Abstract
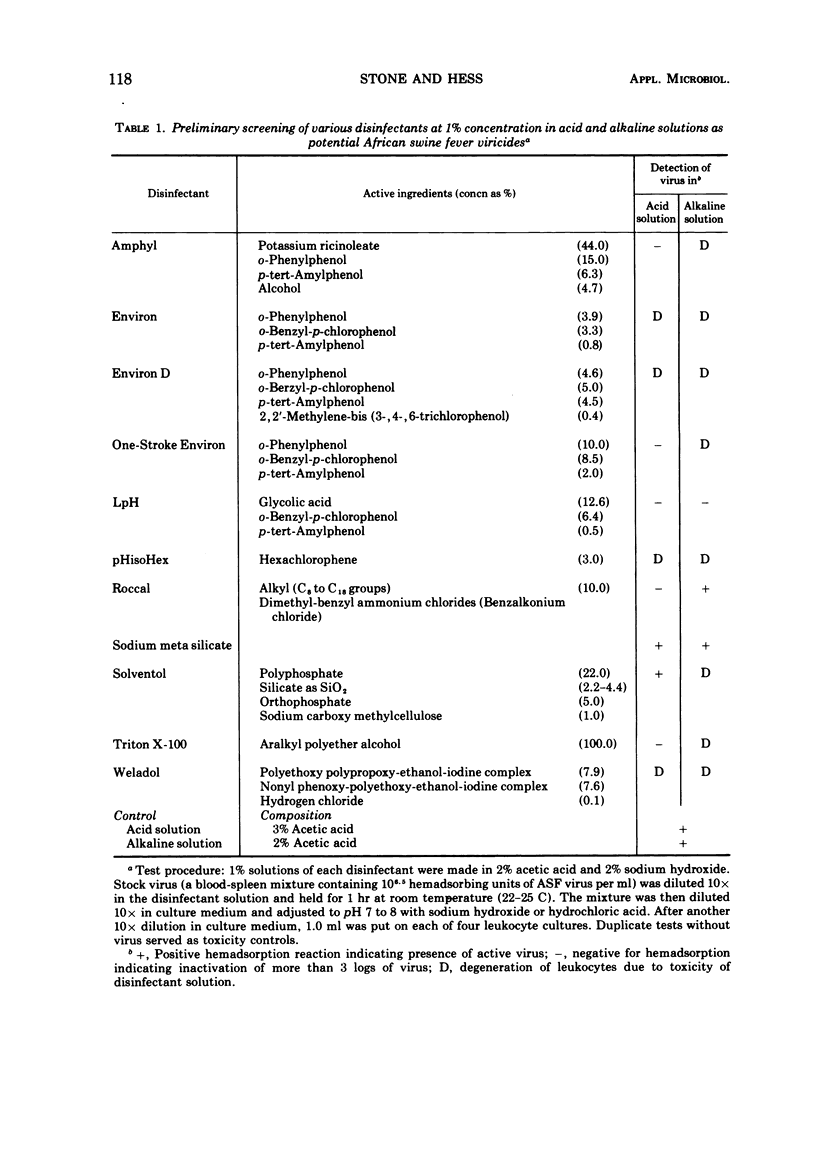
Ten commercially available disinfectants were tested at high pH in 2% sodium hydroxide and low pH in 2% acetic acid as inactivants for African swine fever (ASF) in a protein-rich blood-spleen homogenate. As assayed in leukocyte cultures, sodium hydroxide and acetic acid, sodium meta silicate and Roccal did not inactivate ASF virus in 1 hr at 22 to 25 C. Some viricidal activity as assayed in leukocyte cultures was found with Weladol, Triton X-100 Amphyl, pHisoHex, sodium dodecyl sulfate, LpH, Environ, Environ D, and One-Stroke Environ. Of these, the last four appeared to be most promising. When assayed in pigs, only One-Stroke Environ (1/E) was viricidal. Concentrations of 1.0, 0.75, and 0.5 were effective, but, at 0.25%, virus was not inactivated. The minimal time to inactivate ASF virus by 1% 1/E is 60 min. A room contaminated with ASF virus was made safe for pigs after 1 hr by spraying with 1% 1/E. The most active component of 1/E is o-phenylphenol. Although another component of 1/E, i.e., o-benzyl-p-chlorophenol, also has some activity, the mixture of the active components of 1/E is most effective against ASF virus. One of the soluble antigens associated with ASF virus is destroyed by 1/E.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adldinger H. K., Stone S. S., Hess W. R., Bachrach H. L. Extraction of infectious deoxyribonucleic acid from African swine fever virus. Virology. 1966 Dec;30(4):750–752. doi: 10.1016/0042-6822(66)90184-x. [DOI] [PubMed] [Google Scholar]
- FELLOWES O. N. Chemical inactivation of foot-and-mouth disease virus. Ann N Y Acad Sci. 1960 Jan 13;83:595–608. doi: 10.1111/j.1749-6632.1960.tb40932.x. [DOI] [PubMed] [Google Scholar]
- Greig A., Plowright W. The excretion of two virulent strains of African swine fever virus by domestic pigs. J Hyg (Lond) 1970 Dec;68(4):673–682. doi: 10.1017/s0022172400042613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HESS W. R., COX B. F., HEUSCHELE W. P., STONE S. S. PROPAGATION AND MODIFICATION OF AFRICAN SWINE FEVER VIRUS IN CELL CULTURES. Am J Vet Res. 1965 Jan;26:141–146. [PubMed] [Google Scholar]
- Hess W. R. African swine fever virus. Virol Monogr. 1971;9:1–33. doi: 10.1007/978-3-7091-3987-5_1. [DOI] [PubMed] [Google Scholar]
- MALMQUIST W. A., HAY D. Hemadsorption and cytopathic effect produced by African Swine Fever virus in swine bone marrow and buffy coat cultures. Am J Vet Res. 1960 Jan;21:104–108. [PubMed] [Google Scholar]
- SCHEIDEGGER J. J. Une micro-méthode de l'immuno-electrophorèse. Int Arch Allergy Appl Immunol. 1955;7(2):103–110. [PubMed] [Google Scholar]
- Stone S. S., Hess W. R. Antibody response to inactivated preparations of African swine fever virus in pigs. Am J Vet Res. 1967 Mar;28(123):475–481. [PubMed] [Google Scholar]
- Stone S. S., Hess W. R. Separation of virus and soluble noninfectious antigens in African swine fever virus by isoelectric precipitation. Virology. 1965 Aug;26(4):622–629. doi: 10.1016/0042-6822(65)90325-9. [DOI] [PubMed] [Google Scholar]
- Torrey J. P., Amtower W. C. Inactivation of hog cholera virus in blood and excreta with chemical disinfectants. Proc Annu Meet U S Anim Health Assoc. 1964;68:287–298. [PubMed] [Google Scholar]



