Abstract
A novel picornavirus was isolated from specimens of a diseased European eel (Anguilla anguilla). This virus induced a cytopathic effect in eel embryonic kidney cells and high mortality in a controlled transmission study using elvers. Eel picornavirus has a genome of 7,496 nucleotides that encodes a polyprotein of 2,259 amino acids. It has a typical picornavirus genome layout, but its low similarity to known viral proteins suggests a novel species in the family Picornaviridae.
TEXT
All members of the family Picornaviridae are small, nonenveloped, icosahedral viruses with positive-strand RNA genomes. Genome sizes range from 7,000 to 9,100 nucleotides. The 5′ end of the RNA is covalently linked to a small virus-encoded peptide (3B); the 3′ end is polyadenylated. The genome encodes one or two polyproteins that are processed co- and posttranslationally into 10 to 14 mature polypeptides. Cap-independent translation initiation is stimulated by one or two internal ribosome entry site (IRES) elements. Common to all picornaviruses are homologous capsid proteins and the nonstructural proteins 2C, 3Cpro, and 3Dpol. The sequences of other nonstructural proteins (leader protein, 2A, 2B, 3A, and 3B) are not conserved and may be unique to some picornavirus genera (1, 2).
The family Picornaviridae is currently comprised of 37 species grouped into 17 genera (Aphthovirus, Aquamavirus, Avihepatovirus, Cardiovirus, Cosavirus, Dicipivirus, Enterovirus, Erbovirus, Hepatovirus, Kobuvirus, Megrivirus, Parechovirus, Salivirus, Sapelovirus, Senecavirus, Teschovirus, and Tremovirus; http://ictvonline.org/). However, picornavirus taxonomy is still in flux, as next-generation sequencing technologies facilitate metagenomics studies using viral nucleic acid-containing specimens. Analysis of viral genomes without prior virus isolation led to the identification of more than 30 approved or tentative picornavirus species from humans (cosavirus [3], salivirus [4], saffold virus [5], and rhinovirus-C [6]), rodents (mosavirus, rosavirus, and murine kobuvirus [7]), pinnipeds (seal picornavirus [8] and California sea lion sapelovirus [9]), domestic cats and dogs (feline picornavirus [10], canine picornavirus [11], canine kobuvirus [12], and canine picodicistrovirus [2]), ungulates (bovine hungarovirus, ovine hungarovirus [13], swine pasivirus [14], and porcine kobuvirus [15]), birds (turdiviruses 1 to 3 [16], turkey hepatitis virus [17], pigeon picornaviruses A and B [18], quail picornavirus [19], and gallivirus [20]), and bats (bat picornaviruses 1 to 3 [21], bat kobuvirus-like virus [22], Rhinolophus affinis picornavirus, Ia io picornavirus, and Miniopterus schreibersii picornavirus [23]).
Although all published picornaviruses were identified in mammals and birds, there are reports of picornavirus-like viruses of reptiles (24, 25), fish (26–31), and marine invertebrates (32–35), indicating circulation of such viruses in aquatic ecosystems. Here, we describe a fish picornavirus which proves that picornavirus ecology indeed includes lower vertebrates of freshwater and probably marine ecosystems. Eel picornavirus 1 (EPV-1) was isolated from a European eel (Anguilla anguilla) from Lake Constance on the Rhine River. The viral genome contains an open reading frame (ORF) of 6,777 nucleotides (2,259 amino acids [aa]), and the predicted polyprotein displays the typical organization of a picornavirus. According to the Picornavirus Study Group criteria (www.picornastudygroup.com/definitions/genus_definition.htm), low similarity to the conserved P1 and 3CD precursor proteins of EPV-1 suggests a novel genus in the family Picornaviridae.
Starting in May 2005, an increased number of diseased or dead eels were observed in fish traps and aquacultures in the Lake Constance area. Diseased fish presented with increased mucus production, mucous ulcers, and reddened skin but no hemorrhages. One sample (F15/05) that was sent to the National Reference Laboratory for Fish Diseases, Insel Riems, Germany, included specimens of heart, spleen, kidney, liver, and brain. In routine bacteriology by a regional laboratory, only Aeromonas hydrophila was detected. By PCR, the genome of the anguillid herpesvirus (Herpesvirus anguillae [HVA]) was detected in the kidney. A cytopathogenic, chloroform-resistant virus could be isolated from all four organ samples using eel embryonic kidney (EK-1) cells [catalogue no. CCLV-RIE 809, Collection of Cell Lines in Veterinary Medicine (CCLV) of the Friedrich Loeffler Institut]. This virus exhibited a picornavirus-like appearance in electron microscopy (Fig. 1A) and was designated EPV-1 F15/05. After passaging of the virus, HVA-specific PCR was negative. EPV-1 F15/05 was propagated in EK-1 cells. Infected cell cultures were incubated at 26°C. Three to 4 days postinfection (p.i.), a cytopathic effect was visible (Fig. 1B).
Fig 1.
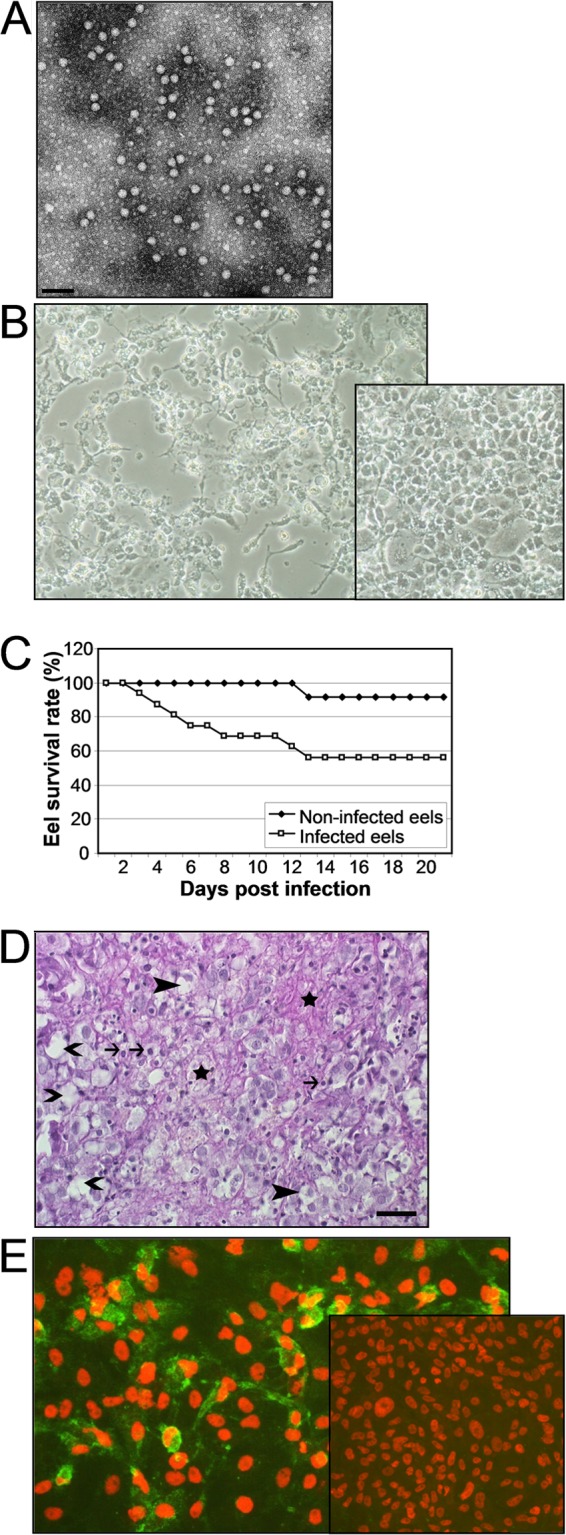
Characterization of eel picornavirus. (A) Electron micrograph of EPV-1 from supernatant of infected EK-1 cells. Bar, 100 nm. (B) Cytopathic effect of EK-1 cells induced by EPV-1 after 3 days p.i. The inset shows uninfected cells. (C) Survival rates of infected and control eels. Glass eels (n = 16) were infected with EPV-1 by bathing for 1 h in virus-containing water. A control group of 12 glass eels was mock infected. The eels were kept in aquaria for 21 days. One glass eel of the control group died after an injury. (D) Histopathological changes in the liver of an experimentally infected glass eel. Hepatocytes are irregularly vacuolated (closed arrowheads). Multiple cell membranes are disrupted (open arrowheads). There are karyopyknosis (arrows) and small areas of necrosis and fibrin exudation (stars). Bar = 25 μm. Hematoxylin-eosin stain. (E) Detection of EPV-1 by indirect immunofluorescence using the rabbit antiserum T51 (in-house production) and goat anti-rabbit IgG–FITC fluorescein isothiocyanate (Sigma), with counterstaining of nuclei with propidium iodide. The inset shows uninfected cells.
As EPV-1 was isolated from an eel with a mixed infection, its virulence was examined in a controlled infection experiment. A group of glass eels (length, approximately 10 cm; n = 16) was infected by bathing in virus-containing water (106 50% tissue culture infective doses [TCID50]/ml water) for 1 h. Subsequently, the eels were kept in 400-liter aquaria at a water temperature of 20°C for 21 days. A control group of eels (n = 12) was treated and kept under the same conditions and the same volume of an analogous cell culture medium without adding virus to the bath. Beginning 3 days p.i., infected eels died without external signs; at the end of the experiment, 7 of the 16 infected eels had died (43%) (Fig. 1C). In the untreated control group, one eel died in consequence of an injury. Histological examination of all organs, performed at the Centre for Fish and Wildlife Health, Bern, Switzerland, showed decreased numbers of fat vacuoles in hepatocytes of infected euthanized and dead eels compared to uninfected controls, increased single-cell necrosis, scattered small necrotic foci, and mild infiltrations of lymphocytes and macrophages in the liver parenchyma (Fig. 1D). In the kidneys, vacuolization and hyaline droplet degeneration of renal tubular epithelium and multiple small necroses in the interstitium were observed. Virus could be reisolated from 10 of 10 infected euthanized or dead eels and was identified as picornavirus by means of indirect immunofluorescence assay (Fig. 1E) and electron microscopy. No virus could be isolated from the control group.
The genome of EPV-1 was sequenced using the Illumina/Solexa method. Viral RNA was purified from EPV-infected EK-1 cells. Virus was released from infected cells showing cytopathic effect by three freeze-thaw steps. After pelleting of cell detritus (centrifugation at 4,000 × g for 20 min), virus was sedimented by ultracentrifugation (100,000 × g, 3 h, 4°C). RNA extraction from the pellets and sample preparation for Illumina sequencing were done as described previously (36). Sequencing was performed on a GAIIx (Illumina) instrument to create reads with lengths of 76 nucleotides (nt). For assembly, the reads were mapped to the NCBI nucleotide sequence database to identify similar picornavirus reads. These were used for de novo assembly using ABySS (37) with a k-mer length of 60. Four internal gaps of 73, 67, 60, and 34 nucleotides as well as the 3′ end (approximately 180 nt) and the very 5′ end were not covered by this approach (Fig. 2). The gaps and the 3′ end were sequenced by conventional Sanger sequencing as described previously (36). All attempts to amplify the very 5′ end by rapid amplification of 5′ cDNA ends (5′ RACE) failed.
Fig 2.
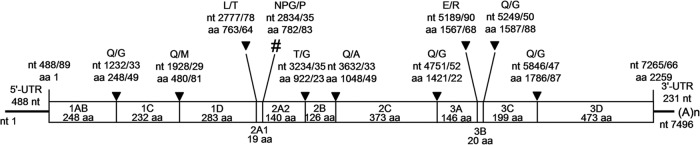
Schematic depiction of the eel picornavirus genome. The 5′ and 3′ UTRs and the ORF (box) are displayed. Filled triangles show the 3Cpro processing sites; the ribosomal skip sequence is indicated by a pound sign. (A)n, poly(A) tail. Nucleotide (nt) and amino acid (aa) positions of putative cleavage sites are given as well as the amino acid positions of the P1/P1′ sites.
As depicted in Fig. 2, the genome of EPV-1 exhibits a typical picornaviral genome organization and is comprised of 7,496 nucleotides with a 5′ untranslated region (UTR) of at least 488 nt, a large ORF of 6,777 nt, and a long 3′ UTR of 231 nt. The genome of EPV-1 has no obvious leader peptide-encoding gene region. Conserved Rhv domains (ranging from aa 130 to 245 and 485 to 630) were found in the capsid proteins 1AB and 1C (http://pfam.sanger.ac.uk/). The P1 precursor is most likely released by an aphthovirus-like 2A1 peptide. The 2A2 gene region exhibits the H-box/NC sequence motifs (H797YGV and N865CE), but the ALTXKAXXXKXXL motif, which is common to the 2A proteins of parechoviruses, is modified (A818TTXKVXXXKXXD). The 2B protein has homology to the 2B protein of parechoviruses (38), duck hepatitis A virus (DHAV-1) (39), and swine pasivirus (14) (Table 1). The 2C gene region exhibits three conserved sequence motifs (A, G1189XXGXGKS; B, Q1244YFHVIDDLAQ; C, K1283GMSYTSRVVIATTN) (reference 40 and literature cited therein). 3A shares some similarity with Ljungan virus (LV) (41) but no obvious homology to any other known picornavirus (Table 1). The N terminus of 3B (RAY1570) is homologous to that of the parechovirus 3B. The putative 3C protease region encodes a GXC1749G active site and a G1765XH substrate-binding pocket (40). Conserved motives of the 3D polymerase are the K1954DELR, G2081GMPSG, Y2122GDD, and F2170LKR sequences (40). Table 1 compares the predicted processing products of EPV-1 with those of its closest relatives. In general, EPV-1 exhibits low similarity to all known picornaviruses.
Table 1.
Pairwise amino acid identitiesa
| EPV-1 proteinb | % Identity with protein from: |
|||||
|---|---|---|---|---|---|---|
| Parechovirus HPeV-1 (L02971) | Parechovirus LV-1 (AF327920) | Avihepatovirus DHAV-1 (DQ249299) | Aquamavirus SePV-1 (EU142040) | Unassigned SPaV-1 (JQ316470) | Unassigned R. affinis PV-1 (JQ814853)c | |
| 1AB | 30.1 | 33.6 | 25.3 | 27.7 | 24.5 | 17.1 |
| 1C | 26.6 | 31.2 | 22.7 | 31.1 | 30.8 | 29.2 |
| 1D | 22.8 | 22.7 | 18.7 | 15.9 | 21.5 | 21.5 |
| 2A1d | 24.0 | 52.2 | 20.7 | 36.4 | 40.0 | |
| 2A2e | 27.9 | 33.8 | 30.0 | |||
| 2B | 28.6 | 25.8 | 21.3 | 13.3 | 19.7 | |
| 2C | 27.6 | 27.3 | 29.9 | 26.5 | 27.3 | |
| 3A | 8.8 | 20.3 | 12.9 | 11.0 | 12.2 | |
| 3C | 18.3 | 19.1 | 21.0 | 26.1 | 19.1 | |
| 3D | 34.6 | 41.6 | 38.6 | 29.0 | 31.3 | |
EPV, eel picornavirus; HPeV, human parechovirus; LV, Ljungan virus; DHAV, duck hepatitis A virus; SePV, seal picornavirus; SPaV, swine pasivirus; R. affinis PV, Rhinolophus affinis picornavirus.
Predicted proteins. 3B was excluded.
Partial sequence comprising 1AB, 1C, 1D, and 2A1.
Aphthovirus-like 2A peptide with cleavage at the NPG/P sequence.
2A protein with H-box/NC motif.
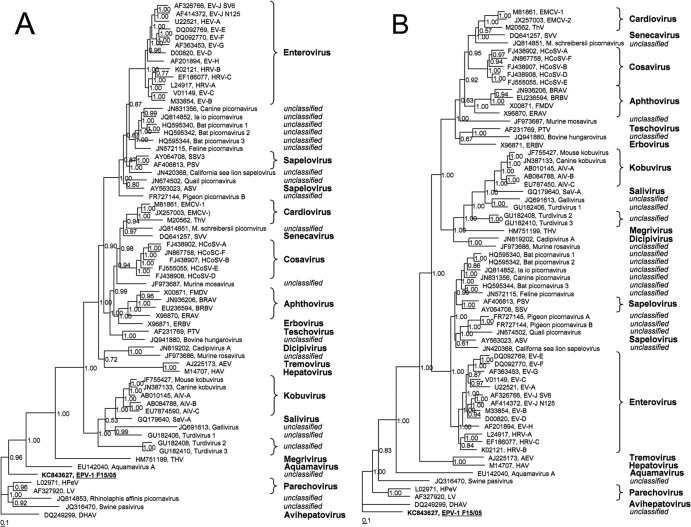
For phylogenetic analyses, the P1 gene region and the 3CD-encoding gene region of EPV-1 were compared to the corresponding gene regions of 64 picornaviruses representing all approved or tentative picornavirus species. Reference picornavirus sequences were retrieved from GenBank and used for comparisons and phylogenetic analyses. Nucleotide sequences were aligned with MEGA5 (42) and adjusted manually. For phylogenetic tree reconstruction, four Bayesian Metropolis-coupled Markov chains were calculated with MrBayes 3.1.2 (43) using an optimal substitution model. Modeltest implemented in MEGA5 suggested GTR+G+I for both analyses. Convergence was reached after 674,000 generations (P1 region) and 322,000 generations (3CD region). Both phylogenetic trees revealed that Parechovirus, Avihepatovirus, Aquamavirus, and the swine pasivirus are the closest relatives of EPV-1. In addition, the P1 gene region of Rhinolaphis affinis picornavirus is also related to EPV-1 (Fig. 3). Low amino acid identity as shown in Table 1 and Fig. 3 suggests that EPV-1 constitutes a novel picornavirus species and probably a novel picornavirus genus. Therefore, we propose the species name “Anguavirus.”
Fig 3.
Phylogenetic analyses of the P1-encoding (1AB-1D) (A) and the 3CD-encoding (B) gene regions of 65 picornavirus strains. Sixty-four sequences obtained from the GenBank and the EPV-1 sequence were included. The tree was inferred with MrBayes 3.1.2 using the GTR substitution model assuming gamma distribution and invariant sites. GenBank accession numbers, (tentative) species names, and strain designations are presented. The scale bar indicates substitutions per site. Numbers at nodes indicate posterior probabilities.
So far, few aspects of picornavirus ecology have been elucidated. Due to the fecal-oral transmission route, picornaviruses can be considered environmental viruses, and detection of enteroviruses, hepatitis A virus, and Aichi virus in shellfish (44, 45) as well as the identification of seal picornavirus (8) and an enterovirus from the bottlenose dolphin (46) confirms this view. However, PCR detection of picornaviruses does not necessarily indicate their replication in bivalve molluscs but suggests contamination with sewage and bioaccumulation. Viruses with picornavirus-like morphology in electron micrographs have been observed repeatedly in fish, reptiles, and invertebrates, but virus isolation and propagation in cultured cells have often failed (for example, see references, 24, 25, 28, 31, 32, 33, 35, 47, and 48). Therefore, isolation of EPV-1 from eels, experimental infection with induction of clinical signs, and reisolation constitute proof that picornaviruses indeed infect lower vertebrates and raise the question of how stable infection chains are maintained in aquatic ecosystems with high dilution and/or continuous outflow.
Nucleotide sequence accession number.
The RNA sequence of EPV-1 F15/05 was submitted to GenBank with accession no. KC843627.
ACKNOWLEDGMENTS
We thank Martina Müller and Ivonne Görlich for excellent technical assistance.
Footnotes
Published ahead of print 24 July 2013
REFERENCES
- 1.Knowles NJ, Hovi T, Hyypia T, King AMQ, Lindberg AM, Pallansch MA, Simmonds P, Skern T, Stanway G, Yamashita T, Zell R. 2012. Family Picornaviridae, p 855–880 In King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ. (ed), Virus taxonomy. Ninth report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press, Amsterdam, The Netherlands [Google Scholar]
- 2.Woo PCY, Lau SKP, Choi GKY, Huang Y, Teng JLL, Tsoi HW, Tse H, Yeung ML, Chan KH, Jin DY, Yuen KY. 2012. Natural occurrence and characterization of two internal ribosome entry site elements in a novel virus, canine picodicistrovirus, in the picornavirus-like superfamily. J. Virol. 86:2797–2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kapoor A, Victoria J, Simmonds P, Slikas E, Chieochansin T, Naeem A, Shaukat S, Sharif S, Alam MM, Angez M, Wang C, Shafter RW, Zaidi S, Delwart E. 2008. A highly prevalent and genetically diversified Picornaviridae genus in South Asian children. Proc. Natl. Acad. Sci. U. S. A. 105:20482–20487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li L, Victoria J, Kapoor A, Blinkova O, Wang C, Babrzadeh F, Mason CJ, Pandey P, Triki H, Bahri O, Oderinde BS, Baba MM, Bukbuk DN, Besser JM, Bartkus JM, Delwart EL. 2009. A novel picornavirus associated with gastroenteritis. J. Virol. 83:12002–12006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones MS, Lukashov VV, Ganac RD, Schnurr DP. 2007. Discovery of a novel human picornavirus in a stool sample from a pediatric patient presenting with fever of unknown origin. J. Clin. Microbiol. 45:2144–2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lau SK, Yip CC, Tsoi HW, Lee RA, So LY, Lau YL, Chan KH, Woo PC, Yuen KY. 2007. Clinical features and complete genome characterization of a distinct human rhinovirus (HRV) genetic cluster, probably representing a previously undetected HRV species, HRV-C, associated with acute respiratory illness in children. J. Clin. Microbiol. 45:3655–3664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phan TG, Kapusinszky B, Wang C, Rose RK, Lipton HL, Delwart EL. 2011. The fecal flora of wild rodents. PLoS Pathog. 7:e1002218. 10.1371/journal.ppat.1002218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kapoor A, Victoria J, Simmonds P, Wang C, Shafer RW, Nims R, Nielsen O, Delwart E. 2008. A highly divergent picornavirus in a marine mammal. J. Virol. 82:311–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li L, Shan T, Wang C, Cote C, Kolman J, Onions D, Gulland FMD, Delwart E. 2011. The fecal flora of California Sea lions. J. Virol. 85:9909–9917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lau SKP, Woo PCY, Yip CCY, Choi GKY, Wu Y, Bai R, Fan RYY, Lai KKY, Chan KH, Yuen KY. 2012. Identification of a novel feline picornavirus from the domestic cat. J. Virol. 86:395–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woo PCY, Lau SKP, Choi GKY, Yip CCY, Huang Y, Tsoi HW, Yuen KY. 2012. Complete genome sequence of a novel picornavirus, canine picornavirus, discovered in dogs. J. Virol. 86:3402–3403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li L, Pesavento PA, Shan T, Leutenegger CM, Wang C, Delwart E. 2011. Viruses in diarrhoeic dogs include novel kobuviruses and sapoviruses. J. Gen. Virol. 92:2534–2541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reuter G, Pankovics P, Knowles NJ, Boros A. 2012. Two closely related novel picornaviruses in cattle and sheep in Hungary from 2008 to 2009, proposed as members of a new genus in the family Picornaviridae. J. Virol. 86:13295–13302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sauvage V, Gouilh MA, Cheval J, Muth E, Pariente K, Burguiere A, Caro V, Manuguerra JC, Eloit M. 2012. A member of the new Picornaviridae genus is shed in pig feces. J. Virol. 86:10036–10046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reuter G, Boldizsár A, Kiss I, Pankovics P. 2008. Candidate new species of kobuvirus in porcine hosts. Emerg. Infect. Dis. 14:1968–1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woo PCY, Lau SKP, Huang Y, Lam CSF, Poon RWS, Tsoi HW, Lee P, Tse H, Chan ASL, Luk G, Chan KH, Yuen KY. 2010. Comparative analysis of six genome sequences of three novel picornaviruses, turdiviruses 1, 2 and 3, in dead wild birds, and proposal of two novel genera, Orthoturdivirus and Paraturdivirus, in the family Picornaviridae. J. Gen. Virol. 91:2433–2448 [DOI] [PubMed] [Google Scholar]
- 17.Honkavuori KS, Shivaprasad LL, Briese T, Street C, Hirschberg DL, Hutchison SK, Lipkin WI. 2011. Novel picornavirus in turkey poults with hepatitis, California, USA. Emerg. Infect. Dis. 17:480–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kofstad T, Jonassen CM. 2011. Screening of feral and wood pigeons for viruses harbouring a conserved mobile viral element: Characterization of novel astroviruses and picornaviruses. PLoS One 6:e25964. 10.1371/journal.pone.0025964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pankovics P, Boros A, Reuter G. 2012. Novel picornavirus in domesticated common quail (Coturnix coturnix) in Hungary. Arch. Virol. 157:525–530 [DOI] [PubMed] [Google Scholar]
- 20.Farkas T, Fey B, Hargitt E, Parcells M, Ladman B, Murgia M, Saif Y. 2012. Molecular detection of novel picornaviruses in chickens and turkeys. Virus Genes 44:262–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lau SK, Woo PC, Lai KK, Huang Y, Yip CC, Shek CT, Lee P, Lam CS, Chan KH, Yuen KY. 2011. Complete genome analysis of three novel picornaviruses from diverse bat species. J. Virol. 85:8819–8828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li L, Victoria JG, Wang C, Jones M, Fellers GM, Kunz TH, Delwart E. 2010. Bat guano virome: predominance of dietary viruses from insects and plants plus novel mammalian viruses. J. Virol. 85:6955–6965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Z, Ren X, Yang L, Hu Y, Yang J, He G, Zhang J, Dong J, Sun L, Du J, Liu L, Xue Y, Wang J, Yang F, Zhang S, Jin Q. 2012. Virome analysis for identification of novel mammalian viruses in bat species from Chinese provinces. J. Virol. 86:10999–11012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Essbauer S, Ahne W. 2001. Viruses of lower vertebrates. J. Vet. Med. B Infect. Dis. Vet. Public Health 48:403–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heldstab A, Bestetti G. 1984. Virus associated gastrointestinal diseases in snakes. J. Zoo Animal Med. 15:118–128 [Google Scholar]
- 26.Ahne W, Anders K, Halder M, Yoshimizu M. 1990. Isolation of picornavirus-like particles from the European smelt, Osmerus eperlanus (L.). J. Fish Dis. 13:167–168 [Google Scholar]
- 27.Bloch B, Gravningen K, Larsen JL. 1991. Encephalomyelitis among turbot associated with a picornavirus-like agent. Dis. Aquat. Org. 10:65–70 [Google Scholar]
- 28.Hetrick FM, Hedrick RP. 1993. New viruses described in finfish from 1988–1992. Annu. Rev. Fish Dis. 3:187–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwanowicz LR, Goodwin AE, Heil N. 2000. A small RNA virus isolated from apparently healthy wild sandbar shiners, Notropis scepticus (Jordan & Gilbert). J. Fish Dis. 23:349–352 [Google Scholar]
- 30.Moore AR, Li MF, McMenemy M. 1988. Isolation of a picorna-like virus from smelt, Osmerus mordax (Mitchill). J. Fish Dis. 11:179–184 [Google Scholar]
- 31.Sano T. 1995. Viruses and viral diseases of salmonids. Aquaculture 132:43–52 [Google Scholar]
- 32.Ahne W. 1994. Viral infections of aquatic animals with special reference to Asian aquaculture. Annu. Rev. Fish Dis. 4:375–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson PT. 1978. Viral diseases of the blue crab, Callinectes sapidus. Mar. Fish. Rev. 40:13–15 [Google Scholar]
- 34.Kuris AM, Poinar GO, Jr, Hess R, Morris TJ. 1979. Virus particles in an internal parasite Portunion conformis (Crustacea: Isopoda), and its marine crab host, Hemigrapsus oregonesis. J. Invertebrate Pathol. 34:26–31 [Google Scholar]
- 35.Rasmussen LPD. 1986. Virus-associated granulocytomas in the marine mussel, Mytilus edulis, from three sites in Denmark. J. Invertebrate Pathol. 48:117–123 [Google Scholar]
- 36.Philipps A, Dauber M, Groth M, Schirrmeier H, Platzer M, Krumbholz A, Wutzler P, Zell R. 2012. Isolation and molecular characterization of a second serotype of the encephalomyocarditis virus. Vet. Microbiol. 161:49–57 [DOI] [PubMed] [Google Scholar]
- 37.Birol I, Jackman SD, Nielsen CB, Qian JQ, Varhol R, Stazyk G, Morin RD, Zhao Y, Hirst M, Schein JE, Horsman DE, Connors JM, Gascoyne RD, Marra MA, Jones SJ. 2009. De novo transcriptome assembly with ABySS. Bioinformatics 25:2872–2877 [DOI] [PubMed] [Google Scholar]
- 38.Hyypiä T, Horsnell C, Maaronen M, Khan M, Kalkinnen N, Auvinen P, Kinnunen L, Stanway G. 1992. A distinct picornavirus group identified by sequence analysis. Proc. Natl. Acad. Sci. U. S. A. 89:8847–8851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tseng CH, Knowles NJ, Tsai HJ. 2007. Molecular analysis of duck hepatitis virus type 1 indicates that it should be assigned to a new genus. Virus Res. 123:190–203 [DOI] [PubMed] [Google Scholar]
- 40.Koonin EV, Dolja VV. 1993. Evolution and taxonomy of positive-strand RNA viruses: implications of comparative analysis of amino acid sequences. Crit. Rev. Biochem. Mol. Biol. 28:375–430 [DOI] [PubMed] [Google Scholar]
- 41.Johansson S, Niklasson B, Maizel J, Gorbalenya AE, Lindberg AM. 2002. Molecular analysis of three Ljungan virus isolates reveals a new, close-to-root lineage of the Picornaviridae with a cluster of two unrelated 2A proteins. J. Virol. 76:8920–8930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tamura K, Peterson D, Peterson M, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574 [DOI] [PubMed] [Google Scholar]
- 44.Di Pinto A, Conversano MC, Forte VT, La Salandra G, Montervino C, Tantillo GM. 2004. A comparison of RT-PCR based assays for the detection of HAV from shellfish. New Microbiol. 27:119–124 [PubMed] [Google Scholar]
- 45.Hansman GS, Oka T, Li TC, Nishio O, Noda M, Takeda N. 2008. Detection of human enteric viruses in Japanese clams. J. Food Prot. 71:1689–1695 [DOI] [PubMed] [Google Scholar]
- 46.Nollens HH, Rivera R, Palacios G, Wellehan JFX, Saliki JT, Caseltine SL, Smith CR, Jensen ED, Hui J, Lipkin WI, Yochem PK, Wells RS, St Leger J, Venn-Watson S. 2009. New recognition of enterovirus infections in bottlenose dolphins (Tursiops truncatus). Vet. Microbiol. 139:170–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glazebrook JS, Heasman MP, de Beer SW. 1990. Picorna-like viral particles associated with mass mortalities in larval barramundi, Lates calcarifer Bloch. J. Fish Dis. 13:245–249 [Google Scholar]
- 48.Yoshikoshi K, Inoue K. 1990. Viral nervous necrosis in hatchery-reared larvae and juveniles of Japanese parrotfish, Oplegnathus fasciatus (Temminck & Schlegel). J. Fish Dis. 13:69–77 [Google Scholar]