Abstract
The influenza virus PA endonuclease, which cleaves capped host pre-mRNAs to initiate synthesis of viral mRNA, is a prime target for antiviral therapy. The diketo acid compound L-742,001 was previously identified as a potent inhibitor of the influenza virus endonuclease reaction, but information on its precise binding mode to PA or potential resistance profile is limited. Computer-assisted docking of L-742,001 into the crystal structure of inhibitor-free N-terminal PA (PA-Nter) indicated a binding orientation distinct from that seen in a recent crystallographic study with L-742,001-bound PA-Nter (R. M. DuBois et al., PLoS Pathog. 8:e1002830, 2012). A comprehensive mutational analysis was performed to determine which amino acid changes within the catalytic center of PA or its surrounding hydrophobic pockets alter the antiviral sensitivity to L-742,001 in cell culture. Marked (up to 20-fold) resistance to L-742,001 was observed for the H41A, I120T, and G81F/V/T mutant forms of PA. Two- to 3-fold resistance was seen for the T20A, L42T, and V122T mutants, and the R124Q and Y130A mutants were 3-fold more sensitive to L-742,001. Several mutations situated at noncatalytic sites in PA had no or only marginal impact on the enzymatic functionality of viral ribonucleoprotein complexes reconstituted in cell culture, consistent with the less conserved nature of these PA residues. Our data provide relevant insights into the binding mode of L-742,001 in the PA endonuclease active site. In addition, we predict some potential resistance sites that should be taken into account during optimization of PA endonuclease inhibitors toward tight binding in any of the hydrophobic pockets surrounding the catalytic center of the enzyme.
INTRODUCTION
The annual influenza epidemics caused by human influenza A and B viruses are associated with substantial morbidity and mortality in susceptible patients and considerable socio-economic and medical burden. Moreover, we are confronted with the eminent threat of unpredictable influenza pandemics, the most recent example being the 2009 pandemic caused by a novel H1N1 influenza virus (1). The existing influenza vaccines require annual updating and are only partially protective in some target populations, such as the elderly (2). Two classes of antiviral drugs are available for prevention or treatment of influenza virus infections: the M2 ion channel blockers, amantadine and rimantadine, and the neuraminidase inhibitors, oseltamivir and zanamivir. Amantadine and rimantadine have limited usefulness, because of their neurological side effects, lack of activity against influenza B virus, and, in particular, the worldwide spread of amantadine-resistant viruses (3). Likewise, during recent years, oseltamivir-resistant influenza viruses were isolated all over the globe (4), raising the urgent need for new anti-influenza virus agents with an entirely different mode of action (5). The influenza virus polymerase is widely recognized as a superior target for antiviral drug development (6).
Within the virion, the influenza virus genome is assembled into eight viral ribonucleoprotein (vRNP) complexes, each containing one of the eight negative-stranded viral RNA (vRNA) segments, multiple copies of the nucleoprotein (NP), and one copy of the viral RNA-dependent RNA polymerase (RdRp) complex. The highly conserved influenza virus polymerase is a large heterotrimeric protein complex composed of three subunits: PB1, PB2, and PA (7). The actual polymerizing function for viral RNA synthesis resides in the PB1 subunit (8). Replication of the vRNPs proceeds via complementary RNP (cRNP) intermediates (made up of cRNA, a single polymerase heterotrimer, and multiple NPs), which act as the template for vRNA synthesis. Recent data from cryogenic electron microscopy studies revealed the precise organization of native vRNPs and support a model in which viral genome replication is carried out by a second polymerase complex (9, 10). The vRNPs also serve as the template for transcription to viral mRNAs. This process is initiated by an endonuclease reaction, referred to as “cap snatching,” in which 5′-capped oligonucleotides are cleaved from cellular pre-mRNAs at 10 to 13 nucleotides from the cap to serve as primers for viral mRNA synthesis (11, 12). While cap binding is performed by an independently folding domain of PB2 (13), the endonuclease activity resides in the N-terminal domain of the PA subunit (PA-Nter; containing residues 1 to 256 or fewer) (14, 15). The crystal structure of PA-Nter shows a unique fold, with five mixed β-strands forming a twisted plane surrounded by seven α-helices. The catalytic core houses one or two divalent metal ions (Mg2+ or Mn2+), coordinated by a histidine (His41), a cluster of acidic residues (Glu80, Asp108, and Glu119), and a conserved lysine (Lys134) implemented in catalysis (14, 15). PA-Nter shares structural characteristics with other nucleases of the PD-(D/E)XK superfamily, which also includes most type II restriction endonucleases (16). Recently, structural similarity was reported for the catalytic domains of PA-Nter and the Prp8 endonuclease component of the yeast spliceosome (17). Intriguingly, influenza virus PA-Nter, the related endonuclease active site of orthobunyavirus L-proteins (18), and the yeast Prp8 endonuclease domain have a histidine in their active site, which appears to be a rather unique feature among the PD-(D/E)XK nucleases.
Its unique structure and crucial role in influenza virus replication make the PA endonuclease a prime target for antiviral therapy. About 2 decades ago, researchers at Merck discovered a series of 4-substituted 2,4-dioxobutanoic acids as selective inhibitors of the influenza virus cap-snatching activity (19, 20). One of the leads, the diketo acid (DKA) compound L-742,001 (Fig. 1), showed strong and dose-dependent inhibition of the influenza virus endonuclease reaction in enzymatic assays with virus-derived vRNPs; moreover, it proved to be active in cell culture and mouse models for influenza virus infection. Another endonuclease inhibitor identified by these investigators is flutimide, a fully substituted 1-hydroxy-3H-pyrazine-2,6-dione isolated from a fungus (21), which served as a lead compound for developing a series of more potent aromatic analogues (22). These discoveries were soon followed by others, resulting in a chemical variety of reported influenza virus endonuclease inhibitors, e.g., N-hydroxamic acid and N-hydroxyimide compounds (23), tetramic acid series and diketobutanoates (24), polyphenolic catechins (25), phenethylphenylphthalimide analogs derived from thalidomide (26), macrocyclic bisbibenzyls (27), a group of compounds bearing distinct pharmacophoric fragments (28), and 3-hydroxyquinolin-2(1H)-ones (29). An effort was also made to define the essential pharmacophore from the available structure-activity relationship (24). However, truly rational and structure-based drug design became possible only after the influenza virus PA protein was identified as the endonuclease subunit and the crystal structure of PA-Nter was available (14, 15). The insight into how endonuclease inhibitors interact with their target was further improved by two recently published crystallization studies of PA-Nter in complex with either L-742,001, closely related analogues, or a few other endonuclease inhibitors (30, 31). The central scaffold of these molecules, composed of a diketo acid, diketo hydroxy, or trihydroxy moiety, binds to the catalytic core of PA-Nter by chelating the divalent metal ion(s) (normally coordinated by His41, Glu80, Asp108, and Glu119) and interacting with the catalytic Lys134 and an ordered water molecule. Both crystallization studies revealed the possibility of exploiting different hydrophobic pockets around the catalytic core of PA-Nter. A logical next step is to design optimized endonuclease inhibitors which are able to fully occupy one or more of these pockets, a criterion that was not fulfilled with the existing compounds used during crystallization.
Fig 1.
Chemical structure and protonation states of L-742,001.
Although these structural data create obvious possibilities for inhibitor design, there is a lack of biological data that support the proposed binding mode of the endonuclease inhibitors in the context of the entire viral polymerase complex, as present in influenza virus-infected cells. To identify which residues within or around the catalytic center of PA-Nter are involved in binding and antiviral activity of L-742,001, here we created a series of influenza viruses carrying specific mutations in the PA-Nter sequence. The mutated sites were selected on the basis of computational docking of L-742,001 within the structure of inhibitor-free PA-Nter (14), as well as by analyzing the published crystal structure of PA-Nter in complex with L-742,001 (30). We included the T20A PA mutation, which was detected in an influenza virus selected after cell culture passage with L-742,001 and shown to afford 3-fold resistance to the compound (32). The impact of all these PA mutations on the inhibitory activity of L-742,001 was studied in two cell culture assays: a virus yield replication assay and a reporter-based vRNP reconstitution assay. Our data shed new light on the binding mode of L-742,001 in the PA protein and provide a more accurate picture of its mode of action. We also evaluated which of the PA mutations reduce the polymerase activity of the vRNP complex and analyzed the naturally occurring variation at the relevant amino acid sites. In this way, we provide a first prediction of potential resistance sites in the PA protein that may be taken into account during development of influenza virus endonuclease inhibitors.
MATERIALS AND METHODS
Cells and media.
Madin-Darby canine kidney (MDCK) cells (a kind gift from M. Matrosovich, Marburg, Germany) and human embryonic kidney 293T (HEK293T) cells (purchased from Thermo Scientific) were cultivated in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal calf serum (FCS), 1 mM sodium pyruvate, and 0.075% sodium bicarbonate. During virus experiments, the MDCK cells were maintained in MDCK infection medium, consisting of Ultra MDCK medium (Lonza) supplemented with 0.0225% sodium bicarbonate, 2 mM l-glutamine, and 2 μg/ml tosylsulfonyl phenylalanyl chloromethyl ketone (TPCK)-treated trypsin (Sigma). The cells were incubated in a humidified atmosphere containing 5% CO2.
Antiviral compounds.
Ribavirin (Virazole; ICN Pharmaceuticals) was included as the reference compound. The procedures for chemical synthesis and analysis of the DKA compound L-742,001 [full chemical name, (Z)-4-(1-benzyl-4-(4-chlorobenzyl)piperidin-4-yl)-2-hydroxy-4-oxobut-2-enoic acid; Fig. 1 shows the chemical structure] are not shown here but will be published in due course. Epigallocatechin gallate (EGCG) was purchased from Sigma. The lipophilic aglycoristocetin derivative SA-19 (33) was synthesized by P. Herczegh (University of Debrecen, Hungary). These test compounds were stored as 25 to 50 mM stock solutions in dimethyl sulfoxide (DMSO). In all cellular assays, the final DMSO concentration was kept below 0.8%, a concentration that was free of aspecific effects on cell viability or luciferase activity.
Plasmids.
A bidirectional eight-plasmid system, generously donated by M. Kim (Korea Research Institute of Chemical Technology, Daejeon, South Korea), was used to generate mutant A/PR/8/34 influenza viruses and perform a vRNP reconstitution assay. These eight plasmids (pVP-HA, pVP-NA, pVP-NP, pVP-NS, pVP-M, pVP-PB1, pVP-PB2, and pVP-PA) contain the A/PR/8/34 genomic sequences flanked, in antisense, by a polymerase (Pol) I promoter and Pol I terminator (obtained from the pHH21 plasmid [34]) and ligated, in the sense orientation, between the cytomegalovirus (CMV) Pol II promoter and bovine growth hormone (BGH) polyadenylation signal of the pVAX1 vector (Invitrogen). To introduce specific mutations into the pVP-PA plasmid, the QuikChange II site-directed mutagenesis kit (Stratagene) was used. The absence of any unwanted mutations was verified by sequencing the entire expression cassette (i.e., from the beginning of the CMV Pol II promoter until the end of the BGH polyadenylation signal) using the BigDye Terminator sequencing kit (Applied Biosystems). Plasmid preparations were made with the PureYield plasmid midiprep system kit (Promega), followed by ethanol precipitation. For each mutant pVP-PA plasmid, two independently prepared midipreps were tested to exclude the possibility that observed reductions in vRNP activity were related to inadvertent impurities in the midiprep which might affect protein expression.
The pHH21-FLuc reporter plasmid, also kindly provided by M. Kim, contains the firefly cDNA (originating from the pEGFPLuc vector; Invitrogen), flanked by the 3′- and 5′-untranslated region (UTR) sequences of the A/PR/8/34 NS gene, and ligated, in antisense orientation, between the Pol I promoter and Pol I terminator of the pHH21 backbone plasmid (34).
Computational docking procedures. (i) Ligand.
Model compound L-742,001 was constructed with standard bond lengths and angles from the fragment database with MacroModel 6.0 (35). Minimization of structures was performed with the MacroModel/BachMin 6.0 program using the AMBER force field. An extensive conformational search was carried out using the Monte Carlo/energy minimization (Ei − Emin < 5 kcal/mol, energy difference between the generated conformation and the current minimum) (36). The atomic charges were assigned using the Gasteiger-Marsili method (37). Representative minimum energy conformations were optimized using the ab initio quantum chemistry program Gaussian 09 with the method B3LYP/6-311G basis set (38), and data were visualized with GaussView 5.0 (39). Considering that the experimentally determined (40) protonation constants of the carboxylic group and the keto-enol function of DKA inhibitors are ∼4 for pKa1 and ∼10 to 11 for pKa2 (where pKa1 is the pKa value of the most acidic proton in a molecule with more than one proton), we presumed that L-742,001 is predominantly in the monodeprotonated form under physiological conditions, and the compound was modeled for docking in this way. Furthermore, since a possible protonation at the piperidine nitrogen can occur at pH 7.4, we also included the zwitterionic form (Fig. 1) in our docking analysis.
(ii) Protein.
From the two published X-ray structures of inhibitor-free PA-Nter (14, 15), we used the 2.05-Å-resolution crystal structure of the PA-Nter protein (residues 1 to 209) of influenza virus A/Victoria/3/1975 (H3N2) containing two Mn2+ ions in the catalytic site (14). This crystal structure (Protein Data Bank [PDB] code 2W69, chain A) was retrieved from the RCSB Protein Data Bank (41). Before docking, the water molecules and sulfate ions were stripped, and hydrogen atoms were added using the ADT module. The Gasteiger charges of Autodock were used, giving special attention to the protonation state of the acidic residues in the active site, i.e., Glu80, Asp108, and Glu119.
(iii) Docking.
Calculations were carried out with the method that we previously used and validated for the HIV-1 integrase protein (42–45), with relevant modifications. Binding of the compound was analyzed using AutoDockTools 1.5.6 (46, 47) and AutoDock 4.2 (47, 48). The structures were docked using the Lamarckian genetic algorithm (LGA) (49) and defined through a centered grid (coordinates: x, 0.0; y, 30.0; z, 85.0) with 100, 100, and 100 grid points in x, y, and z dimensions, respectively. The default grid spacing (0.375 Å) was used and 100 docking runs were performed, treating the docking active site as a rigid or partially flexible structure and the ligand as flexible. The missing residues at positions Leu72 and Asn142 in chain A were incorporated from chain B of the same protein after superimposition of the backbones from His41, Glu80, Pro107, Asp108, Leu109, Glu119, and Ile120 (root mean square [RMS] = 0.2541) and substitution by fitting on Leu71, Lys73, and His74 for Leu72 and Thr143 and His144 for Asn142. The partially solved residues Phe105 and Arg185 were also refined based on chain B, and the protein structure was analyzed with the leap module of AMBER 11 (50) using Amber ff03.r1. To include Mn2+ ions, we inserted the following parameters into the frcmod file: mass Mn 54.938 and nonbonded 6-12 interaction Mn 1.69 (Å) and 0.014 (kcal/mol). Na+ ions were also added to obtain charge neutrality.
Generation of influenza viruses with mutant forms of PA.
Wild-type (WT) and PA mutant viruses were generated from the eight pVP plasmids using the reverse genetics procedure published by Martínez-Sobrido (51), with minor modifications. Briefly, a cosuspension of HEK293T and MDCK cells was prepared in DMEM with 10% FCS at a density of ∼15 × 106 cells per ml for each cell line. The cells were transferred to a 12-well plate (125 μl per well), followed by addition of a 650-μl transfection mixture containing 0.5 μg of each of the eight pVP plasmids, diluted in Opti-MEM I, and preincubated with 4 μl Lipofectamine 2000 (both from Invitrogen). Twenty-four hours posttransfection, the medium was replaced by DMEM containing 0.3% bovine serum albumin and 2 μg/ml TPCK-treated trypsin. Virus-containing supernatants were collected at 5 days posttransfection and centrifuged (650 × g, 10 min, room temperature) to remove cell debris. To propagate the rescued viruses, serial dilutions of the clarified supernatants were added to 96-well plates with fresh MDCK cells, seeded 1 day earlier at 7,500 cells per well in MDCK infection medium. After 3 days, the mutant viruses were harvested from selected wells showing full-blown cytopathic effect (CPE), pooled, and stored in aliquots at −80°C. For all of the mutant viruses, the PA, PB1, and PB2 coding sequences were determined to verify that only the desired mutations in PA-Nter were present and to investigate whether compensatory mutations may have emerged in the polymerase heterotrimeric complex.
Antiviral assay with mutant viruses.
To determine the antiviral susceptibility of the mutant influenza viruses, a virus yield assay was performed as described in Meneghesso et al. (52), with minor modifications.
One day prior to infection, MDCK cells were suspended in MDCK infection medium and seeded into 96-well plates at 25,000 cells per well. At day 0, serial dilutions of the test compounds were added, immediately followed by infection with the mutant viruses, generated as described above. The multiplicity of infection (MOI) was 150 CCID50 per well (50% cell culture infectious dose; determined by the method of Reed and Muench [53]). After 24 h of incubation at 35°C, the supernatants were collected and stored at −80°C. The virus amount in these samples was estimated by determining the viral genome copy number in a one-step quantitative real-time reverse transcription-PCR (qRT-PCR) assay. Four μl of each harvested supernatant was mixed with 20 μl resuspension buffer and 2 μl lysis reagent (CellsDirect one-step qRT-PCR kit; Invitrogen) to disrupt the virus particles. After 10 min of heating at 75°C, 10 μl lysate was transferred to a quantitative PCR (qPCR) plate containing the qRT-PCR enzymes and buffer (CellsDirect one-step qRT-PCR kit; Invitrogen) and influenza virus M1-specific primers and probe (as described in reference 54). The qRT-PCR program was performed on an ABI 7500 fast real-time PCR apparatus (Applied Biosystems) and consisted of 15 min at 50°C, 2 min at 95°C, and 45 cycles of 15 s at 95°C followed by 90 s at 60°C. Absolute quantification of vRNA copies was performed by including an M1-plasmid standard. The EC99 and EC90 values were calculated by interpolation and defined as the compound concentration causing a 2- and 1-log10 reduction, respectively, in vRNA copy number compared to the virus control receiving no compound. Pairwise comparison for statistical significance was assessed on data from at least five experiments using an unpaired two-tailed Student's t test with Welch's correction (GraphPad Prism Software, San Diego, CA). In parallel, compound cytotoxic activity was determined in uninfected MDCK cells, which were incubated with serial dilutions of the compounds for 24 or 72 h, using the MTS cell viability assay (CellTiter 96 AQueous one-solution cell proliferation assay; Promega). The spectrophotometric data were used to calculate the 50% cytotoxic concentration (CC50), i.e., the concentration reducing cell viability by 50%, compared to the wells receiving medium instead of compound.
One-cycle virus replication assay.
A simplified time-of-addition experiment was performed as described in Vanderlinden et al. (54), with minor modifications. MDCK cells were seeded into 24-well dishes at 125,000 cells per well. After 16 h of incubation at 35°C, influenza virus (WT A/PR/8/34) was added at the same MOI as that in the virus yield assay. The compounds were added at either 30 min before or 1 h after virus infection. At 8 h postinfection (p.i.), the supernatant was removed and total cellular RNA extracts were prepared with the RNeasy minikit (Qiagen). In order to quantify the negative-sense vRNA, the samples were analyzed by two-step real-time qRT-PCR (54). cDNA synthesis was performed on 0.5 μg of total cellular RNA using Moloney murine leukemia virus (M-MLV) reverse transcriptase (Invitrogen) and 80 nM M1-FOR primer. Real-time PCR then was performed, using influenza virus M1-specific primers and probe (54) and qPCR MasterMix (Eurogentec). All samples were analyzed in duplicate. The vRNA copy number was quantified by including an M1-plasmid standard.
vRNP reconstitution assay.
To determine the inhibitory effect of the compounds on reconstituted influenza virus vRNPs containing mutant forms of PA, the four relevant plasmids (i.e., pVP-PB1, pVP-PB2, pVP-NP, and pVP-PA [WT or mutant]) were combined with the firefly luciferase reporter plasmid and cotransfected into HEK293T cells. The procedure was derived from the reverse genetics method described above. Specifically, the five plasmids, diluted in Opti-MEM I, were incubated with Lipofectamine 2000, mixed with DMEM plus 10% FCS, and added to a suspension of HEK293T cells. The cells were then transferred to a 96-well plate containing serial dilutions of the test compounds so as to obtain, per 60-μl volume per well, 120,000 HEK293T cells, 0.26 μl Lipofectamine 2000, 11 ng of each of the four pVP plasmids, and 4 ng of the pHH21-FLuc plasmid. After 24 h of incubation at 37°C, luciferase activity was determined using the Dual-Glo assay system from Promega. The 50% effective concentration (EC50) was defined as the compound concentration causing 50% reduction in the vRNP-driven firefly luciferase signal compared to cells receiving medium instead of compound. These EC50s were calculated by interpolation assuming a semilog dose-response effect. Finally, for each mutant form of PA, the firefly signal from the cells receiving no compound was used to calculate the vRNP activity of the mutant PA relative to WT-PA, defining the signal for the WT as 100%.
Protein sequence analysis.
To assess the extent of naturally occurring variation at the relevant amino acid sites in PA, we performed a comprehensive PA protein sequence analysis covering more than 12,000 influenza viruses belonging to various (sub)types from different hosts using the Influenza Research Database online single-nucleotide polymorphism (SNP) tool (55).
RESULTS
Docking of L-742,001 in the catalytic site of the PA endonuclease.
In Fig. 2, the result is shown from our docking study with L-742,001 in the inhibitor-free crystal structure of PA-Nter (14), assuming either a rigid receptor (Fig. 2A and B, structures in cyan) or flexible protein structure (Fig. 2A and B, structures in magenta). A docking study similar to ours was published before (56), but these researchers considered the dianionic form of L-742,001, whereas we assumed that the DKA was in its monodeprotonated form (Fig. 1) (40). Since the catalytic center of PA-Nter is considered to be acidic (57), monodeprotonated L-742,001 appears to be the more relevant form. Also, since the diketo acid moiety of L-742,001 is directly connected to position 4 of a piperidine ring, its pKa2 value is expected to be higher than the value of ∼10 to 11 that we experimentally established for α,γ-diketo acid inhibitors of HIV integrase, which instead contain an electron-withdrawing aromatic ring (40). Finally, since the tertiary amine of the piperidine ring could be partially protonated at pH 7.4, we performed an additional docking experiment with the zwitterionic form (Fig. 1) of L-742,001.
Fig 2.
Binding models of L-742,001 predicted by docking in inhibitor-free PA-Nter. (A and B) Clusterized binding poses obtained by docking L 742,001 into the published (14) structure of inhibitor-free PA Nter (PDB entry 2W69), using a rigid receptor (cyan) or flexible protein (magenta) approach and representing the most favorable binding energies (A) or most diffuse population of conformers (B). (C) Representative pose of L-742,001 when modeled in its zwitterionic form. Metal-binding and catalytic residues are shown in yellow. In case these residues are flexible, the alternative positions are shown in violet (A) or in pale blue (B). The two Mn2+ ions in the catalytic center are shown in orange.
A second difference is that the flexible docking performed by Ishikawa and Fujii (56) was built around the flexible Arg84 residue, whereas in our study, the flexible residues taken into account were the metal binding and catalytic residues His41, Glu80, Asp108, Glu119, Ile120, and Lys134, as well as Cys45, which is close to the catalytic site.
Both of our approaches (i.e., rigid and flexible protein structure) suggested a similar binding mode for L-742,001, giving a largely overlapping orientation in the catalytic site of PA-Nter (Fig. 2A and B) and indicating that the compound interacts with both Mn2+ ions (referred to as Mn1 and Mn2 by DuBois et al. [30]). More specifically, the carboxylate group of the DKA motif chelates the first Mn2+ ion, which agrees with our predictions on the binding mode for DKA inhibitors of HIV integrase (40, 58–60). In addition, our docking predicts that the interaction of L-742,001 with the second Mn2+ ion of PA-Nter involves the lone oxygen pair of its α-hydroxyl group and the oxygen atom of its carboxylate moiety (Fig. 2A and B). In contrast, in the crystal structure model (PDB entry 4E5H), the oxygen atom at the γ-position also participates in metal chelation, which largely determines how L-742,001 is positioned within PA-Nter. This two-metal-binding model involving all three coplanar oxygen atoms was also observed in the docking study of Ishikawa and Fujii, who assumed the dianionic form of L-742,001 (56).
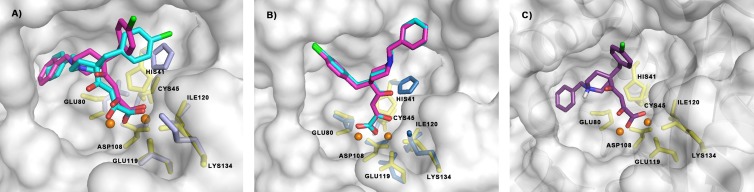
The different binding mode of the DKA moiety in the catalytic center of PA-Nter probably explains why the orientation of the two aromatic wings of L-742,001 (i.e., its benzyl and chlorobenzyl substituents) is clearly different in our docking models (Fig. 3A, structures in cyan and maroon) compared to that observed in the L-742,001-PA-Nter complex (Fig. 3B, pink structure). In our docking study, the two aromatic parts of the compound are reversed by about 180° for the binding pose with the most favorable binding energies (Fig. 3A, cyan structure) compared to that with the most diffuse population (Fig. 3A, maroon structure). The first binding pose (cyan structure) places the benzyl moiety in close proximity to pockets P4 and P5, whereas the chlorobenzyl group is directed toward pockets P1 and P2. In the second pose (maroon structure), L-742,001 is positioned with its chlorobenzyl substituent toward pocket P5 and its benzyl group toward pockets P1 and P2 at another site of the PA-Nter protein. The conformation of both aromatic rings in opposite direction and perpendicularly to the DKA moiety (T-shape), as observed in our docking models, is confirmed in the crystal structure, but in the latter, the compound is tilted about 90° compared to the orientation that we predicted. Thus, in the crystal structure (Fig. 3B, molecule in pink), the chlorobenzyl moiety of L-742,001 is oriented toward pocket P5 around Ala20, whereas the benzyl ring is located in a narrow hydrophobic cavity made up of Arg84, Trp88, Phe105, and Leu106 (pocket P3). Finally, when we docked L-742,001 in its zwitterionic form (Fig. 2C), the binding conformation was similar to that obtained with the monodeprotonated structure (Fig. 2A and B), suggesting that potential protonation of the piperidine ring does not affect either metal binding or orientation of the molecular scaffold within the protein.
Fig 3.
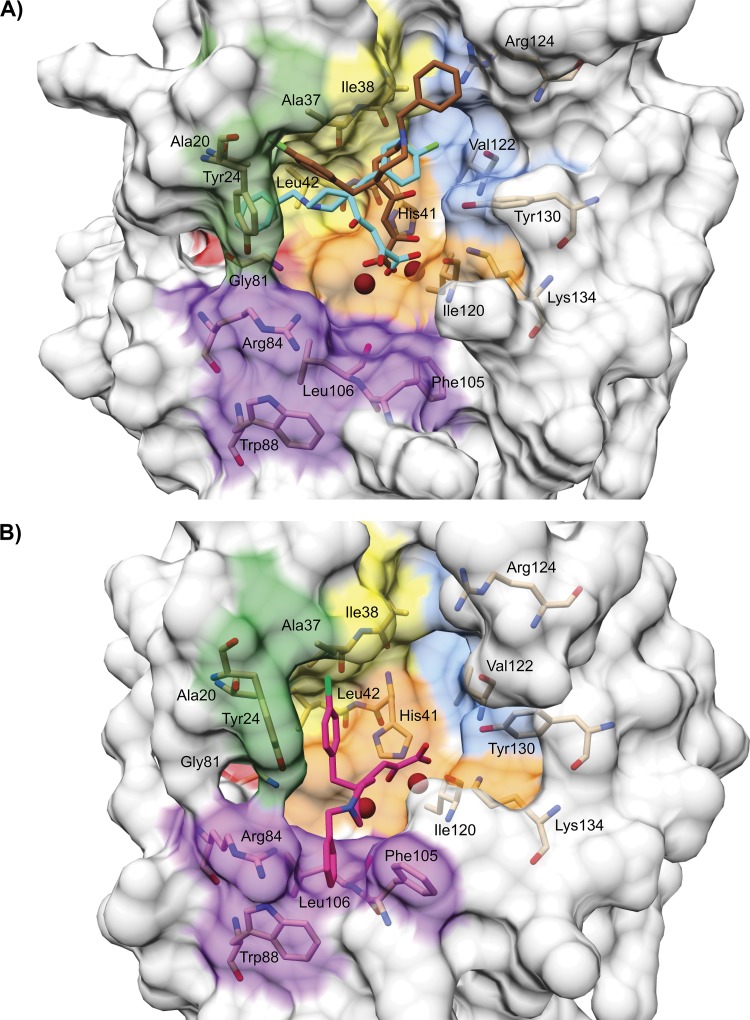
View of the binding pockets for L-742,001 in PA-Nter, as predicted by docking versus X-ray crystallography. (A) Graphical representation of the hypothetical disposition of L-742,001 in PA-Nter. An analogous disposition is seen for the two conformers predicted by docking, i.e., the one representing the most favorable binding energies (in cyan) and that representing the most diffuse population of conformers (in maroon). (B) The structure in pink represents the position of L-742,001 in the crystal structure of the L-742,001-PA-Nter complex (30). The two protein structures are shown in the same orientation after structural alignment using the DALI server (74). The following colors indicate different regions in PA-Nter: orange, metal binding and catalytic residues (His41, Glu80, Asp108, Glu119, Ile120, and Lys134); yellow, pocket P1 (Ala37, Ile38, Leu42, and Lys34); blue, pocket P2 (Thr40, Val122, Arg124, Tyr130, and Phe150); purple, pocket P3 (Arg84, Trp88, Phe105, and Leu106); red, pocket P4 (Leu16 and Gly81); and green, pocket P5 (Ala20, Tyr24, and Glu26). The residues included in our mutational analysis are shown in stick presentation and are labeled. The two metal ions are colored dark red.
Activity and selectivity of L-742,001 in virus yield and vRNP reconstitution assays.
We initially attempted to determine the antiviral activity of L-742,001 in our standard cytopathic effect (CPE) reduction assay, performed in proliferating influenza virus-infected MDCK cells, in which the compound's effect is evaluated at day 3 p.i. (54). Unfortunately, under these conditions, L-742,001 exhibited pronounced cytotoxicity (minimum cytotoxic concentration, as assessed by microscopy, 20 μM; data not shown). We therefore switched to a 24-h virus yield assay in which the number of virus particles released in the supernatant of infected MDCK cells was determined by real-time qRT-PCR. As shown in Table 1, L-742,001 displayed favorable EC90 and EC99 values (i.e., concentrations causing a 10- and 100-fold reduction in virus yield, respectively) of 4.3 and 6.6 μM, respectively, compared to 7.8 and 11 μM for the reference compound ribavirin. The antiviral concentrations we obtained for L-742,001 are higher than the value reported in the initial publication (50% inhibitory concentration in a virus yield assay, 0.35 μM [20]) but in the same range (3 to 11 μM) as that recently published by others (28, 32). For L-742,001 as well as ribavirin, antiviral activity in the virus yield assay nicely correlated with their inhibitory activity in the luciferase reporter-based vRNP reconstitution assay in HEK293T cells, as summarized in Table 1 and displayed in Fig. 4, showing the dose-response curves for both compounds against WT virus and vRNP. Likewise, the CC50 values (50% cytotoxic concentrations assessed by MTS cell viability assay) of L-742,001 were in the same range in MDCK and HEK293T cells (i.e., ∼200 μM after 24 h of incubation).
Table 1.
Activity and selectivity of L-742,001 in the virus yield and vRNP reconstitution assaysa
| Compound | Virus yield assay in MDCK cells |
vRNP reconstitution assay in HEK293T cells |
||||
|---|---|---|---|---|---|---|
| Antiviral activityb |
Cytotoxicity (CC50c) at: |
Activity (EC50d) | Cytotoxicity (CC50) at 24 h |
|||
| EC90 | EC99 | 24 h | 72 h | |||
| L-742,001 | 4.3 ± 0.8 | 6.6 ± 1.3 | 171 ± 23 | 62 ± 26 | 3.8 ± 1.5 | 255 ± 46 |
| Ribavirin | 7.8 ± 0.8 | 11 ± 1 | >400 | >400 | 9.7 ± 1.8 | >400 |
Data shown are the means ± standard errors of the means (SEM) from at least 3 independent tests.
Compound concentration causing a 1-log10 (EC90) or 2-log10 (EC99) reduction in virus yield at 24 h p.i., as determined by real-time RT-PCR.
CC50, 50% cytotoxic concentration, as determined by MTS cell viability assay.
EC50, 50% effective concentration, i.e., compound concentration producing 50% reduction in vRNP-driven firefly reporter signal, estimated at 24 h after transfection.
Fig 4.
Dose-response curves for virus yield reduction in MDCK cells at 24 h p.i. (A) and inhibition of vRNP activity in HEK293T cells (B). Data are means ± standard errors of the means (SEM) from three experiments.
Inhibitory effect of L-742,001 when added after virus entry.
In the past, some experimental anti-HIV compounds with a putative metal-chelating scaffold were initially identified as HIV integrase inhibitors but were later shown to act on HIV entry when evaluated in cell culture (61–63). To exclude that such a dual effect also applies to L-742,001, we determined its anti-influenza virus effect when added at 1 h p.i. We previously demonstrated (33) that, under the experimental conditions used here in MDCK cells, at 1 h p.i., the influenza virus has completed its subsequent stages of viral adsorption, endosomal uptake and escape, and nuclear entry. As shown in Fig. 5, the virus control receiving no compound showed a 200-fold increase in viral RNA copies compared to virus input. In the L-742,001 condition, viral RNA synthesis was inhibited by 100%, whether added at −30 min or 1 h p.i., and the same observation was made for ribavirin. In contrast, the entry inhibitor SA-19 (33) and the polyphenolic compound EGCG (which acts as a PA endonuclease inhibitor in enzymatic assays [25, 31]) were devoid of inhibitory activity when added 1 h p.i. This means that the anti-influenza virus effect of EGCG in cell culture is based on inhibition of virus entry, as also reported for some other unrelated viruses (64). Although EGCG inhibits the PA endonuclease in enzymatic assays quite effectively (25, 31), this effect appears to be irrelevant in virus-infected cell cultures.
Fig 5.
Inhibitory effect of L-742,001 on viral RNA synthesis is situated after viral entry. Light gray bars indicate that the test compound L-742,001 (50 μM), EGCG (50 μM), ribavirin (20 μM), or SA-19 (20 μM) was added to MDCK cells, and after 30 min incubation at 35°C, influenza virus A/PR/8/34 was added. Dark gray bars indicate that virus was added first and allowed to enter during 1 h of incubation, after which the compounds were added at the same concentrations as those described for the light gray bars. In both conditions, total cellular RNA was extracted at 10 h p.i. VC, untreated virus control. The number of vRNA copies was quantified by two-step real-time RT-PCR. On the y axis, the fold increase in vRNA copies is shown relative to the viral copy number added at time zero. Ribavirin and L-742,001 remain fully effective when added after virus entry. In contrast, the reported entry inhibitor SA-19 (33) as well as EGCG are inactive when added at 1 h p.i. Data shown are the means ± SEM from two independent tests.
Activity of L-742,001 against influenza viruses or vRNPs carrying mutant forms of PA.
The models for binding of L-742,001 to the catalytic domain of PA-Nter (Fig. 3) served as the starting point to construct a large series of influenza viruses carrying single amino acid substitutions in PA. A first set of changes was located at sites with a known and crucial role in metal binding or endonuclease cleavage (i.e., H41A, I120T, and K134A). Also, changes near these catalytic residues (i.e., of Gly81, which is adjacent to Glu80; of Leu42, next to His41; and of Val122, close to Ile120) were evaluated. A second set of mutations was introduced to reduce the hydrophobicity in the predicted binding pockets for either the benzyl or chlorobenzyl ring of L-742,001. Aromatic residues were replaced by a smaller hydrophobic amino acid (i.e., Y24L, W88L, F105L, and Y130A). Small hydrophobic residues were replaced by a Thr (i.e., A37T, I38T, L106T, and V122T) to introduce a hydrophilic uncharged moiety that is small enough to have no obvious steric effect on local protein structure. The Arg84 residue, contacting L-742,001 according to the crystal structure model, was replaced by a hydrophobic Leu. The Arg124 residue was replaced by a neutral Gln residue. In a third instance, residue changes found to alter the antiviral activity of L-742,001 (i.e., at positions Gly81, Ile120, and Arg124) were further varied by introducing other nonconservative changes at these sites. Finally, we included the T20A change that was shown to confer 3-fold resistance to L-742,001 in an influenza virus passaged in cell culture in the presence of L-742,001 (32). It should be noted here that the PA-Nter sequence used in our docking study contains an Ala20 residue, whereas the WT pVP-PA plasmid used in our biological experiments contains Thr20.
The impact of these PA mutations on the activity of L-742,001 in cell culture was evaluated both in the context of virus replication (by virus yield assay at 24 h p.i.) and after reconstitution of the vRNP (using the luciferase reporter assay). As shown in Table 2, the antiviral data obtained by both assays showed a nice correlation. The strongest resistance to L-742,001 (EC90s or EC50s increased by up to 20-fold compared to those of WT virus or vRNP) was seen for changes located at or near sites that are involved in metal binding; thus, they are directly relevant for binding of the DKA moiety of L-742,001. Replacement of the metal-binding His41 by Ala conferred 20-fold resistance to L-742,001 in the vRNP assay and 3- to 4-fold resistance in the virus yield assay. Conversion of Ile120, of which the backbone carbonyl function is involved in metal chelation, to Thr caused a >15-fold resistance to L-742,001 in both cell assays, whereas the conservative I120V change had no effect. As expected, the K134A catalytic site mutation rendered the virus and vRNP nonviable and could not be evaluated for resistance to L-742,001. An intriguing observation was made for Gly81, which is adjacent to the metal-chelating residue Glu80. A positively (Lys) or negatively (Asp) charged residue at this site rendered the virus and vRNP nonviable. Three other mutations at this position yielded a viable virus that was >22-fold (G81V), >12-fold (G81F), or > 9-fold (G81T) resistant to L-742,001. The strong antiviral resistance of the G81T and G81F mutants was confirmed in the vRNP assay, whereas the G81V mutant vRNP was not active in the vRNP assay.
Table 2.
Inhibitory activity of L-742,001 and ribavirin against influenza viruses or reconstituted vRNPs carrying mutant forms of PAc
| Mutation in PA | Virus yield assay in MDCK cellsa |
vRNP reconstitution assay in HEK293T cells (EC50b) |
||||
|---|---|---|---|---|---|---|
| L-742,001 |
Ribavirin |
L-742,001 | Ribavirin | |||
| EC90 | EC99 | EC90 | EC99 | |||
| WT | 4.3 ± 0.8 | 6.6 ± 1.3 | 7.8 ± 0.6 | 11 ± 1 | 3.8 ± 1.5 | 9.7 ± 1.8 |
| T20A | 14 ± 1.9 (3) | 21 ± 4 (3) | 8.4 ± 1.2 (1) | 14 ± 3 (1) | 9.6 ± 1.2 (3) | 12 ± 4 (1) |
| Y24L | 6.1 ± 1.3 (1) | 14 ± 4 (2) | 13 ± 2 (2) | 28 ± 5 (2) | 3.7 ± 0.7 (1) | 13 ± 1 (1) |
| A37T | 4.8 ± 0.7 (1) | 7 ± 0.9 (1) | 7.6 ± 0.9 (1) | 11 ± 2 (1) | 5.7 ± 3 (1) | 9.3 ± 3.5 (1) |
| I38T | 4.8 ± 0.7 (1) | 7.7 ± 0.8 (1) | 8.2 ± 1.2 (1) | 12 ± 2 (1) | 8 ± 2.3 (2) | 9.2 ± 2.4 (1) |
| H41A | 14 ± 5 (3) | 28 ± 9 (4) | 12 ± 1 (2) | 19 ± 2 (2) | 77 ± 8 (20) | 10 ± 2 (1) |
| L42T | 9.1 ± 2.2 (2) | 15 ± 3 (2) | 8.6 ± 1.2 (1) | 12 ± 2 (1) | 13 ± 4 (3) | 7.6 ± 1.5 (1) |
| G81D | NV | NV | NV | NV | NV | NV |
| G81F | 49 ± 9 (12) | >90 ± 6 (>14) | 6.7 ± 0.5 (1) | 12 ± 1 (1) | 136 ± 45 (35) | 14 ± 6 (1) |
| G81K | NV | NV | NV | NV | NV | NV |
| G81T | >37 ± 6 (>9) | >81 ± 10 (>12) | 6.6 ± 1.2 (1) | 11 ± 2 (1) | 130 ± 35 (34) | 7.5 ± 2.1 (1) |
| G81V | >96 ± 4 (>22) | >100 ± 0 (>15) | 10 ± 1 (1) | 14 ± 1 (1) | NV | NV |
| R84L | 1.6 ± 0.3 (0.4) | 3.9 ± 0.8 (0.6) | 6.8 ± 0.7 (1) | 12 ± 2 (1) | 2.7 ± 0.8 (0.7) | 4.7 ± 0.3 (0.5) |
| W88L | 3.6 ± 0.5 (1) | 8.7 ± 1.1 (1) | 11 ± 1 (1) | 17 ± 2 (1) | 7.1 ± 0.2 (2) | 9.7 ± 1.7 (1) |
| F105L | 2 ± 0.3 (0.5) | 4.2 ± 0.8 (0.6) | 7.5 ± 0.8 (1) | 12 ± 2 (1) | 1.6 ± 0.4 (0.4) | 6.7 ± 0.8 (1) |
| L106T | 3.6 ± 1.1 (1) | 8.9 ± 1.5 (1) | 7 ± 0.3 (1) | 11 ± 1 (1) | 4.4 ± 1.1 (1) | 6.5 ± 2.3 (1) |
| I120D | NV | NV | NV | NV | NV | NV |
| I120F | NV | NV | NV | NV | NV | NV |
| I120K | NV | NV | NV | NV | NV | NV |
| I120T | >78 ± 9 (>18) | >100 ± 0 (>15) | 5.1 ± 0.5 (1) | 11 ± 2 (1) | 113 ± 29 (29) | 12 ± 3 (1) |
| I120V | 2.7 ± 0.6 (1) | 6.1 ± 1.6 (1) | 6.7 ± 1 (1) | 11 ± 1 (1) | 3 ± 0.9 (1) | 9.8 ± 0.8 (1) |
| V122T | 9.3 ± 1.2 (2) | 15 ± 2 (2) | 6.8 ± 0.5 (1) | 8.8 ± 1.2 (1) | 20 ± 9 (5) | 11 ± 3 (1) |
| R124E | NV | NV | NV | NV | NV | NV |
| R124Q | <1.3 ± 0.2 (0.3) | 3.2 ± 0.6 (0.5) | 6.9 ± 0.5 (1) | 12 ± 1 (1) | 1.3 ± 0.5 (0.3) | 7.1 ± 2.7 (1) |
| Y130A | <1.7 ± 0.3 (0.4) | 5.4 ± 0.5 (0.8) | 10 ± 0 (1) | >29 ± 4 (>3) | 1.3 ± 0.3 (0.3) | 6.7 ± 1.8 (1) |
| K134A | NV | NV | NV | NV | NV | NV |
Antiviral activity expressed as the compound concentrations (in μM) producing a 1-log10 (EC90) or 2-log10 (EC99) reduction in virus yield at 24 h p.i., as determined by real-time RT-PCR. Data in parentheses indicate fold increase compared to the WT.
EC50, 50% effective concentration (in μM), i.e., compound concentration producing 50% reduction in vRNP-driven firefly reporter signal, estimated at 24 h after transfection of HEK293T cells. Data in parentheses indicate fold increase compared to the WT.
NV, nonviable mutation, no virus recovered by reverse genetics, or vRNP activity in HEK293T cells of <2% relative to that of the WT. Data shown are means ± SEM from at least 3 independent tests.
A second site conferring resistance to L-742,001 was the T20A change outside the catalytic center of PA. In the virus yield assay, this substitution caused a 3-fold increase in EC90 and EC99. In the vRNP assay, the increase in EC50 was 2-fold (P < 0.05 for comparison of T20A mutant to WT vRNP). According to our docking results (Fig. 3A), Ala20 was predicted to lie in a hydrophobic pocket binding the benzyl or chlorobenzyl group of L-742,001; in the crystallographic model (30), this pocket is proposed to accommodate the compound's chlorobenzyl group (Fig. 3B). The proximity of Leu42 agrees with the 2- to 3-fold resistance of the L42T mutant. The Y24L, A37T, and I38T changes had no consistent effect, indicating that these residues are located too far away to form relevant hydrophobic interactions with L-742,001. In the crystallographic model (Fig. 3B), the benzyl moiety of L-742,001 binds to a narrow hydrophobic pocket (P3) comprising Arg84, Trp88, Phe105, and Leu106 (30). Although we evaluated only selected changes at these positions (i.e., R84L, W88L, F105L, and L106T), our antiviral data make a role for these residues in the activity of L-742,001 quite ambiguous. The F105L and R84L substitutions rendered the vRNP ∼2-fold more sensitive to L-742,001; in the virus yield assay, these mutants displayed 2-fold decreases in EC90 (P < 0.05), whereas their EC99 values were not significantly changed compared to those of the WT. The W88L mutation had no impact in the virus yield assay but gave a slight (∼2-fold) increase in the vRNP assay. Finally, for the L106T mutant virus and vRNP, the sensitivity to L-742,001 was unchanged.
An intriguing observation was the significant increase in antiviral sensitivity for the PA mutant carrying an alteration of Arg124. The R124Q mutant virus displayed 2- to 3-fold higher sensitivity to L-742,001 (P < 0.05 compared to the WT), and this was confirmed in the vRNP assay. Tyr130 and Val122, which are two residues located near Arg124 (Fig. 3B), also appeared to be associated with sensitivity to L-742,001. The Y130A change caused an ∼3-fold reduction in the EC50 (vRNP assay) or EC90 value (virus yield assay). Regarding Val122, the V122T mutant vRNP was 5-fold resistant to L-742,001; in the virus yield assay, its increase in EC90 and EC99 was 2-fold (P < 0.01 compared to the WT virus). In combination, these data favor a role for Val122, Arg124, and Tyr130 in the activity of L-742,001.
All virus yield data were carefully analyzed to exclude the possibility that the observed resistance for L-742,001 is related to diminished replication fitness of some specific PA mutant viruses. First, the WT and mutant viruses all yielded comparable values for viral genome copy number per ml at 24 h p.i. (i.e., log10 values in the range of 10.2 ± 0.1, which is of the same order of magnitude reported by others [65]). Second, an experiment was performed in which the EC99 values of L-742,001 were determined for WT and G81T mutant virus used at different MOIs (i.e., 40-fold difference between highest and lowest MOI), giving a virus yield at 24 h p.i. in the same range as that described above (i.e., 9.3 to 11.0 log10 viral genome copies per ml). The antiviral activity of L-742,001 was consistent over the MOI range tested, with EC99 values of 7.1 ± 0.3 μM for WT virus and 86.9 ± 6.0 μM for the G81T mutant (data not shown). Finally, ribavirin was consistently included as a reference in every resistance experiment to ensure that any bias due to reduced viral fitness would be recognized. For some mutant viruses or vRNPs, we observed minor increases (up to 2-fold) in the antiviral values for ribavirin. This nucleoside analogue affects viral RNA synthesis, either directly by acting on the viral polymerase or indirectly by depleting the cellular GTP pools (66). This is in agreement with our general observation that mutations in the PB1, PB2, or PA subunit of the influenza virus polymerase complex that are associated with reduced fitness of the virus or vRNP complex often result in an apparent 2- to 3-fold resistance to ribavirin (L. Naesens, unpublished data). We therefore assume that this phenomenon is intrinsic to ribavirin rather than being related to specific mutations in PA.
Polymerase activity of vRNP complexes carrying mutations in PA.
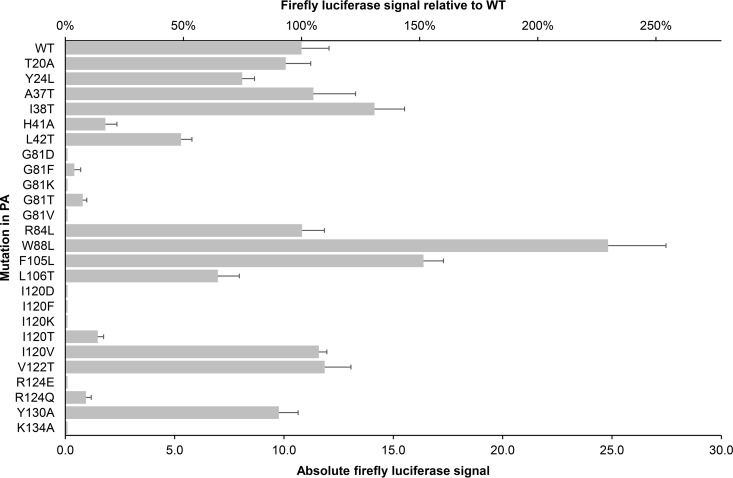
The luciferase-based vRNP reconstitution assay is well suited to estimate the enzymatic activity of vRNP complexes carrying mutations in any of the three polymerase subunits, including PA (67). We therefore calculated the activity of our PA mutant vRNPs relative to the WT. As shown in Fig. 6, the following PA mutations had no or only modest impact on vRNP activity (the range was between 65 and 131% compared to the WT): T20A, Y24L, A37T, I38T, R84L, L106T, V122T, and Y130A. This tolerance is consistent with the fact that neither of these residues has a catalytic function in PA-Nter. Two mutations outside the catalytic domain, i.e., F105L and, in particular, W88L, had a stimulating effect on vRNP activity. The Arg124 residue located outside the catalytic pocket was quite intolerant to mutation, since the R124E mutant was inactive and the R124Q mutant had a relative activity of 9%. In the case of the metal-binding residue Ile120, vRNP activity was reduced to zero for the I120K, I120D, and I120F mutants and to 14% for the I120T mutant; it was unaffected for the conservative I120V change. As expected and given the crucial role of Lys134 in catalysis, the K134A change yielded an inactive vRNP (14, 15). The H41A mutant still had 17% vRNP activity compared to the WT, which is surprising since this His41 is generally considered to have a crucial role in metal chelation, substrate binding, and structural integrity of the polymerase (14, 15, 30, 31, 57, 68, 69). The L42T change in the site adjacent to His41 gave a reduction of 50%. Alterations of the conserved Gly81 (which is adjacent to the catalytic Glu80) caused a dramatic reduction in vRNP activity, i.e., 100% for G81D, G81K, and G81V, 96% for G81F, and 93% for G81T. Several PA mutations studied here elaborate on previous reports, in which the change was limited to an Ala (15, 57, 68).
Fig 6.
Polymerase activity of vRNP complexes carrying mutant forms of PA. HEK293T cells were cotransfected with the four vRNP-reconstituting plasmids (including the WT or mutant form of PA) and the reporter plasmid. On the horizontal axis, the vRNP-driven firefly luciferase signal is shown relative to the WT (top axis) or in absolute values (bottom axis). Data shown are the means ± SEM from at least three independent tests.
DISCUSSION
The study presented here was aimed at revealing the binding mode of the anti-influenza virus compound L-742,001, a DKA inhibitor of the influenza virus endonuclease identified many years ago (19, 20). Rational optimization of this initial lead compound into more potent and selective antiviral agents became possible only after the protein structure of its viral target, the N-terminal part of PA, was revealed. The crystal structure of PA-Nter indicates that its catalytic core contains three acidic residues (Glu80, Asp108, and Glu119) which, together with His41 and Ile120, coordinate two Mn2+ ions (14) or a single Mg2+ ion (15, 69), depending on the crystallization conditions. In biochemical experiments, the endonuclease activation by divalent metal ions is cooperative (70) and the endonuclease activity of isolated PA-Nter is higher in the presence of Mn2+ than Mg2+ (57). We therefore preferred to use the crystal structure with two Mn2+ ions when performing computational docking of L-742,001 in the inhibitor-free PA-Nter protein. Importantly, our rigid and flexible docking approaches (which yielded comparable binding poses) were built on the assumption that L-742,001 is most prevalent in its monodeprotonated or zwitterionic form at the acidic pH inside the catalytic center of PA-Nter (40). In contrast, the docking experiments by Ishikawa and Fujii (56) considered the dianionic form of L-742,001, and the recently published crystallization study of L-742,001 in complex with PA-Nter was performed at pH 8.0 (30). This could explain why the orientation of L-742,001 in our docking models differs from that observed in the crystal structure. According to our predictions, L-742,001 coordinates one of the two Mn2+ ions via its carboxylate group, whereas the second Mn2+ ion is bound by the α-hydroxyl and the carboxylate's oxygen. In the crystal structure of the L-742,001-PA-Nter complex, the third coplanar oxygen (i.e., that in γ-position) is also involved in complexation of the second Mn2+ ion (30). The different binding mode of the DKA motif of L-742,001 in the active center of PA-Nter explains why the molecule is rotated about 90° in our docking models compared to its orientation in the crystal structure model. Consequently, the two approaches also differ in where to locate the aromatic “wings” of L-742,001 in the hydrophobic pockets (Fig. 3) around the catalytic core of PA-Nter. In the crystal structure, the chlorobenzyl of L-742,001 is positioned in pocket P5 (Fig. 3B) around Ala20, whereas the benzyl ring is situated in a cavity (indicated as P3 in Fig. 3B) made up of Arg84, Trp88, Phe105, and Leu106. According to our docking predictions and depending on the type of top-ranked clusterized conformers, the aromatic wings of L-742,001 are located in pockets P1, P2, P4, and P5 (Fig. 3A). Importantly, in neither of the binding models for L-742,001 (i.e., our docking predictions or the published crystal structure), optimal interactions (such as π-stacking) with the hydrophobic residues comprising the presumed binding pockets were observed.
This suboptimal fitting of the compound most likely explains why only a modest (i.e., maximum 5-fold) level of resistance to L-742,001 was seen with the PA mutant viruses and vRNPs bearing mutations within the predicted hydrophobic binding pockets for L-742,001. In contrast, a high level of resistance to L-742,001 was noted for mutations I120T and H41A within the catalytic core of PA-Nter, consistent with the notion that the metal-binding effect of the DKA moiety is a crucial factor in endonuclease inhibition by L-742,001. Since the H41A mutation affects the metal complexation at this site, it likely reduces the ability of L-742,001 to bind in the catalytic center. In the case of Ile120, it is the backbone carbonyl that is involved in metal complexation; this functionality is obviously not changed upon mutation. The strong resistance of the I120T mutant is possibly related to a local change in the protein structure. A Val at position 120 (the common residue in influenza B viruses) did not alter the activity of L-742,001 (Table 3).
Table 3.
Polymorphism of the relevant residues in this study among published influenza virus PA protein sequencesa
| Position and amino acid | Virus (sub)typeb |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human H1N1 (n = 5,681) | 2009 pandemicc (n = 4,352) | Human H3N2 (n = 3,577) | Human H5N1 (n = 246) | Avian H3N8 (n = 295) | Avian H4N6 (n = 209) | Avian H5N1 (n = 333) | Avian H7N3 (n = 150) | Avian H9N2 (n = 122) | Swine H1N1 (n = 726) | Swine H3N2 (n = 331) | B(n = 455) | C(n = 90) | |
| 20 | |||||||||||||
| Ala | 98.1 | 99.6 | 97.1 | 91.1 | 99.7 | 99.5 | 98.5 | 100 | 91.0 | 62.5 | 97.0 | 98.9 | |
| Thr | 1.9 | 0.3 | 2.8 | 8.9 | 0.3 | 1.5 | 9.0 | 37.1 | 1.2 | 100 | |||
| Ile | 0.02 | ||||||||||||
| Val | 0.1 | 0.03 | 0.4 | 1.1 | |||||||||
| Ser | 0.5 | 1.8 | |||||||||||
| 24 | |||||||||||||
| Tyr | 99.9 | 100 | 99.9 | 100 | 98.6 | 100 | 99.7 | 100 | 100 | 74.0 | 89.4 | 100 | |
| His | 0.1 | 0.02 | 0.1 | 1.4 | 0.3 | 26.0 | 10.6 | ||||||
| Cys | 0.02 | ||||||||||||
| Phe | 0.1 | 100.0 | |||||||||||
| 37 | |||||||||||||
| Ala | 99.9 | 99.9 | 100 | 100 | 100 | 100 | 100 | 100 | 91.0 | 100 | 100 | ||
| Thr | 0.02 | 0.02 | |||||||||||
| Ser | 0.02 | 0.02 | 9.0 | 1.1 | |||||||||
| Asn | 100 | ||||||||||||
| Gln | 98.9 | ||||||||||||
| 38 | |||||||||||||
| Ile | 99.9 | 99.9 | 99.9 | 100 | 96.9 | 100 | 100 | 100 | 99.1 | 99.9 | 99.4 | 100 | 98.9 |
| Val | 0.04 | 0.05 | 3.1 | 0.9 | 0.1 | 0.6 | 1.1 | ||||||
| Thr | 0.02 | ||||||||||||
| Met | 0.02 | 0.02 | 0.1 | ||||||||||
| 41 | |||||||||||||
| His | 99.9 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 99.9 | 100 | 100 | 100 |
| Tyr | 0.02 | 0.02 | |||||||||||
| Arg | 0.1 | ||||||||||||
| 42 | |||||||||||||
| Leu | 99.8 | 100 | 100 | 100 | 100 | 100 | 100 | 98.7 | 100 | 61.6 | 98.2 | 100 | |
| Met | 0.2 | 38.3 | 1.8 | ||||||||||
| Phe | 0.7 | 100 | |||||||||||
| Ser | 0.7 | ||||||||||||
| Gln | 0.1 | ||||||||||||
| 81 | |||||||||||||
| Gly | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 84 | |||||||||||||
| Arg | 100 | 100 | 99.9 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Lys | 0.1 | ||||||||||||
| Gly | 0.03 | ||||||||||||
| 88 | |||||||||||||
| Trp | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 99.7 | 100 | 100 | |
| Ser | 0.1 | ||||||||||||
| Arg | 0.1 | ||||||||||||
| Val | 98.9 | ||||||||||||
| Ile | 1.1 | ||||||||||||
| 105 | |||||||||||||
| Phe | 99.6 | 99.8 | 99.4 | 94.4 | 100 | 100 | 95.2 | 100 | 91.9 | 85.6 | 97.0 | 95.6 | |
| Tyr | 0.1 | 1.2 | 8.1 | 13.8 | 1.5 | 100 | |||||||
| Leu | 0.2 | 0.2 | 0.2 | 1.2 | 0.3 | 1.5 | |||||||
| Ser | 0.3 | 3.2 | 4.8 | ||||||||||
| His | 0.4 | ||||||||||||
| Asn | 1.1 | ||||||||||||
| 106 | |||||||||||||
| Leu | 99.9 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Ile | 0.02 | 0.02 | |||||||||||
| Phe | 0.02 | ||||||||||||
| Val | 0.02 | 0.02 | |||||||||||
| 120 | |||||||||||||
| Ile | 99.9 | 100 | 99.9 | 100 | 98.3 | 100 | 100 | 100 | 100 | 99.0 | 99.7 | 0.2 | 98.9 |
| Val | 0.04 | 0.02 | 0.1 | 1.7 | 1.0 | 0.3 | 99.8 | 1.1 | |||||
| 122 | |||||||||||||
| Val | 99.9 | 100 | 99.9 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 0.2 | |
| Ile | 0.04 | 0.03 | 99.8 | 100 | |||||||||
| 124 | |||||||||||||
| Arg | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 96.6 | |
| Lys | 99.8 | 1.1 | |||||||||||
| Ile | 2.3 | ||||||||||||
| Asn | 0.2 | ||||||||||||
| 130 | |||||||||||||
| Tyr | 100.0 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 134 | |||||||||||||
| Lys | 99.9 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 99.7 | 100 | 100 |
| Glu | 0.04 | 0.05 | 0.3 | ||||||||||
| Thr | 0.03 | ||||||||||||
A minority of the sequences contained a deletion or unspecified residue at the selected sites; these were omitted from the analysis.
n, number of sequences analyzed.
The 2009 pandemic H1N1 virus PA segment is derived from an avian influenza virus.
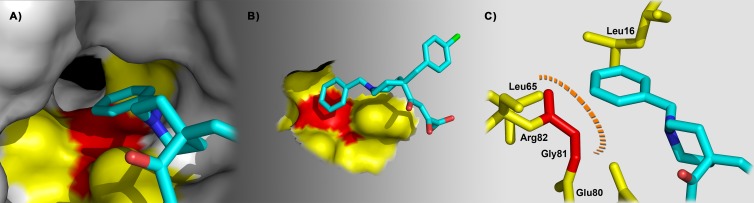
A highly relevant observation is the pronounced resistance (at least 9-fold) for Gly81 mutations near the metal binding residue Glu80. The resistance to L-742,001 was found to decrease in the order G81V > G81F > G81T. A role for Gly81 was proposed in one of our two docking models (Fig. 3B; also shown in closeup in Fig. 7) which suggested that Gly81 forms a hydrophobic interaction with the benzyl moiety of L-742,001. This unsubstituted (Gly) residue may also be required to leave space for the benzyl group when positioning L-742,001 in the tunnel formed by Leu16, Leu65, Ile79, Glu80, Gly81, and Arg82 (Fig. 7A to C). Hence, replacement of Gly81 with a bulkier residue can affect the accommodation of the aromatic wing inside the tunnel. An alternative interpretation is that Gly81 is critical to shape the binding pocket for L-742,001. The finding that, in terms of sensitivity to L-742,001, a Phe at position 81 is less detrimental than a Val could indicate that the resistance is partially reversed by a π-stacking interaction between the aromatic ring of the Phe residue and the benzyl of L-742,001. Alternatively, it could relate to an opposite disposition of the aromatic ring of the Phe residue within the tunnel, limiting its capacity to contribute to the hydrophobic binding site. Whatever the precise role of Gly81 is, our resistance data convincingly show that this residue is directly or indirectly involved in the binding of L-742,001 to PA-Nter.
Fig 7.
Hypothetical interaction between L-742,001 and residue Gly81 in PA-Nter. (A to C) Predicted disposition of L-742,001 into pocket P4. In the best-scoring binding poses predicted by our docking, the benzyl group of L-742,001 is directed toward pocket P4, which contains residue Gly81 (red in all panels) inside the tunnel (A and B). Panel C shows that the benzyl group of L-742,001 may establish a favorable hydrophobic interaction with Gly81 within a distance of ∼3 Å.
Regarding the binding pockets for L-742,001 outside the catalytic center of PA, our antiviral data confirm a role for the cavity around Ala20. The 3-fold resistance of our T20A mutant virus fully agrees with a previous report describing a 3-fold increase in antiviral EC50 for an influenza virus selected in cell culture in the presence of L-742,001 and found to contain this T20A mutation in PA (32). This change is a naturally occurring polymorphism among influenza A viruses, whereas a conserved Thr20 is present in influenza B viruses (Table 3). The slightly larger Thr (compared to Ala) is thought to give a better interaction with one of the two aromatic rings of L-742,001, i.e., the chlorobenzyl group in the crystal structure model (30) or the chlorobenzyl/benzyl group according to our docking models. Although our resistance data with the T20A mutant do not resolve which of the two aromatic wings of L-742,001 is involved, they do support a role for pocket P5 in binding L-742,001. The lack of resistance for the Y24L mutant is noteworthy, since the crystal structure suggested close hydrophobic contact between the compound's chlorobenzyl moiety and the aromatic ring of Tyr24 (30).
Of note, our resistance data implicate a role for Arg124, Val122, and Tyr130 situated in pocket P2 (Fig. 3A) which, according to our docking models, accommodate the chlorobenzyl or benzyl ring of L-742,001. The higher (2- to 3-fold) sensitivity of the R124Q and Y130A mutants and lower (2- to 5-fold) sensitivity of the V122T mutant are consistent with a role for pocket P2 in binding L-742,001. On the other hand, our resistance data with L-742,001 are unclear about the role of hydrophobic pocket P3 (made up of Arg84, Trp88, Phe105, and Leu106), as proposed by the crystal structure model (30). The changes introduced in this pocket caused no change (L106T) or a maximum 3-fold change in sensitivity to L-742,001. Leu106 is situated in the center and Trp88 and Phe105 lie at the borders of the proposed binding cavity, and their narrowest distance to L-742,001 was estimated to be 3 to 4 Å (Fig. 7B). The W88L and F105L changes implicate a dramatic reduction in the size of the hydrophobic moiety, yet they did not markedly alter the activity of L-742,001. Hence, a close binding interaction between L-742,001 and any of these two residues appears unlikely.
Several reasons may account for the apparent discrepancy between the crystallographic data (based on isolated PA-Nter) and our biological data obtained in cell culture. A first hypothesis that, in terms of catalytic functionality, isolated PA-Nter does not adequately reflect the entire PA in the native influenza polymerase complex was contradicted in enzymatic experiments with mutant forms of PA-Nter by Crépin et al. (57). However, Noble et al. (71) produced recombinant full-length PA protein to determine its metal ion dependence and substrate selectivity. These authors hypothesized that the full-length PA protein interacts with inhibitors differently than the truncated PA-Nter, possibly due to stabilization of the active site by the C-terminal domain. Another controversy is related to the number (one or two) and identity (Mn2+ or Mg2+) of the divalent metal ions in the active site of PA (14, 15, 30, 31, 69). This issue is directly relevant for inhibitors such as L-742,001, since the potency of DKA inhibitors of HIV integrase for wild-type or mutant HIV integrase was found to depend on whether the enzymatic assays were performed with Mn2+ or Mg2+; moreover, this metal selectivity was dependent on the structure of the DKA inhibitor (72). Finally, the large difference in the intracellular concentrations of Mg2+ and Mn2+ (mM versus μM ranges) has been used to argue that Mg2+ is a more plausible cofactor for influenza virus endonuclease (69). Although our cell culture data do not answer these specific issues, they draw attention to the importance of cell culture evaluation during early design of influenza virus endonuclease inhibitors.
Though not the first aim of our study, our vRNP data with mutant forms of PA allowed us to estimate the impact of the PA mutations on the overall polymerase activity of the vRNP complexes. The changes which totally destroyed the activity of the reconstituted vRNPs (G81K, G81D and G81V, I120K, I120D and I120F, and K134A) are all located in the catalytic center of PA (14, 15). As far as we know, only the K134A (15, 57, 68) and G81A mutations (68) have been described before. The dramatic impact of R124E and, to a lesser extent, R124Q could be related to a local change in the protein's structure, although a role for Arg124 in binding the RNA substrate or cleavage product might also be a valid hypothesis (15). The following mutations, associated with strong resistance to L-742,001, showed 83 to 96% reduction in vRNP activity: H41A, G81F, G81T, and I120T. This heavily compromised polymerase activity implies that selection of viruses carrying these single mutations, following prolonged exposure to L-742,001 or another PA inhibitor (e.g., in cell culture or patients), is highly unlikely. This concurs with our SNP analysis (Table 3) showing that Gly81 and His41 are 100% conserved among naturally occurring influenza A, B, and C viruses, while for Ile120 and Arg124, only conservative variations are seen (i.e., Val120 and Lys124, the common residues in influenza B viruses). Surprisingly, we were able to recover influenza viruses bearing these highly disadvantageous mutations in PA. During our reverse genetics procedure, we allowed virus breakthrough for as long as 5 days. We therefore postulated that the replication-competent viruses that we obtained might contain some compensatory mutations in the polymerase heterotrimer to partially compensate for the heavily affected endonuclease activity. However, in neither of the mutant viruses did we see evidence for compensatory mutations in PA, PB1, or PB2 when analyzing their entire coding sequences. Further genotyping of other viral proteins (such as the NP or the nuclear export protein [73]) and in-depth phenotypic analysis of these mutant viruses is currently ongoing.
At the other end of the spectrum, we found that the following mutations in PA, lying in the predicted hydrophobic binding pockets for diverse endonuclease inhibitors (including L-742,001) (30, 31), resulted in either no significant change (T20A, Y24L, A37T, I38T, R84L, V122T, and Y130A), a modest decrease (L42T and L106T), or an increase in vRNP activity (W88L and F105L [P < 0.05]). Analysis of the naturally occurring variation of these residues (Table 3) showed that several (i.e., Ile38, Arg84, Leu106, and Tyr130) are ≤99.9% conserved among influenza A, B, and C viruses. As mentioned above, the T20A change is a natural variation in influenza A; in influenza B virus, the common residue is Thr20. The other residues included in our mutational analysis (i.e., Tyr24, Ala37, Leu42, Trp88, Phe105, and Val122) are more prone to intra- or inter(sub)type variation (Table 3). We also examined whether there are species-dependent differences at these sites. Although the analysis included a lower number of PA sequences from avian or swine influenza viruses compared to the number of human virus isolates analyzed, it appears that these residues in PA show hardly any variation among avian influenza viruses. On the other hand, swine viruses display some variation at relevant sites in PA (i.e., A20T, Y24H, L42M, and F105Y). This may be relevant for the development of endonuclease inhibitors, considering that a newly emerging influenza virus can carry a PA protein from avian or swine origin, as exemplified by the 2009 pandemic H1N1 virus, which contains a PA segment derived from an avian virus (1). Our vRNP activity data also indicate that PA mutations at these positions (or at least the changes introduced in our study) do not markedly affect viral polymerase activity. Thus, an endonuclease inhibitor optimized for tight binding to any of these residues might select for escape mutants at the corresponding site. It is impossible to speculate on how this would affect the antiviral efficacy of such an inhibitor. Nevertheless, our biological data demonstrate that structure-based design of PA inhibitors should be accompanied by cell culture evaluation against specific PA mutants to verify the proposed mode of action and anticipate potential resistance sites that might be encountered during future clinical use. Ideally, such mutant viruses should be obtained by repeated cell culture passage of influenza virus with increasing concentrations of the inhibitor. For compounds with a rather narrow window between antiviral activity and cytotoxicity (such as L-742,001), application of high compound concentrations may be impossible; in these cases, intentional and single PA mutations can be introduced, as was done here. Another caveat pointed out by our data is that compounds proposed as PA inhibitors on the basis of enzymatic assays, such as EGCG (25, 31), may have an unrelated antiviral mode of action in cell culture, resulting in erroneous interpretations when investigating their structure-activity relationship (against PA) in virus-infected cells. This can be avoided by performing a one-cycle virus replication assay with compound addition after the virus entry stage (similar to the assay used in Fig. 5) or by switching to the vRNP reconstitution assay. In our experience, the last method is particularly relevant for investigating PA endonuclease inhibitors, provided that the agents have sufficient cell permeability and low cytotoxicity. In this way, the mutational approach and new insights presented here for L-742,001 should serve as a guide for further development of this new class of influenza virus inhibitors.
ACKNOWLEDGMENTS
A.S. is holder of a Ph.D. grant from the Agency for Innovation by Science and Technology (IWT). This study was supported by a grant from the Geconcerteerde Onderzoeksacties (GOA/10/014) from the KU Leuven. M.S. acknowledges the Fondazione Banco di Sardegna for financial support.
We thank Wim van Dam and Stijn Stevens for their dedicated technical assistance and Meehyein Kim for the generous donation of the firefly reporter and reverse genetics plasmids.
Footnotes
Published ahead of print 3 July 2013
REFERENCES
- 1.Smith GJ, Vijaykrishna D, Bahl J, Lycett SJ, Worobey M, Pybus OG, Ma SK, Cheung CL, Raghwani J, Bhatt S, Peiris JS, Guan Y, Rambaut A. 2009. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature 459:1122–1125 [DOI] [PubMed] [Google Scholar]
- 2.Simonsen L, Taylor RJ, Viboud C, Miller MA, Jackson LA. 2007. Mortality benefits of influenza vaccination in elderly people: an ongoing controversy. Lancet Infect. Dis. 7:658–666 [DOI] [PubMed] [Google Scholar]
- 3.Deyde VM, Xu X, Bright RA, Shaw M, Smith CB, Zhang Y, Shu Y, Gubareva LV, Cox NJ, Klimov AI. 2007. Surveillance of resistance to adamantanes among influenza A(H3N2) and A(H1N1) viruses isolated worldwide. J. Infect. Dis. 196:249–257 [DOI] [PubMed] [Google Scholar]
- 4.Moscona A. 2009. Global transmission of oseltamivir-resistant influenza. N. Engl. J. Med. 360:953–956 [DOI] [PubMed] [Google Scholar]
- 5.Hayden F. 2009. Developing new antiviral agents for influenza treatment: what does the future hold? Clin. Infect. Dis. 48(Suppl 1):S3–S13 [DOI] [PubMed] [Google Scholar]
- 6.Das K, Aramini JM, Ma LC, Krug RM, Arnold E. 2010. Structures of influenza A proteins and insights into antiviral drug targets. Nat. Struct. Mol. Biol. 17:530–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boivin S, Cusack S, Ruigrok RW, Hart DJ. 2010. Influenza A virus polymerase: structural insights into replication and host adaptation mechanisms. J. Biol. Chem. 285:28411–28417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Resa-Infante P, Jorba N, Coloma R, Ortín J. 2011. The influenza virus RNA synthesis machine: advances in its structure and function. RNA Biol. 8:207–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moeller A, Kirchdoerfer RN, Potter CS, Carragher B, Wilson IA. 2012. Organization of the influenza virus replication machinery. Science 338:1631–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arranz R, Coloma R, Chichón FJ, Conesa JJ, Carrascosa JL, Valpuesta JM, Ortín J, Martín-Benito J. 2012. The structure of native influenza virion ribonucleoproteins. Science 338:1634–1637 [DOI] [PubMed] [Google Scholar]
- 11.Bouloy M, Plotch SJ, Krug RM. 1978. Globin mRNAs are primers for the transcription of influenza viral RNA in vitro. Proc. Natl. Acad. Sci. U. S. A. 75:4886–4890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plotch SJ, Bouloy M, Ulmanen I, Krug RM. 1981. A unique cap(m7GpppXm)-dependent influenza virion endonuclease cleaves capped RNAs to generate the primers that initiate viral RNA transcription. Cell 23:847–858 [DOI] [PubMed] [Google Scholar]
- 13.Guilligay D, Tarendeau F, Resa-Infante P, Coloma R, Crépin T, Sehr P, Lewis J, Ruigrok RW, Ortín J, Hart DJ, Cusack S. 2008. The structural basis for cap binding by influenza virus polymerase subunit PB2. Nat. Struct. Mol. Biol. 15:500–506 [DOI] [PubMed] [Google Scholar]
- 14.Dias A, Bouvier D, Crépin T, McCarthy AA, Hart DJ, Baudin F, Cusack S, Ruigrok RW. 2009. The cap-snatching endonuclease of influenza virus polymerase resides in the PA subunit. Nature 458:914–918 [DOI] [PubMed] [Google Scholar]
- 15.Yuan P, Bartlam M, Lou Z, Chen S, Zhou J, He X, Lv Z, Ge R, Li X, Deng T, Fodor E, Rao Z, Liu Y. 2009. Crystal structure of an avian influenza polymerase PA(N) reveals an endonuclease active site. Nature 458:909–913 [DOI] [PubMed] [Google Scholar]
- 16.Steczkiewicz K, Muszewska A, Knizewski L, Rychlewski L, Ginalski K. 2012. Sequence, structure and functional diversity of PD-(D/E)XK phosphodiesterase superfamily. Nucleic Acids Res. 40:7016–7045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galej WP, Oubridge C, Newman AJ, Nagai K. 2013. Crystal structure of Prp8 reveals active site cavity of the spliceosome. Nature 493:638–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reguera J, Weber F, Cusack S. 2010. Bunyaviridae RNA polymerases (L-protein) have an N-terminal, influenza-like endonuclease domain, essential for viral cap-dependent transcription. PLoS Pathog. 9:e1001101. 10.1371/journal.ppat.1001101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomassini JE, Selnick H, Davies ME, Armstrong ME, Baldwin J, Bourgeois M, Hastings JC, Hazuda D, Lewis J, McClements W. 1994. Inhibition of cap (m7GpppXm)-dependent endonuclease of influenza virus by 4-substituted 2,4-dioxobutanoic acid compounds. Antimicrob. Agents Chemother. 38:2827–2837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hastings JC, Selnick H, Wolanski B, Tomassini JE. 1996. Anti-influenza virus activities of 4-substituted 2,4-dioxobutanoic acid inhibitors. Antimicrob. Agents Chemother. 40:1304–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomassini JE, Davies ME, Hastings JC, Lingham R, Mojena M, Raghoobar SL, Singh SB, Tkacz JS, Goetz MA. 1996. A novel antiviral agent which inhibits the endonuclease of influenza viruses. Antimicrob. Agents Chemother. 40:1189–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh SB, Tomassini JE. 2001. Synthesis of natural flutimide and analogous fully substituted pyrazine-2,6-diones, endonuclease inhibitors of influenza virus. J. Org. Chem. 66:5504–5516 [DOI] [PubMed] [Google Scholar]
- 23.Cianci C, Chung TDY, Meanwell N, Putz H, Hagen M, Colonno RJ, Krystal M. 1996. Identification of N-hydroxamic acid and N-hydroxy-imide compounds that inhibit the influenza virus polymerase. Antivir. Chem. Chemother. 7:353–360 [Google Scholar]
- 24.Parkes KE, Ermert P, Fässler J, Ives J, Martin JA, Merrett JH, Obrecht D, Williams G, Klumpp K. 2003. Use of a pharmacophore model to discover a new class of influenza endonuclease inhibitors. J. Med. Chem. 46:1153–1164 [DOI] [PubMed] [Google Scholar]
- 25.Kuzuhara T, Iwai Y, Takahashi H, Hatakeyama D, Echigo N. 2009. Green tea catechins inhibit the endonuclease activity of influenza A virus RNA polymerase. PLoS Curr. 1:RRN1052. 10.1371/currents.RRN1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iwai Y, Takahashi H, Hatakeyama D, Motoshima K, Ishikawa M, Sugita K, Hashimoto Y, Harada Y, Itamura S, Odagiri T, Tashiro M, Sei Y, Yamaguchi K, Kuzuhara T. 2010. Anti-influenza activity of phenethylphenylphthalimide analogs derived from thalidomide. Bioorg. Med. Chem. 18:5379–5390 [DOI] [PubMed] [Google Scholar]
- 27.Iwai Y, Murakami K, Gomi Y, Hashimoto T, Asakawa Y, Okuno Y, Ishikawa T, Hatakeyama D, Echigo N, Kuzuhara T. 2011. Anti-influenza activity of marchantins, macrocyclic bisbibenzyls contained in liverworts. PLoS One 5:e19825. 10.1371/journal.pone.0019825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baughman BM, Jake SP, DuBois RM, Boyd VA, White SW, Webb TR. 2012. Identification of influenza endonuclease inhibitors using a novel fluorescence polarization assay. ACS Chem. Biol. 7:526–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sagong HY, Parhi A, Bauman JD, Patel D, Vijayan RSK, Das K, Arnold E, LaVoie EJ. 2013. 3-Hydroxyquinolin-2(1H)-ones as inhibitors of influenza A endonuclease. ACS Med. Chem. Lett. 4:547–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DuBois RM, Slavish PJ, Baughman BM, Yun MK, Bao J, Webby RJ, Webb TR, White SW. 2012. Structural and biochemical basis for development of influenza virus inhibitors targeting the PA endonuclease. PLoS Pathog. 8:e1002830. 10.1371/journal.ppat.1002830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kowalinski E, Zubieta C, Wolkerstorfer A, Szolar OH, Ruigrok RW, Cusack S. 2012. Structural analysis of specific metal chelating inhibitor binding to the endonuclease domain of influenza pH1N1 (2009) polymerase. PLoS Pathog. 8:e1002831. 10.1371/journal.ppat.1002831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakazawa M, Kadowaki SE, Watanabe I, Kadowaki Y, Takei M, Fukuda H. 2008. PA subunit of RNA polymerase as a promising target for anti-influenza virus agents. Antiviral Res. 78:194–201 [DOI] [PubMed] [Google Scholar]
- 33.Vanderlinden E, Vanstreels E, Boons E, ter Veer W, Huckriede A, Daelemans D, Van Lommel A, Röth E, Sztaricskai F, Herczegh P, Naesens L. 2012. Intracytoplasmic trapping of influenza virus by a lipophilic derivative of aglycoristocetin. J. Virol. 86:9416–9431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoffmann E, Webster RG. 2000. Unidirectional RNA polymerase I-polymerase II transcription system for the generation of influenza A virus from eight plasmids. J. Gen. Virol. 81:2843–2847 [DOI] [PubMed] [Google Scholar]
- 35.Mohamadi F, Richards NGJ, Guida WC, Liskamp R, Lipton M, Caufield C, Chang G, Hendrickson T, Still WC. 1990. Macromodel–an integrated software system for modeling organic and bioorganic molecules using molecular mechanics. J. Comput. Chem. 11:440–467 [Google Scholar]
- 36.Chang G, Guida WC, Still WC. 1989. An internal-coordinate Monte Carlo method for searching conformational space. J. Am. Chem. Soc. 111:4379–4386 [Google Scholar]
- 37.Gasteiger J, Marsili M. 1980. Iterative partial equalization of orbital electronegativity–a rapid access to atomic charges. Tetrahedron 36:3219–3228 [Google Scholar]
- 38.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ. 2010. Gaussian 09, revision B. 01; Gaussian, Inc., Wallingford, CT [Google Scholar]
- 39.Dennington R, Keith T, Millam J. 2009. GaussView, version 5 Semichem Inc., Shawnee, Mission, KS [Google Scholar]
- 40.Sechi M, Bacchi A, Carcelli M, Compari C, Duce E, Fisicaro E, Rogolino D, Gates P, Derudas M, Al-Mawsawi LQ, Neamati N. 2006. From ligand to complexes: inhibition of human immunodeficiency virus type 1 integrase by beta-diketo acid metal complexes. J. Med. Chem. 49:4248–4260 [DOI] [PubMed] [Google Scholar]
- 41.Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. 2000. The Protein Data Bank. Nucleic Acids Res. 28:235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sechi M, Angotzi G, Dallocchio R, Dessì A, Carta F, Sannia L, Mariani A, Fiori S, Sanchez T, Movsessian L, Plasencia C, Neamati N. 2004. Design and synthesis of novel dihydroxyindole-2-carboxylic acids as HIV-1 integrase inhibitors. Antivir. Chem. Chemother. 15:67–81 [DOI] [PubMed] [Google Scholar]
- 43.Sechi M, Azzena U, Delussu MP, Dallocchio R, Dessì A, Cosseddu A, Pala N, Neamati N. 2008. Design and synthesis of bis-amide and hydrazide-containing derivatives of malonic acid as potential HIV-1 integrase inhibitors. Molecules 10:2442–2461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sechi M, Carta F, Sannia L, Dallocchio R, Dessì A, Al-Safi RI, Neamati N. 2009. Design, synthesis, molecular modeling, and anti-HIV-1 integrase activity of a series of photoactivatable diketo acid-containing inhibitors as affinity probes. Antiviral Res. 81:267–276 [DOI] [PubMed] [Google Scholar]
- 45.Sechi M, Sannia L, Carta F, Palomba M, Dallocchio R, Dessì A, Derudas M, Zawahir Z, Neamati N. 2005. Design of novel bioisosteres of beta-diketo acid inhibitors of HIV-1 integrase. Antivir. Chem. Chemother. 16:41–61 [DOI] [PubMed] [Google Scholar]
- 46.Sanner MF. 1999. Python: a programming language for software integration and development. J. Mol. Graph. Model. 17:57–61 [PubMed] [Google Scholar]
- 47.Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ. 2009. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J. Comput. Chem. 30:2785–2791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huey R, Morris GM, Olson AJ, Goodsell DS. 2007. A semiempirical free energy force field with charge-based desolvation. J. Comput. Chem. 28:1145–1152 [DOI] [PubMed] [Google Scholar]
- 49.Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ. 1998. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 19:1639–1662 [Google Scholar]
- 50.Case DA, Darden TA, Cheatham TI, Simmerling CL, Wang J, Duke RE, Luo R, Walker RC, Zhang W, Merz KM, Roberts B, Wang B, Hayik S, Roitberg A, Seabra G, Kolossváry I, Wong KF, Paesani F, Vanicek J, Liu J, Wu X, Brozell SR, Steinbrecher T, Gohlke H, Cai Q, Ye X, Wang J, Hsieh M-J, Cui G, Roe DR, Mathews DH, Seetin MG, Sagui C, Babin V, Luchko T, Gusarov S, Kovalenko A, Kollman PA. 2010. Amber 11 University of California, San Francisco, CA [Google Scholar]
- 51.Martínez-Sobrido L, García-Sastre A. 2010. Generation of recombinant influenza virus from plasmid DNA. J. Vis. Exp. 42:2057. 10.3791/2057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meneghesso S, Vanderlinden E, Stevaert A, McGuigan C, Balzarini J, Naesens L. 2012. Synthesis and biological evaluation of pyrimidine nucleoside monophosphate prodrugs targeted against influenza virus. Antiviral Res. 94:35–43 [DOI] [PubMed] [Google Scholar]
- 53.Reed LJ, Muench H. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 27:493–497 [Google Scholar]
- 54.Vanderlinden E, Göktas F, Cesur Z, Froeyen M, Reed ML, Russell CJ, Cesur N, Naesens L. 2010. Novel inhibitors of influenza virus fusion: structure-activity relationship and interaction with the viral hemagglutinin. J. Virol. 84:4277–4288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Squires RB, Noronha J, Hunt V, García-Sastre A, Macken C, Baumgarth N, Suarez D, Pickett BE, Zhang Y, Larsen CN, Ramsey A, Zhou L, Zaremba S, Kumar S, Deitrich J, Klem E, Scheuermann RH. 2012. Influenza research database: an integrated bioinformatics resource for influenza research and surveillance. Influenza. Other Resp. Viruses 6:404–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ishikawa Y, Fujii S. 2011. Binding mode prediction and inhibitor design of anti-influenza virus diketo acids targeting metalloenzyme RNA polymerase by molecular docking. Bioinformation 6:221–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Crépin T, Dias A, Palencia A, Swale C, Cusack S, Ruigrok RW. 2010. Mutational and metal binding analysis of the endonuclease domain of the influenza virus polymerase PA subunit. J. Virol. 84:9096–9104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sechi M, Carcelli M, Rogolino D, Neamati N. 2011. Role of metals in HIV-1 integrase inhibitor design, p 287–307. In HIV-1 integrase: mechanism and inhibitor design. John Wiley & Sons, Inc, Hoboken, NJ [Google Scholar]
- 59.Bacchi A, Carcelli M, Compari C, Fisicaro E, Pala N, Rispoli G, Rogolino D, Sanchez TW, Sechi M, Sinisi V, Neamati N. 2011. Investigating the role of metal chelation in HIV-1 integrase strand transfer inhibitors. J. Med. Chem. 54:8407–8420 [DOI] [PubMed] [Google Scholar]
- 60.Rogolino D, Carcelli M, Sechi M, Neamati N. 2012. Viral enzymes containing magnesium: metal binding as a successful strategy in drug design. Coord. Chem. Rev. 256:3063–3086 [Google Scholar]
- 61.Cherepanov P, Esté JA, Rando RF, Ojwang JO, Reekmans G, Steinfeld R, David G, De Clercq E, Debyser Z. 1997. Mode of interaction of G-quartets with the integrase of human immunodeficiency virus type 1. Mol. Pharmacol. 52:771–780 [DOI] [PubMed] [Google Scholar]
- 62.Pluymers W, Neamati N, Pannecouque C, Fikkert V, Marchand C, Burke TR, Jr, Pommier Y, Schols D, De Clercq E, Debyser Z, Witvrouw M. 2000. Viral entry as the primary target for the anti-HIV activity of chicoric acid and its tetra-acetyl esters. Mol. Pharmacol. 58:641–648 [DOI] [PubMed] [Google Scholar]
- 63.Esté JA, Cabrera C, Schols D, Cherepanov P, Gutierrez A, Witvrouw M, Pannecouque C, Debyser Z, Rando RF, Clotet B, Desmyter J, De Clercq E. 1998. Human immunodeficiency virus glycoprotein gp120 as the primary target for the antiviral action of AR177 (zintevir). Mol. Pharmacol. 53:340–345 [DOI] [PubMed] [Google Scholar]
- 64.Steinmann J, Buer J, Pietschmann T, Steinmann E. 2013. Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br. J. Pharmacol. 5:1059–1073. 10.1111/bph.12009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Youil R, Su Q, Toner TJ, Szymkowiak C, Kwan WS, Rubin B, Petrukhin L, Kiseleva I, Shaw AR, DiStefano D. 2004. Comparative study of influenza virus replication in Vero and MDCK cell lines. J. Virol. Methods 120:23–31 [DOI] [PubMed] [Google Scholar]
- 66.Leyssen P, Balzarini J, De Clercq E, Neyts J. 2005. The predominant mechanism by which ribavirin exerts its antiviral activity in vitro against flaviviruses and paramyxoviruses is mediated by inhibition of IMP dehydrogenase. J. Virol. 79:1943–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mehle A, Dugan VG, Taubenberger JK, Doudna JA. 2012. Reassortment and mutation of the avian influenza virus polymerase PA subunit overcome species barriers. J. Virol. 86:1750–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hara K, Schmidt FI, Crow M, Brownlee GG. 2006. Amino acid residues in the N-terminal region of the PA subunit of influenza A virus RNA polymerase play a critical role in protein stability, endonuclease activity, cap binding, and virion RNA promoter binding. J. Virol. 80:7789–7798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao C, Lou Z, Guo Y, Ma M, Chen Y, Liang S, Zhang L, Chen S, Li X, Liu Y, Bartlam M, Rao Z. 2009. Nucleoside monophosphate complex structures of the endonuclease domain from the influenza virus polymerase PA subunit reveal the substrate binding site inside the catalytic center. J. Virol. 83:9024–9030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Doan L, Handa B, Roberts NA, Klumpp K. 1999. Metal ion catalysis of RNA cleavage by the influenza virus endonuclease. Biochemistry 38:5612–5619 [DOI] [PubMed] [Google Scholar]
- 71.Noble E, Cox A, Deval J, Kim B. 2012. Endonuclease substrate selectivity characterized with full-length PA of influenza A virus polymerase. Virology 433:27–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marchand C, Johnson AA, Karki RG, Pais GC, Zhang X, Cowansage K, Patel TA, Nicklaus MC, Burke TR, Jr, Pommier Y. 2003. Metal-dependent inhibition of HIV-1 integrase by beta-diketo acids and resistance of the soluble double-mutant (F185K/C280S). Mol. Pharmacol. 64:600–609 [DOI] [PubMed] [Google Scholar]
- 73.Manz B, Brunotte L, Reuther P, Schwemmle M. 2012. Adaptive mutations in NEP compensate for defective H5N1 RNA replication in cultured human cells. Nat. Commun. 3:802. [DOI] [PubMed] [Google Scholar]
- 74.Holm L, Sander C. 1996. Mapping the protein universe. Science 273:595–603 [DOI] [PubMed] [Google Scholar]