Abstract
Anaerobic growth and survival are integral parts of the life cycle of many marine bacteria. To identify genes essential for the anoxic life of Dinoroseobacter shibae, a transposon library was screened for strains impaired in anaerobic denitrifying growth. Transposon insertions in 35 chromosomal and 18 plasmid genes were detected. The essential contribution of plasmid genes to anaerobic growth was confirmed with plasmid-cured D. shibae strains. A combined transcriptome and proteome approach identified oxygen tension-regulated genes. Transposon insertion sites of a total of 1,527 mutants without an anaerobic growth phenotype were determined to identify anaerobically induced but not essential genes. A surprisingly small overlap of only three genes (napA, phaA, and the Na+/Pi antiporter gene Dshi_0543) between anaerobically essential and induced genes was found. Interestingly, transposon mutations in genes involved in dissimilatory and assimilatory nitrate reduction (napA, nasA) and corresponding cofactor biosynthesis (genomic moaB, moeB, and dsbC and plasmid-carried dsbD and ccmH) were found to cause anaerobic growth defects. In contrast, mutation of anaerobically induced genes encoding proteins required for the later denitrification steps (nirS, nirJ, nosD), dimethyl sulfoxide reduction (dmsA1), and fermentation (pdhB1, arcA, aceE, pta, acs) did not result in decreased anaerobic growth under the conditions tested. Additional essential components (ferredoxin, cccA) of the anaerobic electron transfer chain and central metabolism (pdhB) were identified. Another surprise was the importance of sodium gradient-dependent membrane processes and genomic rearrangements via viruses, transposons, and insertion sequence elements for anaerobic growth. These processes and the observed contributions of cell envelope restructuring (lysM, mipA, fadK), C4-dicarboxylate transport (dctM1, dctM3), and protease functions to anaerobic growth require further investigation to unravel the novel underlying adaptation strategies.
INTRODUCTION
The Roseobacter clade is one of the most abundant groups of bacteria in oceans. The ecological success of the Roseobacter clade can be attributed to its broad metabolic capabilities (1, 2). One of the model organisms of the Roseobacter clade is Dinoroseobacter shibae. It is a mixotrophic bacterium that can utilize various organic carbon sources, including several carboxylic acids, glucose, glycerol, and succinate (1–3). Fluxome analyses showed that D. shibae lacks phosphofructokinase activity during growth on glucose and preferentially uses the Entner-Doudoroff pathway instead of glycolysis to metabolize sugar (4). Moreover, D. shibae can gain additional energy by aerobic anoxygenic photosynthesis but is unable to grow photoautotrophically. Annotation of the 4.4-Mb genome of D. shibae DFL12T discovered genes that indicated the use of alternative electron acceptors such as nitrate and dimethyl sulfoxide in the absence of molecular oxygen (5). In agreement, anaerobic growth by denitrification was reported recently (6). The bacterium possesses nap, nir, nor, and nos operons encoding the nitrate reductase NapAB, the nitrite reductase NirS, the nitric oxide reductase NorCB, and the nitrous oxide reductase NosZ (7). Notably, D. shibae possesses the genes encoding the periplasmic nitrate reductase NapAB instead of the genes for the membrane-localized nitrate reductase NarGHI (5, 7). Additionally, genes for high-affinity cbb3-type cytochrome c oxidases and various alternative NADH dehydrogenase systems were identified. These might also be involved in energy conversation under low-oxygen conditions (5). Various electron-donating primary dehydrogenase genes were annotated (gcd for glucose, gld for gluconate, lld and dld for lactate, glp for glycerol-3-phosphate, and fda for formate). Moreover, the capacity for substrate level phosphorylation processes, including the arginine deiminase pathway and a mixed-acid-type fermentation, can be deduced from the D. shibae genome (5).
However, the members of the anaerobic modulon remain to be experimentally defined for this important class of marine bacteria. The contribution of the five plasmids of D. shibae to these processes is completely unknown.
Here we present the identification of genes involved in the process of D. shibae adaptation to anaerobic conditions via transposon mutagenesis and combined transcriptome and proteome analyses. Chromosomal and plasmid genes were found to be essential. Only a small overlap between the genes found necessary for anaerobic growth and those induced under these conditions was detected. A novel type of anaerobic adaptation strategy was deduced.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and plasmid transfer.
The type strain D. shibae DFL12T (3) was cultured aerobically in Marine Bouillon (MB; Roth, Karlsruhe, Germany) at 30°C in bottle flasks shaking at 200 rpm in the dark. The mariner transposon located on plasmid pBT20 (8) (see Fig. S1 in the supplemental material) was used for transposon mutagenesis of D. shibae DFL12T. For selection of D. shibae mutants, 80 μg/ml gentamicin was added after conjugation to half-concentrated MB (hMB) (6). Escherichia coli ST18, a ΔhemA mutant of E. coli S17, served as the donor strain for the conjugative transfer of plasmid DNA (9). Luria-Bertani (LB) medium (Roth, Karlsruhe, Germany) supplemented with 50 μg/ml aminolevulinic acid and adjusted to pH 7 was used for its cultivation at 37°C and 200 rpm. For solid medium, agar was added to a final concentration of 1.5% (wt/vol).
Conjugative plasmid transfer into D. shibae DFL12T was performed as described previously (6), with modifications (see the supplemental material). For selection of D. shibae transposon mutants with an anaerobic growth deficiency, all clones were cultivated aerobically and anaerobically at 30°C in 96-well plates with hMB supplemented with 80 μg/μl gentamicin, respectively. For anaerobic cultivation, 25 mM nitrate was added. Growth was monitored by measurement of optical density at 595 nm (OD595) in a microtiter plate reader (model 680; Bio-Rad, Munich, Germany). Strains showing growth deficiencies under anaerobic conditions were isolated for further study. The growth behavior of the selected D. shibae DFL12T transposon mutants was analyzed aerobically and anaerobically in artificial seawater medium (SWM) with 16.9 mM succinate, respectively (10). For anaerobic cultivation, 25 mM nitrate was added. The cultivation occurred in 48-well flower plates (m2p-labs GmbH, Baesweiler/Aachen, Germany) at 30°C for 60 h at 800 rpm in a parallel bioreactor system (Biolector-type Micro Fermentation System; m2p-labs GmbH, Baesweiler/Aachen, Germany). Every hour, the OD620, the pH, and the oxygen partial pressure were measured automatically.
Identification of transposon integration site.
First an arbitrary PCR protocol was established as described by O'Toole and coworkers (11). For this purpose, two different PCR analyses were performed. The first PCR included the genomic DNA from a grown transposon mutant colony and primer 1 (oJG016, 5′-TCT ACG TGC AAG CAG ATT ACG GTG AC-3′), which hybridized to the transposon DNA. Random primer 2 (oJG007, 5′-GGC CAC GCG TCG ACT AGT CAN NNN NNN NNN GAT AT-3′) and primer 3 (oJG008, 5′-GGC CAC GCG TCG ACT AGT CAN NNN NNN NNN GAT CC-3′) were added. The initial incubation at 95°C (5 min) was followed by six cycles of DNA denaturation at 94°C (30 s), annealing at 30°C (30 s), and elongation at 70°C (1 min). In the second part of the PCR, the annealing temperature was increased to 45°C for a further 30 cycles, followed by a final elongation phase at 72°C (5 min). The second PCR involved primer 4 (oJG005, 5′-GAT ATC GAC CCA AGT ACC GCC ACC TA-3′) and primer 5 (oJG009, 5′-GGC CAC GCG TCG ACT AGT AC-3′). The conditions used were chosen according to the first PCR protocol. PCR products were subjected to DNA sequence determination. The resulting FASTA sequences were aligned with the genome sequence of D. shibae DFL12T (GenBank accession numbers NC_009952 and NC_009955-59).
Cultivation of D. shibae in a chemostat.
Continuous cultivation of D. shibae DFL12T for transcriptome and proteome analyses was performed with SWM (10) and an Infors HT Multifor 2 bioreactor (Infors, Bottmingen, Switzerland) at 30°C, pH 8.0, with aeration at 0.7 liter of air/min and a stirring speed of 150 rpm. The bioreactor had a working volume of 1 liter. The pH was adjusted automatically with 500 mM H3PO4 and 500 mM NaOH. At steady state, the oxygen saturation of the culture in the bioreactor was stabilized at approximately 85%. To avoid aerobic anoxygenic photosynthesis of D. shibae during the experiment, the chemostat was protected from light by covering with aluminum foil. The bioreactor was inoculated to a starting OD578 of 0.02 with an appropriate preculture. Feeding with fresh medium was started after the culture reached an OD578 of 0.5. The dilution rate was 0.1 h−1, establishing a half-maximum growth rate of D. shibae in the exponential phase. The anaerobic shift was initialized after 20 h of continuous cultivation by stopping the aeration. The oxygen concentration in the reactor was determined with an InPro 6820 oxygen electrode (Mettler Toledo, Gießen, Germany), as well as with a sensor spot O2 (PreSence, Regensburg, Germany). Anaerobic conditions were reached after approximately 20 min.
DNA microarray experiments and data analysis.
A customized whole-genome DNA microarray (8,000-by-15,000 format; Agilent, Santa Clara, CA) containing three different 60-nucleotide oligonucleotides covering 96% of the genes of D. shibae DFL12T was designed with the eArray platform from Agilent and used as described before (10). The investigated time points were 0 and 30 min after the oxygen supply had been switched off. Two micrograms of isolated total cellular RNA was labeled with either Cy3 or Cy5 with the ULS fluorescent labeling kit for Agilent arrays (Kreatech, Amsterdam, the Netherlands) according to the manufacturer's manual. Subsequently, 300 ng of each labeled RNA was pooled, fragmented, and hybridized according to the “two-color microarray” protocol from Agilent. The DNA microarrays were scanned with an Agilent C scanner with the Agilent scan control 8.4.1 software and the feature extraction 10.7.3.1 software. Data processing was performed in the R environment (http://www.cran.r-project.org/) with the limma package, the BioBASE package, and the gplots package of Bioconductor project q (http://www.bioconductor.org/) (12, 13). Three biological and three technical replicates were performed. Only genes with a logarithmic change of >0.8 in their expression between aerobic (0 min) and anaerobic (30 min) conditions and a P value of <0.05 were considered in subsequent analyses.
Shotgun proteome analysis by nanoliquid chromatography (nanoLC)-electrospray ionization (ESI) tandem mass spectrometry (MS).
Cell pellets of approximately 50 mg (wet weight) from bioreactor growth were resuspended in 200 μl lysis buffer, and cells were disrupted with the PlusOne grinding kit (GE Healthcare, Munich, Germany) as described before (14). Protein concentrations were determined as described before (15). Following the reduction and alkylation of 50 μg total cellular protein, proteolytic digestion was performed overnight with 0.5 μg trypsin GOLD (Promega, Mannheim, Germany). Finally, 1 μg of digested protein was separated with an UltiMate 3000 nanoLC system (Thermo Scientific, Bremen, Germany) by applying a linear gradient of increasing acetonitrile concentrations over 215 min coupled online to an ESI ion trap mass spectrometer (amaZon ETD; Bruker Daltonik GmbH, Bremen, Germany) as described before (14). Three biological replicates were analyzed. Protein identification was performed with ProteinScape (version 3.0; Bruker Daltonik GmbH) on a Mascot server (version 2.3; Matrix Science Ltd., London, United Kingdom) by searching against a genomic database of D. shibae DFL12T translated into amino acid sequences by using a target-decoy strategy. Searching was restricted to doubly and triply charged peptides. A false-discovery rate of <1.0% was set. Only peptides with a mascot score of >25 were considered for protein identification.
Analysis of the membrane protein-enriched fraction by nanoLC-ESI MS.
Preparation and SDS-PAGE separation of the membrane protein-enriched fraction were performed as described recently (14). For each sample, one gel lane was cut into 11 slices that were further cut into smaller pieces for washing, reduction, alkylation, and tryptic digestion as described before (14). Separation of the peptides generated was performed by UltiMate 3000 nanoLC (Thermo Scientific, Bremen, Germany) with a 95-min linear gradient of increasing acetonitrile concentrations (14). Mass spectrometric analysis of the LC eluent was performed with an online-coupled ion trap mass spectrometer (amazon ETD; Bruker Daltonik GmbH) as described before (14). Protein identification was performed as outlined above.
Plasmid curing of D. shibae.
D. shibae DSM 16493T was cured of 191-kb plasmid pDSHI01 (NC_009955.1) as recently described (16). The RepABC-9-type 4,500-bp replication module, which encodes the replicase gene, the origin of replication (oriV), the parAB partitioning operon, and the putative cis-acting palindromic anchor sequence 5′-AAACTCCAATCTTGAACGCGTTCAAGATTGGAGTTT-3′ (17), was amplified with primers P046 (5′-GACCGGCGCTGGCTACTTCAC-3′) and P047 (5′-TCACAAAACCCGAAGGACACT-3′). The PCR product was cloned into the SmaI site of a pBluescript SK+ vector containing an additional gentamicin resistance cassette (18). Complete DNA sequencing of the 4.5-kb insert revealed the integrity of the replication module. The preparation of electrocompetent D. shibae cells and transformation of the plasmid containing the RepABC-9-type replication module construct were conducted as described before (19). The transformants were plated on marine broth medium with 40 μg/ml gentamicin and streaked an additional three times. The successful elimination of the original 191-kb plasmid was verified via PCR with purified plasmid DNA (NucleoSpin Plasmid DNA kit; Macherey-Nagel) and the following primer combinations for all five extrachromosomal elements of D. shibae: pDSHI01 (191 kb), P430 (5′-TCTGGCTGCGTGGTGGCTTTC-3′) and P431 (5′-TGCGCTATAGTGCTCTCAACA-3′); pDSHI02 (153 kb), P252 (5′-CCAAGGGGCGGCGGGAGATGC-3′) and P253 (5′-CGCACGCCGCCCAGTTCTTCG-3′); pDSHI03 (126 kb), P432 (5′-GGCACCATCGTCGGAACCAAT-3′) and P433 (5′-TGGTATCAGGCATTCGCTTCA-3′); pDSHI04 (86 kb), P421 (5′-GATTTTGAAACGGGCATTGAT-3′) and P422 (5′-TATAGAATTCGCGGATAGAAGGGGGTGGTTT-3′); pDSHI05 (72 kb), P562 (5′-ATGGCGACGCAGAAGAAGGTT-3′) and P563 (5′-AAGACACCAGCCCCGCCACAT-3′). Single colonies of strains of interest were streaked onto MB medium without the addition of antibiotics. The procedure was repeated five times for the spontaneous loss of the RepABC-9 replication module-containing vector (17). Loss of the plasmid was confirmed by the absence of the gentamicin cassette. That was verified by PCR with primers P024 (5′-GGAAACGGATGAAGGCACCAA-3′) and P025 (5′-GCCCAGCGCCAGCAGGAAC-3′). The resulting D. shibae Δ191-kb plasmid-cured mutant was subsequently used for growth experiments under aerobic and anaerobic conditions. Curing of the 86-kb plasmid was performed analogously.
Microarray data accession number.
The data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus (20) and are accessible under GEO Series accession number GSE47445.
RESULTS AND DISCUSSION
Rationale of the approach used.
Mariner-based transposon mutagenesis, in combination with PCR-based integration site determination and anaerobic growth phenotype testing, was used to identify genes essential for anaerobic growth of D. shibae under denitrifying conditions. Furthermore, the transposon integration sites of most of the transposon mutants obtained were determined to allow the identification of mutations in known genes involved in anaerobic metabolism with no anaerobic growth phenotype. The resulting representative transposon mutant collection of D. shibae will be made available to other researches in the field. Furthermore, anaerobically expressed genes and formed proteins were identified by a combined transcriptomic and proteomic approach. The results obtained were compared and discussed in light of the currently available literature. A molecular strategy of D. shibae adaptation to anaerobic growth conditions was deduced.
Transposon mutagenesis, chemostat cultivation, and transcriptome and proteome analyses.
Transposon mutagenesis of D. shibae was performed with the mariner transposon localized on plasmid pBT20 (8). The loci of transposon integration into the chromosome and plasmids were determined by a PCR-based approach (11). Only single-transposon-carrying strains were subjected to further analyses. For details, see the supplemental material.
A total of 4,500 D. shibae transposon mutants were isolated and further screened for growth defects under anaerobic denitrifying conditions. Random integration of the transposon was observed (Fig. 1). Of the 1,580 transposon mutants sequenced, 1,134 showed different loci of integration (see Table S1 in the supplemental material). Taking approximately 12% of the essential genes into account, the saturation of mutagenesis reached 82% of the genome. Fifty-three mutants, 35 with transposon integration into chromosomal genes and 18 with transposon integration into plasmid genes, showed significantly decrease or even loss of anaerobic growth (Table 1). Clearly, complementation experiments are required to ultimately confirm that the observed loci of transposon integration are responsible for the observed phenotype. For details, see the supplemental material.
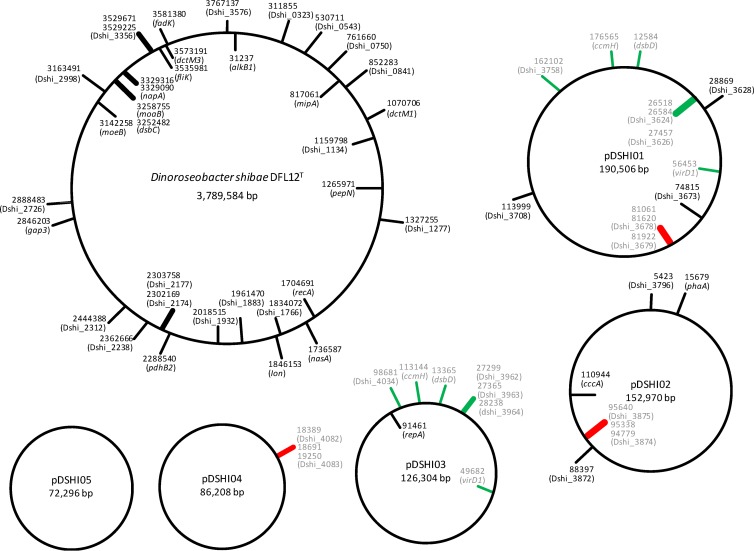
Fig 1.
Genomic distribution of transposon insertion sites. Shown are the chromosomal and extrachromosomal DNAs of D. shibae. Labels on the exterior of a circle specify the loci of insertion in the plus orientation. Marks on the circle interior show insertions in the minus orientation. Numbers denote points of insertion according to the chromosome annotation of D. shibae DFL12T (RefSeq numbers: NC_009952.1, NC_009955.1, NC_009956.1, NC_009957.1, NC_009958.1, and NC_009959.1).
Table 1.
D. shibae DFL12T transposon mutants showing decreased anaerobic growth under denitrifying conditions
| Locus tag | Function of gene product | Gene name | Position in ORFa | Integration position(s) | TDb | Growthc |
|
|---|---|---|---|---|---|---|---|
| Aerobic | Anaerobic | ||||||
| Nitrate reductases and electron transfer | |||||||
| Dshi_1669 | Nitrate reductase | nasA | (283) 2622 | 1736587, chromosome | F | 2 | 0–1 |
| Dshi_3165 | Periplasmic nitrate reductase | napA | (1875) 2496 | 3329316, chromosome | R | 2 | 0 |
| Dshi_3165 | Periplasmic nitrate reductase | napA | (2101) 2496 | 3329090, chromosome | R | 2 | 0 |
| Dshi_0323 | Ferredoxin | (65) 624 | 311855, chromosome | F | 2 | 0 | |
| Molybdopterin biosynthesis and cytochrome c biogenesis | |||||||
| Dshi_1932 | Putative glutathione S-transferase | (703) 870 | 2018515, chromosome | R | 0–1 | 0 | |
| Dshi_2974 | Molybdopterin biosynthesis protein | moeB | (427) 1041 | 3142258, chromosome | R | 2 | 0 |
| Dshi_3089 | Molybdopterin biosynthesis protein | moaB | (475) 543 | 3258755, chromosome | R | 2 | 1 |
| Dshi_3082 | Putative c-type cytochrome biosynthesis protein | dsbC | (709) 810 | 3252482, chromosome | R | 2 | 0 |
| Dshi_3606Dshi_3944 | Cytochrome c biogenesis protein transmembrane region | dsbD | (385) 723 | 12584, pDSHI01; 13365, pDSHI03 | F | 0–1 | 0 |
| Dshi_3887 | Class I cytochrome c | cccA | (150) 432 | 110944, pDSHI02 | R | 2 | 0 |
| Dshi_3777Dshi_4053 | Cytochrome c biogenesis protein | ccmH | (269) 474 | 176565, pDSHI01; 113144, pDSHI03 | F | 2 | 0 |
| Na+-dependent processes | |||||||
| Dshi_0543 | Na+/Pi cotransporter | (765) 1851 | 530711, chromosome | F | 2 | 0 | |
| Dshi_1037 | TRAP dicarboxylate transporter | dctM1 | (1268) 1305 | 1070706, chromosome | F | 2 | 0 |
| Dshi_1195 | TRAP transporter solute receptor | (430) 993 | 1234801, chromosome | R | 2 | 0 | |
| Dshi_2998 | Putative mechanosensitive ion channel | (1158) 2481 | 3163491, chromosome | F | 2 | 0 | |
| Dshi_3395 | C4-dicarboxylate transport system permease (DctM subunit) | dctM3 | (1342) 1449 | 3573191, chromosome | R | 2 | 0–1 |
| Dshi_3708 | AraC-like ligand binding domain | (227) 837 | 113999, pDSHI01 | F | 2 | 0 | |
| Dshi_3805 | NADH dehydrogenase | phaA | (2761) 2910 | 15679, pDSHI02 | F | 2 | 0 |
| Peptidases | |||||||
| Dshi_0841 | Hypothetical protein | (−3) 1029 | 852283, chromosome | F | 2 | 0 | |
| Dshi_1223 | Aminopeptidase N | pepN | (877) 2556 | 1265975, chromosome | R | 2 | 0 |
| Dshi_1777 | ATP-dependent protease | (874) 2409 | 1846153, chromosome | F | 2 | 0 | |
| Dshi_1883 | Putative ClpA/ClpB family protein | (15) 843 | 1961470, chromosome | R | 1 | 1 | |
| Dshi_3625Dshi_3963 | Hypothetical protein | (−17) 566 | 26584, pDSHI01; 27365, pDSHI03 | F | 2 | 0 | |
| Dshi_3872 | Hemolysin-type calcium-binding protein | (43) 5688 | 88397, pDSHI02 | F | 2 | 1 | |
| Central metabolism | |||||||
| Dshi_1134 | 3-Oxo acid-CoA-transferase (B subunit) | (460) 627 | 1159798, chromosome | R | 2 | 0 | |
| Dshi_2159 | Pyruvate dehydrogenase | pdhB2 | (677) 1356 | 2288540, chromosome | F | 1 | 0 |
| Phages, transposons, and DNA restructuring | |||||||
| Dshi_1643 | Bacterial DNA recombination | recA | (1067) 1068 | 1704691, chromosome | R | 0–1 | 0 |
| Dshi_2174 | Putative phage capsid protein | (446) 1218 | 2302169, chromosome | R | 2 | 0 | |
| Dshi_2177 | Phage portal protein, HK97 family | (931) 1191 | 2303758, chromosome | R | 2 | 0–1 | |
| Dshi_2312 | Type I restriction-modification system (R subunit), double-stranded DNase | (511) 516 | 2444388, chromosome | F | 2 | 1 | |
| Dshi_3356 | Transposase | (−424) 267 | 3529671, chromosome | F | 2 | 0 | |
| Dshi_3356 | Transposase | (−870) 267 | 3529225, chromosome | F | 2 | 1 | |
| Dshi_3655 Dshi_3988 | Type VI secretion system protein, TraG/TraD family protein | virD1 | (911) 2004 (912) 2001 | 56453, pDshi01; 49682, pDshi03 | R | 2 | 0 |
| Dshi_3758Dshi_4034 | Transposase | (80) 390 | 162102, pDSHI01; 98681, pDSHI03 | F | 2 | 0 | |
| Dshi_3679Dshi_3875 Dshi_4082 | Integrase catalytic region (transposase) | (1411) 1494 | 81922, pDSHI01 95640, pDSHI02 18389, pDSHI04 | F, R, R | 2 | 0 | |
| Dshi_3678 Dshi_3874 Dshi_4083 | ATP-binding protein, putative transposase | (782)825 | 81061, pDSHI01; 94779, pDSHI02; 19250, pDSHI04 | F, R, R | 2 | 0 | |
| Dshi_3678Dshi_3874 Dshi_4083 | ATP-binding protein, putative transposase | (223) 825 | 81620, pDSHI01; 95338, pDSHI02; 18691, pDSHI04 | F, R, R | 0–1 | 0 | |
| Dshi_4023 | Plasmid partitioning protein RepA | repA | (−173) 1188 | 91461, pDSHI03 | R | 2 | 0 |
| Cell envelope | |||||||
| Dshi_0027 | Fatty acid desaturase | alkB2 | (570) 1155 | 31237, chromosome | R | 2 | 0 |
| Dshi_0808 | Membrane-bound transglycosylase and penicillin-binding protein | mipA | (−11) 744 | 817061, chromosome | R | 2 | 0 |
| Dshi_1766 | Pepidoglycan-binding protein LysM | lysM | (103) 1596 | 1834072, chromosome | R | 2 | 0 |
| Dshi_2238 | Periplasmic binding protein/LacI transcriptional regulator | (676) 1035 | 2362666, chromosome | F | 2 | 1 | |
| Dshi_3403 | AMP-dependent synthetase and ligase | fadK | (1114) 1740 | 3581380, chromosome | F | 2 | 0 |
| Dshi_3576 | Glycosyl transferase family 2 | (1223) 1233 | 3767137, chromosome | F | 2 | 0 | |
| Dshi_3628 | Bacterial outer membrane protein | (113) 696 | 28869, pDshi01 | F | 2 | 0 | |
| Transport | |||||||
| Dshi_3624Dshi_3962 | Co/Zn/Cd efflux system component | (607) 621 | 26518, pDSHI01; 27299, pDSHI03 | F | 2 | 0 | |
| Dshi_3626Dshi_3964 | Co/Zn/Cd resistance protein | (303) 966 | 27457, pDSHI01; 28238, pDSHI03 | F | 2 | 0 | |
| Dshi_3796 | ABC transporter (importer) ATP-binding protein | oppD | (686) 1002 | 5423, pDSHI02 | F | 0–1 | 0 |
| Other | |||||||
| Dshi_0750 | Conserved hypothetical protein | (665) 795 | 761660, chromosome | F | 2 | 1 | |
| Dshi_1277 | Hypothetical protein | (181) 243 | 1327255, chromosome | F | 2 | 0 | |
| Dshi_2726 | Hypothetical protein | (157) 405 | 2888483, chromosome | F | 2 | 1 | |
| Dshi_3364 | Flagellar hook length control protein | fliK | (314) 2337 | 3535981, chromosome | R | 2 | 0 |
| Dshi_3673 | Hypothetical protein | (176) 969 | 74815, pDSHI01 | R | 2 | 0–1 | |
ORF, open reading frame.
TD, transposon direction; F, forward; R, reverse.
The number 2 stands for normal growth, 1 stands for decreased growth, and 0 stands for no growth.
For transcriptome and proteome analyses, chemostat cultivation with a standardized protocol for the shift from aerobic to anaerobic conditions was developed. The transcriptome analysis revealed 474 genes differentially expressed during the shift from aerobic to anaerobic conditions, with 207 showing an increase in expression and 267 showing a decrease. The proteome analyses detected 878 different proteins by the whole-cell protein shotgun approach and 1,215 different proteins in the membrane fraction covering approximately 25% of the predicted D. shibae proteins. The results of the various experimental approaches were interpreted and are discussed in the light of their functional consequences below.
Plasmids are essential for anaerobic growth of D. shibae.
Besides the chromosome, D. shibae DFL12T contains five plasmids (5). The results of transposon mutagenesis revealed an unexpected impact of these plasmids on the anaerobic growth of D. shibae. Both sister plasmids pDSHI01 and pDSHI03 and plasmid pDSHI02 seemed to be essential for anaerobic growth (Table 1). No transposon mutation affecting anaerobic growth was found in plasmid pDSHI05.
In order to unambiguously demonstrate the contribution of the plasmid genes to anaerobic growth, plasmid-deficient D. shibae strains were generated. The strains were cured of plasmids pDSH01 and pDSH04 and tested for aerobic versus anaerobic growth. Both plasmid-cured D. shibae strains had lost the ability to grow anaerobically. These observations clearly demonstrate the requirement of plasmid-provided genetic information for anaerobic growth.
Denitrification is induced, but only nitrate reduction is essential for anaerobic growth.
Under anaerobic conditions, D. shibae is able to grow via denitrification with nitrate, nitrite, NO, and N2O as terminal electron acceptors (6). The first step of denitrification is the reduction of nitrate to nitrite (7). Accordingly, napA (Dshi_3165), which encodes the catalytic subunit of the periplasmic dissimilatory nitrate reductase NapAB, was identified by transposon mutagenesis as one of the essential genes under anaerobic denitrifying conditions (Table 1). Nap is encoded by the napFDAGHBC operon (Dshi_3161 to Dshi_3167). The expression of the operon was found to be slightly induced upon oxygen depletion in the transcriptome analysis. The NapA protein was also detected under aerobic, as well as under anaerobic, conditions in the proteome analyses (see Table S2 in the supplemental material). D. shibae possesses only periplasmic NapAB and not the membrane-spanning NarGHI nitrate reductase (5). Obviously, in the two napA mutants obtained, energy conservation via nitrate respiration became limiting. The later steps of denitrification cannot substitute for the process because nitrite, NO, and N2O production is missing from this mutant. Consequently, mutations in this gene led to a lethal phenotype under anaerobic conditions (Table 1). Similar observations of an essential role for the NapAB nitrate reductase for denitrification and anaerobic growth were recently made for the Magnetospirillum gryphiswaldense enzyme (21).
Several transposon insertions in other genes encoding enzymes of the denitrification pathway were found to have no effect on anaerobic growth (see Table S2). For example, mutations in the nitrite reductase-encoding gene nirS (Dshi_3180) and the nitrous oxide reductase maturation protein-encoding gene nosD (Dshi_3195) failed to cause a lethal phenotype under anaerobic denitrifying conditions. Similarly, the defect in the nitrous oxide regulator-encoding gene nosR2 (Dshi_3181) did not lead to any growth defect. However, all of these genes (napH, napF, nirS, and nosD) were found to be induced under denitrifying conditions. The corresponding proteins were also found to be abundant under anaerobic conditions in the proteomic investigation (see Table S2).
Surprisingly, mutation of the assimilatory NADH-dependent nitrate reductase-encoding gene nasA (Dshi_1669) also led to a significant decrease in anaerobic growth (Table 1). The nasA gene is localized upstream of the nasDE genes, which encode the assimilatory nitrite reductase. During nitrogen assimilation, ammonium is generated through the reduction of nitrate via nitrite in the cytoplasm (22). However, the growth medium was supplemented with sufficient ammonium during the selection process, excluding a general defect in nitrogen metabolism. In agreement, no aerobic phenotype was observed. Furthermore, mutation of the nasD gene (Dshi_1667), which encode one of the assimilatory nitrite reductase subunits, did not result in an anaerobic growth phenotype under denitrifying conditions (see Table S2). Nevertheless, the amount of transcript of the whole nas operon did not change between aerobic and anaerobic conditions. In conclusion, the observed anaerobic growth phenotype of the nasA mutant in the presence of ammonium underscores the importance of this enzyme for dissimilatory denitrifying growth. Alternatively, nitrite may have a novel, as-yet-unknown, function under anaerobic growth conditions.
Molybdopterin cofactor biosynthesis for nitrate reductase formation is essential under anaerobic growth conditions.
The nitrate reductase NapAB contains a molybdopterin cofactor (Moco), iron-sulfur clusters, and a cytochrome c subunit. The nitrate reductase NasA is also an iron-sulfur cluster and a Moco-containing enzyme (23). Consequently, nitrate reductase formation in general requires the biosynthesis of cofactors, including molybdopterin, heme, and iron-sulfur clusters (24, 25). Therefore, it was not surprising that transposon mutants with defects in moeB (Dshi_2974) and moaB (Dshi_3089), which encode enzymes involved in molybdopterin biosynthesis, were not able to grow under denitrifying conditions (Table 1). However, the expression of both genes was found not to be induced under anaerobic conditions. The MoeB protein was observed exclusively under anaerobic conditions in the proteome of D. shibae, suggesting posttranscriptional control of MoeB formation (see Table S2). During Moco biosynthesis, MoeB catalyzes the adenylation of the MoaD subunit of the molybdopterin synthase MoaDE (26, 27). MoaB catalyzes the adenylation of the metal-binding pterin to prepare for molybdenum insertion (28). How is Moco made in D. shibae under anaerobic conditions without MoeB and MoaB? The structural homologue MogA substitutes for MoaB function in other organisms (28). However, the potential mogA gene (Dshi_0119) of D. shibae encodes a Moco-binding protein rather than a real MogA protein. This leaves the question of aerobic Moco biosynthesis without MoeB and MoaB open. The unaffected growth of both mutants under aerobic conditions suggested that other, as-yet-unknown, enzymes of D. shibae complement the defect or that D. shibae does not have an essential Moco-dependent enzyme under the aerobic growth conditions tested.
Mutations affecting cytochrome c and disulfide bond formation.
The nitrate reductase NapAB and the nitrite reductase NirS both require cytochrome c as a cofactor and electron transfer molecule. The nitrate reductase NapAB was shown to be essential for anaerobic growth (see above). Mutations in Dshi_3082 (dsbC), plasmid-carried Dshi_3606/3944 (potential dsbD), and Dshi_3777/4053 (ccmH) had defects in genes involved in disulfide bond formation and cytochrome c formation. The disulfide bond formation machinery is part of cytochrome c formation. Mutations in these genes produced a loss of anaerobic growth, two of them with no influence on aerobic growth (dsbC, ccmH). One mutation (dsbD) was also found to reduce aerobic growth. D. shibae possesses two identical cytochrome c biosynthesis gene clusters (ccmFGHI-dsbD) on sister plasmids pDSHI01 and pDSHI03. Consequently, the exact localization of the transposon insertion site was not possible by the sequencing approach used. As a consequence, the DNA microarray approach used cannot distinguish between the identical dsbD and ccmH genes. However, neither of the clusters was differentially expressed during aerobic and anaerobic growth. In agreement, the protein CcmH was detected under aerobic conditions and under anaerobic denitrifying conditions (see Table S2). The distinct behavior of the isolated mutants indicated that only one of the clusters is functional because they obviously were not able to compensate for each other. For further descriptions of the various systems of disulfide bond and cytochrome c formation, see the supplemental material. Multiple mutations in other genes of the cytochrome c biogenesis pathway did not result in an aerobic or anaerobic phenotype (see Table S1). Obviously, plasmid-carried D. shibae ccmH and most likely dsbC/dsbD are essential for the formation of the anaerobic cytochrome c biogenesis machinery.
Plasmid-encoded cytochrome c is essential for anaerobic growth in D. shibae.
One transposon was found integrated in Dshi_3887 localized on plasmid pDSHI02, which contains the class I cytochrome-encoding gene cccA. cccA gene expression was found to be slightly enhanced under anaerobic growth conditions, indicating a role under denitrifying conditions. Class I cytochrome c molecules are small soluble cytochromes that are needed for electron transfer reactions during denitrification in other bacteria (7). In Neisseria gonorrhoeae, the cccA gene product cytochrome c2 is essential for the shuttling of electrons toward the denitrification machinery (29). Several other unclassified class I cytochromes of D. shibae (Dshi_0508, Dshi_2868) did not influence anaerobic growth (see Table S1). The expression of these genes was found to be downregulated or unaffected (Table S2). Obviously, the transposon has identified an essential cytochrome involved in the initial steps of denitrification.
One of three pyruvate dehydrogenases is essential for anaerobic growth.
A transposon mutation in gene pdh2 (Dshi_2159), which encodes the E1 component of one of the three pyruvate dehydrogenase complexes, resulted in decreased growth under aerobic conditions and no growth under anaerobic conditions (Table 1). In general, pyruvate dehydrogenase is converted into acetyl coenzyme A (acetyl-CoA). D. shibae possesses three loci for pyruvate dehydrogenase, namely, pdhA2B1C2 (Dshi_0534-Dshi_0536), aceEFlpdA (Dshi_1968-1970), and pdhA1B2C1 (Dshi_2158-2160). In contrast to pdhB2, inactivation of pdhB1 (Dshi_0535) did not influence anaerobic growth. However, both the pdhA1B2C1 and pdhA2B1C2 operons were not found to be differentially expressed. Consequently, the functional basis of the observed phenotype remains to be determined.
Sodium-dependent transport processes are essential for anaerobic growth.
The identification of the Na+-dependent NADH dehydrogenase PhaA and Na+-dependent C4-dicarboxylate TRAP transporters (DctM1 and DctM3) as essential for the anaerobic growth of D. shibae pointed toward an important role for the Na+ gradient (Table 2; for a detailed description and discussion, see the supplemental material). Furthermore, Dshi_0543 encodes a type II Na+/Pi cotransporter similar to transporters found in Methylobacter, Campylobacter, and Helicobacter species and Pseudomonas stutzeri (30, 31). Finally, the mechanosensitive ion channel encoded by Dshi_2998 showed 33% amino acid sequence identity to E. coli YbiO (32). YbiO of E. coli revealed NaCl-induced channel activity (33). Finally, Dshi_3675 encodes a Na+-H+ exchange protein. Overall, Na+ gradient-dependent membrane-associated processes are essential for the anaerobic growth of D. shibae. This might reflect an adaptation of D. shibae to its marine habitat.
Table 2.
Comparison of growth phenotypes, fold changes in gene expression after 30 min of oxygen depletion, and presence of cytoplasmic and membrane proteins under anaerobic denitrifying conditionsa
| Locus tag | Gene name | Function of gene product | Transposon insertion | Growth phenotype | Fold change in transcription under anaerobic conditions | Presence of protein under anaerobic conditions |
|---|---|---|---|---|---|---|
| Dshi_3180 | nirS | Nitrite reductase precursor | + | 2 | 31.6 | + |
| Dshi_3192 | Hypothetical protein | + | 2 | 14.2 | 0 | |
| Dshi_3195 | nosD | Nitrous oxide maturation protein | + | 2 | 12.8 | 0 |
| Dshi_2278 | dmsA1 | Dimethyl sulfoxide reductase precursor | + | 2 | 11.7 | 0 |
| Dshi_0542 | Phosphate transporter | + | 2 | 8.3 | 0 | |
| Dshi_3173 | nirJ | Putative nitrite reductase heme biosynthesis J protein | + | 2 | 6.6 | 0 |
| Dshi_2304 | Putative regulator of cell morphogenesis and NO signaling | + | NDb | 6.2 | + | |
| Dshi_0664 | fixP | Cytochrome c oxidase, cbb3 type, subunit III | + | 2 | 5.7 | + |
| Dshi_3165 | napA | Nitrate reductase catalytic subunit | + | − | 4.4 | + |
| Dshi_0543 | Na+/Pi cotransporter | + | − | 3.5 | 0 | |
| Dshi_3152 | Protein DUF1445 of unknown function | + | 1 | 3.5 | 0 | |
| Dshi_1449 | TonB-dependent receptor | + | ND | 3.3 | 0 | |
| Dshi_3163 | napH | Ferredoxin-type protein NapH | + | 2 | 3.2 | 0 |
| Dshi_2233 | phbC | Poly-beta-hydroxybutyrate polymerase | + | 2 | 3.1 | + |
| Dshi_3558 | Hypothetical protein | + | 2 | 3.1 | + | |
| Dshi_1968 | aceE | Pyruvate dehydrogenase subunit E1 | + | 2 | 3.0 | + |
| Dshi_3066 | atoB | Acetyl-CoA acetyltransferase | + | 2 | 2.9 | + |
| Dshi_2363 | ureE | UreE urease accessory domain-containing protein | + | 2 | 2.7 | 0 |
| Dshi_0432 | arcA | Arginine deiminase | + | 2 | 2.5 | 0 |
| Dshi_3590 | NADH dehydrogenase (ubiquinone) | + | 2 | 2.1 | + | |
| Dshi_0563 | irpA | Iron-regulated protein | + | 2 | 2.1 | + |
| Dshi_2052 | Hypothetical protein | + | 2 | 2.1 | 0 | |
| Dshi_2966 | panB | 3-Methyl-2-oxobutanoate hydroxymethyltransferase | + | 2 | 2.1 | 0 |
| Dshi_0426 | Hypothetical protein | + | 2 | 2.1 | 0 | |
| Dshi_3805 | phaA | NADH dehydrogenase | + | − | 2.0 | + |
| Dshi_3249 | fliE | Flagellar hook-basal body protein FliE | + | 2 | 1.9 | 0 |
| Dshi_2965 | Hypothetical protein | + | 2 | 1.8 | 0 | |
| Dshi_3553 | acs | Acetate-CoA ligase | + | 2 | 1.8 | + |
| Dshi_0540 | NnrU family protein | + | 2 | 1.8 | + | |
| Dshi_1399 | acsA | Acetate-CoA ligase | + | 2 | 1.8 | + |
The number 2 stands for normal growth, 1 stands for decreased growth, a minus sign stands for no growth, a 0 stands for not detected, and a plus sign stands for detected. In bold are the induced genes that produced an anaerobic growth phenotype upon transposon insertion.
ND, not done.
Potential genome rearrangement as part of the anaerobic adaptation process.
Unexpectedly, several genes that encode phage-related proteins and transposases were found to be essential for the anaerobic growth of D. shibae. The gene Dshi_2174 encodes a phage capsid protein and is part of a large operon (Dshi_2176 to Dshi_2161) that encodes a complete HK97-type (pro)phage (34). These phages were reported to carry so-called morons, DNA elements that increase host fitness (35). A corresponding lambda prophage of E. coli increased mammalian host cell binding and resistance to killing (36, 37). Salmonella phages Fels-2 and GIFSY-2 carried morons encoding superoxide dismutase, which sustained bacterial fitness during host infection (38). Many morons provide resistance to phage superinfection (39–41). Inspection of the genes downstream of Dshi_2174 identified genes of unknown function between the classical phage genes, however, without providing an explanation for the observed anaerobic growth phenotype.
Another surprising observation was that the transposon mutations found in Dshi_3356, which encodes ISR1 insertion element protein A3 (42), and in Dshi_3655, which encodes a type IV secretory TRAG-type family protein involved in DNA transfer, were located in the vicinity of numerous genes whose products are predicted to be involved in DNA transport function. Similarly, Dshi_3628 and Dshi_3678 are part of inserted transposons. Furthermore, the anaerobically essential gene Dshi_3758 encodes a transposase of an IS4 element. The gene Dshi_2313 encodes an HsdR family type I DNase as part of a restriction-modification system (43). In Mycoplasma, the HsdSMR enzyme system has been shown to be activated by high-frequency gene rearrangements (44) and the whole system is controlled by proteolysis (45). Overall, genetic mobility and rearrangement, most likely involving the highly conserved areas of the plasmids are an integral part of the strategy of D. shibae adaptation to anaerobic conditions.
Anaerobic growth requires proteases, peptide transport, restructuring of the cell envelope, cation efflux proteins, and FliK.
The rest of the transposon mutants found are described and discussed in the supplemental material.
Strategy of D. shibae adaptation to anaerobic growth conditions.
Obviously, solely nitrate reductases and the corresponding cofactor formation (Moco, cytochrome c) are the crucial parts of energy conversation under anaerobic growth conditions. The residual denitrification machinery, which is significantly induced under anaerobic conditions, further sustains anaerobic growth without being essential. Some of the anaerobically essential genes are plasmid encoded. Clear evidence of the importance of a Na+ gradient for anaerobic growth of D. shibae was found. Another surprise was the essential role of genome-restructuring genes localized on phages, transposons, and insertion sequence elements. The cell envelope has to be restructured, and because a set of proteases appears to be required for anaerobic growth, this might suggest that they are linked to cell wall restructuring. Overall, new surprising insights into the adaptation of the marine model bacterium D. shibae to oxygen-limiting conditions were obtained.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by transregional Collaborative Research Consortium 51 of the Deutsche Forschungsgesellschaft.
We thank Vanessa Hering and Annika Michel for support.
Footnotes
Published ahead of print 23 August 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00860-13.
REFERENCES
- 1.Wagner-Döbler I, Biebl H. 2006. Environmental biology of the marine Roseobacter lineage. Annu. Rev. Microbiol. 60:255–280 [DOI] [PubMed] [Google Scholar]
- 2.Brinkhoff T, Giebel HA, Simon M. 2008. Diversity, ecology, and genomics of the Roseobacter clade: a short overview. Arch. Microbiol. 189:531–539 [DOI] [PubMed] [Google Scholar]
- 3.Biebl H, Allgaier M, Tindall BJ, Koblizek M, Lunsdorf H, Pukall R, Wagner-Dobler I. 2005. Dinoroseobacter shibae gen. nov., sp. nov., a new aerobic phototrophic bacterium isolated from dinoflagellates. Int. J. Syst. Evol. Microbiol. 55:1089–1096 [DOI] [PubMed] [Google Scholar]
- 4.Fürch T, Preusse M, Tomasch J, Zech H, Wagner-Döbler I, Rabus R, Wittmann C. 2009. Metabolic fluxes in the central carbon metabolism of Dinoroseobacter shibae and Phaeobacter gallaeciensis, two members of the marine Roseobacter clade. BMC Microbiol. 9:209. 10.1186/1471-2180-9-209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wagner-Döbler I, Ballhausen B, Berger M, Brinkhoff T, Buchholz I, Bunk B, Cypionka H, Daniel R, Drepper T, Gerdts G, Hahnke S, Han C, Jahn D, Kalhoefer D, Kiss H, Klenk HP, Kyrpides N, Liebl W, Liesegang H, Meincke L, Pati A, Petersen J, Piekarski T, Pommerenke C, Pradella S, Pukall R, Rabus R, Stackebrandt E, Thole S, Thompson L, Tielen P, Tomasch J, von Jan M, Wanphrut N, Wichels A, Zech H, Simon M. 2010. The complete genome sequence of the algal symbiont Dinoroseobacter shibae: a hitchhiker's guide to life in the sea. ISME J. 4:61–77 [DOI] [PubMed] [Google Scholar]
- 6.Piekarski T, Buchholz I, Drepper T, Schobert M, Wagner-Doebler I, Tielen P, Jahn D. 2009. Genetic tools for the investigation of Roseobacter clade bacteria. BMC Microbiol. 9:265. 10.1186/1471-2180-9-265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zumft WG. 1997. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev. 61:533–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kulasekara HD, Ventre I, Kulasekara BR, Lazdunski A, Filloux A, Lory S. 2005. A novel two-component system controls the expression of Pseudomonas aeruginosa fimbrial cup genes. Mol. Microbiol. 55:368–380 [DOI] [PubMed] [Google Scholar]
- 9.Thoma S, Schobert M. 2009. An improved Escherichia coli donor strain for diparental mating. FEMS Microbiol. Lett. 294:127–132 [DOI] [PubMed] [Google Scholar]
- 10.Tomasch J, Gohl R, Bunk B, Diez MS, Wagner-Dobler I. 2011. Transcriptional response of the photoheterotrophic marine bacterium Dinoroseobacter shibae to changing light regimes. ISME J. 5:1957–1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Toole GA, Pratt LA, Watnick PI, Newman DK, Weaver VB, Kolter R. 1999. Genetic approaches to study of biofilms. Methods Enzymol. 310:91–109 [DOI] [PubMed] [Google Scholar]
- 12.Smyth GK, Michaud J, Scott HS. 2005. Use of within-array replicate spots for assessing differential expression in microarray experiments. Bioinformatics 21:2067–2075 [DOI] [PubMed] [Google Scholar]
- 13.Miller JM, Alachi P. 1996. Evaluation of new computer-enhanced identification program for microorganisms: adaptation of BioBASE for identification of members of the family Enterobacteriaceae. J. Clin. Microbiol. 34:179–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zech H, Hensler M, Koßmehl S, Drüppel K, Wöhlbrand L, Trautwein K, Hulsch R, Maschmann U, Colby T, Schmidt J, Reinhardt R, Schmidt-Hohagen K, Schomburg D, Rabus R. 24 April 2013, posting date. Adaptation of Phaeobacter gallaeciensis DSM 17395 to growth with complex nutrients. Proteomics (Epub ahead of print.) 10.1002/pmic.201200513 [DOI] [PubMed] [Google Scholar]
- 15.Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254 [DOI] [PubMed] [Google Scholar]
- 16.Petersen J, Frank O, Goker M, Pradella S. 2013. Extrachromosomal, extraordinary and essential—the plasmids of the Roseobacter clade. Appl. Microbiol. Biotechnol. 97:2805–2815 [DOI] [PubMed] [Google Scholar]
- 17.Petersen J, Brinkmann H, Pradella S. 2009. Diversity and evolution of repABC type plasmids in Rhodobacterales. Environ. Microbiol. 11:2627–2638 [DOI] [PubMed] [Google Scholar]
- 18.Petersen J, Brinkmann H, Berger M, Brinkhoff T, Pauker O, Pradella S. 2011. Origin and evolution of a novel DnaA-like plasmid replication type in Rhodobacterales. Mol. Biol. Evol. 28:1229–1240 [DOI] [PubMed] [Google Scholar]
- 19.Dower WJ, Miller JF, Ragsdale CW. 1988. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 16:6127–6145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edgar R, Domrachev M, Lash AE. 2002. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 30:207–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Katzmann E, Borg S, Schuler D. 2012. The periplasmic nitrate reductase nap is required for anaerobic growth and involved in redox control of magnetite biomineralization in Magnetospirillum gryphiswaldense. J. Bacteriol. 194:4847–4856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moreno-Vivián C, Cabello P, Martinez-Luque M, Blasco R, Castillo F. 1999. Prokaryotic nitrate reduction: molecular properties and functional distinction among bacterial nitrate reductases. J. Bacteriol. 181:6573–6584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gates AJ, Luque-Almagro VM, Goddard AD, Ferguson SJ, Roldan MD, Richardson DJ. 2011. A composite biochemical system for bacterial nitrate and nitrite assimilation as exemplified by Paracoccus denitrificans. Biochem. J. 435:743–753 [DOI] [PubMed] [Google Scholar]
- 24.Grimaldi S, Schoepp-Cothenet B, Ceccaldi P, Guigliarelli B, Magalon A. 2013. The prokaryotic Mo/W-bisPGD enzymes family: a catalytic workhorse in bioenergetic. Biochim. Biophys. Acta 1827:1048–1085 [DOI] [PubMed] [Google Scholar]
- 25.Iobbi-Nivol C, Leimkuhler S. 2012. Molybdenum enzymes, their maturation and molybdenum cofactor biosynthesis in Escherichia coli. Biochim. Biophys. Acta 1827:1086–1101 [DOI] [PubMed] [Google Scholar]
- 26.Dahl JU, Urban A, Bolte A, Sriyabhaya P, Donahue JL, Nimtz M, Larson TJ, Leimkuhler S. 2011. The identification of a novel protein involved in molybdenum cofactor biosynthesis in Escherichia coli. J. Biol. Chem. 286:35801–35812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang W, Urban A, Mihara H, Leimkuhler S, Kurihara T, Esaki N. 2010. IscS functions as a primary sulfur-donating enzyme by interacting specifically with MoeB and MoaD in the biosynthesis of molybdopterin in Escherichia coli. J. Biol. Chem. 285:2302–2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bevers LE, Hagedoorn PL, Santamaria-Araujo JA, Magalon A, Hagen WR, Schwarz G. 2008. Function of MoaB proteins in the biosynthesis of the molybdenum and tungsten cofactors. Biochemistry 47:949–956 [DOI] [PubMed] [Google Scholar]
- 29.Hopper AC, Li Y, Cole JA. 2013. A critical role for the cccA gene product, cytochrome c2, in diverting electrons from aerobic respiration to denitrification in Neisseria gonorrhoeae. J. Bacteriol. 195:2518–2529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lebens M, Lundquist P, Soderlund L, Todorovic M, Carlin NI. 2002. The nptA gene of Vibrio cholerae encodes a functional sodium-dependent phosphate cotransporter homologous to the type II cotransporters of eukaryotes. J. Bacteriol. 184:4466–4474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Werner A, Kinne RK. 2001. Evolution of the Na-P(i) cotransport systems. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280:R301–312 [DOI] [PubMed] [Google Scholar]
- 32.Malcolm HR, Maurer JA. 2012. The mechanosensitive channel of small conductance (MscS) superfamily: not just mechanosensitive channels anymore. Chembiochem 13:2037–2043 [DOI] [PubMed] [Google Scholar]
- 33.Edwards MD, Black S, Rasmussen T, Rasmussen A, Stokes NR, Stephen TL, Miller S, Booth IR. 2012. Characterization of three novel mechanosensitive channel activities in Escherichia coli. Channels (Austin) 6:272–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Juhala RJ, Ford ME, Duda RL, Youlton A, Hatfull GF, Hendrix RW. 2000. Genomic sequences of bacteriophages HK97 and HK022: pervasive genetic mosaicism in the lambdoid bacteriophages. J. Mol. Biol. 299:27–51 [DOI] [PubMed] [Google Scholar]
- 35.Cumby N, Edwards AM, Davidson AR, Maxwell KL. 2012. The bacteriophage HK97 gp15 moron element encodes a novel superinfection exclusion protein. J. Bacteriol. 194:5012–5019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barondess JJ, Beckwith J. 1990. A bacterial virulence determinant encoded by lysogenic coliphage lambda. Nature 346:871–874 [DOI] [PubMed] [Google Scholar]
- 37.Barondess JJ, Beckwith J. 1995. bor gene of phage lambda, involved in serum resistance, encodes a widely conserved outer membrane lipoprotein. J. Bacteriol. 177:1247–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Figueroa-Bossi N, Bossi L. 1999. Inducible prophages contribute to Salmonella virulence in mice. Mol. Microbiol. 33:167–176 [DOI] [PubMed] [Google Scholar]
- 39.Uc-Mass A, Loeza EJ, de la Garza M, Guarneros G, Hernández-Sánchez J, Kameyama L. 2004. An orthologue of the cor gene is involved in the exclusion of temperate lambdoid phages. Evidence that Cor inactivates FhuA receptor functions. Virology 329:425–433 [DOI] [PubMed] [Google Scholar]
- 40.Lu MJ, Henning U. 1994. Superinfection exclusion by T-even-type coliphages. Trends Microbiol. 2:137–139 [DOI] [PubMed] [Google Scholar]
- 41.Lu MJ, Stierhof YD, Henning U. 1993. Location and unusual membrane topology of the immunity protein of the Escherichia coli phage T4. J. Virol. 67:4905–4913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Priefer UB, Kalinowski J, Ruger B, Heumann W, Puhler A. 1989. ISR1, a transposable DNA sequence resident in Rhizobium class IV strains, shows structural characteristics of classical insertion elements. Plasmid 21:120–128 [DOI] [PubMed] [Google Scholar]
- 43.Simons M, Szczelkun MD. 2011. Recycling of protein subunits during DNA translocation and cleavage by type I restriction-modification enzymes. Nucleic Acids Res. 39:7656–7666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dybvig K, Sitaraman R, French CT. 1998. A family of phase-variable restriction enzymes with differing specificities generated by high-frequency gene rearrangements. Proc. Natl. Acad. Sci. U. S. A. 95:13923–13928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Makovets S, Doronina VA, Murray NE. 1999. Regulation of endonuclease activity by proteolysis prevents breakage of unmodified bacterial chromosomes by type I restriction enzymes. Proc. Natl. Acad. Sci. U. S. A. 96:9757–9762 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.