Abstract
Current influenza virus vaccines contain H1N1 (phylogenetic group 1 hemagglutinin), H3N2 (phylogenetic group 2 hemagglutinin), and influenza B virus components. These vaccines induce good protection against closely matched strains by predominantly eliciting antibodies against the membrane distal globular head domain of their respective viral hemagglutinins. This domain, however, undergoes rapid antigenic drift, allowing the virus to escape neutralizing antibody responses. The membrane proximal stalk domain of the hemagglutinin is much more conserved compared to the head domain. In recent years, a growing collection of antibodies that neutralize a broad range of influenza virus strains and subtypes by binding to this domain has been isolated. Here, we demonstrate that a vaccination strategy based on the stalk domain of the H3 hemagglutinin (group 2) induces in mice broadly neutralizing anti-stalk antibodies that are highly cross-reactive to heterologous H3, H10, H14, H15, and H7 (derived from the novel Chinese H7N9 virus) hemagglutinins. Furthermore, we demonstrate that these antibodies confer broad protection against influenza viruses expressing various group 2 hemagglutinins, including an H7 subtype. Through passive transfer experiments, we show that the protection is mediated mainly by neutralizing antibodies against the stalk domain. Our data suggest that, in mice, a vaccine strategy based on the hemagglutinin stalk domain can protect against viruses expressing divergent group 2 hemagglutinins.
INTRODUCTION
Influenza caused by pandemic and epidemic influenza virus strains is a public health concern worldwide. Vaccination remains the best countermeasure against influenza virus infections. However, highly effective current influenza virus vaccines are limited in utility because they provide a very narrow breadth of protection (1–3). Since influenza viruses are able to evade the human herd immunity by constantly changing antigenic regions in their surface glycoproteins, the hemagglutinin (HA) and neuraminidase (NA), vaccines have to be reformulated almost every year based on surveillance data of circulating influenza strains and antigenic relatedness (4). This process, however, is not error proof, and mismatches between vaccine strains and circulating viruses affect the efficacy of the vaccines. For example, in the 1997-1998 influenza season, a drifted strain (A/Sydney/05/97, H3N2) caused severe outbreaks because it matched very poorly with the same year's vaccine antigens (A/Nanchang/933/95 or A/Wuhan/359/95, both H3N2) (5). Due to the mismatch, the efficacy of influenza vaccination that year decreased drastically. Different studies reported various efficacies for that annual vaccine, ranging from placebo levels (6) to 35% protection (7). Similarly, in the 2003-2004 season, the H3 component drifted from the predicted A/Panama/2007/99 to the A/Fujian/411/02-like strain, which dominated the season and matched very poorly with the vaccine. Hence, the seasonal vaccinations had suboptimal efficacy; the antibody response against the drifted circulating virus was four times lower. Low vaccine efficacy was also observed in the elderly during the 2012-2013 epidemic (caused mostly by H3N2 strains) (8). Furthermore, mismatch-independent vaccine failure in certain populations (9) and the pandemic threat from avian viruses like H7N9 and other zoonotic influenza viruses (10–12) warrant the development of better, longer-lasting, and broader vaccines.
Most of the neutralizing antibodies against HA are considered to be directed against the highly variable globular head domain of the protein (13). These antibodies inhibit receptor binding and thus have hemagglutination inhibition (HI) activity, which is generally strain specific. The stalk domain of the HA is relatively well conserved; however, it is far less immunogenic and, under normal conditions, antibodies against this domain occur only at a low frequency (14, 15). Recently, broadly neutralizing antibodies against this domain of the HA have been isolated (16–22), suggesting that a vaccine based on the induction of such antibodies would protect from infection with divergent strains within a subtype and also against strains from other subtypes that have similar stalk structures. It is of note that these antibodies are HI negative and that their mechanism of neutralization is likely to be different from the mechanism through which antibodies against the globular head domain work (16, 18–22).
We have recently shown that a vaccine strategy based on chimeric HAs (cHA) (23) expressing H1 HA stalk structures induced broadly protective antibodies against group 1 HA-expressing viruses in mice (24). Considering the extremely low sequence identity of the stalk domains of members from the two groups of HAs, as well as evidence from studies characterizing stalk-directed monoclonal antibodies (19, 20, 22), it seems that cross-protection between group 1 (H1, H2, H5, H6, H8, H9, H11, H12, H13, H16, H17) and group 2 viruses (H3, H4, H7, H10, H14, H15) is limited (24). Also, it has been suggested that stalk-reactive antibodies against group 2 HAs are more rare, and only three monoclonal antibodies that broadly bind to group 2 HAs have been described (18, 20, 21). Therefore, it was unclear if a vaccine strategy based on the group 2 HA stalk is feasible. Here, we describe a vaccination strategy based on cHAs which induces cross-reactive and broadly protective anti-stalk antibodies against group 2 HAs in mice. We show that these polyclonal antibodies neutralize in vitro and are sufficient to protect animals from lethal challenge with a panel of group 2 HA-expressing viruses, including H3N2 and H7N1 strains.
MATERIALS AND METHODS
Cells and viruses.
Madin-Darby canine kidney (MDCK; ATCC CCL-34) and human embryonic kidney 293T (ATCC CRL-11268) cells were purchased from the American Type Culture Collection and were grown in Dulbecco's modified Eagle's medium (DMEM) (Gibco) and minimal essential medium (Gibco), respectively. Both media were supplemented with 10% fetal bovine serum (FBS; HyClone) and 100 units/ml of penicillin and 100 μg/ml of streptomycin (Pen-Strep; Gibco).
Chimeric and recombinant influenza viruses were produced by plasmid-based reverse genetics as described before (3, 23, 24). Virus strains A/Victoria/361/11 (H3N2; Vic11), A/Perth/16/09 (H3N2; Perth09), A/Philippines/2/82 (H3N2; Phil82), X-31 (H3N2; 6:2 reassortant of A/Puerto Rico/8/34 with HA and NA from A/Hong Kong/1/68), A/rhea/North Carolina/39482/93 (H7N1; RheaH7), A/cH5/3N1 (expressing the H5 globular head domain of A/Viet Nam/1203/04, the H3 stalk domain from Perth09, and the NA and internal genes from A/Puerto Rico/8/34), A/Wyoming/03/03 (H3N2; WyoH3), A/Northern shoveler/Alaska/7MP1708/07 (H3N8), A/mallard/Interior Alaska/10BM01929/10 (H10N7), and B/cH7/3 (expressing the H7 globular head from A/mallard/Alberta/24/01 on top of the H3 stalk domain from A/Perth/16/09 in the B/Yamagata/16/88 background) were grown in 8- or 10-day-old embryonated chicken eggs for 48 h at 37°C (for influenza A viruses) or for 72 h at 33°C (for influenza B viruses) (23, 25). A/Indiana/10/11 (H3N2 variant, H3N2v) was grown on MDCK cells. Virus titers were determined on MDCK cells in the presence of tosyl phenylalanyl chloromethyl ketone (TPCK)-treated trypsin. Vic11, Perth09, Phil82, X-31, RheaH7, and A/cH5/3N1 viruses were purified via sucrose density ultracentrifugation for enzyme-linked immunosorbent assays (ELISAs). Purified preparations of strains used as positive controls (Phil82, X-31, RheaH7, and A/cH5/3N1) were inactivated by formalin treatment. All viruses were handled under biosafety level 2 conditions. Viruses expressing cH5/3 and cH7/3 proteins contained the original H3 cleavage sites that are associated with a low-pathogenicity phenotype and were rescued in the A/Puerto Rico/8/34 background, which is usually considered safe for humans (26). The H7N1 isolate we used shows a low pathogenicity phenotype in avian species as well (27). Sf9 cells (ATCC CRL-1711) were maintained in Trichoplusia ni medium-formulation Hink (TNM-FH) (Gemini Bioproducts) supplemented with 10% FBS, 1% Pluronic F68 (Sigma), and Pen-Strep (Gibco). BTI-TN-5B1-4 (High Five) cells (ATCC CRL-10859) were grown in HyClone SFX serum-free insect cell medium (Fisher Scientific) containing Pen-Strep (Gibco).
Recombinant protein expression.
Recombinant baculoviruses for the expression of cH4/3 (H4 globular head domain from A/duck/Czech/56 in combination with the H3 stalk domain from Perth09), cH5/3 (H5 globular head domain from A/Viet Nam/1203/04 in combination with the stalk domain from Perth09), cH7/3 (H7 globular head from A/mallard/Alberta/24/01 on top of the H3 stalk domain of Perth09), and Perth09 HA were generated as described elsewhere (3, 28) and were propagated in Sf9 cells. For expression, High Five cells were infected with recombinant baculoviruses at a multiplicity of infection of approximately 10, transferred into Fernbach flasks, and incubated at 28°C, with shaking. Culture supernatants were harvested 96 h postinfection by low-speed centrifugation (5,500 relative centrifugal force [RCF], 10 min, 4°C) and were then incubated with Ni-nitrilotriacetic acid (NTA) resin (Qiagen) for 3 h at room temperature (RT), with shaking at 75 rpm in a rotational shaker. The resin-supernatant mixture was then passed over 10 ml polypropylene columns (Qiagen), washed four times with washing buffer (50 mM Na2HCO3, 300 mM NaCl, 20 mM imidazole, pH 8), and eluted with elution buffer (50 mM Na2HCO3, 300 mM NaCl, 250 mM imidazole, pH 8). The eluted fractions were concentrated, and the buffer was exchanged against phosphate-buffered saline (PBS; pH 7.4) using Amicon Ultra centrifugal filter units (Millipore; 30-kDa molecular mass cutoff). Purity, identity, and integrity of the purified proteins were assessed by SDS-PAGE and Western blotting or ELISA, and protein concentration was measured using Bradford reagent (Bio-Rad). Soluble A/Perth/16/09 H3, A/Shanghai/1/13 H7, and A/PR/8/34 H1 HAs for ELISAs were expressed in a similar way but as a fusion protein with a GCN4pII trimerization motif and a C-terminal Strep-Tag II via StrepTactin resin (GE Healthcare) to avoid background signal when used in ELISAs to assess stalk-reactive antibodies induced by the vaccine constructs (24).
Animals, vaccination, and challenge.
All animal experiments were performed with 6- to 8-week-old female BALB/c mice (Jackson Laboratories) and in full compliance with the guidelines of the Icahn Sinai School of Medicine at Mount Sinai Institutional Animal Care and Use Committee. Animals had free access to food and water and were kept on a 12-h light-dark cycle. Animals were anesthetized for all intranasal procedures by administering 0.1 ml of a ketamine-xylazine mixture (0.15 mg/kg and 0.03 mg/kg) intraperitoneally.
For the initial experiment, naive 6- to 8-week-old BALB/c mice were vaccinated in the left calf muscle with DNA encoding a cH4/3 HA (3, 24, 29), by in vivo electroporation using a TriGrid delivery system (Ichor Medical Systems). Three weeks later, animals were boosted intramuscularly (i.m.) and intranasally (i.n.) with 5 μg protein adjuvanted with 5 μg poly(I·C) (Invivogen) at each site (n = 9 or 10 animals per group). Prime-only animals (n = 5 per group) received the same amount of irrelevant protein (bovine serum albumin [BSA]), with the same amount of adjuvant, both i.m. and i.n. A second boost was administered 3 weeks later in the same manner but with cH7/3 protein. For the subset of mice dedicated to the H7N1 challenge, the cH7/3 protein was replaced by full-length Perth09 H3 protein. Prime-only animals received the corresponding BSA vaccination again. Positive-control animals (n = 5 per group) were vaccinated with 1 μg of inactivated matched challenge virus i.m., 3 weeks before challenge. Naive mice (n = 5 per group) were included as additional negative-control groups. Four weeks after the last boost, animals were anesthetized and infected with 5 50% lethal doses (LD50) of Phil82, X-31, or RheaH7 viruses. Weight was monitored daily for a period of 14 days, and animals that lost 25% or more of their initial body weight were scored dead and humanely euthanized. Serum samples were collected preprime and prechallenge by submandibular bleeding.
For the second set of experiments, naive 6- to 8-week-old BALB/c mice were infected intranasally with a sublethal dose (106 PFU) of a recombinant influenza B virus vector (based on B/Yamagata/16/88) expressing the cH7/3 HA protein. Control animals received the same dose of wild-type (wt) influenza B virus (Bwt). The mice in the vaccine group were then boosted with cH5/3 protein (5 μg i.n. plus 5 μg i.m., each adjuvanted with 5 μg of poly(I·C), with the exception of a subset of animals dedicated to the cH5/3N1 virus challenge; these animals received recombinant Perth09 full-length H3 HA in the same manner instead of the cH5/3 protein (n = 10 per group). Prime-only and vector control animals (n = 5 per group) received a boost with irrelevant protein instead (BSA; same amount, adjuvant, and administration routes as for the vaccine groups). Finally, animals received a second boost 3 weeks later with cH4/3 protein as described for the first boost. Again, prime-only and vector control animals received irrelevant protein, in the same manner. Naive mice (n = 5 per group) were included as additional negative-control groups. Positive-control animals (n = 5 per group) were vaccinated with 1 μg of inactivated matched challenge virus i.m., 3 weeks before virus challenge. Four weeks after the last boost, animals were anesthetized and challenged with 10 LD50 of either Phil82 or X-31 or with 100 LD50 of cH5/3N1 virus. Weight loss was monitored daily for a period of 14 days, and animals that lost 25% or more of their initial body weight were scored dead and humanely euthanized. Serum samples were collected preprime and prechallenge by submandibular bleeding. For lung titration experiments, mice were vaccinated as described above (cHA and Bwt-BSA-BSA groups, n = 3 mice per group) and were then infected with 5 × 104 PFU of H3N2v or 1 × 105 PFU of H3N8, WyoH3, or H10N7 virus. Lungs were harvested on day 3 postinfection and homogenized, and the 50% tissue culture infectious dose (TCID50) was measured as described before (30).
For passive transfer experiments, sera from the latter set of experiments were collected from the (influenza B-vectored) vaccine group, positive-control group, vector control group, and naive animals. Sera from each group were then transferred by intraperitoneal injection into naive mice (6 to 8 weeks old, n = 5 per group, 300 μl serum per mouse), and mice were challenged with 5 LD50 of Phil82 virus 2 h after the transfer. Weight was monitored daily for a period of 14 days, and animals that lost 30% or more of their initial body weight were scored dead and euthanized.
ELISA.
ELISA plates (Immunolon 4 HBX) were either coated overnight at 4°C with purified virus (4 μg/ml) or purified protein (2 μg/ml) diluted in carbonate/bicarbonate coating buffer (pH 9.4). Plates were blocked for 1 h at RT with PBS containing 0.1% Tween 20 (TPBS) and 3% nonfat dry-milk powder. Mouse serum was prediluted 1:100, serially diluted in 1:2 steps in TPBS containing 1% nonfat dry-milk powder, and incubated on the plates for 1 h at RT. After extensive washing with TPBS (3× 100 μl/well), the plates were incubated for 1 h at RT with an anti-mouse IgG horseradish peroxidase (HRP)-conjugated IgG (Santa Cruz) diluted in TPBS containing 1% nonfat dry-milk powder. After three more washing steps with TPBS, plates were developed using the o-phenylenediamine dihydrochloride (SigmaFast OPD; Sigma) substrate. Reactions were stopped using 3 M HCl, and plates were read at an optical density at 490 nm.
Detection of IgA in nasal washes was performed with a similar assay except that we used an alkaline phosphatase (AP)-linked anti-mouse IgA antibody (Southern Biotech) diluted 1:500 and incubation steps at 37°C for 3 h. Isotype distribution in serum was determined by ELISA using an isotyping kit (Invitrogen), which contains a collection of secondary antibodies specific for each subtype and an AP-conjugated tertiary antibody that allows for detection of binding.
Pseudotyped particle neutralization assay.
The pseudotyped particle production protocol was adapted from previous studies (23, 31, 32). Briefly, 293-T cells were cotransfected with four plasmids encoding a provirus containing a luciferase reporter gene, the HIV Gag-Pol, the Vic11 HA protein, and the neuraminidase from influenza B virus B/Yamagata/16/88. Culture supernatants were collected 48 h posttransfection and filtered through a 0.45-μm-pore-size filter unit to remove cellular debris. The purified pseudotyped particles were incubated with different concentrations of inactivated mouse sera before being added to MDCK cells. The transduction procedure was carried out for 6 h, cells were then washed, and fresh medium was placed over cells. The transduction procedures were performed in the presence of 1 μg/ml Polybrene (Sigma, St. Louis, MO). Luciferase activity was read 48 h after transduction. The stalk-reactive monoclonal antibody 12D1 (17) was used as a positive control with a starting concentration of 123 μg/ml.
Immunofluorescence staining.
MDCK cells were transfected with plasmids expressing HAs from A/Anhui/1/13 (H7N9), A/Shanghai/1/13 (H7N9), A/chicken/Jalisco/12283/12 (H7N3), A/mallard/Gurjev/263/82 (H14N5), and A/wedge tailed shearwater/Western Australia/2576/79 (H15N9). Cells were transfected with the respective plasmid. Sixteen hours posttransfection, cells were fixed with 0.5% paraformaldehyde and stained with 1:200 dilution of sera collected from vaccinated animals (cH4/3DNA-cH5/3-H3) or were naive. Stalk-reactive antibodies FI6 (21) and FBE9 (both used at a concentration of 10 μg/ml) served as positive controls, while serum collected from naive animals was used as a negative control. Secondary antibodies conjugated to Alexa 488 were used to visualize reactivity to the proteins. Images were taken on an LSM 510 Meta confocal microscope (Carl Zeiss MicroImaging GmbH, Jena, Germany) at a magnification of ×10.
Statistical tests, hemagglutinin modeling, and phylogenetic analysis.
Statistical analyses were performed using Prism4 (GraphPad). All values are plotted as averages with standard deviations of the means. Differences in survival were calculated by using Kaplan-Meier survival analysis with log rank significance test. P values at or below 0.05 are considered statistically significant. In order to generate models of the wild-type and chimeric hemagglutinins, structures from the Protein Data Bank (PDB) were modeled by using the PyMol software (Delano Scientific). For modeling of the cH4/3 construct, we used the HA of A/Hong Kong/1/68 (HK68) (PBD identifier [ID] 1MQN) and indicated the H4 head domain with a different color (there is no H4 structure available). The cH5/3 HA was modeled with the stalk from HK68 HA (PBD ID 1MQN) and the globular head of an H5 virus (PBD ID 2FK0); the cH7/3 was modeled with the HK68 stalk domain and a head domain from an avian H7 virus (PDB ID 4DJ6). The chimeric challenge viruses were modeled by using the stalk structure of HK68 (PBD ID 1MQN) and head of H5 HA (PBD ID 4DJ6). Phylogenetic analysis was performed using ClustalW (EMBL-EBI). The protein sequences were downloaded from GenBank, and multiple alignments were performed using the ClustalW algorithm in Mega version 5.1. Phylogenetic trees were constructed using the FigTree software and the neighbor-joining method.
RESULTS
Chimeric HA constructs provide broad protection from challenge with divergent H3N2 viruses.
We aimed to induce broad protection against group 2 HA-expressing influenza viruses in the mouse model, by specifically boosting antibodies against the conserved HA stalk domain. For this reason, we constructed a collection of cHA molecules expressing H3 stalk domains combined with various head domains (3, 23). Mice were primed intramuscularly (i.m.) with plasmid DNA encoding a cH4/3 HA (expressing an H4 globular head domain and an H3 stalk domain) (Fig. 1A). Three weeks later, mice were boosted with soluble cH5/3 protein (H3 stalk domain combined with an H5 globular head domain) via both the i.m. and intranasal (i.n.) routes. We used both routes to ensure induction of systemic and mucosal immunity, since we believe that both responses are important for efficient protection against influenza virus infection. A second boost with cH7/3 protein (H7 globular head on top of an H3 stalk) followed 3 weeks later (Fig. 1A). We speculated that by repeatedly exposing the animals to antigens expressing a common, unfettered (soluble, non-membrane-bound) stalk domain, we could enhance the immunogenicity of this region, to which currently used vaccination strategies induce only a subdominant response (1, 3).
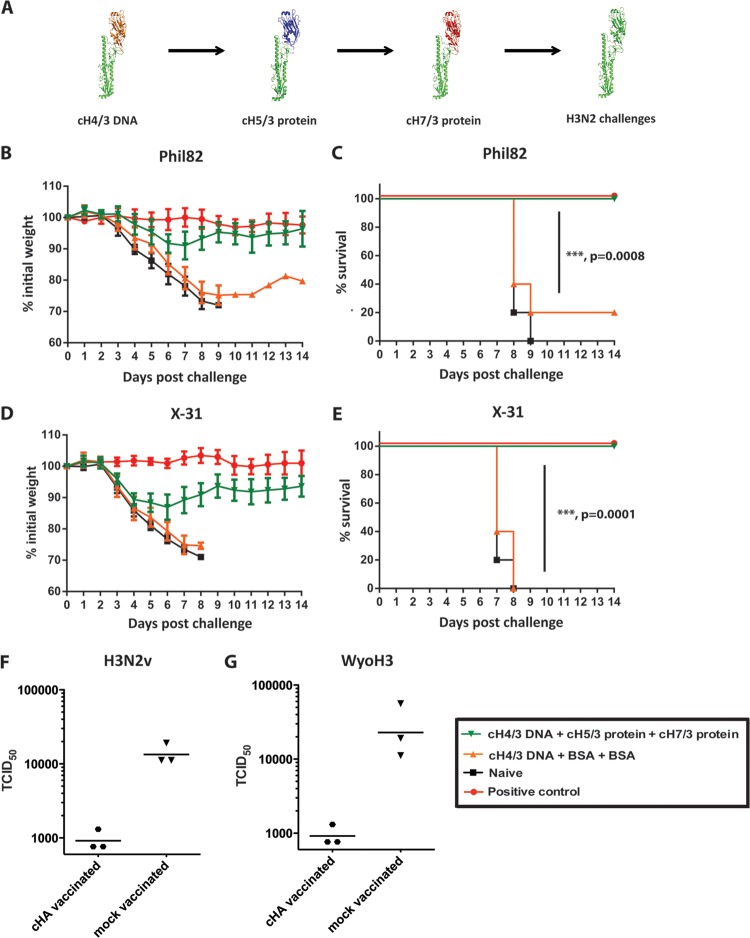
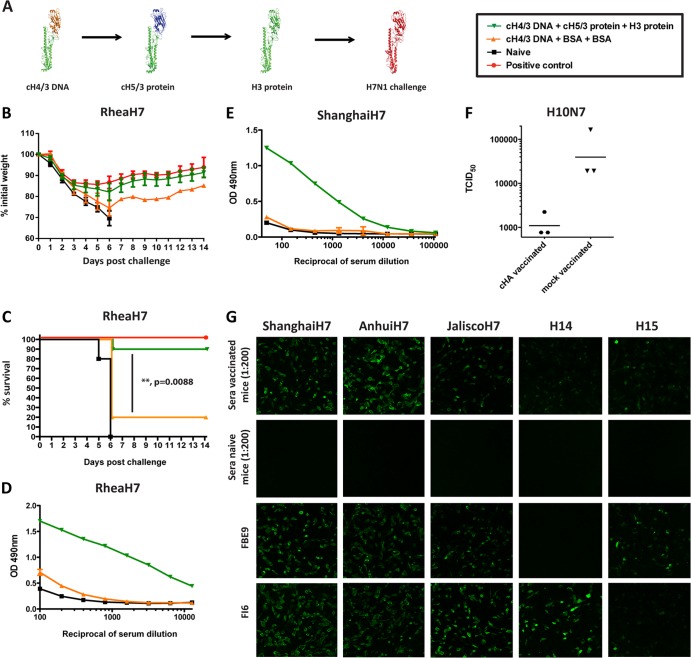
Fig 1.
An HA stalk-based vaccination strategy provides broad protection from challenge with divergent H3N2 viruses. (A) Schematic representation of the vaccination strategy (the monomeric form of each antigen is shown). (B to E) Animals were vaccinated with plasmid DNA coding for cH4/3 HA and subsequently boosted with recombinant soluble cH5/3, followed by cH7/3 proteins (green triangles; n = 9 or 10 animals). Positive-control animals received inactivated vaccine containing the matched challenge strain (red circles; n = 5 animals). Prime-only animals (orange triangles; n = 5 animals) received the DNA prime followed by two irrelevant protein boosts. Naive animals (black squares; n = 5 animals) were used as additional controls for challenge. (B) Weight loss curves upon challenge with the Phil82 (H3N2) virus. (C) Kaplan-Meier survival curve upon Phil82 challenge. (D) Morbidity observed upon challenge with the X-31 (H3N2) virus. (E) Survival curves following the X-31 (H3N2) challenge. Survival of vaccinated (cH4/3DNA-cH5/3-cH7/3) versus control (cH4/3DNA-BSA-BSA) groups is highly significant for both challenge experiments (P = 0.0008 and 0.0001, respectively). (F and G) To further test the protection breadth of the vaccine against viruses that are not lethal in the mouse model, we performed lung titration experiments. Vaccinated animals (BcH7/3-cH5/3-cH4/3) and control animals (Bwt-BSA-BSA) were infected with 5 × 104 PFU of H3N2 variant (F) or 1 × 105 PFU of the human H3N2 A/Wyoming/03/03 (G). On day 3 postinfection, lungs of animals from both groups were harvested and homogenized, and the 50% tissue culture infectious dose (TCID50) was measured.
Control animals either received only the DNA prime (prime-only control) or a matched inactivated challenge virus (positive control) or were naïve. To test the ability of our vaccine to protect against morbidity and mortality associated with influenza, mice were infected with two different H3N2 viruses. It is of note that all cHA-vaccinated animals were HI negative against the respective challenge strains. Upon infection with A/Philippines/2/82 virus (Phil82), the vaccinated animals lost minimal amounts of weight compared to no weight loss in the positive control, did not develop clinical symptoms, and were fully protected against mortality (Fig. 1B and C). Naïve animals, however, lost weight rapidly and succumbed to infection by day 9 (naïve control). Prime-only controls also exhibited rapid weight loss, and only 20% of them survived the viral challenge. A similar outcome was observed upon challenge with X-31, a virus that expresses the glycoproteins of the Hong Kong 1968 H3N2 virus (Fig. 1D and E).
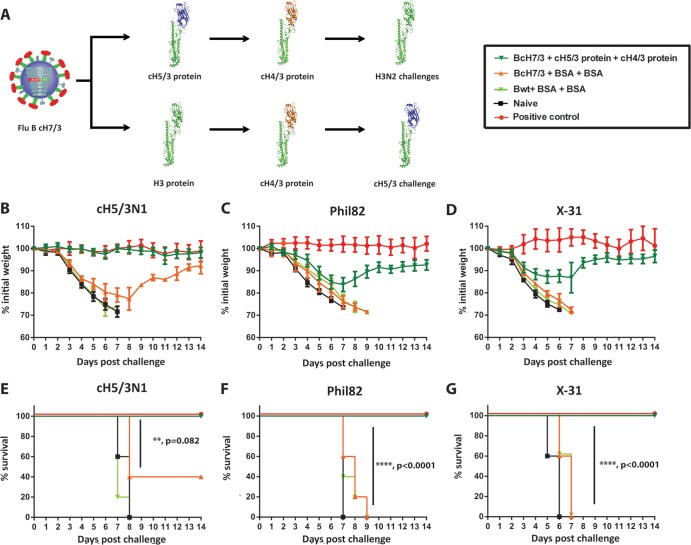
We have previously shown that sublethal infection with influenza viruses is one way to induce stalk-reactive antibodies in mice and humans (3, 32, 33). Since most human individuals are exposed to influenza viruses multiple times throughout their lifetime, they generally have baseline levels of stalk-reactive antibodies. In order to mimic this preexisting immunity in the mouse model, we rescued an influenza B virus expressing a cH7/3 HA instead of the wild-type influenza B virus HA. We used a sublethal dose of this virus to prime mice, which were then vaccinated at 3-week intervals with cH5/3 followed by cH4/3 protein, both i.m. and i.n. (Fig. 2A). When challenged with either Phil82 or X-31, the animals showed no clinical signs of disease and minimal weight loss comparable to that of positive-control animals that received inactivated matched challenge strains (Fig. 2C, D, F, and G). Control animals infected sublethally with wild-type influenza B virus and vaccinated in a similar manner with an irrelevant protein (BSA), as well as naive mice and prime-only controls, lost weight rapidly and succumbed to both infections. We next wanted to test the ability of the stalk-directed immunity to protect against more recent H3N2 isolates. Since contemporary H3N2 viruses are not pathogenic in the mouse model, we used for this purpose a cH5/3N1 virus expressing the H3 stalk domain of the recent vaccine strain A/Perth/16/09 (Perth09) and an NA from A/PR/8/34 (H1N1) (23). To assess the protection conferred by the stalk-reactive antibodies (and to exclude H5-H5 head reactive antibodies), we had to alter the vaccination regimen for this subset of mice and administered full-length H3 instead of the cH5/3 protein (Fig. 2A). When challenging with a high dose (100 mouse LD50 [mLD50]) of the cH5/3N1 virus, we observed robust protection from weight loss, as well as complete protection from mortality of the vaccinated animals. This demonstrated the efficacy of the HA stalk-based vaccine approach against contemporary H3N2 isolates (Fig. 2B and E). To test additional divergent H3 strains that do not induce mortality in the mouse model, we chose to perform lung titration experiments. Animals vaccinated as described above were infected with H3N2 variant virus (H3N2v) (34), an avian H3N8 isolate (H3N8), and the human H3N2 A/Wyoming/03/03 strain (WyoH3). Day 3 postinfection, lung titers of vaccinated animals were low (close to the limit of detection), whereas we detected high virus titers in lungs collected from control animals (Bwt-BSA-BSA) (Fig. 1F and G and data not shown). Taken together, these data clearly show that the stalk-based vaccination strategy can provide robust protection against heterologous and heterosubtypic viruses in the mouse model of influenza.
Fig 2.
Vaccination with cHA constructs can boost preexistent titers of stalk-reactive antibodies to protective levels. (A to G) To mimic the preexisting immunity to the stalk domain present in the human population, animals were sublethaly infected with a recombinant influenza B virus that expresses cH7/3 HA. Subsequently, they were boosted with recombinant soluble cH5/3 (or full-length H3 HA for cH5/3N1-challenged animals) and then cH4/3 protein (green triangles; n = 10 animals). Positive-control animals received inactivated vaccine containing the matched challenge strain (red circles; n = 5 animals). Prime-only animals (orange triangles; n = 5 animals) received the recombinant influenza B prime and then two irrelevant protein boosts. Additional control groups were either infected with wild-type influenza B virus and then received two irrelevant protein boosts (light-green triangles; n = 5) or were naive (black squares; n = 5). (B to D) Weight loss curves following viral challenges. (B) We used a mouse-pathogenic cH5/3N1 virus which expresses the stalk domain of an HA from a recent human H3N2 isolate as the surrogate challenge strain to test efficacy against contemporary stalk domains, since modern human H3N2 isolates are not pathogenic in mice. Weight loss upon infection with the X-31 (H3N2; HA and NA from A/Hong Kong/1/68) (C) and Phil82 (H3N2) (D) viruses; (E) Kaplan-Meier survival curve following the A/cH5/3N1 challenge; (F) survival curves following the Phil82 (H3N2) challenge; (G) survival curves following the X-31 (H3N2) challenge. Statistical analysis revealed high significance for all challenges when comparing BcH7/3-cH5/3-cH4/3 and BcH7/3-BSA-BSA groups (P = 0.082, P < 0.0001, and P < 0.0001).
Vaccination with chimeric HA constructs induces a broad systemic and mucosal stalk-directed humoral response.
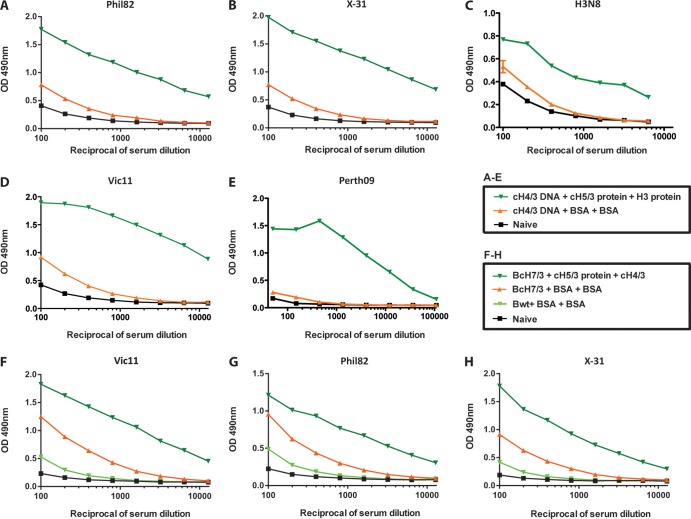
In order to characterize the stalk-directed antibody response induced by our vaccination regimen, we employed ELISAs. Since the animals were exposed only to vector-expressed or recombinant HA but not to any other influenza A proteins, we were able to use purified virus as the substrate to measure the anti-stalk responses. We tested sera from both vaccination regimens (influenza B virus vectored or DNA primed) for their reactivity to the challenge strains Phil82 and X-31, as well as to the current H3N2 vaccine strain A/Victoria/361/11 (Vic11), an H3N8 strain and HA protein from Perth09. While sera collected from animals that were naive, or that were exposed only to the DNA prime, exhibited low background level binding, we detected high reactivity against all five viral strains in sera collected from vaccinated mice (Fig. 3A to E). Similarly, high stalk-directed antibody titers were detected in the sera of animals primed with the cHA-expressing influenza B virus (Fig. 3F to H). Vector control (wild-type influenza B virus) and naive animals again only showed background reactivity, while the prime-only group (c7/3 HA-expressing influenza B virus) had an intermediate binding phenotype. The intermediate titers in the latter group were thought to be the result of replicating virus (3, 33). We also performed an isotype distribution analysis and determined that the profile of the antibody response induced by the vaccine is balanced, with the majority of IgG being of the IgG1, IgG2a, or IgG2b subclass (see Fig. 5C).
Fig 3.
The elicited anti-stalk responses are cross-reactive against multiple H3N2 strains, including the most recent vaccine strain. (A to E) ELISA reactivity against the current influenza vaccine strain Vic11 (H3N2, whole virus) (A), Perth09 (H3 protein) (B), H3N8 virus (C), Phil82 virus (H3N2) (D), or X-31 virus (H3N2; expressing HA and NA from A/Hong Kong/1/68 virus) (E) of sera collected from animals vaccinated with cHA constructs (dark-green triangles, cH4/3DNA-cH5/3-cH7/3), prime-only animals (orange triangles), or naive animals (black squares). (F to H) ELISA reactivity against Vic11 H3N2 (F) or Phil82 H3N2 (G) or X-31 H3N2 (H) virus of sera collected from animals vaccinated with cHA constructs (green triangles, B/cH7/3 virus-cH5/3 protein-cH4/3 protein), prime-only animals (orange triangles), control animals that were infected with wild-type influenza B virus and then received two BSA boosts (light-green triangles), or naive animals (black squares).
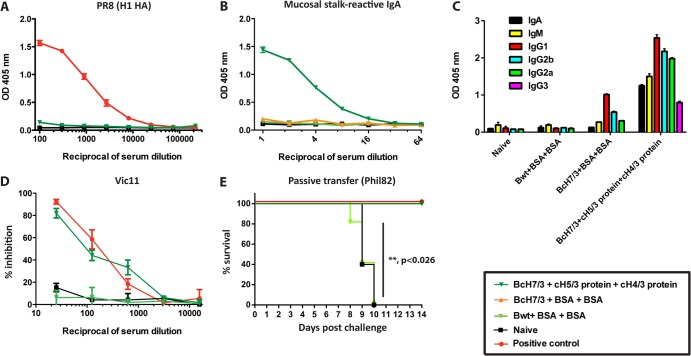
Fig 5.
The polyclonal responses elicited by the chimeric HA vaccination are directed against the stalk domain and neutralize virus infection both in vitro and in vivo. (A) An ELISA against a group 1 HA protein (H1) demonstrates that the cross-reactive responses elicited by the cHA vaccine (dark-green triangles, B/cH7/3-cH5/3-cH4/3) are not directed against conserved parts of the receptor binding site in the HA protein. (B) ELISA reactivity of nasal washes from animals vaccinated with cHA constructs (dark-green triangles, B/cH7/3-cH5/3 protein-cH4/3 protein), prime-only animals (orange triangles, B/cH7/3-irrelevant protein-irrelevant protein), vector controls (light-green squares, Bwt-irrelevant protein-irrelevant protein), and naive animals (black squares). (C) Antibody isotypes in sera from vaccinated (B/cH7/3 virus-cH5/3 protein-cH4/3 protein), naive, prime-only (B/cH7/3 virus-BSA-BSA), and vector control animals (Bwt virus-BSA-BSA). (D) Vic11 (H3) pseudotyped particle neutralization assay with sera from cHA-vaccinated animals (dark-green triangles, B/cH7/3-cH5/3-cH4/3), vector controls (light-green squares, Bwt-irrelevant protein-irrelevant protein), and naive animals (black squares). The reciprocal serum dilution is shown on the x axis. An H3 stalk-reactive monoclonal antibody (12D1) was used as positive control (red triangles), at a starting concentration of 123 μg/ml. (E) Passive transfer challenge experiment (Phil82 H3N2 virus) with sera from animals that were vaccinated (dark-green triangles, BcH7/3-cH5/3-cH4/3, n = 5 animals), vector controls (light-green squares, Bwt-BSA-BSA, n = 5 animals), naive animals (black squares, n = 5 animals), and positive-control animals (received inactivated Phil82 virus vaccine, n = 5 animals). Kaplan-Meier survival curve is shown (P < 0.026 for BcH7/3-cH5/3-cH4/3 versus Bwt-BSA-BSA groups).
In addition to the serum IgG titers, we also assessed levels of secretory IgA on the mucosal surfaces of vaccinated mice. We detected high reactivity to Perth09 H3 HA in nasal washes collected from the group of mice that received the vaccine, whereas nasal washes from control animals did not react to the substrate (see Fig. 5B). Although mucosal anti-stalk IgA antibodies, and their ability to block viral infection, have not been yet formally characterized, we expect that they contribute to the observed protection.
Broadly reactive anti-globular head antibodies have recently been described in the literature (35–37). Though rare in nature, these antibodies tend to recognize conserved regions of the receptor-binding site and recognize divergent globular head domains without closely following phylogenetic relatedness. Binding to head domains of both group 1 and group 2 HAs (e.g., H1-H3 binding) has been described by these antibodies. To assess whether the cHA-based vaccination induces such antibodies, we performed an additional ELISA with full-length H1 HA as the substrate (see Fig. 5A). Sera from cHA-vaccinated animals did not react with this substrate, suggesting that cHA vaccination does not induce detectible levels of broadly reactive anti-head antibodies.
The induced broadly reactive antibodies potently neutralize virus, both in vitro and in vivo.
To further test the in vitro cross-neutralizing nature of the stalk antibodies induced by the vaccination regimen, we performed an entry inhibition assay with Vic11 HA-pseudotyped particles. Serum from vaccinated animals inhibited cell entry of the pseudoparticles in a dose-dependent manner (see Fig. 5D). In contrast, serum from influenza B virus vector-infected control animals and naive mice showed no inhibitory activity in this assay.
In order to show that the vaccine-induced protection we observed in vivo was, at least partially, mediated by neutralizing antibodies in serum, we performed a passive transfer experiment. Sera from vaccinated, positive-control, influenza B virus vector-infected, or naive animals were transferred into naive mice, which were then challenged with the Phil82 virus. Mice that received sera from vaccinated or positive-control groups were completely protected from mortality, whereas none of the animals that received sera from either of the negative-control groups survived (see Fig. 5E). These results suggest that the stalk-directed humoral response is sufficient to protect mice from lethal challenge.
Vaccination with chimeric HA constructs induces stalk-based heterosubtypic immunity.
Antibodies that cross-neutralize divergent group 2 HA influenza viruses have been described in the literature (17, 20). We wanted to test if the stalk-based vaccination regimen was capable of inducing such heterosubtypic antibodies. To test protection against a heterosubtypic H7N1 virus and exclude any involvement of head-directed antibodies in the observed protection, we used the DNA-protein-protein vaccination regimen described above and replaced the cH7/3 HA protein with full-length H3 HA (Fig. 4A). Both vaccinated and positive-control animals challenged with the avian H7N1 A/rhea/North Carolina/39482/93 strain (RheaH7) experienced a similar initial weight loss of approximately 15% (Fig. 4B). This is probably due to the high numbers of PFU/mouse lethal dose needed for this experimental viral challenge. However, mice from both groups regained the weight quickly, and we observed a 90% survival in the vaccine group. Naive and prime-only animals, however, experienced severe weight loss and showed no or low (20%) survival, respectively. The results of the latter experiment demonstrate the true heterosubtypic nature of the immunity afforded by an HA stalk-directed response (Fig. 4C). To further evaluate the level of broadly neutralizing antibodies present in the sera, we performed ELISAs with purified H7N1 challenge virus as well as with recombinant H7 protein from the novel Chinese H7N9 virus strain (10). The fact that we detected high antibody titers in sera collected from these animals to the H7 HAs underlines the cross-reactive nature of the response induced by the H3 stalk domain (Fig. 4D and E, Fig. 5). In addition, we also performed a challenge experiment with an H10N7 virus with lung titers as the assay readout. Day 3 lung titers for vaccinated animals were in the range of 103 TCID50/ml, whereas mock-vaccinated animals showed a 10- to 100-fold-higher titer (Fig. 4F). Efficient binding of sera from cHA-vaccinated animals to cells expressing Eurasian and North American lineage H7 HAs, as well as H14 and H15 HAs, further proves the cross-reactive nature of this immune response (Fig. 4G and 6).
Fig 4.
The breadth of the anti-H3 HA stalk antibodies elicited by the cHA vaccination strategy extends to other members of the group 2 HAs, including the most recent Chinese H7N9 virus. (A) To ensure that anti-H7 globular-head domain antibodies are not involved in the effects observed in this series of experiments, we modified the vaccination scheme presented in Fig. 1. Animals received the same prime (DNA coding for cH4/3 HA) and first boost (cH5/3 protein), but the second boost was replaced with an H3 protein (cH4/3DNA-cH5/3-H3; green triangles, n = 10 animals). Positive-control animals received inactivated RheaH7 virus vaccine (red circles, n = 5). Prime-only animals (orange triangles, n = 5 animals) received the DNA prime followed by two irrelevant protein boosts. Naive animals (black squares, n = 5 animals) were used as additional controls. (B) Weight loss curve upon challenge with RheaH7 (H7N1) virus. (C) Kaplan-Meier survival curve. The vaccine provided good protection against mortality (P = 0.0088, cH4/3DNA-cH5/3-cH7/3 versus controls cH4/3DNA-BSA-BSA). (D, E) ELISA reactivity of sera from vaccinated animals (dark-green triangles, cH4/3DNA-cH5/3-cH7/3), control animals (orange triangles, cH4/3DNA-BSA-BSA), or naive mice (black squares) to RheaH7 virus (D) or the HA protein expressed by the recent Shanghai13 H7N9 (E) virus. (F) We infected mice that received the vaccine (BcH7/3-cH5/3-cH4/3) and controls (Bwt-BSA-BSA) with 1 × 105 PFU of H10N7 virus and measured a 20-fold decrease in viral TCID50 in the lungs of vaccinated mice on day 3 postinfection. (G) Sera collected from vaccinated mice cH4/3DNA-cH5/3-H3 recognize a panel of group 2 HA proteins. MDCK cells were transfected with plasmids encoding the respective HA and were fixed 16 h later with 0.5% paraformaldehyde. Reactivity was detected by immunofluorescence with sera from mice that received the vaccine. Serum collected from naive animals was used as a negative control. A mouse (FBE9 [unpublished, generated in-house]) and a human (FI6) (21) monoclonal anti-stalk antibody were used as positive controls.
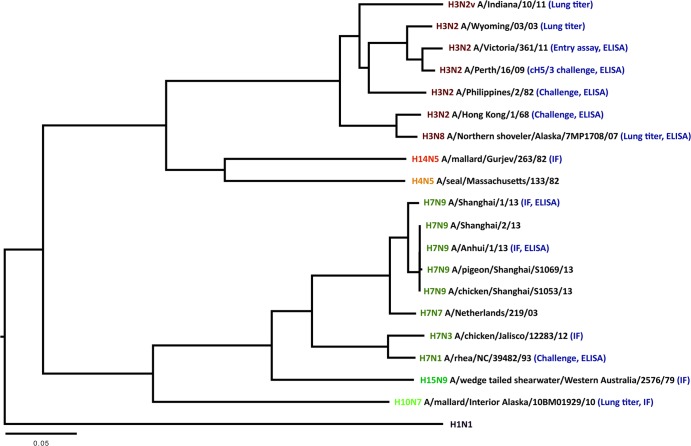
Fig 6.
Phylogeny of select members of group 2 influenza virus hemagglutinins depicting the breadth of the response elicited by the vaccine. The protein sequences were downloaded from GenBank, and multiple alignments were performed using the ClustalW algorithm in Mega version 5.1. Phylogenetic trees were constructed using the FigTree software and the neighbor-joining method. The scale bar represents a 5% amino acid change. Positively tested cross-protection/cross-reactivity of cHA-vaccinated mice to the respective strains is indicated in parenthesis after the strain names (in blue: immunofluorescence [IF], challenge, ELISA, and lung titer). Strain names without indication were not tested.
DISCUSSION
Broadly neutralizing human anti-stalk antibodies provide a rational basis for the design of universal influenza virus vaccines. Although these antibodies can be found in humans who have been exposed to influenza viruses via either vaccination or infection (3, 14, 15), they do seem to be rare in nature, and in vivo levels of these antibodies are likely too low to afford protection. Vaccines able to boost the levels of these broadly neutralizing stalk reactive antibodies could lead to universal protection against circulating human influenza virus strains, as well as potential pandemic avian viruses like the emerging Chinese H7N9 strain (10). Based on the conservation of the stalk domain of the HA, it is likely that such a universal vaccine has to include three components: a group 1, a group 2, and an influenza B stalk-based antigen. We have recently shown that chimeric HA constructs based on the stalk domain of a group 1 HA (H1) induce broad protection against group 1 HA-expressing viruses, including a wide range of H1 strains and avian H5N1 and H6N1 viruses (24). However, the HA stalk-based group 1 vaccine was unable to elicit protection from challenge with a group 2 HA-expressing virus (Phil82) (24). Here, we demonstrate that a similar approach can be successfully applied for group 2 HA-expressing influenza viruses. Animals sequentially vaccinated with chimeric HA constructs expressing an H3 stalk domain and divergent globular heads developed high titers of cross-reactive antibodies against the stalk domain. These antibodies were not only protective against a panel of H3N2 strains and an H3N8 virus but also provided robust protection against a heterosubtypic challenge with avian H7N1 and H10N7 isolates, demonstrating a breadth that spans both clades of the group 2 HA-expressing viruses. This breadth is important in light of growing concerns about the pandemic potential of H3N2 variant (H3N2v) viruses and H3N8 viruses isolated from New England harbor seals and other zoonotic H3 strains (12, 34, 38), as well as H4-, H7-, and H10-expressing viruses that infect humans occasionally (11, 39–43). Importantly, this vaccination strategy also induced high titers of stalk-reactive antibodies against the H7 HA from the emerging Chinese H7N9 virus (10). Recently, broadly reactive anti-head antibodies have also been described in the literature (35–37), and it is theoretically possible that such antibodies are induced by the cHA vaccination regimen. However, we did not detect such antibodies in sera from cHA-immunized animals, suggesting that these antibodies are not induced significantly by this vaccine.
Humans are exposed to influenza viruses multiple times throughout their lifetime and therefore are likely to have preexisting memory B cells with specificities in the stalk domain. We wanted to mimic this situation in the mouse model and see if we could efficiently boost preexisting titers of stalk-reactive antibodies. Mice were preexposed to the H3 stalk domain by being sublethally infected with a recombinant influenza B virus expressing the H3 stalk domain in combination with an irrelevant globular head domain. Upon subsequent vaccination with cHA constructs containing the same stalk domain but different heads, the levels of stalk-reactive antibodies were efficiently boosted and protected mice from challenge with a panel of H3N2 influenza virus strains spanning from 1968 to 2009. Serum from vaccinated mice showed good reactivity to a wide range of H3N2 virus substrates as well as an H7N1 virus and an H7N9 HA protein substrate. Vaccinated animals also showed reduced lung titers after infection with H3N2v, H3N8, and H10N7 infection. Furthermore, the serum showed neutralizing activity and could protect mice in a passive transfer challenge experiment. These findings shed light on the mechanism of neutralization elicited by this vaccination strategy and suggest that an antibody-mediated mechanism, likely based on virus neutralization, is mediating protection. A contribution by CD8+ and CD4+ T cells to protection cannot be ruled out at this point, although transfer of serum alone was sufficient to protect from challenge. Enhanced pathogenicity induced by nonneutralizing cross-reactive anti-influenza antibodies has been proposed as a possible reason for the high pathogenicity of the novel Chinese H7N9 virus in the elderly (44). We did not observe any enhanced pathogenicity in cHA-vaccinated animals that had high titers of cross-neutralizing antibodies. In fact the animals were protected from morbidity and mortality and the virus was cleared faster.
The present experiments were designed as proof of principle to show that protection against group 2 HA-expressing viruses can be mediated by stalk-reactive antibodies alone. A human vaccine strategy, however, would likely be based on either inactivated or attenuated viruses that express cHA structures in combination with a functional neuraminidase and all internal proteins of the influenza virus. We believe that the replacement of the globular head domain of H3 HA, to which humans have memory responses, by an “exotic” irrelevant head domain to which humans are naive would, in addition to boosting stalk-reactive antibodies (24, 30, 32, 45–47), enhance levels of antibodies directed against the NA. Furthermore, the presence of internal proteins with strong T-cell epitopes would ensure that also the cellular arm of the immune response is activated and likely to contribute to protection. As mentioned above, such a vaccine would include a group 1, group 2, and B stalk component to ensure broad protection against all circulating human influenza virus strains as well as against potential pandemic subtypes. Since most humans, with the exception of very young children, have a preexisting immunity to influenza viruses, including low levels of antibodies to the stalk domain of the HA, we would imagine that vaccination with a trivalent chimeric HA vaccine could sufficiently boost these titers to protective levels.
ACKNOWLEDGMENTS
We thank Juan Ayllon for assistance with imaging, Natalie Pica for helpful discussions, Nicole Bouvier for the H3N2v virus, and Chen Wang for excellent technical support.
F. Krammer was supported by an Erwin Schrödinger fellowship (J 3232) from the Austrian Science Fund (FWF). N. S. Heaton was supported by training grant T32 AI07647-13. This work was partially supported by Centers for Excellence for Influenza Research and Surveillance (CEIRS) grant HHSN26620070010C, NIH program project grant 1P01AI097092-01A1, and PATH.
Footnotes
Published ahead of print 31 July 2013
REFERENCES
- 1.Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, Zheng NY, Mays I, Garman L, Helms C, James J, Air GM, Capra JD, Ahmed R, Wilson PC. 2008. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature 453:667–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moody MA, Zhang R, Walter EB, Woods CW, Ginsburg GS, McClain MT, Denny TN, Chen X, Munshaw S, Marshall DJ, Whitesides JF, Drinker MS, Amos JD, Gurley TC, Eudailey JA, Foulger A, DeRosa KR, Parks R, Meyerhoff RR, Yu JS, Kozink DM, Barefoot BE, Ramsburg EA, Khurana S, Golding H, Vandergrift NA, Alam SM, Tomaras GD, Kepler TB, Kelsoe G, Liao HX, Haynes BF. 2011. H3N2 influenza infection elicits more cross-reactive and less clonally expanded anti-hemagglutinin antibodies than influenza vaccination. PLoS One 6:e25797. 10.1371/journal.pone.0025797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Margine I, Hai R, Albrecht RA, Obermoser G, Harrod AC, Banchereau J, Palucka K, García-Sastre A, Palese P, Treanor JJ, Krammer F. 2013. H3N2 influenza virus infection induces broadly reactive hemagglutinin stalk antibodies in humans and mice. J. Virol. 87:4728–4737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerdil C. 2003. The annual production cycle for influenza vaccine. Vaccine 21:1776–1779 [DOI] [PubMed] [Google Scholar]
- 5.Klimov A, Simonsen L, Fukuda K, Cox N. 1999. Surveillance and impact of influenza in the United States. Vaccine 17(Suppl 1):S42–S46 [DOI] [PubMed] [Google Scholar]
- 6.Bridges CB, Thompson WW, Meltzer MI, Reeve GR, Talamonti WJ, Cox NJ, Lilac HA, Hall H, Klimov A, Fukuda K. 2000. Effectiveness and cost-benefit of influenza vaccination of healthy working adults: a randomized controlled trial. JAMA 284:1655–1663 [DOI] [PubMed] [Google Scholar]
- 7.Nordin J, Mullooly J, Poblete S, Strikas R, Petrucci R, Wei F, Rush B, Safirstein B, Wheeler D, Nichol KL. 2001. Influenza vaccine effectiveness in preventing hospitalizations and deaths in persons 65 years or older in Minnesota, New York, and Oregon: data from 3 health plans. J. Infect. Dis. 184:665–670 [DOI] [PubMed] [Google Scholar]
- 8.CDC 2013. Interim adjusted estimates of seasonal influenza vaccine effectiveness—United States, February 2013. MMWR Morb. Mortal. Wkly. Rep. 62:119–123 [PMC free article] [PubMed] [Google Scholar]
- 9.Ohmit SE, Petrie JG, Malosh RE, Cowling BJ, Thompson MG, Shay DK, Monto AS. 2013. Influenza vaccine effectiveness in the community and the household. Clin. Infect. Dis. 56:1363–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao R, Cao B, Hu Y, Feng Z, Wang D, Hu W, Chen J, Jie Z, Qiu H, Xu K, Xu X, Lu H, Zhu W, Gao Z, Xiang N, Shen Y, He Z, Gu Y, Zhang Z, Yang Y, Zhao X, Zhou L, Li X, Zou S, Zhang Y, Yang L, Guo J, Dong J, Li Q, Dong L, Zhu Y, Bai T, Wang S, Hao P, Yang W, Han J, Yu H, Li D, Gao GF, Wu G, Wang Y, Yuan Z, Shu Y. 2013. Human infection with a novel avian-origin influenza A (H7N9) virus. N. Engl. J. Med. 368:1888–1897 [DOI] [PubMed] [Google Scholar]
- 11.Runstadler J, Hill N, Hussein IT, Puryear W, Keogh M. 2013. Connecting the study of wild influenza with the potential for pandemic disease. Infect. Genet. Evol. 17:162–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baz M, Paskel M, Matsuoka Y, Zengel J, Cheng X, Jin H, Subbarao K. 2013. Replication and immunogenicity of swine, equine and avian H3 subtype influenza viruses in mice and ferrets. J. Virol. 87:6901–6910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yewdell JW, Webster RG, Gerhard WU. 1979. Antigenic variation in three distinct determinants of an influenza type A haemagglutinin molecule. Nature 279:246–248 [DOI] [PubMed] [Google Scholar]
- 14.Sui J, Sheehan J, Hwang WC, Bankston LA, Burchett SK, Huang CY, Liddington RC, Beigel JH, Marasco WA. 2011. Wide prevalence of heterosubtypic broadly neutralizing human anti-influenza A antibodies. Clin. Infect. Dis. 52:1003–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corti D, Suguitan AL, Pinna D, Silacci C, Fernandez-Rodriguez BM, Vanzetta F, Santos C, Luke CJ, Torres-Velez FJ, Temperton NJ, Weiss RA, Sallusto F, Subbarao K, Lanzavecchia A. 2010. Heterosubtypic neutralizing antibodies are produced by individuals immunized with a seasonal influenza vaccine. J. Clin. Invest. 120:1663–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan GS, Krammer F, Eggink D, Kongchanagul A, Moran TM, Palese P. 2012. A pan-h1 anti-hemagglutinin monoclonal antibody with potent broad-spectrum efficacyin vivo. J. Virol. 86:6179–6188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang TT, Tan GS, Hai R, Pica N, Petersen E, Moran TM, Palese P. 2010. Broadly protective monoclonal antibodies against H3 influenza viruses following sequential immunization with different hemagglutinins. PLoS Pathog. 6:e1000796. 10.1371/journal.ppat.1000796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dreyfus C, Laursen NS, Kwaks T, Zuijdgeest D, Khayat R, Ekiert DC, Lee JH, Metlagel Z, Bujny MV, Jongeneelen M, van der Vlugt R, Lamrani M, Korse HJ, Geelen E, Sahin Ö, Sieuwerts M, Brakenhoff JP, Vogels R, Li OT, Poon LL, Peiris M, Koudstaal W, Ward AB, Wilson IA, Goudsmit J, Friesen RH. 2012. Highly conserved protective epitopes on influenza B viruses. Science 337:1343–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ekiert DC, Bhabha G, Elsliger MA, Friesen RH, Jongeneelen M, Throsby M, Goudsmit J, Wilson IA. 2009. Antibody recognition of a highly conserved influenza virus epitope. Science 324:246–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ekiert DC, Friesen RH, Bhabha G, Kwaks T, Jongeneelen M, Yu W, Ophorst C, Cox F, Korse HJ, Brandenburg B, Vogels R, Brakenhoff JP, Kompier R, Koldijk MH, Cornelissen LA, Poon LL, Peiris M, Koudstaal W, Wilson IA, Goudsmit J. 2011. A highly conserved neutralizing epitope on group 2 influenza A viruses. Science 333:843–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corti D, Voss J, Gamblin SJ, Codoni G, Macagno A, Jarrossay D, Vachieri SG, Pinna D, Minola A, Vanzetta F, Silacci C, Fernandez-Rodriguez BM, Agatic G, Bianchi S, Giacchetto-Sasselli I, Calder L, Sallusto F, Collins P, Haire LF, Temperton N, Langedijk JP, Skehel JJ, Lanzavecchia A. 2011. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science 333:850–856 [DOI] [PubMed] [Google Scholar]
- 22.Sui J, Hwang WC, Perez S, Wei G, Aird D, Chen LM, Santelli E, Stec B, Cadwell G, Ali M, Wan H, Murakami A, Yammanuru A, Han T, Cox NJ, Bankston LA, Donis RO, Liddington RC, Marasco WA. 2009. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat. Struct. Mol. Biol. 16:265–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hai R, Krammer F, Tan GS, Pica N, Eggink D, Maamary J, Margine I, Albrecht RA, Palese P. 2012. Influenza viruses expressing chimeric hemagglutinins: globular head and stalk domains derived from different subtypes. J. Virol. 86:5774–5781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krammer F, Pica N, Hai R, Margine I, Palese P. 2013. Chimeric hemagglutinin influenza virus vaccine constructs elicit broadly protective stalk-specific antibodies. J. Virol. 87:6542–6550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hai R, García-Sastre A, Swayne DE, Palese P. 2011. A reassortment-incompetent live attenuated influenza virus vaccine for protection against pandemic virus strains. J. Virol. 85:6832–6843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beare AS, Schild GC, Craig JW. 1975. Trials in man with live recombinants made from A/PR/8/34 (H0 N1) and wild H3 N2 influenza viruses. Lancet ii:729–732 [DOI] [PubMed] [Google Scholar]
- 27.Joseph T, McAuliffe J, Lu B, Jin H, Kemble G, Subbarao K. 2007. Evaluation of replication and pathogenicity of avian influenza a H7 subtype viruses in a mouse model. J. Virol. 81:10558–10566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krammer F, Margine I, Tan GS, Pica N, Krause JC, Palese P. 2012. A carboxy-terminal trimerization domain stabilizes conformational epitopes on the stalk domain of soluble recombinant hemagglutinin substrates. PLoS One 7:e43603. 10.1371/journal.pone.0043603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steel J, Lowen AC, Wang TT, Yondola M, Gao Q, Haye K, García-Sastre A, Palese P. 2010. Influenza virus vaccine based on the conserved hemagglutinin stalk domain. mBio 1(1):e00018–10. 10.1128/mBio.00018-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller MS, Tsibane T, Krammer F, Hai R, Rahmat S, Basler CF, Palese P. 2013. 1976 and 2009 H1N1 influenza virus vaccines boost anti-hemagglutinin stalk antibodies in humans. J. Infect. Dis. 207:98–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evans MJ, von Hahn T, Tscherne DM, Syder AJ, Panis M, Wolk B, Hatziioannou T, McKeating JA, Bieniasz PD, Rice CM. 2007. Claudin-1 is a hepatitis C virus coreceptor required for a late step in entry. Nature 446:801–805 [DOI] [PubMed] [Google Scholar]
- 32.Pica N, Hai R, Krammer F, Wang TT, Maamary J, Eggink D, Tan GS, Krause JC, Moran T, Stein CR, Banach D, Wrammert J, Belshe RB, García-Sastre A, Palese P. 2012. Hemagglutinin stalk antibodies elicited by the 2009 pandemic influenza virus as a mechanism for the extinction of seasonal H1N1 viruses. Proc. Natl. Acad. Sci. U. S. A. 109:2573–2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krammer F, Pica N, Hai R, Tan GS, Palese P. 2012. Hemagglutinin stalk-reactive antibodies are boosted following sequential infection with seasonal and pandemic H1N1 influenza virus in mice. J. Virol. 86:10302–10307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.CDC 2012. Notes from the field: outbreak of influenza A (H3N2) virus among persons and swine at a county fair—Indiana, July 2012. MMWR Morb. Mortal. Wkly. Rep. 61:561. [PubMed] [Google Scholar]
- 35.Ekiert DC, Kashyap AK, Steel J, Rubrum A, Bhabha G, Khayat R, Lee JH, Dillon MA, O'Neil RE, Faynboym AM, Horowitz M, Horowitz L, Ward AB, Palese P, Webby R, Lerner RA, Bhatt RR, Wilson IA. 2012. Cross-neutralization of influenza A viruses mediated by a single antibody loop. Nature 489:526–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee PS, Yoshida R, Ekiert DC, Sakai N, Suzuki Y, Takada A, Wilson IA. 2012. Heterosubtypic antibody recognition of the influenza virus hemagglutinin receptor binding site enhanced by avidity. Proc. Natl. Acad. Sci. U. S. A. 109:17040–17045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krause JC, Tsibane T, Tumpey TM, Huffman CJ, Albrecht R, Blum DL, Ramos I, Fernandez-Sesma A, Edwards KM, García-Sastre A, Basler CF, Crowe JE. 2012. Human monoclonal antibodies to pandemic 1957 H2N2 and pandemic 1968 H3N2 influenza viruses. J. Virol. 86:6334–6340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anthony SJ, St. Leger JA, Pugliares K, Ip HS, Chan JM, Carpenter ZW, Navarrete-Macias I, Sanchez-Leon M, Saliki JT, Pedersen J, Karesh W, Daszak P, Rabadan R, Rowles T, Lipkin WI. 2012. Emergence of fatal avian influenza in New England harbor seals. mBio 3(4):e00166–12. 10.1128/mBio.00166-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kayali G, Barbour E, Dbaibo G, Tabet C, Saade M, Shaib HA, Debeauchamp J, Webby RJ. 2011. Evidence of infection with H4 and H11 avian influenza viruses among Lebanese chicken growers. PLoS One 6:e26818. 10.1371/journal.pone.0026818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arzey GG, Kirkland PD, Arzey KE, Frost M, Maywood P, Conaty S, Hurt AC, Deng YM, Iannello P, Barr I, Dwyer DE, Ratnamohan M, McPhie K, Selleck P. 2012. Influenza virus A (H10N7) in chickens and poultry abattoir workers, Australia. Emerg. Infect. Dis. 18:814–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.CDC 2012. Notes from the field: highly pathogenic avian influenza A (H7N3) virus infection in two poultry workers—Jalisco, Mexico, July 2012. MMWR Morb. Mortal. Wkly. Rep. 61:726–727 [PubMed] [Google Scholar]
- 42.Fouchier RA, Schneeberger PM, Rozendaal FW, Broekman JM, Kemink SA, Munster V, Kuiken T, Rimmelzwaan GF, Schutten M, Van Doornum GJ, Koch G, Bosman A, Koopmans M, Osterhaus AD. 2004. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc. Natl. Acad. Sci. U. S. A. 101:1356–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tweed SA, Skowronski DM, David ST, Larder A, Petric M, Lees W, Li Y, Katz J, Krajden M, Tellier R, Halpert C, Hirst M, Astell C, Lawrence D, Mak A. 2004. Human illness from avian influenza H7N3, British Columbia. Emerg. Infect. Dis. 10:2196–2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Skowronski D, Janjua N, Kwindt T, De Serres G. 2013. Virus-host interactions and the unusual age and sex distribution of human cases of influenza A(H7N9) in China, April 2013. Euro Surveill. 18:pii=20465 http://www.eurosurveillance.org/View Article.aspx? ArticleId=20465 [PubMed] [Google Scholar]
- 45.Li GM, Chiu C, Wrammert J, McCausland M, Andrews SF, Zheng NY, Lee JH, Huang M, Qu X, Edupuganti S, Mulligan M, Das SR, Yewdell JW, Mehta AK, Wilson PC, Ahmed R. 2012. Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc. Natl. Acad. Sci. U. S. A. 109:9047–9052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wrammert J, Koutsonanos D, Li GM, Edupuganti S, Sui J, Morrissey M, McCausland M, Skountzou I, Hornig M, Lipkin WI, Mehta A, Razavi B, Del Rio C, Zheng NY, Lee JH, Huang M, Ali Z, Kaur K, Andrews S, Amara RR, Wang Y, Das SR, O'Donnell CD, Yewdell JW, Subbarao K, Marasco WA, Mulligan MJ, Compans R, Ahmed R, Wilson PC. 2011. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J. Exp. Med. 208:181–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomson CA, Wang Y, Jackson LM, Olson M, Wang W, Liavonchanka A, Keleta L, Silva V, Diederich S, Jones RB, Gubbay J, Pasick J, Petric M, Jean F, Allen VG, Brown EG, Rini JM, Schrader JW. 2012. Pandemic H1N1 influenza infection and vaccination in humans induces cross-protective antibodies that target the hemagglutinin stem. Front. Immunol. 3:87. [DOI] [PMC free article] [PubMed] [Google Scholar]