Abstract
Outer membrane vesicles (OMVs) of Gram-negative bacteria receive increasing attention because of various biological functions and their use as vaccines. However, the mechanisms of OMV release and selective sorting of proteins into OMVs remain unclear. Comprehensive quantitative proteome comparisons between spontaneous OMVs (SOMVs) and the outer membrane (OM) have not been conducted so far. Here, we established a protocol for metabolic labeling of neisserial proteins with 15N. SOMV and OM proteins labeled with 15N were used as an internal standard for proteomic comparison of the SOMVs and OMs of two different strains. This labeling approach, coupled with high-sensitivity mass spectrometry, allowed us to comprehensively unravel the proteome of the SOMVs and OMs. We quantified the relative distribution of 155 proteins between SOMVs and the OM. Complement regulatory proteins, autotransporters, proteins involved in iron and zinc acquisition, and a two-partner secretion system were enriched in SOMVs. The highly abundant porins PorA and PorB and proteins connecting the OM with peptidoglycan or the inner membrane, such as RmpM, MtrE, and PilQ, were depleted in SOMVs. Furthermore, the three lytic transglycosylases MltA, MltB, and Slt were less abundant in SOMVs. In conclusion, SOMVs are likely to be released from surface areas with a low local abundance of membrane-anchoring proteins and lytic transglycosylases. The enrichment of complement regulatory proteins, autotransporters, and trace metal binding and transport proteins needs to be explored in the context of the pathogenesis of meningococcal disease.
INTRODUCTION
Gram-negative bacteria release OM vesicles (OMVs, blebs) into the environment (1). OMVs are spherical bodies with diameters of 50 to 200 nm that are composed of a phospholipid bilayer containing OM proteins, lipopolysaccharide (LPS), and a cargo of periplasmic proteins (2).
Bacterial OMVs contribute to intraspecies and interspecies delivery of proteins (3, 4) and to nutrient acquisition (2). OMVs of pathogenic bacteria add to virulence, e.g., by delivery of toxins to host cells via membrane fusion (5).
OMV release has been proposed as a novel trait for protein secretion distinct from type I to VI secretion (6). Oxidative stress triggers vesiculation (7), which might be a mechanism to shed OM contaminated with antimicrobials or misfolded proteins (6). So far, no conserved mechanisms of bacterial vesiculation have been defined.
Global qualitative proteomic profiling has been conducted for OMVs of, e.g., Pseudomonas aeruginosa, Escherichia coli, Neisseria meningitidis, Myxococcus xanthus, and Acinetobacter baumannii primarily addressing the qualitative protein composition (8–13). Reports addressing the selective sorting of distinct proteins into OMVs are rare (14). A comprehensive quantitative comparison of the protein contents of OMVs and OMs has not been conducted so far.
N. meningitidis is restricted to humans and frequently colonizes the upper respiratory tract. Progression of meningococcal sepsis is exceptionally quick (15). Fulminant meningococcal sepsis (FMS) is accompanied by high death rates (summarized in: reference 16). During FMS, N. meningitidis releases large amounts of OMVs (spontaneous OMVs [SOMVs]), which are assumed to contribute to disease progression (17, 18). SOMVs have also been shown to protect meningococci from binding to neutrophil extracellular traps and subsequent killing in vitro (19).
Meningococcal OMVs are also components of a vaccine against N. meningitidis serogroup B strains (20). Detergent-extracted OMVs (DOMVs) (21), so-called native OMVs (NOMVs) induced by chelating agents (22), and SOMVs(12), which are regarded as the natural form of OMV release, are used as vaccine components. Initial comparative studies using two-dimensional gel electrophoresis coupled with liquid chromatography (LC)-tandem mass spectrometry (MS/MS) revealed qualitative protein differences between DOMVs and SOMVs (12). More recently, qualitative and quantitative differences among SOMVs, NOMVs, and DOMVs were addressed by using a gel-free approach (23). The comparison revealed enrichment of lipoproteins in SOMVs and NOMVs, whereas DOMVs were enriched with cytoplasmic proteins (23).
Here, we report a comprehensive quantitative proteomic comparison of SOMVs with the meningococcal OM. By applying a novel approach to the metabolic labeling of meningococcal proteins with 15N, we were able to precisely determine the protein compositions of SOMV and OM fractions. We observed an enrichment of numerous minor OM proteins in SOMVs, whereas the major OM proteins porins A and B and OM-anchoring proteins were depleted from SOMVs. This study shows the enrichment and depletion of proteins in SOMVs, shedding light on possible mechanisms of SOMV formation and on pathogenesis.
MATERIALS AND METHODS
Bacterial growth and metabolic labeling.
The unencapsulated mutants of strains MC58 (sequence type 74 [ST-74], disease isolate, 1985, United Kingdom [24]) and 2120 (ST-11, disease isolate, 1997, Germany [25]) were used. Bacteria were cultivated at 37°C in a CO2-enriched atmosphere on the novel minimal medium MMM (minimal medium for metabolic labeling of meningococci) containing 8 g/liter agarose. MMM is based on previously described minimal media (26, 27). MMM contains 140 mM NaCl, 40 mM Tris, 35 mM glucose monohydrate, 25 mM 14/15NH4Cl, 1 mM NaH2PO4, 0.5 mM CaCl2 · 2H2O, 0.2 mM MgSO4, 0.4 mM Na2S2O3 · 5H2O, 0.1 mM FeCl3 · 6H2O, 0.34 μM ZnSO4 · 7H2O, 0.16 μM Na2MoO4 · 2H2O, 0.08 μM MnCl2 · 4H2O, 0.08 μM CoCl2 · 6H2O, 0.08 μM CuSO4 · 5H2O, and 5 mM NaHCO3. The pH was adjusted to 7.6. For metabolic labeling of meningococcal proteins with 15N, unencapsulated strains MC58csb− and 2120csc− were passaged five times over MMM agar containing [15N]H4Cl (Cambridge Isotope Laboratories) to ensure the replacement of more than 99% of the 14N with 15N atoms within proteins. Of note, csb and csc are the new names of the polysialyltransferase genes of serogroups B and C, respectively (28).
Preparation of SOMVs and OM.
MC58csb− and 2120csc− grown on gonococci agar plates at 37°C and 5% CO2 for 10 h was resuspended in phosphate-buffered saline (PBS), pH 7.4, and adjusted to an optical density at 600 nm (OD600) of 0.1. The bacterial suspension was streaked onto agar plates with cotton swabs to achieve confluent growth. After 12 h of incubation at 37°C and 5% CO2, the bacteria were collected from the plates and resuspended in PBS. After centrifugation at 15,000 × g for 30 min, protease inhibitor (Roche Diagnostics) was added to the supernatant, which was subsequently filtered through 0.2-μm-pore-size filters (Sarstedt) to obtain SOMVs.
OM material was obtained from the bacterial pellet by the detergent-free “shake-and-bake” method (29, 30). In brief, the bacterial pellet was dissolved in 0.2 M lithium chloride–0.1 M lithium acetate (pH 6.0) and non-acid-washed glass beads (0.5 mm) (Sigma-Aldrich). The suspension was incubated at 45°C for 2 h while shaking at 250 rpm and subsequently centrifuged at 15,000 × g and 4°C for 30 min. Protease inhibitor (Roche Diagnostics) was added to the supernatant, which was subsequently filtered through 0.2-μm-pore-size filters (Sarstedt) to yield OM particles.
The SOMVs and OM from both filtrates were pelleted by ultracentrifugation (200,000 × g, 90 min, 4°C) with a Beckman L7-65 ultracentrifuge and a 70.1 Ti rotor. The SOMV and OM pellets were resuspended in PBS and centrifuged for another 90 min at 200,000 × g. This washing step was repeated twice. The final SOMV and OM pellets were resuspended in deionized H2O. A 500-μl portion of either SOMV or OM suspension was mixed with 500 μl of 25% sucrose containing 1 mM EDTA. These samples were carefully loaded onto a density gradient composed of 1-ml portions of 25, 30, 35, 40, 45, 50, and 55% sucrose in 1 mM EDTA and then ultracentrifuged (30,000 rpm, 16 h, 4°C) in a Beckman L7-65 ultracentrifuge with a 40.1 Ti rotor. Pure SOMV and OM particles were obtained from the 45 and 50% sucrose fractions. Residual sucrose was removed by serial washing with deionized water and subsequent ultracentrifugation. Final SOMV and OM suspensions were stored at −80°C.
14N-15N mixing and proteomic measurement.
A sample containing equal amounts of 15N-labeled SOMV and the OM proteins was used for quantitative assessment of the amounts of peptides within SOMV and OM fractions according to reference 31. By the addition of equal amounts of an internal standard to the samples compared, any possible bias incurred during sample preparation was kept to a minimum. Following the preparation of SOMV and OM fractions, samples were subjected to one-dimensional SDS-gel electrophoresis, followed by tryptic in-gel digestion according to previously described methods (32). Protein concentrations of SOMV and OM preparations were quantified with the Pierce bicinchoninic acid protein assay kit.
MS measurement.
The digests were measured by LC-MS/MS as described in reference 33. In brief, the peptide samples were subjected to reversed-phase C18 column chromatography on the nanoACQUITY UPLC system (Waters Corporation) with a two-column setup by which the sample was first concentrated and desalted and then eluted on a separation column. MS/MS data were acquired with an LTQ-Orbitrap mass spectrometer (Thermo Fisher) coupled on line to the chromatography system.
Data analysis and 14N/15N quantification.
Acquired MS/MS data were processed to yield *.dta files from *.raw files by using Sorcerer v3.5 (Sage-N Research, Inc.) with no charge state deconvolution or deisotoping performed. The data were then searched by using Sorcerer-SEQUEST (ThermoFinnigan; version v.27, rev. 11) against the N. meningitidis strain MC58 (34) and FAM18 (35) target decoy protein sequence databases (complete proteome set of N. meningitidis strain MC58 or FAM18 with a set of common laboratory contaminants) compiled with BioEdit (36). The searches were performed in two iterations, i.e., with search parameters light (enzyme type, trypsin [KR]; peptide tolerance, 10 ppm; tolerance for fragment ions, 1 amu; b- and y-ion series; variable modification, methionine [15.99 Da]; a maximum of three modifications per peptide allowed) and with search parameters heavy (parameters identical to those used for 14N/light with the mass shift of all amino acids completely labeled with 15N taken into account).
Resulting *.dta and *.out files were assembled and filtered with DTASelect (revision 2.0.25) (parameters GeLC-MS: −y 2 −c 2 −C 4 −here −decoy Rev_ −p 2 −t 2 −u −MC 2 −i 0.3 −fp 0.005). The resulting protein identification data were cured with an in-house java script to ensure a minimum of two different peptides for each protein identified.
The cured search results were then used for parsing by the Census software (37) to obtain the relative quantitative data of 14N peaks (sample) versus 15N peaks (pooled reference). Quantification results were exported (R2 values of >0.7; only unique peptides; proteins failing to be relatively quantified were checked manually in the graphical user interface for on/off proteins). Proteins relatively quantified with at least two peptides were taken into account in the subsequent analysis.
Data analysis: normalized spectral abundance factors (NSAFs).
For determination of NSAFs, MS/MS spectra were extracted from *.raw files by Sorcerer v3.5 (Sage-N Research Inc.). Database searching was carried out by Sorcerer-SEQUEST matching the same parameters as for the relative quantification but leaving out the second iteration of searches for the reference mass trace. Scaffold (version 3.4.3; Proteome Software Inc.) was then used to filter the peptide and protein identifications: For reliable peptide identifications, deltaCn scores of greater than 0.1 and XCorr scores of greater than 2.2, 3.3, and 3.8 for z = 2, 3, and 4 were required. Protein identifications were accepted if at least two identified peptides were detected with the peptide identification criteria just described. NSAFs were then calculated according to Zybailov et al. (38).
Prediction of protein localization.
Subcellular localization of proteins was predicted according to the NeMeSys database containing eight (re)annotated N. meningitidis genomes (39). For all proteins without annotation of subcellular localization in the NeMeSys database, the PSORTb algorithm was used for prediction (40).
Protein determination, Western blotting, and enzyme-linked immunosorbent assay (ELISA).
SDS-PAGE and immunoblot analysis were done as described previously (41). Antibodies against PorA (P.1.7., NIBSC), PorB (polyclonal antiserum against PorB), ZnuD (1042, kindly provided by Martine Bos, University of Utrecht, Utrecht, The Netherlands), NMB0801 (kindly provided by Mariagrazia Pizza, Novartis Vaccines and Diagnostics, Siena, Italy), FetA (42), Opc (B306; MPI for Infection Biology, Berlin, Germany), fHbp (741 antiserum against fHbp variant 1, kindly provided by Maurizio Comanducci, Novartis Vaccines and Diagnostics, Siena, Italy), and NadA (kindly provided by Nikolaus Ackermann, Max von Pettenkofer Institut, Munich, Germany). Gels were scanned with a GS-800 calibrated densitometer (Bio-Rad) and analyzed by the PDQuest Advanced software (version 8.0; Bio-Rad).
ELISA for quantification of NspA was performed as described previously (43), with the following exceptions. Twenty-microliter aliquots containing 1, 5, 10, 20, or 40 μg of total SOMV or OM fraction protein were added to wells of flat-bottom 96-well microtiter plates (Greiner bio-One). Anti-NspA antibody 14C7 (43) was used for detection. The OD405 was determined with a microtiter plate reader (Thermo Labsystems).
Transmission electron microscopy (TEM).
For the visualization of SOMVs and OMs by TEM, vesicles were purified as described above and set to a final concentration of 400 μg protein per ml of PBS. Thirty microliters of the SOMV or OM suspension was dropped onto Parafilm (Brand). Glow-discharged 300-mesh carbon-coated copper grids were carefully placed on top of the SOMV or OM drops for 5 min to allow the adsorption of vesicles to the grids. The grids were then air dried for 10 min and fixed with ethanol. After two washes with PBS, the grids were negatively stained with 2% uranyl acetate for 1 min. Micrographs were generated with an accelerating voltage of 80 kV and a 906 E electron microscope (Carl Zeiss), a Proscan slow-scan camera, and the corresponding iTEM software (Soft Imaging System).
RESULTS
Conditions for stable isotope labeling with 15N and SOMV and OM isolation.
In this study, a quantitative comparison of the SOMV and OM proteins of N. meningitidis is reported. Quantitative assessment required the development of a metabolic-labeling approach for N. meningitidis to produce internal standards, which greatly increase the precision of quantitative comparisons (33).
To achieve reliable labeling of N. meningitidis proteins with 15N, we developed a minimal medium for stable isotope labeling of N. meningitidis called MMM.
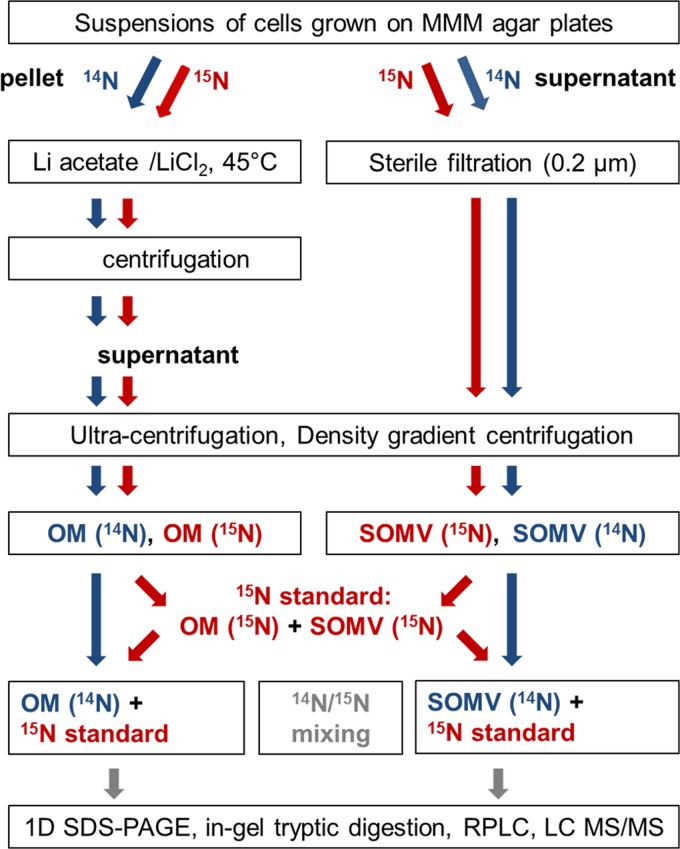
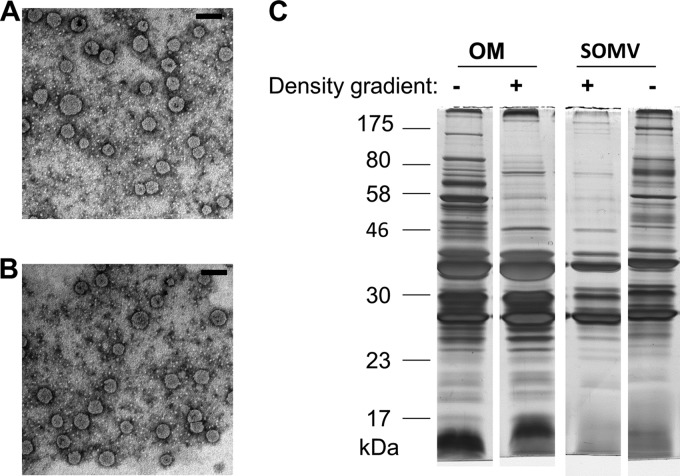
Figure 1 summarizes the work flow for the preparation and analysis of SOMVs and the OM. SOMVs were obtained from supernatants of N. meningitidis cells grown on MMM agar plates, followed by resuspension and centrifugation steps. The N. meningitidis OM was obtained from the cell pellet by the so-called “shake-and-bake” method, which applies lithium chloride-lithium acetate at 45°C. The mild procedure (free of detergents and EDTA) is regarded as the gold standard for neisserial OM purification because of its high specificity for the OM compartment (29, 30, 44) and high yields of OM (45). Unlike methods using lysozyme-EDTA to induce spheroplasting during OM purification, such as the “Osborne procedure” (46), the “shake-and bake” procedure does not significantly alter the protein composition of OMs by the release of major membrane proteins such as PorA (47). Both SOMVs and OM particles were obtained by ultracentrifugation. Contaminating protein aggregates were removed from the preparations by density gradient centrifugation. For quality control, SOMVs and the OM were subjected to TEM and denaturing SDS-PAGE (Fig. 2). TEM of both SOMVs (Fig. 2A) and the OM (Fig. 2B) showed membranous round bodies with diameters of 50 to 100 nm. SDS-PAGE of SOMV and OM preparations (Fig. 2C) revealed a strong reduction of the number of protein bands in comparison with whole-cell lysates and an enrichment of proteins of 23 to 46 kDa. The banding patterns very much resembled those described previously (12).
Fig 1.
Work flow of proteome analysis. OMs were obtained with lithium chloride-lithium acetate at 45°C. Density gradient centrifugation of the SOMV and OM fractions was performed to reduce cytosolic protein contamination. A 15N-labeled standard was mixed with the unlabeled samples. Proteins were digested in gel and then subjected to reversed-phase LC (RPLC). LC-MS/MS was performed with high resolution and high mass accuracy. 1D, one dimensional.
Fig 2.
SOMV and OM visualization and purity analysis. (A) SOMVs and (B) OM particles obtained with lithium chloride-lithium acetate were negatively stained with uranyl acetate and visualized by TEM. The scale bars represent 100 nm. (C) Comparison of OM and SOMV protein profiles by SDS-PAGE (12.5%). Samples before and after density gradient centrifugation are shown.
Quantification of SOMV and OM proteins against the 15N-labeled internal standard.
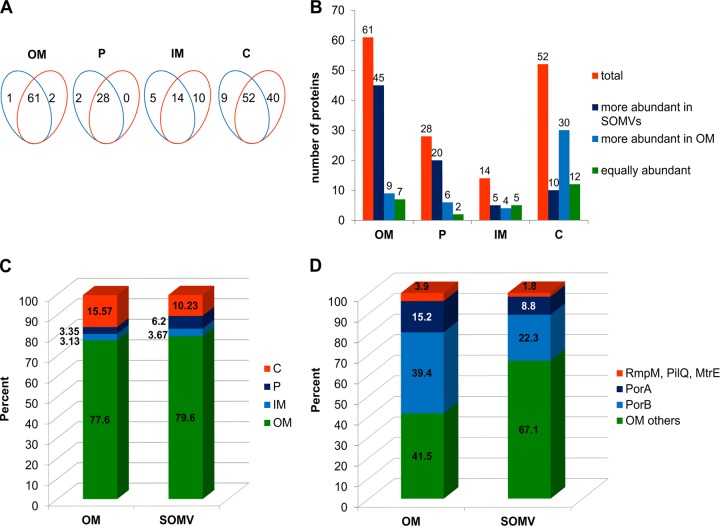
To determine differences in protein abundance between the SOMV and OM fractions, metabolically 15N-labeled mixtures of SOMV and OMV preparations were used as internal standards. Equal protein amounts of either unlabeled (light, 14N) SOMV or OM preparations were mixed with equal amounts of the metabolically labeled (heavy, 15N) internal standard. By mixing 14N- and 15N-labeled proteins, every light protein species of unknown amount in either SOMV or OM preparations corresponded to a heavy protein species counterpart that was obtained by running the preparation procedure (Fig. 1). Using equal amounts of internal standard in the samples compared, any possible bias incurred from sample preparation to MS was kept to a minimum. Unlabeled peptides from either the SOMV or the OM fraction were quantified by highly accurate MS against the respective 15N-labeled peptides from the internal standard by discriminating the mass shift between the light (14N) and heavy (15N) labeled peptides of the same peptide species. For each peptide of SOMV and OM samples, the proportions of heavy and light variants were determined, which allowed the calculation of relative protein abundance between SOMVs and the OM. Only 14N-labeled proteins, which were quantified in at least two out of three biological replicates against the 15N internal standard, were included in the data analysis. One hundred seventy-two proteins from SOMV fractions and 207 proteins from OM fractions were quantified against the 15N standard, with an overlap of 155 proteins (Fig. 3A). We observed an almost complete overlap of quantified OM and periplasmic proteins between OM and SOMV fractions, while the overlap of inner membrane (IM) proteins and cytosolic proteins was about 50% (Fig. 3A). For a comprehensive list of all of the proteins quantified against the metabolic standard, see Table S1 in the supplemental material. For 155 proteins found in both the SOMV and OM fractions, a ratio of abundance relative to the 15N standard was calculated (Fig. 3B). Of the 61 OM proteins, 45 were significantly more abundant in the SOMV fraction whereas 9 were more abundant in the OM fraction. Of the 28 periplasmic proteins, 20 were more abundant in the SOMV fraction. Thirty of 52 cytoplasmic proteins were enriched in the OM fraction (Fig. 3B). Using the NSAFs (Table 1), which are a measure of the relative portion of a protein in proteomic analysis, we determined the relative amounts of proteins with the respective subcellular localizations (Fig. 3C). Almost 80% of the protein in the SOMV and OM fractions were in the OM, which suggests a high purity of the preparations. SOMVs contained about twice as much periplasmic as OM protein. Periplasmic proteins are very likely packed into SOMVs during natural vesiculation, while the lithium chloride-acetate-induced OM release does not include such a biological packing step. The OM fraction comprised about 50% (16% versus 10%) more cytosolic protein mass than SOMVs. This difference might be due to cell lysis during OM preparation or a selective binding of cytosolic proteins to certain OM areas.
Fig 3.
Comparative proteome analysis of SOMV and OM with a metabolic standard. OM and from SOMV proteins were quantified against a metabolic standard. (A) Venn diagrams showing the numbers of proteins from either SOMVs (blue) or the OM (orange) that could be quantified against the metabolic standard. The proteins were sorted according to predicted subcellular localization. P, periplasm; C, cytosol. (B) Distribution of quantified proteins according to enrichment or depletion in SOMVs. (C) Relative amounts of proteins with distinct subcellular localizations. The sum of the NSAFs was calculated for each subcellular localization. (D) Proportions of all OM proteins that are major OM proteins (PorA, PorB) and minor OM proteins (RmpM, PilQ, MtrE) according to NSAFs. SOMVs were depleted of PorA, PorB, and RmpM plus PilQ plus MtrE in favor of the other 56 OM proteins.
Table 1.
Functional categorization of OM and periplasmic proteins statistically significantly over- and underrepresented in SOMVs
| Functional category and protein name or NMB designation | Function | Fold SOMV/OM changea | Relative amt in SOMVsb |
|---|---|---|---|
| OM proteins overrepresented | |||
| Autotransporter proteins | |||
| App | Adhesion and penetration protein | 4.72 | 213.33 |
| AusI | Autotransporter, serine-type peptidase | 4.10 | 116.33 |
| NalP | Autotransporter protease | 3.10 | 19 |
| Iga | IgA-specific serine endopeptidase | 2.71 | 278 |
| NadA | Putative adhesin/invasin | 2.04 | 19 |
| Complement regulatory proteins | |||
| fHbp | Factor H-binding protein | 3.78 | 84 |
| Opc | Class 5 OM protein | 3.16 | 2,132 |
| NspA | OM protein | 3.10 | 36.67 |
| NhbA | Heparin-binding protein A | 2.32 | 5.67 |
| Two-partner secretion proteins | |||
| HprA | TPSS,c secreted component | 2.71 | 6 |
| HprB | TPSS, transporter protein | 1.94 | 16.67 |
| Tr metal (iron, zinc, others) binding and uptake proteins | |||
| Tbp2 | Transferrin-binding protein 2 | 3.80 | 10.67 |
| Tbp1 | Transferrin-binding protein 1 | 1.99 | 130.33 |
| LbpA | Lactoferrin-binding protein A | 1.78 | 5 |
| HpuB | Hemoglobin receptor | 1.38 | 36 |
| ZnuD | Zinc uptake component D | 1.66 | 220.33 |
| FetA | Iron-regulated OM protein FrpB | 1.56 | 17.67 |
| FetB | Iron(III) ABC transporter, periplasmic binding protein | 1.06 | 4 |
| NMB1829 | TonB-dependent receptor | 1.32 | 7.33 |
| Autolysin OMPLA | OM phospholipase A | 1.49 | 30.67 |
| Unknown function | |||
| NMB1578 | Conserved hypothetical protein | 1.96 | |
| NMB2139 | Conserved hypothetical protein | 1.79 | 7.67 |
| NMB0035 | Conserved hypothetical protein | 1.13 | 25.67 |
| NMB2134 | Conserved hypothetical protein | 1.06 | 13.67 |
| NMB1212 | Hypothetical protein | 1.85 | 2.33 |
| NMB0841 | Hypothetical protein | 1.68 | 5 |
| NMB1084 | Hypothetical protein | 1.63 | 43.67 |
| NMB1035 | Hypothetical protein | 1.46 | 22.67 |
| NMB1213 | Putative lipoprotein | 1.74 | 14.33 |
| NMB0204 | Putative lipoprotein | 1.73 | 17 |
| NMB0873 | Putative OM protein | 1.63 | 10.67 |
| NMB2091 | Putative hemolysin | 1.58 | 40.67 |
| NMB0707 | Putative rare lipoprotein B | 1.53 | 20.67 |
| NMB1898 | Lipoprotein | 1.40 | 21.67 |
| Other transporters | |||
| PotD-3 | Spermidine/putrescine ABC transporter | 2.06 | 5.67 |
| PotD-1 | Spermidine/putrescine ABC transporter | 1.61 | 34 |
| NMB0787 | Amino acid ABC transporter | 1.23 | 46 |
| NMB0623 | Spermidine/putrescine ABC transporter | 1.18 | 22.67 |
| OM proteins underrepresented | |||
| Porins | |||
| PorA | Major OM protein PorA | 0.70 | 621.33 |
| PorB | Major OM protein PorB | 0.69 | 1,604 |
| Membrane integrity protein RmpM | OM protein class 4 | 0.58 | 46.67 |
| Drug efflux protein MtrE | Multidrug efflux pump channel protein | 0.31 | 22 |
| Pilus-related protein PilQ | Pilus pore protein PilQ | 0.44 | 69 |
| Other | |||
| Mip | Macrophage infectivity potentiator | 0.77 | 24.33 |
| Unknown function | |||
| NMB0086 | Hypothetical protein | 0.92 | 2.67 |
| NMB1369 | Hypothetical protein | 0.82 | 2.33 |
| NMB1592 | Putative lipoprotein | 0.81 | 7.67 |
| Periplasmic proteins overrepresented | |||
| Transporter proteins | |||
| NMB0041 | ABC transporter, periplasmic solute binding protein | 3.43 | 17 |
| FbpA | Iron(III) ABC transporter, periplasmic binding protein | 3.27 | 74.33 |
| ZnuA | Zinc uptake component A | 2.05 | 14 |
| NMB1017 | Sulfate ABC transporter, periplasmic sulfate-binding protein | 1.20 | 22.67 |
| Functional maturation of proteins | |||
| DsbC | Thiol-disulfite interchange protein | 3.41 | 10.67 |
| NMB0006 | Thioredoxin-related protein | 3.05 | 5.67 |
| DsbA3 | Thiol-disulfite interchange protein | 2.85 | 2 |
| Unknown function | |||
| NMB1030 | Conserved hypothetical protein | 3.44 | 17 |
| NMB1963 | Conserved hypothetical protein | 3.39 | 11.67 |
| NMB0355 | Conserved hypothetical protein | 3.35 | 8.33 |
| NMB0783 | Conserved hypothetical protein | 3.33 | 5.33 |
| NMB0345 | Putative cell-binding factor | 3.27 | 52.67 |
| NMB1475 | Conserved hypothetical protein | 3.13 | 55.33 |
| NMB2074 | Hypothetical protein | 1.85 | 11.67 |
| NMB1557 | Conserved hypothetical protein | 1.83 | 2 |
| NMB0088 | Putative OM protein P1 | 1.5 | 157.33 |
| NMB0872 | Conserved hypothetical protein | 1.28 | 5.67 |
| NMB0313 | Conserved hypothetical protein | 1.26 | 25.33 |
| Cell wall biogenesis protein NMB1620 | Putative muramoyltetrapeptide carboxypeptidase | 1.35 | 3 |
| Periplasmic proteins underrepresented | |||
| Cell wall remodeling | |||
| MltA | Membrane-bound lytic murein transglycosylase A | 0.89 | 6 |
| MltB | Membrane-bound lytic murein transglycosylase B | 0.75 | 0 |
| Slt | Putative soluble lytic murein transglycosylase | 0.75 | 22.33 |
| Functional maturation of proteins | |||
| DsbA1 | Thiol-disulfite interchange protein | 0.81 | 3.33 |
| DsbA2 | Thiol-disulfite interchange protein | 0.78 | 14 |
| Membrane integrity protein NMB0109 | Putative PG-binding periplasmic protein | 0.79 | 27 |
Ratio of relative SOMV and OM protein abundances obtained by MS quantification against the metabolically labeled internal standard.
Relative amount of protein within the SOMV fraction based on NSAFs. Statistical significance was calculated with Student's t test. P < 0.05 was defined as statistically significant.
TPSS, two-partner secretion system.
Functional categorization of differentially distributed OM and periplasmic proteins.
Among the 40 OM proteins significantly enriched in SOMVs, we identified 5 of the 6 known autotransporter proteins of strain MC58, i.e., App, Iga, AusI, NadA, and NalP (Table 1). The complement regulatory proteins fHbp, Opc, NspA, and NhbA, as well as proteins of the two-partner secretion system (HprA, HprB), were also enriched in SOMVs. Furthermore, trace metal binding and uptake proteins (Tbp1/2, LbpA, FetA/B, ZnuD, HpuB), the autolysin OMPLA, other transporters, and a group of 14 protein of unknown function were enriched in SOMVs. The two porins PorA and PorB, the peptidoglycan (PG)-binding protein RmpM, the multidrug efflux pump channel protein MtrE, the pilus pore protein PilQ, the macrophage infectivity potentiator, and three proteins of unknown function were depleted in SOMVs compared with those in the OM (Table 1). Of the 20 periplasmic proteins more abundant in SOMVs, 7 are involved in transport, functional maturation of proteins, and cell wall biogenesis. However, the functions of 11 of these 20 proteins remain unclear. The three putative lytic transglycosylases MltA, MltB, and Slt, which are likely to be involved in cell wall remodeling and have been suggested to be involved in SOMV release (48), were depleted in SOMVs. Since the total amounts of PorA and PorB make up more than 50% of the OM protein mass (Fig. 3D), the subtle but statistically significant 1.4-fold depletion of PorA and PorB in SOMVs (Table 1) has a considerable impact on the total protein composition.
Verification of proteomic data.
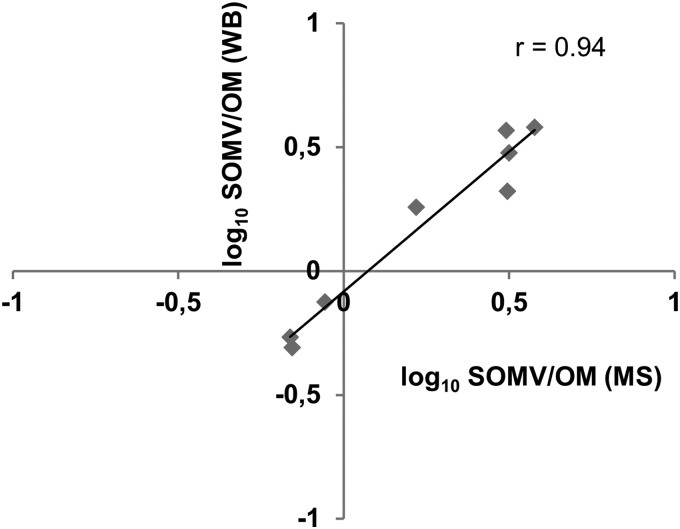
To verify differences in the relative abundance of proteins in SOMVs and the OM, we quantified fHbp, NMB0801, NMB1475, Opc, ZnuD, PorA, and PorB by Western blotting and NspA by ELISA. Subsequently, we calculated the SOMV/OM relative protein abundance ratios. We observed a good correlation between the SOMV/OM values obtained from MS and Western blotting or ELISA, respectively (Fig. 4).
Fig 4.
Verification of proteomic data by Western blotting and ELISA. PorA, PorB, ZnuD, Opc, fHbp, NMB0801, and NMB1475 from SOMVs and the OM were detected with specific antibodies. The relative abundance of a certain protein in both fractions was calculated from the respective band intensities. SOMV/OM protein abundances obtained by MS (x axis) and Western blotting (WB, y axis) are shown. The Pearson correlation coefficient (r) was 0.94. For NspA, the SOMV/OM value was calculated from ELISA.
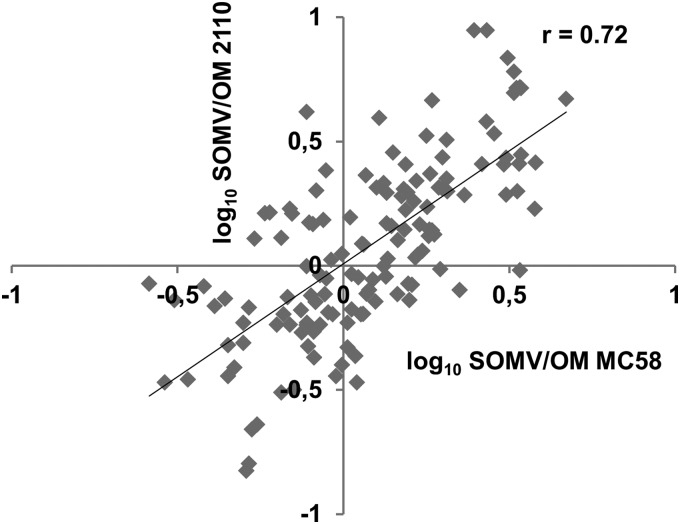
We finally asked whether the relative abundance of proteins in SOMVs and the OM is a general property of meningococci. For that purpose, we also purified the SOMVs and OM from strain 2120csc−. For as-yet-unknown reasons, we were not able to sufficiently separate OM particles of strain 2120csc− from contaminating cytosolic proteins by gradient centrifugation. The bands were repeatedly fuzzy and difficult to retain. This probably led to an increased proportion of cytosolic proteins (22% versus 16%) and an increased number of quantified cytosolic proteins (164 versus 52) Therefore, we compared the SOMV/OM values of only 155 proteins obtained by MS from strain MC58csb− against the SOMV/OM values of the same proteins from strain 2120csc− (Fig. 5). We observed a good correlation between the SOMV/OM ratios of the two strains. This finding indicates that the differential distribution of proteins between SOMVs and the OM is a general trait of genetically distant lineages of the genetically very heterogeneous species N. meningitidis. However, of the 73 over- and underrepresented OM and periplasmic proteins in SOMVs of strain MC58csb−, 12 showed the contrary distribution in strain 2120csc−, i.e., NMB1497, NMB1578, NMB0035, NMB2091, PotD-1, NMB0787, NMB0623, PorA, NMB1592, NMB0872, MltA, and NMB0109.
Fig 5.
Distribution of proteins between SOMVs and the OM in different isolates. Included are all of the proteins for which an SOMV/OM ratio was calculated against the metabolic standard in both strains MC58csb− (x axis) and 2120csc− (y axis). The Pearson correlation coefficient (r) was 0.73.
DISCUSSION
Proteomic studies of meningococcal SOMVs.
The focus of this study was a comprehensive quantitative comparison of the SOMVs and OM of N. meningitidis. The use of state-of-the-art metabolic labeling and highly accurate MS allowed us to reliably determine the number and abundance of proteins and the enrichment of several proteins in either fraction. In this work, we were able to quantify 155 proteins, including 61 OM proteins from both the SOMV and OM fractions, against a metabolic standard composed of a mixture of the SOMV and OM fractions. Thus, this study provides the most comprehensive view of protein expression in SOMVs of N. meningitidis. Post et al. identified 48 unique proteins comprising 27 OM proteins in SOMVs, while Ferrari et al. identified 65 unique proteins comprising 35 OM proteins according to the criteria used in this study (12, 13). Recently, van de Waterbeemd et al. compared the distribution of proteins in SOMVs and two different vaccine preparations (EDTA-induced NOMVs and sodium deoxycholate-induced DOMVs) also with the help of a labeled internal standard (49). They identified 140 unique proteins in SOMVs, but only 113 or 86 of those proteins, respectively, could be quantified against NOMVs or DOMVs. Both sodium deoxycholate and EDTA treatments led to the enrichment of OMV preparations with cytosolic proteins (23). However, EDTA has been shown to cause the depletion of major OM proteins from OM preparations (47). Aiming at qualitative and quantitative comparisons of SOMVs with the OM, we therefore decided to obtain the OM fraction by the mild “shake-and-bake” method. This is the first proteomic study of SOMVs that also determined the relative amount of each quantified protein within the protein samples. The sums of the relative quantities of all proteins (NSAFs) from both the SOMV and OM fractions belonging to a distinct subcellular localization showed that the proteomic composition with regard to subcellular localization was very similar between the SOMV and OM fractions (Fig. 3B). Both SOMV and OM preparations contained a large proportion of OM proteins but only small proportions of cytosolic proteins. We are therefore confident that OM preparations obtained by the “shake-and-bake” method resemble the natural OM with regard to protein composition.
Protein composition of OMVs with regard to subcellular localization.
We identified and quantified 62 OM proteins but also high numbers of IM (n = 14), periplasmic (n = 28), and cytosolic (n = 52) proteins. Periplasmic proteins are a natural cargo packed into the blebs during vesiculation. Cytosolic proteins found in OMV fractions have been regarded as an artifact of the OMV preparation process (for a review, see reference 50). Since high levels of cytosolic proteins (30 to 40%) and IM proteins are constantly found in highly purified OMV preparations of different species (9–11), their identification might prove them to be a natural constituent of OMVs. Neisserial SOMVs contain DNA (51). In the same way, as it is counterintuitive that cytosolic proteins are constituents of OMVs, one may ask how bacterial DNA is packed into OMVs. The recently discovered OM-IM vesicles (O-IMVs) of Shewanella vesiculosa might explain the presence of both DNA and cytosolic proteins. During the formation of O-IMVs, in addition to OM and periplasmic content, IM and portions of the cytosol are also included (52). Future studies have to clarify whether the formation of O-IMVs is also applicable to N. meningitidis.
Differential distribution of proteins between SOMVs and the OM.
We identified and quantified 61 OM proteins, which represent about 80% of the protein mass of SOMVs according to the NSAFs. Of those proteins, 45 were enriched and 9 were depleted in SOMVs. Among the enriched OM proteins, we identified five out of six autotransporter proteins encoded in N. meningitidis strain MC58 (53). Autotransporters are involved in autoaggregation (54) and contribute to the adhesion (55) and biofilm formation of N. meningitidis (56). Furthermore, the meningococcal two-partner secretion system contributing adhesion (57) was enriched in SOMVs. It remains to be determined what impact the enrichment of the above-mentioned proteins has on nasopharyngeal colonization or pathogenesis.
Among the OM proteins enriched in SOMVs, we found complement regulatory proteins fHbp, Opc, NspA, and NhhA (55, 58–60). Why does N. meningitidis sort complement regulatory proteins into SOMVs, although these proteins modulate complement activation on the bacterial surface? Opc- and NhbA-mediated elevated vitronectin and heparin binding might enable SOMVs to act as an anticoagulant counteracting blood coagulation to contain the systemic spread of meningococci during systemic infection. Furthermore, effective heparin or vitronectin binding (55) might elevate SOMV binding to host structures.
OMVs mediate intraspecies communication (4). The fusion of meningococci with SOMVs might explain why DNA-containing SOMVs contribute to natural transformation (51). In this study, several factors involved in iron or zinc acquisition and transport were enriched in SOMVs. It remains to be determined whether iron- or zinc-loaded SOMVs might be conducive to trace element acquisition. The altruistic sacrifice of subpopulations in favor of surviving subpopulations is a well-known phenomenon among prokaryotes (61). SOMVs might contribute to trace element acquisition under zinc- or iron-depleted conditions for a surviving meningococcal subpopulation, whereas the SOMV-donating subpopulation is expected to die.
The largest group of overrepresented OM proteins in SOMVs (n = 14) was without any known function. Furthermore, 11 out of 18 overrepresented periplasmic proteins, which resemble the SOMV cargo, lack any function so far. Among the proteins depleted in SOMVs, we identified the porins PorA and PorB, which contribute more than 50% of the mass of all of the OM proteins according to the NSAFs. Since PorA and PorB are the most abundant proteins within the OM, a moderate relative depletion within SOMVs of both proteins already has a substantial impact on protein composition. Proteins anchoring the OM with the PG or the IM, such as the pilus pore PilQ, RmpM, and the multidrug efflux pump channel protein MtrE, were less abundant in SOMVs.
Mechanisms of SOMV formation.
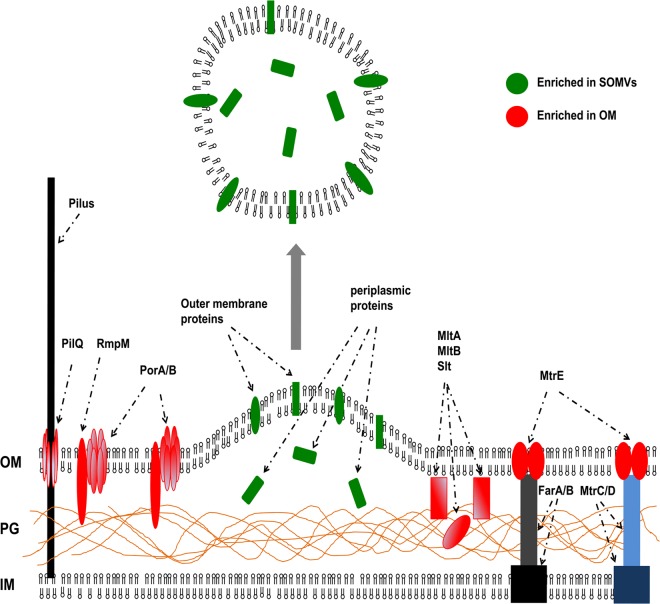
Well-established theories on the mechanism of OMV formation in different bacteria include the reduction of OM linkage with PG and a local PG fragment-mediated increase of turgor, which both might induce OMV release (2). In N. meningitidis, the knockout of membrane-bound lytic transglycosylase A (MltA), which is involved in cell wall recycling and remodeling, elevated SOMV release (48). Here, we demonstrate that the membrane-bound lytic transglycosylases MltA and MltB and the soluble lytic transglycosylase Slt were depleted from SOMVs. Therefore, SOMVs might be shed from OM regions with reduced lytic transglycosylase concentration and activity. Recently, elevated levels of SOMV release were shown for a mutant form of RmpM (62), which is an OM protein with PG-binding capacity (63). In this study, RmpM, PilQ, and MtrE were depleted from SOMVs. Since MtrE belongs to two different transmembrane multidrug efflux systems (MtrCDE, FarABmtrE) and the pilus pore PilQ is part of the transmembrane pilus apparatus, we furthermore suggest that SOMVs are released from OM areas with reduced linkage to PG or IM. The depletion of PorA and PorB from SOMVs is explained by complex formation with RmpM. Complexes of RmpM with PorA and PorB are the most abundant protein complexes within the OM of N. meningitidis (64). A hypothesis of SOMV release that we derived from the proteomic data is shown in Fig. 6.
Fig 6.
Proposed model of SOMV release based on the proteomic approach. SOMVs are released from OM areas where proteins or protein complexes are underrepresented (red) or from areas with low lytic transglycosylase activity, and they link the OM with the PG or the IM. Therefore, proteins such as PorA, PorB, RmpM, PilQ, Slt, MltA, and MltB are underrepresented in SOMVs. Instead, SOMVs are enriched with other OM proteins such as complement inhibitory proteins and autotransporters (green).
We provide evidence of quantitative proteomic differences between SOMVs and the OM the vesicles are derived from. The SOMVs were enriched for autotransporters, complement regulatory proteins, and trace metal uptake and transport components, which implies as-yet-unknown functions of SOMVs during the onset and progression of meningococcal disease. The depletion of OM-anchoring proteins and lytic transglycosylases points to the involvement of two distinct mechanisms of SOMV formation in N. meningitidis.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by Bundesministerium für Bildung und Forschung grant Medizinische Infektionsgenomik: Proteomics von Meningokokken und Pneumokokken, Teilprojekt Würzburg, Foerderkennzeichen 0315828D, to U.V.
We thank Janos Groh and Heinrich Blazyka, Würzburg, Germany, for expert help with electron microscopy.
Footnotes
Published ahead of print 26 July 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00625-13.
REFERENCES
- 1.Ellis TN, Kuehn MJ. 2010. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol. Mol. Biol. Rev. 74:81–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kulp A, Kuehn MJ. 2010. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu. Rev. Microbiol. 64:163–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berleman J, Auer M. 2013. The role of bacterial outer membrane vesicles for intra- and interspecies delivery. Environ. Microbiol. 15:347–354 [DOI] [PubMed] [Google Scholar]
- 4.Pathak DT, Wei X, Bucuvalas A, Haft DH, Gerloff DL, Wall D. 2012. Cell contact-dependent outer membrane exchange in myxobacteria: genetic determinants and mechanism. PLoS Genet. 8:e1002626. 10.1371/journal.pgen.1002626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kesty NC, Mason KM, Reedy M, Miller SE, Kuehn MJ. 2004. Enterotoxigenic Escherichia coli vesicles target toxin delivery into mammalian cells. EMBO J. 23:4538–4549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McBroom AJ, Kuehn MJ. 2007. Release of outer membrane vesicles by Gram-negative bacteria is a novel envelope stress response. Mol. Microbiol. 63:545–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van de Waterbeemd B, Zomer G, van den Ijssel J, van Keulen L, Eppink MH, van der Ley P, van der Pol LA. 2013. Cysteine depletion causes oxidative stress and triggers outer membrane vesicle release by Neisseria meningitidis; implications for vaccine development. PLoS One 8:e54314. 10.1371/journal.pone.0054314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwon SO, Gho YS, Lee JC, Kim SI. 2009. Proteome analysis of outer membrane vesicles from a clinical Acinetobacter baumannii isolate. FEMS Microbiol. Lett. 297:150–156 [DOI] [PubMed] [Google Scholar]
- 9.Choi DS, Kim DK, Choi SJ, Lee J, Choi JP, Rho S, Park SH, Kim YK, Hwang D, Gho YS. 2011. Proteomic analysis of outer membrane vesicles derived from Pseudomonas aeruginosa. Proteomics 11:3424–3429 [DOI] [PubMed] [Google Scholar]
- 10.Lee EY, Bang JY, Park GW, Choi DS, Kang JS, Kim HJ, Park KS, Lee JO, Kim YK, Kwon KH, Kim KP, Gho YS. 2007. Global proteomic profiling of native outer membrane vesicles derived from Escherichia coli. Proteomics 7:3143–3153 [DOI] [PubMed] [Google Scholar]
- 11.Kahnt J, Aguiluz K, Koch J, Treuner-Lange A, Konovalova A, Huntley S, Hoppert M, Sogaard-Andersen L, Hedderich R. 2010. Profiling the outer membrane proteome during growth and development of the social bacterium Myxococcus xanthus by selective biotinylation and analyses of outer membrane vesicles. J. Proteome Res. 9:5197–5208 [DOI] [PubMed] [Google Scholar]
- 12.Post DM, Zhang D, Eastvold JS, Teghanemt A, Gibson BW, Weiss JP. 2005. Biochemical and functional characterization of membrane blebs purified from Neisseria meningitidis serogroup B. J. Biol. Chem. 280:38383–38394 [DOI] [PubMed] [Google Scholar]
- 13.Ferrari G, Garaguso I, Adu-Bobie J, Doro F, Taddei AR, Biolchi A, Brunelli B, Giuliani MM, Pizza M, Norais N, Grandi G. 2006. Outer membrane vesicles from group B Neisseria meningitidis delta gna33 mutant: proteomic and immunological comparison with detergent-derived outer membrane vesicles. Proteomics 6:1856–1866 [DOI] [PubMed] [Google Scholar]
- 14.Haurat MF, Aduse-Opoku J, Rangarajan M, Dorobantu L, Gray MR, Curtis MA, Feldman MF. 2011. Selective sorting of cargo proteins into bacterial membrane vesicles. J. Biol. Chem. 286:1269–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrick WW. 1919. Extrameningeal meningococcus infection. Arch. Intern. Med. 23:409–418 [Google Scholar]
- 16.van Deuren M, Brandtzaeg P, van der Meer JW. 2000. Update on meningococcal disease with emphasis on pathogenesis and clinical management. Clin. Microbiol. Rev. 13:144–166, table of contents [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brandtzaeg P, Kierulf P, Gaustad P, Skulberg A, Bruun JN, Halvorsen S, Sorensen E. 1989. Plasma endotoxin as a predictor of multiple organ failure and death in systemic meningococcal disease. J. Infect. Dis. 159:195–204 [DOI] [PubMed] [Google Scholar]
- 18.Brandtzaeg P, Bryn K, Kierulf P, Ovstebo R, Namork E, Aase B, Jantzen E. 1992. Meningococcal endotoxin in lethal septic shock plasma studied by gas chromatography, mass-spectrometry, ultracentrifugation, and electron microscopy. J. Clin. Invest. 89:816–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lappann M, Danhof S, Guenther F, Olivares-Florez S, Mordhorst IL, Vogel U. 2013. In vitro resistance mechanisms of Neisseria meningitidis against neutrophil extracellular traps. Mol. Microbiol. 89:433–449 [DOI] [PubMed] [Google Scholar]
- 20.Sierra GV, Campa HC, Varcacel NM, Garcia IL, Izquierdo PL, Sotolongo PF, Casanueva GV, Rico CO, Rodriguez CR, Terry MH. 1991. Vaccine against group B Neisseria meningitidis: protection trial and mass vaccination results in Cuba. NIPH Ann. 14:195–207; discussion 208–210 [PubMed] [Google Scholar]
- 21.Claassen I, Meylis J, van der Ley P, Peeters C, Brons H, Robert J, Borsboom D, van der Ark A, van Straaten I, Roholl P, Kuipers B, Poolman J. 1996. Production, characterization and control of a Neisseria meningitidis hexavalent class 1 outer membrane protein containing vesicle vaccine. Vaccine 14:1001–1008 [DOI] [PubMed] [Google Scholar]
- 22.Keiser PB, Gibbs BT, Coster TS, Moran EE, Stoddard MB, Labrie JE, III, Schmiel DH, Pinto V, Chen P, Zollinger WD. 2010. A phase 1 study of a group B meningococcal native outer membrane vesicle vaccine made from a strain with deleted lpxL2 and synX and stable expression of opcA. Vaccine 28:6970–6976 [DOI] [PubMed] [Google Scholar]
- 23.van de Waterbeemd B, Mommen GP, Pennings JL, Eppink MH, Wijffels R, van der Pol LA, de Jong AP. 15 February 2013, posting date Quantitative proteomics reveals distinct differences in the protein content of outer membrane vesicle vaccines. J. Proteome Res. (Epub ahead of print.) 10.1021/pr301208g [DOI] [PubMed] [Google Scholar]
- 24.Dunn KL, Virji M, Moxon ER. 1995. Investigations into the molecular basis of meningococcal toxicity for human endothelial and epithelial cells: the synergistic effect of LPS and pili. Microb. Pathog. 18:81–96 [DOI] [PubMed] [Google Scholar]
- 25.Vogel U, Morelli G, Zurth K, Claus H, Kriener E, Achtman M, Frosch M. 1998. Necessity of molecular techniques to distinguish between Neisseria meningitidis strains isolated from patients with meningococcal disease and from their healthy contacts. J. Clin. Microbiol. 36:2465–2470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baart GJ, Zomer B, de Haan A, van der Pol LA, Beuvery EC, Tramper J, Martens DE. 2007. Modeling Neisseria meningitidis metabolism: from genome to metabolic fluxes. Genome Biol. 8:R136. 10.1186/gb-2007-8-7-r136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lappann M, Haagensen JA, Claus H, Vogel U, Molin S. 2006. Meningococcal biofilm formation: structure, development and phenotypes in a standardized continuous flow system. Mol. Microbiol. 62:1292–1309 [DOI] [PubMed] [Google Scholar]
- 28.Harrison OB, Claus H, Jiang Y, Bennett JS, Bratcher HB, Jolley KA, Corton C, Care R, Poolman JT, Zollinger WD, Frasch CE, Stephens DS, Feavers I, Frosch M, Parkhill J, Vogel U, Quail MA, Bentley SD, Maiden MC. 2013. Description and nomenclature of Neisseria meningitidis capsule locus. Emerg. Infect. Dis. 19:566–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heckels JE. 1977. The surface of Neisseria gonorrhoeae: isolation of the major components of the outer membrane. J. Gen. Microbiol. 99:333–341 [DOI] [PubMed] [Google Scholar]
- 30.Tsai CM, Frasch CE. 1980. Chemical analysis of major outer membrane proteins of Neisseria meningitidis: comparison of serotypes 2 and 11. J. Bacteriol. 141:169–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacCoss MJ, Wu CC, Liu H, Sadygov R, Yates JR., III 2003. A correlation algorithm for the automated quantitative analysis of shotgun proteomics data. Anal. Chem. 75:6912–6921 [DOI] [PubMed] [Google Scholar]
- 32.Dreisbach A, Otto A, Becher D, Hammer E, Teumer A, Gouw JW, Hecker M, Volker U. 2008. Monitoring of changes in the membrane proteome during stationary phase adaptation of Bacillus subtilis using in vivo labeling techniques. Proteomics 8:2062–2076 [DOI] [PubMed] [Google Scholar]
- 33.Otto A, Bernhardt J, Meyer H, Schaffer M, Herbst FA, Siebourg J, Mader U, Lalk M, Hecker M, Becher D. 2010. Systems-wide temporal proteomic profiling in glucose-starved Bacillus subtilis. Nat. Commun. 1:137. 10.1038/ncomms1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tettelin H, Saunders NJ, Heidelberg J, Jeffries AC, Nelson KE, Eisen JA, Ketchum KA, Hood DW, Peden JF, Dodson RJ, Nelson WC, Gwinn ML, DeBoy R, Peterson JD, Hickey EK, Haft DH, Salzberg SL, White O, Fleischmann RD, Dougherty BA, Mason T, Ciecko A, Parksey DS, Blair E, Cittone H, Clark EB, Cotton MD, Utterback TR, Khouri H, Qin H, Vamathevan J, Gill J, Scarlato V, Masignani V, Pizza M, Grandi G, Sun L, Smith HO, Fraser CM, Moxon ER, Rappuoli R, Venter JC. 2000. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science 287:1809–1815 [DOI] [PubMed] [Google Scholar]
- 35.Bentley SD, Vernikos GS, Snyder LA, Churcher C, Arrowsmith C, Chillingworth T, Cronin A, Davis PH, Holroyd NE, Jagels K, Maddison M, Moule S, Rabbinowitsch E, Sharp S, Unwin L, Whitehead S, Quail MA, Achtman M, Barrell B, Saunders NJ, Parkhill J. 2007. Meningococcal genetic variation mechanisms viewed through comparative analysis of serogroup C strain FAM18. PLoS Genet. 3:e23. 10.1371/journal.pgen.0030023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41:95–98 [Google Scholar]
- 37.Park SK, Venable JD, Xu T, Yates JR., III 2008. A quantitative analysis software tool for mass spectrometry-based proteomics. Nat. Methods 5:319–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zybailov B, Mosley AL, Sardiu ME, Coleman MK, Florens L, Washburn MP. 2006. Statistical analysis of membrane proteome expression changes in Saccharomyces cerevisiae. J. Proteome Res. 5:2339–2347 [DOI] [PubMed] [Google Scholar]
- 39.Rusniok C, Vallenet D, Floquet S, Ewles H, Mouze-Soulama C, Brown D, Lajus A, Buchrieser C, Medigue C, Glaser P, Pelicic V. 2009. NeMeSys: a biological resource for narrowing the gap between sequence and function in the human pathogen Neisseria meningitidis. Genome Biol. 10:R110. 10.1186/gb-2009-10-10-r110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu NY, Wagner JR, Laird MR, Melli G, Rey S, Lo R, Dao P, Sahinalp SC, Ester M, Foster LJ, Brinkman FS. 2010. PSORTb 3.0: improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics 26:1608–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lappann M, Claus H, van Alen T, Harmsen M, Elias J, Molin S, Vogel U. 2010. A dual role of extracellular DNA during biofilm formation of Neisseria meningitidis. Mol. Microbiol. 75:1355–1371 [DOI] [PubMed] [Google Scholar]
- 42.Thompson EA, Feavers IM, Maiden MC. 2003. Antigenic diversity of meningococcal enterobactin receptor FetA, a vaccine component. Microbiology 149:1849–1858 [DOI] [PubMed] [Google Scholar]
- 43.Hou VC, Moe GR, Raad Z, Wuorimaa T, Granoff DM. 2003. Conformational epitopes recognized by protective anti-neisserial surface protein A antibodies. Infect. Immun. 71:6844–6849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams JN, Skipp PJ, Humphries HE, Christodoulides M, O'Connor CD, Heckels JE. 2007. Proteomic analysis of outer membranes and vesicles from wild-type serogroup B Neisseria meningitidis and a lipopolysaccharide-deficient mutant. Infect. Immun. 75:1364–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnston KH, Holmes KK, Gotschlich EC. 1976. The serological classification of Neisseria gonorrhoeae. I. Isolation of the outer membrane complex responsible for serotypic specificity. J. Exp. Med. 143:741–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Osborn MJ, Gander JE, Parisi E, Carson J. 1972. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J. Biol. Chem. 247:3962–3972 [PubMed] [Google Scholar]
- 47.Frasch CE, Gotschlich EC. 1974. An outer membrane protein of Neisseria meningitidis group B responsible for serotype specificity. J. Exp. Med. 140:87–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adu-Bobie J, Lupetti P, Brunelli B, Granoff D, Norais N, Ferrari G, Grandi G, Rappuoli R, Pizza M. 2004. GNA33 of Neisseria meningitidis is a lipoprotein required for cell separation, membrane architecture, and virulence. Infect. Immun. 72:1914–1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van de Waterbeemd B, Mommen GP, Pennings JL, Eppink MH, Wijffels RH, van der Pol LA, de Jong AP. 15 February 2013, posting date Quantitative proteomics reveals distinct differences in the protein content of outer membrane vesicle vaccines. J. Proteome Res. (Epub ahead of print.) 10.1021/pr301208g [DOI] [PubMed] [Google Scholar]
- 50.Lee EY, Choi DS, Kim KP, Gho YS. 2008. Proteomics in gram-negative bacterial outer membrane vesicles. Mass Spectrom. Rev. 27:535–555 [DOI] [PubMed] [Google Scholar]
- 51.Dorward DW, Garon CF, Judd RC. 1989. Export and intercellular transfer of DNA via membrane blebs of Neisseria gonorrhoeae. J. Bacteriol. 171:2499–2505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pérez-Cruz C, Carrion O, Delgado L, Martinez G, Lopez-Iglesias C, Mercade E. 2013. A new type of outer membrane vesicles produced by the Gram-negative bacterium Shewanella vesiculosa M7T: implications for DNA content. Appl. Environ. Microbiol. 79:1874–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Ulsen P, Adler B, Fassler P, Gilbert M, van Schilfgaarde M, van der Ley P, van Alphen L, Tommassen J. 2006. A novel phase-variable autotransporter serine protease, AusI, of Neisseria meningitidis. Microbes Infect. 8:2088–2097 [DOI] [PubMed] [Google Scholar]
- 54.Klemm P, Vejborg RM, Sherlock O. 2006. Self-associating autotransporters, SAATs: functional and structural similarities. Int. J. Med. Microbiol. 296:187–195 [DOI] [PubMed] [Google Scholar]
- 55.Serruto D, Spadafina T, Ciucchi L, Lewis LA, Ram S, Tontini M, Santini L, Biolchi A, Seib KL, Giuliani MM, Donnelly JJ, Berti F, Savino S, Scarselli M, Costantino P, Kroll JS, O'Dwyer C, Qiu J, Plaut AG, Moxon R, Rappuoli R, Pizza M, Arico B. 2010. Neisseria meningitidis GNA2132, a heparin-binding protein that induces protective immunity in humans. Proc. Natl. Acad. Sci. U. S. A. 107:3770–3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arenas J, Nijland R, Rodriguez FJ, Bosma TN, Tommassen J. 2013. Involvement of three meningococcal surface-exposed proteins, the heparin-binding protein NhbA, the alpha-peptide of IgA protease and the autotransporter protease NalP, in initiation of biofilm formation. Mol. Microbiol. 87:254–268 [DOI] [PubMed] [Google Scholar]
- 57.Schmitt C, Turner D, Boesl M, Abele M, Frosch M, Kurzai O. 2007. A functional two-partner secretion system contributes to adhesion of Neisseria meningitidis to epithelial cells. J. Bacteriol. 189:7968–7976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Griffiths NJ, Hill DJ, Borodina E, Sessions RB, Devos NI, Feron CM, Poolman JT, Virji M. 2011. Meningococcal surface fibril (Msf) binds to activated vitronectin and inhibits the terminal complement pathway to increase serum resistance. Mol. Microbiol. 82:1129–1149 [DOI] [PubMed] [Google Scholar]
- 59.Lewis LA, Ngampasutadol J, Wallace R, Reid JE, Vogel U, Ram S. 2010. The meningococcal vaccine candidate neisserial surface protein A (NspA) binds to factor H and enhances meningococcal resistance to complement. PLoS Pathog. 6:e1001027. 10.1371/journal.ppat.1001027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Madico G, Welsch JA, Lewis LA, McNaughton A, Perlman DH, Costello CE, Ngampasutadol J, Vogel U, Granoff DM, Ram S. 2006. The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J. Immunol. 177:501–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mai-Prochnow A, Webb JS, Ferrari BC, Kjelleberg S. 2006. Ecological advantages of autolysis during the development and dispersal of Pseudoalteromonas tunicata biofilms. Appl. Environ. Microbiol. 72:5414–5420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van de Waterbeemd B, Streefland M, van der Ley P, Zomer B, van Dijken H, Martens D, Wijffels R, van der Pol L. 2010. Improved OMV vaccine against Neisseria meningitidis using genetically engineered strains and a detergent-free purification process. Vaccine 28:4810–4816 [DOI] [PubMed] [Google Scholar]
- 63.Grizot S, Buchanan SK. 2004. Structure of the OmpA-like domain of RmpM from Neisseria meningitidis. Mol. Microbiol. 51:1027–1037 [DOI] [PubMed] [Google Scholar]
- 64.Sánchez S, Arenas J, Abel A, Criado MT, Ferreiros CM. 2005. Analysis of outer membrane protein complexes and heat-modifiable proteins in Neisseria strains using two-dimensional diagonal electrophoresis. J. Proteome Res. 4:91–95 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.