Abstract
A central feature of herpesvirus biology is the ability of herpesviruses to remain latent within host cells. Classically, exposure to inducing agents, like activating cytokines or phorbol esters that stimulate host cell signal transduction events, and epigenetic agents (e.g., butyrate) was thought to end latency. We recently showed that Kaposi's sarcoma-associated herpesvirus (KSHV, or human herpesvirus-8 [HHV-8]) has another, alternative emergency escape replication pathway that is triggered when KSHV's host cell undergoes apoptosis, characterized by the lack of a requirement for the replication and transcription activator (RTA) protein, accelerated late gene kinetics, and production of virus with decreased infectivity. Caspase-3 is necessary and sufficient to initiate the alternative replication program. HSV-1 was also recently shown to initiate replication in response to host cell apoptosis. These observations suggested that an alternative apoptosis-triggered replication program might be a general feature of herpesvirus biology and that apoptosis-initiated herpesvirus replication may have clinical implications, particularly for herpesviruses that almost universally infect humans. To explore whether an alternative apoptosis-initiated replication program is a common feature of herpesvirus biology, we studied cell lines latently infected with Epstein-Barr virus/HHV-4, HHV-6A, HHV-6B, HHV-7, and KSHV. We found that apoptosis triggers replication for each HHV studied, with caspase-3 being necessary and sufficient for HHV replication. An alternative apoptosis-initiated replication program appears to be a common feature of HHV biology. We also found that commonly used cytotoxic chemotherapeutic agents activate HHV replication, which suggests that treatments that promote apoptosis may lead to activation of latent herpesviruses, with potential clinical significance.
INTRODUCTION
Herpesviruses can productively infect cells or remain latent within the host cell for long periods of time, enabling herpesviruses to cause lifelong infections (reviewed in reference 1). For some herpesviruses, notably gammaherpesviruses, latency is typically the default replication program. Latent virus can reactivate, even after many years, and replicate lytically. Classically, the end of latency and the initiation of lytic replication can be triggered by activation signals, such as proinflammatory cytokines (2, 3), and more potent activators of signal transduction cascades, such as the diacyl glycerol homolog tetradecanoyl phorbol acetate (TPA) (4), and by agents that alter chromatin structure, like histone deacetylase inhibitors such as butyrate (5).
Host cell apoptosis is clearly a threat to a latent herpesvirus: if the host cell completes the apoptotic process before virus progeny is produced, the virus has no chance of infecting a new cell. Presumably because host cell apoptosis poses an extreme threat, herpesviruses have evolved many mechanisms that aim to prevent host cell apoptosis. For example, the Kaposi's sarcoma-associated herpesvirus (KSHV, or human herpesvirus-8 [HHV-8]) genome encodes viral homologs of Flice-inhibitory protein (vFLIP) (open reading frame [ORF] K13/vFLIP) (6), antiapoptotic homologs of Bcl-2 (7), and a glycoprotein homologous to survivin (8), among others. The genomes of other herpesviruses encode proteins with analogous antiapoptotic functions (6, 9, 10).
While herpesviruses devote considerable genomic resources to preventing their host cells from undergoing apoptosis, these efforts may sometimes fail. If apoptosis proceeds, the latent virus will not be able to successfully reproduce, but recent data suggest that herpesviruses have another way to cope with the challenges posed by host cell apoptosis. At least two herpesviruses apparently can replicate via an alternative, accelerated replication pathway that offers the virus some chance of reproducing before the completion of host cell apoptosis makes viral reproduction impossible. We recently found, for KSHV, that when the virus detects that the host cell is undergoing apoptosis, it adopts an emergency escape alternative replication program (ARP) (11). The KSHV ARP is characterized by an absence of a requirement for the replication and transcription activator (RTA) protein, the product of open reading frame (ORF) 50, a protein that was previously believed to be essential for KSHV replication, an accelerated pattern of late gene expression, and the production of large amounts of virus with decreased infectivity. Herpes simplex virus 1 (HSV-1) has also recently been found to have an alternative replication program triggered by host cell apoptosis in a caspase-3-dependent manner, in the case of HSV-1, in latently infected ganglion cells when nerve growth factor (NGF) activity was withdrawn by exposure to anti-NGF monoclonal antibody (12, 13). The alternative apoptosis-associated replication program of HSV-1 also has a dysregulated pattern of gene expression.
Given that HSV-1, an alphaherpesvirus, apparently has an apoptosis-initiated ARP (12, 13) and our recent findings that the gammaherpesvirus KSHV had an ARP (11), we hypothesized that an ARP may be a universal feature of herpesvirus biology and decided to explore that hypothesis by determining if human betaherpesviruses, represented by HHV-6A, -6B, and -7, and another human gammaherpesvirus, Epstein-Barr virus (EBV, or HHV-4), had apoptosis-initiated replication programs. In addition to the fact that these viruses represent a cross-section of human herpesvirus families, we also chose these herpesviruses because good latently infected cell line model systems exist for them and because they cause close to universal infections in humans by an early age (14–17), making the clinical and translational implications of the studies more compelling. These viruses have been associated with significant adverse clinical outcomes in clinical situations in which substantial numbers of cells may be undergoing apoptosis, such as treatment with cytotoxic cancer chemotherapeutic agents, solid organ and bone marrow transplantation (18, 19), and severe hypersensitivity and autoimmune disorders (20–24), and in patients critically ill from a variety of causes (25). Human herpesviruses have been implicated in a variety of incompletely understood clinical disorders (26), some of which may be associated with apoptosis. EBV is also known to cause lymphomas and nasopharyngeal carcinoma (reviewed in references 27 and 28).
We found that apoptosis, or more specifically, caspase-3 activity, triggers the replication of EBV and of HHV-6A, -6B, and -7, which along with the previously published studies (11, 13) showing that caspase-3 activity triggers the replication of KSHV and HSV-1, suggests that apoptosis initiation of viral replication via a caspase-3-dependent mechanism is a widespread feature of herpesvirus biology. We also found that apoptosis triggered herpesvirus replication when the cells latently infected with the viruses were treated with commonly employed cytotoxic cancer chemotherapeutic agents. Since several herpesviruses, including EBV, and HHV-6A, -6B, and -7, cause close to universal infections in humans, the findings imply that exposure to conditions that promote apoptosis may broadly activate latent herpesviruses within most or all humans, with potentially significant negative clinical consequences.
MATERIALS AND METHODS
Cell culture.
BCBL-1 cells (29) latently infected with KSHV were obtained from the NIH AIDS Reference Reagent Program. LCLa cells (30) latently infected with EBV were the kind gift of Jeff Cohen, NIAID, NIH. HSB2 cells (31) latently infected with HHV-6A, Sup-T1/Z29 cells (32) latently infected with HHV-6B, and Sup-T1/JI cells (33) latently infected with HHV-7 were obtained from the NIH AIDS Reference Reagent Program, with the kind help of Dharam Ablashi, HHV-6 Foundation. Cells were grown and maintained in RPMI 1640 medium enriched with 10% fetal bovine serum (FBS), glutamine, penicillin-streptomycin, and β-mercaptoethanol (standard growth medium) in 5% CO2 at 37°C. The cell densities were maintained at between 0.25 × 106 and 0.5 × 106 cell/ml. Cell viability and density were monitored using a hemocytometer and trypan blue staining, and viability was maintained at >90%.
Lytic replication was induced by adding tetradecanoyl phorbol-13-acetate (TPA) (Sigma-Aldrich) at a final concentration of 20 ng/ml to the medium. BCBL-1 cells were incubated with TPA for 1 h, LCLa cells were incubated for 3 h, and HSB2 cells, Z29/SupT-1, and SupT-1/JI cells were incubated for 6 h at 37°C. Following incubation with TPA, cells were washed in medium and returned to the incubator.
Protected viral DNA isolation.
Protected viral DNA was quantitated using reverse transcription-PCR (RT-PCR) assays, using a modification of a previously described procedure (11). In brief, supernatants from each treatment condition were centrifuged at 5,000 × g for 5 min, and the clarified supernatant was incubated with RQ DNase 1 (Promega) at a final concentration of 100 U/ml and incubated at 37°C for 1 h to digest free DNA unprotected in virions. The samples were then treated with 0.5 M EDTA to a final concentration of 10 mM and incubated at 65°C for 30 min to inactivate DNase. The samples were treated with 10% SDS to a final concentration of 0.5% and with proteinase K to a final concentration of 200 μg/ml and incubated at 65°C for 2 h to disrupt the envelope and digest capsid protein. These samples were extracted with phenol-chloroform-isoamyl alcohol (25:24:1) (Invitrogen), followed by precipitation with ethanol, dried, and dissolved in Tris-EDTA (TE) buffer.
Quantitation of protected viral DNA.
Protected viral DNA was quantified using a TaqMan real-time PCR assay for conserved viral sequences. For KSHV we used primers and probes specific for ORF57: ORF57 TaqMan probe 57TM (5′-FAM-AGAAACCGCAGCCGCCGGAG-TAMRA-3′, where FAM is 6-carboxyfluorescein and TAMRA is 6-carboxytetramethylrhodamine), the forward primer 5′-TTTGTGACCAGTTTGTTCCTCCACGAAAGCCCC-3′, and the reverse primer 5′-TCATTTGTTCCTCCACGAAAGCCCC-3′ (Applied Biosystems). For EBV we used sequences in BALF5: TaqMan probe BALF5TM (5′-FAM-TGTACACGCACGAGAAATGCGCC-TAMRA-3′), the forward primer 5′-CGGAAGCCCTCTGGACTTC-3′, and the reverse primer 5′-CCCTGTTTATCCGATGGAATG-3′ (Integrated DNA Technologies, IDT). For HHV-6A and -6B we used sequences in U38. For HHV-6A we used TaqMan Probe U38TM (5′-FAM-TGCAGCCATTTCTTTGGAAAGC-TAMRA-3′), and for HHV-6B we used TaqMan probe U38TM (5′-FAM-TGCAGCCACCTCCTTGGAAAG-TAMRA-3′). For HHV-6A and HHV-6B we used the forward primer 5′-GGAGTGCCTGTGGGTATTC-3′ and the reverse primer 5′-CTAAGGTGACCAGATTCG-3′ (IDT). For HHV-7 we used sequences in U100: TaqMan probe for HHV-7 U100TM (5′-FAM-ATGAAAACATGCACAACGCAAGCTCT-TAMRA-3′), the forward primer5′-AGCTTTGTCTTTCCTCGGAAC-3′, and the reverse primer 5′-ACGCACGGCAATAACTCTAG-3′ (IDT). Real-time PCR assays were performed using an Applied Biosystems 7900 HT Fast Real-Time PCR System. All assays were performed in triplicate.
Assays for second-round production of infectious virions.
We collected supernatants from the cell lines latently infected with HHV-6A, HHV-6B, or HHV-7 after inducing viral replication with either TPA or the apoptosis inducer DCPE [2-(3-(2,3-dichlorophenoxy)propylamino) ethanol] and added 200 μl of supernatant from each condition to Jurkat cells, which can support productive infection of HHV-6 (34). After 24 h, supernatants from the Jurkat cells were subsequently assayed for protected viral DNA, as described above.
Induction and determination of apoptosis.
BCBL-1, LCLa, HSB2, Z29/SupT-1, and SupT-1/JI cells were seeded overnight at a concentration of 0.25 × 106 cells/ml. Apoptosis was induced by adding DCPE (2,3-DCPE HCl; Enzo Life Sciences) at a final concentration of 50 nM, and cells were incubated at 37°C for 24 h. The cells were harvested by centrifugation at ∼150 × g after assessment of cell number and viability. Supernatants were used for protected viral DNA isolation and quantitation as described above. Cell pellets were washed with phosphate-buffered saline (PBS) (without calcium and magnesium) at pH 7.4 and analyzed by flow cytometry or stained for immunofluorescence assays.
Apoptosis was assessed using a fluorescein isothiocyanate (FITC) Annexin V Apoptosis Detection Kit I (BD Pharmingen) to determine cell surface phosphatidylserine with flow cytometry. In these experiments, 106 cells from each treatment condition were resuspended in 100 μl of 1× binding buffer, followed by the addition of fluorescein-conjugated annexin V and propidium iodide (annexin-PI; 50 μg/ml in 1× PBS). The cells were incubated for 15 min at room temperature (RT) and analyzed by flow cytometry (FACSCalibur; BD Biosciences). Untreated cells were used to establish forward and side scatter gates for compensation baselines. Data were analyzed using FlowJo, version 8.5.2, software (FlowJo, Ashland, OR).
Confocal microscopy for EBV p52, HHV-6A gp116, HHV-6B gp116, HHV-7 KR4, and the KSHV late gene ORFK8.1.
Detection of viral protein expression was performed by immunofluorescence using a confocal Zeiss LSM 510 microscope. Coverslips in 24-well plates were coated with poly-l-lysine and incubated at RT overnight. Cells (0.5 × 106 to 1.0 × 106) from each treatment condition were pelleted and then resuspended in RPMI 1640 medium. The cells were transferred to coated coverslips in 24-well plates and allowed to settle for 15 min, washed with PBS, and fixed with 4% paraformaldehyde at 4°C for 30 min. The cells were washed twice with PBS and permeabilized with PBS containing 0.1% Triton X-100 at RT for 10 min. The cells were washed twice with PBS and incubated with mouse monoclonal antibodies against KSHV ORFK8.1 (Advanced Biotechnologies, Inc.), EBV p52, HHV-6A gp116, HHV-6B gp116, and HHV-7 KR4 (NIH AIDS Research and Reference Reagent Program), each at a 1:400 dilution in PBS, and incubated overnight at 4°C. The cells were washed twice with PBS and incubated with goat anti-mouse secondary antibody conjugated to peridinin chlorophyll protein (PerCP; Santa Cruz Biotechnology) at a dilution of 1:1,000 in PBS for 2 h at room temperature. Cells from each treatment condition were resuspended in 100 μl of 1× binding buffer, followed by incubation with allophycocyanin-conjugated annexin V (APC Annexin V; Life Technologies) in the dark at RT for 15 min to stain for apoptotic cells. The coverslips were mounted on slides with ProlongGold antifade reagent with 4′,6′-diamidino-2-phenylindole (DAPI) (Invitrogen) as a nuclear stain. A magnification of ×40 (oil) was used to acquire all images.
Transfection of cells with a plasmid expressing a caspase-3–GFP fusion protein.
Cell lines latently infected with herpesviruses were transfected with a plasmid, pcasp3-WT-GFP, that expresses functional wild-type (WT) caspase-3 fused to green fluorescent protein (GFP), a kind gift from Shinji Kamada, Biosignal Research Center, Kobe University (35). pUC19 was used as a negative control. APC-annexin V (BD Pharmingen) was used to detect apoptosis. Monoclonal antibodies against EBV p52, HHV-6A gp116, HHV-6B gp116, and HHV-7 KR4 (NIH AIDS Research and Reference Reagent Program) and KSHV ORFK8.1 (Advanced Biotechnologies, Inc.), each at 1:400 dilution, and goat anti-mouse secondary antibody labeled with PerCP (Advanced Biotechnologies) were used to detect viral protein expression. Cells were seeded in 24-well plates, and 2 h prior to transfection, the medium was replaced by RPMI 1640 medium with no FBS and antibiotics. Lipofectamine 2000 (Invitrogen) was mixed with Opti-MEM I medium (Invitrogen) at a 1:25 dilution and kept for 10 min at RT. This mixture was then complexed with 500 ng of pcasp3-WT-GFP at a ratio of 1:1 and kept at RT for 30 min. Two hundred microliters of this complex was added for transfection in each well. The plates were gently rocked at 37°C for 5 h. TPA (20 ng/ml) was added to selected wells and incubated for 1 h. DCPE (final concentration, 50 nM) was added to selected samples.
Inhibition of apoptosis and viral DNA replication by a caspase-3 inhibitor.
To study the effects of a caspase-3 inhibitor on cell apoptosis and viral replication, cell lines latently infected with herpesviruses were treated with the caspase-3 inhibitor acetyl (Ac)-AAVALLPAVLLAPDGVD-CHO (Enzo Life Sciences). The caspase-3 inhibitor was reconstituted in dimethyl sulfoxide (DMSO) (Fisher Scientific) to a stock concentration of 10 mM. Cells were seeded in six-well plates at a density of 0.25 × 106 cells/ml and treated with the caspase-3 inhibitor at final concentrations of 50 to 250 μM in the presence of DCPE (50 nM). Some cells were treated with TPA (20 ng/ml) as a positive control to induce viral replication. The cells were harvested after 24 h. Supernatants were used to assay for herpesvirus replication as described above. Approximately 106 cells from each treatment condition were resuspended in 100 μl of 1× binding buffer, followed by staining with fluorescein-conjugated annexin V and propidium iodide (FITC Annexin V Apoptosis Detection Kit I) (BD Pharmingen). The cells were incubated in the dark for 15 min at room temperature (RT) and analyzed by flow cytometry (FACSCalibur; BD Biosciences). Data were analyzed using FlowJo, version 8.5.2, software.
Induction of apoptosis and viral replication by cytotoxic chemotherapy agents.
Cell lines latently infected with herpesviruses were treated with the cytotoxic chemotherapeutic agents doxorubicin, prednisone, and vincristine (Sigma-Aldrich). Doxorubicin was reconstituted in molecular-grade water at a stock concentration of 100 μM, prednisone was reconstituted at a stock concentration of 100 μM in absolute ethanol (Sigma-Aldrich), and vincristine was reconstituted in molecular biology-grade water at a stock concentration of 100 μM. Cells were seeded in 24-well plates at a density of 0.25 × 106 cells/ml 12 h prior to treatment. The cells were treated with chemotherapy agents at final concentrations of 0.1 μM, 0.5 μM, 1.0 μM, 5.0 μM, and 10.0 μM. Cells were harvested after 24 h by centrifugation at 5,000 × g for 5 min. The amounts of protected viral DNA in the supernatants were assayed by TaqMan quantitative PCR (qPCR) as described above. Cell pellets were washed in PBS and stained for apoptosis assay as described above using an FITC Annexin V Apoptosis Detection Kit I (BD Pharmingen).
RESULTS
Apoptosis-associated induction of viral replication in cells latently infected with EBV, HHV-6A, HHV-6B, HHV-7, and KSHV.
To test the hypothesis that apoptosis broadly activates replication among clinically significant Herpesviridae, we studied LCLa cells (30) latently infected with EBV, HSB2 cells (31) latently infected with HHV-6A, Sup-T1/Z29 cells (32) latently infected with HHV-6B, and Sup-T1/JI cells (33) latently infected with HHV-7. As a positive control, we also studied BCBL-1 cells latently infected with KSHV (29). We treated aliquots of the cells with DCPE to induce apoptosis and treated additional aliquots of the cells with TPA as a positive control for induction of viral replication through a non-apoptosis-mediated pathway.
After the treatments, we examined the cells with confocal microscopy by staining nuclei with DAPI, assessing apoptosis using an annexin stain, and evaluating induction of viral protein expression by staining for a viral protein for each virus (KSHV ORFK8.1, EBV p52, HHV-6A gp116, HHV-6B gp116, and HHV-7 KR4) using a primary mouse antiviral monoclonal antibody and a PerCP-conjugated goat anti-mouse secondary antibody. Figure 1 shows that TPA did not induce apoptosis but did induce viral protein expression in all of the latently infected cells, so the cell lines were fully capable of being induced and expressing viral proteins. When we treated the cells with the apoptosis inducer DCPE, we found (Fig. 2) that DCPE induced both apoptosis and viral protein expression for all the cell lines latently infected with herpesviruses that we studied.
Fig 1.

Induction of viral protein expression in herpesvirus latently infected cells treated with TPA. BCBL-1 cells latently infected KSHV, LCLa cells latently infected with EBV, HSB2 cells latently infected with HHV-6A, Z29/SupT-1 cells latently infected with HHV-6B, and SupT-1/JI cells latently infected with HHV-7 were treated with TPA and examined at 24 h. Cells were stained with the nuclear stain DAPI (blue), stained with annexin V-APC (red) to assay for apoptosis, and incubated with mouse monoclonal antibodies against viral proteins (EBV p52, HHV-6A gp116, HHV-6B gp116, HHV-7 KR4, and the KSHV late gene ORFK8.1) followed by incubation with secondary antibodies conjugated to PerCP (yellow). Cells were examined using confocal microscopy. Upon TPA treatment, all the cell lines showed minimal evidence of apoptosis (red) and high viral protein expression (yellow).
Fig 2.

Apoptosis induces viral protein expression in herpesvirus latently infected cells. BCBL-1 cells latently infected with KSHV, LCLa cells latently infected with EBV, HSB2 cells latently infected with HHV-6A, Z29/SupT-1 cells latently infected with HHV-6B, and SupT-1/JI cells latently infected with HHV-7 were treated with the proapoptotic agent DCPE and examined at 24 h. Cell nuclei were stained with DAPI (blue), and cells were stained with annexin V-APC (red) to detect cells undergoing apoptosis. Cells were incubated with mouse monoclonal antibodies against specific viral proteins (KSHV ORFK8.1, EBV p52, HHV-6A gp116, HHV-6B gp116, and HHV-7 KR4), followed by incubation with goat anti-mouse secondary antibody conjugated to PerCP (yellow), and examined with confocal microscopy. Almost all the cells appeared to be undergoing apoptosis, which was associated with viral protein expression (yellow).
Caspase-3-associated induction of viral replication in cells latently infected with EBV, HHV-6A, HHV-6B, HHV-7, and KSHV.
Since the apoptosis inducer DCPE might have induced viral protein expression through some other mechanism than one linked to apoptosis and since we previously showed that apoptosis triggered KSHV replication in a caspase-3-dependent manner (11), we conducted experiments in which we transfected the cells latently infected with the different herpesviruses with a plasmid, pcasp3-WT-GFP (35), that expresses a functional caspase-3–GFP fusion protein. The results of these experiments are shown in Fig. 3. We observed expression of the caspase-3–GFP fusion protein in all of the herpesvirus latently infected cell lines studied, and expression was associated with induction of apoptosis, as judged by annexin V binding; the expression of the caspase-3–GFP fusion protein and apoptosis were associated with induction of viral protein expression, suggesting that apoptosis induced the replication of the different herpesviruses through a caspase-3-dependent pathway.
Fig 3.
Caspase-3 induces apoptosis in cell lines latently infected with herpesviruses and is associated with herpesvirus protein expression. BCBL-1 cells latently infected with KSHV, LCLa cells latently infected with EBV, HSB2 cells latently infected with HHV-6A, Z29/SupT-1 cells latently infected with HHV-6B, and SupT-1 cells latently infected with HHV-7 were transfected with pcas3-WT-GFP, which expresses caspase-3 as a GFP fusion protein, or pUC19 as a negative control. Cells were stained with annexin V-APC (red) to assay apoptosis. Cell nuclei were stained with DAPI (blue). Cells were incubated with mouse monoclonal antibodies against specific viral proteins (KSHV ORFK8.1, EBV p52, HHV-6A gp116, HHV-6B gp116, and HHV-7 KR4), followed by incubation with goat anti-mouse secondary antibody conjugated to PerCP (yellow), and examined with confocal microscopy. Caspase-3 expression, shown as GFP expression (green), was associated with high levels of apoptosis (red) and also with viral protein expression (yellow) in all of the herpesvirus latently infected cell lines.
Apoptosis-associated, caspase-3-dependent virion production from cells latently infected with EBV, HHV-6A, HHV-6B, HHV-7, and KSHV.
To show that apoptosis induced not only the expression of viral protein but also the formation of virion particles, we used a TaqMan qPCR assay for protected viral DNA (11, 36, 37), as we previously described for KSHV, modified using the appropriate primers and probes for the other herpesviruses (Fig. 4). We found that the apoptosis inducer DCPE induced the production of herpesvirus virions in all of the herpesvirus latently infected cell lines studied, suggesting that host cell apoptosis triggers not only the expression of herpesvirus proteins but also the production of herpesvirus virions across a broad selection of human herpesviruses. We treated the cells with the caspase-3 inhibitor (Ac)-AAVALLPAVLLAPDGVD-CHO and found that the caspase-3 inhibitor blocked both host cell apoptosis and production of virions in a parallel fashion for all of the herpesviruses studied (EBV, HHV-6A, HHV-6B, HHV-7, and KSHV), providing another line of evidence that activation of the viruses in association with apoptosis depends on the activity of caspase-3.
Fig 4.
The proapoptotic agent DCPE induces cell death and viral replication in cell lines latently infected with herpesviruses in a caspase-3-dependent process. Cell lines latently infected with herpesviruses, BCBL-1 cells latently infected KSHV, LCLa cells latently infected with EBV, HSB2 cells latently infected with HHV-6A, Z29/SupT-1 cells latently infected with HHV-6B, and SupT-1/JI cells latently infected with HHV-7 were treated with TPA to induce viral replication through the conventional pathway as a positive control and with the proapoptotic agent DCPE to induce apoptosis. Aliquots of the cells treated with the apoptotic inducer DCPE were also treated with various concentrations of a caspase-3 inhibitor. Cells were stained with annexin V-FITC and propidium iodide (PI) and assayed for apoptosis using flow cytometry. Cell supernatants were assayed for viral DNA replication using a viral DNA TaqMan assay. DCPE induced apoptosis, which was blocked by the caspase-3 inhibitor in a dose-dependent manner. All of the herpesvirus latently infected cell lines induced into apoptosis by DCPE produced large amounts of virus, which was blocked in a dose-dependent manner by the caspase-3 inhibitor.
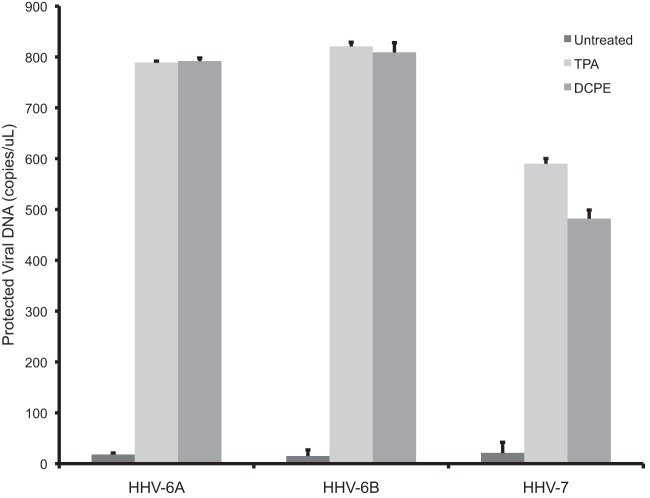
To show that the virions produced in association with apoptosis were, in fact, functional infectious virions, we assayed supernatants from cells latently infected with viruses for which there are efficient acute viral replication systems. We collected supernatants from cells latently infected with HHV-6A, HHV-6B, and HHV-7 and exposed Jurkat cells to these supernatants. Following exposure to the cell supernatants, we assayed for the production of a second round of virions, using the assay for protected viral DNA (Fig. 5). We found that for each of the viruses studied, HHV-6A, HHV-6B, and HHV-7, the supernatants following induction of viral replication either by the control inducer TPA or the inducer of apoptosis DCPE contained infectious virus, so apoptosis can induce not only the production of viral DNA but also the production of infectious virions.
Fig 5.
Production of infectious virions following induction of viral replication by the apoptotic inducer DCPE. Supernatants from the cell lines latently infected with HHV-6A, HHV-6B, or HHV-7, induced into replication with either the conventional inducer, TPA, or the inducer of apoptosis, DCPE, were added to uninfected Jurkat cells, and subsequently supernatants from the Jurkat cells were assayed for protected viral DNA. The Jurkat cells produced virus.
Apoptosis and caspase-3-associated induction of viral replication in cells latently infected with EBV, HHV-6A, HHV-6B, HHV-7, and KSHV after treatment with cytotoxic chemotherapy agents.
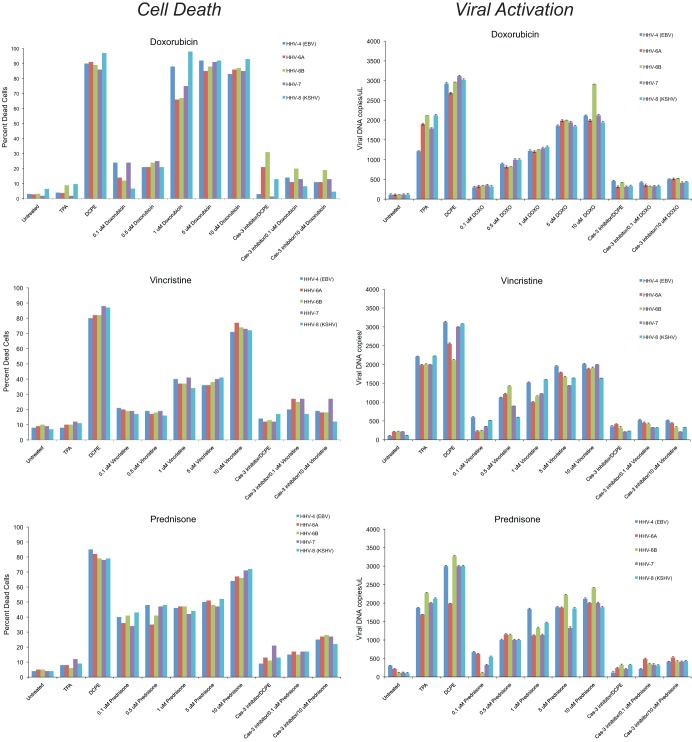
Since the herpesviruses we studied in these experiments, particularly HHV-6A, -6B, and -7, infect humans almost universally in the first years of life, it may be important to consider whether proapoptotic agents and disorders that humans may be exposed to later in life are also capable of inducing the replication of these herpesviruses. Some of the kinds of proapoptotic agents that patients may be exposed to are cancer chemotherapeutic agents. We therefore tested three different agents commonly used in cancer chemotherapy that act by different mechanisms, the anthracycline DNA intercalator doxorubicin, the vinca alkaloid microtubule inhibitor vincristine, and the glucocorticoid prednisone. In these experiments (Fig. 6), we assessed apoptosis flow cytometrically and in parallel evaluated herpesvirus replication by assaying for protected herpesvirus DNA in the cell supernatants. We found that doxorubicin, vincristine, and prednisone induced apoptosis in all of the herpesvirus latently infected cell lines and induced herpesvirus replication in those cell lines in a caspase-3-dependent fashion. These studies also confirmed, using a different flow-cytometric approach than the approach used in the experiments shown in Fig. 1 to 4, that TPA induced viral replication without inducing apoptosis, that DCPE induced both apoptosis and viral replication, and that the induction of viral replication by apoptosis required caspase-3 activity.
Fig 6.
Cytotoxic chemotherapeutic agents induce apoptosis and herpesvirus replication in a caspase-3-dependent manner. Cell lines latently infected with herpesviruses, BCBL-1 cells latently infected KSHV, LCLa cells latently infected with EBV, HSB2 cells latently infected with HHV-6A, Z29/SupT-1 cells latently infected with HHV-6B, and SupT-1/JI cells latently infected with HHV-7 were treated with TPA as a positive control for induction of the conventional herpesvirus replication pathway, with DCPE as positive control for the induction of apoptosis, and with cytotoxic chemotherapeutic agents acting through different mechanisms (doxorubicin, vincristine and prednisone). Aliquots of the cells treated with DCPE or the cytotoxic chemotherapeutic agents were also treated with a caspase-3 (Cas-3) inhibitor. Cells were harvested after 24 h and stained with annexin V-FITC and propidium iodide to assay for apoptosis by flow cytometry. Supernatants from the aliquots were assayed for protected viral DNA using a TaqMan qPCR assay. DCPE induced apoptosis in all herpesvirus latently infected cell lines and induced herpesvirus replication, which was blocked by the caspase-3 inhibitor. The cytotoxic chemotherapeutic drugs also induced apoptosis in the herpesvirus latently infected cell lines, which was associated with the induction of viral replication. Both induction of apoptosis and viral replication by the cytotoxic chemotherapeutic agents occurred in a caspase-3-dependent manner. DOXO, doxorubicin.
DISCUSSION
Overall, the results show that several different human herpesviruses have a caspase-3-dependent apoptosis-initiated viral alternative replication program (ARP). These findings reinforce our prior findings (11) that KSHV has an ARP characterized by the lack of a requirement for the KSHV RTA transactivator, an accelerated pattern of late gene expression, caspase-3 dependence, and the production of virus with still present but decreased infectivity and the findings that HSV-1 has an ARP that is caspase-3 dependent and results in a dysregulated pattern of gene expression (12, 13). These previously published findings and the findings we present here suggest that all members of the family Herpesviridae may have an apoptosis-initiated ARP. It is important to note that all of the cells latently infected with herpesviruses examined in these studies were transformed cell lines. It is possible that latent herpesviruses in transformed cell lines may be particularly sensitive to activation by apoptosis or that the apoptotic pathways active in cell lines may exhibit some important differences compared to primary cells. However, experiments involving HSV-1 (12, 13) used a primary cell model system, ganglion explants, so it is unlikely that apoptosis activation of herpesvirus replication is an artifactual phenomenon restricted to the special case of transformed cell lines latently infected with herpesviruses.
The existence of an apoptosis-initiated ARP makes sense evolutionarily since an ARP would be the only chance for a herpesvirus to replicate when apoptosis threatens the host cell, but a firm conclusion that all herpesviruses have an apoptosis-induced ARP must await future studies on many other herpesviruses. The findings likewise suggest that caspase-3 plays a key role in the induction of the ARP for all herpesviruses. The apoptosis-inducing agents that we studied, DCPE, and the chemotherapeutic agents doxorubicin, prednisone, and vincristine, act through several different initial pathways and can have pleiotropic effects on cells, raising the possibility that at least some herpesvirus activation may result from effects other than those mediated through induction of apoptosis. However, the ability of the caspase-3 inhibitor to block replication following treatment with these agents argues that apoptosis and caspase activation at least play important roles for all of the cytotoxic agents studied. A complete understanding of how caspase-3 activity mediates the induction of the ARP similarly must await many future detailed additional mechanistic studies, but one plausible hypothesis would be that there is some viral or host cell protein that is sensitive to caspase-3-mediated cleavage that leads to its activation as a potent transactivator. At least one herpesvirus protein, the HSV-1 ICP-22 (38), has been shown to be cleaved by caspase-3, but whether this protein or another protein or other analogous proteins mediate herpesvirus activation remains to be determined.
Our findings that the cytotoxic chemotherapeutic agents we studied, doxorubicin, vincristine, and prednisone, which act through distinct mechanisms, activate EBV, HHV-6A, -6B, -7, and KSHV in a caspase-3-dependent manner confirm the model that caspase-3 constitutes an essential factor in herpesvirus apoptosis sensing and the initiation of the apoptosis-initiated ARP. The findings further suggest the possibility that these drugs may activate the herpesviruses in vivo and that other cytotoxic chemotherapeutic agents may also activate herpesviruses latent within patients. While cytotoxic agents are known to activate herpesviruses in tumors believed to be caused by herpesviruses, the possibility of a general activation of latent herpesviruses by cytotoxic agents is less well appreciated. If cytotoxic chemotherapeutic agents do generally activate latent herpesviruses within patients via an apoptosis-associated ARP, the exposure of a patient to large amounts of circulating herpesviruses replicating as a result of an ARP induced by therapeutic interventions may have significant clinical consequences. If cytotoxic chemotherapeutic agents do activate latent herpesviruses in potentially deleterious ways, for example, by causing culture-negative sepsis-like syndromes or by infecting hematopoietic cells, then it would conceivably be helpful to treat patients at a high risk of experiencing substantial herpesvirus activation in association with apoptosis induced by chemotherapeutic agents with antiviral agents in addition to their antineoplastic cytotoxic chemotherapy. Similarly, since prednisone induced the replication of the herpesviruses via the ARP, it may be important to consider whether treatments with high doses of glucocorticoids generally induce herpesvirus replication in patients treated for a variety of inflammatory and autoimmune disorders and whether the activation of latent herpesviruses in these disorders might have negative clinical consequences. Indeed, it has been a long-standing clinical observation that treatment with glucocorticoids can worsen KS (39). In most instances of herpesvirus activation by glucocorticoids, the herpesvirus activation has been ascribed to immunosuppression although in the instance of a prior in vitro study in which treatment of KSHV latently infected BCBL-1 cells with hydrocortisone was shown to activate KSHV, activation was ascribed to immunosuppression or direct activation of the virus through an unknown mechanism (40).
Clinically, a large number of sometimes serious states and disorders that affect the host can produce apoptosis, in addition to cytotoxic cancer chemotherapy or treatment with high doses of glucocorticoids. Some of these have been associated with HHV activation. These associations range from advanced age and the activation of varicella-zoster virus (VZV) as shingles, to stress and UV radiation and the activation of HSV-1, to cancer chemotherapy or neoplastic diseases themselves, bone marrow transplantation, drug-induced hypersensitivity syndrome/drug reaction/rash with eosinophilia and systemic symptoms (DIHS/DRESS) (21–24, 41), and the activation of cytomegalovirus (CMV), EBV, HHV-6, and HHV-7. Our findings would suggest that some, if not many, of the HHV activation phenomena observed in association with these disorders result from a caspase-3-dependent activation of a herpesvirus ARP.
In some instances, herpesvirus activation has been associated with adverse outcomes, for example, activation of CMV and EBV, and poor outcomes following bone marrow transplantation (42) and activation of HHV-6 and DIHS/DRESS. Activation of herpesviruses in these states and disorders has previously been variably attributed to general immune suppression, suppression of specific arms of the immune system, and increased concentrations of inflammatory and activating cytokines. Our results would also indicate that, at least in some cases, conditions and disorders that induce apoptosis may themselves induce herpesvirus replication via the apoptosis-associated emergency escape ARP. The existence of the apoptosis-associated ARP may help to explain some of the pathogenic features that accompany disorders associated with apoptosis in host cells. For example, in DIHS/DRESS, activation of HHV-6 has been proposed as one of the defining features of the syndrome. One interpretation of our findings might then be that if a hypersensitivity reaction is severe enough to induce significant apoptosis in cells latently infected with HHV-6, apoptosis will trigger detectable HHV-6 replication.
Our findings suggest that many, and perhaps all, herpesviruses, as they exist latently within their host cells, sense the health of the host cell and evaluate whether the host cell is threatened with apoptosis by detecting activation of the end effector caspase, caspase-3, and either choose to replicate in a careful, orderly fashion if they do not detect caspase-3 activation or select an emergency escape ARP as the only option for effective replication if they do. If confirmed, the findings would suggest that there is a dialog between a host cell and a latent herpesvirus within that host cell that was not previously appreciated and that could ultimately have important clinical consequences.
ACKNOWLEDGMENTS
We thank Dharam Ablashi and Jeff Cohen for helpful discussions and the gift of latently infected cell lines and Jeff Dome and Bernard Roizman for helpful discussions. We thank the HHV-6 Foundation and the NIH AIDS Reference Reagent Program for providing cell lines and antibodies. We thank Shinji Kamada, Kobe University, for the kind gift of pcasp3-WT-GFP. We thank the imaging core facility of Children's National Medical Center, Washington, DC, for help with confocal microscopy and Lina Chakrabarti at Children's National Medical Center for assistance in flow cytometry.
This work was supported in part by the District of Columbia Developmental Center for AIDS Research (P30AI087714 from NIAID, NIH) and in part by R01-AI090571 from NIAID and R56 DE021570, NIDCR, NIH, to S.L.Z.
Footnotes
Published ahead of print 24 July 2013
REFERENCES
- 1.Speck SH, Ganem D. 2010. Viral latency and its regulation: lessons from the gamma-herpesviruses. Cell Host Microbe 8:100–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monini P, Colombini S, Sturzl M, Goletti D, Cafaro A, Sgadari C, Butto S, Franco M, Leone P, Fais S, Melucci-Vigo G, Chiozzini C, Carlini F, Ascherl G, Cornali E, Zietz C, Ramazzotti E, Ensoli F, Andreoni M, Pezzotti P, Rezza G, Yarchoan R, Gallo RC, Ensoli B. 1999. Reactivation and persistence of human herpesvirus-8 infection in B cells and monocytes by Th-1 cytokines increased in Kaposi's sarcoma. Blood 93:4044–4058 [PubMed] [Google Scholar]
- 3.Amon W, Farrell PJ. 2005. Reactivation of Epstein-Barr virus from latency. Rev. Med. Virol. 15:149–156 [DOI] [PubMed] [Google Scholar]
- 4.Wang SE, Wu FY, Chen H, Shamay M, Zheng Q, Hayward GS. 2004. Early activation of the Kaposi's sarcoma-associated herpesvirus RTA, RAP, and MTA promoters by the tetradecanoyl phorbol acetate-induced AP1 pathway. J. Virol. 78:4248–4267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller G, Heston L, Grogan E, Gradoville L, Rigsby M, Sun R, Shedd D, Kushnaryov VM, Grossberg S, Chang Y. 1997. Selective switch between latency and lytic replication of Kaposi's sarcoma herpesvirus and Epstein-Barr virus in dually infected body cavity lymphoma cells. J. Virol. 71:314–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thome M, Schneider P, Hofmann K, Fickenscher H, Meinl E, Neipel F, Mattmann C, Burns K, Bodmer JL, Schroter M, Scaffidi C, Krammer PH, Peter ME, Tschopp J. 1997. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature 386:517–521 [DOI] [PubMed] [Google Scholar]
- 7.Sarid R, Sato T, Bohenzky RA, Russo JJ, Chang Y. 1997. Kaposi's sarcoma-associated herpesvirus encodes a functional Bcl-2 homologue. Nat. Med. 3:293–298 [DOI] [PubMed] [Google Scholar]
- 8.Wang HW, Sharp TV, Koumi A, Koentges G, Boshoff C. 2002. Characterization of an anti-apoptotic glycoprotein encoded by Kaposi's sarcoma-associated herpesvirus which resembles a spliced variant of human survivin. EMBO J. 21:2602–2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen JI. 1999. The biology of Epstein-Barr virus: lessons learned from the virus and the host. Curr. Opin. Immunol. 11:365–370 [DOI] [PubMed] [Google Scholar]
- 10.Kofod-Olsen E, Ross-Hansen K, Schleimann MH, Jensen DK, Moller JM, Bundgaard B, Mikkelsen JG, Hollsberg P. 2012. U20 is responsible for human herpesvirus 6B inhibition of tumor necrosis factor receptor-dependent signaling and apoptosis. J. Virol. 86:11483–11492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prasad A, Lu M, Lukac DM, Zeichner SL. 2012. An alternative Kaposi's sarcoma-associated herpesvirus replication program triggered by host cell apoptosis. J. Virol. 86:4404–4419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du T, Zhou G, Roizman B. 2011. HSV-1 gene expression from reactivated ganglia is disordered and concurrent with suppression of latency-associated transcript and miRNAs. Proc. Natl. Acad. Sci. U. S. A. 108:18820–18824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du T, Zhou G, Roizman B. 2012. Induction of apoptosis accelerates reactivation of latent HSV-1 in ganglionic organ cultures and replication in cell cultures. Proc. Natl. Acad. Sci. U. S. A. 109:14616–14621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Staras SA, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ. 2006. Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clin. Infect. Dis. 43:1143–1151 [DOI] [PubMed] [Google Scholar]
- 15.Stanberry LR, Rosenthal SL, Mills L, Succop PA, Biro FM, Morrow RA, Bernstein DI. 2004. Longitudinal risk of herpes simplex virus (HSV) type 1, HSV type 2, and cytomegalovirus infections among young adolescent girls. Clin. Infect. Dis. 39:1433–1438 [DOI] [PubMed] [Google Scholar]
- 16.Emery VC, Clark DA. 2007. HHV-6A, 6B, and 7: persistence in the population, epidemiology and transmission, p 875–884 In Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley G, Yamanishi K. (ed), Human herpesviruses: biology, therapy, and immunoprophylaxis. Cambridge University Press, Cambridge, United Kingdom: [PubMed] [Google Scholar]
- 17.Roush KS, Domiati-Saad RK, Margraf LR, Krisher K, Scheuermann RH, Rogers BB, Dawson DB. 2001. Prevalence and cellular reservoir of latent human herpesvirus 6 in tonsillar lymphoid tissue. Am. J. Clin. Pathol. 116:648–654 [DOI] [PubMed] [Google Scholar]
- 18.Dulery R, Salleron J, Dewilde A, Rossignol J, Boyle EM, Gay J, de Berranger E, Coiteux V, Jouet JP, Duhamel A, Yakoub-Agha I. 2012. Early human herpesvirus type 6 reactivation after allogeneic stem cell transplantation: a large-scale clinical study. Biol. Blood Marrow Transplant 18:1080–1089 [DOI] [PubMed] [Google Scholar]
- 19.Lautenschlager I, Razonable RR. 2012. Human herpesvirus-6 infections in kidney, liver, lung, and heart transplantation: review. Transpl Int. 25:493–502 [DOI] [PubMed] [Google Scholar]
- 20.Peppercorn AF, Miller MB, Fitzgerald D, Weber DJ, Groben PA, Cairns BA. 2010. High-level human herpesvirus-6 viremia associated with onset of Stevens-Johnson syndrome: report of two cases. J. Burn Care Res. 31:365–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kano Y, Hiraharas K, Sakuma K, Shiohara T. 2006. Several herpesviruses can reactivate in a severe drug-induced multiorgan reaction in the same sequential order as in graft-versus-host disease. Br. J. Dermatol. 155:301–306 [DOI] [PubMed] [Google Scholar]
- 22.Seishima M, Yamanaka S, Fujisawa T, Tohyama M, Hashimoto K. 2006. Reactivation of human herpesvirus (HHV) family members other than HHV-6 in drug-induced hypersensitivity syndrome. Br. J. Dermatol. 155:344–349 [DOI] [PubMed] [Google Scholar]
- 23.Tohyama M, Hashimoto K, Yasukawa M, Kimura H, Horikawa T, Nakajima K, Urano Y, Matsumoto K, Iijima M, Shear NH. 2007. Association of human herpesvirus 6 reactivation with the flaring and severity of drug-induced hypersensitivity syndrome. Br. J. Dermatol. 157:934–940 [DOI] [PubMed] [Google Scholar]
- 24.Aihara Y, Ito SI, Kobayashi Y, Yamakawa Y, Aihara M, Yokota S. 2003. Carbamazepine-induced hypersensitivity syndrome associated with transient hypogammaglobulinaemia and reactivation of human herpesvirus 6 infection demonstrated by real-time quantitative polymerase chain reaction. Br. J. Dermatol. 149:165–169 [DOI] [PubMed] [Google Scholar]
- 25.Cook CH, Trgovcich J. 2011. Cytomegalovirus reactivation in critically ill immunocompetent hosts: a decade of progress and remaining challenges. Antiviral Res. 90:151–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tselis A. 2011. Evidence for viral etiology of multiple sclerosis. Semin. Neurol. 31:307–316 [DOI] [PubMed] [Google Scholar]
- 27.Saha A, Robertson ES. 2011. Epstein-Barr virus-associated B-cell lymphomas: pathogenesis and clinical outcomes. Clin. Cancer Res. 17:3056–3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raab-Traub N. 2012. Novel mechanisms of EBV-induced oncogenesis. Curr. Opin. Virol. 2:453–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Renne R, Zhong W, Herndier B, McGrath M, Abbey N, Kedes D, Ganem D. 1996. Lytic growth of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat. Med. 2:342–346 [DOI] [PubMed] [Google Scholar]
- 30.Zou P, Kawada J, Pesnicak L, Cohen JI. 2007. Bortezomib induces apoptosis of Epstein-Barr virus (EBV)-transformed B cells and prolongs survival of mice inoculated with EBV-transformed B cells. J. Virol. 81:10029–10036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ablashi DV, Balachandran N, Josephs SF, Hung CL, Krueger GR, Kramarsky B, Salahuddin SZ, Gallo RC. 1991. Genomic polymorphism, growth properties, and immunologic variations in human herpesvirus-6 isolates. Virology 184:545–552 [DOI] [PubMed] [Google Scholar]
- 32.Mayne M, Cheadle C, Soldan SS, Cermelli C, Yamano Y, Akhyani N, Nagel JE, Taub DD, Becker KG, Jacobson S. 2001. Gene expression profile of herpesvirus-infected T cells obtained using immunomicroarrays: induction of proinflammatory mechanisms. J. Virol. 75:11641–11650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ablashi DV, Berneman ZN, Kramarsky B, Whitman J, Jr, Asano Y, Pearson GR. 1995. Human herpesvirus-7 (HHV-7): current status. Clin. Diagn. Virol. 4:1–13 [DOI] [PubMed] [Google Scholar]
- 34.Lusso P, Malnati M, De Maria A, Balotta C, DeRocco SE, Markham PD, Gallo RC. 1991. Productive infection of CD4+ and CD8+ mature human T cell populations and clones by human herpesvirus 6. Transcriptional down-regulation of CD3. J. Immunol. 147:685–691 [PubMed] [Google Scholar]
- 35.Kamada S, Kikkawa U, Tsujimoto Y, Hunter T. 2005. Nuclear translocation of caspase-3 is dependent on its proteolytic activation and recognition of a substrate-like protein(s). J. Biol. Chem. 280:857–860 [DOI] [PubMed] [Google Scholar]
- 36.Lu M, Suen J, Frias C, Pfeiffer R, Tsai MH, Chuang E, Zeichner SL. 2004. Dissection of the Kaposi's sarcoma-associated herpesvirus gene expression program by using the viral DNA replication inhibitor cidofovir. J. Virol. 78:13637–13652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paulose-Murphy M, Ha NK, Xiang C, Chen Y, Gillim L, Yarchoan R, Meltzer P, Bittner M, Trent J, Zeichner S. 2001. Transcription program of human herpesvirus 8 (Kaposi's sarcoma-associated herpesvirus). J. Virol. 75:4843–4853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Munger J, Hagglund R, Roizman B. 2003. Infected cell protein no. 22 is subject to proteolytic cleavage by caspases activated by a mutant that induces apoptosis. Virology 305:364–370 [DOI] [PubMed] [Google Scholar]
- 39.Harwood AR, Osoba D, Hofstader SL, Goldstein MB, Cardella CJ, Holecek MJ, Kunynetz R, Giammarco RA. 1979. Kaposi's sarcoma in recipients of renal transplants. Am. J. Med. 67:759–765 [DOI] [PubMed] [Google Scholar]
- 40.Hudnall SD, Rady PL, Tyring SK, Fish JC. 1999. Hydrocortisone activation of human herpesvirus 8 viral DNA replication and gene expression in vitro. Transplantation 67:648–652 [DOI] [PubMed] [Google Scholar]
- 41.Cacoub P, Musette P, Descamps V, Meyer O, Speirs C, Finzi L, Roujeau JC. 2011. The DRESS syndrome: a literature review. Am. J. Med. 124:588–597 [DOI] [PubMed] [Google Scholar]
- 42.Razonable RR, Paya CV. 2003. Herpesvirus infections in transplant recipients: current challenges in the clinical management of cytomegalovirus and Epstein-Barr virus infections. Herpes 10:60–65 [PubMed] [Google Scholar]