Abstract
Salmonella pathogenicity island 1 (SPI-1) carries genes required for the formation of a type 3 secretion system, which is necessary for the invasion process of Salmonella. Among the proteins encoded by SPI-1 is IacP, a homolog of acyl carrier proteins. Acyl carrier proteins are mainly involved in fatty acid biosynthesis, and they require posttranslational maturation by addition of a 4′-phosphopantetheine prosthetic group to be functional. In this study, we analyzed IacP maturation in vivo. By performing matrix-assisted laser desorption ionization–time-of-flight (MALDI-TOF) mass spectrometry analysis of intact purified proteins, we showed that IacP from Salmonella enterica serovar Typhimurium was matured by addition of 4′-phosphopantetheine to the conserved serine 38 residue. Therefore, we searched for the phosphopantetheinyl transferases in charge of IacP maturation. A bacterial two-hybrid approach revealed that IacP interacted with AcpS, an enzyme normally required for the maturation of the canonical acyl carrier protein (ACP), which is involved in fatty acid biosynthesis. The creation of a conditional acpS mutant then demonstrated that AcpS was necessary for the maturation of IacP. However, although IacP was similar to ACP and matured by using the same enzyme, IacP could not replace the essential function of ACP in fatty acid synthesis. Hence, the demonstration that IacP is matured by AcpS establishes a cross-connection between virulence and fatty acid biosynthesis pathways.
INTRODUCTION
Salmonella enterica serovar Typhimurium is a facultative intracellular pathogen with a broad host spectrum that is a common cause of gastroenteritis in humans. Invasion of the nonphagocytic cells of the intestinal epithelium requires a type 3 secretion system (T3SS) encoded by Salmonella pathogenicity island 1 (SPI-1). The SPI-1 T3SS allows translocation of bacterial effector proteins into the host cytoplasm, which trigger engulfment of the bacterial pathogen (1). Among the 35 genes harbored by SPI-1 is iacP, encoding an invasion acyl carrier protein. Although SPI-1 has been identified and studied for decades (2, 3), little is known about IacP and its role in the invasion process.
Carrier proteins involved in diverse biosynthesis processes, such as fatty acid (FA), polyketide (PK), and nonribosomal peptide (NRP) synthesis, are small (80 to 100 residues), discrete proteins (type II) or modular domains of multienzymatic complexes (type I). These carrier proteins are first produced as apoproteins, and posttranslational modification, consisting of the attachment of a 4′-phosphopantetheine (4′-PP) prosthetic group on a conserved serine residue, occurs, generating holoproteins. The 4′-PP prosthetic group provides a terminal sulfhydryl group to tether intermediate metabolites (see Fig. S1 in the supplemental material). The transfer of 4′-PP from coenzyme A to the hydroxyl group of a conserved serine residue is catalyzed by phosphopantetheinyl transferases (PPTases). The best-studied member of this family of carrier proteins is the acyl carrier protein, ACP, involved in FA biosynthesis. In Escherichia coli, ACP is an essential small acidic protein that shuttles the growing acyl chain from enzyme to enzyme during FA biosynthesis (4, 5). When acyl chains attain their final length, they are transferred by acyltransferases to phospholipid or lipid A precursors. Acyl-ACP is also used for acylation and maturation of the prohemolysin pro-HlyA (6, 7).
In E. coli, maturation of ACP is carried out by AcpS, an essential PPTase due to its crucial function on ACP (8–10). AcpS was the first PPTase to be characterized, and homologs were not readily identified by sequence similarity. Refinement of the search motif finally revealed the existence of a PPTase superfamily, including AcpT and EntD in E. coli (11). AcpT has been shown to partially compensate for the absence of AcpS (10, 12). However, if AcpT has another specific role in the cell, it remains mysterious. EntD is specifically involved in the biosynthesis of the siderophore molecule enterobactin and adds a 4′-PP group to the aryl and peptidyl carrier domains of EntB and EntF, respectively. Specificity between PPTases and their cognate substrates was revealed by the observation that EntD was efficient at transferring 4′-PP to apo-EntB and apo-EntF but not to apo-ACP in vitro. Reciprocally, AcpS was efficient at transferring 4′-PP to apo-ACP but not to apo-EntB and apo-EntF in vitro (11, 13, 14).
Although the canonical ACP involved in FA biosynthesis has been extensively studied, much less is known about the role and function of ACP-like proteins sometimes found in multiple copies in microorganisms (15). IacP and ACP are homologous and are both present in S. Typhimurium (Fig. 1). A recent study has shown that IacP is a cytoplasmic protein necessary for invasion and full virulence of S. Typhimurium (16). The proposed explanation for this phenotype was that IacP might promote secretion and translocation of specific protein effectors: SopA, SopB, and SopD (16). It has also been reported that IacP might be involved in the regulation of flagellin expression (17). In the two studies, the conserved serine 38 residue was required for the observed roles of IacP, suggesting that maturation was occurring on IacP and was necessary for its activity. If true, this raises the question of the identity of the enzyme responsible for this maturation.
Fig 1.

Alignment of IacP and ACP proteins. Identical amino acid residues between the two proteins are indicated in the middle sequence, and similar residues are indicated by a plus sign. The conserved serine residue is in boldface, and the four α-helices present in the ACP structure are underlined (5).
In this study, we demonstrated that IacP is indeed matured through phosphopantetheinylation on the conserved serine 38 residue, and we identified the essential PPTase AcpS, normally associated with FA biosynthesis, as responsible for IacP maturation.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
E. coli and S. Typhimurium strains used in this study are listed in Table 1. Bacteria were grown in 2YT or Luria-Bertani (LB) (Sigma-Aldrich). Ampicillin (100 μg/ml), kanamycin (25 or 50 μg/ml), spectinomycin (100 μg/ml), and chloramphenicol (30 μg/ml) were added when necessary. To trigger overexpression from the PTET promoter and to control the overproduction of the recombinant PPTases, 400 ng/ml anhydrotetracycline was added at the start of the culture. Crude extracts were made 6 h later and analyzed by red Ponceau staining and Western blotting against the 6His tag.
Table 1.
Bacterial strains used in this study
| Strain | Laboratory no. | Description | Reference(s) or source |
|---|---|---|---|
| Salmonella enterica serovar Typhimurium | |||
| WT | JV01 | NCTC 12023 | Laboratory stock |
| IacP_TAP | JV48 | Kanr | This study |
| IacPS38T_TAP | JV56 | Kanr | This study |
| PBADacpS IacP_TAP | JV68 | Kanr Spcr | This study |
| pPTET | JV95 | Kanr Spcr Ampr | This study |
| pPTETacpS | JV96 | Kanr Spcr Ampr | This study |
| pPTETentD | JV97 | Kanr Spcr Ampr | This study |
| pPTETacpT | JV98 | Kanr Spcr Ampr | This study |
| pPTETyieE | JV99 | Kanr Spcr Ampr | This study |
| Escherichia coli | |||
| DH5α | EB070 | fhuA2 Δ(argF-lacZ)U169 phoA glnV44 Φ80 Δ(lacZ)M15 gyrA96 recA1 relA1 endA1 thi-1 hsdR17 | Laboratory stock |
| BTH101 | EB003 | F−, cya99, araD139, galE15, galK16, rpsL1, hsdR2, mcrA1, mcrB1; Strr | 25 |
| ACPts | EB337 | MG1655 acpP(ts) ΔfabF::Cmr | 24, 32 |
| ΔacpP::kanR pKO3acpP | EB689 | MG1655 ΔacpP::Kanr pEB1334 | 31 |
DNA manipulations and plasmids.
Gene modifications on the chromosome were carried out by using PCR products transformed into S. Typhimurium pKD46, from which lambda red recombinase was expressed and mediated recombination (18). Mutations were transferred to the wild-type strain using P22 transduction. To introduce a tandem affinity purification (TAP) tag by homologous recombination at the 3′-end chromosomal loci of iacP, a PCR product was made using pJL72 as the template (19) and primers Ebm732 and Ebm734 (see Table S1 in the supplemental material). To create the IacPS38T_TAP-producing strain, a PCR product corresponding to the sequence of IacP-TAP was amplified using primers Ebm735 and Ebm736 and cloned into pUC18 at the SmaI restriction site, creating pJV21 (see Table S2 in the supplemental material). Site-directed mutagenesis was performed on this plasmid using primers Ebm798 and Ebm799 and the QuickChange site-directed mutagenesis kit (Stratagene) to create pJV22. This construction was used as the template to produce a PCR product allowing lambda red-mediated recombination into the ΔiacP pKD46 strain in order to generate the IacPS38T_TAP strain. To introduce a PBAD promoter upstream of the chromosomal coding sequence of acpS, a PCR product, including aadA7 (conferring spectinomycin resistance), araC, and the PBAD promoter, was amplified from E. coli TG1 strain spec-araC (20) using primers Ebm904 and Ebm905.
For the bacterial two-hybrid technique, pairs of proteins to be tested were fused to the C-terminal side of the two catalytic domains, T18 and T25, of adenylate cyclase. PCR products obtained using primers indicated in Table S1 in the supplemental material were inserted into EcoRI/XhoI restriction sites of pT18Clink and pKT25link (21).
For the PPTase activity complementation tests, constructions were made into the pASK-IBA37plus vector (IBA), which is subsequently abbreviated pPTET. PPTase coding sequences were amplified using primers indicated in Table S1 in the supplemental material and were cloned into EcoRI/XhoI restriction sites, generating a translational fusion with an N-terminal 6His tag. Expression of the recombinant gene was under the control of the tetracycline promoter/operator that could be induced upon addition of 200 ng/ml anhydrotetracycline.
To construct pKO3iacP, splicing by overlap extension PCR (22) using primers ebm133/ebm960 and ebm959/ebm675 was used to fuse the E. coli acpP promoter to the iacP gene. The corresponding DNA fragment was inserted into pKO3 (23) at the BamHI/SalI compatible restriction sites.
E. coli and S. Typhimurium ACP have identical protein sequences, and previously made pT18-ACP and pKO3acpP were used (21, 31).
Bacterial two-hybrid method.
We used the adenylate cyclase-based two-hybrid technique (25) as described previously (26). β-Galactosidase activity reported the interaction between pairs of proteins.
Western blotting.
Western blotting was performed on whole-cell extracts using peroxidase–anti-peroxidase complex to detect ProtA-tagged proteins (PAP; Sigma), CyaA antibody 3D1 to detect T18-fused proteins (Santa Cruz Biotechnology), and anti-His-horseradish peroxidase to detect 6His-tagged proteins (Santa Cruz Biotechnology).
Tandem affinity purification.
IacP_TAP and IacPS38T_TAP were purified from S. Typhimurium soluble extracts using the TAP procedure adapted to E. coli (27). Briefly, using sonication and centrifugation for 30 min at 15,000 × g, cytoplasmic protein extracts from cultures of the corresponding strains were made in buffer IPP150-ProtA (10 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.1% NP-40) and were incubated on IgG-Sepharose beads (Pharmacia) for 2 h at 4°C. After washes with buffer IPP150-ProtA, the beads were incubated for 1 h at room temperature in recombinant tobacco etch virus protease (rTEV) buffer with 100 U of rTEV (Gibco). The eluted fraction was diluted with IPP150-calmodulin binding buffer (10 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.1% NP-40, 1 mM Mg-acetate, 2 mM CaCl2) and was incubated for 1 h at 4°C on calmodulin beads (Stratagene). After washes with IPP150-calmodulin binding buffer, the purified protein was eluted with the elution buffer containing 2 mM EGTA instead of 2 mM CaCl2.
Mass spectrometry analysis of intact proteins.
Matrix-assisted laser desorption ionization–time-of-flight (MALDI-TOF) analyses were performed on a Microflex II mass spectrometer (Bruker Daltonics, Germany). Ten to 20 μl of protein solution was desalted by following the ZipTip C18 Millipore protocol. A saturated solution of sinapinic acid made in acetonitrile-water-trifluoroacetic acid (50:50:0.1) was used as the matrix. Samples were treated according to the dry droplet method; mixtures were allowed to dry at room temperature. Data were acquired in a positive linear mode; the range was set from 5 to 20 kDa, and pulsed ion extraction was fixed to 180 ns. External mass calibration was done just before the acquisition of the sample using protein calibration standard I (Bruker Daltonics, Germany). The mass of intact proteins was determined with an accuracy of 200 ppm in this mass range (i.e., ±2.8 Da at 14 kDa). Mass spectra were examined in Flex Analysis software, and no smoothing or baseline subtraction was applied.
RESULTS AND DISCUSSION
Phosphopantetheinylation of IacP.
To assess if IacP is posttranslationally modified, mass spectrometry analysis was performed to determine the mass of the purified protein. For that purpose, a strain harboring a wild-type allele of iacP fused to the tandem affinity purification tag was engineered. The tagged version of iacP was placed at the original chromosomal locus, allowing physiological expression of the recombinant protein (strain JV48). The TAP tag sequence consists of two IgG binding domains of Staphylococcus aureus protein A (ProtA) and a calmodulin binding peptide (CBP) separated by a TEV protease cleavage (28). Two-step purification was realized based on these tags to finally recover IacP_CBP (see Fig. S2 in the supplemental material). In parallel, a strain harboring a mutant allele of iacP carrying the serine 38-to-threonine substitution fused to the TAP tag was also created in order to purify a mutated form of IacP that could not be modified (strain JV56). Indeed, replacement of the equivalent conserved serine 36 of ACP by the similar amino acid residue threonine prevents the possibility of 4′-PP addition (29). The mass of purified IacP_CBP and IacPS38T_CBP was analyzed by MALDI-TOF mass spectrometry. Spectra showed a major peak at 14,589.6 Da for IacP_CBP (Fig. 2, black line), which, given an accuracy of ±2.8 Da for the mass measurement, corresponded to the expected theoretical mass [M + H]+ of 14,591.5 Da for IacP_CBP bound to 4′-PP, i.e., holo-IacP_CBP. In contrast, spectra showed a major peak at 14,265.6 Da for IacPS38T_CBP (Fig. 2, gray line), which corresponded to the expected theoretical mass [M + H]+ of 14,266.3 Da for the unmodified IacPS38T_CBP. In conclusion, these results showed that 4′-PP was attached to IacP_CBP but not to IacPS38T_CBP, indicating that phosphopantetheinylation was occurring on serine 38 of IacP.
Fig 2.
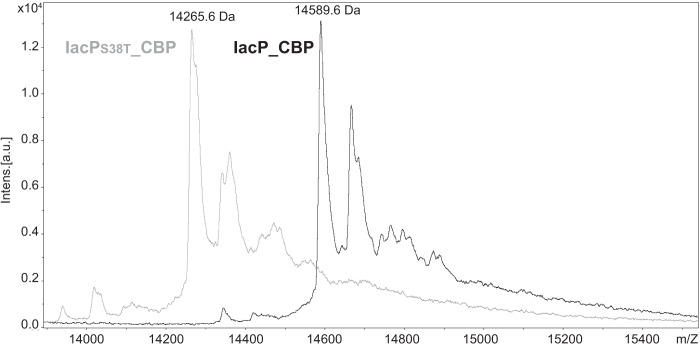
Phosphopantetheinylation of IacP on serine 38. MALDI-TOF mass spectrometry of purified IacP_CBP (black line) and IacPS38T_CBP (gray line). The lower molecular mass (14,265.6 Da) indicated for the major gray peak corresponds to the mass of the intact IacPS38T_CBP protein ([M + H]+ theoretical mass of 14,266.3 Da for apo-IacPS38T_CBP), while the higher molecular mass (14,589.6 Da) indicated for the major black peak corresponds to the mass of the intact IacP_CBP protein onto which a 4′-PP prosthetic group had been attached ([M + H]+ theoretical mass of 14,591.5 Da for holo-IacP_CBP).
Mass spectra showed additional peaks in a window of +10 to +500 Da larger than the major peaks (Fig. 2). However, those peaks could not represent IacP loaded with specific molecules attached to the 4′-PP group. Indeed, identical adducts were observed for the IacPS38T form, which is theoretically unable to load intermediate metabolites. The main additional peaks corresponding to adducts of +76 and +96 Da might result from technical issues and might correspond to molecules of β-mercaptoethanol and a trifluoroacetyl ion, respectively, that are present during the experiment and might be bound to the analyzed proteins.
Interaction between IacP and the PPTase AcpS.
Attachment of the 4′-PP prosthetic group onto carrier proteins is carried out by PPTases. There is no gene coding for a putative PPTase in the genomic environment of iacP, suggesting that IacP does not have an exclusively dedicated PPTase. Based on what was published about E. coli PPTases, we found the same three PPTase genes, acpS, acpT, and entD, annotated in the genome of S. Typhimurium (http://www.genome.jp/kegg/). Each of the three PPTase protein sequences were used to blast the S. Typhimurium protein library to search for additional putative PPTases. We identified the YieE protein of unknown function, most similar to AcpT, as a fourth putative PPTase (see Fig. S3 in the supplemental material).
A bacterial two-hybrid approach was chosen to test protein-protein interactions between IacP and each of the four PPTases (25, 30). As a proof of concept, we first tested the interaction between ACP and AcpS, which is the PPTase-maturing ACP (8–10). However, no interaction was detected between ACP and AcpS (Fig. 3) or between ACP and the other PPTases (data not shown). We reasoned that the bacterial two-hybrid test was not sensitive enough to detect transient interaction between an enzyme and its substrate that would be quickly modified. We hypothesized that by mutating the conserved serine residue where modification occurs, the reaction of the enzyme on its substrate would be prevented but the recognition between the two proteins would be maintained, and the interaction between the enzyme and the inactive substrate would even been prolonged. Therefore, bacterial two-hybrid tests were repeated using the ACPS36T version, which is an inactive substrate of AcpS (10). Under these conditions, a clear interaction was revealed between ACPS36T and AcpS but not between ACPS36T and the other PPTases (Fig. 3). Next, interactions between IacP, IacPS38T, and PPTases were tested. Only the coexpression of pT25-IacPS38T and pT18-AcpS gave significant β-galactosidase activity (Fig. 3). Interaction could not be detected between IacPS38T and the other PPTases (Fig. 3) or between wild-type IacP and the other PPTases (data not shown). Overall, these results showed an interaction between IacP and AcpS but not between IacP and the other PPTases, suggesting that AcpS is the PPTase responsible for the posttranslational modification occurring on IacP.
Fig 3.
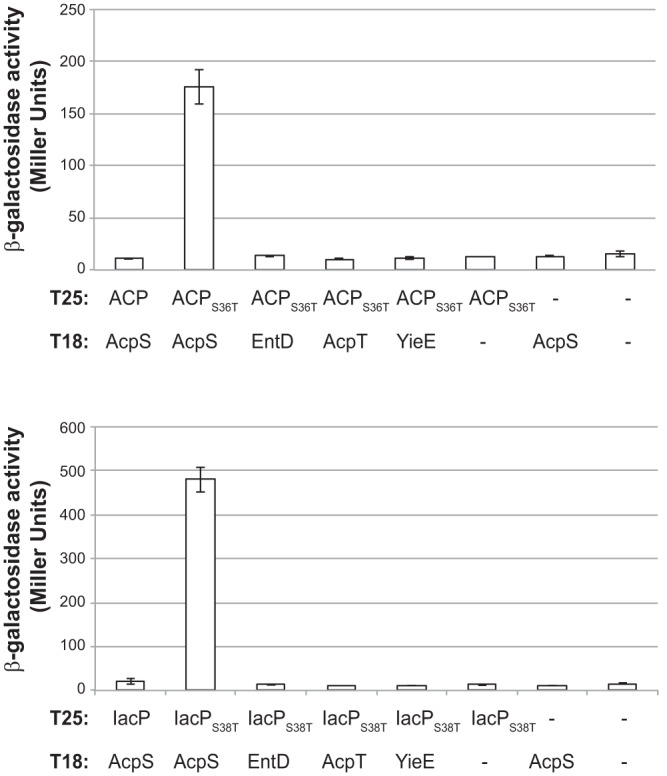
Interactions between ACPs and PPTases assayed by bacterial two-hybrid method. Interactions between pairs of hybrid proteins, resulting from the fusion of the indicated protein with the T25 and T18 fragments of Bordetella pertussis adenylate cyclase, were assayed using the bacterial two-hybrid method in E. coli BTH101. Interactions were assayed by β-galactosidase activity measurement. The values are the means from three independent assays. Similar results were obtained with the reverse combination of plasmids, i.e., when ACPS36T and IacPS38T were fused to the T18 domain and coproduced with PPTases fused to the T25 domain (data not shown).
The PPTase AcpS is essential in Salmonella.
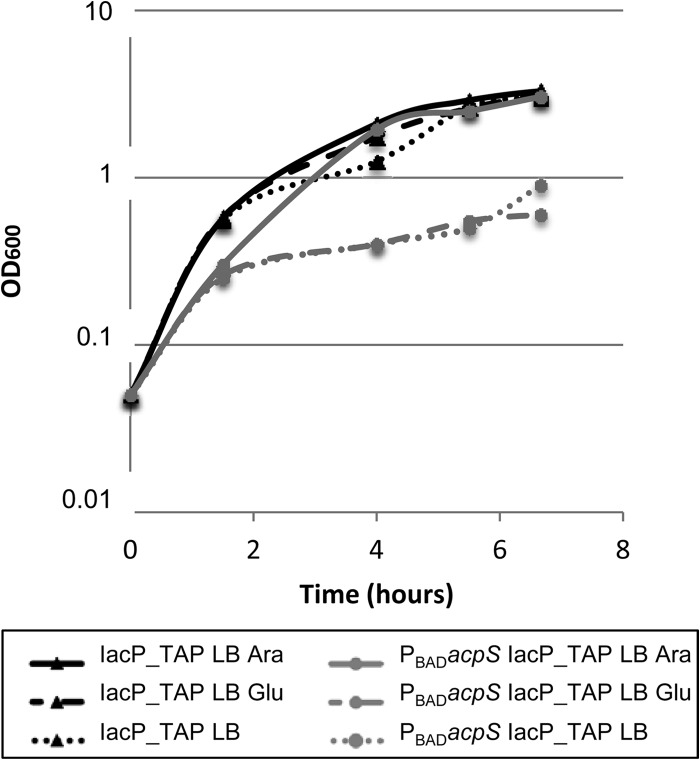
In E. coli, acpS is an essential gene, suggesting that this is also the case in Salmonella. Therefore, deletion of the acpS gene, to further demonstrate the role of AcpS in the phosphopantetheinylation process of IacP, may be inappropriate. Instead, we chose to insert a PBAD promoter at the chromosomal locus upstream of acpS, using the RExBAD cassette described by Roux et al. (20). Under the control of PBAD, the expression of acpS could be induced by addition of arabinose into the culture medium. This genetic manipulation was realized in the strain expressing IacP-TAP to create PBAD acpS IacP_TAP (strain JV68).
Growth of the PBAD acpS IacP_TAP strain (JV68) was tested on agar LB plates. The PBAD acpS IacP_TAP strain was only able to grow in the presence of arabinose (inducing conditions for PBAD). On the other hand, the parental control strain IacP_TAP (JV48) was able to grow on LB media with or without arabinose (Table 2; also see Fig. S4A in the supplemental material). Growth of the two strains was also monitored in liquid LB media with arabinose or glucose (inducing and repressing conditions for PBAD, respectively) (Fig. 4). From various growth experiments, we observed that about eight generations were necessary to deplete AcpS and to observe growth defects of the conditional acpS mutant. Hence, Fig. 4 shows growth curves of cultures that were first grown for about 5 generations before being back diluted to an OD600 of 0.05, and then the optical density was monitored. Those results showed that AcpS is essential in Salmonella.
Table 2.
Capacity of the conditional acpS mutants to grow as isolated coloniesa
| Strain | Growth on: |
|
|---|---|---|
| LB agar (arabinose 0.2%) | LB agar | |
| Iacp_TAP | + | + |
| PBADacpS IacP_TAP | + | − |
| PBADacpS IacP_TAP pPTET | + | − |
| PBADacpS IacP_TAP pPTETacpS | + | + |
| PBADacpS IacP_TAP pPTETentD | + | +/− |
| PBADacpS IacP_TAP pPTETacpT | + | − |
| PBADacpS IacP_TAP pPTETyieE | + | − |
Strains were isolated on LB agar media with or without 0.2% arabinose and incubated overnight at 37°C.
Fig 4.
Growth curves of the conditional acpS mutant in liquid LB media. Cultures of IacP_TAP and PBADacpS IacP_TAP were grown for 3 h in LB to expand for about 5 generations of growth. Cultures then were diluted to an OD600 of 0.05 in LB-0.1% arabinose, LB-0.1% glucose, or LB, and the optical density was monitored. Cultures were incubated at 37°C with shaking.
Complementation of the lethal effect of AcpS depletion.
Complementation experiments were performed to rescue growth of the conditional acpS mutant. Each PPTase-encoding gene was cloned into pPTET. This vector allows expression of recombinant protein under the control of the TET promoter, which is inducible by addition of anhydrotetracycline. The strain PBAD acpS IacP_TAP was transformed by each of the pPTETPPTase constructions and tested for the capacity to grow on an agar LB plate with or without arabinose. Only PBAD acpS IacP_TAP pPTETacpS grew normally in the absence of arabinose (Table 2; also see Fig. S4B in the supplemental material). Growth complementation conferred by the presence of pPTETacpS was effective even without addition of anhydrotetracycline to the LB plate, indicating that the amount of AcpS produced by leakage of the PTET promoter was sufficient for growth complementation. Interestingly, PBAD acpS IacP_TAP, carrying pPTETentD, was able to grow in the absence of arabinose, but small colonies were produced (Table 2; also see Fig. S4B). PBAD acpS IacP_TAP harboring pPTETacpT or pPTETyieE did not show any growth rescue. The overproduction of the recombinant proteins by addition of anhydrotetracycline in LB agar did not change the overall growth pattern of each strain (data not shown). A control production test, however, indicated that PPTases were indeed produced upon addition of anhydrotetracycline to liquid cultures (data not shown). As acpT has been proposed to function as a backup for acpS in E. coli (10, 12), we were surprised to see no complementation using pPTETacpT. However, we cannot exclude that production of a recombinant PPTase fused to a N-terminal 6His tag (present in pPTET) is detrimental to AcpT activity. These results showed that the growth defect due to depletion of AcpS could be fully rescued by expression of the corresponding gene in trans and partially rescued by overexpression of entD.
IacP is matured by the PPTase AcpS.
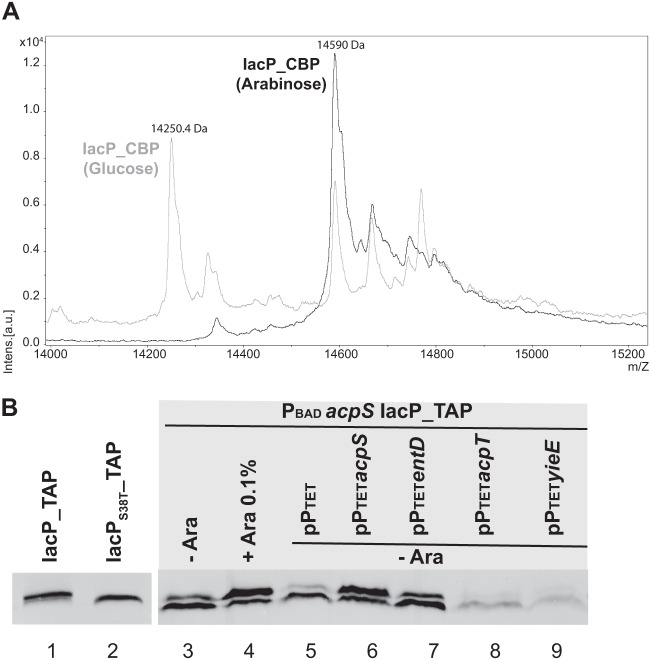
Maturation of IacP and detection of an interaction between IacP and AcpS by the bacterial two-hybrid method suggested that AcpS was the enzyme responsible for IacP maturation. To test this hypothesis, we studied the effect of the depletion of AcpS on the posttranslational modification of IacP. IacP_CBP was purified from strain PBAD acpS IacP_TAP (JV68) grown with (plus arabinose) or without (plus glucose) AcpS production. MALDI-TOF mass spectrometry analysis of intact proteins then was performed. The spectrum of IacP_CBP, purified while AcpS was produced (with arabinose), showed a major peak at 14,590 ± 2.8 Da that corresponded to IacP_CBP bound to 4′-PP, for which the theoretical mass [M + H]+ was 14,591.5 Da (Fig. 5A, black spectrum). However, when IacP_CBP was purified while AcpS was depleted (with glucose), the major peak for IacP_CBP shifted from 14,590 ± 2.8 Da to 14,250.4 ± 2.8 Da (Fig. 5A, gray spectrum). The peak at 14,250.4 ± 2.8 Da corresponded to unmodified IacP_CBP, for which the theoretical mass [M + H]+ was 14,252.2 Da. This indicated a maturation defect of IacP in the absence of AcpS.
Fig 5.
Phosphopantetheinylation of IacP by AcpS. (A) Analysis by MALDI-TOF mass spectrometry of the intact IacP_CBP protein recovered after TAP when purified from strain PBADacpS IacP_TAP (JV68), which was grown while AcpS was produced (0.1% arabinose; black spectrum) or depleted (0.1% glucose; gray spectrum). The lower molecular mass (14,250.4 Da) indicated for the major gray peak corresponds to the global mass of apo-IacP_CBP ([M + H]+ theoretical mass of 14,252.2 Da for apo-IacP_CBP), while the higher molecular mass (14,590 Da) indicated for the major black peak corresponds to the global mass of holo-IacP_CBP ([M + H]+ theoretical mass of 14,591.5 Da for holo-IacP_CBP). (B) Western blot on protein crude extracts using PAP to reveal the ProtA part of TAP-tagged IacP. Excepted if specified (+ Ara 0.1%), strains were grown under noninducing conditions of PBAD, meaning that AcpS production from the chromosome was off when we used the strain PBADacpS IacP_TAP (JV68). When specified, strains harbored a plasmid carrying one of the PPTases under the control of PTET.
To determine if a PPTase other than AcpS could promote maturation of IacP, protein crude extracts were prepared from PBAD acpS IacP_TAP grown under conditions where expression of chromosomal acpS was off and while one of the 4 PPTases was present in trans under the control of PTET. IacP_TAP was then detected by Western blotting (Fig. 5B). Previous experiments with the control strains JV48 and JV56, producing IacP_TAP and IacPS38T_TAP, respectively, had shown that IacP_TAP typically ran on SDS-PAGE as a doublet, with the upper band being more intense (Fig. 5B, lane 1), while IacPS38T_TAP typically ran on SDS-PAGE as a single lower band (Fig. 5B, lane 2). We took advantage of this mobility pattern on SDS-PAGE to conveniently analyze the maturation status of IacP_TAP from the PBAD acpS IacP_TAP strain. First, we observed that IacP_TAP ran predominantly as the apo form when chromosomal expression of acpS was off (absence of arabinose) (Fig. 5B, lane 3) and ran predominantly as the holo form when chromosomal expression of acpS was on (presence of arabinose) (Fig. 5B, lane 4), which is in agreement with the mass spectrometry results (Fig. 5A). We then analyzed IacP_TAP from PBAD acpS IacP_TAP, carrying one of the pPTETPPTase plasmids, while chromosomal expression of acpS was off. IacP_TAP showed the characteristic pattern of holo_IacP only when PBAD acpS IacP_TAP carried pPTETacpS (Fig. 5B, compare lane 6 to lanes 5, 7, 8, and 9).
The presence of acpT or yieE in trans seemed detrimental for IacP production, since less protein was detected by Western blotting. This was possibly linked to disturbed growth. Indeed, we noticed repeatedly that expression of acpT, and especially yieE, was detrimental to growth (data not shown). In conclusion, only AcpS seemed to promote IacP maturation.
IacP does not complement the lethal phenotype associated with ACP defect.
Although ACP is essential, it is possible to isolate acpP temperature-sensitive (ACPts) mutant strains (24). To determine if IacP could substitute for ACP, we tried to complement the temperature-sensitive phenotype of an E. coli ACPts strain with IacP. For that purpose, the E. coli ACPts strain (EB337) was transformed with pT18-IacP as well as pT18-ACP as a positive control and pT18 as a negative control. Transformants were spread on selective LB plates and incubated at 30 or 42°C for 3 days. While all strains grew at 30°C, only the ACPts strain complemented by pT18-ACP was able to grow at 42°C, indicating that the T18-ACP hybrid protein was functional and that IacP could not replace ACP to rescue cell growth (Table 3; also see Fig. S5A in the supplemental material). As we had no certainty that the T18-IacP protein was functional or optimal, we also developed a second genetic assay to test if the ΔacpP::Kanr allele could be transduced in a strain where the wild-type acpP or iacP gene was carried in trans (31). The acpP or iacP gene was cloned into the low-copy pKO3 vector, both under the control of the E. coli acpP promoter, and the constructions were introduced into E. coli MG1655. The capacity to transduce the ΔacpP::Kanr allele (from EB689) into MG1655 harboring pKO3, pKO3acpP, or pKO3iacP was assayed on LB agar plates supplemented with kanamycin. Clones were obtained only when the E. coli strain harbored pKO3acpP (see Fig. S5B). Overall, two different tests indicated that IacP could not replace the essential functions of ACP.
Table 3.
Capacity of ACPts transformants to form colonies at nonpermissive temperaturea
| Strain | Growth at: |
|
|---|---|---|
| 30°C | 42°C | |
| ACPts pT18 | + | − |
| ACPts pT18-ACP | + | + |
| ACPts pT18-IacP | + | − |
The E. coli ACPts strain was transformed with the indicated plasmids, spread on selective LB agar plates, and incubated for 3 days at 30 or 42°C.
Conclusions.
Whereas E. coli K-12 harbors only one gene encoding ACP, bacteria with more complex metabolisms or lifestyles often possess multiple ACP elements. This study shows for the first time the maturation process of an acyl carrier protein associated with the invasion machinery of a bacterial pathogen. Using a conditional acpS mutant, we were able to show in vivo the unambiguous involvement of AcpS in the maturation process of IacP. The use of an essential function as the one performed by AcpS may ensure that the bacterial pathogen maintains the capacity to mature the virulence-associated protein IacP. As mentioned earlier, IacP has been proposed to promote the secretion of the protein effectors SopA, SopB, and SopD (16) and to influence the Hin DNA invertase-mediated flagellar phase variation (17). However, in light of what is known about the mode of action of ACPs, it is unclear at this stage how IacP would regulate these events. Now that we have shown that phosphopantetheinylation occurs on IacP thanks to AcpS, the existence of further connections between FA biosynthesis and virulence pathways has to be explored. The next challenge will be to demonstrate that IacP is loaded with FA and to determine the nature and final destination of the FA carried by IacP.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Ghigo group from Institut Pasteur, France, for the kind gift of materials that made possible the construction with the chromosomal insertion of the PBAD promoter. We thank Régine Lebrun and other members from the proteomic platform from IMM for technical help and fruitful discussions.
This work was funded by the Université Aix-Marseille (AMU), the Centre National de la Recherche Scientifique (CNRS), and the Agence Nationale de la Recherche (ANR), grant LipidStress (ANR-09-JCJC-0018). L.M. is a recipient of a fellowship from the Fondation pour la Recherche Médicale.
Footnotes
Published ahead of print 26 July 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00472-13.
REFERENCES
- 1.Agbor TA, McCormick BA. 2011. Salmonella effectors: important players modulating host cell function during infection. Cell Microbiol. 13:1858–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galan JE, Curtiss RR. 1989. Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc. Natl. Acad. Sci. U. S. A. 86:6383–6387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mills DM, Bajaj V, Lee CA. 1995. A 40 kb chromosomal fragment encoding Salmonella typhimurium invasion genes is absent from the corresponding region of the Escherichia coli K-12 chromosome. Mol. Microbiol. 15:749–759 [DOI] [PubMed] [Google Scholar]
- 4.White SW, Zheng J, Zhang YM, Rock CO. 2005. The structural biology of type II fatty acid biosynthesis. Annu. Rev. Biochem. 74:791–831 [DOI] [PubMed] [Google Scholar]
- 5.Chan DI, Vogel HJ. 2010. Current understanding of fatty acid biosynthesis and the acyl carrier protein. Biochem. J. 430:1–19 [DOI] [PubMed] [Google Scholar]
- 6.Byers DM, Gong H. 2007. Acyl carrier protein: structure-function relationships in a conserved multifunctional protein family. Biochem. Cell Biol. 85:649–662 [DOI] [PubMed] [Google Scholar]
- 7.Stanley P, Koronakis V, Hughes C. 1998. Acylation of Escherichia coli hemolysin: a unique protein lipidation mechanism underlying toxin function. Microbiol. Mol. Biol. Rev. 62:309–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polacco ML, Cronan JEJ. 1981. A mutant of Escherichia coli conditionally defective in the synthesis of holo-[acyl carrier protein]. J. Biol. Chem. 256:5750–5754 [PubMed] [Google Scholar]
- 9.Lambalot RH, Walsh CT. 1995. Cloning, overproduction, and characterization of the Escherichia coli holo-acyl carrier protein synthase. J. Biol. Chem. 270:24658–24661 [DOI] [PubMed] [Google Scholar]
- 10.Flugel RS, Hwangbo Y, Lambalot RH, Cronan JEJ, Walsh CT. 2000. Holo-(acyl carrier protein) synthase and phosphopantetheinyl transfer in Escherichia coli. J. Biol. Chem. 275:959–968 [DOI] [PubMed] [Google Scholar]
- 11.Lambalot RH, Gehring AM, Flugel RS, Zuber P, LaCelle M, Marahiel MA, Reid R, Khosla C, Walsh CT. 1996. A new enzyme superfamily–the phosphopantetheinyl transferases. Chem. Biol. 3:923–936 [DOI] [PubMed] [Google Scholar]
- 12.De Lay NR, Cronan JE. 2006. A genome rearrangement has orphaned the Escherichia coli K-12 AcpT phosphopantetheinyl transferase from its cognate Escherichia coli O157:H7 substrates. Mol. Microbiol. 61:232–242 [DOI] [PubMed] [Google Scholar]
- 13.Gehring AM, Bradley KA, Walsh CT. 1997. Enterobactin biosynthesis in Escherichia coli: isochorismate lyase (EntB) is a bifunctional enzyme that is phosphopantetheinylated by EntD and then acylated by EntE using ATP and 2,3-dihydroxybenzoate. Biochemistry 36:8495–8503 [DOI] [PubMed] [Google Scholar]
- 14.Walsh CT, Gehring AM, Weinreb PH, Quadri LE, Flugel RS. 1997. Post-translational modification of polyketide and nonribosomal peptide synthases. Curr. Opin. Chem. Biol. 1:309–315 [DOI] [PubMed] [Google Scholar]
- 15.Geiger O, Lopez-Lara IM. 2002. Rhizobial acyl carrier proteins and their roles in the formation of bacterial cell-surface components that are required for the development of nitrogen-fixing root nodules on legume hosts. FEMS Microbiol. Lett. 208:153–162 [DOI] [PubMed] [Google Scholar]
- 16.Kim JS, Eom JS, Jang JI, Kim HG, Seo DW, Bang IS, Bang SH, Lee IS, Park YK. 2011. Role of Salmonella pathogenicity island 1 protein IacP in Salmonella enterica serovar Typhimurium pathogenesis. Infect. Immun. 79:1440–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eom JS, Kim JS, Jang JI, Kim HG, Bang IS, Park YK. 2012. Effect of iacP mutation on flagellar phase variation in Salmonella enterica serovar Typhimurium strain UK-1. J. Bacteriol. 194:4332–4341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeghouf M, Li J, Butland G, Borkowska A, Canadien V, Richards D, Beattie B, Emili A, Greenblatt JF. 2004. Sequential peptide affinity (SPA) system for the identification of mammalian and bacterial protein complexes. J. Proteome Res. 3:463–468 [DOI] [PubMed] [Google Scholar]
- 20.Roux A, Beloin C, Ghigo JM. 2005. Combined inactivation and expression strategy to study gene function under physiological conditions: application to identification of new Escherichia coli adhesins. J. Bacteriol. 187:1001–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gully D, Bouveret E. 2006. A protein network for phospholipid synthesis uncovered by a variant of the tandem affinity purification method in Escherichia coli. Proteomics 6:282–293 [DOI] [PubMed] [Google Scholar]
- 22.Horton RM, Cai ZL, Ho SN, Pease LR. 1990. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. Biotechniques 8:528–535 [PubMed] [Google Scholar]
- 23.Link AJ, Phillips D, Church GM. 1997. Methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: application to open reading frame characterization. J. Bacteriol. 179:6228–6237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Lay NR, Cronan JE. 2006. Gene-specific random mutagenesis of Escherichia coli in vivo: isolation of temperature-sensitive mutations in the acyl carrier protein of fatty acid synthesis. J. Bacteriol. 188:287–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karimova G, Pidoux J, Ullmann A, Ladant D. 1998. A bacterial two-hybrid system based on a reconstituted signal transduction pathway. Proc. Natl. Acad. Sci. U. S. A. 95:5752–5756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Battesti A, Bouveret E. 2006. Acyl carrier protein/SpoT interaction, the switch linking SpoT-dependent stress response to fatty acid metabolism. Mol. Microbiol. 62:1048–1063 [DOI] [PubMed] [Google Scholar]
- 27.Gully D, Moinier D, Loiseau L, Bouveret E. 2003. New partners of acyl carrier protein detected in Escherichia coli by tandem affinity purification. FEBS Lett. 548:90–96 [DOI] [PubMed] [Google Scholar]
- 28.Puig O, Caspary F, Rigaut G, Rutz B, Bouveret E, Bragado-Nilsson E, Wilm M, Seraphin B. 2001. The tandem affinity purification (TAP) method: a general procedure of protein complex purification. Methods 24:218–229 [DOI] [PubMed] [Google Scholar]
- 29.Keating DH, Carey MR, Cronan JEJ. 1995. The unmodified (apo) form of Escherichia coli acyl carrier protein is a potent inhibitor of cell growth. J. Biol. Chem. 270:22229–22235 [DOI] [PubMed] [Google Scholar]
- 30.Battesti A, Bouveret E. 2012. The bacterial two-hybrid system based on adenylate cyclase reconstitution in Escherichia coli. Methods 58:325–334 [DOI] [PubMed] [Google Scholar]
- 31.Angelini S, My L, Bouveret E. 2012. Disrupting the acyl carrier protein/SpoT interaction in vivo: identification of ACP residues involved in the interaction and consequence on growth. PLoS One 7:e36111. 10.1371/journal.pone.0036111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Battesti A, Bouveret E. 2009. Bacteria possessing two RelA/SpoT-like proteins have evolved a specific stringent response involving the acyl carrier protein-SpoT interaction. J. Bacteriol. 191:616–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.