, and BM were measured by qRT-PCR for 10 min at 1 dpi and every 3 days until 30 dpi. Ten minutes postinjection, viral RNA was detected in the serum, liver, and spleen but not in the BM (Fig. 2E). Viral RNA was detected only in the spleen at 1 dpi, and none was detected in any of the tissues at 3 to 30 dpi, suggesting that infection with NGC virus occurs only in the presence of human cells in humice. We further validated DENV infection in humice with a clinical isolate from the early DENV infection and outcome (EDEN) study, DENV2 07K2861 (22). Humice were injected i.v. with 1 × 107 PFU of 07K2861 or NGC virus and analyzed for virus levels in various tissues 7 dpi. Among the five 07K2861 virus-infected mice, although viremia was detected only in three mice, DENV RNA was detected in all five liver samples and four of the five spleen samples (Fig. 2F). Infectious viral particles were amplifiable in the serum of infected humice 7 dpi (Fig. 2G to I), and the viral E protein was detected in the infected cells in the liver at 7 dpi (Fig. 2J). Together, these data show that a systemic infection can be established in humice with both lab-adapted and clinical strains of DENV, and the productive infection occurs only in the presence of human cells.
Fig 2.
Dengue virus infection in humanized mice. (A and B) Systemic DENV infection in humanized mice. Humice were injected with 1 × 107 PFU of NGC virus i.v. Mice were sacrificed at the indicated dpi, and RNA was isolated from serum, liver, spleen, LN, and BM. The levels of viral RNA in different tissues were measured by qRT-PCR. Shown are numbers of viral RNA copies/ml of serum at different dpi (A) and numbers of viral RNA copies/mg of tissues or per femur at 7 dpi (B). (C and D) Mice with undetectable viremia in serum have detectable DENV RNA in organs and vice versa. RNA was isolated from serum, spleen, liver, and LN from infected mice on 7 dpi and measured by qRT-PCR. Shown are viral RNA levels in different tissues from mice with undetectable viremia in the serum (C) and from mice with detectable viremia in the serum (D). Different symbols represent different mice, and the same symbol is used to indicate the different tissue samples of the same mouse. (E) NGC virus does not cause infection of nonhumanized NSG mice. Plain NSG mice were injected i.v. with 1 × 107 PFU of NGC viruses. Three mice were sacrificed 10 min postinfection, 1 or every 3 dpi until 30 dpi. RNA was isolated from serum, liver, spleen, and BM and used to measure viral RNA levels by qRT-PCR as described above. Numbers of viral RNA copies per milliliter of serum or per milligram of spleen or liver or per femur are shown. (F) Comparison of infection of humice by clinical (07K2861) and lab-adapted (NGC) strains. Humice (5 per group) were injected with 1 × 107 PFU of DENV2 07K2861 or NGC virus. Seven days postinfection, RNA was isolated from serum, liver, and spleen and used for measuring viral RNA levels as described above. Shown are numbers of viral RNA copies per milliliter of serum or per milligram of spleen or liver. (G to J) Productive dengue virus infection in humanized mice. Humanized mice were injected with 1 × 107 PFU of NGC virus i.v. Seven days postinfection, sera were collected and added into C6/36 cells and cultured for 4 to 5 days (1st amplification). Culture supernatants were concentrated and blind-passaged twice in C6/36 cell cultures. (G) Comparison of viral RNA levels in sera or supernatants after first amplification. (H) Two culture supernatants from the third amplification (open triangle and closed circle) were used in the plaque assay to detect the presence of infectious viruses. Shown is a photograph of the plaque assay plate. The numbers indicate dilution of culture supernatants used in the plaque assay. (I) Cells from the third amplification were lysed, and the presence of viral NS1 protein was detected by Western blotting. The positive control (PC) was the lysate of C3/36 cells cultured with NGC virus (104 PFU). The negative control (NC) was the lysate of C3/36 cells cultured with serum from an uninfected humanized mouse. Liver sections from one of the infected humanized mice (open triangle) were stained with antibody specific for the viral E protein (green) and DAPI (blue). (J) A representative immunofluorescence image with a scale bar of 500 μm and a higher amplification of the boxed area with a scale bar of 50 μm is shown. Each symbol represents serum or one tissue from one mouse. The horizontal lines indicate the average viral copies in each tissue or serum collected from mice at the same day postinfection. The dotted lines indicate the sensitivity of PCR at 100 copies. The numbers in the parentheses indicate the number of mice where viral copies were below the detection limit. Data in panels A, B, C, and D are from at least three independent experiments. Data in panels E to I are from one experiment each.
Characteristic responses in dengue virus-infected humanized mice.
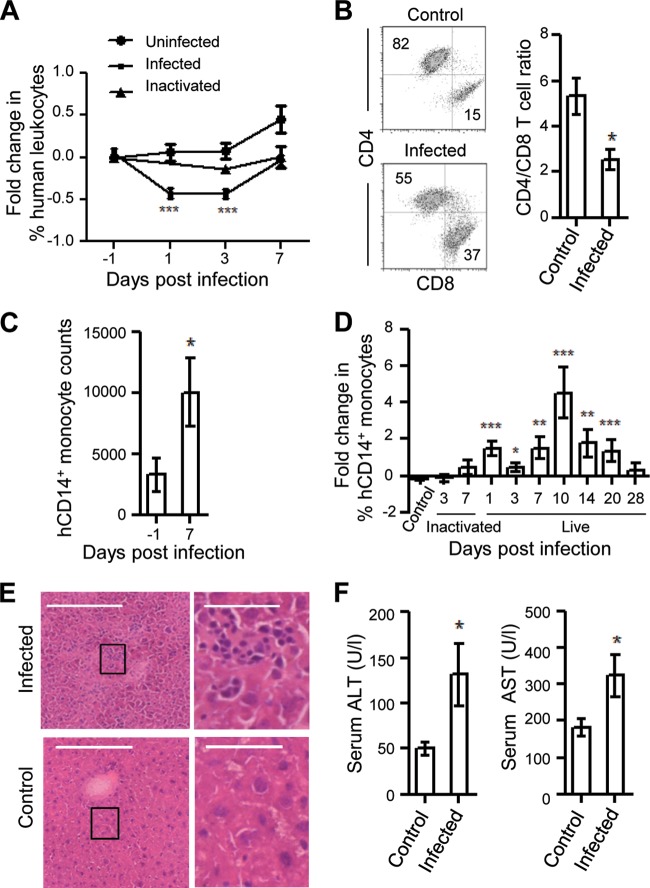
The responses of human immune cells to DENV infection were characterized in the infected humice. One consistent observation was a transient decrease in the percentages of human CD45+ cells in the blood of the infected mice 1 and 3 dpi (Fig. 3A). This transient leukopenia was not seen in the blood of uninfected or heat-inactivated virus-injected humice. At the peak of infection (7 dpi), there was no significant change in the percentages of human CD3+ T, CD19+ B, and CD56+ NK cells in most tissues (Fig. 4A to C), except for a significant decrease in the CD4-to-CD8 ratio in the BM (Fig. 3B) and in the percentage of B cells in the blood (Fig. 4C). However, both the number and the percentage of hCD14+ monocytes were significantly increased in the blood at 7 dpi (Fig. 3C and Fig. 4C), whereas the percentage of hCD14+ monocytes was decreased significantly in the BM (Fig. 4C). Further analysis during the entire course of infection showed that the increase in the percentage of hCD14+ monocytes in the blood was detected as early as 1 dpi and lasted until 20 dpi (Fig. 3D). As controls, uninfected humice and humice injected with heat-inactivated virus did not show any increase in the percentage of hCD14+ monocytes in the blood on 1 and 3 days postinjection.
Fig 3.
Dengue virus-induced leukopenia, hepatitis, and increase in monocyte counts in infected humanized mice. (A) Transient leukopenia of human CD45+ cells in the blood of the infected mice. Humice were not infected (n = 15), infected with live NGC virus (n = 15), or injected with heat-inactivated virus (n = 5). PBMCs were obtained from each mouse before infection and at 1, 3, and 7 dpi and stained for hCD45 and mCD45, followed by flow cytometry. The percentage of hCD45+ cells of each mouse at various days postinfection was normalized to the percentage of hCD45+ cells in the same mouse before infection. Shown are the averages over time. (B) Decrease in the CD4-to-CD8 T cell ratio in the BM. Single-cell suspensions were prepared from the BM of the infected mice at 7 dpi and from age-matched, uninfected humice, stained for human CD3+, CD4+, and CD8+, and analyzed by flow cytometry. Shown are representative CD4 versus CD8 staining profiles of CD3+ human T cells (left) and the ratio (means ± SEM) of CD4+ to CD8+ T cells in uninfected (n = 5) and infected (n = 7) mice (right). The numbers in the plots show the percentages of cells in the gated regions. (C) Increase in hCD14+ monocyte numbers in the blood of infected humice. The number of hCD14+ monocytes per milliliter of blood was quantified at 7 dpi by multiplying the percentages of hCD14+ cells with the total number of mononuclear cells. Shown are average numbers of hCD14+ cells before and 7 dpi of the same group of humice (n = 13). (D) Increase in percentages of the hCD14+ monocytes in the blood of infected mice during the course of infection. Humice were not infected (n = 20), infected with live NGC virus (n = 10 to 15), or injected with heat-inactivated NGC virus (n = 5). PBMCs were stained for hCD14 and hCD45 and analyzed by flow cytometry before and at the indicated days postinfection. The percentage of hCD14+ cells of each mouse at various days postinfection was normalized to the percentage of hCD14+ cells in the same mouse before infection. Shown are the averages over time. (E) Infiltration of immune cells in the liver of the infected humice. Liver sections from the infected humice (n = 3) at 7 dpi and an uninfected humanized mouse were stained with H&E. Representative images of the liver sections from one infected and uninfected humice are shown (left). The boxed areas are shown in higher magnification (right). Scale bars are 200 μm (left) and 50 μm (right). (F) Increase of liver transaminases in the serum of infected humice. The levels of ALT and AST in the sera of the uninfected humice (n = 5) and infected humice (n = 7) at 7 dpi were measured. The average levels of ALT (left) and AST (right) are shown as units per liter of serum. Data for panels A and D were a compilation from at least four different experiments with humice reconstituted with CD34+ cells from two different fetal livers. Data in panels B, C, E, and F are from one experiment each. *, P < 0.05; **, P < 0.01; ***, P < 0.001, compared to control or the −1 dpi sample.
Fig 4.
Characterization of human cells in infected humanized mice. Single-cell suspensions were prepared from the indicated tissues of the infected humanized mice at 7 dpi, stained for various combinations of cell surface markers, and analyzed by flow cytometry. (A) Representative hCD45 versus mCD45 staining profiles of blood, liver, spleen, LN, and BM gating on live cells (DAPI negative). (B) Representative staining profiles for CD14 versus CD45, CD19 versus CD45, CD56 versus CD3, and CD8 versus CD4 gating on hCD45+ cells in various tissues. (C) Average percentages of human CD56+ NK cells, CD14+ monocytes/macrophages, CD19+ B cells, CD3+ T cells, CD8+ T cells, and CD4+ T cells in various tissues. Human leukocyte chimerism is also shown for each tissue (n = 10 to 15). Data for panels A to C are a compilation from three different experiments with humanized mice reconstituted with CD34+ cells from at least two different fetal liver samples. The numbers in the plots indicate percentages of cells in the gated regions. Error bars are SEM. *, P < 0.05 compared to the corresponding uninfected samples in Fig. 1C.
Associated with immune responses to DENV infection, mild infiltration of immune cells was detected in the liver sections of the infected but not uninfected humice at 7 dpi (Fig. 3E). Consistently, the levels of liver transaminases (ALT and AST) were significantly increased in the serum of the infected mice 7 dpi, indicating liver damage (Fig. 3F). However, hematocrit values did not change after infection, indicating the absence of plasma leakage. These data show that as in humans, DENV infection induces transient leukopenia, elevation of monocytes, and liver damage in humice.
DENV infection induces human thrombocytopenia.
To determine if thrombocytopenia occurred in infected humice, blood was collected from the infected mice before infection and at 1, 3, 7, and 10 dpi, and human and mouse platelets, specifically identified by hCD41 and mCD41, respectively, were counted by flow cytometry (Fig. 5A). Notably, the percentages of human platelets in the blood dropped from 0.37% before infection to 0.03% (10-fold) by 7 dpi in the same mouse. Correspondingly, the counts of human platelets/μl of blood decreased significantly at all four time points compared to the counts before infection (Fig. 5B). The observed thrombocytopenia was specific for the human platelets, as the mouse platelet counts in the same mouse did not change significantly (Fig. 5C). There was also a significant reduction in human platelet counts in mice having below-detectable viremia in their serum at 7 dpi, compared to that of the uninfected control. This reduction was not seen in the mice injected with heat-inactivated virus (Fig. 5D). When human platelet counts (at 3 and 7 dpi) were plotted as a function of viremia level of the same mouse at 7 dpi, there was a negative correlation between the level of viremia and the human platelet counts as observed in dengue patients. The correlation between viremia and human platelet was more significant with the counts at 3 dpi than at 7 dpi (Fig. 5E). These data suggest that DENV infection in humice induces a human-specific thrombocytopenia and that thrombocytopenia appears to be a more sensitive indication of DENV infection in humice.
Fig 5.
Thrombocytopenia of human platelets in the dengue-infected humanized mice. (A to C) Significant decrease in human platelets but not mouse platelets in the blood of the infected humice. Ten microliters of whole blood from humice before infection and 1, 3, 7, and 10 dpi was stained for hCD41 and mCD41, and human and mouse platelets counted by flow cytometry using fluorescent beads as described in Materials and Methods. (A) Representative hCD41 versus mCD41 staining profiles of the whole blood of a humanized mouse before and 3 and 7 dpi are shown. The numbers indicate percentages of cells in the gated regions. The average counts (±SEM) of human (B) and mouse (C) platelets per microliter of blood of humice (n = 15) before and at different days postinfection. (D) Induction of human thrombocytopenia in mice with below-detectable viremia. The average counts (±SEM) of human platelets per microliter of blood at 7 dpi are shown for the uninfected mice (control) (n = 25), infected mice with detectable levels of viremia in serum (n = 17), infected mice with below-detectable levels of viremia in serum (n = 15), and mice injected with heat-inactivated virus (n = 5). (E) Correlation between human platelet counts and viremia. The human platelet counts per microliter of blood at 3 dpi and 7 dpi are plotted as a function of viremia (viral RNA copies/ml of serum) of the same mice at 7 dpi with round and square symbols, respectively. Each symbol represents one mouse. The straight and dotted lines are the linear regression plotted between the viremia and human platelet counts at 3 and 7 dpi with Pearson's r values of −0.6 (P value of 0.04) and −0.5 (P value of 0.09), respectively. The dark line is the average of the human platelet count per microliter of blood in uninfected mice. Data for panels A to E are a compilation from at least three different experiments, with humice reconstituted with CD34+ cells from two different fetal liver samples. *, P < 0.05; **, P < 0.01; ***, P < 0.001; n.s., not significant, compared to control or the −1 dpi sample.
Specific inhibition of production of human megakaryocytes in the bone marrow.
To test whether the observed human thrombocytopenia was caused by peripheral clearance mediated by antibodies, the presence of dengue-specific human IgM and IgG antibodies was measured in the sera of the infected mice 1, 3, 10, 14, 21, 28, 42, 62, and 84 dpi (3 mice per time point). Dengue-specific IgM was detected in 5 of the 27 mice: two mice each at 3 and 42 dpi and one mouse at 84 dpi (data not shown). None of the mice were positive for dengue-specific IgG. These results are consistent with previous reports showing very poor antibody responses in humanized NSG mice (17, 18, 23). Thus, antibody-mediated peripheral clearance is unlikely to be a significant cause of human thrombocytopenia in the infected humice.
Generation of human platelets in the humice might require human TPO (hTPO), which is produced mainly by hepatocytes (6). The observed thrombocytopenia could be because of a decreased production of hTPO by human hepatocytes in the mouse liver due to DENV-induced liver damage and inflammation in the infected mice. To test this possibility, the levels of hTPO transcript were assayed by real-time PCR in the liver of uninfected humanized mice, infected humanized mice (7 dpi), nonhumanized NSG mice, and human fetal liver, using hGAPDH as an internal control. The PCR amplification product of hTPO was detected in the liver of all five uninfected humanized mice and all six infected humanized mice (Fig. 6A), and there was no significant difference in the level of hTPO transcripts between the infected and uninfected humanized mice (Fig. 6B). Thus, human thrombocytopenia following DENV infection is unlikely due to a reduced expression of hTPO.
Fig 6.

Comparison of human TPO transcript levels in humanized mice with and without infection. (A and B) Livers were harvested from uninfected and 7-dpi humanized mice for RNA isolation. The relative levels of hTPO and hGAPDH transcripts were quantified using qRT-PCR. As controls, RNA from human fetal liver (FL) and from the liver of nonhumanized NSG mice (NSG) was used or qRT-PCR was done without the addition of the RNA template (NTC). (A) PCR products were separated on an agarose gel, showing a 172-bp hGAPDH product and an 80-bp hTPO product. The multiple samples represent uninfected humanized mice (n = 4), infected humanized mice (n = 6), human fetal liver (n = 1), NSG mice (n = 2), and no template control (NTC). The first lane is the molecular weight marker. (B) Comparison of the relative hTPO transcript level (normalized to hGAPDH) in the liver of the infected and uninfected humanized mice. Shown are the average fold change in the infected mice (n = 6) and the fold change in uninfected mice (which is equal to 1). Error bars are means ± SEM.
To investigate if the observed thrombocytopenia could be due to inhibition of platelet production in the BM of the infected humice, we analyzed the frequencies of megakaryocytes (platelet precursors) in the BM of uninfected and infected humice. Human megakaryocytes were identified by their large size (high forward scatter [FSC]) and expression of hCD41 (Fig. 7A). Compared to that of hCD41+ FSChigh megakaryocytes in uninfected mice, the frequencies of human megakaryocytes were significantly decreased 1, 3, 7, and 10 dpi in the BM of infected mice (Fig. 7B). Further, human hematopoietic progenitor cells in the BM cells were analyzed by staining for human CD45, CD34, and CD41. Although the frequencies of the total hCD45low hCD34+ progenitor cells in the BM remained similar before and after infection, the frequencies of hCD45low hCD34+ hCD41+ cells, which give rise to CFU-MK (4), decreased significantly 1, 3, 7, and 10 dpi (Fig. 7C and D). Thus, the dengue-induced thrombocytopenia is associated with a specific depletion of human megakaryocytes and their progenitors in the BM.
Fig 7.

Depletion of human megakaryocyte and their progenitors in the BM of infected humanized mice. Single-cell suspensions were prepared from the BM of uninfected and infected humice before infection and 1, 3, 7, and 10 dpi, stained for human CD45, CD34, and CD41, and analyzed by flow cytometry. (A) Shown are representative profiles of hCD41 versus FSC of all live cells, hCD34 versus hCD45 of all live cells, and hCD34 versus hCD41 gating on hCD34+ hCD45+ cells. The numbers indicate percentages of cells in the gated regions. (B to D) Average percentages of FSChigh hCD41+ human megakaryocytes (B), hCD45low hCD34+ human progenitor cells (C), and hCD45low hCD34+ hCD41+ human megakaryocyte progenitors (D) in the BM of the uninfected mice (n = 10 to 15) and infected mice (n = 10 to 15) on the indicated days postinfection. Data for panels A to D are a compilation from two different experiments with humice reconstituted with CD34+ cells from two different fetal liver samples. *, P < 0.05; **, P < 0.01; n.s., not significant, compared to control sample.
DISCUSSION
In this study, we have constructed humice with significant levels of human platelets, hepatocytes, monocytes/macrophages, and other immune cells. Injection of both lab-adapted and clinical strains of DENV2 leads to a systemic infection with characteristic symptoms, such as transient leukopenia, elevation of blood monocyte levels, thrombocytopenia, and liver damage. In particular, we show that the specific depletion of human platelets is not mediated by antibodies or a reduced production of hTPO in the liver but by reduction of human megakaryocytes and progenitor cells in the BM of the infected mice. These findings identify the likely mechanism of thrombocytopenia and suggest the utility of humice in studying DENV infection in a human cell context.
Compared to the previous reports of DENV infection in humanized NSG mice (16–18), our humanized mouse model of DENV infection not only shares many common features but also has some unique advantages. Jaiswal et al. used the DENV2 NGC strain (18), where viremia was detected till 21 dpi in 80% of the mice and the DENV RNA was detected in spleen, BM, and liver, while Mota and Rico-Hesse used different strains of DENV2 (16, 17), where viremia was detected up to 18 dpi in all the infected mice as well as in the BM and spleen. Similar to the previous studies, i.v. administration of both a lab-adapted and a clinical isolate of DENV2 induced systemic infection in the spleen, LN, BM, and liver in our model. Viremia was detected in a significant proportion of mice even at 28 dpi in NGC-infected humice, where human cells but not mouse cells were infected, in contrast to NGC's original isolation by serial passaging in the brains of suckling mice (24). This may be due to the decades of lab adaptation of the virus in mosquito cells. In humans, DENV causes an acute infection, and viremia disappears in about a week. The much longer period of viremia in the humice could be due to the slow clearance of the infection associated with the poor immune responses in humice as indicated by the lack of significant levels of dengue-specific antibodies.
Engraftment of NSG mice with human fetal liver CD34+ cells is the key difference between our and previous studies (where engraftment was done with human cord blood CD34+ cells), and this had resulted in the generation of human hepatocytes in the mouse liver (19). It has been reported that in fetuses, the liver accounts for up to 95% of TPO production (25). Thus, it is likely that production of TPO by human hepatocytes leads to a higher level of human platelets in mice reconstituted with human fetal liver CD34+ cells as opposed to mice reconstituted with cord blood CD34+ cells. This major advantage in our model leads to the induction of thrombocytopenia in infected mice which is specific to human platelets despite the presence of numerous mouse platelets. This is in contrast to a drop of total platelets in infected humice reported by Mota and Rico-Hesse (16, 17), while Jaiswal et al. (18) did not observe any change in platelets even though the same strain of DENV was used. Interestingly, in our model, thrombocytopenia was observed in humice even with viremia levels below the detection limit. This raises the possibility that thrombocytopenia could be used as a more sensitive marker for monitoring infection during testing of potential therapeutics in our model. Also, DENV infection induces additional hematological changes such as transient leukopenia in the blood and elevated levels of blood monocytes in our model, as in humans. We observed liver damage indicated by elevated levels of liver transaminases (ALT and AST) in the circulation of infected humice. Liver damage is believed to be caused by the infiltrating immune cells (26), which was also observed in our model. Finally, we observed a transient decrease of the CD4-to-CD8 ratio in human T cells in the BM of infected mice, which has also been reported in the blood of patients during the severe phase of infection (27). Thus, our humanized mouse model of DENV infection captures some key clinical features of DENV infection, including thrombocytopenia.
The depletion of human platelets in our model enabled us to investigate the underlying mechanisms of thrombocytopenia. Two mechanisms, antibody-mediated platelet clearance in the periphery and decreased platelet production in the BM, have been proposed to account for thrombocytopenia in humans. Although the involvement of the BM has been suggested nearly 5 decades ago (11), until recently, the focus has been primarily on the mechanism of peripheral clearance mediated by dengue virus-specific or cross-reactive antibodies, which mark the platelets for clearance. We excluded antibody-mediated depletion as a significant contributing factor to thrombocytopenia in humice because of poor antibody induction, consistent with previous reports in humice (23, 28–30). Our result, however, does not exclude the involvement of antibodies in platelet depletion in humans, as dengue-specific antibodies are induced following infection. We did not detect plasma leakage in the infected mice, and thus the loss of platelets due to plasma leakage can also be excluded.
Our study points to reduction of platelet production as the major cause of thrombocytopenia in humice. Following infection, we detected a significant drop in the level of megakaryocytes and the CD34+ CD41+ progenitors that give rise to megakaryocytes in the BM. In contrast, the level of hTPO transcripts in the liver did not change after DENV infection, suggesting that the reduction of megakaryocytes and their progenitors in the BM was not due to a lack of human TPO in infected mice. We did not detect viral RNA in the purified human megakaryocytes (data not shown), suggesting that either direct infection of megakaryocytes may not be a major cause of their depletion in our model or our qRT-PCR assay was not sufficiently sensitive (100 copies per reaction) to detect the infected megakaryocytes. We observed direct DENV infection and immune responses in the BM as indicated by the change of the CD4-to-CD8 T cell ratio, which might alter the BM microenvironment, resulting in inhibition of human megakaryocytopoiesis and therefore thrombocytopenia. While the precise details still need to be worked out, our findings show that the decreased production of platelets due to inhibition of megakaryocyte development is a key mechanism underlying DENV-induced thrombocytopenia in humice.
In summary, we have established a humanized mouse model of DENV infection that recaptures some of the key clinical symptoms in humans, including thrombocytopenia. Using this model, we have elucidated the mechanism underlying the depletion of human platelets, helping to gain insight into DENV-induced thrombocytopenia in humans. Although absence of a strong antibody response is a drawback of the current model, it has significant advantages over other DENV infection models, including the induction of thrombocytopenia and studying DENV infection in the context of human cells. Improving antibody response in the humanized mice will likely make the model even more useful for studying other aspects of DENV infection, such as antibody-dependent enhancement.
ACKNOWLEDGMENTS
We thank Jerry Chan for providing human fetal liver samples.
This research was supported in part by the National Research Foundation, Singapore, through the Singapore-MIT Alliance for Research and Technology's Infectious Disease IRG research program.
All authors have no conflicting financial or commercial interests.
Footnotes
Published ahead of print 21 August 2013
REFERENCES
- 1.World Health Organization 2009. Dengue guidelines for diagnosis treatment prevention and control. World Health Organization, Geneva, Switzerland: [PubMed] [Google Scholar]
- 2.Thon JN, Italiano JE. 2012. Platelets: production, morphology and ultrastructure. Handb. Exp. Pharmacol. 210:3–22 [DOI] [PubMed] [Google Scholar]
- 3.Avecilla ST, Hattori K, Heissig B, Tejada R, Liao F, Shido K, Jin DK, Dias S, Zhang F, Hartman TE, Hackett NR, Crystal RG, Witte L, Hicklin DJ, Bohlen P, Eaton D, Lyden D, de Sauvage F, Rafii S. 2004. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat. Med. 10:64–71 [DOI] [PubMed] [Google Scholar]
- 4.Tomer A. 2004. Human marrow megakaryocyte differentiation: multiparameter correlative analysis identifies von Willebrand factor as a sensitive and distinctive marker for early (2N and 4N) megakaryocytes. Blood 104:2722–2727 [DOI] [PubMed] [Google Scholar]
- 5.Kaushansky K. 2006. Lineage-specific hematopoietic growth factors. N. Engl. J. Med. 354:2034–2045 [DOI] [PubMed] [Google Scholar]
- 6.Wolber EM, Jelkmann W. 2002. Thrombopoietin: the novel hepatic hormone. News Physiol. Sci. 17:6–10 [DOI] [PubMed] [Google Scholar]
- 7.Wang S, He R, Patarapotikul J, Innis BL, Anderson R. 1995. Antibody-enhanced binding of dengue-2 virus to human platelets. Virology 213:254–257 [DOI] [PubMed] [Google Scholar]
- 8.Saito M, Oishi K, Inoue S, Dimaano EM, Alera MTP, Robles AMP, Estrella BD, Kumatori A, Moji K, Alonzo MT, Buerano CC, Matias RR, Morita K, Natividad FF, Nagatake T. 2004. Association of increased platelet-associated immunoglobulins with thrombocytopenia and the severity of disease in secondary dengue virus infections. Clin. Exp. Immunol. 138:299–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin CF, Lei HY, Liu CC, Liu HS, Yeh TM, Wang ST, Yang TI, Sheu FC, Kuo CF, Lin YS. 2001. Generation of IgM anti-platelet autoantibody in dengue patients. J. Med. Virol. 63:143–149 [PubMed] [Google Scholar]
- 10.Sun DS, King CC, Huang HS, Shih YL, Lee CC, Tsai WJ, Yu CC, Chang HH. 2007. Antiplatelet autoantibodies elicited by dengue virus nonstructural protein 1 cause thrombocytopenia and mortality in mice. J. Thromb Haemost. 5:2291–2299 [DOI] [PubMed] [Google Scholar]
- 11.Nelson ER, Bierman HR, Chulajata R. 1964. Hematologic findings in the 1960 hemorrhagic fever epidemic (dengue) in Thailand. Am. J. Trop. Med. Hyg. 13:642–649 [DOI] [PubMed] [Google Scholar]
- 12.La Russa VF, Innis BL. 1995. Mechanisms of dengue virus-induced bone marrow suppression. Baillieres Clin. Haematol. 8:249–270 [DOI] [PubMed] [Google Scholar]
- 13.Noisakran S, Onlamoon N, Hsiao HM, Clark KB, Villinger F, Ansari AA, Perng GC. 2012. Infection of bone marrow cells by dengue virus in vivo. Exp. Hematol. 40:250–259.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishikawa F, Yasukawa M, Lyons B, Yoshida S, Miyamoto T, Yoshimoto G, Watanabe T, Akashi K, Shultz LD, Harada M. 2005. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood 106:1565–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, Kotb M, Gillies SD, King M, Mangada J, Greiner DL, Handgretinger R. 2005. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J. Immunol. 174:6477–6489 [DOI] [PubMed] [Google Scholar]
- 16.Mota J, Rico-Hesse R. 2009. Humanized mice show clinical signs of dengue fever according to infecting virus genotype. J. Virol. 83:8638–8645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mota J, Rico-Hesse R. 2011. Dengue virus tropism in humanized mice recapitulates human dengue fever. PLoS One 6:e20762. 10.1371/journal.pone.0020762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaiswal S, Pearson T, Friberg H, Shultz LD, Greiner DL, Rothman AL, Mathew A. 2009. Dengue virus infection and virus-specific HLA-A2 restricted immune responses in humanized NOD-scid IL2rgammanull mice. PLoS One 4:e7251. 10.1371/journal.pone.0007251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Q, Khoury M, Limmon G, Choolani M, Chan JK, Chen J. 2013. Human fetal hepatic progenitor cells are distinct from, but closely related to, hematopoietic stem/progenitor cells. Stem Cells. [Epub ahead of print.] 10.1002/stem.1359 [DOI] [PubMed] [Google Scholar]
- 20.Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, Ueyama Y, Koyanagi Y, Sugamura K, Tsuji K, Heike T, Nakahata T. 2002. NOD/SCID/gamma(c) (null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood 100:3175–3182 [DOI] [PubMed] [Google Scholar]
- 21.Lepus CM, Gibson TF, Gerber SA, Kawikova I, Szczepanik M, Hossain J, Ablamunits V, Kirkiles-Smith N, Herold KC, Donis RO, Bothwell AL, Pober JS, Harding MJ. 2009. Comparison of human fetal liver, umbilical cord blood, and adult blood hematopoietic stem cell engraftment in NOD-scid/gammac-/-, Balb/c-Rag1-/-gammac-/-, and C.B-17-scid/bg immunodeficient mice. Hum. Immunol. 70:790–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Low JGH, Ooi EE, Tolfvenstam T, Leo YS, Hibberd ML, Ng LC, Lai YL, Yap GSL, Li CSC, Vasudevan SG, Ong A. 2006. Early dengue infection and outcome study (EDEN)—study design and preliminary findings. Ann. Acad. Med. Singapore 35:783–789 [PubMed] [Google Scholar]
- 23.Chen Q, He F, Kwang J, Chan JKY, Chen J. 2012. GM-CSF and IL-4 stimulate antibody responses in humanized mice by promoting T, B, and dendritic cell maturation. J. Immunol. 189:5223–5229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sabin AB, Schlesinger RW. 1945. Production of immunity to dengue with virus modified by propagation in mice. Science 101:640–642 [DOI] [PubMed] [Google Scholar]
- 25.Wolber EM, Dame C, Fahnenstich H, Hofmann D, Bartmann P, Jelkmann W, Fandrey J. 1999. Expression of the thrombopoietin gene in human fetal and neonatal tissues. Blood 94:97–105 [PubMed] [Google Scholar]
- 26.Sung JM, Lee CK, Wu-Hsieh BA. 2012. Intrahepatic infiltrating NK and CD8 T cells cause liver cell death in different phases of dengue virus infection. PLoS One 7:e46292. 10.1371/journal.pone.0046292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu CC, Huang KJ, Lin YS, Yeh TM, Liu HS, Lei HY. 2002. Transient CD4/CD8 ratio inversion and aberrant immune activation during dengue virus infection. J. Med. Virol. 68:241–252 [DOI] [PubMed] [Google Scholar]
- 28.Carballido JM, Namikawa R, Carballido-Perrig N, Antonenko S, Roncarolo MG, de Vries JE. 2000. Generation of primary antigen-specific human T- and B-cell responses in immunocompetent SCID-hu mice. Nat. Med. 6:103–106 [DOI] [PubMed] [Google Scholar]
- 29.Legrand N, Weijer K, Spits H. 2006. Experimental models to study development and function of the human immune system in vivo. J. Immunol. 176:2053–2058 [DOI] [PubMed] [Google Scholar]
- 30.Shultz LD, Ishikawa F, Greiner DL. 2007. Humanized mice in translational biomedical research. Nat. Rev. Immunol. 7:118–130 [DOI] [PubMed] [Google Scholar]