Abstract
Bacterial dual-function small RNAs regulate gene expression by RNA-RNA base pairing and also code for small proteins. SgrS is a dual-function small RNA in Escherichia coli and Salmonella that is expressed under stress conditions associated with accumulation of sugar-phosphates, and its activity is crucial for growth during stress. The base-pairing function of SgrS regulates a number of mRNA targets, resulting in reduced uptake and enhanced efflux of sugars. SgrS also encodes the SgrT protein, which reduces sugar uptake by a mechanism that is independent of base pairing. While SgrS base-pairing activity has been characterized in detail, little is known about how base pairing and translation of sgrT are coordinated. In the current study, we utilized a series of mutants to determine how translation of sgrT affected the efficiency of base pairing-dependent regulation and vice versa. Mutations that abrogated sgrT translation had minimal effects on base-pairing activity. Conversely, mutations that impaired base-pairing interactions resulted in increased SgrT production. Furthermore, while ectopic overexpression of sgrS mutant alleles lacking only one of the two functions rescued cell growth under stress conditions, the SgrS base-pairing function alone was indispensable for growth rescue when alleles were expressed from the native locus. Collectively, the results suggest that during stress, repression of sugar transporter synthesis via base pairing with sugar transporter mRNAs is the first priority of SgrS. Subsequently, SgrT is made and acts on preexisting transporters. The combined action of these two functions produces an effective stress response.
INTRODUCTION
In the past decade, a vast number of small RNAs (sRNAs) have been identified as important posttranscriptional regulators. In bacteria, sRNAs typically do not encode proteins. Rather, they regulate gene expression by binding to target mRNAs via base-pairing interactions, resulting in enhanced (positive regulation) or reduced (negative regulation) translation. With the identification of new sRNAs, it is increasingly clear that some sRNAs, dubbed dual-function sRNAs, not only act as canonical sRNAs by regulating targets via a base-pairing mechanism but also encode regulatory proteins (1). While the noncoding, base-pairing sRNA mechanisms have been studied extensively (reviewed in references 2 and 3), much remains to be learned about the dual-function sRNA regulators. For example, the molecular functions of the proteins encoded by bifunctional sRNAs and the roles of these small proteins in cellular physiology are not well understood. Moreover, the interplay between translation and base-pairing activity has not been characterized for any dual-function sRNA. In this study, we use a dual-function sRNA from Escherichia coli and Salmonella as a model for understanding how a single RNA can serve two mechanistically distinct purposes.
SgrS is a bifunctional sRNA expressed during a specific metabolic stress condition called glucose-phosphate stress (4–6). This stress causes growth inhibition when sugar-phosphates accumulate in cells during the uptake of nonmetabolizable glucose analogs such as α-methyl glucoside (αMG). SgrS can utilize both RNA and protein functions to combat this stress (7). The RNA regulatory function (referred to as riboregulation) involves base pairing with mRNAs encoding sugar transporters (ptsG, encoding the major glucose transporter [4, 8] and manXYZ, encoding a mannose and auxiliary glucose transporter [9]) and an mRNA encoding a sugar phosphatase (yigL) (10). SgrS pairing with ptsG and manXYZ mRNAs results in translational repression, mRNA degradation, and therefore reduced sugar transporter synthesis (8, 9, 11), whereas pairing with yigL mRNA enhances mRNA stability and promotes phosphatase synthesis (10). All these interactions are facilitated by the RNA chaperone Hfq (12, 13). The riboregulation function allows decreased uptake of sugars via repression of transporters. It also increases sugar efflux by promoting sugar phosphatase synthesis, which is a prerequisite for efflux (10). The SgrT protein that is translated from the sgrS transcript also prevents uptake of sugar-phosphates, but by a different mechanism that acts at the level of sugar transporter (PtsG) activity (7). Of the bifunctional sRNAs, SgrS is the only one known thus far whose RNA and protein functions contribute to the same stress response (1). While SgrS-target mRNA base-pairing interactions and SgrT function have been characterized individually (7, 12), little is known about how these two functions interact with or influence one another.
In a previous study, we tested the ability of SgrS orthologs from other enteric bacteria to rescue E. coli cell growth during glucose-phosphate stress (14). Despite limited conservation of sgrT at the amino acid and nucleotide sequence levels (apart from the conserved, ∼20-nucleotide [nt] base-pairing region), SgrS orthologs promoted growth recovery of E. coli sgrS mutants under stress conditions. A striking result of that study was that significantly more SgrT was produced from the Salmonella enterica serovar Typhimurium SgrS ortholog than from the E. coli SgrS ortholog due to a hairpin structure present in E. coli SgrS (and absent in S. Typhimurium) that inhibits sgrT translation (14). Given the increased production of SgrT from S. Typhimurium SgrS and the demonstrated functionality of both base pairing (10, 14, 15) and SgrT (14) for this ortholog, we sought to investigate how these two functions are coordinated on the S. Typhimurium SgrS molecule.
A dual-function sRNA like SgrS must associate with different sets of protein and RNA cofactors to carry out each of its two activities. For riboregulation, SgrS associates with its target mRNA as well as the RNA chaperone Hfq and components of the RNase E degradosome complex (16). In order to be translated, SgrS must bind ribosomes. It seems likely that a given molecule of SgrS can associate with only one of these large RNA-protein complexes at a time. Thus, SgrS riboregulation and sgrT translation might compete with one another in the sense that if an SgrS molecule is ribosome associated for the purpose of sgrT translation, it is unavailable for riboregulation and vice versa. In this study, we utilized SgrS as a model dual-function sRNA to investigate how base-pairing and translation activities affect one another. We utilized sgrS mutants impaired for either base pairing or translation initiation at sgrT to gain insight into whether the two functions can act in tandem (i.e., with a single SgrS molecule simultaneously being translated and acting as a riboregulator) or, instead, are mutually exclusive. If riboregulation and translation can take place simultaneously, we postulated that mutations that impair one function might have little effect on the other. On the other hand, if the two functions are mutually exclusive, we hypothesized that mutation of one might enhance the other by increasing the total SgrS pool available to perform the other activity. In this study, we present evidence suggesting that the SgrS base-pairing function and SgrT production are mutually exclusive. Furthermore, we demonstrate that the SgrS RNA is produced rapidly in response to initial stress in S. Typhimurium and that the base-pairing activity is critical for recovery of cells from αMG stress. In contrast, the SgrT protein is produced only later after the onset of stress and seems to act as an accessory in the S. Typhimurium response to glucose-phosphate stress.
MATERIALS AND METHODS
Strain and plasmid construction.
All strains and plasmids used in this study are summarized in Table S1 in the supplemental material, and the oligonucleotides (obtained from IDT) are listed in Table S2.
The plasmids used in this study were derived from three different parental plasmids. The pBRCS plasmids (14) were derived from the medium-copy-number plasmid pBR322 (maintained at 15 to 20 copies/cell), which has the pMB1 replicon. The expression of sgrS alleles cloned into pBRCS plasmids is driven by the PLlac-O1 promoter (17). The pZA plasmids (18) were derived from the medium-copy-number plasmid pZA31luc (17), which contains the p15A replicon. The expression of sgrS alleles cloned into pZA plasmids is driven by the PLtet-O1 promoter (17). The pZE plasmids (18) were derived from the pZE12luc plasmid (17) and are maintained at 50 to 70 copies/cell. The expression of known SgrS targets (ptsG, manXYZ, and yigL) cloned into pZE plasmids is driven by the PLlac-O1 promoter.
The wild-type S. Typhimurium sgrS sequence was amplified using oligonucleotides O-DB151 and O-DB152 and cloned into the BamHI and NdeI cloning sites of the pZA31-R plasmid (18), yielding pZADB01. The sgrS14 (pZADB05), sgrS19 (pZADB12), sgrS21 (pZADB19), sgrS22 (pZADB20), and sgrS23 (pZADB21) alleles were created using QuikChange mutagenesis (Agilent Technologies) on the pZADB01 template, using the oligonucleotides listed in Table S2 in the supplemental material. QuikChange mutagenesis was also used on the sgrS19 (pZADB12) template to create the sgrS20 allele (pZADB18).
For construction of translational reporter fusions, the superfolder gfp gene from pXG-10 (19) was subcloned into pZE12S (18) by Maksym Bobrovskyy, in our laboratory, yielding pZEMB8. S. Typhimurium ptsG (from the +1 transcription start site to 30 nt after the start codon) was amplified by a PCR using oligonucleotides O-DB163 and O-DB164. The E. coli manX region (from position +1 to 132 nt after the start codon) was amplified using oligonucleotides MBP2L and MBP2R44, and the E. coli yigL sequences were amplified using oligonucleotides MBP16F2 and MBPR41. Plasmids pZEDB03 (ptsG′-′gfp), pZEMB11 (manX′-′gfp), and pZEMB15 (yigL′-′gfp) were constructed by cloning the appropriate PCR products into EcoRI and KpnI sites on the pZEMB8 plasmid. Plasmids pZEMB11 and pZEMB15 were created in our laboratory by Maksym Bobrovskyy.
The rne131 mutant (DB148) was constructed by P1 transduction of the λatt::lacIq tetR cassette into the rne131 mutant strain (EM1264) (20). lacIq encodes the superrepressor of the lac operon, such that multicopy Plac promoters remain repressed until addition of IPTG (isopropyl-β-d-thiogalactopyranoside). Similarly, TetR represses multicopy Ptet promoters until the addition of the inducer anhydrotetracycline (aTc). The Δhfq mutant (DB138) was created by P1 transduction of an hfq::cat allele from EM1264 (20) into a strain carrying the λatt::lacIq Spcr tetR cassette (JH111) (9). The Δhfq ptsG′-′lacZ fusion (DB151) was created by P1 transduction of the ptsG′-′lacZ fusion (linked to kanamycin) from JH258 (hfq+ ptsG′-′lacZ) (9) into DB138.
Mutations in the S. Typhimurium chromosome were made by first inserting a PBAD-Kanr-ccdB cassette (generated using oligonucleotides O-DB173 and O-DB174) into S. Typhimurium LT2, with deletion of nucleotides targeted for mutagenesis by lambda red recombination (21). This resulted in strain DB139. Second, PCR products generated using oligonucleotides O-DB109 and -110 (to make the sgrS1 mutant), O-DB106 and -107 (to make the sgrS2 mutant), and O-DB110 and -106 (to construct the sgrS3 mutant), and containing the corresponding mutations, were recombined independently into DB139. Recombinants were subjected to counterselection on arabinose (for loss of the toxic ccdB gene product) and subsequently screened for arabinose resistance and kanamycin sensitivity. This led to the generation of the following strains with unmarked sgrS mutations: DB140 (sgrS1), DB142 (sgrS2), and DB143 (sgrS3). The sgrS::tet (DB111) strain was created by lambda red recombination as described previously (21), using a PCR product generated using oligonucleotides O-DB104 and O-DB105.
β-Galactosidase assays.
Strains were grown overnight in LB medium (with 100 μg/ml ampicillin while carrying plasmids) and were subcultured into fresh medium with antibiotics and grown to mid-logarithmic phase. When cultures reached an optical density at 600 nm (OD600) of ∼0.3, IPTG (Sigma-Aldrich) was added to a final concentration of 1 mM. Samples were harvested 20 min after IPTG induction and assayed for β-galactosidase activity as described previously (22).
Kinetic fluorescence assays.
Strains harboring two plasmids were grown overnight in defined MOPS (morpholinepropanesulfonic acid) rich medium (Teknova) with 0.2% fructose, 100 μg/ml ampicillin, and 25 μg/ml chloramphenicol. Cultures were subcultured 1:100 in fresh medium with antibiotics and with IPTG and/or aTc (Sigma) as an inducer. Triplicate samples of induced cultures were then grown with shaking in 48-well plates in a BMG Omega FLUOstar spectrophotometer at 37°C. Measurements of the OD600 and fluorescence were made every 20 min until the cells reached an OD600 of ∼0.4. The ratio of relative fluorescence units (RFU) to OD600 was calculated at the same OD600 across 3 to 5 replicates (18).
Protein extraction and Western blot analysis.
Strains were grown overnight in LB or LB containing 100 μg/ml ampicillin (if they carried plasmids). They were subcultured in fresh medium and grown to mid-log phase. To assay for SgrT levels in cells carrying plasmids, 1 mM IPTG was added to cultures at mid-log phase. Samples were taken 20 min after IPTG addition, and proteins were harvested by trichloroacetic acid (TCA) precipitation as described previously (7). For experiments monitoring SgrT production from chromosomal alleles, cultures at an OD600 of ∼0.1 were treated with 0.5% αMG. Total protein was harvested at various time points by TCA precipitation. Samples were resuspended in SDS-PAGE loading buffer (Invitrogen) in volumes normalized to the OD600 of the culture.
Western blot analyses were performed by running equal volumes of normalized protein samples on 4 to 12% Bis-Tris gels (Invitrogen) with 1× morpholineethanesulfonic acid (MES)-SDS running buffer. Proteins were then transferred to Immobilon-PSQ membranes (Millipore) by electrophoresis using 1× transfer buffer. SgrT was probed by use of a 1:1,000 dilution of anti-SgrT antiserum (raised in rabbits; Fisher) and a 1:5,000 dilution of secondary anti-rabbit antiserum. ImageJ software was used to quantify the intensities of SgrT normalized to the cross-reacting bands that served as internal loading controls.
Growth assays. (i) αMG rescue.
Strains harboring plasmids or with chromosomal mutations were grown in minimal M63 glycerol medium overnight. Following a 1:200 subculture into fresh medium, cultures were grown to an OD600 of 0.1, and 0.5% αMG was added. Growth was monitored by measuring the optical density of the cultures at 600 nm.
(ii) Glucose growth inhibition.
Strains carrying plasmids were grown in minimal M63 glycerol medium with ampicillin overnight. Cells were then subcultured 1:200 into minimal glucose medium with ampicillin. Growth was monitored over time by measuring the OD600 of cultures until they reached stationary phase.
RNA extraction and Northern blot analysis.
Strains carrying plasmids were grown overnight in LB with ampicillin. They were then subcultured into fresh medium with antibiotics and grown to mid-log phase. When cultures reached an OD600 of ∼0.3, IPTG was added, and samples were harvested at different time points. To measure SgrS expression from the S. Typhimurium chromosome, 0.5% αMG was added to cultures at an OD600 of ∼0.4. RNA was extracted by the hot phenol method as described previously (23).
Northern blot analysis was carried out as described previously (24). Briefly, 3 μg total RNA (for SgrS) or 12 μg total RNA (for ptsG mRNA) was run in acrylamide gels and 1% agarose gels, using 1× Tris-acetate-EDTA (TAE) and 1× MOPS buffer, respectively. RNA in acrylamide gels was transferred to a 0.2-μm membrane (Whatman) in 0.5× TAE buffer by electrophoresis. RNA in agarose gels was transferred to a membrane by capillary transfer using 20× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). Following transfer, the membranes were probed overnight with biotinylated DNA oligonucleotides (IDT) complementary to SgrS or ptsG mRNA. Detection was carried out according to the instructions for a Brightstar Biodetect kit (Ambion). ImageJ software was used to quantify the intensities of SgrS and ptsG mRNA bands normalized to the intensity of the loading control, ssrA RNA.
RESULTS
In S. Typhimurium, SgrS base pairing and SgrT independently rescue cell growth when SgrS and SgrT are expressed ectopically.
The S. Typhimurium sgrS sequence is shown in Fig. 1. It is 43% identical to the E. coli sgrS sequence. Since S. Typhimurium SgrS produces SgrT in greater abundance than that with E. coli SgrS (14) and has a base-pairing activity that participates in the glucose-phosphate stress response (10), we studied the interplay between translation and riboregulation, using S. Typhimurium sgrS alleles. We began this investigation by testing the individual contributions of the two functions to the glucose-phosphate stress response in S. Typhimurium. Plasmid constructs containing S. Typhimurium wild-type sgrS or sgrS mutants were transformed into an S. Typhimurium ΔsgrS host and assayed for growth. The mutants lacked either the base-pairing function or the ability to produce SgrT (Fig. 2A). The sgrS1 allele contains mutations that disrupt the ability of SgrS1 to base pair with several targets, including ptsG and yigL (10, 12, 14); regulation of these targets is known to be important for the response to stress in E. coli and Salmonella (10, 37). The sgrS2 allele has a mutation in the sgrT start codon but retains a functional base-pairing region. Lastly, mutations in the negative-control sgrS3 allele combine both the start codon and base-pairing mutations (Fig. 2A). Growth of S. Typhimurium strains constitutively expressing these mutant alleles was monitored before and after stress induction by addition of αMG. As expected, ectopic expression of wild-type SgrS rescued cells from growth inhibition during αMG stress (Fig. 2B), whereas ΔsgrS cells with the empty vector or sgrS3 were severely growth inhibited during stress. Mutants that lacked either function individually (sgrS1 and sgrS2) grew similarly to the wild-type strain, suggesting that either SgrS function is capable of providing stress relief when overexpressed.
Fig 1.
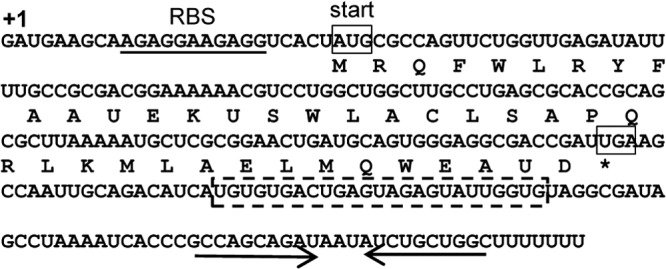
S. Typhimurium sgrS and sgrT sequences. S. Typhimurium sgrS is 239 nt long. The 41-amino-acid SgrT sequence is shown below the sgrS RNA sequence. The transcription start site of sgrS is denoted “+1.” The ribosome binding site of sgrT is underlined and marked (RBS). The start and stop codons of sgrT are boxed. The highly conserved sequences involved in base pairing with ptsG mRNA are indicated by the dashed box. The inverted repeat of the SgrS intrinsic terminator is indicated by arrows. The asterisk indicates the stop codon of sgrT.
Fig 2.
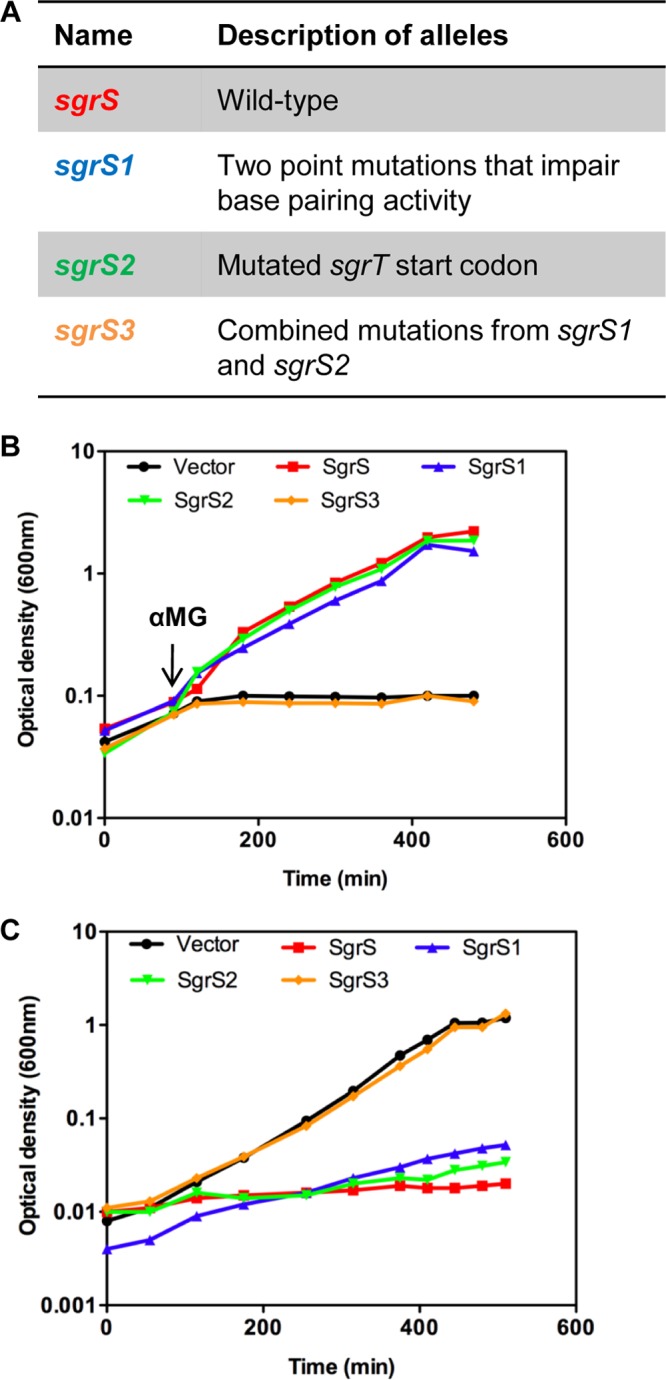
Description and phenotypic analysis of sgrS mutants. (A) sgrS alleles used in this study. Construction of the alleles is described in detail in Materials and Methods. Functional features of different sgrS mutants are described in detail in the text. (B) S. Typhimurium ΔsgrS strains (DB111) carrying the plasmid vector (pBRCS12) or a plasmid with the S. Typhimurium wild-type sgrS (pBRCS22), sgrS1 (pBRCS27), sgrS2 (pBRCS30), or sgrS3 (pBRCS31) allele (described in panel A and in the text) were grown to early logarithmic phase in M63 minimal medium with glycerol. αMG was added to a final concentration of 0.5%, and growth was monitored over time until cultures reached stationary phase. The graph shown is representative of 4 independent experiments. (C) The constructs described in panel A were grown in M63 medium with glycerol overnight and subcultured into M63 glucose medium. Growth was monitored over time until cultures reached stationary phase. The graph shown is representative of 3 independent experiments.
Another SgrS-associated phenotype is growth inhibition of cells overexpressing SgrS on minimal glucose media (4). To characterize the individual roles of the base-pairing function and SgrT in this phenotype, sgrS, sgrS1, sgrS2, and sgrS3 alleles were constitutively expressed in an S. Typhimurium ΔsgrS strain grown in glucose minimal liquid medium. As shown in Fig. 2C, cells overexpressing either sgrS1 or sgrS2 showed growth inhibition comparable to that of cells expressing wild-type SgrS. In contrast, cells harboring the negative-control sgrS3 allele or vector did not exhibit growth inhibition under these conditions. These data implied that either base-pairing or SgrT activity is sufficient to prevent glucose uptake. Consistent with our previous work in E. coli (7), the data suggest that SgrT and the base-pairing function can act independently and that either can rescue cell growth during stress.
Translation of sgrT has minimal effects on SgrS riboregulation.
The E. coli sgrT coding sequence is located at the 5′ end of SgrS (nt 22 through 153), while the sequences involved in base pairing with mRNA targets are located downstream of sgrT (nt 167 through 187). These two regions are separated by only 18 nt, which encompass the region that a ribosome located at the sgrT stop codon would occupy on each side (25, 26). Thus, terminating ribosomes might sterically interfere with base-pairing activity. We tested whether altering the distance between the sgrT stop codon and the base-pairing region would have any impact on efficiency of riboregulation. The sgrS14 allele contains a premature stop codon at the 6th codon of sgrT (see Fig. S1A in the supplemental material), such that sgrT translation terminates 107 nt upstream of the normal stop codon. SgrS14 regulated ptsG′-′gfp similarly to wild-type SgrS, suggesting that altering the spacing between the sgrT open reading frame and the base-pairing region does not affect the base-pairing activity of SgrS (see Fig. S1B).
To further examine whether translation affects the efficiency of riboregulation, we tested the effects of several different mutations in the sgrT translation initiation region on the efficiency of base pairing-dependent translational regulation. A ptsG′-′gfp translational fusion was used to monitor SgrS-dependent translational repression. Mutations in the sgrT translation initiation region (Fig. 3A) included some expected to severely impair sgrT translation (sgrS19 and sgrS20), some expected to moderately reduce sgrT translation (sgrS21 and sgrS22), and one that should yield a minor reduction in translation (sgrS23). Steady-state SgrS levels from these translation-deficient sgrS alleles were similar as measured by Northern blotting (see Fig. S2 in the supplemental material). Consistent with previous studies (14), wild-type SgrS caused a 5-fold repression of ptsG′-′gfp activity (Fig. 3B). All mutant alleles expressing SgrS with minor (sgrS23), moderate (sgrS21 or sgrS22) or severe (sgrS19 or sgrS20) defects in sgrT translation still repressed ptsG translation to a degree similar to that with wild-type SgrS (Fig. 3B). We also examined how these mutants regulated two other SgrS targets: manXYZ and yigL. As shown in Fig. S3 and S4, sgrS mutant alleles regulated expression of both manXYZ and yigL similarly to wild-type SgrS. These results are consistent with observations by other groups, where mutations in sgrT translation sequences had neutral effects on regulation of SgrS targets in S. Typhimurium, i.e., yigL and sopD (which encodes a secreted virulence factor in S. Typhimurium) (10, 15). In sum, these data indicate that mutations that impair sgrT translation or change the distance between sgrT and the base-pairing region have no major effect on the ability of SgrS to regulate mRNA targets via base pairing.
Fig 3.
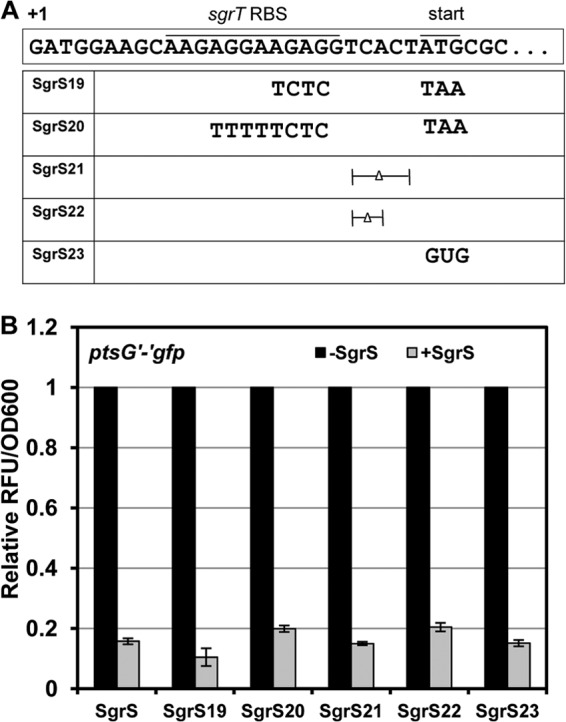
Description and analysis of sgrS alleles with mutations in the sgrT translation initiation region. (A) Mutations in the sgrT translation initiation region are indicated. The sgrS19 and sgrS20 alleles have an extensively mutated sgrT RBS and start codon. The sgrS21 and sgrS22 alleles have deletions in the spacer region between the RBS and the start codon, and sgrS23 has the AUG start codon mutated to a GUG start codon. (B) Cultures of JH111 (ΔsgrS lacIq tetR) harboring a plasmid carrying Plac-ptsG′-′gfp (pZEDB03) and a compatible plasmid (pZA plasmid series) with the Ptet-sgrS alleles indicated in panel A were grown to mid-logarithmic phase, and expression of ptsG′-′gfp and SgrS was induced with 0.75 mM IPTG and 30 ng/ml aTc, respectively. Black bars represent cultures with only the translational fusion induced (−SgrS). Gray bars represent cultures having both the translational fusion and the sgrS allele induced (+SgrS). Kinetic fluorescence assays were performed, and the relative fluorescence units (RFU) were measured and normalized to the culture density (OD600). The RFU/OD600 value for cultures induced only for the translational fusion was set to 1.
Mutations in the SgrS base-pairing region lead to increased SgrT production.
To determine how the base-pairing function affects SgrT protein production, we monitored SgrS RNA and SgrT protein levels in cells expressing wild-type sgrS and sgrS1 (defective for base pairing) (Fig. 2A), using Northern and Western blots, respectively. Northern blots demonstrated that SgrS and SgrS1 RNAs accumulated to similar levels by 20 min after induction of the plasmid-borne sgrS alleles (Fig. 4A). In contrast, Western blots (using an anti-SgrT antibody) revealed that ∼2-fold more SgrT was produced from the base-pairing-deficient SgrS1 RNA than from wild-type SgrS (Fig. 4B). Since so little SgrT is produced by the wild-type SgrS allele, accurate quantitation was difficult, and the 2-fold difference is likely an underestimate. In fact, SgrT was often not detectable in samples from cells expressing wild-type SgrS when blots were exposed for short times, whereas it was always readily detected in samples from SgrS1-expressing cells (see Fig. S5 in the supplemental material). These data imply that while mutation of sgrT translation signals does not significantly affect SgrS base-pairing properties, mutation of base-pairing residues results in enhanced SgrT production.
Fig 4.
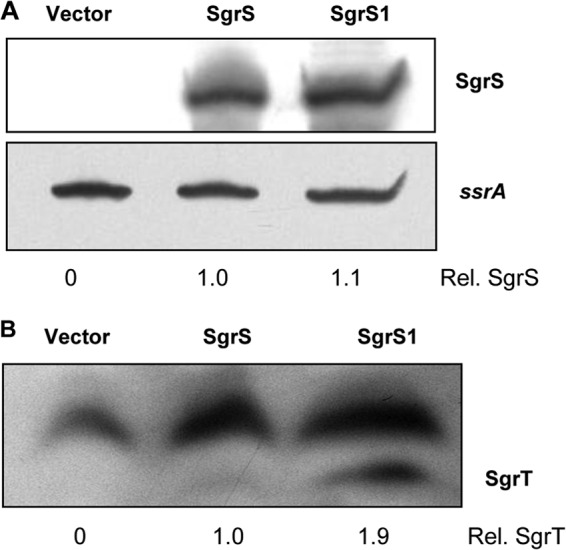
Comparison of SgrS and SgrT levels produced by wild-type and base-pairing-deficient sgrS alleles. (A) Cultures of JH111 containing the plasmid vector (pBRCS12) or the S. Typhimurium wild-type sgrS plasmid (pBRCS22) or sgrS1 plasmid (pBRCS27) were grown to mid-logarithmic phase. Transcription of Plac-sgrS alleles was induced with 1 mM IPTG, and total RNA was harvested 20 min later. Total RNA was subjected to Northern blot analysis, with probing for SgrS and subsequently for SsrA RNA (loading control). (B) Total proteins were harvested from the same cultures at the same time and analyzed by Western blotting using an anti-SgrT antibody. The cross-reacting bands above SgrT served as loading controls for quantification purposes. ImageJ software was used to quantitate SgrS and SgrT expression from the different alleles, and values were normalized to the wild-type SgrS and SgrT levels.
Next, we examined the role of the RNase E degradosome complex (involved in SgrS-dependent target mRNA destabilization) in SgrS riboregulation and translation functions. Coupled degradation of sRNAs with their mRNA targets was noted for RyhB (20), and we observed the same phenomenon for SgrS. When SgrS stability was monitored by the rifampin chase method, in which synthesis of all cellular transcripts (including SgrS and all its targets) is halted, SgrS has a long half-life (see Fig. S6A in the supplemental material). In contrast, when we tested the stability of SgrS in the presence of ongoing target mRNA synthesis (by pulse expression of SgrS with the IPTG inducer, followed by removal of the inducer), SgrS was much less stable, implying that SgrS is turned over along with newly synthesized targets (see Fig. S6B). SgrS-mediated degradation of target mRNAs such as ptsG and manXYZ requires the RNase E degradosome (16). The rne131 allele encodes a truncated RNase E that is defective for degradosome assembly (27). It was shown previously that SgrS still represses translation of targets in an rne131 mutant, even though mRNA degradation is defective in this background (8, 9, 28, 29). In other words, in the absence of degradosome assembly, coupled degradation of SgrS-mRNA complexes is abolished, while base pairing and translational silencing proceed. We hypothesized that increased stability of SgrS in an rne131 host compared with a wild-type rne+ host might allow increased SgrT production in the former strain background. To test this hypothesis, wild-type SgrS and SgrS1 were ectopically produced in rne+ and rne131 strains, and SgrS RNA and SgrT levels were measured. As shown in Fig. 5A, similarly high levels of SgrS RNA were detected from wild-type SgrS and SgrS1 in both strains (likely because the alleles were overexpressed from a heterologous promoter). Western blot analyses again revealed ∼2-fold more SgrT production from SgrS1 than from wild-type SgrS in the rne+ background (Fig. 5B). In the rne131 mutant, SgrT was still produced in ∼2-fold greater abundance in sgrS1-expressing cells than in sgrS-expressing cells (Fig. 5B). Disrupting the base-pairing function of SgrS in the rne131 background still increased SgrT production, suggesting that even in a situation where SgrS is not codegraded with mRNA targets, the riboregulation function limits SgrT synthesis. This observation is consistent with the idea that SgrS molecules engaged in riboregulation are unavailable for translation, and thus that impairing base-pairing activity increases the pool of SgrS molecules available to ribosomes for translation of sgrT. We noted that in the rne131 mutant, there were reduced levels of SgrT overall (Fig. 5B), even though SgrS RNA levels appeared to be similar to those in the rne+ strain (Fig. 5A). In a previous study, we observed decreased translation of reporter gene fusions in the rne131 background (28). We hypothesize that reduced protein production in these cases may be due to pleiotropic effects of the degradosome mutation. RNase E plays a key role in ribosome biogenesis due to its role in processing ribosomal RNAs. Moreover, RNase E has been implicated in general ribosome function (30, 31), and specifically in regulation of mRNA targets by sRNAs (reviewed in reference 32). While the precise mechanism underlying reduced SgrT production in the rne131 background is unclear, the consistent difference between wild-type and base-pairing-deficient SgrS molecules implies that active riboregulation makes less SgrS available for SgrT synthesis.
Fig 5.
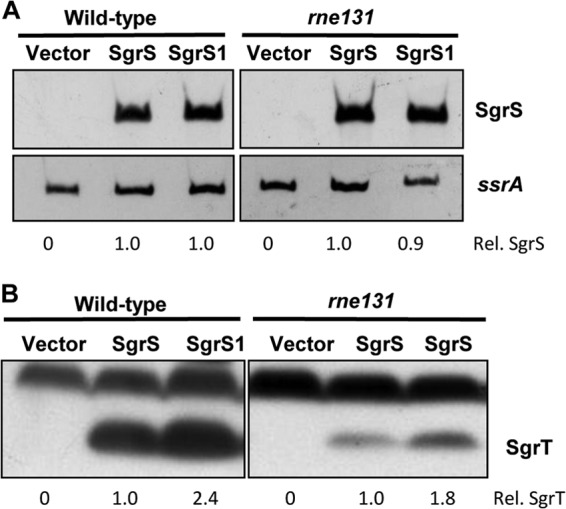
Comparison of SgrS and SgrT levels produced by wild-type and base-pairing-deficient sgrS alleles in a degradosome mutant host. Cultures of the wild-type (JH111) or rne131 mutant (DB148) strain containing the Plac plasmid vector or a plasmid with S. Typhimurium wild-type sgrS (pBRCS22) or sgrS1 (pBRCS27) were grown to mid-logarithmic phase, and transcription of sgrS was induced with 1 mM IPTG. (A) Total RNA was harvested 20 min after IPTG addition and analyzed as described in the legend to Fig. 4A. (B) Total protein was extracted at the same time as RNA and was subjected to Western blotting as described in the legend to Fig. 4B. The cross-reacting bands above SgrT served as loading controls for quantification purposes. ImageJ software was used to quantitate SgrS and SgrT expression. Amounts produced by strains carrying SgrS1 were normalized to amounts produced by strains carrying wild-type SgrS (set at 1.0).
SgrT production increases when base-pairing activity is impaired in the absence of Hfq.
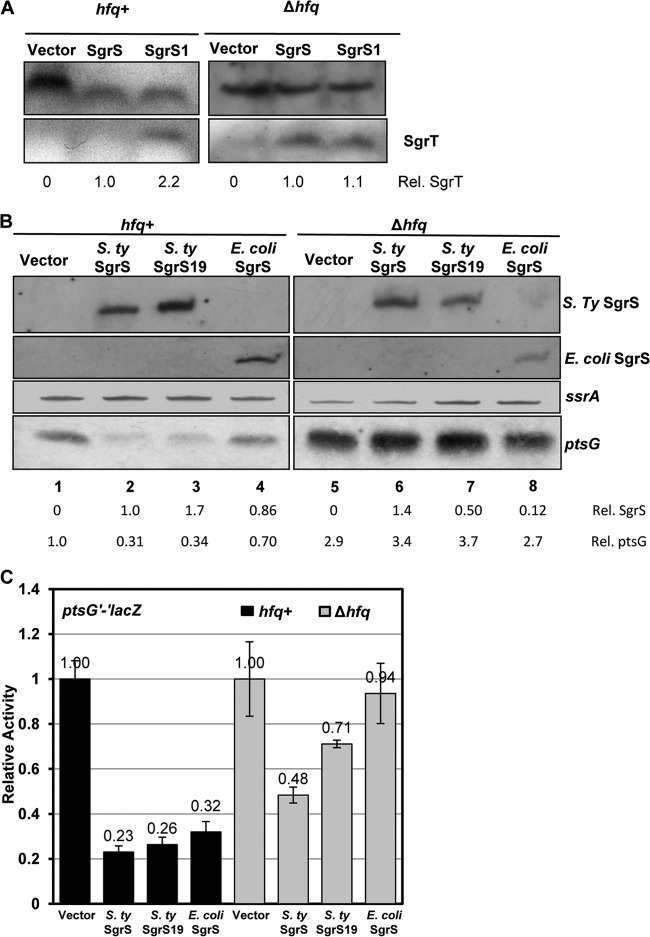
Aiba and coworkers showed that the SgrS-ptsG mRNA duplex exists as a complex with Hfq and the degradosome (16). Thus, in the rne131 mutant, while the degradosome is not formed, SgrS will still be in complex with Hfq and targets such as ptsG mRNA, likely rendering it unavailable to ribosomes. Therefore, we postulated that impairing the ability of SgrS to base pair with targets by mutation of hfq might further increase the number of SgrS molecules available for SgrT translation. Consistent with this hypothesis, when SgrT levels were measured in cells expressing wild-type SgrS or SgrS1 in a Δhfq background, equal amounts of SgrT were observed in both strains (Fig. 6A). This contrasts with the higher levels of SgrT production from SgrS1 than from wild-type SgrS in the hfq+ background (Fig. 6A). This result is consistent with the idea that when SgrS is prevented from base pairing with mRNA targets, more SgrS molecules are available for translation into SgrT.
Fig 6.
Analysis of SgrS levels and the efficiency of riboregulation in wild-type and hfq mutant strains. (A) Wild-type (JH111) and Δhfq (DB138) strains containing the Plac plasmid vector (pBRCS12) or a plasmid with S. Typhimurium wild-type sgrS (pBRCS22) or sgrS19 (pBRDB12) were grown to mid-logarithmic phase. Transcription of sgrS alleles was induced with 1 mM IPTG, and total protein was harvested after 20 min and subjected to Western blot analysis using an anti-SgrT antibody. The cross-reacting bands above SgrT served as loading controls for quantification purposes. ImageJ software was used to quantify SgrS and SgrT levels as described in the legend to Fig. 5B. (B) Strains JH111 and DB138 carrying the vector (pBRCS12), S. Typhimurium wild-type sgrS (pBRCS22), translation-deficient sgrS19 (pBRDB12), or E. coli wild-type sgrS (pLCV1) were grown to mid-logarithmic phase. Transcription of sgrS was induced as described for panel A, and total RNA was harvested and subjected to Northern blot analysis, with probing for SgrS, ptsG, or SsrA RNA (loading control). ImageJ was used to quantify the intensities of SgrS and ptsG bands. The amount of wild-type S. Typhimurium SgrS in the hfq+ strain was set at 1.0, and SgrS levels in other samples were normalized to this. The amount of ptsG mRNA in hfq+ cells carrying the vector control was set at 1.0, and levels in all other samples were normalized to this. (C) Strains containing the ptsG′-′lacZ fusion in an hfq+ (JH258) or Δhfq (DB151) background carrying the vector, S. Typhimurium wild-type sgrS (pBRCS22), sgrS19 (pBRDB12), or E. coli wild-type sgrS (pLCV1) were grown to mid-logarithmic phase. Transcription of sgrS was induced with 0.1 mM IPTG for 60 min, and then cultures were assayed for β-galactosidase activity as described in Materials and Methods.
Previous studies demonstrated that E. coli SgrS is present at much lower levels in an hfq mutant, presumably due to reduced stability (33, 34). However, the fact that SgrT was still produced from S. Typhimurium SgrS and SgrS1 in an hfq mutant host suggested that S. Typhimurium SgrS molecules might be more stable than E. coli SgrS in this background. To examine this, we measured levels of E. coli and S. Typhimurium SgrS (and mutant variants) produced from inducible plasmids in wild-type and hfq mutant strains. Strains expressing S. Typhimurium wild-type sgrS, sgrS19 (translation-deficient S. Typhimurium sgrS) (Fig. 3A), and E. coli wild-type sgrS (naturally translation deficient owing to the 5′-end secondary structure [14]) were assessed for SgrS levels by Northern blotting. Steady-state levels of S. Typhimurium wild-type SgrS were similar in the hfq+ and Δhfq strains (Fig. 6B, compare lanes 2 and 6), whereas SgrS19 levels were reduced ∼3.5-fold in the Δhfq strain compared to the wild-type strain (Fig. 6B, compare lanes 3 and 7). As seen previously (33), E. coli SgrS was significantly less abundant (∼7-fold) in the Δhfq background than in the hfq+ background (Fig. 6B, compare lanes 4 and 8). These results suggest that translation of Salmonella sgrT contributes partially to the stability of SgrS and that there are likely other determinants (perhaps differences in structure) that make Salmonella SgrS more stable than E. coli SgrS in the hfq mutant background.
Since S. Typhimurium SgrS was expressed stably in the Δhfq background, we tested whether it could still regulate ptsG mRNA at the level of target mRNA stability or translation. SgrS-mediated changes in ptsG mRNA levels and translational regulation of ptsG′-′lacZ in hfq+ and Δhfq hosts were monitored by Northern blotting and β-galactosidase assays, respectively (Fig. 6B and C). Salmonella SgrS and SgrS19 (translation deficient) both efficiently reduced ptsG mRNA levels (Fig. 6B, lanes 2 and 3) and repressed ptsG translation (Fig. 6C) in the hfq+ background. In contrast, in the Δhfq background, S. Typhimurium SgrS failed to significantly reduce ptsG mRNA levels (Fig. 6B, lane 6) and was impaired in its ability to repress ptsG translation (Fig. 6C). The S. Typhimurium translation-deficient SgrS19 allele also failed to reduce ptsG mRNA levels (Fig. 6B, lane 7) and was even more impaired for translational regulation (Fig. 6C). These results suggest that while Salmonella SgrS is stable in the absence of Hfq, owing to translation of sgrT, it does not efficiently regulate mRNA targets. In sum, these data indicate that in a Δhfq background, S. Typhimurium SgrS is stabilized by translation of sgrT but functions poorly as a riboregulator in the absence of the RNA chaperone. Failure to efficiently pair with mRNA targets likely leaves more SgrS molecules available for translation, accounting for increased SgrT production from wild-type SgrS in the Δhfq mutant background than in the hfq+ strain (Fig. 6A). Consistent with these observations, E. coli SgrS, which naturally produces very little SgrT due to an sgrT translation-inhibitory hairpin structure (14), strictly required Hfq for regulation of ptsG mRNA stability (Fig. 6B, lane 8) and translation (Fig. 6C).
The riboregulation activity of SgrS is necessary for growth rescue of stressed S. Typhimurium cells.
The data presented so far suggest that translation of sgrT has a minimal impact on riboregulation, whereas base-pairing activity limits SgrT production. We wanted to further examine how mutants impaired for either the riboregulation or translation function coped with stress when sgrS alleles were expressed from the S. Typhimurium chromosome in response to typical stress signals. Previously, our group showed that E. coli cells require the base-pairing function to cope with stress because very little SgrT is made from E. coli SgrS (14). Since SgrT is produced more abundantly by S. Typhimurium SgrS and since, when SgrS and SgrT are expressed ectopically, either base pairing or SgrT individually can rescue cells from stress, we suspected that S. Typhimurium cells may require only one of the two functions for growth recovery during stress. To test this hypothesis, wild-type S. Typhimurium and strains with the mutations described in Fig. 2—sgrS1 (base-pairing mutant), sgrS2 (sgrT start codon mutant), and the negative-control sgrS3 strain (base-pairing and start codon mutant)—were grown in minimal glycerol medium and treated with αMG. Wild-type S. Typhimurium cells and cells expressing SgrS2 from the chromosome grew equally well in the presence of αMG (Fig. 7). In contrast, the base-pairing-deficient sgrS1 mutant strain exhibited severe growth inhibition comparable to that of the ΔsgrS and sgrS3 mutant strains (Fig. 7). These results indicated that the base-pairing function of SgrS is necessary and sufficient for rescue of cells from αMG stress in minimal glycerol medium, whereas natural levels of SgrT produced under these conditions could not rescue cells. This result suggests that SgrS riboregulation plays the primary role in growth recovery from stress induced by αMG.
Fig 7.
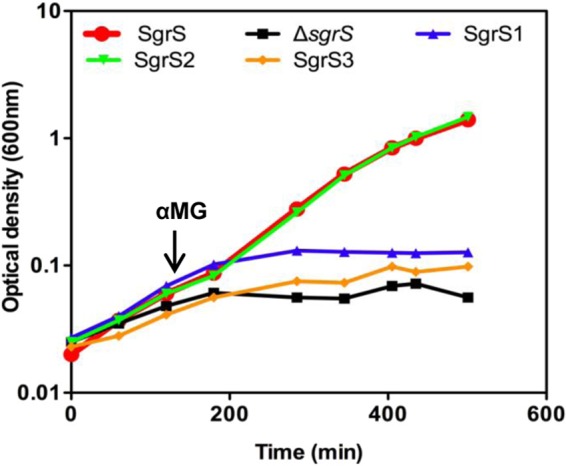
Expression of wild-type and mutant sgrS alleles from the Salmonella chromosome and analysis of growth during stress. Cultures of S. Typhimurium with sgrS alleles at the native locus were grown to mid-logarithmic phase in M63 glycerol medium. The alleles were wild-type SgrS (DB108), ΔsgrS (DB111), sgrS1 (DB140), sgrS2 (DB142), and sgrS3 (DB143). All cultures were treated with αMG at an OD600 of ∼0.1, and growth was monitored over time.
Production of SgrT lags behind synthesis of SgrS RNA.
In Fig. 2B, we showed that SgrT produced constitutively from a plasmid was able to rescue cells from stress, independent of the base-pairing function, but the results in Fig. 7 demonstrated that SgrT produced from chromosomal sgrS could not confer stress relief. One possible explanation for the discrepancy between phenotypes yielded by ectopically produced SgrT and SgrT made from the chromosomal locus is that constitutive expression provides SgrT prior to the onset of stress. However, when SgrT is produced from chromosomally encoded sgrS, perhaps there is not enough SgrT present at the beginning of stress to prevent αMG entry via PtsG. To gain insight into this issue, we measured SgrT protein and SgrS RNA levels produced from the S. Typhimurium chromosome before and after stress induction. We were unable to detect SgrT made from the single chromosomal copy of sgrS with the SgrT antibody, indicating a lower abundance of SgrT produced here than under ectopic expression conditions. However, we could detect a functional SgrT-FLAG protein when an allele expressing the epitope-tagged variant was integrated at the native chromosomal locus (suggesting a higher sensitivity of the anti-FLAG antibody than that of the anti-SgrT antibody). SgrT-FLAG protein and SgrS RNA levels were monitored via Western and Northern blot analyses, respectively. As shown in Fig. 8A and quantified in Fig. 8B, SgrS RNA levels dramatically increased upon addition of αMG (4.9 × 109 to 1.2 × 1012 molecules/ml of culture) and remained steady from 20 to 60 min after stress induction. While we did not take samples from early time points in this experiment, we have shown previously that SgrS accumulates rapidly within 2 min of αMG addition (4). After 60 min, SgrS continued to accumulate until cells reached early stationary phase. SgrT levels were similar to SgrS levels before addition of αMG (5.6 × 109 molecules/ml of culture) (Fig. 8A and C). However, upon αMG addition, the amount of SgrT did not significantly increase until 40 min after stress induction. At 40 min, SgrT levels increased 10-fold compared to those at the 20-min time point, and they remained steady 60, 120, and 150 min after αMG addition. Interestingly, SgrT levels declined significantly (10-fold) at the 180-min time point (7.3 × 109 molecules/ml of culture), even though SgrS RNA levels remained elevated at this time point (Fig. 8B and C). Altogether, the data indicated that steady-state SgrS/SgrT ratios vary significantly over time following induction of stress. Soon after stress induction, SgrS RNA accumulates rapidly relative to SgrT. This is also the time frame during which riboregulation is active (4, 9, 10). Later, between 40 and 150 min after stress induction, SgrT accumulates. After prolonged stress, SgrT levels fall again, even though SgrS RNA levels remain very high.
Fig 8.
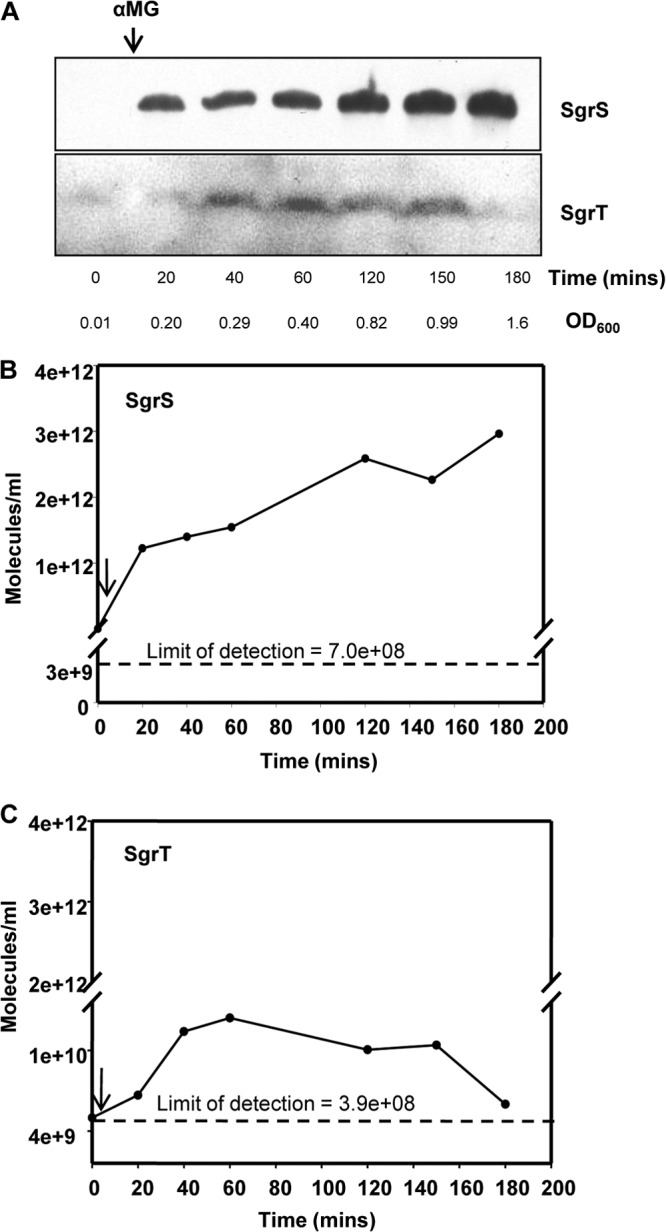
Quantitation of SgrS and SgrT levels over a time course following stress induction. (A) S. Typhimurium cells containing an sgrT-3X FLAG construct (DB153) were treated with αMG at an OD600 of ∼0.1, and total RNA and protein samples were simultaneously extracted at the time points indicated. The RNA and protein samples were subjected to Northern and Western blot analyses, with probing for SgrS and FLAG, respectively. (B) Serial dilutions of known amounts of in vitro-transcribed SgrS (in μg) were loaded on gels simultaneously with total RNA from each time point shown in panel A, subjected to Northern blotting, probed with a biotinylated SgrS probe, and quantified using ImageJ. Standard curves were first derived from the known quantities of in vitro-transcribed SgrS. Levels of SgrS from each time point in panel A were quantified (in μg) based on the standard curve, and then the number of molecules of SgrS/ml of culture was calculated. The arrow indicates addition of αMG after the 0-min time point. (C) Serial dilutions of known amounts of the BAP-FLAG protein (in ng) were loaded on gels simultaneously with protein from each time point shown in panel A, subjected to Western blotting, probed with a FLAG probe, and quantified using ImageJ. Standard curves were first derived from the known quantities of BAP-FLAG protein. Levels of SgrT from each time point in panel A were quantified (in ng) based on the standard curve, and then the number of SgrT molecules/ml of culture was calculated. The arrow indicates addition of αMG after the 0-min time point.
DISCUSSION
This study describes interactions between the two separate functions of the sRNA SgrS. Ectopic expression of sgrS alleles that possessed only base-pairing activity or only produced SgrT could rescue cells from glucose-phosphate stress. When we assessed how each activity affected the efficiency of the other, we found that impairing sgrT translation had no real effect on the ability of SgrS to translationally regulate its three known mRNA targets (Fig. 3B; see Fig. S3 and S4 in the supplemental material). In contrast, impairing base-pairing activity by mutating sgrS nucleotides important for target mRNA interactions (Fig. 4B) or by mutating the RNA chaperone Hfq (Fig. 6A) enhanced SgrT production. These results suggest that the base-pairing function is the more robust or primary activity of SgrS, as it is just as efficient with or without translation, whereas production of SgrT is limited by the competing riboregulation activity. Consistent with the concept of riboregulation as the primary activity of SgrS, we found that when sgrS mutant alleles were expressed from the native chromosomal locus in response to stress, mutants defective for riboregulation were severely growth inhibited, similar to sgrS null mutants, while mutants defective for SgrT production recovered from stress as well as wild-type cells (Fig. 7).
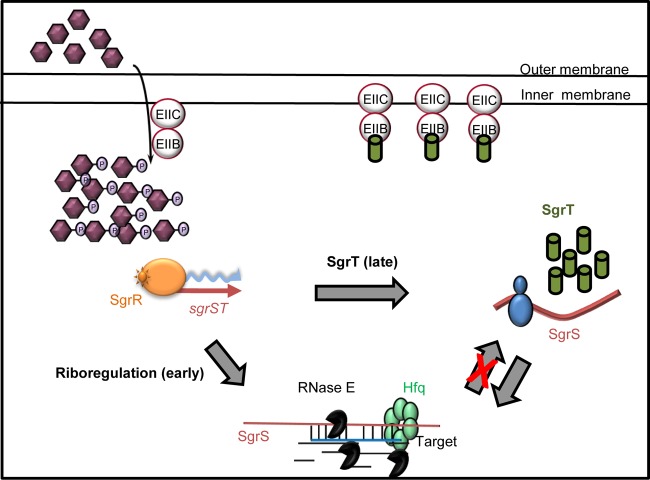
One possible molecular explanation for the more robust riboregulation activity is illustrated in Fig. 9. After an SgrS molecule is synthesized in response to stress, it can act as a substrate for translation or as a riboregulator. An SgrS that is translated to produce SgrT can presumably subsequently go on to act as a riboregulator. However, the reverse is not true—instead, an SgrS that becomes a riboregulator (at least for repressing targets such as ptsG and manXYZ mRNAs) is degraded along with its targets (see Fig. S6B in the supplemental material). Thus, when an SgrS molecule is synthesized, it can be utilized in one of two ways: (i) it may act as a riboregulator, base pair, and be codegraded along with certain targets; or (ii) it may act as an mRNA and produce the SgrT protein. The first pathway is a dead end for that SgrS molecule. It cannot later be used as a substrate for translation. Thus, disrupting the base-pairing function significantly increases the pool of SgrS available to be translated. The second pathway is not terminal. An SgrS molecule that is translated to make SgrT protein can subsequently go on and serve as a riboregulator. This fits with the observation that altering translation properties does not have major effects on SgrS riboregulation.
Fig 9.
Model for SgrS and SgrT activities in S. Typhimurium. The model is discussed in detail in the text.
Constitutively expressed ectopic SgrT rescued cells from αMG stress in the absence of base-pairing activity (Fig. 2B), whereas SgrT expressed alone from the chromosome (from the base-pairing-deficient sgrS1 allele) was unable to rescue cells from glucose-phosphate stress (Fig. 7). This result underscores the importance of the SgrS riboregulation activity with respect to promoting growth recovery during stress. Quantitation of SgrS and SgrT levels produced from the chromosomal sgrS locus during stress showed that although SgrS and SgrT levels are similar before stress induction, upon addition of αMG, SgrS RNA levels increase immediately (in less than 20 min) and dramatically (>100-fold), whereas SgrT levels remain lower until a later time point (40 min) and only reach ∼10-fold higher than the initial levels. Taken together, our results are congruent with a model in which SgrS RNA first acts primarily on target mRNAs to regulate their translation and stability during early stages of glucose-phosphate stress. SgrT is produced during later stages of the stress response, presumably when riboregulation has reached a steady state. To put this model in the context of cellular physiology during stress, it is worth noting that base-pairing and SgrT activities both ultimately act to limit uptake of nonmetabolizable sugar-phosphates by reducing transport capacity. For αMG, the PtsG (EIICBGlc) protein is the relevant transporter, and we have shown in E. coli that repression of ptsG translation via SgrS riboregulation is absolutely indispensable for recovery from αMG stress (37). Paradoxically, exposure to αMG actually increases ptsG transcription via derepression by the Mlc protein (35, 36). Thus, SgrS has a formidable task: to silence ptsG transcripts that are actually being synthesized at an enhanced rate under stress conditions. Given this, perhaps it makes sense that the cell's first priority is stopping new transporter synthesis via SgrS riboregulation repressing ptsG translation. When this is accomplished, SgrS can accumulate, and molecules not engaged in riboregulation can be translated to produce SgrT. We have shown that SgrT acts by inhibiting the activity of existing PtsG (EIICBGlc) transporters (7), though the exact molecular mechanism of SgrT activity remains unclear. Our current hypothesis is that SgrT interacts directly with PtsG, and if this is so, its activity probably critically depends on the PtsG-SgrT stoichiometry. Perhaps SgrT is not sufficient to rescue cell growth during stress in the absence of base-pairing activity because it cannot accumulate quickly enough to inhibit an ever-increasing number of active PtsG transporters.
While SgrS-dependent reduction of ptsG mRNA is evident within 2 min following stress induction, with the full disappearance of ptsG mRNA occurring within 10 min (4), the riboregulation of other targets takes longer. For manXYZ mRNA, reduced levels are not seen until 10 to 15 min poststress, and full degradation takes up to ∼30 min (9, 28). Likewise, yigL mRNA does not increase significantly until ∼10 min following stress, and increased levels of YigL protein do not appear until 20 min poststress (10; C. S. Wadler and C. K. Vanderpool, unpublished data). Moreover, SgrS also regulates other mRNA targets by base pairing, and the kinetics of these other regulatory events have not yet been elucidated (M. Bobrovskyy and C. K. Vanderpool, unpublished data). Nevertheless, given the time frame required for regulation of targets such as manXYZ and yigL, it is reasonable to postulate that the delay in SgrT production is due to SgrS engagement as a riboregulator. However, we cannot rule out the possibility that another regulatory mechanism is responsible for impairing SgrT synthesis until later time points. This is an issue that is under investigation.
When SgrT produced from the S. Typhimurium chromosomal sgrS locus was measured, we noticed that SgrT levels remained highest between 40 and 150 min after stress induction (Fig. 8A). However, at the 180-min time point, SgrT levels decreased back to the basal prestress levels (Fig. 8A and C). Decreased SgrT in later stages of stress may reflect reduced PtsG levels at this time if SgrT stability is tied to its interaction with PtsG. In support of the idea that SgrT is unstable in the absence of PtsG, we could not detect ectopically expressed SgrT in a ΔptsG mutant strain (data not shown), even though it was readily detected in ptsG+ strains (Fig. 4B, 5B, and 6A). Along with our collaborators, we recently observed that upon αMG treatment of S. Typhimurium cells, although SgrS accumulated immediately and stopped synthesis of new PtsG, PtsG protein levels had declined by only ∼2-fold 80 min after αMG treatment (10), suggesting that the reduction in PtsG levels was not due to active turnover but, instead, passive dilution by cell growth. We suggest that the later time points in our experiments (after 150 min) correspond to greatly reduced PtsG protein levels leading to instability of SgrT.
The implication that SgrS base pairing and SgrT act at different stages of the stress response raises questions about the nature of glucose-phosphate stress itself. Our previous hypothesis was that SgrT would be produced immediately to inhibit uptake of potentially toxic sugar-phosphates, while the base-pairing activity would provide a longer-term adaptation to stress by preventing continued synthesis of transporters. Instead, our data suggest that stopping new PtsG synthesis by regulating ptsG mRNA is the first order of business for SgrS and that PtsG transporters present at the onset of stress might remain active until SgrT is produced after prolonged stress. The idea that stopping new PtsG synthesis is essential for growth rescue is upheld by other recent work from our laboratory, in which we demonstrated that an sgrS mutant defective specifically for ptsG silencing (but competent for regulation of other mRNA targets) had the same growth-inhibited phenotype as an sgrS null mutant during αMG stress (37). Other work aiming to uncover the metabolic deficits experienced by stressed sgrS mutant cells revealed that growth inhibition is not strictly tied to intracellular αMG concentrations (G. Richards, M. Patel, C. Lloyd, and C. K. Vanderpool, submitted for publication). Rather, stress-related growth inhibition of sgrS mutants seems to be due to depletion of glycolytic intermediates, particularly phosphoenolpyruvate (PEP), during unregulated transport of αMG, a substrate that cannot be metabolized to replenish these intermediates (Richards et al., submitted). Taking these observations in the context of the current work, it seems possible that the cell can tolerate some level of αMG uptake and depletion of metabolic intermediates, as long as SgrS base-pairing activity prevents the problem from getting worse, i.e., by immediately limiting production of new transporters. Viewed this way, perhaps it makes sense that SgrT is produced only later, once the base-pairing activity has reached its maximal effect, in order to more completely shut down the uptake of αMG. Work is ongoing to test these hypotheses regarding the timing of the two SgrS functions and their relative roles in reducing αMG uptake and central metabolite depletion. These studies provide a foundation for understanding how a small RNA regulator with two functions coordinates these functions to produce a successful stress response.
Supplementary Material
ACKNOWLEDGMENTS
We thank Erel Levine, Nadim Majdalani, and Eric Massé for strains and plasmids. We are grateful to James Slauch, Maksym Bobrovskyy, and Chelsea Lloyd for critical readings of the manuscript and to members of the Vanderpool and Slauch laboratory for stimulating discussions.
This work was supported by an American Cancer Society research scholar grant (ACS2008-01868), a National Institutes of Health grant (RO1-GM092830), and the University of Illinois Department of Microbiology James R. Beck Fellowship to D.B.
Footnotes
Published ahead of print 9 August 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00586-13.
REFERENCES
- 1.Vanderpool C, Balasubramanian D, Lloyd C. 2011. Dual-function RNA regulators in bacteria. Biochimie 93:1943–1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Storz G, Vogel J, Wassarman KM. 2011. Regulation by small RNAs in bacteria: expanding frontiers. Mol. Cell 43:880–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Repoila F, Darfeuille F. 2009. Small regulatory non-coding RNAs in bacteria: physiology and mechanistic aspects. Biol. Cell 101:117–131 [DOI] [PubMed] [Google Scholar]
- 4.Vanderpool CK, Gottesman S. 2004. Involvement of a novel transcriptional activator and small RNA in post-transcriptional regulation of the glucose phosphoenolpyruvate phosphotransferase system. Mol. Microbiol. 54:1076–1089 [DOI] [PubMed] [Google Scholar]
- 5.Vanderpool CK. 2007. Physiological consequences of small RNA-mediated regulation of glucose-phosphate stress. Curr. Opin. Microbiol. 10:146–151 [DOI] [PubMed] [Google Scholar]
- 6.Richards GR, Vanderpool CK. 2012. Induction of the Pho regulon suppresses the growth defect of an Escherichia coli sgrS mutant, connecting phosphate metabolism to the glucose-phosphate stress response. J. Bacteriol. 194:2520–2530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wadler CS, Vanderpool CK. 2007. A dual function for a bacterial small RNA: SgrS. Proc. Natl. Acad. Sci. U. S. A. 104:20454–20459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morita T, Maki K, Yagi M, Aiba H. 2008. Analyses of mRNA destabilization and translational inhibition mediated by Hfq-binding small RNAs. Methods Enzymol. 447:359–378 [DOI] [PubMed] [Google Scholar]
- 9.Rice JB, Vanderpool CK. 2011. The small RNA SgrS controls sugar-phosphate accumulation by regulating multiple PTS genes. Nucleic Acids Res. 39:3806–3819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papenfort K, Sun Y, Miyakoshi M, Vanderpool CK, Vogel J. 2013. Small RNA-mediated activation of sugar phosphatase mRNA regulates glucose homeostasis. Cell 153:426–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morita T, Aiba H. 2011. RNase E action at a distance: degradation of target mRNAs mediated by an Hfq-binding small RNA in bacteria. Genes Dev. 25:294–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawamoto H, Koide Y, Morita T, Aiba H. 2006. Base-pairing requirement for RNA silencing by a bacterial small RNA and acceleration of duplex formation by Hfq. Mol. Microbiol. 61:1013–1022 [DOI] [PubMed] [Google Scholar]
- 13.Vogel J, Luisi BF. 2011. Hfq and its constellation of RNA. Nat. Rev. Microbiol. 9:578–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wadler CS, Vanderpool CK. 2009. Characterization of homologs of the small RNA SgrS reveals diversity in function. Nucleic Acids Res. 37:5477–5485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papenfort K, Podkaminski D, Hinton JC, Vogel J. 2012. The ancestral SgrS RNA discriminates horizontally acquired Salmonella mRNAs through a single G-U wobble pair. Proc. Natl. Acad. Sci. U. S. A. 109:E757–E764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morita T, Maki K, Aiba H. 2005. RNase E-based ribonucleoprotein complexes: mechanical basis of mRNA destabilization mediated by bacterial noncoding RNAs. Genes Dev. 19:2176–2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lutz R, Bujard H. 1997. Independent and tight regulation of transcriptional units in Escherichia coli via the LacR/O, the TetR/O and AraC/I1-I2 regulatory elements. Nucleic Acids Res. 25:1203–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levine E, Zhang Z, Kuhlman T, Hwa T. 2007. Quantitative characteristics of gene regulation by small RNA. PLoS Biol. 5:e229. 10.1371/journal.pbio.0050229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urban JH, Vogel J. 2007. Translational control and target recognition by Escherichia coli small RNAs in vivo. Nucleic Acids Res. 35:1018–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Massé E, Escorcia FE, Gottesman S. 2003. Coupled degradation of a small regulatory RNA and its mRNA targets in Escherichia coli. Genes Dev. 17:2374–2383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellermeier CD, Janakiraman A, Slauch JM. 2002. Construction of targeted single copy lac fusions using lambda Red and FLP-mediated site-specific recombination in bacteria. Gene 290:153–161 [DOI] [PubMed] [Google Scholar]
- 22.Miller JH. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 23.Aiba H, Adhya S, de Crombrugghe B. 1981. Evidence for two functional gal promoters in intact Escherichia coli cells. J. Biol. Chem. 256:11905–11910 [PubMed] [Google Scholar]
- 24.Majdalani N, Chen S, Murrow J, St John K, Gottesman S. 2001. Regulation of RpoS by a novel small RNA: the characterization of RprA. Mol. Microbiol. 39:1382–1394 [DOI] [PubMed] [Google Scholar]
- 25.Alexeeva EV, Shpanchenko OV, Dontsova OA, Bogdanov AA, Nierhaus KH. 1996. Interaction of mRNA with the Escherichia coli ribosome: accessibility of phosphorothioate-containing mRNA bound to ribosomes for iodine cleavage. Nucleic Acids Res. 24:2228–2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beyer D, Skripkin E, Wadzack J, Nierhaus KH. 1994. How the ribosome moves along the mRNA during protein synthesis. J. Biol. Chem. 269:30713–30717 [PubMed] [Google Scholar]
- 27.Lopez PJ, Marchand I, Joyce SA, Dreyfus M. 1999. The C-terminal half of RNase E, which organizes the Escherichia coli degradosome, participates in mRNA degradation but not rRNA processing in vivo. Mol. Microbiol. 33:188–199 [DOI] [PubMed] [Google Scholar]
- 28.Rice JB, Balasubramanian D, Vanderpool CK. 2012. Small RNA binding-site multiplicity involved in translational regulation of a polycistronic mRNA. Proc. Natl. Acad. Sci. U. S. A. 109:E2691–E2698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maki K, Uno K, Morita T, Aiba H. 2008. RNA, but not protein partners, is directly responsible for translational silencing by a bacterial Hfq-binding small RNA. Proc. Natl. Acad. Sci. U. S. A. 105:10332–10337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thiele I, Fleming RM, Bordbar A, Schellenberger J, Palsson BO. 2010. Functional characterization of alternate optimal solutions of Escherichia coli's transcriptional and translational machinery. Biophys. J. 98:2072–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsai YC, Du D, Dominguez-Malfavon L, Dimastrogiovanni D, Cross J, Callaghan AJ, Garcia-Mena J, Luisi BF. 2012. Recognition of the 70S ribosome and polysome by the RNA degradosome in Escherichia coli. Nucleic Acids Res. 40:10417–10431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Lay N, Schu DJ, Gottesman S. 2013. Bacterial small RNA-based negative regulation: Hfq and its accomplices. J. Biol. Chem. 288:7996–8003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andrade JM, Pobre V, Matos AM, Arraiano CM. 2012. The crucial role of PNPase in the degradation of small RNAs that are not associated with Hfq. RNA 18:844–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Otaka H, Ishikawa H, Morita T, Aiba H. 2011. PolyU tail of rho-independent terminator of bacterial small RNAs is essential for Hfq action. Proc. Natl. Acad. Sci. U. S. A. 108:13059–13064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kimata K, Inada T, Tagami H, Aiba H. 1998. A global repressor (Mlc) is involved in glucose induction of the ptsG gene encoding major glucose transporter in Escherichia coli. Mol. Microbiol. 29:1509–1519 [DOI] [PubMed] [Google Scholar]
- 36.Plumbridge J. 1998. Expression of ptsG, the gene for the major glucose PTS transporter in Escherichia coli, is repressed by Mlc and induced by growth on glucose. Mol. Microbiol. 29:1053–1063 [DOI] [PubMed] [Google Scholar]
- 37.Sun Y, Vanderpool CK. Physiological consequences of multiple-target regulation by the small RNA SgrS in Escherichia coli. J. Bacteriol., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.