Abstract
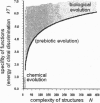
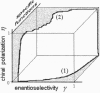
Reasoning from two basic principles of molecular physics, P invariance of electromagnetic interaction and the second law of thermodynamics, one would conclude that mirror symmetry retained in the world of chiral molecules. This inference is fully consistent with what is observed in inorganic nature. However, in the bioorganic world, the reverse is true. Mirror symmetry there is definitely broken. Is it possible to account for this phenomenon without going beyond conventional concepts of the kinetics of enantioselective processes? This study is an attempt to survey all existing hypotheses containing this phenomenon.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avetisov V. A., Goldanskii V. I., Kuz'min V. V. Handedness, origin of life and evolution. Phys Today. 1991 Jul;44(7):33–41. doi: 10.1063/1.881264. [DOI] [PubMed] [Google Scholar]
- Avetisov V. V., Goldanskii V. I. Homochirality and stereospecific activity: evolutionary aspects. Biosystems. 1991;25(3):141–149. doi: 10.1016/0303-2647(91)90002-3. [DOI] [PubMed] [Google Scholar]
- Beaudry A. A., Joyce G. F. Directed evolution of an RNA enzyme. Science. 1992 Jul 31;257(5070):635–641. doi: 10.1126/science.1496376. [DOI] [PubMed] [Google Scholar]
- Bonner W. A. The origin and amplification of biomolecular chirality. Orig Life Evol Biosph. 1991;21(2):59–111. doi: 10.1007/BF01809580. [DOI] [PubMed] [Google Scholar]
- Broda E. Dissident remarks on the origin of optical activity. An intrabiological explanation. Orig Life. 1984;14(1-4):391–396. doi: 10.1007/BF00933682. [DOI] [PubMed] [Google Scholar]
- Cech T. R. RNA chemistry. Ribozyme self-replication? Nature. 1989 Jun 15;339(6225):507–508. doi: 10.1038/339507a0. [DOI] [PubMed] [Google Scholar]
- Cech T. R. RNA. Fishing for fresh catalysts. Nature. 1993 Sep 16;365(6443):204–205. doi: 10.1038/365204a0. [DOI] [PubMed] [Google Scholar]
- Craig D. P., Mellor D. P. Discriminating interactions between chiral molecules. Top Curr Chem. 1976;0(63):1–48. doi: 10.1007/BFb0046191. [DOI] [PubMed] [Google Scholar]
- FRANK F. C. On spontaneous asymmetric synthesis. Biochim Biophys Acta. 1953 Aug;11(4):459–463. doi: 10.1016/0006-3002(53)90082-1. [DOI] [PubMed] [Google Scholar]
- Goldanskii V. I., Avetisov V. A., Kuz'min V. V. Chiral purity of nucleotides as a necessary condition of complementarity. FEBS Lett. 1986 Oct 20;207(1):181–183. doi: 10.1016/0014-5793(86)80036-9. [DOI] [PubMed] [Google Scholar]
- Harrison L. G. The possibility of spontaneous resolution of enantiomers on a catalyst surface. J Mol Evol. 1974 Nov 28;4(1):99–101. doi: 10.1007/BF01732775. [DOI] [PubMed] [Google Scholar]
- Joyce G. F. RNA evolution and the origins of life. Nature. 1989 Mar 16;338(6212):217–224. doi: 10.1038/338217a0. [DOI] [PubMed] [Google Scholar]
- Joyce G. F., Schwartz A. W., Miller S. L., Orgel L. E. The case for an ancestral genetic system involving simple analogues of the nucleotides. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4398–4402. doi: 10.1073/pnas.84.13.4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce G. F., Visser G. M., van Boeckel C. A., van Boom J. H., Orgel L. E., van Westrenen J. Chiral selection in poly(C)-directed synthesis of oligo(G). Nature. 1984 Aug 16;310(5978):602–604. doi: 10.1038/310602a0. [DOI] [PubMed] [Google Scholar]
- Keszthelyi L. Origin of the homochirality of biomolecules. Q Rev Biophys. 1995 Nov;28(4):473–507. doi: 10.1017/s0033583500003309. [DOI] [PubMed] [Google Scholar]
- Kondepudi D. K., Kaufman R. J., Singh N. Chiral symmetry breaking in sodium chlorate crystallizaton. Science. 1990 Nov 16;250(4983):975–976. doi: 10.1126/science.250.4983.975. [DOI] [PubMed] [Google Scholar]
- Morozov L. Mirror symmetry breaking in biochemical evolution. Orig Life. 1979 Jul;9(3):187–217. doi: 10.1007/BF00932495. [DOI] [PubMed] [Google Scholar]
- Nicolis G., Prigogine I. Symmetry breaking and pattern selection in far-from-equilibrium systems. Proc Natl Acad Sci U S A. 1981 Feb;78(2):659–663. doi: 10.1073/pnas.78.2.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orgel L. E. Molecular replication. Nature. 1992 Jul 16;358(6383):203–209. doi: 10.1038/358203a0. [DOI] [PubMed] [Google Scholar]
- Pace N. R., Marsh T. L. RNA catalysis and the origin of life. Orig Life Evol Biosph. 1985;16(2):97–116. doi: 10.1007/BF01809465. [DOI] [PubMed] [Google Scholar]
- Schuster P. RNA based evolutionary optimization. Orig Life Evol Biosph. 1993 Dec;23(5-6):373–391. doi: 10.1007/BF01582087. [DOI] [PubMed] [Google Scholar]
- Seelig F. F., Zielke R. Five simultaneous steady states in a flipping two-compartment-system with optical antipodes. J Mol Evol. 1975 Oct 29;6(2):117–129. doi: 10.1007/BF01732292. [DOI] [PubMed] [Google Scholar]
- Topiol S. A general criterion for molecular recognition: implications for chiral interactions. Chirality. 1989;1(1):69–79. doi: 10.1002/chir.530010113. [DOI] [PubMed] [Google Scholar]
- Visscher J., Schwartz A. W. Selective cleavage of pyrophosphate linkages. Nucleic Acids Res. 1992 Nov 11;20(21):5749–5752. doi: 10.1093/nar/20.21.5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A. L. The triose model: glyceraldehyde as a source of energy and monomers for prebiotic condensation reactions. Orig Life Evol Biosph. 1987;17(2):107–119. doi: 10.1007/BF01808239. [DOI] [PubMed] [Google Scholar]
- Zaug A. J., Cech T. R. The intervening sequence RNA of Tetrahymena is an enzyme. Science. 1986 Jan 31;231(4737):470–475. doi: 10.1126/science.3941911. [DOI] [PubMed] [Google Scholar]