Abstract
Lassa fever (LF) is a potentially lethal human disease that is caused by the arenavirus Lassa virus (LASV). Annually, around 300,000 infections with up to 10,000 deaths occur in regions of Lassa fever endemicity in West Africa. Here we demonstrate that mice lacking a functional STAT1 pathway are highly susceptible to infection with LASV and develop lethal disease with pathology similar to that reported in humans.
TEXT
Lassa virus (LASV) is a member of the family Arenaviridae that is endemic to West Africa (1). LASV is an enveloped virus with a genome consisting of two single-stranded RNA segments, referred to as small (S) and large (L). Each segment uses an ambisense coding strategy to direct the synthesis of two viral gene products. The S segment encodes the glycoprotein precursor protein (GPC) and the nucleoprotein (NP). The L segment encodes the viral RNA-dependent RNA polymerase (L) and a small RING finger protein, Z, that has bona fide matrix functions (2). LASV is the etiologic agent of Lassa fever (LF), a severe hemorrhagic fever disease associated with high morbidity and mortality rates (up to 25% in hospitalized patients) (3, 4). Individuals that succumb to LF develop either limited or no humoral immune response to LASV (5), and convalescence in survivors begins only weeks after infection has been cleared (6). Histopathologically, LF is characterized by mild immune cell infiltration and only modest signs of tissue damage (7). A high level of viremia is a strong predictor of a poor disease outcome in LASV-infected patients (8).
Currently, outbred Hartley and inbred strain 13 guinea pigs are available for experimental LASV infection. However, LASV is myocardiotropic and myocardiophatic in guinea pigs, phenotypes which have not been reported in humans, and does not exhibit the florid hepatotropic phenotype characteristic of human LF (9). The development of a mouse model of LASV infection would represent a significant advance for the investigation of LASV pathogenesis and development of antiviral strategies. Most laboratory mouse strains are resistant to the Josiah strain of LASV commonly used in research and rapidly clear the virus. Notably, HHD mice expressing human/mouse-chimeric HLA-A2.1 instead of murine major histocompatibility complex class I (MHC-I) molecules have been reported to develop severe illness following LASV infection. Depletion of T cells prevents disease development in LASV-infected HHD mice despite high-level viremia, suggesting the importance of immunopathology in LF (10). These findings indicate that suboptimal induction of an immune response to LASV infection may serve as a causative factor in the development of pathological lesions. However, this phenomenon has not been confirmed in nonhuman primate models of LASV infection and human cases of LF. Moreover, only one of several tested LASV strains administered intravenously at a high dose resulted in disease symptoms in HHD mice (10). Therefore, the relevance of this model to human disease is still unclear. Recently, we established that disruption of type I and type II interferon (IFN) signaling results in enhanced, nonlethal, disseminated LASV infection in mice (11). Here, we present an improved mouse model, in which LASV causes severe disease with lethal outcome and pathology with similarities to human LF.
Recombinant Lassa virus generated from cloned cDNAs via reverse genetics and its parental natural isolate have similar in vitro growth properties.
To ensure the nature of the genetic makeup and biological properties of the LASV used in our studies and prevent the confounding factors due to mutations accumulated during propagation in cell culture of the parental natural isolate, we developed an RNA polymerase I/polymerase II (pol-I/II) reverse genetics system for the rescue of a molecular clone of the highly pathogenic Josiah strain of LASV using methods we have previously described for Junin virus (12). Briefly, intracellular synthesis of both the L and S genome RNA segments (Fig. 1A) is driven by a pol-I promoter using appropriate plasmid constructs (13), whereas the minimal viral trans-acting factors, NP and L, required for virus RNA replication and gene expression are supplied by pol-II-driven expression plasmids (14, 15). Intriguingly, we found that the orientation of the insertion dramatically influenced the genetic stability in bacteria of the pol-I plasmid coding for the full-length L RNA (Fig. 1B). Thus, the construct that contains the cDNA for the L RNA in an antigenomic orientation with respect to the pol-I promoter (Fig. 1B, upper left) was significantly more prone to large deletions than the construct containing the same insert in the opposite genomic orientation (Fig. 1B, upper left). However, pol-I plasmids with either orientation were successfully used to rescue recombinant LASV (rLASV) with similar levels of efficiency (data not shown). To rescue rLASV, BHK-21 cells were transfected with the four plasmids described above, and tissue culture supernatants (TCS) collected at 72 h posttransfection (Fig. 1C, P0) were used to infect fresh monolayers of Vero cells to produce stocks with increased virus titers. TCS collected at 72 h postinfection (p.i.) from Vero cells (P1) consistently had titers of ∼106 PFU/ml (Fig. 1C, P1). The genome of the rescued virus was fully sequenced, and we did not find any mutations between rLASV and parental LASV Josiah. We next assessed whether the rescued rLASV exhibited growth properties in cultured cells similar to those of the parental natural LASV isolate. For this, we infected Vero cells (multiplicity of infection [MOI] = 0.1) with either LASV or rLASV and, at the indicated times after infection, determined titers of infectious virus in TCS. LASV and rLASV displayed similar growth properties (Fig. 1D). Likewise, rLASV exhibited plaquing efficiency, as well as plaque size and morphology, similar to that of its wild-type counterpart (Fig. 1E).
Fig 1.
Rescue of recombinant LASV from cloned cDNAs. (A) Schematic representation of LASV genome (not drawn to scale). (B) Pol-I promoter-driven constructs containing full-length L and S RNA genome segments. (C) P0 and P1 titers of rLASV were determined by plaque assay at 3 days posttransfection (dpt) or postinoculation (dpi). (D) TCS of Vero cells infected with a 0.1 MOI of either wild-type LASV (wtLASV) or rLASV were collected at the indicated times, and titers were determined by plaque assay. (E) Monolayers of Vero cells were infected with either rLASV or wtLASV and then overlaid with culture medium containing 0.5% agarose. Cells were fixed with formaldehyde at 6 days p.i. and stained with crystal violet.
rLASV and its parental natural isolate LASV exhibited similar in vivo biological properties.
To ensure that the rescued rLASV exhibited in vivo biological properties similar to those displayed by the parental natural LASV isolate, we infected mice deficient for alpha/beta IFN receptor (IFNAR−/−) with rLASV and compared the outcomes to our previous experiments where we used LASV to infect IFNAR−/− mice (11). Mice of matching ages and sexes were infected with the same dose by the same route of administration (104 PFU administered intraperitoneally [i.p.]), as described previously (9). Consistent with our published results, all IFNAR−/− mice survived the rLASV challenge but showed signs of disease, including ruffled fur and hypoactivity, and developed a persistent infection. As with the results seen with LASV, mice infected with rLASV experienced mild weight loss, but fever was not observed in any of the experimental animals (reference 11 and data not shown).
Mice lacking functional Stat1 signaling were susceptible to lethal LASV infection.
Mice lacking functional type I or both type I and II IFN receptors developed enhanced but nonlethal disease upon infection with LASV. To further investigate immune pathways that would allow us to develop a lethal mouse model of LASV, we infected 6-to-8-week-old mice with homozygous disruption of the Stat1 gene (Stat1−/−) with 104 PFU of rLASV (n = 12) or a mock infection (n = 9) via the i.p. route and monitored the animals daily for disease development and survival. In contrast to the mild disease observed in rLASV-infected type I/II IFN receptor knockout (KO) mice, all rLASV-infected Stat1−/− mice rapidly lost weight (∼18%) between days 4 and 6 and by day 7 p.i. either succumbed to infection or reached the monitoring criteria that called for euthanasia (>20% body weight loss and hypothermia) (Fig. 2). Temperature measurements at day 3 and 6 p.i. did not detect fever, but we cannot exclude the possibility that infected mice experienced a temporary fever between these days that was followed by hypothermia (Fig. 2C).
Fig 2.
Survival and changes in body weight and temperature of LASV-infected Stat1−/− mice. (A) rLASV-, LF2384-, and mock-infected Stat1−/− mice were monitored for survival for 16 days. (B and C) Body weight (B) and temperature (C) changes were recorded throughout the course of study. Average values and standard deviations (SD) are shown. LASV, Josiah strain of LASV; LF2384, human isolate LF2384-NS-DIA-1 of LASV.
To assess the occurrence and levels of viremia and viral spread into the visceral organs and brains, three randomly selected mice in each group were bled and euthanized on day 3 p.i. Also, blood samples were collected from all animals that were euthanized and subjected to necropsy on day 7 p.i. Viral loads in tissue homogenates and serum samples were determined by plaque assay (16). All rLASV-infected Stat1−/− mice developed disseminated infection by day 3 p.i. (Fig. 3A). Titers of infectious virus in the brain and all visceral organs of rLASV-infected mice increased over time, with the highest titers being observed at 7 days p.i. (Fig. 3B). We detected early viremia on day 3 p.i. at a level that remained high until mice were terminally ill (Fig. 3C).
Fig 3.
Organ virus titers and levels of serum viremia in Stat1−/− mice infected with LASV. (A and B) Necropsy was performed on mice infected with either rLASV or human isolate LF2384-NS-DIA-1 (LF2384) and euthanized on day 3 (A) or 7 (B) p.i. Organs were homogenized, and virus titers were determined by plaque assay. (C) Blood was collected at the indicated days postinfection, and virus titers in sera were determined by plaque assay. Filled and open symbols represent samples obtained from mice infected with rLASV and LF2384, respectively. Horizontal dashes indicate average titer values.
To evaluate the pathological changes in rLASV-infected Stat1−/− mice, we examined hematoxylin-eosin-stained tissue sections from mice euthanized on day 7 p.i. (Fig. 4A). Prominent histopathological lesions were observed in the spleen and liver of rLASV-infected Stat1−/− mice. The spleen sections showed accumulation of eosinophilic material in the red pulp surrounding the periarteriolar lymphocytic sheaths. Portions of the eosinophilic material were fibrillar and suggestive of fibrin in Stat1−/− mice. The splenic periarteriolar sheaths contained numerous macrophages containing fragments of apoptotic nuclei. Further examination of ultrathin spleen sections by transmission electron microscopy (17) revealed increased numbers of apoptotic/necrotic cells in the red pulp (Fig. 4Aii and 5A) and fibrin deposits (Fig. 5), which were similar to the findings in human cases (18). Liver sections showed marked microvesicular steatosis and the presence of apoptotic nuclear fragments in the hepatic sinusoids (Fig. 4Aiv). None of the lesions observed in rLASV-infected animals were detected in mock-infected mice (Fig. 4Ai and 4Aiii). Histopathological results were in agreement with our hematological findings showing that white blood cell and monocyte counts were increased in rLASV-infected Stat1−/− mice as early as 3 days p.i. Decreased production of albumin and increased levels of alanine aminotransferase confirmed hepatic involvement in these animals. Moderately decreased platelet counts were also observed (Fig. 4B).
Fig 4.
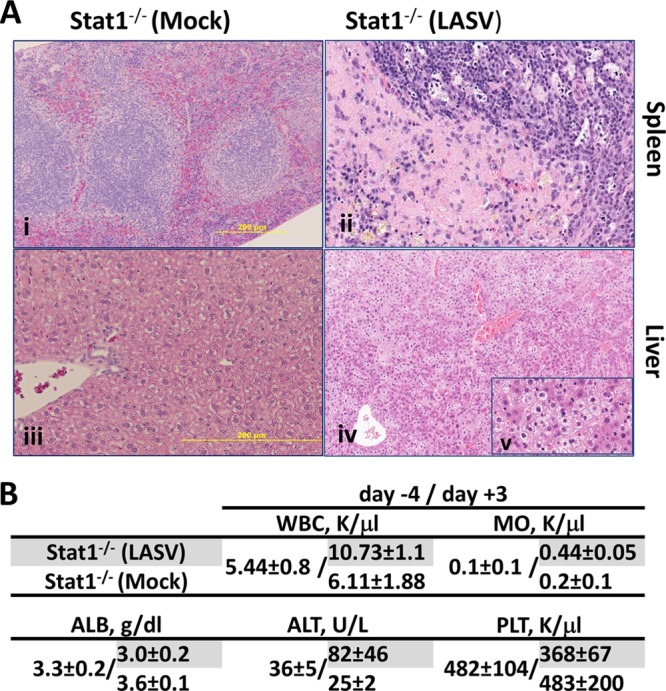
Comparative histopathology and hematological parameters of Stat1−/− mice infected with rLASV. (A) Representative hematoxylin-and-eosin-stained tissue sections from animals euthanized on day 7 p.i. are shown. Magnification, (i) ×20, (ii) ×34, (iii) ×40, (iv) ×9, and (v) ×40. (B) Whole-blood samples were collected preinfection (day −4) and postinfection (day +3). White blood cell (WBC), monocyte (MO), and platelet (PLT) counts were determined using a Hemavet 1700 system (Drew Scientific, Inc.). Levels of albumin (ALB) and alanine aminotransferase (ALT) were determined using VetScan VS2 (Abaxis).
Fig 5.
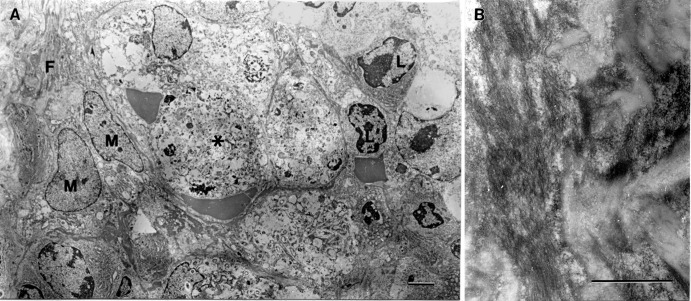
Ultrastructure of spleen in LASV-infected Stat1−/− mice. (A) Area of spleen showing fibrin accumulation (F) and adjacent zone of necrosis (asterisk). L, nuclei of normal lymphocytes; M, nuclei of normal macrophages surrounding the necrotic area. Bar = 2 um. (B) High power magnification of a fibrin accumulation area. Bar = 1 um.
The Josiah strain of LASV used to generate the rLASV was associated with a history of passages in cultured cells. We therefore examined whether a human LASV isolate with no passage history in cultured cells would also induce a severe disease in Stat1−/− mice. For this, we inoculated (n = 3) Stat1−/− mice with 104 PFU of the recent LASV isolate LF2384-NS-DIA-1 that we obtained directly from the serum sample of a patient who succumbed to LF in Sierra Leone. All infected animals exhibited disease signs and developed fever about day 4 to 5 p.i. and, similarly to Josiah strain-infected mice, had high titers of infectious virus in the brain, visceral organs, and blood (Fig. 3B and C) and became terminally ill on day 7 after inoculation (Fig. 2).
In summary, we have reported here that Stat1−/− mice infected with LASV developed a disseminated infection that was associated with lethal disease. These results allowed us to speculate that these animals develop more severe disease and pathology in organs than IFN receptor KO mice upon infection with LASV. Importantly, these mice had necrosis, apoptosis, and fibrin deposits in spleen and extensive hepatocellular injury manifesting as microsteatosis in liver, and some of the lesions were highly similar to those observed in human cases of LF (18, 19). The final experiment also demonstrated that this model is highly permissive for human isolates of LASV that have not been highly passaged in cell culture, enabling scientists to utilize it for testing of vaccines and/or antiviral drugs.
ACKNOWLEDGMENTS
N.E.Y. was supported by funding from the Institute for Human Infections and Immunity, University of Texas Medical Branch. A.V.S. was supported by the Biodefense Training Program, NIH grant T32-AI060549. This work was supported in part by National Institute of Allergy and Infectious Diseases Public Health Service grant UC7AI094660 (N.E.Y., S.P., and M.M.).
Footnotes
Published ahead of print 31 July 2013
REFERENCES
- 1.Salvato MS, Clegg JCS, Buchmeier MJ, Charrel RN, Gonzales JP, Lukashevich IS, Peters CJ, Rico-Hesse R, Romanowski V. 2012. Family Arenaviridae, p 714–723 In King AMQ, Adams MJ, Carstens EB, Lefkowitz EF. (ed), Virus taxonomy: ninth report of the International Committee on Taxonomy of Viruses. Academic Press, New York, NY [Google Scholar]
- 2.Buchmeier MJ, Peters CJ, de la Torre JC. 2007. Arenaviridae: the viruses and their replication, p 1792–1827 In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE. (ed), Fields virology 5th ed, vol 2 Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 3.Fisher-Hoch SP, McCormick JB. 1987. Pathophysiology and treatment of Lassa fever. Curr. Top. Microbiol. Immunol. 134:231–239 [DOI] [PubMed] [Google Scholar]
- 4.Fisher-Hoch SP, Tomori O, Nasidi A, Perez-Oronoz GI, Fakile Y, Hutwagner L, McCormick JB. 1995. Review of cases of nosocomial Lassa fever in Nigeria: the high price of poor medical practice. BMJ 311:857–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baize S, Kaplon J, Faure C, Pannetier D, Georges-Courbot MC, Deubel V. 2004. Lassa virus infection of human dendritic cells and macrophages is productive but fails to activate cells. J. Immunol. 172:2861–2869 [DOI] [PubMed] [Google Scholar]
- 6.Jahrling PB, Frame JD, Rhoderick JB, Monson MH. 1985. Endemic Lassa fever in Liberia. IV. Selection of optimally effective plasma for treatment by passive immunization. Trans. R. Soc. Trop. Med. Hyg. 79:380–384 [DOI] [PubMed] [Google Scholar]
- 7.Yun NE, Walker DH. 2012. Pathogenesis of Lassa fever. Viruses 4:2031–2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson KM, McCormick JB, Webb PA, Smith ES, Elliott LH, King IJ. 1987. Clinical virology of Lassa fever in hospitalized patients. J. Infect. Dis. 155:456–464 [DOI] [PubMed] [Google Scholar]
- 9.Walker DH, Wulff H, Lange JV, Murphy FA. 1975. Comparative pathology of Lassa virus infection in monkeys, guinea pigs, and Mastomys natalensis. Bull. World Health Organ. 52:523–535 [PMC free article] [PubMed] [Google Scholar]
- 10.Flatz L, Rieger T, Merkler D, Bergthaler A, Regen T, Schedensack M, Bestmann L, Verschoor A, Kreutzfeldt M, Brück W, Hanisch U-K, Günther S, Pinschewer DD. 2010. T cell-dependence of Lassa fever pathogenesis. PLoS Pathog. 6:e1000836. 10.1371/journal.ppat.1000836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yun NE, Poussard AL, Seregin AV, Walker AG, Smith JK, Aronson JF, Smith JN, Soong L, Paessler S. 2012. Functional interferon system is required for clearance of Lassa virus. J. Virol. 86:3389–3392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emonet SF, Seregin AV, Yun NE, Poussard AL, Walker AG, de la Torre JC, Paessler S. 2011. Rescue from cloned cDNAs and in vivo characterization of recombinant pathogenic Romero and live-attenuated Candid #1 strains of Junin virus, the causative agent of Argentine hemorrhagic fever disease. J. Virol. 85:1473–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flatz L, Bergthaler A, de la Torre JC, Pinschewer DD. 2006. Recovery of an arenavirus entirely from RNA polymerase I/II-driven cDNA. Proc. Natl. Acad. Sci. U. S. A. 103:4663–4668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee KJ, Novella IS, Teng MN, Oldstone MB, de La Torre JC. 2000. NP and L proteins of lymphocytic choriomeningitis virus (LCMV) are sufficient for efficient transcription and replication of LCMV genomic RNA analogs. J. Virol. 74:3470–3477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee KJ, Perez M, Pinschewer DD, de la Torre JC. 2002. Identification of the lymphocytic choriomeningitis virus (LCMV) proteins required to rescue LCMV RNA analogs into LCMV-like particles. J. Virol. 76:6393–6397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yun NE, Linde NS, Dziuba N, Zacks MA, Smith JN, Smith JK, Aronson JF, Chumakova OV, Lander HM, Peters CJ, Paessler S. 2008. Pathogenesis of XJ and Romero strains of Junin virus in two strains of guinea pigs. Am. J. Trop. Med. Hyg. 79:275–282 [PMC free article] [PubMed] [Google Scholar]
- 17.Moncayo AC, Hice CL, Watts DM, Travassos de Rosa APA, Guzman H, Russell KL, Calampa C, Gozalo A, Popov VL, Weaver SC, Tesh RB. 2001. Allpahuayo virus: a newly recognized arenavirus (Arenaviridae) from arboreal rice rats (Oecomys bicolor and Oecomys paricola) in northeastern Peru. Virology 284:277–286 [DOI] [PubMed] [Google Scholar]
- 18.Walker DH, McCormick JB, Johnson KM, Webb PA, Komba-Kono G, Elliott LH, Gardner JJ. 1982. Pathologic and virologic study of fatal Lassa fever in man. Am. J. Pathol. 107:349–356 [PMC free article] [PubMed] [Google Scholar]
- 19.McCormick JB, Walker DH, King IJ, Webb PA, Elliott LH, Whitfield SG, Johnson KM. 1986. Lassa virus hepatitis: a study of fatal Lassa fever in humans. Am. J. Trop. Med. Hyg. 35:401–407 [DOI] [PubMed] [Google Scholar]