Abstract
Understanding the factors governing host species barriers to virus transmission has added significantly to our appreciation of virus pathogenesis. Jaagsiekte sheep retrovirus (JSRV) is the causative agent of ovine pulmonary adenocarcinoma (OPA), a transmissible lung cancer of sheep that has rarely been found in goats. In this study, in order to further clarify the pathogenesis of OPA, we investigated whether goats are resistant to JSRV replication and carcinogenesis. We found that JSRV induces lung tumors in goats with macroscopic and histopathological features that dramatically differ from those in sheep. However, the origins of the tumor cells in the two species are identical. Interestingly, in experimentally infected lambs and goat kids, we revealed major differences in the number of virus-infected cells at early stages of infection. These differences were not related to the number of available target cells for virus infection and cell transformation or the presence of a host-specific immune response toward JSRV. Indeed, we also found that goats possess transcriptionally active endogenous retroviruses (enJSRVs) that likely influence the host immune response toward the exogenous JSRV. Overall, these results suggest that goat cells, or at least those cells targeted for viral carcinogenesis, are not permissive to virus replication but can be transformed by JSRV.
INTRODUCTION
Jaagsiekte sheep retrovirus (JSRV) is the causative agent of a transmissible lung cancer (ovine pulmonary adenocarcinoma [OPA]) of the domestic sheep (Ovis aries) (1–6). The envelope (Env) glycoprotein of JSRV functions as a dominant oncoprotein, and its expression is sufficient to induce cell transformation in vitro (7–13) and in vivo (14, 15) via the induction of several signal transduction pathways, including phosphatidylinositol 3-kinase (PI-3K)/Akt and Ras–MEK–mitogen-activated protein kinase (MAPK) (10, 11, 16–18). The oncoprotein of JSRV is therefore a structural protein, rather than a nonstructural protein as in almost all other oncogenic viruses. This constitutes an evolutionary paradox, since abundant JSRV replication appears to be entirely dependent on tumor development in the host, a unique paradigm for oncogenic viruses.
Several studies of JSRV support the notion that this virus has found a unique strategy for survival during evolution. Experimental infection of young lambs but not adult animals results almost invariably in the induction of lung adenocarcinoma after a short (several weeks to months) incubation period (5, 19). On the other hand, in naturally occurring OPA, lung adenocarcinoma develops slowly after a long incubation period (20). Interestingly, high levels of JSRV antigens are found only in the lung tumor cells in both experimentally induced and naturally occurring OPA cases (21–23). An apparent inconsistency is that in the field, the majority of JSRV-infected animals do not have macroscopically or microscopically detectable lung neoplasms and viral nucleic acids are detectable in lymphoid cells rather than in the lungs (20). However, we have recently shown that the target cells for JSRV productive infection and transformation are rare for most of the life span of the host (24). Although JSRV can infect a variety of cell types (21, 25), abundant viral replication and cell transformation occur predominantly, if not exclusively, in lung alveolar proliferating cells (LAPCs) (characterized by the expression of the type II pneumocyte marker SPC+ and the proliferation marker Ki67+), a dividing precursor of the type 2 pneumocyte lineage (24). These cells are abundant in young lambs during postnatal development (hence the age-related susceptibility to JSRV infection) or in adults as a result of damage to the bronchioalveolar epithelium, when LAPCs become active in order to repair the injury. JSRV preferentially infects dividing cells (a property shared with most retroviruses), and indeed, in vivo the virus does not infect mature type II pneumocytes (24). In addition, the JSRV long terminal repeats (LTRs) (where the retroviral promoter and enhancer are located) have been found to be preferentially expressed in cell lines derived from type II pneumocytes and Clara cells as opposed to other cell lines (26). Thus, it appears that JSRV has only a small window of opportunity to infect the cell targets of viral carcinogenesis and this occurs in a minority of naturally infected animals (20).
Interestingly, sheep infected with JSRV (with or without clinical OPA) do not mount a humoral or cellular response against the virus. The apparent immunological tolerance of sheep toward JSRV appears to be due to the presence in the genome of small ruminants of transcriptionally active endogenous retroviruses (enJSRVs) highly related to JSRV (27–32). It is likely that the abundant expression of enJSRVs during ontogeny makes sheep tolerant toward their exogenous counterpart (31, 33, 34). Notably, enJSRVs play a number of additional biological functions in their host, since they are essential for the reproductive biology of sheep and interfere with the replication of related exogenous viruses (27, 35–39).
Thus, small ruminants represent a fascinating system with which to investigate the interaction between retroviruses and their hosts. OPA has been found almost exclusively in the domestic sheep and in farmed European moufflons (Ovis orientalis; a primitive ancestor of the domestic sheep readapted to feral life in some Mediterranean islands) (1). A few reports on the natural occurrence of OPA in goats (Capra hircus) in the 1960s contained little information on the disease and did not distinguish between alveolar epithelization and adenocarcinoma (40, 41). Later, more-convincing studies described OPA in goats at very low incidence (42, 43). Two separate studies have investigated the experimental transmission of OPA in goats in the 1980s using lung secretions from OPA-affected animals as an inoculum. Experimental transmission of OPA in a single goat kid (of the three originally inoculated) was reported by Sharp et al. (44). Tustin and colleagues (45) reproduced tumor lesions in 2 of 6 inoculated goat kids, although the inoculum was contaminated with a small-ruminant lentivirus.
In this study, we investigated whether goats display a degree of resistance to JSRV replication and cell transformation, in order to further clarify the pathogenesis of OPA and understand host species barriers to virus transmission.
MATERIALS AND METHODS
Cells and virus production.
293T cells, 293-GP cells, and H322/oH2 cells were grown in Iscove's modified Dulbecco's medium with 10% fetal bovine serum at 37°C and 5% CO2. H322/oH2 cells were generated by transduction of parent NCI-H322 cells (a human adenocarcinoma cell line; HPA Cell Culture Collection) with a murine leukemia virus (MLV) vector (LNCX2) expressing ovine hyaluronidase 2 (HYAL-2), the cellular receptor for JSRV (13). Stably transduced cells were selected in 500 μg/ml G418 (Sigma) and used as a polyclonal population. Virus for neutralization assays was produced by transfection of 293-GP, a packaging cell line expressing MLV Gag and Pol, with pLNCX-LacZ (a plasmid encoding a transfer vector and beta-galactosidase) and either pCMV3JS21-ΔGP (12) (encoding the JSRV Env) or pVPACK-10A1 (Stratagene) (encoding the MLV Env) using Fugene HD reagent (Promega). Supernatants were collected 48 and 72 h posttransfection, clarified by centrifugation (5 min, 350 × g), filtered (0.2 μm), and ultracentrifuged (2 h, 80,000 × g) before being resuspended at a 10× concentration in serum-free medium and stored at −80°C until use. Viral titers were expressed as LacZ focus-forming units (FFU) per ml.
208FJSRV21 cells derive from the 208F rat cell line (46) and stably express the JSRV21 infectious molecular clone under the control of the human cytomegalovirus immediate early promoter as previously described (5, 47). For the production of infectious virus, cells were cultured in 2-liter rolling bottles (Corning) using Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum at 37°C, 5% CO2, and 95% humidity. Cell culture supernatant containing infectious JSRV was collected every 12 h for 3 days. Virus was then concentrated (200-fold) by ultracentrifugation as described above and resuspended in TNE buffer (100 mM NaCl, 10 mM Tris, and 1 mM EDTA). Various virus preparations were pooled into a single stock, aliquoted, and stored at −80°C until use.
In vivo studies.
Animal experiments were carried out at the Istituto Zooprofilattico Sperimentale dell'Abruzzo e Molise G. Caporale (Teramo, Italy) (studies A, B, and C) or at Texas A&M University (study D). Animal studies were carried out in accordance with local and national approved protocols regulating animal experimental use (protocol no. 3315/10) in Italy and by the Institutional Animal Care and Use Committee of Texas A&M University in the United States. Prior to experimental infections, all animals were anesthetized with sodium pentobarbital.
Study A.
In study A (long-term study), 4 lambs and 4 kids (2 days old) were inoculated intratracheally with 1.5 ml of JSRV virus stock, while 2 lambs and 3 kids, used as negative controls, were inoculated with uninfected cell culture medium. Blood from these animals was collected at the time of virus infection and at 14, 30, 60, 90, 120, and 150 days postinoculation (p.i.). Animals were euthanized at 5 months p.i. when two lambs showed signs of respiratory distress. At necropsy, animals were examined for the presence of macroscopic lesions and tissues were collected from seven distinct anatomical regions of the lungs: the cranial part of the left cranial lobe, caudal part of the left cranial lobe, left diaphragmatic lobe, right diaphragmatic lobe, right middle lobe, right cranial lobe, and accessory lobe.
Study B.
In study B (short-term study), in order to facilitate the detection of infected cells, animals were inoculated into the accessory bronchus of the cranial lobe by fiber-optic bronchoscopy. Four lambs and four kids (2 days old) were inoculated with 1.5 ml of virus stock, while one lamb and two kids were used as mock-infected negative controls and inoculated with uninfected cell culture medium. All animals were euthanized 9 days p.i. At postmortem, tissues were collected from 8 distinct anatomical regions of the cranial lobe, fixed in 10% buffered formalin, and embedded in paraffin.
Study C.
Two healthy 4-day-old kids were euthanized, and lung samples from 8 anatomical distinct regions were collected postmortem, fixed in 10% buffered formalin, and embedded in paraffin for further analysis.
Studies D.
Mature does were observed daily for estrus (designated day 0) using vasectomized bucks. All does exhibited at least two estrous cycles of normal duration. At estrus, some does were bred to intact bucks at 12 h and 24 h postestrus. Cyclic and pregnant does were assigned randomly to be ovariohysterectomized (n = 4 does/day) on days 5, 11, 15, and 23 of the cycle or pregnancy (the latter time point only in pregnant does) (day 0 = mating). At hysterectomy, the uterus was trimmed free of cervix and oviduct. Several sections (∼0.5 cm) from the midportion of each uterine horn were fixed in fresh 4% paraformaldehyde in phosphate-buffered saline (PBS) (pH 7.2). Samples were frozen in liquid nitrogen and stored at −80°C for RNA extraction.
Virus neutralization assays.
The presence of neutralizing antibodies against JSRV in infected animals was assessed by neutralization assays as follows. H322/oH2 cells were seeded in 48-well plates at 5 × 104 cells per well. The next day, sheep and goat sera (heat inactivated at 56°C for 30 min) were mixed at a 1:10 dilution with 100 to 150 infectious units of MLV-LacZ vectors in a total volume of 50 μl and incubated for 30 min at 37°C. The virus-serum mixtures were then added to H322/ovH2 cells with 200 μl medium and 4 μg/ml Polybrene for 4 h before removing and replacing with 500 μl of fresh medium. Two days postinfection, cells were fixed with 1% glutaraldehyde solution and stained for beta-galactosidase activity as described previously (48). Assays were performed in triplicate in at least 2 independent experiments. A reduction of infectivity of 50% or greater was taken as evidence for neutralizing activity of the tested sera.
Histopathology, immunohistochemistry, and immunofluorescence.
Each tissue specimen was divided into two samples in order to be fixed in either immunohistochemistry zinc fixative (BD PharMingen), a zinc-based fixative, or 10% buffered formalin. The use of a zinc-based fixative favors in some cases the subsequent detection of ovine cellular markers in fixed tissues (49). Tissues were then embedded in paraffin, and tissue sections were deparaffinized, hydrated, and stained with hematoxylin and eosin using standard procedures.
Immunohistochemistry was performed as previously described (23, 24, 49). JSRV was detected using antibodies against the viral matrix or Env as already described (15, 39, 50). Mouse monoclonal antibodies employed to detect cell markers were purchased from Serotec unless stated otherwise. The following antibodies raised against the following ovine molecules were used: CD4 (44.38, Th cells), major histocompatibility complex (MHC) class II (VPM54), gamma interferon (IFN-γ) (CC302), WC1 antigen (CC15, γδ T cells), and CD8 (SBU-T8, cytotoxic T cells; kindly gifted by John Hopkins, University of Edinburgh). Slides were processed using the EnVision Plus system (Dako) as recommended by the manufacturer.
Immunofluorescence was carried out as previously described (24). Primary antibodies used were polyclonal rabbit anti pro-Sp-C (Seven Hills Bioreagents), bovine CC-10 (24), Ki67 (Dako Agilent Technologies), JSRV Env (15), and appropriate secondary antibodies conjugated with Alexa-488 or Alexa-555 (Molecular Probes). Negative controls, for both immunohistochemistry and immunofluorescence, included the analysis of samples with no primary antibodies or with normal serum or unrelated monoclonal antibodies. After the addition of tyramide signal amplification (TSA) (Perkin-Elmer Life Science Products), slides were mounted with medium containing 4′,6-diamidino-2-phenylindole (DAPI) (Vectashield, Vector Laboratories). Images were analyzed using confocal microscopy (TCS SP2; Leica), and images were analyzed and merged using Image-pro Analyzer 7 software (MediaCybernetics). Analysis of proliferation of type II pneumocytes and Clara cells in healthy 4-day-old goat kids (study C above) was performed by counting, respectively, SP-C/Ki67 and CC-10/Ki67 doubly positive cells from samples collected from 10 lung sections. Numbers of doubly positive cells were normalized to the sectioned area using Image-pro Analyzer 7 software. The number of CC10/Ki67 doubly positive cells was determined by analyzing 100 terminal bronchioli for each animal.
In situ hybridization.
Localization of enJSRV RNA was performed essentially as previously described from uterine sections collected from cyclic and pregnant does (31). After deparaffinization and rehydration, uterine sections were hybridized with an ovine JSRV env radiolabeled antisense or sense cRNA probe generated from linearized plasmid template (DD54) using in vitro transcription with [α-35S]UTP (3,000 Ci/nmol; Amersham-Pharmacia) as already described (31, 51). Autoradiographs were prepared using Kodak NTB-2 liquid photographic emulsion. Slides were kept at 4°C for up to 1 week and developed in Kodak D-19 developer. Slides were then counterstained with Harris' modified hematoxylin in acetic acid (Fisher) and dehydrated before the addition of a coverslip. Photomicrographs were taken under bright-field and dark-field illumination using a Carl Zeiss Axioplan2 microscope fitted with a Hamamatsu chilled 3CCD camera.
RESULTS
JSRV induces lung tumors in both goats and sheep but with different macroscopic and histological features.
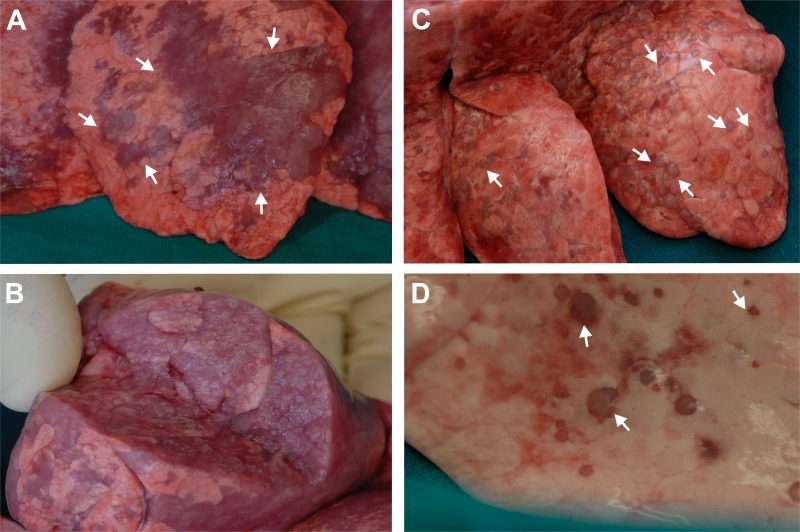
Initially, we assessed whether JSRV was able to induce tumors in goat kids as reported for older studies (45). Four lambs and four goat kids were inoculated intratracheally with JSRV21. At 5 months postinoculation (p.i.), two JSRV-infected lambs showed respiratory clinical signs (audible breathing and progressive dyspnea) and mild cachexia, suggesting that they had developed pulmonary lesions. At this stage, none of the goat kids or the mock-infected lambs showed any clinical signs. All animals were then euthanized and subjected to necropsy. Pulmonary lesions were observed in all JSRV-infected animals of both species, but there were clear morphological differences between the lesions developed by lambs and goat kids (Fig. 1). All four of the infected lambs developed tumors that were diffusely infiltrative, especially within the cranioventral lobes. Specifically, there was marked consolidation of cranioventral lung lobes, which were diffusely purple to gray in color and firm to cut. The infiltrative nature of the neoplasms resulted in an indistinct border between neoplastic and normal lung tissue (Fig. 1A and B).
Fig 1.
Macroscopic appearance of lung tumors induced by JSRV in lambs and goat kids. (A) Lungs from experimentally infected lambs at 5 months postinfection show extensive tumor lesions (some indicated by arrows), purple to greyish in color, infiltrating different lobes of the lungs. (B) Cross-section of lungs from an infected lamb reveals extensive infiltration of the neoplasm throughout the organ. (C) Lungs from an experimentally infected goat kid show well-circumscribed pale small nodules (arrows indicate some of them) well delimitated from the surrounding healthy pulmonary parenchyma. (D) Higher-magnification picture of lesions from an infected kid.
Conversely, lungs from infected goat kids contained multifocal, well-circumscribed pale firm nodules (<1 cm in diameter), which were located predominantly within cranioventral lobes. Lung tissue surrounding these nodules was of normal color and consistency (Fig. 1C and D). The confined nature of the pulmonary lesions may explain why the function of the organ was not compromised.
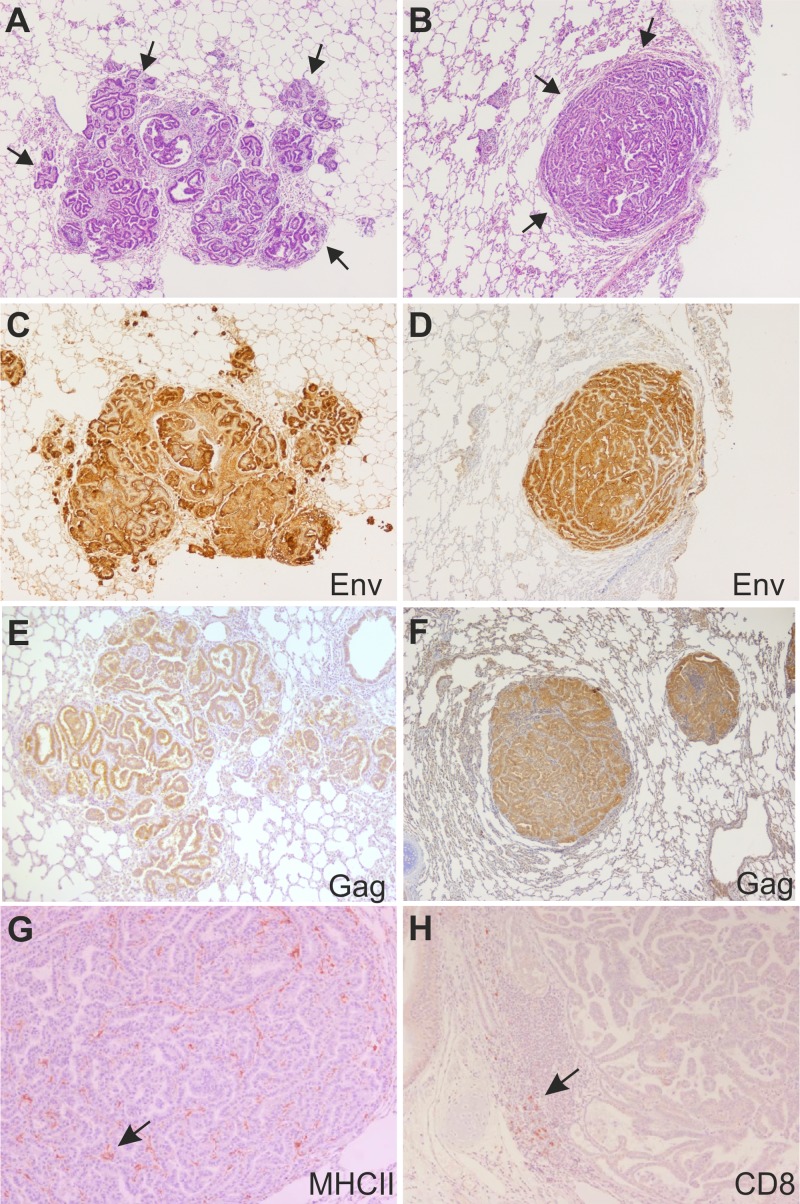
Histological examination of multiple lung sections from infected lambs revealed a neoplasia with the classic appearance of a bronchioloalveolar carcinoma. This was characterized by proliferating epithelial cells, forming tumor nodules with an acinar or papillary pattern in close proximity to one another and infiltrating adjacent alveoli (Fig. 2A). In contrast, histological examination of sections from infected goat kids showed large, well-circumscribed nodules of bronchioloalveolar carcinoma compressing the surrounding normal parenchyma (Fig. 2B). These lesions were well demarcated from healthy parenchyma but not surrounded by a significant inflammatory cell infiltrate. Indeed, by immunohistochemistry, we determined that as shown previously for OPA lesions in sheep (49), there were relatively few cells surrounding or infiltrating the tumors and they were MHC-II-expressing cells and in some cases a few CD8 T and γ/δ T cells (Fig. 2G and H and not shown). As expected, JSRV Env and Gag were expressed at high levels in the neoplastic cells of tumors in both experimentally infected lambs and goat kids (Fig. 2C to F). Mock-infected animals (2 lambs and 3 kids) showed no macroscopic or microscopic lesions in the lungs (data not shown).
Fig 2.
Histopathology of lung sections collected from lambs and goat kids experimentally infected with JSRV. (A) Tissue section (stained with hematoxylin and eosin) collected from the lungs of an experimentally infected lamb showing the presence of numerous neoplastic foci (indicated by arrows) in close proximity. (B) Tissue section (stained with hematoxylin and eosin) collected from the lungs of an experimentally infected goat kid with a well-isolated expanding neoplastic nodule (indicated by arrows). (C) Immunohistochemistry of lung section from an experimental infected lamb (same as panel A) showing labeling of JSRV Env in tumor cells (characterized by the intracytoplasmic brown color). (D) Immunohistochemistry of lung section from an experimental infected goat kid (same as panel B) showing labeling of JSRV Env in tumor cells. (E and F) Immunohistochemistry of lung section from an experimental infected lamb (E) or goat kid (F) showing labeling of JSRV Gag in tumor cells. (G and H) Immunohistochemistry of lung section from an experimental infected goat kid shows expression in a few cells (see arrows) infiltrating the tumor expressing MHC-II (E) or CD8 (F) cell markers.
JSRV replication but not oncogenesis is restricted in goats.
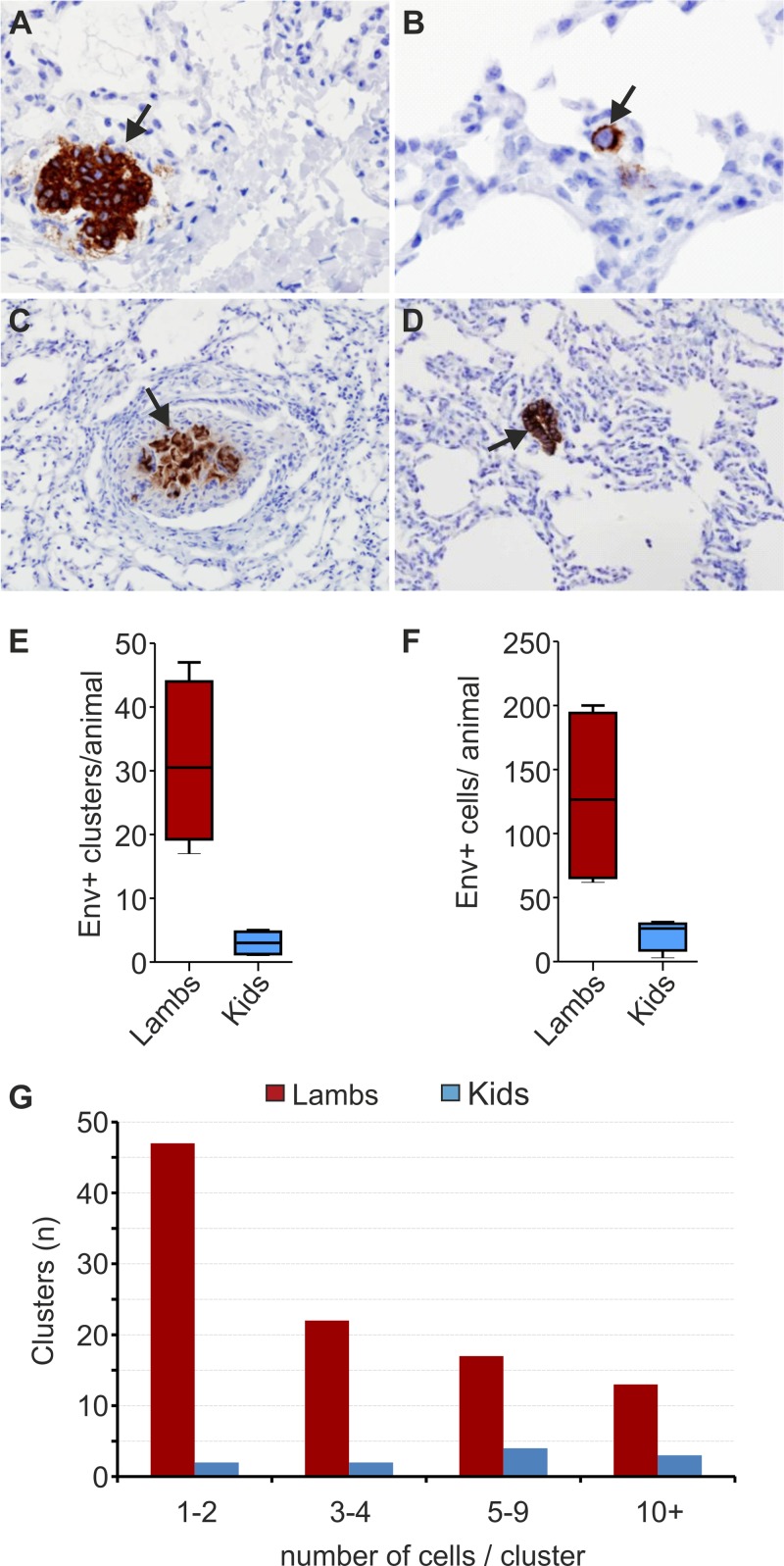
Taking into consideration the data presented above, we can conclude that in goats, JSRV induces neoplastic lesions that appear to be more circumscribed than those observed in infected sheep. This finding could be due to (i) a decreased susceptibility of goat cells to JSRV-induced transformation, (ii) a reduced ability of the virus to infect and replicate in the target cells for virus transformation, or (iii) a difference in the grade of malignancy of the neoplasm induced in the two different species. To address these possibilities experimentally, we carried out an additional infection of newborn lambs (n = 4) and goat kids (n = 4) with JSRV and analyzed virus infection of lung cells 9 days p.i. We also used 1 lamb and 2 kids as mock-infected controls. In order to facilitate subsequent virus detection, animals were infected by bronchoscopy in the accessory bronchus of the cranial lobe of the lungs. We assessed the presence of JSRV-infected cells by immunohistochemistry in 8 sections collected from 8 regions of the cranial lobe of the lungs to maximize our chances of detecting the relatively small number of infected cells (Fig. 3). For each section, we quantified both the total number of virus-infected cells (i.e., expressing the JSRV Env) and the number of infected isolated “clusters” of Env+ cells (Fig. 3A to D). We scored as an infected “cluster” either an individual Env+ cell surrounded by uninfected cells (Fig. 3B) or multiple Env+ cells grouping together (Fig. 3A, C, and D).
Fig 3.
JSRV more readily infects lung cells in experimentally infected lambs than in goat kids. (A to D) Immunohistochemistry of JSRV Env+ cells in lung sections collected from experimentally infected lambs (A and B) and kids (C and D) 9 days postinfection. JSRV-infected cells are characterized by dark intracytoplasmic brown staining. (E) Box-and-whisker plot showing the number of JSRV Env+ clusters per animal as detected by immunohistochemistry in four lambs and four goat kids at 9 days postinfection. Data represent analysis of 8 lung sections for each animal. (F) Box-and-whisker plot showing the total number of JSRV Env+ cells per animal as detected by immunohistochemistry. Lung sections (n = 8) were analyzed for each animal (as with panel E). (G) Graph indicating the number of clusters in infected lambs and goat kids formed by either 1 to 2, 3 to 4, 5 to 9, or more than 10 JSRV Env+ cells.
In the four infected lambs, we detected 8.6 times more virus-positive clusters than in infected goat kids. Indeed, 75% (i.e., 24/32) of the sections collected from the infected lambs, compared to only 28% of the sections from goats, contained at least one Env+ cluster. We detected a total of 104 Env+ cell clusters in infected lambs. We counted in each cluster between 1 and 36 infected cells, for a total of 515 JSRV Env+ cells in all the lung sections collected from the four lambs. On the other hand, in the lung sections analyzed from goat kids, we counted only 12 Env+ cell clusters, each containing between 1 and 21 infected cells, for a total of 86 virus-positive cells (Fig. 3E).
Interestingly, we noticed that approximately 70% of the 104 Env+ clusters in lambs contained 4 or fewer Env+ cells (presumably representing recent infection events), while the remaining 30% contained more than 5 Env+ cells. Only 13 clusters contained 10 or more cells (neoplastic lesions representing earlier infection events). In contrast, in infected kids, only approximately 30% of the clusters were formed by 4 or fewer Env+ cells, while the remaining 70% clusters contained 5 or more Env+ cells. Three clusters contained more than 10 infected cells (Fig. 3F and G).
Characterization of lungs cells infected by JSRV in goat kids.
Our results suggested that the target cells for JSRV infection and transformation in the lungs of goat kids are somewhat restrictive to JSRV replication. In previous studies (24, 25), we described that lung tumors in OPA-affected sheep are formed mainly by type II pneumocytes. In vivo, JSRV can infect multiple cell types upon infection, but the cells targeted for transformation are a subset of proliferating type II pneumocytes (lung alveolar proliferating cells [LAPCs]) (24). Thus, it was possible that the differences observed in the phenotypes of OPA tumors in goats and sheep could be due merely to differences in either the cell type targeted by the virus or their relative abundances between these two animal species.
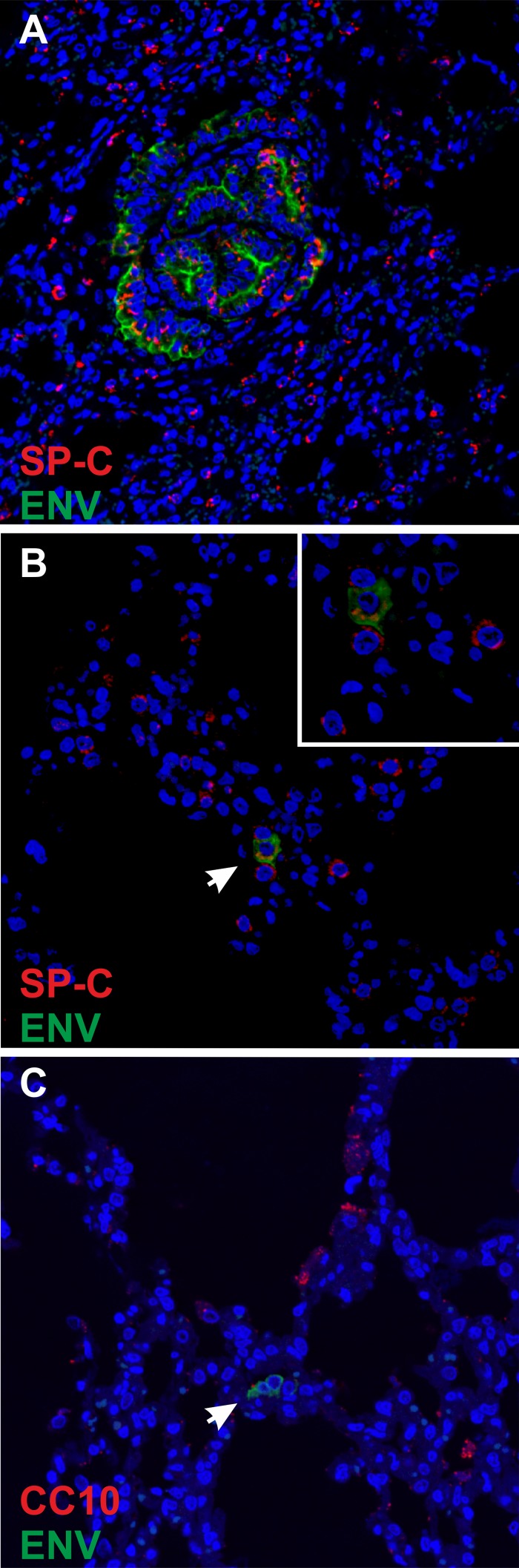
In order to address these hypotheses, we first performed confocal microscopy on lung sections of goats at both late and early times postinfection. We consistently found that late-stage tumors induced by JSRV in goats are formed by type II pneumocytes, as shown by confocal microscopy using antibodies against JSRV Env and the surfactant protein C (SP-C) (specifically expressed by type II pneumocytes) (Fig. 4A). Thus, tumors in sheep and goats are formed by the same cell type. At early time points in goat kids, we detected JSRV in type 2 pneumocytes and not Clara cells (Fig. 4B and C), similar to what we had already observed in lambs (24).
Fig 4.
Phenotype of JSRV-infected cells in experimentally inoculated goat kids. (A to C) Confocal microscopy of lung sections from goat kids experimentally infected with JSRV and collected at either 5 months (A) or 9 days (B and C) postinfection. In panels A and B, immunofluorescence was carried out using antibodies toward SP-C (shown in red) and JSRV Env (shown in green), while in panel C, antibodies toward the Clara cell marker CC10 and JSRV Env were used. Nuclei were stained with DAPI and are shown in blue. Arrows indicate cells expressing JSRV Env.
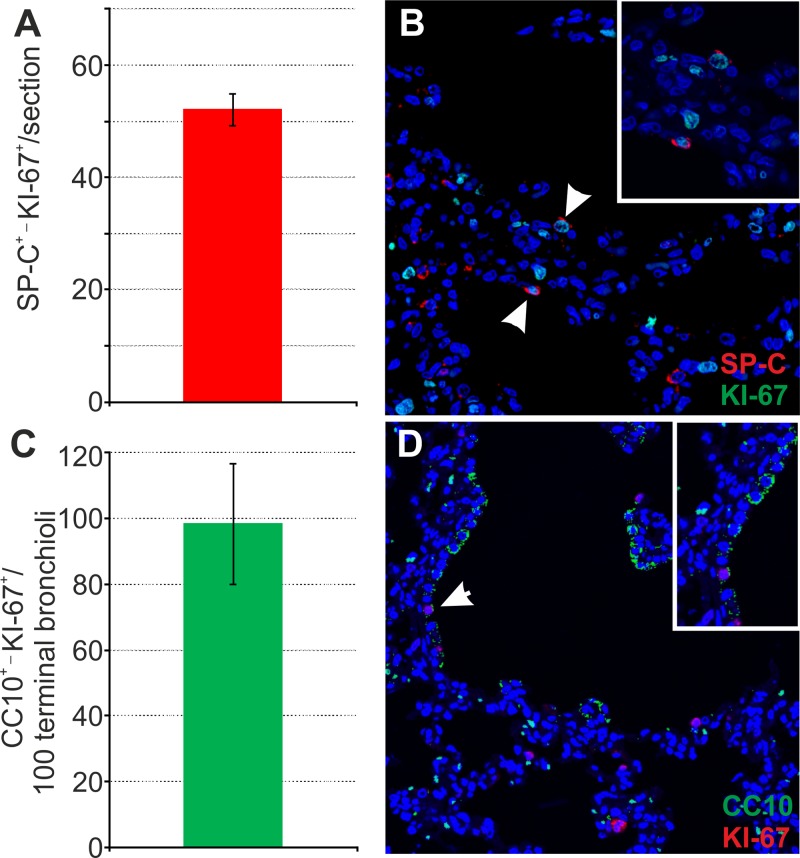
We also checked for the relative abundance of LAPCs in lung sections from goat kids (Fig. 5). The LAPCs are SPC+/Ki67C+ cells abundant in young animals, or in adults after lung injury. We counted LAPCs in 10 lung sections in healthy goat kids (n = 2). We were able to identify on average approximately 50 LAPCs per section, a value essentially identical to what we found in lambs in a previous study (24). In addition, we also counted the relative abundance of proliferating Clara cells in goat kids. These cells have been found to be infected by JSRV in one study but not in others (24, 25), and it is unclear whether they can contribute to OPA tumors in rare occasions. On average, we found approximately 100 proliferating Clara cells (characterized by expression of CC10 and Ki-67) per 100 terminal bronchioli, a value very similar to what we found previously in lambs (24). Thus, the distal respiratory tracts of sheep and goats contain the same relative abundances of the target cells for JSRV infection and cell transformation.
Fig 5.
Number of proliferating SP-C+ and CC10+ cells in healthy goat kids. (A and B) Analysis of proliferating type 2 pneumocytes (LAPCs) was performed by counting SP-C/Ki67 doubly positive cells in 4-day-old goat kids by confocal microscopy as described in Materials and Methods. Sections (n = 10) for each animal were analyzed by confocal microscopy, and numbers of doubly positive cells were normalized to the sectioned area. Results shown are the average numbers of SP-C+ Ki67+ cells (± SD) per section for both groups of animals. (B) Representative image of a lung section from a 4-day-old goat kid analyzed by confocal microscopy using antibodies toward SP-C (shown in red) and Ki67 (shown in green). Nuclei were stained with DAPI and are shown in blue. Note that Ki67 is a nuclear marker, and therefore the positive signal appears in turquoise in the merged image. Arrows indicate SP-C+ Ki67+ cells. (C) Analysis of proliferating Clara cells was performed by counting the number of CC10+ Ki67+ cells in 100 terminal bronchioli per each animal. Results shown are the average numbers of CC10+ Ki67+ cells (± SD) per 100 terminal bronchioli for both groups of animals. (D) Representative image of a lung section from a 2-day-old goat kid analyzed by confocal microscopy using antibodies toward CC10 (shown in green) and Ki67 (shown in red). Nuclei were stained with DAPI and are shown in blue. Arrows indicate CC10+ Ki67+ cells.
No immune response in kids as result of JSRV infection.
We next investigated whether goats mounted an immune response toward JSRV that could explain both the reduced number of virus infected cells and the more restricted distribution of the neoplastic lesions observed in experimentally infected kids. Sheep do not mount a strong humoral or cell-mediated immune response against JSRV infection (33). The exact mechanism underlying this apparent tolerance of sheep toward JSRV is not clear, although it is likely related to the expression, during both ontogeny and adult life, of the multiple copies of endogenous betaretrotroviruses (enJSRVs) present in the sheep genome, which are highly related to exogenous JSRV (31, 34, 52). Most of the cloned enJSRV loci entered the host genome after the divergence of the genera Ovis (including domestic sheep and related species) and Capra (including domestic goats and related species) (27), and therefore it was feasible that domestic goats could be better equipped to respond immunologically toward JSRV infection.
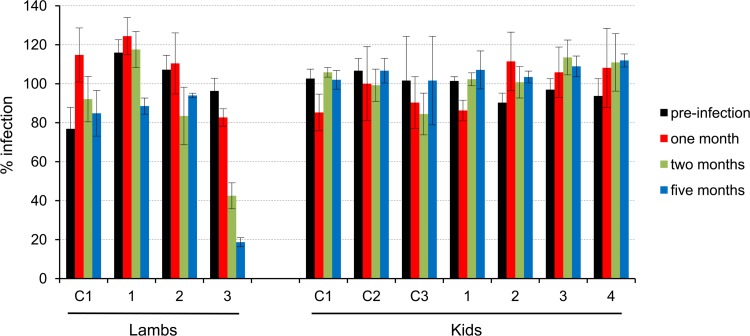
In a previous study, low-titer neutralizing antibodies to JSRV were found in some of the lambs experimentally coinfected with JSRV and ovine lentivirus (53). Thus, we tested sera from the experimentally infected lambs and goats for the presence of neutralizing antibodies. We used 150 focus-forming units (FFU) of a retroviral vector expressing LacZ pseudotyped with JSRV Env and scored sera as positive when they induced plaque reduction of >50%. We found no neutralizing antibodies toward JSRV in any of the experimentally infected kids. Only one of the experimentally infected lambs had neutralizing antibodies toward JSRV (Fig. 6). In this lamb, neutralizing antibody titers in serum samples were relatively low (1:10 to 1:20), but they were consistently found at different time points. In addition, serum samples from this lamb did not neutralize retroviral vectors pseudotyped with the MLV envelope used as a negative control, suggesting that this was a specific reaction (data not shown).
Fig 6.
Neutralization activities of lamb and kid sera against retroviral vectors pseudotyped with JSRV Env. Sera collected prior to infection and at the indicated times postinfection were tested at a 1:10 dilution against MLV-LacZ vectors pseudotyped with JSRV Env. The infectivity of the serum-treated vector is shown relative to the infectivity of the untreated vector (100%). Sera were tested in triplicate. Error bars show standard deviations. C1, C2, and C3 are mock-inoculated control animals.
enJSRV expression in goat reproductive tract.
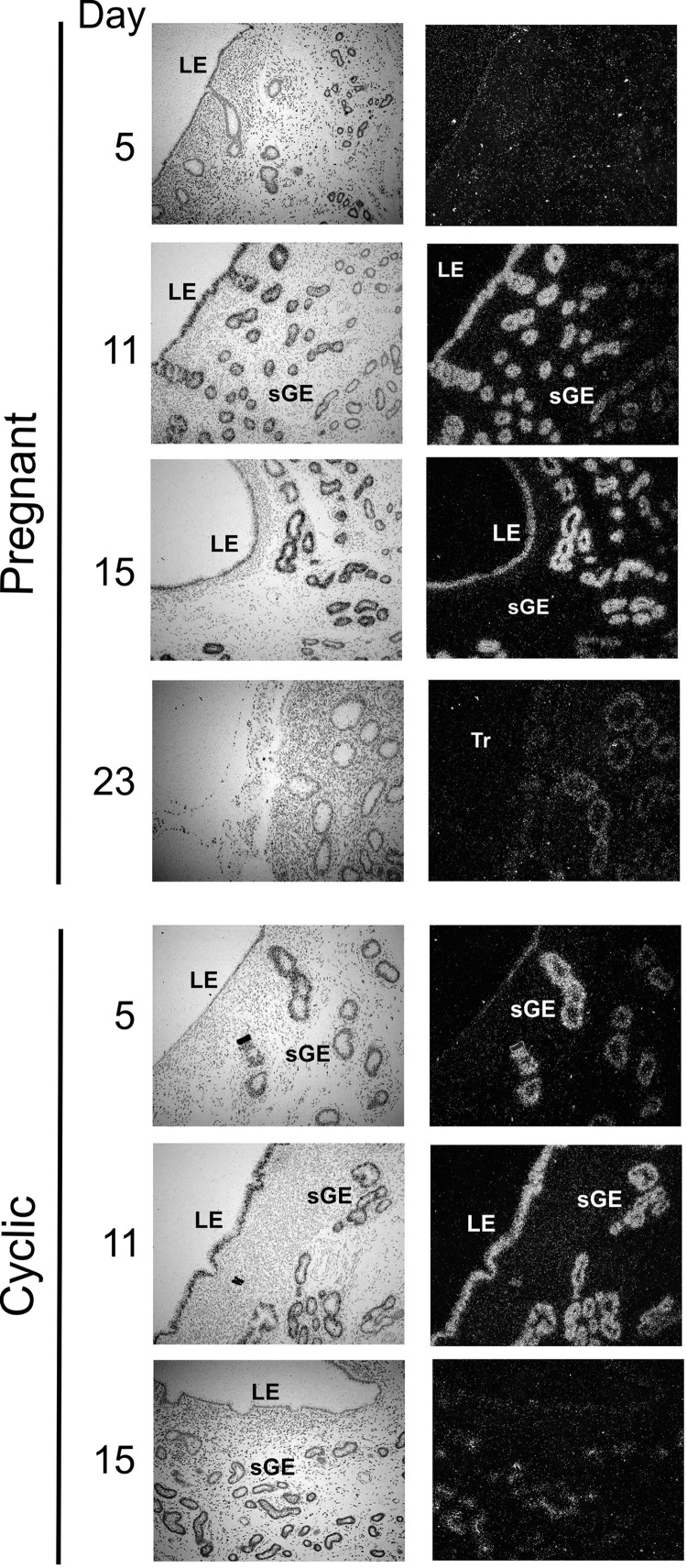
Since neutralizing antibodies toward JSRV were not detected in experimentally infected goats, we assessed enJSRV expression in healthy goats by in situ hybridization in the uterus of cyclic and pregnant does. We have shown previously that in both cyclic and pregnant sheep, enJSRVs are highly expressed in the uterus and reproductive tissues (29, 31, 51). In goats, enJSRV RNA was present predominantly in the luminal epithelium (LE) and in the glandular epithelium (GE) of the endometrium, while no expression was detected in the endometrial stroma or myometrium (Fig. 7). In pregnant does, enJSRV RNAs were abundant in the LE on day 11 but declined to almost undetectable levels at day 15. Also, in pregnant does, expression of enJSRV RNA was most abundant in the glandular epithelium of the upper stroma. Thus, enJSRV expression patterns in goats appear to be very similar to those observed in sheep (29, 31, 51).
Fig 7.
Expression of enJSRVs in the goat uterus. In situ localization of enJSRV RNA expression in the endometrium of pregnant and cyclic does as indicated in the figure. Cross sections of the endometrium were hybridized with radiolabeled antisense or sense ovine enJSRV env cRNA probes. Transcripts were visualized by liquid emulsion autoradiography for 1 week and imaged under bright-field (left) or dark-field (right) illumination. Numbers indicate the gestational or cyclic day when samples were collected. LE, luminal epithelium; GE, glandular epithelium; Tr, trophectoderm; sGE, superficial glandular epithelium.
DISCUSSION
Studies of cross-species barriers to virus transmission have unveiled fundamental aspects of virus biology and pathogenesis. JSRV is the etiological agent of ovine pulmonary adenocarcinoma, a transmissible lung cancer that has been found in sheep in most regions of the world (5). OPA was found in goats in the 1960s, mainly in India (40–43). Some experimental evidence on the induction of OPA in goat kids was also obtained in the 1980s (44, 45). However, there is a paucity of reports of naturally occurring OPA in goats as opposed to those for sheep. Thus, we evaluated the pathogenesis of OPA in goats in order to determine whether domestic small ruminants are differentially susceptible to JSRV infection and carcinogenesis.
We found that JSRV induces lung tumors in goats with macroscopic and histopathological features that differ from those induced in sheep. In experimentally infected lambs, the tumor occupies extensive areas of the lungs, causing a significant increase in their volume and consistency. Histologically, lung sections from infected lambs revealed multiple neoplastic foci coalescing into each other and involving single or multiple alveoli. On the other hand, in infected goat kids, lungs were of normal volume and consistency and tumors appeared as small hard nodules well isolated from the surrounding healthy pulmonary parenchyma. These differences in the tumor phenotype between the two species explain why before necropsy only the experimentally infected lambs, and not goat kids, displayed clinical signs of respiratory distress indicative of reduced lung functionality.
We also found that in goats, as shown previously in sheep, type II pneumocytes are the cells originating OPA (1, 2, 24). In late-stage tumors of both animal species, we found high levels of JSRV expression only in the tumor cells, and we observed a limited number of infiltrating cells, similar to what we previously described for sheep (49). However, we found major differences between lambs and kids in the numbers of virus-infected cells at the early stages after infection (9 days p.i.). Indeed, we found that experimentally infected lambs displayed approximately 10 times more clusters of JSRV Env+ cells than goat kids. Interestingly, infected lambs possessed on average 20 times more clusters formed by 1 or 2 isolated JSRV Env+ cells than infected goat kids. Moreover, the numbers of clusters formed by 3 or 4 Env+ cells differed by 10-fold between infected lambs and goats but only by 4-fold for either clusters formed by 5 to 9 Env+ cells or for those with more than 10 Env+ cells. It is most likely that clusters formed by isolated Env+ cells represent recent infection events of LAPCs, while clusters formed by several Env+ cells derive from earlier infection events followed by several rounds of cell division induced by the JSRV oncoprotein. Collectively, these data strongly support the idea that goat cells (or at least goat LAPCs) are not permissive for JSRV replication, since there were very few clusters of Env+ cells in kids (especially those formed by isolated Env+ cells). We found no differences in the relative abundances of LAPCs in healthy lambs or goat kids, demonstrating therefore that there is no difference in the availability of target cells for viral infection and subsequent transformation between these two animal species. In addition, we have also shown that goats, similarly to lambs, do not mount an immune response against JSRV that could protect the animals from the infection. Indeed, we found that enJSRVs are highly active and expressed in the female genital tract of goats, which is the same as findings for sheep (31, 51). These data suggest that in goats as well, JSRV proteins are probably recognized as self-antigens and explain the absence of antibody responses after infection.
JSRV possesses a structural protein (its envelope glycoprotein) that functions as a dominant oncoprotein both in vitro and in vivo (8, 10, 12–15). Thus, the virus does not need a productive infection in order to induce cell transformation, but it only needs to enter a target cell, reverse transcribe its genome, and integrate its provirus into the cellular DNA to initiate env expression that will eventually lead to cell transformation.
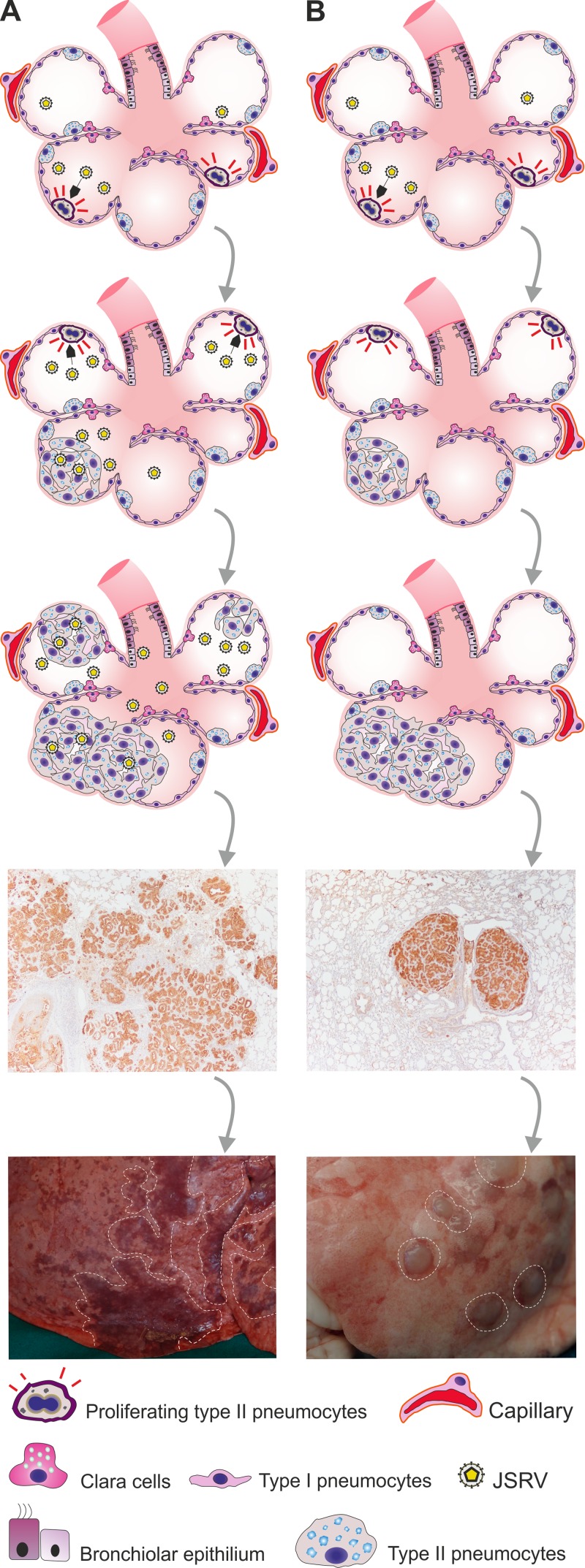
The lesions observed in experimentally infected goats are strikingly similar to those induced in lambs by a replication-defective JSRV-based virus (JS-RD) that we developed in a previous study (14). JS-RD expresses the viral Env under the control of its own long terminal repeats, and it was produced, like any other standard retroviral vector, by transiently transfecting 293T with a JSRV-based packaging plasmid that provides Gag, Pro, and Pol in trans and a “vector” deleted of its structural genes with the exception of env. Thus, JS-RD is phenotypically identical to JSRV but can express only Env after proviral integration. Lesions induced by JS-RD appear as well isolated neoplastic foci compressing the surrounding alveoli. These lesions essentially have a morphology identical to that of those induced in goats by replication-competent JSRV (Fig. 8). Interestingly, lesions induced in mice by a replication-defective adeno-associated virus (AAV) vector expressing the JSRV Env also result in well-isolated adenocarcinomas that compress the surrounding alveoli with a phenotype similar to that of JSRV-induced lung tumors in goats (15, 50). Thus, we would expect the tumor nodules in lambs induced by JS-RD and those in goats induced by JSRV (like the ones in mice induced by AAV vectors expressing JSRV Env) to be monoclonal.
Fig 8.
Model for pathogenesis of OPA lesions in sheep and goats. (A) Young lambs have many available target cells for JSRV-induced cell transformation (LAPCs) that are dividing and consequently can be infected and transformed by the virus. Transformed cells produce infectious virus that can then infect and transform other target cells, resulting in many satellite and coalescing lesions giving rise to tumors with an invasive phenotype. This is also known as the “classic” OPA phenotype. (B) Experimental inoculation of young lambs with a replication-incompetent JSRV (JS-RD) or goat kids with wild-type JSRV results in infection and transformation of target cells that do not produce infectious virus, hence the “expanding” phenotype, where tumor nodules are derived from a single transformed cell. The tumor phenotype in panel B is similar to the “atypical” tumor phenotype observed occasionally in adult sheep.
In lambs experimentally infected with JSRV, neoplastic foci of different sizes are adjacent to each other, resulting in large tumors with a “spreading” or “invasive” appearance. We believe that this is due to the ability of JSRV, upon infection of LAPCs, to produce new viral progeny that can then infect and transform adjacent LAPCs, resulting in multiple tumor foci of polyclonal origin that subsequently coalesce into larger tumor masses (Fig. 8A). On the contrary, JS-RD can complete only a single round of replication, and therefore, infected cells will then expand by cell division, resulting in lesions growing centripetally and compressing the surrounding alveoli (Fig. 8B). We suggest that in the experimental model of OPA in goats, JSRV behaves essentially as a replication-defective virus able to transform cells but not produce viable viral progeny. Thus, all the available data suggest that goat cells are somewhat restrictive for one or more steps of the JSRV replication cycle. Only relatively few LAPCs can be infected upon experimental infection (when animals are infected with a high viral dose), and there is little spreading infection within the lung, resulting in isolated neoplastic foci. It is reasonable to expect that under natural conditions, goats are exposed to relatively low infectious doses of JSRV and therefore rarely develop lung adenocarcinoma.
Interestingly, two morphological forms of naturally occurring OPA have been described in the literature as “classical” or “atypical” depending on the macroscopic appearance of the tumors (54). Classical OPA, comprising the majority of OPA cases, is characterized by enlargement of the lungs, development of greyish extended neoplastic lesions, and production of excess lung fluids. Instead, the atypical form of OPA is usually present in a subclinical form of the disease. Lesions consist of a limited number of tumor nodules that are dry and pale. Occasionally, both classic and atypical lesions are present in the same cases. It is possible that these two forms represent different scenarios when LAPCs are either abundant or scarce in the infected host. LAPCs are abundant only in lambs during postnatal development or in the adult during tissue repair (24). JSRV infection at a time when LAPCs are not abundant in the infected sheep would result in transformation of isolated cells, giving origin to isolated neoplastic nodules. On the other hand, JSRV infection coinciding with abundant availability of LAPCs results in multiple neoplastic foci and productive virus infection that “seeds” transformation in adjacent LAPCs.
We do not know which step of the JSRV replication cycle is restricted in goat cells. Unfortunately, there is no tissue culture system that supports a robust and productive JSRV infection in order to readily investigate a cellular block for JSRV in goat cells in vitro. The cellular receptor for JSRV is HYAL-2, a glycosylphosphatidylinositol (GPI)-anchored protein that is expressed in a variety of cell types (13, 55, 56). In a previous study, we have shown using retroviral vectors pseudotyped with JSRV Env that the virus utilizes both the goat and sheep orthologs of HYAL-2 equally well (27). Thus, the block in goat cells for JSRV is likely to be after virus entry, unless there is a role played by a yet-unidentified cellular coreceptor (57). In theory, any of the replication steps can be affected by a restriction factor that JSRV might have evolved to overcome in sheep but not in goats. Several cellular restriction factors, such as APOBEC3G (aprolipoprotein B editing catalytic subunit-like 3G), Trim-5/TRIMCyp, tetherin, and others, have been shown to be effective against retrovirus infections and provide barriers to cross-species transmission (58).
It could be hypothesized that restriction could be at the posttranscriptional level, since Env is also expressed efficiently by JSRV in goat cells. We have previously described a late block in JSRV replication mediated by an enJSRV locus with a transdominant Gag protein (27, 28, 35, 36, 38, 39). It is therefore possible that transdominant enJSRV loci expressed in lung cells are also present in goats. We cannot exclude that restriction occurs at early steps of JSRV replication. Thus, Env expression in tumor cells in goats could be simply the result of few incoming virions that escaped an early block. This is feasible considering that experimental infection of goat kids was carried out with relatively large amounts of virus.
Interestingly, there is another oncogenic retrovirus, the enzootic nasal tumor virus (ENTV), that is highly related to JSRV and causes an adenocarcinoma of the ethmoid turbinates in small ruminants (59–64). ENTV possesses many features in common with JSRV. ENTV Env is also a dominant oncoprotein, and the virus uses HYAL-2 as a cellular receptor (7, 11, 16). Unlike JSRV, ENTV has been found in two phylogenetically distinct groups that are commonly associated with nasal tumors in either sheep (ENTV-1) or goats (ENTV-2) (59, 60). ENTV and JSRV, despite being simple retroviruses, have an additional open reading frame (orf-x) (65) that appears to be expressed by a viral spliced mRNA (66). Orf-x does not appear to be required for virus particle formation or cell transformation, but there are no known functions for this protein (9). In future studies, it will be interesting to determine whether cellular restriction factors from small-ruminant species block ovine betaretrovirus replication and to assess the role of Orf-X in virus host species tropism.
ACKNOWLEDGMENTS
We are grateful to Gianpaolo Foschini, Massimo Gentile, and Doriano Ferrari for excellent animal care and to Sandro Martella and Federica Lopes for sample collection and processing. We are also grateful to Dusty Miller for providing monoclonal antibodies against JSRV Env.
This work was funded by the BBSRC, the Italian Ministry of Health, the Scottish Funding Council, and the Scottish Government's Rural and Environment Science and Analytical Services Division.
Footnotes
Published ahead of print 31 July 2013
REFERENCES
- 1.Fan H. 2003. Jaagsiekte sheep retrovirus and lung cancer, vol 275 Springer-Verlag, Berlin, Germany [Google Scholar]
- 2.Griffiths DJ, Martineau HM, Cousens C. 2010. Pathology and pathogenesis of ovine pulmonary adenocarcinoma. J. Comp. Pathol. 142:260–283 [DOI] [PubMed] [Google Scholar]
- 3.Hofacre A, Fan H. 2010. Jaagsiekte sheep retrovirus biology and oncogenesis. Viruses 2:2618–2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palmarini M, Fan H. 2001. Retrovirus-induced ovine pulmonary adenocarcinoma, an animal model for lung cancer. J. Natl. Cancer Inst. 93:1603–1614 [DOI] [PubMed] [Google Scholar]
- 5.Palmarini M, Sharp JM, De las Heras M, Fan H. 1999. Jaagsiekte sheep retrovirus is necessary and sufficient to induce a contagious lung cancer in sheep. J. Virol. 73:6964–6972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanna MP, Sanna E, De Las Heras M, Leoni A, Nieddu AM, Pirino S, Sharp JM, Palmarini M. 2001. Association of jaagsiekte sheep retrovirus with pulmonary carcinoma in Sardinian moufflon (Ovis musimon). J. Comp. Pathol. 125:145–152 [DOI] [PubMed] [Google Scholar]
- 7.Alberti A, Murgia C, Liu SL, Mura M, Cousens C, Sharp M, Miller AD, Palmarini M. 2002. Envelope-induced cell transformation by ovine betaretroviruses. J. Virol. 76:5387–5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen TE, Sherrill KJ, Crispell SM, Perrott MR, Carlson JO, DeMartini JC. 2002. The jaagsiekte sheep retrovirus envelope gene induces transformation of the avian fibroblast cell line DF-1 but does not require a conserved SH2 binding domain. J. Gen. Virol. 83:2733–2742 [DOI] [PubMed] [Google Scholar]
- 9.Cousens C, Maeda N, Murgia C, Dagleish MP, Palmarini M, Fan H. 2007. In vivo tumorigenesis by Jaagsiekte sheep retrovirus (JSRV) requires Y590 in Env TM, but not full-length orfX open reading frame. Virology 367:413–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu SL, Miller AD. 2007. Oncogenic transformation by the jaagsiekte sheep retrovirus envelope protein. Oncogene 26:789–801 [DOI] [PubMed] [Google Scholar]
- 11.Maeda N, Fan H. 2008. Signal transduction pathways utilized by enzootic nasal tumor virus (ENTV-1) envelope protein in transformation of rat epithelial cells resemble those used by jaagsiekte sheep retrovirus. Virus Genes 36:147–155 [DOI] [PubMed] [Google Scholar]
- 12.Maeda N, Palmarini M, Murgia C, Fan H. 2001. Direct transformation of rodent fibroblasts by jaagsiekte sheep retrovirus DNA. Proc. Natl. Acad. Sci. U. S. A. 98:4449–4454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rai SK, Duh FM, Vigdorovich V, Danilkovitch-Miagkova A, Lerman MI, Miller AD. 2001. Candidate tumor suppressor HYAL2 is a glycosylphosphatidylinositol (GPI)-anchored cell-surface receptor for jaagsiekte sheep retrovirus, the envelope protein of which mediates oncogenic transformation. Proc. Natl. Acad. Sci. U. S. A. 98:4443–4448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caporale M, Cousens C, Centorame P, Pinoni C, De las Heras M, Palmarini M. 2006. Expression of the Jaagsiekte sheep retrovirus envelope glycoproteins is sufficient to induce lung tumor in sheep. J. Virol. 80:8030–8037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wootton SK, Halbert CL, Miller AD. 2005. Sheep retrovirus structural protein induces lung tumours. Nature 434:904–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu SL, Duh FM, Lerman MI, Miller AD. 2003. Role of virus receptor Hyal2 in oncogenic transformation of rodent fibroblasts by sheep betaretrovirus Env proteins. J. Virol. 77:2850–2858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maeda N, Fu W, Ortin A, de las Heras M, Fan H. 2005. Roles of the Ras-MEK-mitogen-activated protein kinase and phosphatidylinositol 3-kinase-Akt-mTOR pathways in Jaagsiekte sheep retrovirus-induced transformation of rodent fibroblast and epithelial cell lines. J. Virol. 79:4440–4450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmarini M, Maeda N, Murgia C, De-Fraja C, Hofacre A, Fan H. 2001. A phosphatidylinositol 3-kinase docking site in the cytoplasmic tail of the Jaagsiekte sheep retrovirus transmembrane protein is essential for envelope-induced transformation of NIH 3T3 cells. J. Virol. 75:11002–11009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharp JM, Angus KW, Gray EW, Scott FM. 1983. Rapid transmission of sheep pulmonary adenomatosis (jaagsiekte) in young lambs. Brief report. Arch. Virol. 78:89–95 [DOI] [PubMed] [Google Scholar]
- 20.Caporale M, Centorame P, Giovannini A, Sacchini F, Di Ventura M, De las Heras M, Palmarini M. 2005. Infection of lung epithelial cells and induction of pulmonary adenocarcinoma is not the most common outcome of naturally occurring JSRV infection during the commercial lifespan of sheep. Virology 338:144–153 [DOI] [PubMed] [Google Scholar]
- 21.Holland MJ, Palmarini M, Garcia-Goti M, Gonzalez L, McKendrick I, de las Heras M, Sharp JM. 1999. Jaagsiekte retrovirus is widely distributed both in T and B lymphocytes and in mononuclear phagocytes of sheep with naturally and experimentally acquired pulmonary adenomatosis. J. Virol. 73:4004–4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmarini M, Cousens C, Dalziel RG, Bai J, Stedman K, DeMartini JC, Sharp JM. 1996. The exogenous form of Jaagsiekte retrovirus is specifically associated with a contagious lung cancer of sheep. J. Virol. 70:1618–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palmarini M, Dewar P, De las Heras M, Inglis NF, Dalziel RG, Sharp JM. 1995. Epithelial tumour cells in the lungs of sheep with pulmonary adenomatosis are major sites of replication for Jaagsiekte retrovirus. J. Gen. Virol. 76:2731–2737 [DOI] [PubMed] [Google Scholar]
- 24.Murgia C, Caporale M, Ceesay O, Di Francesco G, Ferri N, Varasano V, de las Heras M, Palmarini M. 2011. Lung adenocarcinoma originates from retrovirus infection of proliferating type 2 pneumocytes during pulmonary post-natal development or tissue repair. PLoS Pathog. 7:e1002014. 10.1371/journal.ppat.1002014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martineau HM, Cousens C, Imlach S, Dagleish MP, Griffiths DJ. 2011. Jaagsiekte sheep retrovirus infects multiple cell types in the ovine lung. J. Virol. 85:3341–3355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmarini M, Datta S, Omid R, Murgia C, Fan H. 2000. The long terminal repeat of Jaagsiekte sheep retrovirus is preferentially active in differentiated epithelial cells of the lungs. J. Virol. 74:5776–5787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arnaud F, Caporale M, Varela M, Biek R, Chessa B, Alberti A, Golder M, Mura M, Zhang Y, Yu L, Pereira F, DeMartini JC, Leymaster K, Spencer TE, Palmarini M. 2007. A paradigm for virus-host coevolution: sequential counter-adaptations between endogenous and exogenous retroviruses. PLoS Pathogens. 3:e170. 10.1371/journal.ppat.0030170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arnaud F, Murcia PR, Palmarini M. 2007. Mechanisms of late restriction induced by an endogenous retrovirus. J. Virol. 81:11441–11451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arnaud F, Varela M, Spencer TE, Palmarini M. 2008. Coevolution of endogenous betaretroviruses of sheep and their host. Cell. Mol. Life Sci. 65:3422–3432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chessa B, Pereira F, Arnaud F, Amorim A, Goyache F, Mainland I, Kao RR, Pemberton JM, Beraldi D, Stear MJ, Alberti A, Pittau M, Iannuzzi L, Banabazi MH, Kazwala RR, Zhang YP, Arranz JJ, Ali BA, Wang Z, Uzun M, Dione MM, Olsaker I, Holm LE, Saarma U, Ahmad S, Marzanov N, Eythorsdottir E, Holland MJ, Ajmone-Marsan P, Bruford MW, Kantanen J, Spencer TE, Palmarini M. 2009. Revealing the history of sheep domestication using retrovirus integrations. Science 324:532–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palmarini M, Gray CA, Carpenter K, Fan H, Bazer FW, Spencer TE. 2001. Expression of endogenous betaretroviruses in the ovine uterus: effects of neonatal age, estrous cycle, pregnancy, and progesterone. J. Virol. 75:11319–11327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palmarini M, Mura M, Spencer TE. 2004. Endogenous betaretroviruses of sheep: teaching new lessons in retroviral interference and adaptation. J. Gen. Virol. 85:1–13 [DOI] [PubMed] [Google Scholar]
- 33.Ortin A, Minguijon E, Dewar P, Garcia M, Ferrer LM, Palmarini M, Gonzalez L, Sharp JM, De las Heras M. 1998. Lack of a specific immune response against a recombinant capsid protein of Jaagsiekte sheep retrovirus in sheep and goats naturally affected by enzootic nasal tumour or sheep pulmonary adenomatosis. Vet. Immunol. Immunopathol. 61:229–237 [DOI] [PubMed] [Google Scholar]
- 34.Spencer TE, Mura M, Gray CA, Griebel PJ, Palmarini M. 2003. Receptor usage and fetal expression of ovine endogenous betaretroviruses: implications for coevolution of endogenous and exogenous retroviruses. J. Virol. 77:749–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Armezzani A, Arnaud F, Caporale M, di Meo G, Iannuzzi L, Murgia C, Palmarini M. 2011. The signal peptide of a recently integrated endogenous sheep betaretrovirus envelope plays a major role in eluding Gag-mediated late restriction. J. Virol. 85:7118–7128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arnaud F, Black SG, Murphy L, Griffiths DJ, Neil SJ, Spencer TE, Palmarini M. 2010. Interplay between ovine bone marrow stromal cell antigen 2/tetherin and endogenous retroviruses. J. Virol. 84:4415–4425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dunlap KA, Palmarini M, Varela M, Burghardt RC, Hayashy K, Farmer JL, Spencer TE. 2006. Endogenous retroviruses regulate peri-implantation conceptus growth and differentiation. Proc. Natl. Acad. Sci. U. S. A. 103:14390–14395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mura M, Murcia P, Caporale M, Spencer TE, Nagashima K, Rein A, Palmarini M. 2004. Late viral interference induced by transdominant Gag of an endogenous retrovirus. Proc. Natl. Acad. Sci. U. S. A. 101:11117–11122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murcia PR, Arnaud F, Palmarini M. 2007. The transdominant endogenous retrovirus enJS56A1 associates with and blocks intracellular trafficking of Jaagsiekte sheep retrovirus Gag. J. Virol. 81:1762–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nobel TA. 1958. Pulmonary adenomatosies (jaagsiekte) in sheep with special reference to its occurrence in Israel. Refu. Vet. (Israel) 15:98–101 [Google Scholar]
- 41.Rajya BS, Singh CM. 1964. The pathology of pneumonia and associated respiratory disease of sheep and goats. I. Occurrence of jagziekte and maedi in sheep and goats in India. Am. J. Vet. Res. 25:61–67 [PubMed] [Google Scholar]
- 42.Banerjee M, Gupta PP. 1979. Note on maedi and jaagsiekte in sheep and goats in Ludhiana area of Punjab. Indian J. Anim. Sci. 12:1102–1105 [Google Scholar]
- 43.Stamp JT, Nisbet DI. 1963. Pneumonia of sheep. J. Comp. Pathol. 73:319–328 [DOI] [PubMed] [Google Scholar]
- 44.Sharp JM, Angus KW, Jassim FA, Scott FM. 1986. Experimental transmission of sheep pulmonary adenomatosis to a goat. Vet. Rec. 119:245. 10.1136/vr.119.10.245 [DOI] [PubMed] [Google Scholar]
- 45.Tustin RC, Williamson AL, York DF, Verwoerd DW. 1988. Experimental transmission of jaagsiekte (ovine pulmonary adenomatosis) to goats. Onderstepoort J. Vet. Res. 55:27–32 [PubMed] [Google Scholar]
- 46.Quade K. 1979. Transformation of mammalian cells by avian myelocytomatosis virus and avian erythroblastosis virus. Virology 98:461–465 [DOI] [PubMed] [Google Scholar]
- 47.Caporale M, Arnaud F, Mura M, Golder M, Murgia C, Palmarini M. 2009. The signal peptide of a simple retrovirus envelope functions as a posttranscriptional regulator of viral gene expression. J. Virol. 83:4591–4604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clapham PR, McKnight A, Weiss RA. 1992. Human immunodeficiency virus type 2 infection and fusion of CD4-negative human cell lines: induction and enhancement by soluble CD4. J. Virol. 66:3531–3537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Summers C, Benito A, Ortin A, Garcia de Jalon JA, Gonzalez L, Norval M, Sharp JM, De las Heras M. 2012. The distribution of immune cells in the lungs of classical and atypical ovine pulmonary adenocarcinoma. Vet. Immunol. Immunopathol. 146:1–7 [DOI] [PubMed] [Google Scholar]
- 50.Wootton SK, Metzger MJ, Hudkins KL, Alpers CE, York D, DeMartini JC, Miller AD. 2006. Lung cancer induced in mice by the envelope protein of jaagsiekte sheep retrovirus (JSRV) closely resembles lung cancer in sheep infected with JSRV. Retrovirology 3:94. 10.1186/1742-4690-3-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dunlap KA, Palmarini M, Adelson DL, Spencer TE. 2005. Sheep endogenous betaretroviruses (enJSRVs) and the hyaluronidase 2 (HYAL2) receptor in the ovine uterus and conceptus. Biol. Reprod. 73:271–279 [DOI] [PubMed] [Google Scholar]
- 52.Spencer TE, Stagg AG, Joyce MM, Jenster G, Wood CG, Bazer FW, Wiley AA, Bartol FF. 1999. Discovery and characterization of endometrial epithelial messenger ribonucleic acids using the ovine uterine gland knockout model. Endocrinology 140:4070–4080 [DOI] [PubMed] [Google Scholar]
- 53.Hudachek SF, Kraft SL, Thamm DH, Bielefeldt-Ohmann H, DeMartini JC, Miller AD, Dernell WS. 2010. Lung tumor development and spontaneous regression in lambs coinfected with Jaagsiekte sheep retrovirus and ovine lentivirus. Vet. Pathol. 47:148–162 [DOI] [PubMed] [Google Scholar]
- 54.De las Heras M, Gonzalez L, Sharp JM. 2003. Pathology of ovine pulmonary adenocarcinoma. Curr. Top. Microbiol. Immunol. 275:25–54 [DOI] [PubMed] [Google Scholar]
- 55.Miller AD. 2008. Hyaluronidase 2 and its intriguing role as a cell-entry receptor for oncogenic sheep retroviruses. Semin. Cancer Biol. 18:296–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Palmarini M, Sharp JM, Lee C, Fan H. 1999. In vitro infection of ovine cell lines by Jaagsiekte sheep retrovirus. J. Virol. 73:10070–10078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van Hoeven NS, Miller AD. 2005. Improved enzootic nasal tumor virus pseudotype packaging cell lines reveal virus entry requirements in addition to the primary receptor Hyal2. J. Virol. 79:87–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hatziioannou T, Bieniasz PD. 2011. Antiretroviral restriction factors. Curr. Opin. Virol. 1:526–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cousens C, Minguijon E, Dalziel RG, Ortin A, Garcia M, Park J, Gonzalez L, Sharp JM, de las Heras M. 1999. Complete sequence of enzootic nasal tumor virus, a retrovirus associated with transmissible intranasal tumors of sheep. J. Virol. 73:3986–3993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De las Heras M, Ortin A, Cousens C, Minguijon E, Sharp JM. 2003. Enzootic nasal adenocarcinoma of sheep and goats. Curr. Top. Microbiol. Immunol. 275:201–223 [DOI] [PubMed] [Google Scholar]
- 61.De las Heras M, Sharp JM, Garcia de Jalon JA, Dewar P. 1991. Enzootic nasal tumour of goats: demonstration of a type D-related retrovirus in nasal fluids and tumours. J. Gen. Virol. 72:2533–2535 [DOI] [PubMed] [Google Scholar]
- 62.Ortin A, Cousens C, Minguijon E, Pascual Z, Villarreal MP, Sharp JM, De las Heras M. 2003. Characterization of enzootic nasal tumour virus of goats: complete sequence and tissue distribution. J. Gen. Virol. 84:2245–2252 [DOI] [PubMed] [Google Scholar]
- 63.Vitellozzi G, Mughetti L, Palmarini M, Mandara MT, Mechelli L, Sharp JM, Manocchio I. 1993. Enzootic intranasal tumour of goats in Italy. Zentralbl. Vet. B 40:459–468 [DOI] [PubMed] [Google Scholar]
- 64.Walsh SR, Linnerth-Petrik NM, Laporte AN, Menzies PI, Foster RA, Wootton SK. 2010. Full-length genome sequence analysis of enzootic nasal tumor virus reveals an unusually high degree of genetic stability. Virus Res. 151:74–87 [DOI] [PubMed] [Google Scholar]
- 65.Rosati S, Pittau M, Alberti A, Pozzi S, York DF, Sharp JM, Palmarini M. 2000. An accessory open reading frame (orf-x) of jaagsiekte sheep retrovirus is conserved between different virus isolates. Virus Res. 66:109–116 [DOI] [PubMed] [Google Scholar]
- 66.Palmarini M, Murgia C, Fan H. 2002. Spliced and prematurely polyadenylated Jaagsiekte sheep retrovirus (JSRV)-specific RNAs from infected or transfected cells. Virology 294:180–188 [DOI] [PubMed] [Google Scholar]