Abstract
In the present study, we report the identification of a putative enoyl-coenzyme A (CoA) hydratase/isomerase that is required for synthesis of the biofilm dispersion autoinducer cis-2-decenoic acid in the human pathogen Pseudomonas aeruginosa. The protein is encoded by PA14_54640 (PA0745), named dspI for dispersion inducer. The gene sequence for this protein shows significant homology to RpfF in Xanthomonas campestris. Inactivation of dspI was shown to abolish biofilm dispersion autoinduction in continuous cultures of P. aeruginosa and resulted in biofilms that were significantly greater in thickness and biomass than those of the parental wild-type strain. Dispersion was shown to be inducible in dspI mutants by the exogenous addition of synthetic cis-2-decenoic acid or by complementation of ΔdspI in trans under the control of an arabinose-inducible promoter. Mutation of dspI was also shown to abolish cis-2-decenoic acid production, as revealed by gas chromatography-mass spectrometry (GC-MS) analysis of cell-free spent culture medium. The transcript abundance of dspI correlated with cell density, as determined by quantitative reverse transcriptase (RT) PCR. This regulation is consistent with the characterization of cis-2-decenoic acid as a cell-to-cell communication molecule that regulates biofilm dispersion in a cell density-dependent manner.
INTRODUCTION
Biofilm dispersion is the terminal stage of the biofilm developmental cycle, where bacteria regulate their escape from a biofilm and transition to a mobile planktonic lifestyle (1, 2). Induction of biofilm dispersion in Pseudomonas aeruginosa occurs naturally when biofilm microcolonies attain a critical size, releasing bacteria as free cells into the surrounding environment (2, 3). Recently, we have reported that the small messenger fatty acid molecule cis-2-decenoic acid (cis-DA), produced by P. aeruginosa in batch and continuous cultures, acts as the autoinducer of biofilm dispersion for P. aeruginosa (3). This molecule has also been shown to induce biofilm dispersion in a range of Gram-negative and Gram-positive bacteria and in the fungal pathogen Candida albicans (3).
The autoinducer cis-DA is a fatty acid cell-to-cell communication molecule with structural homology to cis-11-methyl-2-dodecenoic acid (DSF), isolated from Xanthomonas campestris (4, 5). Analogs of DSF have been identified in Burkholderia cenocepacia (cis-2-dodecenoic acid [BDSF]), Streptococcus mutans (trans-2-decenoic acid [SDSF]), and Xylella fastidiosa (trans-2-tetradecenoic acid [XyDSF]) (6–8). Additional, structurally related fatty acid signals have been identified in the genera Burkholderia, Xanthomonas, and Stenotrophomonas (6, 8–13). Fatty acid signals have been shown to regulate a wide range of bacterial behaviors, including virulence, motility, biofilm development, and dispersion (4, 8–10, 12, 14–23).
The mechanism of fatty acid signal biosynthesis appears to be widely conserved. DSF biosynthesis in X. campestris is dependent on the gene rpfF, which encodes a putative enoyl-coenzyme A (CoA) hydratase (4, 24). The role of RpfF homologs in fatty acid signal biosynthesis has subsequently been established in B. cenocepacia (6), Xanthomonas oryzae pv. oryzae (10), X. fastidiosa (18), Stenotrophomonas maltophilia (13), and Xanthomonas axonopodis pv. glycines (20).
In the present work, we report that the gene PA14_54640 (PA0745), named dspI (dispersion inducer), is required for production of the dispersion-inducing cell-to-cell signal cis-DA, synthesized by P. aeruginosa. The gene dspI encodes a putative enoyl-CoA hydratase/isomerase responsible for catalyzing the formation of α,β-unsaturated fatty acids. We further demonstrate that expression of dspI is correlated with cell density during planktonic and biofilm growth.
MATERIALS AND METHODS
Bacterial strains, plasmids, media, and culture conditions.
All bacterial strains and plasmids used in this study are listed in Table 1. P. aeruginosa strain PA14 was used as a parental strain for all work in the present study. Planktonic cultures were grown aerobically at 22°C in modified EPRI medium containing 0.005% ammonium nitrate, 0.00019% KH2PO4, 0.00063% K2HPO4 (pH 7.0), and 0.001% Hutner salts (25) supplemented with 0.2% glucose or in Luria-Bertani (LB) broth (BD, Sparks, MD) in flasks with shaking at 220 rpm. Continuous-culture biofilms were grown at 22°C in modified EPRI medium or 5% LB broth in tube reactors. Semi-batch culture biofilms were grown in 20% LB broth in 24-well culture plates. Gene complementation experiments were performed in modified EPRI medium or 5% LB broth with or without 0.1% arabinose. Antibiotics were used at the following concentrations: 75 μg/ml gentamicin (Gm), 250 μg/ml carbenicillin (Cb), and 50 μg/ml tetracycline (Tet) for P. aeruginosa; 50 μg/ml ampicillin (Amp), 25 μg/ml kanamycin (Km), and 20 μg/ml Tet for E. coli. Cb at a concentration of 10 μg/ml was used for maintenance of the pMJT1 plasmid in P. aeruginosa continuous-culture biofilm reactors.
Table 1.
Bacterial strains and plasmids
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5α | λ− ϕ80dlacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17(rK− mK−) supE44 thi-1 gyrA relA1 | Invitrogen Corp. |
| P. aeruginosa PA14 | ||
| PA14 | Wild type | 26 |
| PA14Δ19740 | PA14 19740::MAR2 × T7; Gmr | 26 |
| PA14Δ26690 | PA14 26690::MAR2 × T7; Gmr | 26 |
| PA14Δ28310 | PA14 28310::MAR2 × T7; Gmr | 26 |
| PA14Δ40640 | PA14 40640::MAR2 × T7; Gmr | 26 |
| PA14Δ40980 | PA14 40980::MAR2 × T7; Gmr | 26 |
| PA14Δ43440 | PA14 43440::MAR2 × T7; Gmr | 26 |
| PA14Δ51110 | PA14 51110::MAR2 × T7; Gmr | 26 |
| PA14ΔdspI | PA14 54640::MAR2 × T7; Gmr | 26 |
| PA14ΔdspI/pMJT | PA14 ΔdspI bearing empty pMJT-1 vector; Gmr Cbr | This study |
| PA14ΔdspI/pMJT-dspI | Complementation of ΔdspI; Gmr Carbr; arabinose inducible | This study |
| PA14/dspI-lacZ | pCTX-dspI-lacZ conjugated into PA14; Tetr | This study |
| PA14/lacZ | pCTX conjugated into PA14; Tetr | This study |
| Plasmids | ||
| pCR2.1-TOPO | TA cloning vector; Kmr Ampr | Invitrogen Corp. |
| pRK2013 | Helper plasmid for triparental mating; mob tra; Kmr | 31 |
| pMJT1 | araC-PBAD casette of pJN105 cloned into pUCP18; Ampr Cbr | 27 |
| pCTX | mini-CTX-lacZ; Tetr | 28 |
| pMJT-dspI | dspI cloned into pMJT1 using primers dspI_NheI_for/dspI_SacI_rev; Cbr | This study |
| pCTX-dspI-lacZ | dspI promoter reporter construct in mini-CTX-lacZ using primers dspI-PROM_XhoI_for/dspI-PROM_EcoRI_rev; Tetr | This study |
Strain construction.
Complementation of ΔdspI (26) was accomplished by placing the gene under the control of an arabinose-inducible PBAD promoter in the pMJT1 vector (27). Briefly, the open reading frame of dspI was amplified by PCR using primers listed in Table S1 in the supplemental material and cloned into pMJT1 at restriction sites indicated in Table S1. Plasmids were mobilized into P. aeruginosa from E. coli via electroporation, and transformants were selected by growth on LB medium with 250 μg/ml Cb. Strains were confirmed to contain vector constructs following amplification by PCR using MCS primers for pMJT1 listed in Table S1 in the supplemental material.
Reporter strain construction.
A transcriptional reporter for dspI was constructed by placing the promoter region of dspI upstream of the lacZ gene in the mini-CTX-lacZ vector (28). We found that dspI was cotranscribed with the upstream genes PA14_54620 and PA14_54630 (see Fig. S1 in the supplemental material). A 500-bp region of DNA upstream of the gene PA14_54620 was selected as including the putative promoter region of dspI based on the observation that most promoters are between 100 and 200 bp long and recognizing that multiple promoters are possible in P. aeruginosa, as is the case for algD (29, 30). This sequence was amplified by PCR using primers listed in Table S1 in the supplemental material, cloned into the mini-CTX-lacZ vector at restriction sites indicated in Table S1, and introduced into P. aeruginosa via triparental mating (31). Transformants were selected by growth on Vogel-Bonner minimal medium (VBMM) containing 0.3% citrate as the sole carbon source (32) and supplemented with Tet. Chromosomal vector integration was confirmed via PCR amplification using primers for the attB integration site listed in Table S1.
Dispersion phenotype screen.
Biofilms were grown in semi-batch culture on the submerged surfaces of 24-well cell culture plates inoculated with 250 μl/well overnight P. aeruginosa culture diluted 1:100 in 20% LB growth medium and incubated at 37°C with shaking at 220 rpm for 24 h. The plates were incubated at a 45° angle to allow biofilm development within each well. The medium in the wells was replaced every 24 h for 6 days to promote biofilm growth and remove planktonic cells. Images of biofilm microcolonies were viewed by transmitted light using an Olympus BX60 microscope and 20× and 50× UPlanF Olympus objectives. Images used to evaluate biofilm dispersion in wild-type and mutant bacterial strains were recorded using a ProgRes CF camera (Jenoptik, Jena, Thuringia, Germany) and processed with ProgRes CapturePro 2.7.7 software.
Microscopic analysis.
A continuous-culture once-through flow cell (BioSurface Technologies, Bozeman, MT) was configured to observe biofilm growth, architecture, and development on a glass substratum as described previously (3). Biofilms grown in flow cells were observed by transmitted light as described above. The biofilms were also analyzed by confocal laser scanning microscopy (CLSM) using a Leica TCS SP5 confocal microscope and Syto 40 nucleic acid stain (Invitrogen Corp.). The CLSM images were processed using LAS AF software v. 2.4.1, and quantitative analysis was performed by COMSTAT using MATLab software to determine the biofilm biomass, average thickness, and total thickness (33). All microscopy experiments were performed in triplicate.
Biofilm dispersion assays.
P. aeruginosa biofilm cultures were grown in continuous-flow tube reactors as described previously (2, 3, 34). Briefly, the interior surfaces of silicone tubing (81.5 cm long by 14-mm inner diameter; Masterflex; Cole Parmer, Inc.) of a continuous-culture once-through reactor system were used to culture biofilms. The tubing was connected to an influent medium reservoir and effluent waste reservoir. Medium was pumped through the closed and sterilized reactor system using an eight-head peristaltic pump (Cole Parmer, Inc.) at a flow rate of 0.2 ml/min. Silicone tubes were inoculated with 6 ml of log-phase cultures of P. aeruginosa by syringe through a rubber septum immediately upstream from each reactor tube. Bacterial cells were allowed to attach to the surface of the tubing for 1 h under static conditions prior to initiation of medium flow. Biofilms were grown at 22°C for a period of 6 days.
Biofilm dispersion assays were performed on 6-day biofilm cultures by addition of cis-DA or sterile medium under static flow conditions. Synthetic cis-DA (310 nM) or sterile medium was added to test or control tubes, respectively, via a rubber septum upstream of the biofilm reactor. Following a 2-h incubation, both the liquid fraction, containing released bacterial cells, and the remaining biofilm from each tube were collected separately on ice. CFU were determined by serial dilution and plating. Dispersion efficacy was calculated as follows: dispersion efficacy = (CFU in liquid fraction × 100)/(CFU in liquid fraction + CFU in biofilm fraction).
Preparation of spent medium.
Preparation of cell-free spent culture medium was performed as described previously (3) with the following modifications. Batch cultures of P. aeruginosa wild type PA14 or the dspI mutant were grown in 4 liters of modified EPRI medium for 10 days at 22°C with stirring at 220 rpm. Batch cultures of P. aeruginosa ΔdspI/pMJT-dspI or ΔdspI/pMJT were grown in 4 liters of arabinose-supplemented LB medium for 10 days at 22°C with stirring at 220 rpm. Cell-free spent culture medium was prepared by centrifugation (16,000 × g; 20 min; 4°C), followed by prefiltration using a 0.45-μm nitrile filter (Millipore, Billerica, MA) and secondary filtration using a 0.2-μm syringe filter (Millipore). The cell-free spent culture medium was stored at 4°C.
Preparation of CSM.
Chloroform-extracted spent-medium (CSM) samples were prepared as described previously (3) with the following modifications. The organic compounds contained in 3 liters of spent medium were extracted in 0.96 liters of chloroform. The chloroform was evaporated using a Rotavapor R-3000 rotovap (Buchi, Switzerland), and the remaining organic material was resuspended in 2 ml of 18 mΩ water. The CSM contained a final concentration of cis-DA 250-fold greater than in cell-free spent culture medium.
GC-MS.
Preparation of CSM and synthetic cis-DA (Carbosynth Limited, Compton-Berkshire, United Kingdom) samples for gas chromatography-mass spectrometry (GC-MS) and tandem mass spectrometry (MS-MS) analyses was carried out as previously described (3). Spectra were obtained with a Shimadzu (Columbia, MD) QP5050A GC-MS system, and analysis was performed using the Lab Solutions program GC-MS Solution (version 1.2).
Batch culture growth curve.
To determine cell density throughout planktonic growth, a growth curve of P. aeruginosa PA14 was performed on cultures grown in shake flasks at 23°C. Overnight cultures were grown in LB broth, optical density at 600 nm (OD600) adjusted, and diluted 1:100 in fresh LB broth. Absorbance readings (OD600) were taken at 12 time points throughout 32 h of growth. Experiments were carried out in triplicate with independent overnight cultures.
qRT-PCR.
Quantitative reverse transcriptase PCR (qRT-PCR) was used to determine the expression levels of dspI using 1 μg of total RNA isolated from wild-type P. aeruginosa cells grown as planktonic cultures (6.5 h, 10 h, 12.5 h, 15 h, and 24 h) and as biofilm cultures (1-, 3-, and 5-day cultures). qRT-PCR was also used to determine the effect of the exogenous addition of 310 nM cis-DA on dspI transcript abundance in 12.5-h-old PA14 planktonic cultures. Isolation of mRNA and subsequent cDNA synthesis were performed as described previously (35–38). Transcript amplification by qRT-PCR was performed according to the manufacturer's specifications with an Eppendorf Mastercycler ep realplex instrument (Eppendorf AG, Hamburg, Germany) and a KAPA SYBR FAST qPCR Kit (KAPA Biosystems, Woburn, MA), using oligonucleotide primers listed in Table S1 in the supplemental material. The gene mreB was used as a housekeeper control. Relative transcript abundances were determined using the ep realplex software (Eppendorf AG). Transcript quantification was normalized (based on the threshold cycle [CT] value) to mreB transcripts, followed by determination of transcript abundance ratios. The fold change in dspI abundance for planktonic and biofilm cells was determined relative to dspI abundance of early-exponential planktonic samples. Melting curve analyses were performed to ensure specific single-product amplification.
dspI transcription assays.
The β-galactosidase specific activities of strains harboring the dspI reporter construct were determined using the Miller assay (39) modified to determine specific β-galactosidase activity and normalized to cell protein extracts, as described previously (38, 40). An extinction coefficient of 4,500 nl/nmol/cm for o-nitrophenyl-β-galactoside (ONPG) cleavage at 420 nm was used. Specific-activity values were calculated following subtraction of background levels of β-galactosidase activity in a promoterless lacZ control strain.
β-Galactosidase activity was also assessed by fluorescence microscopy (2). Microscopic analysis of dspI expression during planktonic and biofilm growth was performed for cultures grown in medium containing 0.02 g/liter methylumbelliferyl β-d-galactopyranoside (MUG) dissolved in N,N-di-methylformamide. β-Galactosidase activity was assessed by microscopy by examination under long-wave UV excitation (2, 41). Samples were analyzed using an exposure time of 1,500 ms, with UV illumination only during image collection. β-Galactosidase activity was determined for both planktonic (early-exponential, mid-exponential, late-exponential, and early- and late-stationary-phase) and biofilm (1-, 3-, and 5-day) cells grown in batch culture or continuous-culture flow cells, respectively.
Statistical analysis.
Student's t test was performed for pairwise comparisons of groups, and multivariate analyses were performed using one-way analysis of variance (ANOVA).
RESULTS
Identification of the P. aeruginosa fatty acid synthase gene required for native biofilm dispersion.
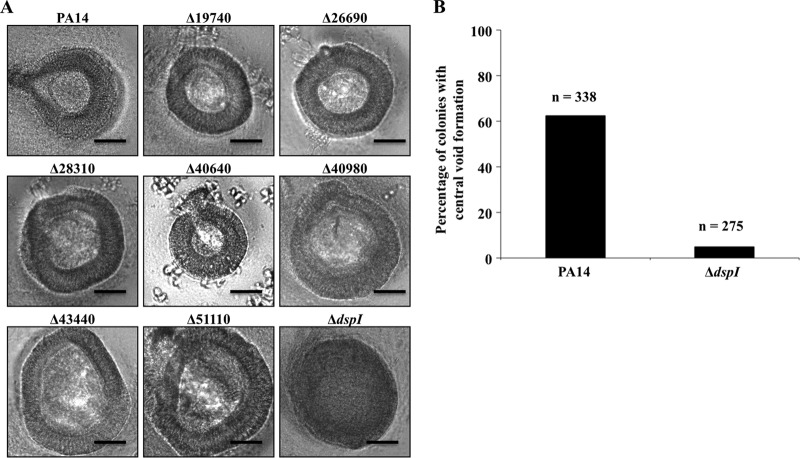
We have previously reported that the cell-to-cell communication molecule cis-DA was capable of inducing a biofilm dispersion response in P. aeruginosa (3). To identify a key enzyme required for the production of cis-DA, we focused on novel enoyl-CoA hydratase proteins with potential to be required for native biofilm dispersion. A query of the P. aeruginosa database (www.pseudomonas.com) revealed 15 putative enoyl-CoA hydratases encoded within the genome. Knockout mutations were found to be nonlethal in 8 of these genes (PA14_19740/PA3426, PA14_26690/PA2890, PA14_28310/PA2767, PA14_40640/PA1846, PA14_40980/PA1821, PA14_43440/PA1629, PA14_51110/PA1021, and PA14_54640/PA0745). Transposon mutants of these genes were selected for analysis by dispersion phenotype screening (see Materials and Methods) to determine whether they naturally formed central voids within microcolonies following 6 days of biofilm growth. Central-void formation in biofilm microcolonies is a characteristic consequence of natural dispersion, inducible with endogenously produced cis-DA. These voids result from the release of bacteria from the interiors of mature biofilm microcolonies (3, 42). All of the mutants tested, with the exception of PA14_54640/PA0745 (dspI), formed a central void in the majority of microcolonies observed by microscopic analysis (Fig. 1A). Void formation was observed in only 5% of microcolonies of dspI mutant biofilms compared to 63% of wild-type biofilm microcolonies (Fig. 1B). The presence of void spaces in dspI mutant biofilms may have resulted from P. aeruginosa dispersion in response to factors other than cis-DA. Thus, complete loss of the dispersion phenotype may not be possible with a single mutation. Interestingly, of all the enoyl-CoA hydratase proteins investigated, DspI had the highest homology (5.0E−14) to RpfF, the synthase for DSF in X. campestris (4).
Fig 1.
Microcolonies of P. aeruginosa PA14 biofilms grown in 24-well cell culture plates demonstrating the native dispersion response. (A) Transmitted-light images showing the presence and absence of interior voids formed within microcolonies of wild-type PA14 and 8 putative enoyl-CoA hydratase mutants. Biofilms of the dspI (PA14_54640) mutant showed no evidence of void formation. All images are shown at the same relative size at ×200 magnification. Scale bars, 50 μm. (B) Quantification of microcolony voids formed as a percentage of the total number of microcolonies observed for biofilms of PA14 and ΔdspI strains.
dspI is required for native biofilm dispersion and is restored by complementation of dspI in trans.
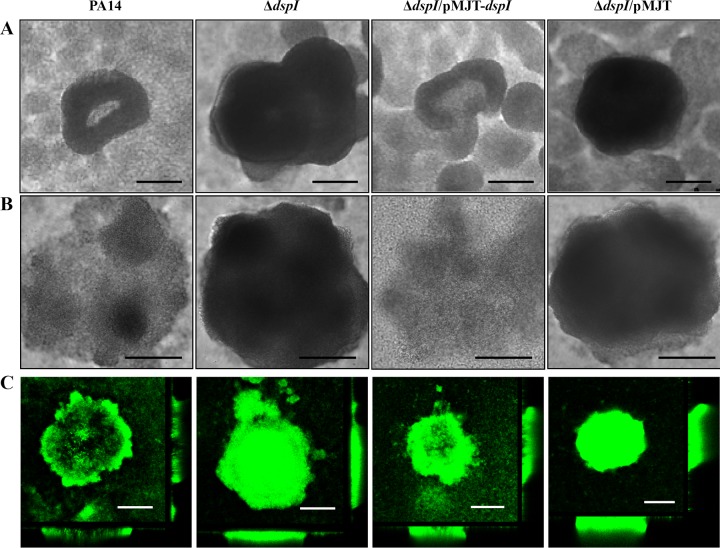
To further investigate the native dispersion phenotype of dspI mutant biofilms, the architecture of 6-day biofilms was analyzed using a microscope-mounted continuous-culture flow cell reactor system. Previous work in our laboratories has shown the dispersion stage of P. aeruginosa biofilm development to occur at day 6 under the continuous-culture conditions used in this work. We hypothesized that loss of native dispersion by mutation of dspI would result in increased biomass and microcolony size of 6-day biofilms compared to the wild type. Both the wild type and the dspI mutant formed biofilm microcolonies with distinct three-dimensional architecture; however, the dspI mutant biofilm displayed microcolonies with greater thickness and diameter in both modified EPRI and 5% LB media (Fig. 2A and B). Quantitative analysis of biofilm architecture using COMSTAT confirmed these observations, indicating that the dspI mutant 6-day biofilms had greater average and maximum thickness, as well as greater total biomass, than the wild-type strain (Table 2).
Fig 2.
dspI is required for native biofilm dispersion. Transmitted-light images (A and B) and confocal laser scanning microscopy images (C) at a magnification of ×500 of P. aeruginosa wild-type and dspI mutant biofilms. The photomicrographs show microcolonies of biofilms grown in modified EPRI medium (A) or 5% LB medium (B and C) for 6 days, with continuous dspI induction in the complemented dspI mutant strain. Microcolonies of the dspI mutant remained solid, whereas wild-type and complemented mutant biofilms showed dispersion. Experiments were completed in triplicate. Scale bars, 50 μm.
Table 2.
Quantitative analysis of biofilm architecture using COMSTATa
| Strain and conditions | Total biomass (μm3/μm2) | Avg biofilm thickness (μm) | Maximum biofilm thickness (μm) |
|---|---|---|---|
| Continuous dspI induction (5% LB; 6-day-old biofilms) | |||
| PA14 | 9.4 ± 3.8 | 13.4 ± 5.0 | 61.9 ± 21.8 |
| ΔdspI | 22.0 ± 7.0c | 35.5 ± 11.5c | 127.1 ± 35.6c |
| ΔdspI/pMJT-dspI | 6.5 ± 3.9d,e | 10.4 ± 5.1d,e | 64.2 ± 23.0d,e |
| ΔdspI/pMJT | 25.4 ± 10.8c | 39.3 ± 16.8c | 97.4 ± 35.7c |
| Before dspI induction (EPRI; 5-day-old biofilms) | |||
| PA14 | 9.3 ± 2.9 | 9.7 ± 3.4 | 25.7 ± 7.8 |
| ΔdspI | 14.8 ± 2.4c | 16.0 ± 2.6c | 43.0 ± 14.0c |
| ΔdspI/pMJT-dspI | 14.5 ± 5.6c,f | 16.4 ± 6.0c,f | 38.7 ± 18.0c,f |
| ΔdspI/pMJT | 16.7 ± 3.8c | 18.3 ± 4.4c | 38.8 ± 12.1c |
| After dspI induction (EPRI; 6-day-old biofilms)b | |||
| PA14 | 9.2 ± 4.7 | 10.5 ± 5.4 | 30.23 ± 13.4 |
| ΔdspI | 20.2 ± 3.5c | 22.5 ± 3.7c | 53.8 ± 16.3c |
| ΔdspI/pMJT-dspI | 6.0 ± 3.8d,e | 7.0 ± 4.3d,e | 20.4 ± 8.3d,e |
| ΔdspI/pMJT | 21.8 ± 7.3c | 23.5 ± 8.1c | 40.0 ± 12.4c |
COMSTAT analysis was carried out on biofilms grown in replicate (n = 2) from at least 8 images per replicate.
dspI expression was induced by growth in arabinose-containing culture medium for 24 h.
Significantly different from the wild-type PA14 (P < 0.01), as determined by single-variant ANOVA.
Significantly different from the dspI mutant (P < 0.01), as determined by single-variant ANOVA.
Not significantly different from the wild type (P > 0.01), as determined by single-variant ANOVA.
Not significantly different from the dspI mutant (P > 0.01), as determined by single-variant ANOVA.
We investigated whether activation of a plasmid-borne dspI gene in ΔdspI biofilms would result in restoration of the dispersion phenotype. Complementation of dspI for the duration of biofilm development resulted in biofilm architecture not observably different from that of the wild type and restored void formation associated with the natural-dispersion phenotype (Fig. 2A and B and Table 2). To determine the effect of dspI induction in mature ΔdspI biofilms, dspI gene expression was induced between days 5 and 6 in the complemented mutant strain. Quantitative analysis of biofilm architecture using COMSTAT was performed for 5-day (preinduction) biofilms and again for 6-day (postinduction) biofilms for the wild type, dspI mutant, and dspI-inducible complement grown in flow cell reactors. At 5 days, both the dspI mutant and the dspI inducible complement formed biofilms with greater average and maximum thickness, as well as greater total biomass, than the wild type. However, at 6 days, induction of dspI resulted in biofilms with average and maximum thickness and total biomass more similar to those of wild-type biofilms than those of biofilms of the uninduced dspI mutant (Table 2).
Mutation of dspI does not impair growth.
We tested whether mutation of dspI would have an impact on cellular growth rates to ensure that there was no difference in growth between wild-type and mutant strains in the study. Growth curves of both the wild type and the dspI mutant were found to be superimposable, indicating no difference in growth kinetics between the two strains (see Fig. S2 in the supplemental material).
Exogenous addition of cis-2-decenoic acid restores dispersion in dspI mutant biofilms.
To investigate whether dspI mutants (deficient in native dispersion) disperse in response to exogenous addition of synthetic cis-DA, 6-day biofilms grown in continuous-culture tube reactors were exposed to medium containing synthetic cis-DA (310 nM) or carrier control for a period of 2 h. Exogenous addition of cis-DA to dspI mutant cultures resulted in the release of 51% of the total biofilm population into the bulk liquid. This number is comparable to the release of 47% of biofilm cells by the wild-type strain and 55% by the complemented dspI mutant strain. Carrier controls showed 4%, 3%, and 3% cell release, respectively (Fig. 3A). When viewed by CLSM, biofilms of the dspI mutant were observed to become significantly reduced in biomass following treatment for 1 h with synthetic cis-DA. The results from a representative experiment are shown in Fig. 3B.
Fig 3.
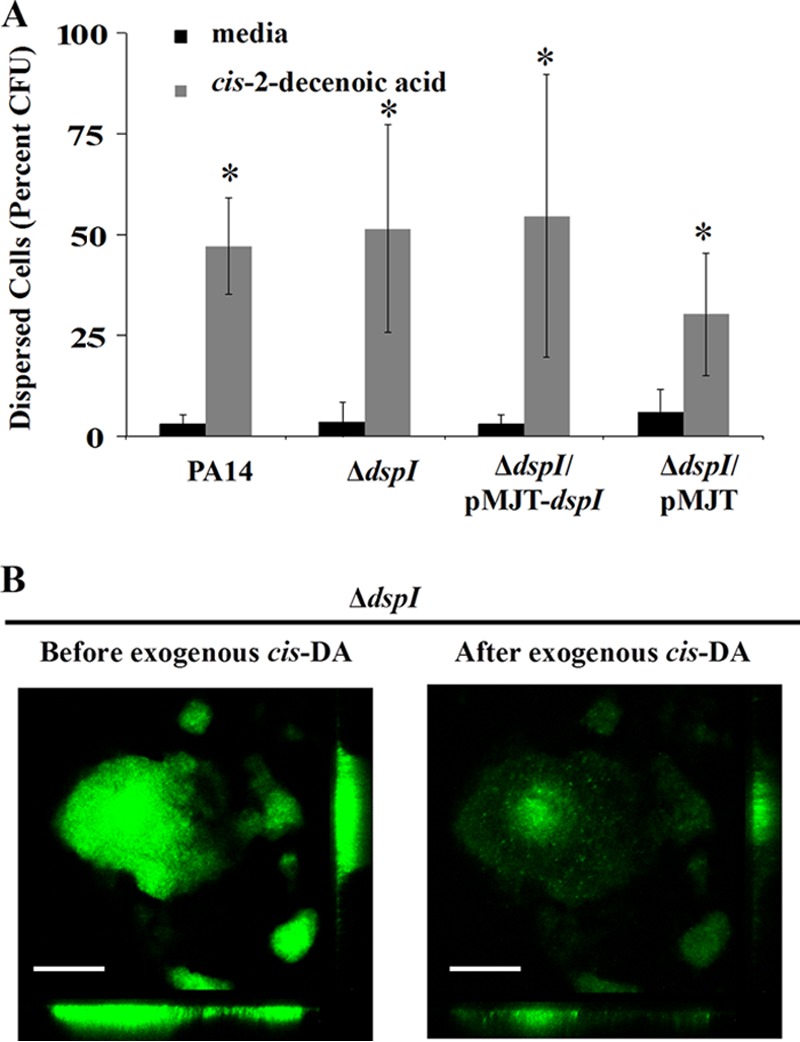
dspI mutant biofilms disperse in the presence of exogenous cis-DA. (A) Biofilms of wild-type PA14, dspI mutants, or complemented dspI mutants were grown in continuous-culture tube reactors for 6 days and switched to fresh medium or cis-DA for 2 h under static conditions. The numbers of released cells in the bulk liquid of each tube and of the remaining biofilm cells in each tube were determined by viable count (CFU). Percent dispersion was calculated as a function of released cells (CFU) divided by the total number of CFU from each tube (released cells plus remaining biofilm cells). Error bars indicate one standard deviation. (B) CLSM images of mature dspI mutant biofilm microcolonies grown in continuous culture in a microscope-mounted flow cell before and after the addition of cis-DA. Microcolony disaggregation is shown following treatment under static conditions for 1 h. Control biofilms treated with fresh medium showed no disaggregation (not shown). The images are the same relative size at ×500 magnification. Scale bars, 50 μm. Experiments were completed in triplicate. *, values significantly different from the respective negative control (P < 0.01).
dspI is essential for production of cis-2-decenoic acid.
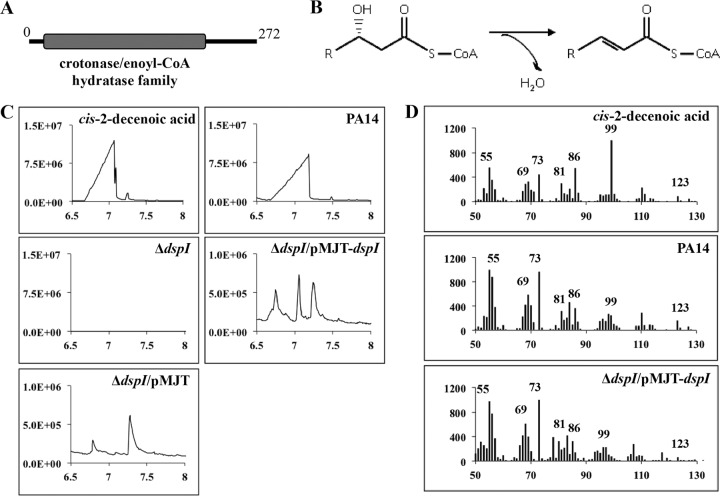
The dspI gene encodes a 272-amino-acid peptide harboring a crotonase/enoyl-CoA hydratase-like conserved domain (www.ncbi.nlm.nih.gov), similar to other enoyl-CoA hydratase/isomerase proteins involved in fatty acid metabolism (Fig. 4A). Enoyl-CoA hydratase/isomerase proteins are known to catalyze the dehydration reaction in short-chain fatty acids, resulting in the formation of a double bond at the 2,3 carbon (Fig. 4B). These proteins are also known to catalyze the cis/trans isomerization of double bonds. To investigate whether dspI mutants produce the cis-DA signal, samples of cell-free CSM were chemically analyzed using GC-MS. A single major peak with a retention time of 7.0 min was detected for CSM of the wild type and the complemented dspI mutant, identical to that of synthetic cis-DA compound, but was absent in the dspI mutant (Fig. 4C). The presence of cis-DA in the samples was confirmed by MS-MS; the fragmentation patterns for all three peaks were shown to be identical at the 95% confidence interval (Fig. 4D). These findings indicated that dspI is required for production of cis-DA in P. aeruginosa and that DspI is most likely the terminal enzyme in the synthesis pathway, responsible for double-bond formation and cis/trans isomerization.
Fig 4.
dspI is required for synthesis of cis-2-decenoic acid in P. aeruginosa. (A) DspI contains a conserved domain (gray) belonging to the crotonase/enoyl-CoA hydratase family, which includes many diverse enzymes involved in fatty acid metabolism. (B) The predicted enzymatic reaction performed by the enoyl-CoA hydratase dspI includes the formation of a double bond at the β-carbon of small fatty acids. (C) Spectral analysis of synthetic cis-DA and CSM prepared from the P. aeruginosa PA14 wild type and mutants with dspI inactivated or complemented was performed using gas chromatography-mass spectrometry. The y axes indicate intensity; the x axes indicate time in minutes. (D) MS-MS fragmentation patterns of the 7.0-min peak from the GC-MS spectra of cis-DA, PA14 CSM, and CSM of the complemented dspI mutant. The y axes indicate intensity; the x axes indicate m/z.
Transcription of dspI is correlated with cell density during planktonic and biofilm growth.
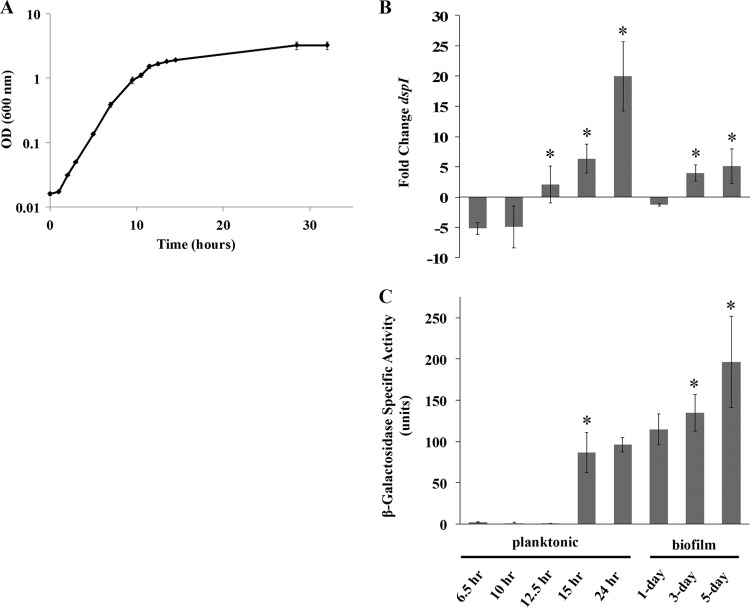
We sought to characterize the transcriptional regulation of dspI throughout planktonic and biofilm growth. During planktonic growth, cellular dspI transcript abundance was observed to increase throughout the 24-h period of batch growth (Fig. 5A). A 7-fold increase was detected between 10 h and 12.5 h, followed by a 4-fold increase between 12.5 h and 15 h. Finally, a 14-fold increase between 15 h and 24 h was observed (Fig. 5B). Similarly, under biofilm conditions, dspI transcript levels increased from 1 to 5 days, with a 5-fold increase between day 1 and day 3 and a 1-fold increase between day 3 and day 5. These findings suggested that expression of dspI, under both planktonic and biofilm growth conditions, is correlated with cell density.
Fig 5.
Expression and transcript abundance levels of dspI in planktonic and biofilm cells. (A) Growth curve of P. aeruginosa PA14 in LB medium. The curve represents the average of 3 replicates. The error bars indicate standard deviations. (B) Fold change in dspI mRNA levels in P. aeruginosa planktonic and biofilm cells compared to lag phase planktonic cells. Experiments were performed in triplicate. (C) Transcriptional reporter fusion assay for dspI expression in P. aeruginosa wild-type 6.5-h-, 10-h-, 12.5-h-, 15-h-, and 24-h-old planktonic cells and 1-day-, 3-day-, and 5-day-old biofilm cells. The values indicated by asterisks differ significantly from the values of the preceding growth phase (P < 0.05).
A transcriptional reporter for dspI.
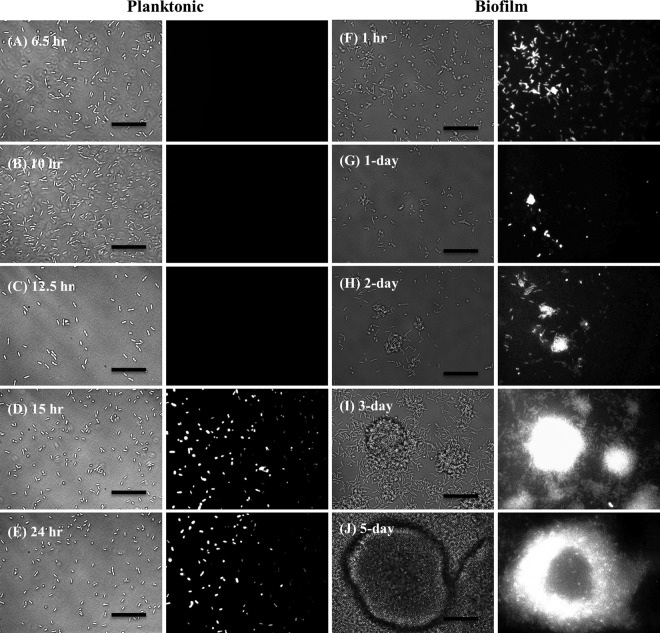
A chromosomal transcriptional lacZ fusion for the dspI promoter was used to determine whether dspI promoter activity supported the finding of increasing dspI transcript levels with increasing cell density. The β-galactosidase activity of the dspI reporter construct was determined for planktonic cells at 6.5 h, 10 h, 12.5 h, 15 h, and 24 h and for biofilm cells at 1 day, 3 days, and 5 days. β-Galactosidase activity was also monitored by bright-field and fluorescence microscopy to determine when and where in the biofilm dspI promoter activity occurred. The β-galactosidase specific activity of 6.5-h, 10-h, and 12.5-h planktonic cells was below the level of detection but increased significantly in 15-h and 24-h cells (Fig. 5B and 6). Cell numbers in the images in Fig. 6 do not reflect the actual cell density in the sample. Similarly, β-galactosidase specific activity increased throughout biofilm development, with the highest level observed at 5 days (Fig. 5B). Microscopic observation of biofilm cells carrying the dspI reporter revealed β-galactosidase activity as early as 1 h after attachment to a glass substratum, with continued expression though 6 days of growth (Fig. 7). Individual cell reporter activity was not observed to be location specific within the biofilm. Control biofilms carrying the lacZ transcriptional reporter without an upstream dspI promoter did not show fluorescence at the single-cell level (see Fig. S3 in the supplemental material). Detectable fluorescence in cell clusters at 3 and 5 days was likely due to either autofluorescence of the cells or readthrough of RNA polymerase into the lacZ structural gene downstream from the attB integration site of the chromosome.
Fig 6.
Microscopic analysis of dspI transcriptional-reporter activity during planktonic and biofilm growth. P. aeruginosa PA14 harboring a dspI-lacZ reporter construct was grown in batch or continuous culture in medium supplemented with MUG. The indicated planktonic (A to E) and biofilm (F to J) conditions are shown (bright-field images [left] and fluorescent cells displaying β-galactosidase activity [right]). Scale bars, 20 μm.
Fig 7.
Multiple-sequence alignment of DspI, RpfF homologs, and rat mitochondrial enoyl-CoA hydratase. The sequences were obtained from the NCBI (http://www.ncbi.nlm.nih.gov/) and were aligned using ClustalW. Fully conserved (*), strongly conserved (:), and weakly conserved (.) amino acid residues are indicated. (A) The 29 amino acid residues of the predicted ligand binding site for RpfF in X. oryzae pv. oryzae are boxed (47). DspI contains 15 out of 29 conserved amino acid residues of the predicted DSF ligand binding site of RpfF. (B) Conserved glutamate residues at the enoyl-CoA active site of rat mitochondrial enoyl-CoA hydratase, Glu144 and Glu164, align with Glu126 and Glu146 of DspI (shaded in red and indicated by triangles).
DISCUSSION
The structures of many fatty acid signals have been elucidated; however, the synthase genes for these signals are in many cases not identified. For those fatty acid signals whose synthase has been identified, all have been shown to be dependent on enoyl-CoA hydratase enzymes encoded by rpfF or rpfF-like homologs. In X. campestris, mutation of rpfF abolishes DSF production and results in reduced virulence of the plant pathogen. The gene rpfF is encoded by the rpf operon, which also includes the genes rpfC (sensor kinase) and rpfG (response regulator) (4).
Homologs of RpfF have been identified in B. cenocepacia (6), X. oryzae pv. oryzae (10), X. fastidiosa (18), S. maltophilia (13), and X. axonopodis pv. glycines (20). Thus, synthesis of fatty acid signaling molecules appears to be widely conserved. A BLAST search (www.pseudomonas.com) revealed homologs of RpfF (greater than 35% identity) in over 10 Pseudomonas species, indicating that production of small fatty acid signaling molecules may be widespread among members of the genus. Here, we report that DspI is a previously uncharacterized enoyl-CoA hydratase/isomerase that is required for production of the communication molecule cis-DA in P. aeruginosa (3). DspI is homologous (>30%) to the synthase RpfF in X. campestris, X. oryzae, S. maltophilia, and X. fastidiosa, as well as the synthase Bcam0581 in B. cenocepacia (Fig. 7A).
DspI is a putative member of the crotonase superfamily (Fig. 4A), which includes enzymes that catalyze the reversible addition of water to α,β-unsaturated enoyl-CoA thioesters. Previous work has determined that rat mitochondrial enoyl-CoA hydratase contains two conserved catalytic glutamate residues, Glu144 and Glu164, that are required for complex formation between the enzyme, a catalytic water, and the bound substrate at the active site (43–45). Sequence alignment of DspI with rat mitochondrial enoyl-CoA hydratase (NCBI accession number CAA34080) reveals that Glu144 and Glu164 align with Glu126 and Glu146 of DspI, supporting the role of DspI as a putative enoyl-CoA hydratase (Fig. 7B). These catalytic Glu residues are also conserved in RpfF of X. campestris, and mutation of either Glu residue abolished DSF synthesis (46).
Recently, Reddy et al. (47) identified a putative active site for the RpfF protein in X. oryzae pv. oryzae, which elucidated 29 amino acid residues involved in ligand binding: Leu84, Gly85, Gly86, Leu88, Phe91, Ile95, Tyr106, Ala107, Cys110, Val111, Leu136, Gly137, Gly138, Glu141, Pro160, Glu161, Leu163, Leu164, Leu166, Pro168, Gly169, Met170, Thr255, Trp258, Aal262, Leu265, Thr272, Met273, and Leu276. Interestingly, the catalytic Glu144 and Glu164 residues for enoyl-CoA hydratase activity are included among those predicted to be involved in the active site (Fig. 7A). An alignment with X. oryzae RpfF reveals that P. aeruginosa DspI contains 15/29 of the predicted active-site amino acid residues, indicating that these are related enzymes that bind similar substrates and produce structurally related products (Fig. 7A).
It has been shown that mutation of rpfC results in increased DSF production and a significant increase in rpfF transcription (24, 48). This indicates that DSF detection by RpfC results in negative regulation of rpfF. This is in contrast to other quorum-sensing (QS) signaling systems, in which signal detection exhibits transcriptional positive feedback on the signal synthase. For example, in the P. aeruginosa QS LasR/I system, the 3-oxo-C12-homoserine lactone (3OC12-HSL) autoinducer molecule is produced by the LasI acylhomoserine lactone (AHL) synthase (49). Transcription of the lasI gene is positively regulated when the LasR regulator is bound to the signal, 3OC12-HSL (50). Interestingly, in P. aeruginosa, we have found no evidence of the cis-DA signal autoregulating the expression of its cognate synthase gene, dspI. Addition of synthetic cis-DA to late-exponential-stage planktonic cultures resulted in less than a 2-fold change in dspI transcript levels. These results do not support the idea that autoregulation of cis-DA synthesis occurs at the transcriptional level.
We have previously demonstrated the role of cis-DA as an autoinducer of biofilm dispersion in P. aeruginosa and that dispersion of mature biofilms is inducible by the exogenous addition of naturally or synthetically produced cis-DA (3). However, the full range of phenotypes regulated by this signaling molecule in P. aeruginosa has not been characterized. Recently, Feinbaum et al. have shown that production of the virulence factor pyoverdine is reduced in P. aeruginosa when the gene PA14_54640 (dspI) is mutated (51). These mutants were also shown to be defective in swarming motility, a phenotype inversely correlated with biofilm formation (51). Together, these findings suggest that loss of cis-DA-induced dispersion may be associated with reduced pathogenicity and loss of swarming motility.
The discovery that DspI is required for cis-DA production has important implications for the future characterization of cis-DA signal transduction in P. aeruginosa. A two-component regulatory system for signal transduction of cis-DA has not yet been identified; however, several homologs of the DSF two-component regulatory system, RpfC/G, exist in P. aeruginosa. Elucidation of the signal transduction of cis-DA poses a significant challenge, considering that the P. aeruginosa genome encodes many predicted sensor kinase and response regulator proteins (63 and 64, respectively). A dspI mutant strain in which cis-DA production is abrogated yet that still disperses upon exogenous addition of cis-DA, may be a useful tool for future work to characterize cis-DA signal transduction- and dispersion-related phenotypes, including acute virulence and antimicrobial tolerance.
Supplementary Material
ACKNOWLEDGMENTS
We thank Karin Sauer, Olga Petrova, and Tim Lowenstein for their valuable assistance and for the use of their laboratory facilities. We also thank Allison Ferreira, Courtney Kleeschulte, Joycy Samson, and Ian Silverman for their contributions to this work.
This study was supported in part by NIH grant 1 R15 AI094485-01 2011.
Footnotes
Published ahead of print 9 August 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00707-13.
REFERENCES
- 1.Davies DG. 1999. Regulation of matrix polymer in biofilm formation and dispersion, p 93–112 In Wingender J, Neu TR, Flemming H-C. (ed), Microbial extrapolymeric substances, characterization, structure and function. Springer-Verlag, Berlin, Germany [Google Scholar]
- 2.Sauer K, Camper AK, Ehrlich GD, Costerton JW, Davies DG. 2002. Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J. Bacteriol. 184:1140–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies DG, Marques CNH. 2009. A fatty acid messenger is responsible for inducing dispersion in microbial biofilms. J. Bacteriol. 191:1393–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barber CE, Tang JL, Feng JX, Pan MO, Wilson TJG, Slater H, Dow JM, Williams P, Daniels MJ. 1997. A novel regulatory system required for pathogenicity of Xanthomonas campestris is mediated by a small diffusible signal molecule. Mol. Microbiol. 24:555–566 [DOI] [PubMed] [Google Scholar]
- 5.Wang LH, He Y, Gao Y, Wu JE, Dong YH, He C, Wang SX, Weng LX, Xu J-L, Tay L, Fang RX, Zhang LH. 2004. A bacterial cell-cell communication signal with cross-kingdom structural analogues. Mol. Microbiol. 51:903–912 [DOI] [PubMed] [Google Scholar]
- 6.Boon C, Deng Y, Wang LH, He Y, Xu JL, Fan Y, Pan SQ, Zhang L-H. 2008. A novel DSF-like signal from Burkholderia cenocepacia interferes with Candida albicans morphological transition. ISME J. 2:27–36 [DOI] [PubMed] [Google Scholar]
- 7.Vílchez R, Lemme A, Ballhausen B, Thiel V, Schulz S, Jansen R, Sztajer H, Wagner-Döbler I. 2010. Streptococcus mutans inhibits Candida albicans hyphal formation by the fatty acid signaling molecule trans-2-decenoic acid (SDSF). Chembiochem 11:1552–1562 [DOI] [PubMed] [Google Scholar]
- 8.Beaulieu ED, Ionescu M, Chatterjee S, Yokota K, Trauner D, Lindow S. 2013. Characterization of a diffusible signaling factor from Xylella fastidiosa. mBio 4:e00539–00512. 10.1128/mBio.00539-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fouhy Y, Scanlon K, Schouest K, Spillane C, Crossman L, Avison MB, Ryan RP, Dow JM. 2007. Diffusible signal factor-dependent cell-cell signaling and virulence in the nosocomial pathogen Stenotrophomonas maltophilia. J. Bacteriol. 189:4964–4968 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.He YW, Wu J, Cha JS, Zhang LH. 2010. Rice bacterial blight pathogen Xanthomonas oryzae pv. oryzae produces multiple DSF-family signals in regulation of virulence factor production. BMC Microbiol. 10:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryan RP, Fouhy Y, Garcia BF, Watt SA, Niehaus K, Yang L, Tolker-Nielsen T, Dow JM. 2008. Interspecies signalling via the Stenotrophomonas maltophilia diffusible signal factor influences biofilm formation and polymyxin tolerance in Pseudomonas aeruginosa. Mol. Microbiol. 68:75–86 [DOI] [PubMed] [Google Scholar]
- 12.Deng Y, Wu J, Eberl L, Zhang LH. 2010. Structural and functional characterization of diffusible signal factor family quorum-sensing signals produced by members of the Burkholderia cepacia complex. Appl. Environ. Microbiol. 76:4675–4683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang TP, Lee Wong AC. 2007. Extracellular fatty acids facilitate flagella-independent translocation by Stenotrophomonas maltophilia. Res. Microbiol. 158:702–711 [DOI] [PubMed] [Google Scholar]
- 14.Dow JM, Crossman L, Findlay K, He YQ, Feng JX, Tang JL. 2003. Biofilm dispersal in Xanthomonas campestris is controlled by cell-cell signaling and is required for full virulence to plants. Proc. Natl. Acad. Sci. U. S. A. 100:10995–11000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He YW, Xu M, Lin K, Ng YJ, Wen CM, Wang LH, Liu ZD, Zhang HB, Dong YH, Dow JM, Zhang LH. 2006. Genome scale analysis of diffusible signal factor regulon in Xanthomonas campestris pv. campestris: identification of novel cell-cell communication-dependent genes and functions. Mol. Microbiol. 59:610–622 [DOI] [PubMed] [Google Scholar]
- 16.He YW, Wang C, Zhou L, Song H, Dow JM, Zhang LH. 2006. Dual signaling functions of the hybrid sensor kinase RpfC of Xanthomonas campestris involve either phosphorelay or receiver domain-protein interaction. J. Biol. Chem. 281:33414–33421 [DOI] [PubMed] [Google Scholar]
- 17.Ryan RP, McCarthy Y, Andrade M, Farah CS, Armitage JP, Dow JM. 2010. Cell-cell signal-dependent dynamic interactions between HD-GYP and GGDEF domain proteins mediate virulence in Xanthomonas campestris. Proc. Natl. Acad. Sci. U. S. A. 107:5989–5994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chatterjee S, Wistrom C, Lindow SE. 2008. A cell-cell signaling sensor is required for virulence and insect transmission of Xylella fastidiosa. Proc. Natl. Acad. Sci. U. S. A. 105:2670–2675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chatterjee S, Sonti RV. 2002. rpfF mutants of Xanthomonas oryzae pv. oryzae are deficient for virulence and growth under low iron conditions. Mol. Plant Microbe Interact. 15:463–471 [DOI] [PubMed] [Google Scholar]
- 20.Thowthampitak J, Shaffer BT, Prathuangwong S, Loper JE. 2008. Role of rpfF in virulence and exoenzyme production of Xanthomonas axonopodis pv. glycines, the causal agent of bacterial pustule of soybean. Phytopathology 98:1252–1260 [DOI] [PubMed] [Google Scholar]
- 21.Ryan RP, McCarthy Y, Watt SA, Niehaus K, Dow JM. 2009. Intraspecies signaling involving the diffusible signal factor BDSF (cis-2-dodecenoic acid) influences virulence in Burkholderia cenocepacia. J. Bacteriol. 191:5013–5019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng Y, Boon C, Eberl L, Zhang LH. 2009. Differential modulation of Burkholderia cenocepacia virulence and energy metabolism by the quorum-sensing signal BDSF and its synthase. J. Bacteriol. 191:7270–7278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCarthy Y, Yang L, Twomey KB, Sass A, Tolker-Nielsen T, Mahenthiralingam E, Dow JM, Ryan RP. 2010. A sensor kinase recognizing the cell-cell signal BDSF (cis-2-dodecenoic acid) regulates virulence in Burkholderia cenocepacia. Mol. Microbiol. 77:1220–1236 [DOI] [PubMed] [Google Scholar]
- 24.Slater H, Alvarez-Morales A, Barber CE, Daniels MJ, Dow JM. 2000. A two-component system involving an HD-GYP domain protein links cell-cell signalling to pathogenicity gene expression in Xanthomonas campestris. Mol. Microbiol. 38:986–1003 [DOI] [PubMed] [Google Scholar]
- 25.Cohen-Bazire G, Sistrom WR, Stanier RY. 1957. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J. Cell Physiol. 49:25–68 [DOI] [PubMed] [Google Scholar]
- 26.Liberati NT, Urbach JM, Miyata S, Lee DG, Drenkard E, Wu G, Villanueva J, Wei T, Ausubel FM. 2006. An ordered, nonredundant library of Pseudomonas aeruginosa strain PA14 transposon insertion mutants. Proc. Natl. Acad. Sci. U. S. A. 103:2833–2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaneko Y, Thoendel M, Olakanmi O, Britigan BE, Singh PK. 2007. The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity. J. Clin. Invest. 117:877–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Becher A, Schweizer HP. 2000. Integration-proficient Pseudomonas aeruginosa vectors for isolation of single-copy chromosomal lacZ and lux gene fusions. Biotechniques 29:948–950, 952 [DOI] [PubMed] [Google Scholar]
- 29.Mohr CD, Leveau JHJ, Krieg DP, Hibler NS, Deretic V. 1992. AlgR-binding sites within the algD promoter make up a set of inverted repeats separated by a large intervening segment of DNA. J. Bacteriol. 174:6624–6633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohr CD, Hibler NS, Deretic V. 1991. AlgR, a response regulator controlling mucoidy in Pseudomonas aeruginosa, binds to the FUS sites of the algD promoter located unusually far upstream from the mRNA start site. J. Bacteriol. 173:5136–5143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Figurski DH, Helinski DR. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. U. S. A. 76:1648–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoang TT, Schweizer HP. 1997. Fatty acid biosynthesis in Pseudomonas aeruginosa: cloning and characterization of the fabAB operon encoding beta-hydroxyacyl-acyl carrier protein dehydratase (FabA) and beta-ketoacyl-acyl carrier protein synthase I (FabB). J. Bacteriol. 179:5326–5332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heydorn A, Nielsen AT, Hentzer M, Sternberg C, Givskov M, Ersbøll BK, Molin S. 2000. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 146:2395–2407 [DOI] [PubMed] [Google Scholar]
- 34.Davies DG, Parsek MR, Pearson JP, Iglewski BH, Costerton JW, Greenberg EP. 1998. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295–298 [DOI] [PubMed] [Google Scholar]
- 35.Allegrucci M, Sauer K. 2007. Characterization of colony morphology variants isolated from Streptococcus pneumoniae biofilms. J. Bacteriol. 189:2030–2038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allegrucci M, Sauer K. 2008. Formation of Streptococcus pneumoniae non-phase-variable colony variants is due to increased mutation frequency present under biofilm growth conditions. J. Bacteriol. 190:6330–6339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petrova OE, Sauer K. 2009. A novel signaling network essential for regulating Pseudomonas aeruginosa biofilm development. PLoS Pathog. 5:e1000668. 10.1371/journal.ppat.1000668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Southey-Pillig CJ, Davies DG, Sauer K. 2005. Characterization of temporal protein production in Pseudomonas aeruginosa biofilms. J. Bacteriol. 187:8114–8126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller J. 1972. Experiments in molecular genetics, p 352–355 Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 40.Davies DG, Geesey GG. 1995. Regulation of the alginate biosynthesis gene algC in Pseudomonas aeruginosa during biofilm development in continuous culture. Appl. Environ. Microbiol. 61:860–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davies DG, Chakrabarty AM, Geesey GG. 1993. Exopolysaccharide production in biofilms: substratum activation of alginate gene expression by Pseudomonas aeruginosa. Appl. Environ. Microbiol. 59:1181–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stoodley P, Wilson S, Hall-Stoodley L, Boyle JD, Lappin-Scott HM, Costerton JW. 2001. Growth and detachment of cell clusters from mature mixed-species biofilms. Appl. Environ. Microbiol. 67:5608–5613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bahnson BJ, Anderson VE, Petsko GA. 2002. Structural mechanism of enoyl-CoA hydratase: three atoms from a single water are added in either an E1cb stepwise or concerted fashion. Biochemistry 41:2621–2629 [DOI] [PubMed] [Google Scholar]
- 44.Engel CK, Mathieu M, Zeelen JP, Hiltunen JK, Wierenga RK. 1996. Crystal structure of enoyl-coenzyme A (CoA) hydratase at 2.5 angstroms resolution: a spiral fold defines the CoA-binding pocket. EMBO J. 15:5135–5145 [PMC free article] [PubMed] [Google Scholar]
- 45.Engel CK, Kiema TR, Hiltunen JK, Wierenga RK. 1998. The crystal structure of enoyl-CoA hydratase complexed with octanoyl-CoA reveals the structural adaptations required for binding of a long chain fatty acid-CoA molecule. J. Mol. Biol. 275:847–859 [DOI] [PubMed] [Google Scholar]
- 46.Cheng Z, He Y-W, Lim SC, Qamra R, Walsh MA, Zhang L-H, Song H. 2010. Structural basis of the sensor-synthase interaction in autoinduction of the quorum sensing signal DSF biosynthesis. Structure 18:1199–1209 [DOI] [PubMed] [Google Scholar]
- 47.Reddy VS, Kumar YN, Raghavendra A, Sowjenya G, Kumar S, Ramyasree G, Reddy GR. 2012. In silico model of DSF synthase RpfF protein from Xanthomonas oryzae pv. oryzae: a novel target for bacterial blight of rice disease. Bioinformation 8:504–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.An S-Q, Febrer M, McCarthy Y, Tang D-J, Clissold L, Kaithakottil G, Swarbreck D, Tang J-L, Rogers J, Dow JM, Ryan RP. 2013. High-resolution transcriptional analysis of the regulatory influence of cell-to-cell signaling reveals novel genes that contribute to Xanthomonas phytopathogenesis. Mol. Microbiol. 88:1058–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pearson JP, Pesci EC, Iglewski BH. 1997. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J. Bacteriol. 179:5756–5767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seed PC, Passador L, Iglewski BH. 1995. Activation of the Pseudomonas aeruginosa lasI gene by LasR and the Pseudomonas autoinducer PAI: an autoinduction regulatory hierarchy. J. Bacteriol. 177:654–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feinbaum RL, Urbach JM, Liberati NT, Djonovic S, Adonizio A, Carvunis A-R, Ausubel FM. 2012. Genome-wide identification of Pseudomonas aeruginosa virulence-related genes using a Caenorhabditis elegans infection model. PLoS Pathog. 8:e1002813. 10.1371/journal.ppat.1002813 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.