Abstract
The clinical relevance of gene therapy using the recombinant adeno-associated virus (rAAV) vectors often requires widespread distribution of the vector, and in this case, systemic delivery is the optimal route of administration. Humoral blood factors, such as antibodies or complement, are the first barriers met by the vectors administered systemically. We have found that other blood proteins, galectin 3 binding protein (G3BP) and C-reactive protein (CRP), can interact with different AAV serotypes in a species-specific manner. While interactions of rAAV vectors with G3BP, antibodies, or complement lead to a decrease in vector efficacy, systemic transduction of the CRP-deficient mouse and its respective control clearly established that binding to mouse CRP (mCRP) boosts rAAV vector 1 (rAAV-1) and rAAV-6 transduction efficiency in skeletal muscles over 10 times. Notably, the high efficacy of rAAV-6 in CRP-deficient mice can be restored by reconstitution of the CRP-deficient mouse with mCRP. Human CRP (hCRP) does not interact with either rAAV-1 or rAAV-6, and, consequently, the high efficiency of mCRP-mediated muscle transduction by these serotypes in mice cannot be translated to humans. No interaction of mCRP or hCRP was observed with rAAV-8 and rAAV-9. We show, for the first time, that serum components can significantly enhance rAAV-mediated tissue transduction in a serotype- and species-specific manner. Bioprocessing in body fluids should be considered when transfer of a preclinical proof of concept for AAV-based gene therapy to humans is planned.
INTRODUCTION
Adeno-associated virus (AAV) vectors attract great attention as a promising tool for a wide range of applications in gene therapy. The process of cell transduction by recombinant AAVs (rAAVs) has been studied in detail, and cellular receptors responsible for the virus entry have been identified. Most of these studies were accomplished in cell culture (1–3) without taking into account the exposure of rAAVs to components of body fluids in the in vivo situation. Interestingly, in many cases, protein classes having specific posttranslational modifications, such as α-2,3 and α-2,6 sialic acids, N-linked glycoproteins, or heparan sulfate proteoglycan, were identified as primary cell receptors for efficient rAAV transduction (4–6). These posttranslational modifications are common between mammalian species, giving hope to the possibility that rAAV efficiency could be similar across species and that animal data are predictive of the human situation.
Nevertheless, some recent data indicate that interactions of cellular receptors or blood proteins with rAAVs can be species specific. Thus, adeno-associated virus vector 3 (rAAV-3), which efficiently transduces human hepatocytes through the hepatocyte growth factor receptor (HGFR), failed to transduce murine hepatocytes, suggesting that AAV-3 specifically uses human HGFR, but not murine HGFR, as a cellular coreceptor for transduction (7–9). In human and dog blood, but neither mouse nor monkey blood, galectin 3 binding protein (G3BP) interacts with and decreases rAAV-6 efficiency (10). In this study, we also observed that C-reactive protein (CRP) interacts with rAAV-6 in mouse but not human sera. In the present work, we demonstrate that the mouse CRP (mCRP) interacts with rAAV-1 and rAAV-6 but not with rAAV-8 or rAAV-9 and study the role played by this protein in the efficiency and biodistribution of rAAV vectors. CRP, named for its capacity to precipitate the somatic C-polysaccharide of Streptococcus pneumoniae, is an acute-phase protein and is an exquisitely sensitive systemic marker of inflammation and tissue damage in humans. It belongs to the pentraxin family of calcium-dependent ligand-binding plasma proteins, composed of five identical nonglycosylated polypeptide subunits, each containing 206 amino acid residues. The protomers are noncovalently associated in an annular configuration with cyclic pentameric symmetry (11).
We took advantage of a CRP knockout (KO) mouse model (12) to elucidate the role of CRP's interactions with rAAVs under systemic delivery conditions. We demonstrate that the presence of the mCRP in the bloodstream increases the efficiencies of rAAV-1 and rAAV-6 muscle transduction in mice more than 10-fold. Since the interactions of rAAV-1 and rAAV-6 with mCRP are mouse specific, the high transduction efficiencies of these vectors reported in a mouse model (13, 14) could be inadequate when translating this concept to humans. These data provide further evidence that species-specific interactions of rAAV with components of body fluids must be appropriately taken into account when planning regulatory toxicology and translational gene therapy studies.
MATERIALS AND METHODS
rAAV production.
Adenovirus-free vectors (rAAV-1, -6, -8, and -9) were generated by using a three-plasmid transfection of HEK293 cells (15, 16) and purified by standard procedures, including two cycles of cesium chloride centrifugation (17). The number of viral genomes (vg) was estimated by quantitative PCR (qPCR) of extracted vector DNA. The number of vector physical particles (pp) was estimated either by an enzyme-linked immunosorbent assay (ELISA)-based method or by quantification of VP3 protein after SDS-PAGE analysis stained with Coomassie G250 with bovine serum albumin (BSA) as a standard. The contents of full capsids were very similar for the different serotypes used in the study: the ratio of viral genomes to physical particles varied from 1/3 to 1/10.
Animals.
Healthy, 4-week-old C57BL/6 mice with CRP knockout (CRP KO mice) on a C57BL/6 background and C57BL/6 male mice were used in the study. The C57BL/6 CRP KO mice were generated with BRUCE4 embryonic stem (ES) cells derived from C57BL/6 mice (18) and always maintained on C57BL/6 mice. All procedures involving animals were performed according to the guidelines of the Animal Ethical Committee of our institute. All experiments were performed independently at least 2 times, and each experiment comprised 3 to 4 mice for each condition. Results, if not otherwise stated, are given as means ± standard deviations (SD). For systemic delivery, rAAV vectors coding for the murine secreted embryonic alkaline phosphatase (MuSEAP) (19) or firefly luciferase under the cytomegalovirus (CMV) promoter were injected into the lateral tail vein. Two weeks after injection, MuSEAP expression was evaluated by chemoluminescence reporter assay (Tropix, Inc., Bedford, MA) and luciferase expression was evaluated by in vivo imaging or by measuring luciferase protein activity in tissue extracts. To study blood vector clearance, blood samples (50 μl) were collected from the tail vein or retro-orbital plexus at 3, 6, 24, and 48 h postinjection using heparin-coated capillary tubes.
To test the impact of CRP on rAAV-6 efficiency and distribution in C57BL/6 CRP knockout mice, 5 × 10E10 vg of rAAV vectors were incubated either with 200 μl of serum from C57BL/6 mice or with 200 μl of CRP-depleted serum for 1 h at ambient temperature before injections.
Serum depletion of CRP.
Mouse serum was depleted of CRP by incubation with biotinylated anti-mCRP antibodies (R&D Systems) bound to streptavidin-agarose beads (Pierce) for 2 h at room temperature. One hundred micrograms of antibodies was used in a reaction with 1 ml of mouse serum.
In vivo bioluminescence imaging.
Mice were anesthetized with isoflurane, and the d-luciferin substrate (Molecular Imaging Products) was injected intraperitoneally at a dose of 200 μg/g of body weight. Images were taken with an IVIS-100 live image instrument (Xenogen, Hopkinton, MA) over 2 min with 1-by-1 binning and analyzed using Living Image software (Xenogen). The visual output represents the number of photons emitted/s/cm2 as a false-color image, where the maximum is red and the minimum is blue. All animals were imaged on day 14 after rAAV injections.
Ex vivo biodistribution. (i) Luciferase protein activity.
Mice were sacrificed 14 days after rAAV injection. The following organs were dissected: liver, lung, spleen, heart, kidney, and different muscles (quadriceps, hamstrings, gastrocnemius, and tibialis anterior). Luciferase activity was analyzed in 50-mg tissue samples homogenized in 250 μl of lysis buffer (12.5 mM Tris-phosphate [pH 7.8], 7.5% glycerol, 0.05 M EDTA [pH 8], 10 mM dithiothreitol [DTT], 4 mM MgCl2, 1% Triton X-100). Homogenates were centrifuged at 18,000 × g for 10 min at 4°C, and 20 μl of each supernatant was aliquoted in duplicate in an opaque 96-well plate. A Wallac VICTOR2 luminometer (PerkinElmer/Life Sciences, Waltham, MA) was used to measure luminescence over a 10-s interval after simultaneous addition of 2 μM ATP in 100 μl of the lysis buffer without Triton X-100 and 100 μl of 170 mM d-luciferin. The Bradford assay was used to determine the protein concentration. Luciferase levels are shown as relative light units (RLU) normalized to the protein content.
DNA isolation and real-time PCR.
To determine the numbers of AAV genome copies in injected mice, homogenized samples were supplemented with 1% SDS and 400 μg/ml proteinase K and digested overnight. DNA was isolated by the phenol-chloroform-iodoacetamide (IAA) method, and the DNA concentration was estimated with a NanoDrop spectrophotometer (ThermoScientific). One hundred nanograms of DNA was analyzed by qPCR on an ABI 7900HT (Applied Biosystems) according to the manufacturer's instructions. Primer pairs and 6-carboxyfluorescein (6-FAM) 5′-end- and 6-carboxytetramethylrhodamine (TAMRA) 3′-end-labeled probes were designed for the CMV promoter region of the vector and for the endogenous titin gene as follows: CMV F (forward), 5′-CATCAATGGGCGTGGATAGC, CMV R (reverse), 5′-GGAGTTGTTACGACATTTTGGAAA, and CMV probe, 5′-6-FAM-ATTTCCAAGTCTCCC-TAMRA; and titin F, 5′-AAAACGAGCAGTGTGAGC, titin R, 5′-TTCAGTCATGCTGCTAGCGC, and titin probe, 5′-6-FAM-ACGGAAGCGTCTCGTCTCAGTC-TAMRA.
Sera.
Murine sera were obtained from healthy 6-week-old C57BL/6 mice. The sera used in this study were assessed for the presence of antibodies according to reference 20 and were seronegative for the respective AAV serotypes administered.
Coimmunoprecipitation.
Coimmunoprecipitation of serum proteins was performed with rAAV vectors immobilized on NHS-activated Sepharose (GE Healthcare Life Sciences, Piscataway, NJ). Immobilized rAAV vectors (10 μl of Sepharose with 1× 10E11 vector particles) were incubated with 100 μl of serum from C57BL/6 mice for 1 h. Beads were collected by centrifugation and washed four times with 1× phosphate-buffered saline (PBS)–0.5% Triton X-100. The immunoprecipitate was dissolved in the Laemmli sample buffer for further analysis by one-dimensional (1D) electrophoresis.
Western blot analysis of mCRP.
Protein samples were separated by electrophoresis onto a 4 to 12% gradient SDS-polyacrylamide gel and transferred onto a polyvinylidene difluoride (PVDF-Plus) membrane (Millipore). A goat polyclonal antibody to mouse CRP (1:1,000; R&D Systems) followed by donkey anti-goat IRDye-800CW-conjugated antibodies (1:10,000) were used according to the manufacturer's instructions (LI-COR Bioscience). Infrared fluorescence of the secondary antibodies was read on an Odyssey imaging system.
RESULTS
Recently we have shown, using an immunoprecipitation assay and 2D gel analysis, that mouse but not human CRP binds to the rAAV-6 vector (10). In the present study, we further analyze the role of mCRP on rAAV vectors' transduction efficiency and biodistribution under systemic delivery by taking advantage of the CRP-deficient mouse model on an inbred C57BL/6 background (12).
rAAV-6 is more efficient in C57BL/6 than CRP-deficient mice under systemic delivery.
To study the impact of mCRP on transduction efficiency in vivo, rAAV-6 antibody-seronegative CRP-deficient mice and control C57BL/6 mice intravenously received increasing doses of rAAV-6 coding for murine secreted embryonic alkaline phosphatase (MuSEAP) as a reporter protein. Two weeks after injections, mice were sacrificed and the level of MuSEAP in sera was quantified. At all doses tested, rAAV-6 transduction was more efficient in the control mice than in CRP-deficient mice (77 ± 22, 391 ± 25, and 1,374 ± 283 ng/ml for the control mice versus 8 ± 4, 107 ± 14, and 293 ± 65 ng/ml for CRP-deficient mice at doses of 1 × 10E10, 5 × 10E10, and 2.5 × 10E11 vg, respectively) (Fig. 1).
Fig 1.
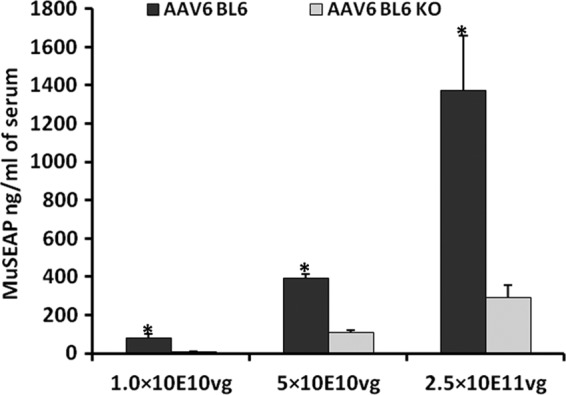
rAAV-6 is more efficient in the control C57BL/6 mice than in CRP-deficient mice. Mice received intravenous injections of increasing doses of rAAV-6 (1 × 10E10, 5 × 10E10, and 2.5 × 10E11 vg of rAAV-6 coding for MuSEAP). Three mice were used for each condition; transduction is expressed as mean MuSEAP serum levels (ng/ml) ± SD 2 weeks after tail vein injection. Levels of significance were determined using Student's t test. *, P < 0.01. BL6, C57BL/6 mice; BL6 KO, CRP-deficient mice.
rAAV-8 and rAAV-9 serotypes do not bind mCRP and transduce C57BL/6 and CRP-deficient mice equally, while rAAV-1 and rAAV-6, which bind mCRP, are more efficient in C57BL/6 mice.
Other serotypes that bind to or do not interact with CRP provide an additional opportunity to confirm specificity of mCRP action on rAAVs. The association of CRP with rAAV-1, rAAV-8, and rAAV-9 was tested by immunoprecipitation via rAAV vectors immobilized on NHS-activated Sepharose, and the resulting mCRP binding was detected by Western blotting. These experiments demonstrated that rAAV-1 and rAAV-6, the capsids of which differ by only 6 amino acids, interacted with mCRP similarly. Neither rAAV-8 nor rAAV-9 was able to bind CRP in mouse serum (Fig. 2).
Fig 2.
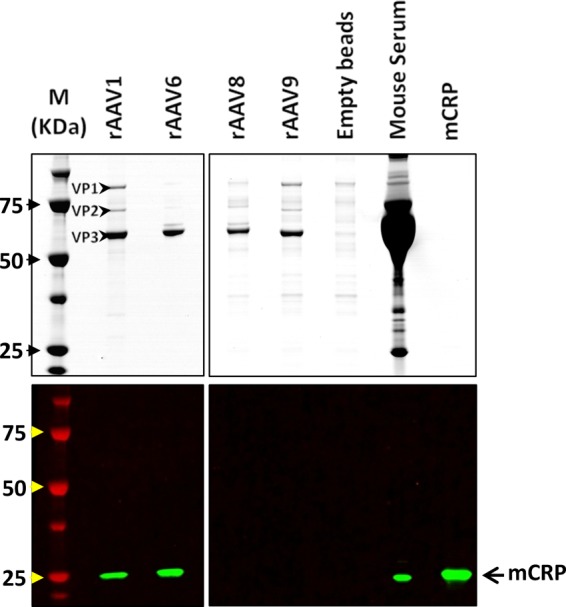
rAAV-1 and rAAV-6, but neither rAAV-8 nor -9, bind CRP in mouse serum. Interactions of mCRP with rAAV-1, -6, -8, and -9 were evaluated by coprecipitation of serum proteins with rAAV vectors immobilized on NHS-activated Sepharose. The resulting mCRP binding was detected by Western blotting. (Top panel) Coomassie staining of VPs of rAAV. Additional bands seen in the lanes loaded with coprecipitated proteins resulted from nonspecific binding of serum proteins to the Sepharose and can also be seen in the control lane with empty beads. (Bottom panel) Western blot analysis of CRP bound to AAVs. Lanes (from left to right): M, molecular mass marker; rAAV-1, -6, -8, and -9, respective immobilized rAAVs after incubation with C57BL/6 mouse serum; Empty beads, serum proteins retained by NHS-activated Sepharose alone; Mouse Serum, 0.7 μl of mouse serum; mCRP, recombinant CRP (10 ng).
We next assessed the transduction efficiency and biodistribution of rAAV-1, rAAV-6, and rAAV-8 serotypes in C57BL/6 and CRP-deficient mice by in vivo imaging. C57BL/6 and CRP-deficient mice received intravenous injections of either rAAV-1, rAAV-6, or rAAV-8 coding for luciferase, and 2 weeks after administration, the number of photons emitted from the ventral image of each mouse was evaluated. Consistent with the results obtained with rAAV-6 coding for MuSEAP, rAAV-6 efficiency in these experiments was considerably higher in C57BL/6 than in CRP-deficient mice (Fig. 3).
Fig 3.
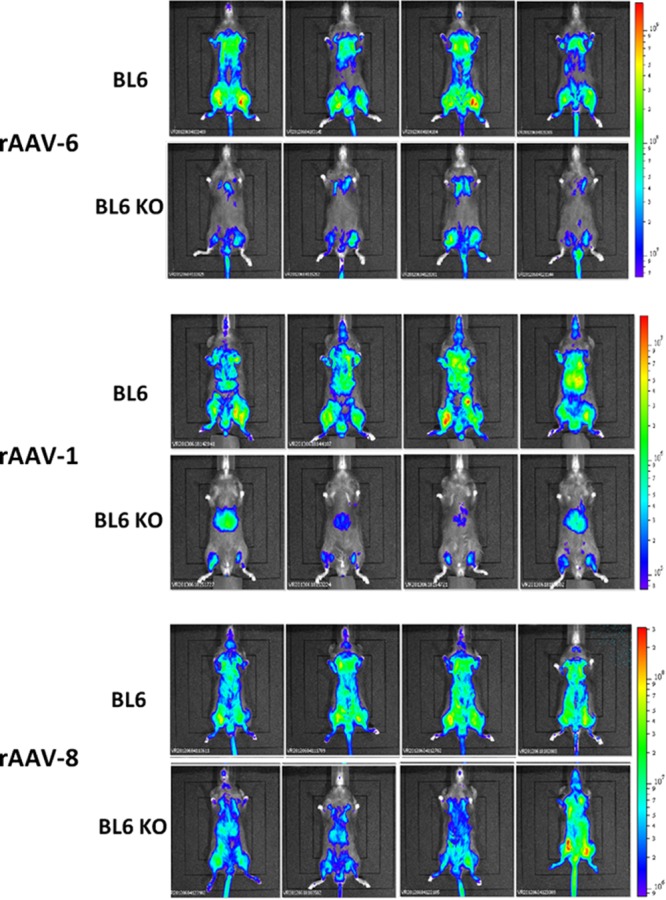
In vivo imaging of luciferase transgene expression in C57BL/6 and CRP KO mice. rAAV-1 and rAAV-6, but not rAAV-8, are more efficient in the control C57BL/6 mice than the CRP KO mice. Mice were injected intravenously with rAAV-1, rAAV-6, or rAAV-8 CMV-Luc vectors (dose of 5 × 10E10 vg). Photographs and bioluminescent images were obtained 2 weeks after injection. The overlay demonstrates decreased transduction efficiency for rAAV-1 and rAAV-6 in CRP-deficient mice compared to C57BL/6 mice, while no difference was seen for rAAV-8. The bioluminescence logarithmic scales range from 6.4 × 10E6 to 1.8 × 10E9 relative light units (RLU: photons/s/cm2) for rAAV-6, from 8.0 × 10E4 to 2.5 × 10E7 RLU for rAAV-1, and from 8.4 × 10E5 to 3.0 × 10E8 RLU for rAAV-8 serotypes. BL6, C57BL/6 mice; BL6 KO, CRP-deficient mice.
Importantly, the efficiency of the other serotype that binds mCRP, rAAV-1, was also higher in C57BL/6 mice (Fig. 3). Besides, in C57BL/6 mice, rAAV-1 and rAAV-6 displayed a systemic and body-wide transduction profile, while in CRP-deficient mice, the overall transduction levels of these vectors were substantially lower and luciferase expression was preferentially seen in the limbs (rAAV-6) or limbs and liver (rAAV-1). As expected, the rAAV-8 serotype, which does not interact with mCRP, had similar efficacies and biodistributions in both C57BL/6 and CRP-deficient mice (Fig. 3). These results further validate the hypothesis that interactions with CRP determine different behaviors of rAAV vectors in C57BL/6 mice compared to CRP-deficient mice.
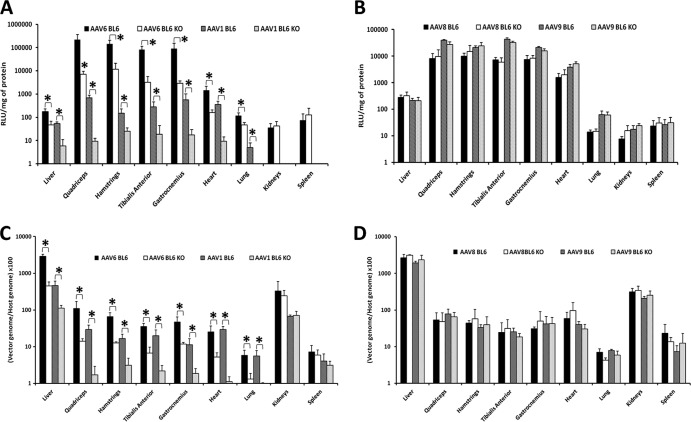
In order to further evaluate the impact and specificity of CRP action on different rAAV serotypes, we quantified luciferase enzyme activity and vector genome copy numbers in various tissues (Fig. 4). For this, C57BL/6 and CRP-deficient mice received intravenously either rAAV-1, -6, -8, or -9 and different organs (spleen, heart, lung, liver, kidneys, and the skeletal muscles gastrocnemius, hamstrings, tibialis anterior, and quadriceps) were taken for analysis 2 weeks after injection. The level of luciferase enzyme activity in examined tissues from C57BL/6 mice and CRP-deficient mice confirmed the pattern of localization observed by in vivo imaging. Transduction with either rAAV-1 or rAAV-6 resulted in higher luciferase enzyme activity in C57BL/6 mice than in CRP-deficient mice, with the most intense disparity in muscles (level of transduction ∼10 to 30 times greater in C57BL/6 mice than the level observed in CRP-deficient mice), heart (1,400 ± 740 versus 160 ± 48 relative light units [RLU]/mg total protein for rAAV-6 and 359 ± 137 versus 9 ± 4 RLU/mg for rAAV-1), liver (178 ± 49 versus 47 ± 18 RLU/mg for rAAV-6 and 55 ± 8 versus 6 ± 5 RLU/mg for rAAV-1), and lung (117 ± 38 versus 46 ± 13 RLU/mg for rAAV-6 and 5 ± 3 versus 1 ± 0.01 RLU/mg for rAAV-1). No statistically significant difference was observed in the transduction efficiencies of kidney and spleen between the C57BL/6 mouse and the CRP-deficient mouse (Fig. 4A). The overall lower transduction efficiency of rAAV-1 than rAAV-6 seen in these experiments has been previously reported (14, 21, 22).
Fig 4.
rAAV-1 and rAAV-6, but not rAAV-8 or rAAV-9, are more efficient in the control C57BL/6 mice than in CRP KO mice. (A and B) Luciferase protein expression profile after intravenous administration of 5 × 10E10 vg of rAAV-6 and rAAV-1 (A) or rAAV-8 and rAAV-9 (B) in C57BL/6 and CRP-deficient mice. The level of luciferase activity (RLU/mg of protein) was determined in different tissues 2 weeks after injection of the corresponding rAAVs. Levels of significance were determined using Student's t test. The data are presented as mean values ± SD; four mice were used for each condition. *, P < 0.05. (C and D) Vector genome copy numbers in selected tissues 2 weeks after intravenous administration of 5 × 10E10 vg of rAAV-6 and rAAV-1 (C) or rAAV-8 and rAAV-9 (D) in C57BL/6 and CRP-deficient mice. Primers to the CMV promoter were used to quantify vectors, and the titin gene was used for normalization. Levels of significance were determined using Student's t test. The data are presented as mean values ± SD; four mice were used for each condition. *, P < 0.01. BL6, C57BL/6 mice; BL6 KO, CRP-deficient mice.
In agreement with previously published data (14), the transduction efficiency of rAAV-9 was higher than that of rAAV-8. Importantly, both rAAV-8 and rAAV-9, serotypes that do not interact with CRP, have shown similar levels of efficiency in all tested organs of C57BL/6 and CRP-deficient mice (Fig. 4B).
The vector genome copy number was analyzed by real-time PCR. Primers to the CMV promoter were used to quantify vector DNA, and the titin gene was used for normalization. Corroborating the results of luciferase enzyme activity measurement, rAAV-1 and rAAV-6 copy numbers were ∼5 to 10 times higher in the muscles of C57BL/6 mice than those in CRP-deficient mice (quadriceps, 1.1 ± 0.6 versus 0.1 ± 0.02 for rAAV-6 and 0.3 ± 0.09 versus 0.02 ± 0.01 for rAAV-1; hamstrings, 0.7 ± 0.2 versus 0.1 ± 0.01 for rAAV-6 and 0.16 ± 0.05 versus 0.03 ± 0.017 for rAAV-1; tibialis anterior, 0.4 ± 0.07 versus 0.06 ± 0.03 for rAAV-6 and 0.2 ± 0.09 versus 0.02 ± 0.009 for rAAV-1; gastrocnemius, 0.5 ± 0.02 versus 0.1 ± 0.01 for rAAV-6 and 0.1 ± 0.05 versus 0.02 ± 0.007 for rAAV-1; heart, 0.3 ± 0.1 versus 0.05 ± 0.2 for rAAV-6 and 0.3 ± 0.01 versus 0.01 ± 0.004 for rAAV-1), as well as in liver (28.8 ± 3.7 versus 4.5 ± 1.3 for rAAV-6 and 5 ± 1 versus 1 ± 0.02 for rAAV-1) and lung (0.06 ± 0.02 versus 0.01 ± 0.006 for rAAV-6 and 0.05 ± 0.02 versus 0.01 ± 0.0003 for rAAV-1). No statistically significant difference in rAAV-6 copy numbers was found for kidney and spleen between the C57BL/6 mouse and the CRP-deficient mouse (Fig. 4A).
Importantly, no difference in vector genome copy number was found for rAAV-8 and rAAV-9 when C57BL/6 and CRP-deficient mice were compared (Fig. 4B).
mCRP restores transduction efficiency of rAAV-6 in the CRP-deficient mouse.
Finally, we sought to determine whether reconstitution of the AAV-6/CRP complex could restore rAAV-6 efficiency in knockout mice. CRP is composed of five identical nonglycosylated polypeptide subunits noncovalently associated in an annular configuration with cyclic pentameric symmetry. This complex structure makes purification of the functional protein difficult. Thus, in order to reconstitute the rAAV-6 interaction with mCRP, the vector was preincubated with unfractionated serum from C57BL/6 mice, or in the control experiments, with CRP-depleted serum and then injected into the tails of CRP knockout mice. To ensure that preincubation with sera per se had no effect on the vector efficiency, rAAV-6 preincubated with either CRP-depleted or unfractionated serum from C57BL/6 mice was injected into C57BL/6 mice.
Analysis of in vivo luciferase expression demonstrated that incubation with either CRP-depleted or unfractionated serum did not change the efficiency of rAAV-6 in C57BL/6 mice (Fig. 5A, rows 1 and 2). Consistent with the results obtained with rAAV-6 formulated in PBS, rAAV-6 preincubated with CRP-depleted serum demonstrated lower efficiency in CRP-deficient mice than in C57BL/6 controls (Fig. 5A, rows 1, 2, and 3). Finally, preincubation with unfractionated serum led to the restoration of rAAV-6 efficiency in CRP knockout mice to the same level observed in C57BL/6 mice (Fig. 5A, row 4), confirming that mCRP is essential for the high efficiency of rAAV-6 in mice.
Fig 5.
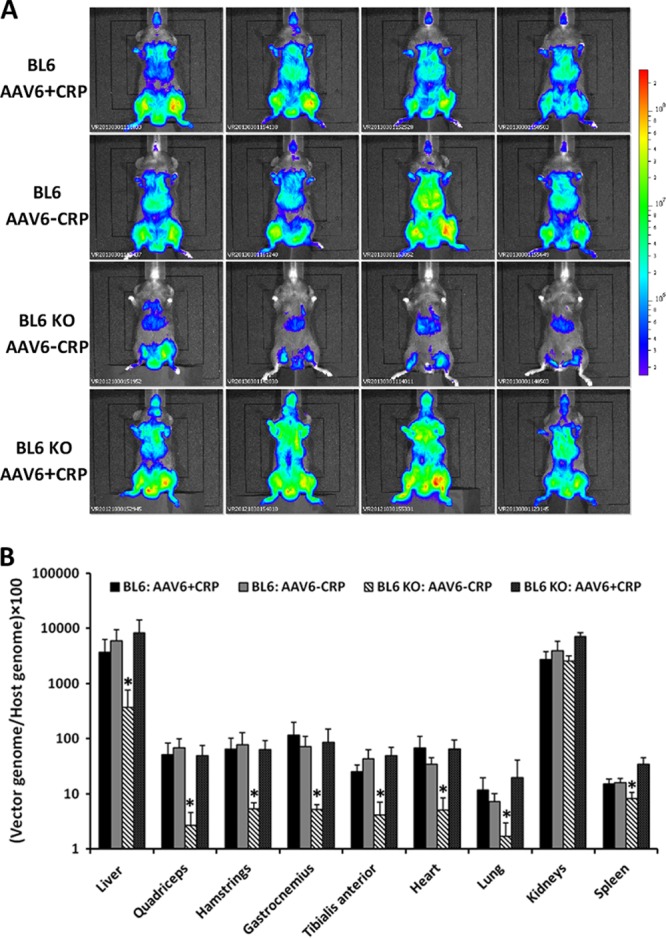
mCRP restores transduction efficiency of rAAV-6 in CRP-deficient mice. (A) C57BL/6 (BL6) and C57BL/6 CRP KO (BL6 KO) mice were injected intravenously with rAAV-6 CMV-Luc vector (dose of 5 × 10E10 vg) preincubated with either CRP-depleted or unfractionated serum from C57BL/6 mice. Photographs and bioluminescent images were obtained 2 weeks after injection. Incubation with either unfractionated or CRP-depleted serum did not change the efficiency of rAAV-6 in C57BL/6 mice (rows 1 and 2, BL6 AAV6 + CRP and BL6 AAV6 − CRP, respectively). rAAV-6 preincubated with CRP-depleted serum had lower efficiency in CRP-deficient mice than in C57BL/6 controls (row 3, BL6 KO AAV6 − CRP; compare with rows 1 and 2). Preincubation with unfractionated serum restored rAAV-6 efficiency in CRP knockout mice to the same level as that observed in C57BL/6 controls (row 4, BL6 KO AAV6 + CRP). The bioluminescence logarithmic scale ranges from 1.6 × 10E5 to 2.8 × 10E8 relative light units (RLU; photons/s/cm2). The bioluminescence scale is indicated in RLU. (B) Vector genome copy numbers in selected tissues. Tissues from the same experiment were used for DNA extraction and analyzed by real-time PCR. Primers to the CMV promoter were used to quantify the vector, and the titin gene was used for normalization. The data are presented as mean values ± SD; four mice were used for each condition. *, P < 0.05.
Restoration of rAAV-6 efficiency in CRP-deficient mice after preincubation with mCRP was further confirmed by the analysis of the viral genome copy numbers in selected tissues. The results showed that preincubation with mCRP leads to the restoration of rAAV-6 efficiency in all tested tissues (Fig. 5B), strengthening a key role of mCRP in efficient transduction of mice with rAAV-6.
Blood vector clearance following intravenous injection is more rapid in CRP-deficient mice.
Less-efficient transduction across multiple tissues in CRP knockout mice could imply that CRP might play a role in prolonging the circulation half-life of rAAV particles (14, 23, 24). In order to compare the rates of rAAV-6 clearance in C57BL6 and CRP knockout mice, we determined the blood vector concentrations at 3, 6, 24, and 48 h postinjection. The concentrations of rAAV particles in the blood were 4.7-, 3.7-, and 2.1-fold higher in C57BL6 mice than those in CRP knockout mice at 3, 6, and 24 h, respectively (8.1 × 10E5, 3.1 × 10E5, and 1.6 × 10E4 versus 1.7 × 10E5, 8.1 × 10E4, and 7.3 × 10E3 vg/μl), while no difference was found at 48 h postinjection (Fig. 6).
Fig 6.
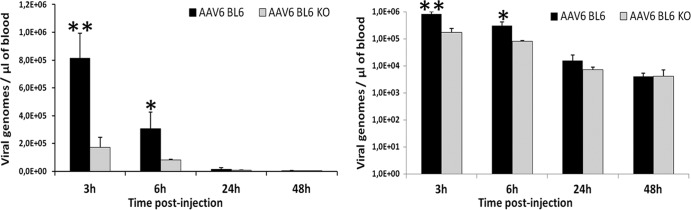
Blood vector clearance following intravenous injection is more rapid in CRP-deficient mice. C57BL/6 (BL6) and C57BL/6 CRP knockout (BL6 KO) mice were injected through the tail vein (n = 3) with rAAV-6 CMV-Luc vector (dose of 5 × 10E10 vg). Whole-blood DNA was extracted 3, 6, 24, and 48 h postinjection and analyzed by qPCR with primers against the CMV promoter region of the vector. The concentrations of rAAV particles in the blood are shown as a function of time after injection on a linear scale (left) or a log scale (right). Known titers of virus were used as a positive control and noninjected mice as a negative control. The data are presented as mean values ± SD. **, P < 0.01; *, P < 0.05.
DISCUSSION
Several naturally occurring AAV serotypes and variants have been used as gene therapy vectors. These vectors are different in tissue tropism and in their capacity to evade preexisting neutralizing antibodies. It is presumed that the specificity and efficiency of transduction and the biodistribution of the AAV particles are dependent on the vector capsid. In most of the studies, rAAV vectors were assembled through transencapsidation of distinct capsid particles, with the transgene cassette flanked by AAV2 inverted terminal repeats. Examination in vivo of nine serotypes of AAV (pseudotypes 1 to 9) packaging the same transgene and produced and purified in the same manner demonstrated profound differences in their transduction of mouse organs after systemic delivery (14). Importantly, while thorough studies identified primary receptors and coreceptors for rAAV1, -2, -3, -4, -5, -6, -8, and -9 in cell culture (25–30), their relative contributions to in vivo vector efficiency are less clear.
Based on mouse studies, rAAV-6 was recognized as one of the serotypes suitable for efficient whole-body gene transduction (14), and it was successfully applied to restore body-wide expression of a dystrophin-based protein for the treatment of mdx mice (31, 32). Nevertheless, some recent data demonstrated that the process of rAAV transduction can be species specific at both cellular (7–9) and systemic (10) levels, complicating translation of animal studies to humans. This species specificity can be of crucial importance when either preclinical toxicology studies are conducted to characterize specific risks associated with one vector or when widespread distribution of a transgene is needed and gene therapy has to be applied at a systemic level. Animal studies argue that systemic treatment requires very high vector doses to be efficient. The risk is that doses calculated in such a way could be inappropriate if the vector's efficiency is different in humans from those in the animal models.
Humoral blood factors such as antibodies (20, 33, 34) or complement (35) are the first barriers met by the vectors administered systemically. Recently, we have found that blood proteins G3BP and CRP interact with different AAV serotypes in a species-specific manner. In this study, we wanted to elucidate the role of mCRP interactions with rAAVs for the vector efficiency and biodistribution under systemic delivery. To achieve this goal, we compared the levels of efficiency and biodistributions of two AAV serotypes interacting with mCRP (rAAV-1 and rAAV-6) and the two others which do not interact with mCRP (rAAV-8 and rAAV-9) in CRP-deficient mice (12) with those in the C57BL/6 control mice. Our results demonstrate, for the first time, that a serum protein can have an enhancing effect on rAAV efficiency. While interactions of rAAVs with previously described serum factors like antibodies, complement, or G3BP, led to a decrease in vector efficacy, binding to mCRP substantially increased the performance of rAAV-1 and rAAV-6 in mice, boosting its transduction efficiency in skeletal muscles, heart, lung, and liver up to 10 times. Despite the high degree of sequence homology between serotype 1 and 6 capsids, these vectors have different liver transduction profiles and rAAV-1 has lower overall transduction efficiency (14, 21). This difference is most probably due to the lysine-to-glutamate change (K531E), which suppresses the heparin binding ability of AAV6 (22). Interestingly, while this change was important for human G3BP (huG3BP) interaction with the respective serotypes (10), no difference was found in the binding of rAAV-1 or rAAV-6 to mCRP, thus indicating that mCRP and huG3BP interact with different epitopes on the rAAV surface.
Serotypes AAV-8 and AAV-9 that do not react with mCRP have shown similar efficiencies in both C57BL/6 and CRP-deficient mice, thus confirming the specific mechanism of mCRP action on rAAV-6 and rAAV-1 efficacies in mice. Interestingly, despite the presence of relatively high vector genome copies in the kidney, the expression of the transgene was low in this organ for all tested serotypes. This phenomenon has been previously reported (14) and could be linked to a low efficiency of the CMV promoter in this organ. Importantly, the transduction efficiencies of rAAV-8 and rAAV-9 were comparable to the efficiency of rAAV-6 in C57BL/6 mice (Fig. 4B), indicating that these serotypes utilize different mechanisms to achieve efficient systemic transduction in mice.
CRP was discovered in 1930 as a protein that could precipitate the “C” polysaccharide from the pneumococcal cell wall (36) by recognizing its specific ligand, phosphocholine (37). This protein was mostly studied in humans where, during the acute phase response, its plasma concentration increases 1,000 times. CRP belongs to the pentraxin family of calcium-dependent ligand-binding plasma proteins, the other member of which in humans is the serum amyloid P (SAP) component (38). The human CRP molecule is composed of five identical nonglycosylated polypeptide subunits (molecular weight [MW], 25,125), each containing 224 amino acid residues. These protomers are noncovalently associated in an annular configuration with cyclic pentameric symmetry (39). This pentameric molecule has two faces: a recognition face, which binds with the highest affinity to phosphocholine residues and also to a variety of ligands on apoptotic cells, cell debris, and several microorganisms, and a reverse effector face, which interacts with host receptors, such as Fcγ receptors (40) or complement C1q (41).
Mouse CRP of 225 amino acids has 64% homology with the human protein. Even if substantial functional differences exist between human and animal proteins (42), the evolutionary conservation of sequence presumes similar subunit organizations and protein folds (43). Nevertheless, canine or macaque CRP, like a human CRP, did not interact with rAAV-6 in the coimmunoprecipitation assay (data not shown). Interestingly, mCRP is not an acute-phase protein, and this role is played in mice by another member of the pentraxin family, the serum amyloid P (SAP) component. The concentration of mCRP in sera was estimated at a level below 2 μg/ml (11, 44). Our evaluation of serum mCRP by Western blotting using recombinant mouse protein as a control gave mCRP concentrations between 2 to 5 μg/ml or 1 × 10E14 to 3 × 10E14 molecules/ml (data not shown). This discrepancy could be due to the strain-to-strain variations in mCRP levels (45). If we consider that one vector particle interacts with 10 mCRP molecules (10), then one 25-g mouse with 1.8 ml of blood could provide enough CRP to react with 10E14 rAAV-6 pp.
What could be a mechanism for rAAV efficiency enhancement by mCRP? Considering the two faces of the pentameric CRP molecule (39), we hypothesize that mCRP interacts with rAAV through its recognition face and interaction of the opposite, effector face with some components of vascular endothelium facilitate transfer of the complexes to the targeted cells. Human CRP binds with highest affinity to phosphocholine residues (43), and we performed competition experiments with phosphocholine in order to check if rAAV-6 interacts with mCRP through this site. Presence of up to 160 μg/ml (∼500 μM) phosphocholine in the immunoprecipitation experiments did not interfere with mCRP binding, thus indicating rAAV-6 does not interact with mCRP through its phosphocholine binding site. Variability of the CRP ligands suggests that other mCRP sites can be involved in rAAV binding.
The opposite, effector face of the pentamer binds human and mouse Fcγ receptors (40, 46–49) and complement C1q (41, 50, 51). In our coimmunoprecipitation experiments, we did not notice specific complement binding to rAAV-6. Possible interactions of CRP with vessel endothelial cells (52, 53) through Fcγ receptors leave open the possibility that these interactions could be a part of the mechanism boosting rAAV transduction in the mouse.
Recently, several groups demonstrated that the efficiency of heart and systemic transduction by rAAVs correlated with the delayed blood clearance of the vector (14, 23, 24). To explain this phenomenon, the authors proposed that the delayed blood clearance that sustains the slow process of transvascular transport of the vector (24) could be due to masking not-yet-defined blood clearance-accelerating motifs present on the surface of rAAVs (24). A similar mechanism—masking of blood clearance-accelerating motifs by CRP—could be proposed to explain the enhancing effect of CRP on rAAV-1 and rAAV-6 transduction.
Importantly, human CRP does not interact with rAAV-1 or rAAV-6, and, consequently, the higher efficiency of muscle transduction by rAAV-6 compared to several other tested serotypes in mice (13, 14) cannot be translated to humans. The complexity of these host-vector interactions slows translation of rAAV technology to the clinic. Without having the possibility to conduct direct comparative studies of animal models and humans, useful information for the prediction of AAV vector efficiency in humans upon systemic delivery can be obtained by comparing molecular mechanisms of AAV vector bioprocessing in the respective body fluids, like blood or cerebrospinal fluid.
ACKNOWLEDGMENTS
We thank Philippe Moullier for critical reading of the manuscript, Valérie Rouffiac (Plate-Forme Imagerie Cellulaire of the Institute Gustave Roussy), Adeline Miranda, Sébastien Plault (Genethon In Vivo Evaluation Core), and Jeremy Rouillon for technical support, and Anatolii Kokoza for proofreading the manuscript.
This work was supported by the Association Française contre les Myopathies (AFM).
Footnotes
Published ahead of print 31 July 2013
REFERENCES
- 1.Bartlett JS, Wilcher R, Samulski RJ. 2000. Infectious entry pathway of adeno-associated virus and adeno-associated virus vectors. J. Virol. 74:2777–2785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schuhmann NK, Pozzoli O, Sallach J, Huber A, Avitabile D, Perabo L, Rappl G, Capogrossi MC, Hallek M, Pesce M, Buning H. 2010. Gene transfer into human cord blood-derived CD34(+) cells by adeno-associated viral vectors. Exp. Hematol. 38:707–717 [DOI] [PubMed] [Google Scholar]
- 3.Seiler MP, Miller AD, Zabner J, Halbert CL. 2006. Adeno-associated virus types 5 and 6 use distinct receptors for cell entry. Hum. Gene Ther. 17:10–19 [DOI] [PubMed] [Google Scholar]
- 4.O'Donnell J, Taylor KA, Chapman MS. 2009. Adeno-associated virus-2 and its primary cellular receptor—cryo-EM structure of a heparin complex. Virology 385:434–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Summerford C, Samulski RJ. 1998. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J. Virol. 72:1438–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Z, Miller E, Agbandje-McKenna M, Samulski RJ. 2006. Alpha2,3 and alpha2,6 N-linked sialic acids facilitate efficient binding and transduction by adeno-associated virus types 1 and 6. J. Virol. 80:9093–9103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng B, Ling C, Dai Y, Lu Y, Glushakova LG, Gee SW, McGoogan KE, Aslanidi GV, Park M, Stacpoole PW, Siemann D, Liu C, Srivastava A, Ling C. 2012. Development of optimized AAV3 serotype vectors: mechanism of high-efficiency transduction of human liver cancer cells. Gene Ther. 19:375–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ling C, Lu Y, Cheng B, McGoogan KE, Gee SW, Ma W, Li B, Aslanidi GV, Srivastava A. 2011. High-efficiency transduction of liver cancer cells by recombinant adeno-associated virus serotype 3 vectors. J. Vis. Exp. 49:e2538. 10.3791/2538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ling C, Lu Y, Kalsi JK, Jayandharan GR, Li B, Ma W, Cheng B, Gee SW, McGoogan KE, Govindasamy L, Zhong L, Agbandje-McKenna M, Srivastava A. 2010. Human hepatocyte growth factor receptor is a cellular coreceptor for adeno-associated virus serotype 3. Hum. Gene Ther. 21:1741–1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denard J, Beley C, Kotin R, Lai-Kuen R, Blot S, Leh H, Asokan A, Samulski RJ, Moullier P, Voit T, Garcia L, Svinartchouk F. 2012. Human galectin 3 binding protein interacts with recombinant adeno-associated virus type 6. J. Virol. 86:6620–6631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Black S, Kushner I, Samols D. 2004. C-reactive protein. J. Biol. Chem. 279:48487–48490 [DOI] [PubMed] [Google Scholar]
- 12.Teupser D, Weber O, Rao TN, Sass K, Thiery J, Fehling HJ. 2011. No reduction of atherosclerosis in C-reactive protein (CRP)-deficient mice. J. Biol. Chem. 286:6272–6279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zincarelli C, Soltys S, Rengo G, Koch WJ, Rabinowitz JE. 2010. Comparative cardiac gene delivery of adeno-associated virus serotypes 1–9 reveals that AAV6 mediates the most efficient transduction in mouse heart. Clin. Transl. Sci. 3:81–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zincarelli C, Soltys S, Rengo G, Rabinowitz JE. 2008. Analysis of AAV serotypes 1–9 mediated gene expression and tropism in mice after systemic injection. Mol. Ther. 16:1073–1080 [DOI] [PubMed] [Google Scholar]
- 15.Drittanti L, Jenny C, Poulard K, Samba A, Manceau P, Soria N, Vincent N, Danos O, Vega M. 2001. Optimised helper virus-free production of high-quality adeno-associated virus vectors. J. Gene Med. 3:59–71 [DOI] [PubMed] [Google Scholar]
- 16.Wright JF. 2009. Transient transfection methods for clinical adeno-associated viral vector production. Hum. Gene Ther. 20:698–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hermens WT, ter Brake O, Dijkhuizen PA, Sonnemans MA, Grimm D, Kleinschmidt JA, Verhaagen J. 1999. Purification of recombinant adeno-associated virus by iodixanol gradient ultracentrifugation allows rapid and reproducible preparation of vector stocks for gene transfer in the nervous system. Hum. Gene Ther. 10:1885–1891 [DOI] [PubMed] [Google Scholar]
- 18.Kontgen F, Suss G, Stewart C, Steinmetz M, Bluethmann H. 1993. Targeted disruption of the MHC class II Aa gene in C57BL/6 mice. Int. Immunol. 5:957–964 [DOI] [PubMed] [Google Scholar]
- 19.Wang M, Orsini C, Casanova D, Millan JL, Mahfoudi A, Thuillier V. 2001. MUSEAP, a novel reporter gene for the study of long-term gene expression in immunocompetent mice. Gene 279:99–108 [DOI] [PubMed] [Google Scholar]
- 20.Boutin S, Monteilhet V, Veron P, Leborgne C, Benveniste O, Montus MF, Masurier C. 2010. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum. Gene Ther. 21:704–712 [DOI] [PubMed] [Google Scholar]
- 21.Arnett AL, Beutler LR, Quintana A, Allen J, Finn E, Palmiter RD, Chamberlain JS. 2013. Heparin-binding correlates with increased efficiency of AAV1- and AAV6-mediated transduction of striated muscle, but negatively impacts CNS transduction. Gene Ther. 20:497–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Z, Asokan A, Grieger JC, Govindasamy L, Agbandje-McKenna M, Samulski RJ. 2006. Single amino acid changes can influence titer, heparin binding, and tissue tropism in different adeno-associated virus serotypes. J. Virol. 80:11393–11397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asokan A, Conway JC, Phillips JL, Li C, Hegge J, Sinnott R, Yadav S, DiPrimio N, Nam HJ, Agbandje-McKenna M, McPhee S, Wolff J, Samulski RJ. 2010. Reengineering a receptor footprint of adeno-associated virus enables selective and systemic gene transfer to muscle. Nat. Biotechnol. 28:79–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kotchey NM, Adachi K, Zahid M, Inagaki K, Charan R, Parker RS, Nakai H. 2011. A potential role of distinctively delayed blood clearance of recombinant adeno-associated virus serotype 9 in robust cardiac transduction. Mol. Ther. 19:1079–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akache B, Grimm D, Pandey K, Yant SR, Xu H, Kay MA. 2006. The 37/67-kilodalton laminin receptor is a receptor for adeno-associated virus serotypes 8, 2, 3, and 9. J. Virol. 80:9831–9836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hauck B, Xiao W. 2003. Characterization of tissue tropism determinants of adeno-associated virus type 1. J. Virol. 77:2768–2774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaludov N, Brown KE, Walters RW, Zabner J, Chiorini JA. 2001. Adeno-associated virus serotype 4 (AAV4) and AAV5 both require sialic acid binding for hemagglutination and efficient transduction but differ in sialic acid linkage specificity. J. Virol. 75:6884–6893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kashiwakura Y, Tamayose K, Iwabuchi K, Hirai Y, Shimada T, Matsumoto K, Nakamura T, Watanabe M, Oshimi K, Daida H. 2005. Hepatocyte growth factor receptor is a coreceptor for adeno-associated virus type 2 infection. J. Virol. 79:609–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen S, Bryant KD, Brown SM, Randell SH, Asokan A. 2011. Terminal N-linked galactose is the primary receptor for adeno-associated virus 9. J. Biol. Chem. 286:13532–13540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen S, Bryant KD, Sun J, Brown SM, Troupes A, Pulicherla N, Asokan A. 2012. Glycan binding avidity determines the systemic fate of adeno-associated virus type 9. J. Virol. 86:10408–10417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gregorevic P, Allen JM, Minami E, Blankinship MJ, Haraguchi M, Meuse L, Finn E, Adams ME, Froehner SC, Murry CE, Chamberlain JS. 2006. rAAV6-microdystrophin preserves muscle function and extends lifespan in severely dystrophic mice. Nat. Med. 12:787–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gregorevic P, Blankinship MJ, Allen JM, Chamberlain JS. 2008. Systemic microdystrophin gene delivery improves skeletal muscle structure and function in old dystrophic mdx mice. Mol. Ther. 16:657–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Veron P, Leborgne C, Monteilhet V, Boutin S, Martin S, Moullier P, Masurier C. 2012. Humoral and cellular capsid-specific immune responses to adeno-associated virus type 1 in randomized healthy donors. J. Immunol. 188:6418–6424 [DOI] [PubMed] [Google Scholar]
- 34.Zaiss AK, Muruve DA. 2008. Immunity to adeno-associated virus vectors in animals and humans: a continued challenge. Gene Ther. 15:808–816 [DOI] [PubMed] [Google Scholar]
- 35.Zaiss AK, Cotter MJ, White LR, Clark SA, Wong NC, Holers VM, Bartlett JS, Muruve DA. 2008. Complement is an essential component of the immune response to adeno-associated virus vectors. J. Virol. 82:2727–2740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Francis T, Tillett WS. 1930. Cutaneous reactions in pneumonia. The development of antibodies following the intradermal injection of type-specific polysaccharide. J. Exp. Med. 52:573–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Volanakis JE, Kaplan MH. 1971. Specificity of C-reactive protein for choline phosphate residues of pneumococcal C-polysaccharide. Proc. Soc. Exp. Biol. Med. 136:612–614 [DOI] [PubMed] [Google Scholar]
- 38.Ohnishi S, Maeda S, Nishiguchi S, Arao T, Shimada K. 1988. Structure of the mouse C-reactive protein gene. Biochem. Biophys. Res. Commun. 156:814–822 [DOI] [PubMed] [Google Scholar]
- 39.Thompson D, Pepys MB, Wood SP. 1999. The physiological structure of human C-reactive protein and its complex with phosphocholine. Structure 7:169–177 [DOI] [PubMed] [Google Scholar]
- 40.Shrive AK, Cheetham GM, Holden D, Myles DA, Turnell WG, Volanakis JE, Pepys MB, Bloomer AC, Greenhough TJ. 1996. Three dimensional structure of human C-reactive protein. Nat. Struct. Biol. 3:346–354 [DOI] [PubMed] [Google Scholar]
- 41.Bang R, Marnell L, Mold C, Stein MP, Clos KT, Chivington-Buck C, Clos TW. 2005. Analysis of binding sites in human C-reactive protein for Fc{gamma}RI, Fc{gamma}RIIA, and C1q by site-directed mutagenesis. J. Biol. Chem. 280:25095–25102 [DOI] [PubMed] [Google Scholar]
- 42.Pepys MB, Hirschfield GM, Tennent GA, Gallimore JR, Kahan MC, Bellotti V, Hawkins PN, Myers RM, Smith MD, Polara A, Cobb AJ, Ley SV, Aquilina JA, Robinson CV, Sharif I, Gray GA, Sabin CA, Jenvey MC, Kolstoe SE, Thompson D, Wood SP. 2006. Targeting C-reactive protein for the treatment of cardiovascular disease. Nature 440:1217–1221 [DOI] [PubMed] [Google Scholar]
- 43.Pepys MB, Hirschfield GM. 2003. C-reactive protein: a critical update. J. Clin. Invest. 111:1805–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ballou SP, Kushner I. 1992. C-reactive protein and the acute phase response. Adv. Intern. Med. 37:313–336 [PubMed] [Google Scholar]
- 45.Siboo R, Kulisek E. 1978. A fluorescent immunoassay for the quantification of C-reactive protein. J. Immunol. Methods 23:59–67 [DOI] [PubMed] [Google Scholar]
- 46.Marnell LL, Mold C, Volzer MA, Burlingame RW, Du Clos TW. 1995. C-reactive protein binds to Fc gamma RI in transfected COS cells. J. Immunol. 155:2185–2193 [PubMed] [Google Scholar]
- 47.Mold C, Gresham HD, Du Clos TW. 2001. Serum amyloid P component and C-reactive protein mediate phagocytosis through murine Fc gamma Rs. J. Immunol. 166:1200–1205 [DOI] [PubMed] [Google Scholar]
- 48.Muller H, Fehr J. 1986. Binding of C-reactive protein to human polymorphonuclear leukocytes: evidence for association of binding sites with Fc receptors. J. Immunol. 136:2202–2207 [PubMed] [Google Scholar]
- 49.Stein MP, Mold C, Du Clos TW. 2000. C-reactive protein binding to murine leukocytes requires Fc gamma receptors. J. Immunol. 164:1514–1520 [DOI] [PubMed] [Google Scholar]
- 50.Agrawal A, Shrive AK, Greenhough TJ, Volanakis JE. 2001. Topology and structure of the C1q-binding site on C-reactive protein. J. Immunol. 166:3998–4004 [DOI] [PubMed] [Google Scholar]
- 51.Gaboriaud C, Juanhuix J, Gruez A, Lacroix M, Darnault C, Pignol D, Verger D, Fontecilla-Camps JC, Arlaud GJ. 2003. The crystal structure of the globular head of complement protein C1q provides a basis for its versatile recognition properties. J. Biol. Chem. 278:46974–46982 [DOI] [PubMed] [Google Scholar]
- 52.Devaraj S, Kumaresan PR, Jialal I. 2004. Effect of C-reactive protein on chemokine expression in human aortic endothelial cells. J. Mol. Cell. Cardiol. 36:405–410 [DOI] [PubMed] [Google Scholar]
- 53.Pasceri V, Willerson JT, Yeh ET. 2000. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation 102:2165–2168 [DOI] [PubMed] [Google Scholar]