Abstract
The Enterococcus faecalis cell wall-anchored protein Ace is an important virulence factor involved in cell adhesion and infection. Expression of Ace on the cell surface is affected by many factors, including stage of growth, culture temperature, and environmental components, such as serum, urine, and collagen. However, the mechanisms that regulate or modulate Ace display are not well understood. With interest in identifying genes associated with Ace expression, we utilized a whole-cell enzyme-linked immunosorbent assay (ELISA)-based screening method to identify mutants from a transposon insertion mutant library which exhibited distinct Ace surface expression profiles. We identified a ccpA insertion mutant which showed significantly decreased levels of Ace surface expression at early growth phase versus those of wild-type OG1RF. Confirmation of the observation was achieved through flow cytometry and complementation analysis. Compared to the wild type, the E. faecalis ccpA mutant had an impaired ability to adhere to collagen when grown to early exponential phase, consistent with the lack of Ace expression in the early growth phase. As a key component of carbon catabolite regulation, CcpA has been previously reported to play a critical role in regulating expression of proteins involved in E. faecalis carbohydrate uptake and utilization. Our discovery is the first to associate CcpA with the production of a major E. faecalis virulence factor, providing new insights into the regulation of E. faecalis pathogenesis.
INTRODUCTION
Enterococcus faecalis, a commensal organism of the gastrointestinal tract, is also known to be an opportunistic pathogen, a major cause of hospital-acquired infections, and a growing public health concern due to its increasing resistance to multiple antibiotics. A group of surface proteins, the microbial surface components recognizing adhesive matrix molecules (MSCRAMMs), play an important role in the virulence of E. faecalis by encouraging adherence and colonization to host tissue. Among them, Ace (adhesin of collagen from E. faecalis) plays an important role in adherence, presumably by mediating binding to extracellular matrix proteins, including both collagen and laminin (1). Ace has significant sequence homology with and a structural organization similar to that of Cna, a known collagen binding virulence factor of Staphylococcus aureus (2), and homology to Acm, a collagen adhesion protein in Enterococcus faecium (3). The association of Ace expression with virulence has been reported in many publications, including recent reports that an ace deletion mutant was significantly attenuated in both a rat endocarditis model and a murine urinary tract infection model, suggesting that Ace is involved in the pathogenesis of E. faecalis in both infectious endocarditis and urinary tract infections (4–6).
The surface expression of Ace has been shown to be regulated by many factors, including growth phase, temperature, and medium components, such as serum and collagen. As previously reported, Ace is maximally displayed on the surface of E. faecalis OG1RF in exponential phase and decreases to undetectable levels by stationary phase (7, 8). Growth at 46°C also significantly increases Ace expression compared to growth at 37°C; furthermore, reverse transcription-quantitative PCR (qRT-PCR) revealed an 18-fold increase in ace mRNA levels in cells grown in the presence of collagen IV compared to levels in the control (9). However, many of the genetic determinants that regulate Ace expression have yet to be evaluated.
Recently, we developed a whole-cell enzyme-linked immunosorbent assay (ELISA)-based library screening method to identify genetic elements that are involved in the display of E. faecalis cell surface proteins (8, 10). From a 536-membered E. faecalis OG1RF transposon (Tn) insertion mutant library, one mutant, an fsrB insertion mutant, was identified with significantly higher Ace expression levels at late growth phase. Further analysis demonstrated that E. faecalis mutants with disruption or deletion of fsrB or gelE, known to be regulated by fsrB, demonstrated higher Ace surface expression levels than the wild type and that gelatinase encoded by gelE is responsible for cleavage of Ace from the cell wall. These findings revealed one mechanism which E. faecalis utilizes to regulate Ace surface display. At high cell density, activation of the fsr quorum-sensing system leads to elevated production of gelatinase, which in turn cleaves Ace from the cell surface.
In this study, we utilized the same screening strategy to identify genes which affect Ace expression in early growth phase. An E. faecalis transposon insertion mutant with disruption of its ccpA gene was identified from the library screen with significantly lower levels of Ace surface expression than those in other mutant strains. The ccpA gene encodes the catabolite control protein A, which is a key regulator for carbon catabolite repression (CCR) (11). The involvement of ccpA in the regulation of Ace expression demonstrates a role for this gene in E. faecalis virulence factor surface presentation and, subsequently, the infection process.
MATERIALS AND METHODS
Chemicals.
Unless otherwise indicated, all culture media were purchased from Difco and all chemicals were purchased from Sigma (St. Louis, MO). Bacto brain heart infusion (BHI), Bacto tryptic soy broth without glucose (TSB), and Luria broth (LB) were prepared as described by the manufacturer (Becton, Dickinson). Bacto agar was used as a solidifying agent for all semisolid media. Oligonucleotides were purchased from Invitrogen (La Jolla, CA).
Bacteria strains, plasmids, and culture conditions.
Bacterial strains and plasmids used in this study are listed in Table 1. Escherichia coli cell cultures were grown at 37°C in LB medium, while E. faecalis strains were grown at 37°C in BHI, TSB, or tryptic soy agar. When required, the growth medium was supplemented with antibiotics at the following concentrations: 100 μg/ml ampicillin, 200 μg/ml erythromycin, and 10 μg/ml tetracycline. For culture conditions specific for in-frame deletion mutant construction, refer to the work of Kristich et al. (12).
Table 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Reference or source |
|---|---|---|
| Strains | ||
| E. faecalis | ||
| OG1RF | Wild-type strain, Fusr Rifr p-Cl-Pher | 32 |
| OG1RF Tn library | OG1RF Tn insertion mutants | 13 |
| CK111 | Conjugative donor | 12 |
| ΔccpA mutant | Deletion of ccpA from OG1RF | This study |
| pMSP/ccpA::Tn917 strain | ccpA::Tn917-containing plasmid pMSP3535-tet | This study |
| pMSP-ccpA/ccpA::Tn917 strain | ccpA::Tn917-containing plasmid pMSP-ccpA | This study |
| TX5467 | OG1RF ace (Δace) deletion mutant | 6 |
| JH2-2 | ||
| CL14 | JH2-2 ccpA insertion mutant | 33 |
| E. coli | ||
| XL1-Blue | Cloning host | Stratagene |
| EC1000 | Host for pCJK47 | 12 |
| Plasmids | ||
| pGEM-T Easy | Cloning plasmid | Promega |
| pMSP3535 | Nisin-inducible expression vector, Emr | 34 |
| pMSP3535-tet | Nisin-inducible expression vector | This study |
| pMSP-ccpA | pMSP3535-tet carrying a ccpA fragment encoding residues 1–333 | This study |
| pCJK47 | Plasmid for markerless exchange | 12 |
| pMC1 | Deletion of ccpA allele in pCJK47 | This study |
Whole-cell ELISA library screen.
The whole-cell ELISA screen was performed as previously described (10), with some modifications. The Tn library of cells was cultured for 24 h before being diluted 1:10 into new wells containing BHI medium and subsequently cultured at 37°C for 1.5 h. Anti-Ace monoclonal antibody (MAb) 70 at 2 μg/ml was used to label cells, followed by the addition of 100 μl of a 1:3,000 dilution of goat anti-mouse IgG-horseradish peroxidase (HRP) (Jackson ImmunoResearch). After 1 h of incubation at 37°C, the wells were washed three times with phosphate-buffered saline (PBS)–0.2% Tween 20 (PBST). Tetra-methylbenzidine (TMB) substrate was added, and the mixture was incubated at room temperature for 10 min before the reaction was stopped with the addition of 1 M H2SO4. The absorbance of each well was measured at an optical density at 450 nm (OD450).
Flow cytometry analysis.
Briefly, cells grown in BHI broth were collected in the early log phase of growth, centrifuged at 10,000 × g, and resuspended to an OD600 of 0.5 in PBS-bovine serum albumin (BSA) at 100 μl in a 1.5-ml Eppendorf tube. Diluted (PBS-BSA), purified anti-Ace MAb 70 at 10 μg/ml (100 μl) was added, and the mixture was allowed to stand for 30 min at room temperature. Following centrifugation and washing with 1 ml PBS-BSA, 100 μl of R-phycoerythrin (PE)-labeled goat F(ab′)2 anti-mouse IgG (Fc) (Jackson ImmunoResearch) was added, and the mixture was incubated for 30 min at room temperature. The bacteria were washed twice with 1 ml PBS-BSA/tube and resuspended in 0.5 ml PBS-BSA, followed by 0.5 ml 2% paraformaldehyde/tube in PBS. Fluorescence analysis was carried out with a Becton, Dickinson FACSCalibur flow cytometer.
Complementation of E. faecalis ccpA mutant.
To create the pMSP3535-tet vector, the tetM gene from HH22 was amplified by PCR using the primer pair AB262/AB263, shown in Table 2. The resulting 2,594-bp fragment was subcloned into pGEM-T Easy (Promega), digested using BglII, and ligated with pMSP3535 digested with BglII to yield the plasmid pMSP3535-tet. A 1,017-bp fragment containing the E. faecalis ccpA gene and its ribosome binding site was PCR amplified from E. faecalis OG1RF (primers are listed in Table 2) and cloned under the control of the nisin promoter of the shuttle vector pMSP3535-tet. The resulting plasmid, pMSP-ccpA, was transformed into the ccpA insertion mutant by electroporation. Nisin was added to the culture medium to induce exogenous expression of the ccpA gene. The cells were cultured in BHI with 25 ng/ml nisin overnight before being diluted to an OD of 0.1 in the same medium and subsequently cultured at 37°C for 1 h and harvested for flow cytometry analysis using anti-Ace MAb 70.
Table 2.
Primers designed and used in this study
| Primer function and name | Sequence (5′–3′) |
|---|---|
| ccpA cloning | |
| Forward primer | GCGCGGATCCATAGGAGAAGAAACATGG |
| Reverse primer | CCGGCTCGAGTTATTTTGTTGAACC |
| ccpA deletion | |
| ccpAFlank1F | CGGAATTCCGCCATTCATCACCAATTTTGATGGC |
| ccpAFlank1R | GCCGTGTCAATGCGCTTAT' |
| ccpAFlank2F | CTTTGTCATTAGTTTTGTTAATAAGCGCATTGACACGGCGACAACACGAGAAACAGTAGCC |
| ccpAFlank2R | GCTCTAGAGCCGGTTGACTGATGGCCTC |
| ccpAUpF | ATAAGCGCATTGACACGGC |
| ccpAdownR | CTGTTTCTCGTGTTGTCAATGGTA |
| ccpAOuterF | CAAGATGTTCTTTCGGCGTCA |
| ccpAOuterR | GTGTGCCTTGTGTTCGT |
| ace qRT-PCR set 1 | |
| Forward primer | AACAGCAACGGCGACTCAAC |
| Reverse primer | AACGGATAGTCTCGCTCAATTTG |
| ace qRT-PCR set 2 | |
| Forward primer | GGCAACGAGAACTTCCCAAA |
| Reverse primer | ACACCTAAAACGGCAAATGTACTTG |
| gelE qRT-PCR set | |
| Forward primer | CTAATCCAGAAATTGGTGCGGA |
| Reverse primer | AGCCATGGTTTCTGGTTGTCC |
| gyrB qRT-PCR | |
| Forward primer | ACCAACACCGTGCAAGCC |
| Reverse primer | CAAGCCAAAACAGGTCGCC |
| srtA qRT-PCR set | |
| Forward primer | ATTGATGACGTTCCTGGTCA |
| Reverse primer | TTTAATAGGCGTCGTTGCTG |
Construction of the ccpA in-frame deletion mutant.
The primer pairs ccpAFlank1F/ccpAFlank1FR and ccpAFlank2F/ccpAFlank2FR were used to amplify the sequences upstream and downstream of the ccpA gene. The forward primer of flank 1 was designed to incorporate an EcoRI site, and the reverse primer of flank 2 was designed to incorporate an XbaI site. These two amplicons were joined by crossover PCR, resulting in a 5′ EcoRI site and 3′ XbaI site, which were used for insertion into the recipient pCJK47 vector to generate pMC1. The plasmid pMC1 was then introduced into E. faecalis CKIII by electroporation and then conjugated into E. faecalis OG1RF. Allelic exchange was performed as described by Kristich et al. (12).
The mutant resulted in an in-frame deletion of nucleotides 57 to 916 out of a total open reading frame of 1,059 nucleotides. The mutant was verified using two sets of primers (Table 2) outside the deleted region.
Quantitative real-time PCR.
Cells grown to an OD600 equivalent to ∼1 in BHI were collected at early log phase and mid-log phase for RNA extraction. Total RNA was prepared using a NucleoSpin RNA II kit (Macherey-Nagel, Bethlehem, PA) and reverse transcribed to cDNA using SuperScript II reverse transcriptase and random primers (Invitrogen, CA) according to the method provided by the manufacturer. Quantitative PCR of cDNA was performed using a SYBR green PCR master mix kit and an ABI 7900 real-time PCR system (Applied Biosystems). The expression of ace and the reference gene gyrB were analyzed using primer pairs listed in Table 2. For each primer set, a reference curve was established using the genomic DNA purified from wild-type OG1RF cells. The amount obtained for ace transcripts was normalized with the amount of gyrB transcripts.
Gelatinase plate assay.
Briefly, gelatinase activity analysis was performed by inoculating overnight-cultured E. faecalis strains on Todd-Hewitt agar plates containing 3% gelatin, and the strains were grown overnight at 37°C. The gelatinase activities of different strains were determined by their halo formation.
Cell fractionation and Western blotting.
Cell wall-associated proteins were prepared and cell lysates and culture media were generated by following previously published protocols, with some modification. Briefly, OG1RF and ΔccpA cells grown in BHI broth were collected in exponential phase (OD, 0.4) and stationary phase (OD, 1.8) by centrifugation at 10,000 × g for 5 min. Culture medium supernatants were collected, concentrated to a 1/10 volume using a YM-10 spin column, and subjected to SDS-PAGE analysis. Two OD600 units of cells were washed twice with 20 mM Tris-HCl, 10 mM MgCl2, 0.5 M sucrose (pH 7.0) (TMS) buffer and incubated in 100 μl of the same buffer supplemented with 1 mM phenylmethylsulfonyl fluoride (PMSF)–EDTA-free Complete protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN) and 10 U mutanolysin at 37°C for 1 h. After centrifugation at 12,000 × g for 10 min, the supernatant cell wall extracts were collected for SDS-PAGE analysis. The protoplasts were resuspended in 100 μl of lysis buffer (20 mM Tris-HCl [pH 8.0], 200 mM NaCl, 1 mM EDTA, 1× protease inhibitor cocktail [Roche]) and mixed with 50 μl 0.1-mm-diameter zirconia beads (BioSpec Products, Bartlesville, OK), and the mixture was disrupted using a mini-beadbeater for 1 min. The cell lysates were centrifuged at 12,000 × g for 30 min to remove cell debris. The supernatants (cell lysate) were collected for SDS-PAGE analysis. The samples were loaded on 4% to 12% NuPAGE Novex Bis-Tris gels (Invitrogen) under reducing conditions in MOPS (morpholinepropanesulfonic acid) buffer and transferred to an Immobilon-P PVDF membrane (Millipore, Billerica, MA) according to the manufacturer's protocol. Membranes were then probed with anti-Ace MAb 70 (8) followed by IR800-conjugated goat anti-mouse IgG antibody (LI-COR Biosciences, Lincoln, NE), and DNA was detected using the LI-COR Odyssey scanner (LI-COR Biosciences).
Collagen adhesion assay.
A collagen adhesion assay was carried out as previously described (8), with some modification. Briefly, Microlon 600 ELISA plates were coated with human collagen IV (Sigma) at 10 μg/ml in PBS overnight at 4°C. The ELISA plates were blocked with 200 μl PBS-BSA/well. The overnight cultures of OG1RF, ΔccpA, ccpA::Tn917, and Δace cells were diluted into 125-ml centrifuge tubes containing 20 ml BHI broth and incubated for 1 or 4 h at 37°C. Each cell strain at a density equivalent to an OD600 of 1 was resuspended in 1 ml of PBS-BSA, and 100 μl/well was added to the collagen-coated plates for 1 h at room temperature under static conditions. The wells were washed, and the remaining attached bacterial cells were fixed with Bouin's solution. After additional washes, a 1% solution of crystal violet was applied and removed by using water flushes. The absorption of the solubilized (80:20, ethanol-acetone) crystal violet-stained cells was determined with a Multiskan EX plate reader with a 595-nm filter.
RESULTS
A library screen identified a gene which regulates Ace surface display in early growth phase.
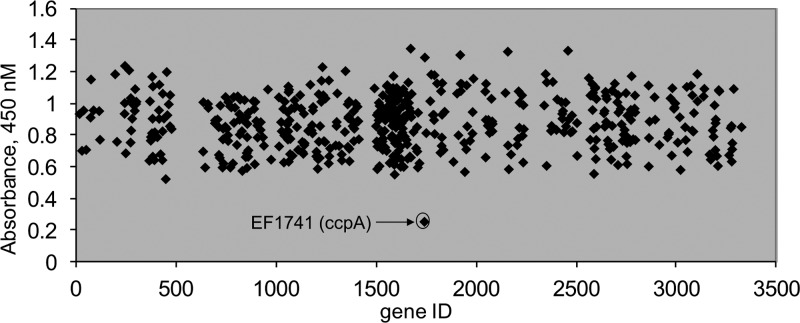
Based on the characteristic surface expression profile of Ace in wild-type OG1RF cells, namely, high levels of Ace at a low cell density (early growth phase), with the cell density significantly decreasing in later phases of growth, screening for mutants affecting Ace surface expression can be used to detect increases as well as decreases of Ace surface expression. We have previously reported that screening stationary-phase-grown E. faecalis cells identified fsrB as a gene negatively associated with Ace surface display (8). In this study, we applied a similar strategy to an E. faecalis OG1RF insertion library with 536 unique gene disruption mutants (13) to identify factors that influence Ace surface display during exponential phase (1.5 h). As shown in Fig. 1, the ELISA readouts for the vast majority of the mutants were between OD450s of 0.6 and 1.2, indicating a high level of Ace surface expression on cells cultured to exponential phase. The only mutant in this library that exhibited a significantly lower ELISA reading was a ccpA (EF_1741) insertion mutant with an OD450 of 0.26. The cell densities of all mutants grown under these conditions were determined, with no significant growth variability observed. These results indicated that disruption of the ccpA gene in this insertion (ccpA::Tn917) mutant altered the Ace surface expression profile.
Fig 1.
Identification of genes involved in the surface expression of Ace at early growth phase. The 540 Tn917 insertion mutants of OG1RF in the Tn library are represented by the gene identification (gene ID) number (EF number) on the x axis. Relative whole-cell ELISA signals are represented on the y axis. An early-growth-phase culture of each mutant was labeled with anti-Ace MAb and then with a goat anti-mouse IgG-HRP conjugate and developed with the TMB substrate. Absorbance values were determined by using a Thermo Multiskan EX plate reader.
ccpA is required for Ace surface expression.
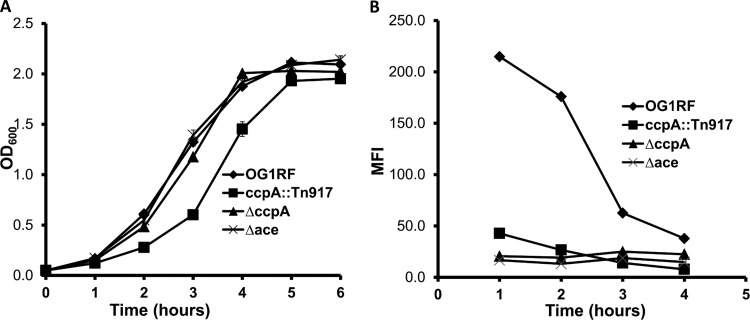
To confirm the whole-cell ELISA results, we analyzed the growth and Ace expression profile of the ccpA insertion mutant. Compared to the wild type or an ace deletion mutant, the ccpA::Tn917 strain has a slightly longer lag time to exponential phase but has ODs equal to those of the wild type and ace deletion mutant by 4 h of growth (Fig. 2A). Since Ace surface display is higher for cells at low cell density than at high cell density due to the absence of gelatinase cleavage early on, this lower growth rate of the ccpA::Tn917 mutant than that of wild-type cells cultured for same period in theory might be predicted to positively affect surface Ace. However, the ccpA::Tn917 strain exhibited an expression profile similar to that of the Ace deletion mutant throughout all growth phases by flow cytometry analysis (Fig. 2B). In comparison, wild-type strain OG1RF demonstrated much higher levels of Ace expression at early time points (mean fluorescent intensities [MFIs] of 215 and 176 at 1 and 2 h of culture, compared to MFIs of 43 and 27 for the ccpA::Tn917 strain collected at the same time points). These results clearly demonstrate that insertion of the transposon in the ccpA gene altered Ace surface display.
Fig 2.
Growth and Ace surface expression of wild-type OG1RF and ccpA mutants. (A) Growth curves of E. faecalis OG1RF, the ccpA::Tn917 strain, the ΔccpA mutant, and the Δace mutant were determined by measuring OD600s at various time points. Cells were grown in BHI broth medium at a starting OD600 of 0.05. (B) Levels of the bacterial-surface Ace expression of OG1RF, the ccpA::Tn917 strain, the ΔccpA mutant, and the Δace mutant were determined via flow cytometry at various time points using anti-Ace MAb 70 as the primary antibody and monitoring the mean fluorescence intensity of the population using R-phycoerythrin (PE)-labeled goat F(ab′)2 anti-mouse IgG (Fc). Plotted data are representative of 3 independent experiments.
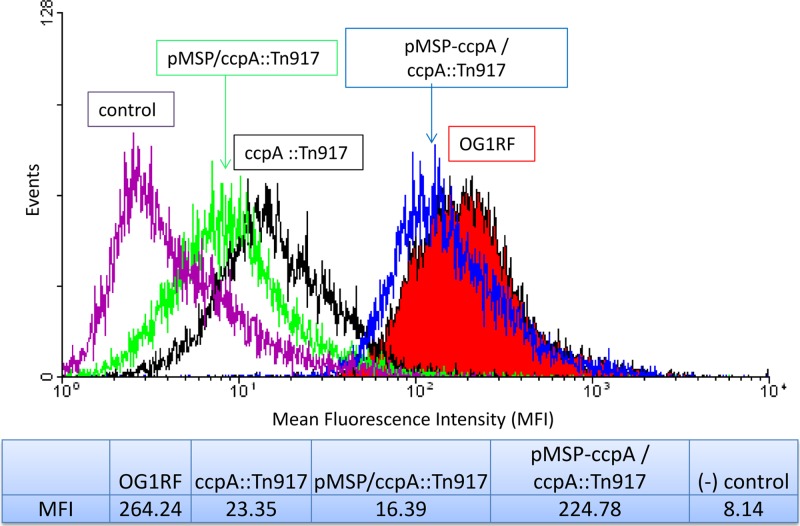
To confirm that the ccpA gene is associated with E. faecalis expression of Ace, we genetically complemented the ccpA::Tn917 strain using the plasmid pMSP-ace, a nisin-controlled expression vector carrying the E. faecalis ccpA gene. As shown in Fig. 3, the complemented pMSP-ccpA/ccpA::Tn917 strain exhibited an Ace expression phenotype that resembled that of wild-type OG1RF (MFI, 225 versus 264 by flow cytometry), which was significantly higher than that of the ccpA::Tn917 mutant strain alone or the ccpA::Tn917 mutant with the empty vector (MFIs, 23 and 16, respectively). This result demonstrated that ccpA is required for E. faecalis Ace surface expression in early growth phase.
Fig 3.
Complementation of ccpA restores Ace surface display. The nisin-inducible vector pMSP3535-tet alone and with a ccpA gene insertion were introduced into the ccpA::Tn917 mutant strain, and cells were analyzed for Ace expression using flow cytometry. Plotted data are representative of 3 independent experiments.
To ensure that the effects of the transposon insertion mutant were indeed due to loss of ccpA and not due to polar effects on surrounding genes and to further confirm the effect of ccpA on Ace expression, an in-frame deletion of ccpA was generated in the wild-type OG1RF strain (ΔccpA mutant). As demonstrated in Fig. 2A, the ΔccpA strain shows a growth profile similar to that of wild-type OG1RF. In addition, its Ace surface expression resembles that of the insertion mutant, which is consistently low in all growth phases. As shown in Fig. 2B, the Ace expression level of the ΔccpA strain in early growth (1 h of culture) is about 13-fold lower than that of the wild-type strain (MFI, 215 versus 16).
Ace surface expression is glucose independent.
Since ccpA plays an important role in carbon catabolite repression (CCR), we evaluated whether Ace expression is regulated through a CCR-mediated mechanism. To test this possibility, we studied whether different carbon sources affected Ace expression. As shown in Table 3, similar levels of Ace surface expression were detected by flow cytometry on wild-type OG1RF cells grown in TSB without sugar (MFI, 314.97), 0.5% glucose (phosphoenolpyruvate-sugar-phosphotransferase system [PTS] sugar) (MFI, 291.62), and 0.5% galactose (non-PTS) (14) (MFI, 258.4). In comparison, Ace surface expression levels on both ccpA mutants are consistently lower in all three media. All together, these results suggest that the regulatory effect of CcpA on Ace production may be through a CCR-independent mechanism.
Table 3.
Ace surface expression with different carbon sourcesa
| Strain | Medium | MFI |
|---|---|---|
| OG1RF | TSB | 314.97 |
| TSB + Glu | 291.62 | |
| TSB + Gal | 258.4 | |
| ccpA::Tn917 strain | TSB | 39.88 |
| TSB + Glu | 53.55 | |
| TSB + Gal | 49.21 | |
| ΔccpA mutant | TSB | 44.28 |
| TSB + Glu | 43.4 | |
| TSB + Gal | 44.69 |
Bacterial surface Ace expression was determined via flow cytometry using 1-h-cultured cells grown in various media at a starting OD600 of 0.05.
The regulation on Ace expression by ccpA is not at the transcriptional level.
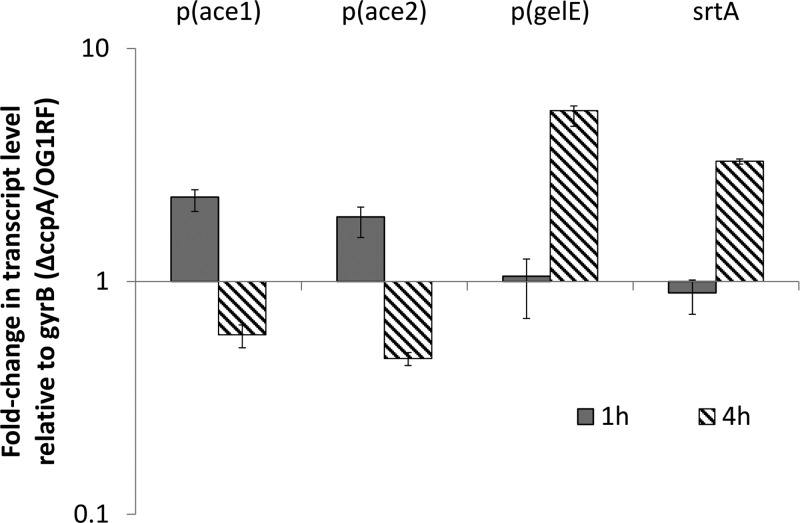
CcpA has a well-defined function as a transcriptional regulator via binding to target DNA at catabolite-responsive element (CRE) sites to alter gene transcription (15). To test whether loss of ccpA results in regulation of Ace expression at the transcriptional level, we utilized qRT-PCR to quantitatively determine ace mRNA levels in wild-type and ΔccpA strains. Two sets of primers targeting each end of ace mRNA were chosen to ensure detection of the full-length mRNA product. As shown in Fig. 4, no significant reduction of ace mRNA levels in the ccpA deletion mutant can be observed using either primer pair at analyzed time points. In fact, we observed the ace mRNA levels in the ΔccpA mutant to be about 1-fold higher than those of the wild-type strain in early-stage culture (1 h). Nonetheless, these results indicate that the phenotype of decreased Ace expression in the ΔccpA mutant is not due to regulation at the transcriptional level.
Fig 4.
Comparison of Ace and GelE mRNA levels in wild-type OG1RF and the ΔccpA mutant by qRT-PCR analysis. RNA was isolated from the early-log and early-stationary-phase-cultured wild-type and ΔccpA cells. Two sets of ace primers were used to target both the 5′ and 3′ ends of ace. The fold change in gene expression corresponds to the ratio of the transcript level of the ace gene in the ΔccpA strain to that of the wild type. The level of the ace transcript was determined in triplicate and normalized using gyrB transcript levels. Data shown here are averaged from three independent experiments.
As an LPXTG motif cell wall anchor protein, Ace requires the function of sortases for surface display, specifically, for attachment to peptidoglycan (16). It has been shown that OG1RF contains only one class A sortase, which is responsible for Ace anchoring to the cell wall (17). To study whether loss of ccpA affects Ace display through the function of sortase A, we compared the srtA mRNA levels of the wild-type and ΔccpA strains using qRT-PCR analysis. As shown in Fig. 4, no significant change was observed in the mRNA levels, suggesting that CcpA does not affect srtA transcription.
CcpA affects Ace protein production.
Since Ace RNA levels are not reduced in the ccpA deletion mutant, the regulation of Ace surface expression may occur at the translational level or during posttranslational events, including secretion, cell wall anchoring, or protease digestion. In our previous study of Ace surface expression (8), we demonstrated that Ace surface display is associated with the FSR quorum-sensing system through gelatinase, which functions as an extracellular protease to cleave the cell wall-anchored Ace from the enterococcal cell surface. Since deletion of ccpA does not affect Ace expression at the transcriptional level, it is possible that it alters Ace surface display through regulation of gelE expression. To test this hypothesis, we first compared the mRNA levels of gelE in the wild-type and ΔccpA strains. Although the ΔccpA strain showed higher levels of gelE mRNA than OG1RF at the late growth phase (4 h of culture), this still could not explain the Ace-deficient phenotype observed in the ΔccpA mutant at the early growth phase since no significant difference was observed in gelE mRNA levels in the cells cultured to early growth phase (Fig. 4). At the protein level, both the wild-type and ΔccpA strains are gelatinase positive, as demonstrated by a gelatinase plate assay whose results are shown in Fig. 5A, suggesting that gelE expression is not affected by a mutation in ccpA in OG1RF.
Fig 5.
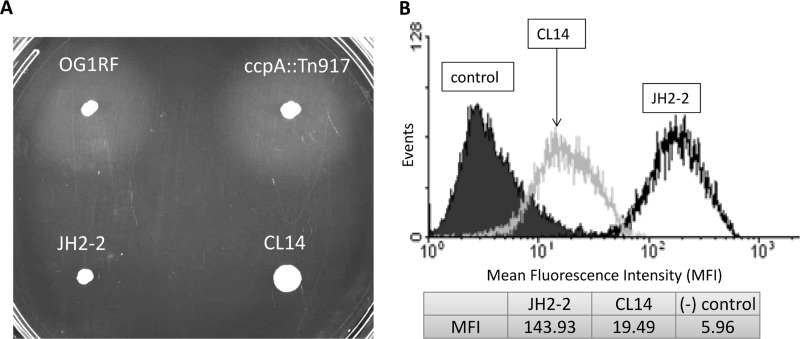
Gelatinase production and Ace expression of JH2-2 and the corresponding ccpA mutant. (A) The GelE activities of E. faecalis OG1RF, OG1RF ccpA::Tn917, JH2-2, and CL14 were determined by a gelatinase plate assay. The gelatinase activities of different strains were determined by assessing halo formation. (B) Surface Ace expression of E. faecalis JH2-2 and its ccpA insertion mutant CL14 was determined by flow cytometry after labeling Ace with anti-Ace MAb 70 primary antibody and R-phycoerythrin (PE)-labeled goat F(ab′)2 anti-mouse IgG (Fc) secondary antibody using 1-h-cultured cells grown in BHI at a starting OD600 of 0.05. The control population represents JH2-2 culture labeled with secondary antibody alone. Plotted data are representative of 3 independent experiments.
To further confirm whether gelatinase plays a role in ccpA-dependent Ace expression, we analyzed the Ace expression of a ccpA mutant, CL14 (generously provided by Axel Hartke), derived from an fsr-deficient strain, JH2-2 (18, 19); this deficiency results in attenuated gelatinase production. As demonstrated by the gelatinase plate assay whose results are shown in Fig. 5A, both JH2-2 and CL14 are gelatinase negative. The Ace surface expression levels during the early growth phase of JH2-2 and CL14 were compared, as shown in Fig. 5B. Like OG1RF and the ccpA::Tn917 mutant, the ccpA mutant strain of JH2-2 (CL14) showed significantly decreased Ace surface expression compared to that of JH2-2 (MFI, 19.49 versus 143.93), suggesting that gelatinase is indeed not involved in the ccpA-dependent Ace regulation process.
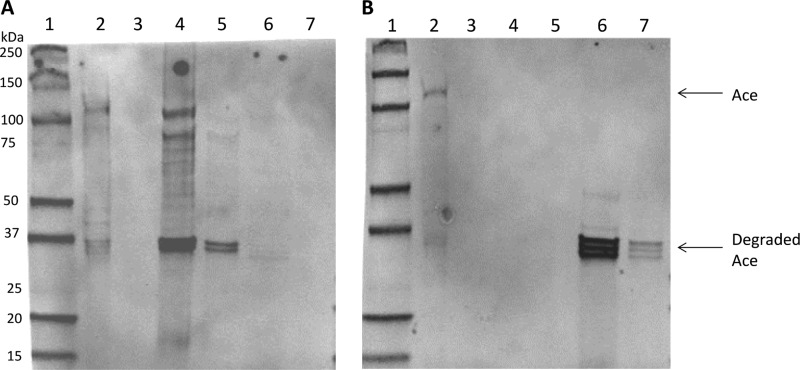
To test if CcpA affects Ace secretion or cell wall anchoring, we further explored the presence of Ace in protoplast lysates, mutanolysin-treated cell wall extracts, and conditioned culture media collected at the exponential and stationary phases of growth of OG1RF and the ΔccpA mutant using SDS-PAGE and anti-Ace MAb 70 probes (8). High-molecular-weight Ace bands were detected in the cytoplasmic and cell wall extracts of exponential-phase OG1RF cells (Fig. 6A, lanes 2 and 4), consistent with our previous flow cytometry results showing that Ace is readily detected on OG1RF cells grown to exponential phase. In comparison, no high-molecular-weight Ace band was detected in the cytoplasmic or cell wall extracts of exponential-phase ΔccpA cells (Fig. 6A, lanes 3 and 5), suggesting less Ace production. Ace degradation products (around 37 kDa) were also detected in the cell wall fractions, which may result from protease cleavage of Ace during mutanolysin processing, and is consistent with previously published results (16). Although Ace degradation products were seen in both ΔccpA and OG1RF cell wall extracts, it was more abundant in the OG1RF extracts, representing OG1RF's higher level of Ace surface expression. In the stationary phase, intact Ace is still detected in the cytoplasm of OG1RF but not in the ΔccpA mutant (Fig. 6B, lanes 2 and 3). Minimal levels of Ace can be detected in the cell wall extracts of either OG1RF or the ΔccpA mutant, consistent with the result of our flow cytometry, in which very little Ace was detected on stationary-phase cells. Cleaved products of Ace were detected in the culture media of both OG1RF and the ΔccpA mutant, and again, they were more abundant in OG1RF. Altogether, less Ace is detected in all fractions of the ΔccpA mutant than in OG1RF, suggesting that Ace protein production is lower in the ΔccpA mutant than in OG1RF.
Fig 6.
Comparison of Ace protein production levels by E. faecalis OG1RF and ΔccpA. Fractions of wild-type OG1RF and ΔccpA mutant cells were prepared at exponential phase (A) or stationary phase (B) and analyzed by Western blotting using anti-Ace MAb 70. Lanes: 1, prestained protein markers; 2, OG1RF cytosolic lysates; 3, ΔccpA cytosolic lysates; 4, OG1RF cell wall extracts; 5, ΔccpA cell wall extracts; 6, OG1RF culture medium; 7, ΔccpA cell culture medium.
Loss of ccpA impacts the ability of E. faecalis to adhere to collagen.
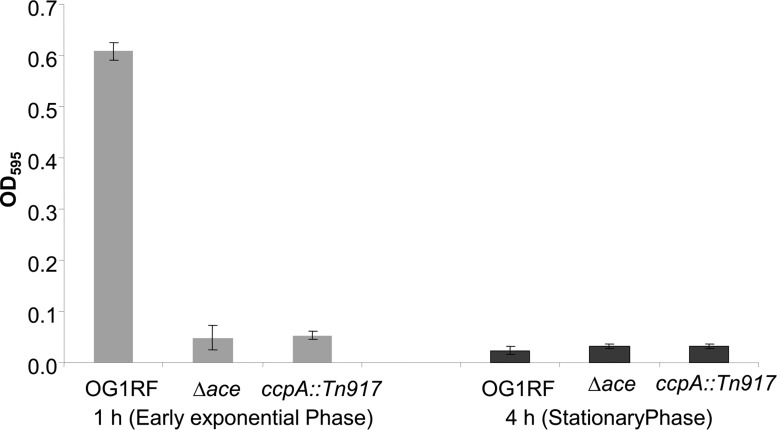
Since loss of Ace has been shown to decrease cellular adhesion to collagen, we evaluated the direct effect of the ccpA mutants on collagen adhesion. Compared to the wild type, the ccpA deletion mutant, the insertion mutant, and an ace deletion mutant all demonstrated significant reductions of collagen adhesion when cells were evaluated in early exponential phase (1 h) (Fig. 7). This is as expected, since only at early growth phase do wild-type OG1RF cells have high levels of Ace on their surfaces. In the late growth phase, all the tested strains showed reduced adhesion due to the absence of Ace on the surface (Fig. 7).
Fig 7.
Collagen adhesion of E. faecalis OG1RF and mutant strains at different growth phases. Net adherence values were determined (ANet = Acollagen − APBS). Adhesion and standard deviation calculations were averaged from triplicate samples. The data provided are from one of three representative experiments.
DISCUSSION
Carbon catabolite repression (CCR) is a common negative regulatory mechanism that bacteria utilize to inhibit catabolite-repressive genes in the presence of a rapidly metabolized carbon source (20). In Gram-positive bacteria, such as Bacillus subtilis, the repression of gene transcription is mediated through the interaction of three components: CcpA of the LacI/GalR family of transcriptional regulators, the phosphocarrier HPr protein of the phosphoenolpyruvate-sugar-phosphotransferase system (PTS), and the CRE site of the target genes (21). In the presence of glucose, HPr kinase is activated, resulting in the phosphorylation of HPr at Ser46 [Hpr(Ser46-P)]. The complex of CcpA and Hpr(Ser46-P) is then formed and binds to CREs of catabolite-sensitive operons to block gene transcription. The majority of the catabolite-sensitive operons are systems involved in the uptake and utilization of alternative carbohydrates. As an example, the expression of the B. subtilis gluconate operon (gnt) is repressed upon association of the CcpA-HPr complex with the CRE site located far downstream of the gnt transcription initiation site (22).
Despite the fact that CcpA is a key component of the CCR machinery, its role is not limited to repression of catabolite-repressive genes. In fact, CcpA, as a global transcriptional regulator, acts as both a repressor and an activator. Genes are activated by CcpA, including ackA and pta for the formation of acetate (23) and the ilv-leu operon for amino acid biosynthesis (24), suggesting the involvement of CcpA in anabolism as well. Moreover, in S. aureus, a large amount of CcpA-dependent gene regulation occurs in the absence of glucose, suggesting a glucose-independent CcpA regulatory mechanism (25).
Recently, the involvement of CcpA in bacterial virulence has been unveiled, as several reports directly associate CcpA with expression of virulence factors in S. aureus, group A streptococci (GAS), and other human pathogens (26–30). In S. aureus, it has been demonstrated that ccpA regulates the expression of a number of virulence factors, including the agr quorum-sensing system, protein A, and alpha-hemolysin (26). In GAS, CcpA is reported to influence the transcription of several well-characterized virulence factors, including cytolysin, streptolysin S, and a number of other virulence-related genes (30). An in vitro binding assay demonstrated binding between recombinant GAS CcpA and the streptolysin S-encoding gene pel (sagA) promoter region (27), suggesting the direct involvement of CcpA in streptolysin S transcriptional regulation. All these new discoveries suggest another important functional role of CcpA as a modulator of the expression of virulence-related genes as well, although mechanisms involved in these regulation processes are still largely unknown.
In E. faecalis, the role of CcpA in regulating the expression of catabolite-repressive and starvation-inducible proteins has been documented (18, 31). Our discovery in this report demonstrates for the first time a direct link between E. faecalis ccpA and virulence factor production. Although the mechanism by which ccpA regulates Ace surface expression is still not clear, we have demonstrated that the Ace mRNA level is not decreased in the ccpA mutant compared to that of the wild type, indicating that the regulation of Ace expression does not occur at the transcriptional level. Western blot analysis demonstrated that Ace protein is less in the ccpA mutant than in the wild type in all fractions, including cytosolic lysate, cell wall extracts, and culture media, suggesting that the loss of ccpA affects Ace protein production rather than secretion or cell wall anchoring. In addition, our results using the gelatinase-negative strain CL14 demonstrated that the decrease of Ace expression in the ccpA mutants is not associated with the function of gelatinase. Furthermore, the presence of various carbon sources in the culture media did not significantly change Ace surface display, suggesting that the regulatory effect of CcpA on Ace production in not through a CCR-mediated mechanism.
Our library screen method once again (8, 10) proved to be of great value in studying genetic determinants involved in bacterial surface protein expression, since it can detect changes that occur at any level from transcription to surface anchoring. Since Ace mRNA levels are not affected by deletion of ccpA, the link between these two factors would not have been discovered through traditional genetic or transcriptome analysis. Further application of this screening strategy will yield enhanced understanding of the regulation of protein production in the postgenomic era.
In conclusion, our study demonstrated that ccpA is required for Ace surface display in E. faecalis. Disruption of the ccpA gene leads to significant decreases of Ace expression at early growth phase, which in turn diminished the ability of the mutant strains to adhere to collagen. Our discovery that ccpA is required for the surface expression of a major E. faecalis virulence factor demonstrates a role for ccpA in the regulation of enterococcal pathogenesis.
ACKNOWLEDGMENTS
We thank Axel Hartke for providing the JH2-2 ccpA mutant CL14.
This work was supported in part by NIH grant R01 AI047923-14 from the NIAID to B.E.M. and NIH grants R01AI076406 and R56AI093699 to D.A.G. from the NIAID.
Footnotes
Published ahead of print 23 August 2013
REFERENCES
- 1.Nallapareddy SR, Qin X, Weinstock GM, Hook M, Murray BE. 2000. Enterococcus faecalis adhesin, ace, mediates attachment to extracellular matrix proteins collagen type IV and laminin as well as collagen type I. Infect. Immun. 68:5218–5224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Visai L, Xu Y, Casolini F, Rindi S, Hook M, Speziale P. 2000. Monoclonal antibodies to CNA, a collagen-binding microbial surface component recognizing adhesive matrix molecules, detach Staphylococcus aureus from a collagen substrate. J. Biol. Chem. 275:39837–39845 [DOI] [PubMed] [Google Scholar]
- 3.Nallapareddy SR, Weinstock GM, Murray BE. 2003. Clinical isolates of Enterococcus faecium exhibit strain-specific collagen binding mediated by Acm, a new member of the MSCRAMM family. Mol. Microbiol. 47:1733–1747 [DOI] [PubMed] [Google Scholar]
- 4.Lebreton F, Riboulet-Bisson E, Serror P, Sanguinetti M, Posteraro B, Torelli R, Hartke A, Auffray Y, Giard JC. 2009. ace, which encodes an adhesin in Enterococcus faecalis, is regulated by Ers and is involved in virulence. Infect. Immun. 77:2832–2839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nallapareddy SR, Singh KV, Duh RW, Weinstock GM, Murray BE. 2000. Diversity of ace, a gene encoding a microbial surface component recognizing adhesive matrix molecules, from different strains of Enterococcus faecalis and evidence for production of ace during human infections. Infect. Immun. 68:5210–5217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh KV, Nallapareddy SR, Sillanpaa J, Murray BE. 2010. Importance of the collagen adhesin ace in pathogenesis and protection against Enterococcus faecalis experimental endocarditis. PLoS Pathog. 6:e1000716. 10.1371/journal.ppat.1000716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall AE, Gorovits EL, Syribeys PJ, Domanski PJ, Ames BR, Chang CY, Vernachio JH, Patti JM, Hutchins JT. 2007. Monoclonal antibodies recognizing the Enterococcus faecalis collagen-binding MSCRAMM Ace: conditional expression and binding analysis. Microb. Pathog. 43:55–66 [DOI] [PubMed] [Google Scholar]
- 8.Pinkston KL, Gao P, Diaz-Garcia D, Sillanpaa J, Nallapareddy SR, Murray BE, Harvey BR. 2011. The Fsr quorum-sensing system of Enterococcus faecalis modulates surface display of the collagen-binding MSCRAMM Ace through regulation of gelE. J. Bacteriol. 193:4317–4325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nallapareddy SR, Murray BE. 2006. Ligand-signaled upregulation of Enterococcus faecalis ace transcription, a mechanism for modulating host-E. faecalis interaction. Infect. Immun. 74:4982–4989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao P, Pinkston KL, Nallapareddy SR, van Hoof A, Murray BE, Harvey BR. 2010. Enterococcus faecalis rnjB is required for pilin gene expression and biofilm formation. J. Bacteriol. 192:5489–5498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chauvaux S. 1996. CcpA and HPr(ser-P): mediators of catabolite repression in Bacillus subtilis. Res. Microbiol. 147:518–522 [DOI] [PubMed] [Google Scholar]
- 12.Kristich CJ, Chandler JR, Dunny GM. 2007. Development of a host-genotype-independent counterselectable marker and a high-frequency conjugative delivery system and their use in genetic analysis of Enterococcus faecalis. Plasmid 57:131–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garsin DA, Urbach J, Huguet-Tapia JC, Peters JE, Ausubel FM. 2004. Construction of an Enterococcus faecalis Tn917-mediated-gene-disruption library offers insight into Tn917 insertion patterns. J. Bacteriol. 186:7280–7289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heller K, Roschenthaler R. 1978. β-d-Phosphogalactoside galactohydrolase of Streptococcus faecalis and the inhibition of its synthesis by glucose. Can. J. Microbiol. 24:512–519 [DOI] [PubMed] [Google Scholar]
- 15.Ramseier TM, Reizer J, Kuster E, Hillen W, Saier MH. 1995. In vitro binding of the CcpA protein of Bacillus megaterium to cis-acting catabolite responsive elements (CREs) of gram-positive bacteria. FEMS Microbiol. Lett. 129:207–213 [DOI] [PubMed] [Google Scholar]
- 16.Rich RL, Kreikemeyer B, Owens RT, LaBrenz S, Narayana SV, Weinstock GM, Murray BE, Hook M. 1999. Ace is a collagen-binding MSCRAMM from Enterococcus faecalis. J. Biol. Chem. 274:26939–26945 [DOI] [PubMed] [Google Scholar]
- 17.Kemp KD, Singh KV, Nallapareddy SR, Murray BE. 2007. Relative contributions of Enterococcus faecalis OG1RF sortase-encoding genes, srtA and bps (srtC), to biofilm formation and a murine model of urinary tract infection. Infect. Immun. 75:5399–5404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leboeuf C, Leblanc L, Auffray Y, Hartke A. 2000. Characterization of the ccpA gene of Enterococcus faecalis: identification of starvation-inducible proteins regulated by ccpA. J. Bacteriol. 182:5799–5806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeng J, Teng F, Murray BE. 2005. Gelatinase is important for translocation of Enterococcus faecalis across polarized human enterocyte-like T84 cells. Infect. Immun. 73:1606–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorke B, Stulke J. 2008. Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat. Rev. Microbiol. 6:613–624 [DOI] [PubMed] [Google Scholar]
- 21.Deutscher J, Kuster E, Bergstedt U, Charrier V, Hillen W. 1995. Protein kinase-dependent HPr/CcpA interaction links glycolytic activity to carbon catabolite repression in gram-positive bacteria. Mol. Microbiol. 15:1049–1053 [DOI] [PubMed] [Google Scholar]
- 22.Fujita Y, Miwa Y. 1994. Catabolite repression of the Bacillus subtilis gnt operon mediated by the CcpA protein. J. Bacteriol. 176:511–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grundy FJ, Waters DA, Allen SH, Henkin TM. 1993. Regulation of the Bacillus subtilis acetate kinase gene by CcpA. J. Bacteriol. 175:7348–7355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ludwig H, Meinken C, Matin A, Stulke J. 2002. Insufficient expression of the ilv-leu operon encoding enzymes of branched-chain amino acid biosynthesis limits growth of a Bacillus subtilis ccpA mutant. J. Bacteriol. 184:5174–5178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seidl K, Muller S, Francois P, Kriebitzsch C, Schrenzel J, Engelmann S, Bischoff M, Berger-Bachi B. 2009. Effect of a glucose impulse on the CcpA regulon in Staphylococcus aureus. BMC Microbiol. 9:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seidl K, Stucki M, Ruegg M, Goerke C, Wolz C, Harris L, Berger-Bachi B, Bischoff M. 2006. Staphylococcus aureus CcpA affects virulence determinant production and antibiotic resistance. Antimicrob. Agents Chemother. 50:1183–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shelburne SA, III, Keith D, Horstmann N, Sumby P, Davenport MT, Graviss EA, Brennan RG, Musser JM. 2008. A direct link between carbohydrate utilization and virulence in the major human pathogen group A Streptococcus. Proc. Natl. Acad. Sci. U. S. A. 105:1698–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kietzman CC, Caparon MG. 2010. CcpA and LacD1 affect temporal regulation of Streptococcus pyogenes virulence genes. Infect. Immun. 78:241–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abranches J, Nascimento MM, Zeng L, Browngardt CM, Wen ZT, Rivera MF, Burne RA. 2008. CcpA regulates central metabolism and virulence gene expression in Streptococcus mutans. J. Bacteriol. 190:2340–2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kinkel TL, McIver KS. 2008. CcpA-mediated repression of streptolysin S expression and virulence in the group A streptococcus. Infect. Immun. 76:3451–3463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suarez CA, Blancato VS, Poncet S, Deutscher J, Magni C. 2011. CcpA represses the expression of the divergent cit operons of Enterococcus faecalis through multiple cre sites. BMC Microbiol. 11:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murray BE, Singh KV, Ross RP, Heath JD, Dunny GM, Weinstock GM. 1993. Generation of restriction map of Enterococcus faecalis OG1 and investigation of growth requirements and regions encoding biosynthetic function. J. Bacteriol. 175:5216–5223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacob AE, Hobbs SJ. 1974. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J. Bacteriol. 117:360–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bryan EM, Bae T, Kleerebezem M, Dunny GM. 2000. Improved vectors for nisin-controlled expression in gram-positive bacteria. Plasmid 44:183–190 [DOI] [PubMed] [Google Scholar]