Abstract
We have previously reported that photoirradiation of retinyl palmitate (RP), a storage and ester form of vitamin A (retinol), with UVA light resulted in the formation of photodecomposition products, generation of reactive oxygen species, and induction of lipid peroxidation. In this paper, we report our results following the photoirradiation of RP in ethanol by an UV lamp with approximately equal UVA and UVB light. The photodecomposition products were separated by reversed-phase HPLC and characterized spectroscopically by comparison with authentic standards. The identified products include: 4-keto-RP, 11-ethoxy-12-hydroxy-RP, 13-ethoxy-14-hydroxy-RP, anhydroretinol (AR), and trans- and cis-15-ethoxy-AR. Photoirradiation of RP in the presence of a lipid, methyl linoleate, resulted in induction of lipid peroxidation. Lipid peroxidation was inhibited when sodium azide was present during photoirradiation which suggests free radicals were formed. Our results demonstrate that, similar to irradiation with UVA light, RP can act as a photosensitizer leading to free radical formation and induction of lipid peroxidation following irradiation with UVB light.
Keywords: Retinyl palmitate, UVB, Photoirradiation, Lipid peroxidation
Introduction
Retinyl palmitate (RP) (Figure 1) is an ester and the storage form of vitamin A (retinol) (Figure 1) which is required in many essential biological processes, including regulation of epidermal cell growth, normal cell differentiation, and cell maintenance [1, 2]. RP is more thermally stable than retinol, and therefore is frequently used as a retinoid in cosmetic products [3]. The number of cosmetic products containing RP has increased rapidly in the last two decades, with more than 700 RP-containing cosmetic products in the U.S. market in 2004 [4]. The individuals using these products are unavoidably exposed to sunlight. There is a paucity of data regarding the photostability of RP-containing cosmetics in sunlight, and potential deleterious effects resulting from the use of RP-containing cosmetics on sun-exposed skin [5].
Figure 1.
Structures, names, abbreviations, and numbering of retinol, retinyl palmitate (RP), and anhydroretinol (AR).
RP has been shown to be more photochemically labile than the parent retinol [6]. In order to estimate if any risk is associated with concomitant exposure to RP-containing cosmetics and sunlight, the photodecomposition products of RP following irradiation with UVA, UVB, or solar light need to be identified and their possible toxicological activities need to be determined. RP has an absorption maximum at 326 nm [2], which is within the wavelength range of UVA light (315–400 nm). Hypothetically, RP could absorb UVA light, and the excited RP (either triplet or singlet states) could transfer energy or an electron to molecular oxygen leading to the formation of singlet oxygen and superoxide (reactive oxygen species; ROS), respectively. We have confirmed the above hypothesis and demonstrated that photoirradiation of RP with UVA light generated several photodecomposition products, and also formed superoxide and singlet oxygen [7, 8]. In addition, we have shown that photoirradiation of RP with UVA light in the presence of a lipid, methyl linoleate, resulted in the formation of ROS-induced lipid peroxidation [8].
While UVA light causes DNA damage indirectly through photoactivation of endogeneous and exogeneous photosensitizers [5], UVB light (between 280 to 315 nm), possessing higher energy than UVA light, is capable of causing DNA damage directly. UVB-induced DNA damage can result in cytotoxicity, mutagenicity, initiation of tumorigenicity, and formation of skin cancer in humans [5, 9, 10, 11]. In this paper, we provide evidence that, similar to UVA light irradiation, photoirradiation of RP with UVB light results in the formation of photodecomposition products, generation of ROS, and induction of lipid peroxidation.
Materials and Methods
Materials
RP and sodium azide (NaN3) were purchased from the Sigma Chemical Co. (St. Louis, MO). All other reagents were obtained through commercial sources and were the highest quality available. All solvents used were HPLC grade.
Light Source
The UVB and UVA light boxes were custom made 4-lamp units with 2ft fluorescent lamps spaced approximately 7 mm apart. The emissions from each source were unfiltered, and the high output UVB and UVA lamps were obtained from National Biologics (Twinsburg, OH). The irradiance of the UVB and UVA light sources was determined using an Optronics OL-754 Spectroradiometer (Optronics Laboratories, Orlando, FL), and the light dose was routinely checked using a Solar Light (Philadelphia, PA) PMA-2200 radiometer equipped with either a Solar Light PMA-2110 UVA detector or Solar Light PMA-2101 UVB detector.
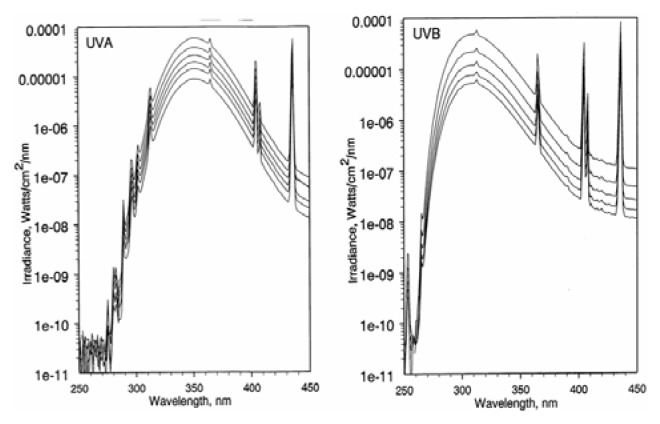
As shown in Figure 2, the maximum emission of the high output UVA fluorescent lamps was 351–352 nm, with a spectral distribution (left spectrum in Figure 2) as follows: UVC (250–280 nm), 7.80 × 10−10 Watts/cm2; UVB (280–315 nm), 4.03 × 10−6 Watts/cm2; UVA (315–400 nm), 3.68 × 10−4 Watts/cm2. Thus, the distribution of ultraviolet radiation in the UVA source was 0.0002% UVC, 1.1% UVB, and 98.9% UVA.
Figure 2.
The light wavelength distribution of the (A) UVA and (B) UVB light sources
The maximum emission of the unfiltered high output UVB fluorescent lamps was 313 nm, with a spectral distribution (right spectrum in Figure 2) as follows: UVC (250–280 nm), 1.01 × 10−5 Watts/cm2; UVB (280–315 nm), 1.16 × 10−3 Watts/cm2; UVA (315–400 nm), 9.95 × 10−4 Watts/cm2. Thus, the distribution of ultraviolet radiation was 0.5% UVC, 53.6% UVB, and 46.0% UVA.
Photoirradiation of RP
A solution (2–3 mL) of 0.5 % RP dissolved in ethanol was placed in a UV-transparent cuvette and photoirradiated under the high output UVB lamps for a period of time required to deliver a UVB light dose of 7, 14, or 21 J/cm2. The reaction mixture was then concentrated to about 200 μL under reduced pressure. Reversed-phase HPLC separation of the resulting photodecomposition products was accomplished using a Prodigy 5 μ ODS column (4.6 × 250 mm, Phenomenex, Torrance, CA) eluted isocratically with 10% methylene chloride in methanol (v/v) at 1 mL/min.
Peroxidation of Methyl Linoleate Initiated by Photoirradiation of RP with the High Output UVB Source
Samples containing 100 mM methyl linoleate and 1.0 mM RP in ethanol were placed in a UV-transparent cuvette and irradiated under the high output UVB lamps for a period of time required to deliver UVB dose of 0, 7, 14, 21, or 35 J/cm2 of UVB light for the first set of experiments and with 0, 2.5, 5.0, 7.5, or 10 J/cm2 of UVB light for the second set of experiments. The levels of lipid peroxidation were measured by quantification of the methyl linoleate hydroperoxides from the HPLC peak area at 235 nm [7, 8, 12]. Methyl linoleate hydroperoxides were separated by HPLC using a Prodigy 5 μ ODS column (46 × 250 mm, Phenomenex, Torrance, CA) stationary phase and 10% water in methanol (v/v) as the mobile phase at 1 mL/min [7, 8].
The effect of 20 mM NaN3, a singlet oxygen and free radical scavenger, on the levels of lipid hydroperoxide formation induced by photoirradiation of RP with UVB light was investigated using irradiation conditions described above.
NaN3 is a singlet oxygen and free radical scavenger. To further confirm the formation of singlet oxygen, one of the ROS species, involved in the lipid peroxidation, photoirradiation of RP with UVB light was determined in the presence of H2O or deuterium oxide (D2O) (enhancement of singlet oxygen lifetime) under experimental conditions as described above. The amount of deuterium oxide and water was 20% in methanol.
Statistical comparison between experimental groups was performed with the General Linear Model (GLM) using the SAS software, version 8.0 (SAS Institute Inc., Cary, NC). Statistically significant differences were identified when p ≤ 0.05.
Instrumentation
A Waters HPLC system consisting of a Model 600 controller, a Model 996 photodiode array detector, and a pump was used for the separation and purification of photodecomposition products of RP.
Results
Photoirradiation of RP
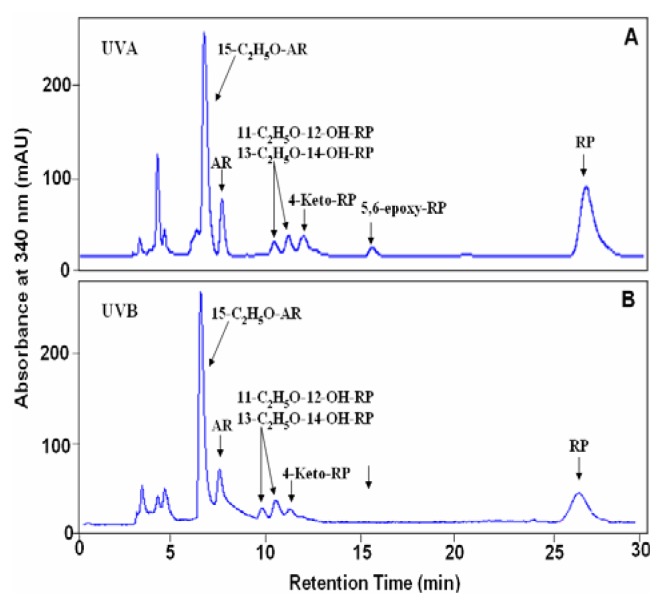
Photoirradiation of RP in ethanol with 7, 14, or 21 J/cm2 UVB light was conducted and the photodecomposition products were separated by reversed phase HPLC. The HPLC profile of RP irradiated with 14 J/cm2 UVB light is shown in Figure 3 (Panel B). For structural identification of the photodecomposition products, photoirradiation of RP with UVA light under similar conditions as previously reported (7) was performed in parallel and the resulting HPLC profile is shown in Figure 3 (Panel A).
Figure 3.
Reversed-phase HPLC profile of the photodecomposition products of RP (0.5% in ethanol) after irradiation with 14 J/cm2 of (A) UVA or (B) UVB light. HPLC analysis was conducted on a Prodigy 5 μ ODS column (4.6 × 250 mm) eluted isocratically with methylene chloride in methanol (1/9; v/v) at 1 mL/min.
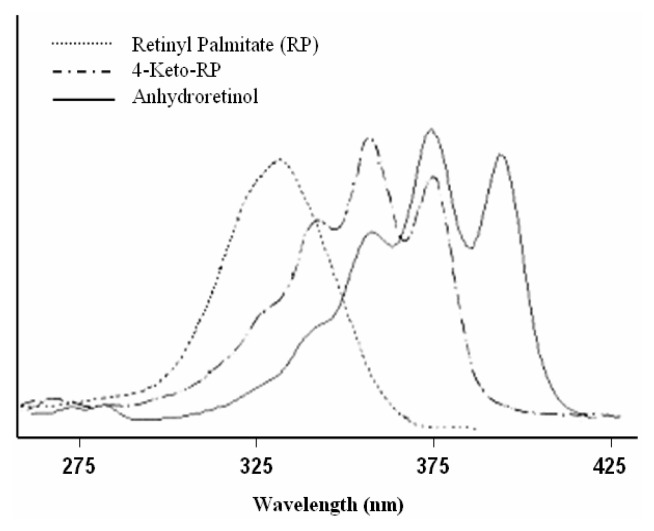
As such, the profiles of the photodecomposition products formed from photoirradiation of RP with the high output UVB light source and the high output UVA light source can be compared. Based on comparison of the HPLC retention time, UV-absorption spectrum and mass spectrum with those of RP, the material contained in the chromatographic peak that eluted at 26.5 min in Figure 3B was identified as the recovered substrate, RP. The material eluting at 12.7 min in Figure 3 following irradiation of RP with the high output UVB light source was 4-keto-RP as evidenced by the HPLC retention time (Figure 3), UV-visible absorption spectrum (with maximal absorptions at 335, 349, and 369 nm, respectively) (Figure 4), and mass spectrum (molecular ion at m/z 540) (data not shown). These results are identical to those of 4-keto-RP formed from photoirradiation of RP with the high output UVA light source (Figure 3A) and formation of 4-keto-RP prepared synthetically by oxidation of RP with m-chloroperbenzoic acid [7].
Figure 4.
UV-visible absorption spectra of RP (--------), 4-keto-RP (–·–·–·–), and anhydroretinol (AR) (———).
Following irradiation of RP with the high output UVB light, the compounds that eluted at 9.8 and 10.2 min (Figure 3) each contained more than one compound, which is similar to the results previously reported for the photoirradiation of RP with UVA light [7]. Mass spectral analysis indicated that each peak contained molecular ions at m/z 586 and characteristic fragment ions at m/z 571 (loss of a methyl group), m/z 540 (loss of a molecule of ethanol), m/z 287 and 247 (data not shown). By comparison of their HPLC retention times (Figure 3A and B), mass spectra, and UV absorption spectra with the standards characterized previously from photoirradiation of RP with UVA light [7], these photodecomposition products were identified as 11-ethoxy-12-hydroxy-RP and 13-ethoxy-14-hydroxy-RP, with each peak containing a mixture of the trans- and cis isomers [7].
The material contained in the chromatographic peak eluted at 7.8 min (Figure 3) following irradiation of RP with ultraviolet light had a molecular ion at m/z 268 (data not shown). The mass spectral profile, UV-visible absorption spectrum and HPLC retention time are identical to those of the synthetic standard anhydroretinol (AR) prepared from reaction of RP with hydrochloric acid [7]. Upon further separation by HPLC using a Vydac C18 column, this AR was determined to be a mixture of all-trans-AR, 6Z-cis-AR, 8Z-cis-AR, and 12Z-cis-AR, with the 12Z-AR isomer as the predominant product (Figure 5).
Figure 5.
Reversed-phase HPLC profile and UV-visible absorption spectra of RP photodecomposition products identified as all-trans-AR (AR) and cis-ARs (6Z-, 8Z-, and 12Z-AR). HPLC analysis was conducted on a Vydac C18 column (4.6 × 250 mm) eluted isocratically with water in methanol (14/86; v/v) at 1 mL/min.
The material contained in the chromatographic peak eluted at 6.8 min in Figure 3 had a mass spectrum with a molecular ion at m/z 314 (data not shown) which is consistent with ethoxy derivatives of AR [7]. The peak was collected and separated using a different HPLC system (Vydac C18 column; isocratic 14% water in methanol mobile phase; data not shown) into four major chromatographic peaks which had nearly identical UV-visible absorption spectra (data not shown) and identical molecular ions at m/z, 314. These products are isomeric 15-ethoxy-ARs, with each tentatively assigned as 8Z-15-ethoxy-AR, 12Z-15-ethoxy-AR, 6Z-15-ethoxy-AR, and all-trans-15-ethoxy-AR, respectively. These results suggest that these compounds are formed from addition of one molecule of ethanol to AR.
The products formed when RP was irradiated with 7 and 21 J/cm2 UVB light were similarly analyzed. The same photoirradiation products were identified, with differences only in the yields of each photodecomposition product (data not shown).
Photoirradiation of RP in the Presence of Methyl Linoleate
Photoirradiation of RP in the presence of methyl linoleate with UVB light of different light doses (0, 7, 14, 21, 28, 35, and 70 J/cm2) was conducted. Photoirradiation of RP and photoirradiation of methyl linoleate alone were also performed in parallel. As previously described, we have determined the extent of lipid peroxide formation by quantification of the amount of methyl linoleate hydroperoxides using HPLC methods [7, 12]. Photoirradiation of RP with UVB light in the absence of methyl linoleate did not produce any methyl linoleate hydroperoxides. Photoirradiation of methyl linoleate with 7.5 and 10 J/cm2 UVB light produced measurable methyl linoleate hydroperoxides (Figure 6). This differs from our previous studies where irradiation of methyl linoleate with up to 35 J/cm2 of UVA did not produce methyl linoleate hydroperoxides (7). Nonetheless, inclusion of RP in the methyl linoleate solution undergoing UVB irradiation resulted in increased methyl linoleate hydroperoxide formation (Figure 6). The level of lipid peroxidation induction increased linearly with the light dose up to 10 J/cm2 and is significantly higher (p ≤ 0.05) than that induced by irradiating methyl linoleate in the absence of RP.
Figure 6.
Lipid peroxidation induced by photoirradiation of RP with UVB light, and inhibitory effect of NaN3 on the peroxidation of methyl linoleate. The levels of peroxidation were measured by HPLC analysis in which the eluate was monitored at 235 nm.
Similar to our previous study of photoirradiation of RP with UVA light [7], the involvement of free radical intermediates in the peroxidation of methyl linoleate initiated by photoirradiation of RP with UVB light was examined by adding the free radical scavenger NaN3. As shown in Figure 6, NaN3 significantly inhibited lipid peroxidation initiated by photoexcited RP (p ≤ 0.05).
Our results indicated that lipid peroxidation induced by photoirradiation of RP with UVB was increased by 12–15% when H2O was replaced by D2O (p ≤ 0.05) (data not shown). In addition to being a free radical scavenger, NaN3 also effectively reacts with singlet oxygen (1O2) and hydroxyl radical [7] preventing their reaction with other molecules (e.g. methyl lineolate). Therefore, NaN3 alone cannot be relied upon to determine whether singlet oxygen or free radicals are involved in peroxidation. Since singlet oxygen has a longer half-life in deuterium water (D2O), use of both NaN3 and D2O should provide a reliable approach for determining whether singlet oxygen is involved in peroxidation. Thus, our results from the inhibition of lipid peroxidation by NaN3 and the enhancement by D2O indicate that singlet oxygen, formed from photoirradiation of RP with UVB light, is involved in the induction of lipid peroxidation.
Discussion
ROS have been shown to damage nuclear DNA, cell membranes, and proteins, and initiate lipid peroxidation which can lead to aging, inflammation, cardiovascular diseases, and cancer [13–18]. ROS can also alter signal transduction systems that direct gene expression controlling the balance of cell proliferation, differentiation and apoptosis [19, 20]. Lipid peroxidation produces lipid alkoxy radicals and aldehydes of low molecular weight, such as malondialdehyde and 4-hydroxy-2-nonenal that bind covalently with cellular DNA forming DNA adducts which is associated with cancer development in experimental animals [7, 21, 22].
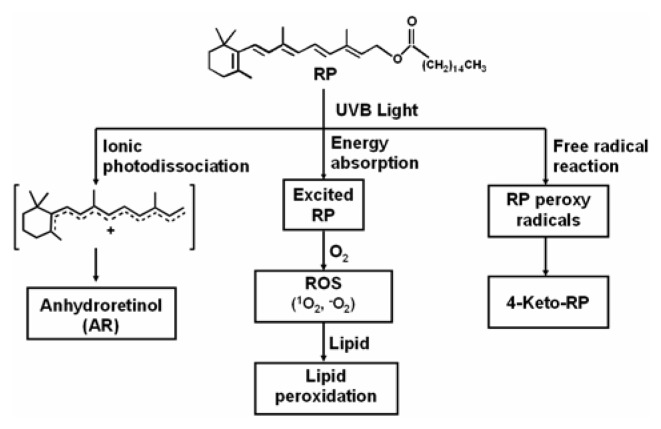
In this paper, we report that photoirradiation of RP in ethanol with light that was a mixture of 53.6% UVB light and 46% UVA light (referred to as UVB light) resulted in the formation of photodecomposition products similar to those formed with a light source that was 98.9% UVA light. The photodecomposition products that were identified following UVB irradiation included 4-keto-RP, 11-ethoxy-12-hydroxy-RP, 13-ethoxy-14-hydroxy-RP, and AR, and the tentatively identified isomeric 15-ethoxy-ARs. Our previous study on photoirradiation of RP with UVA light has shown that the formation of 4-keto-RP, 11-ethoxy-12-hydroxy-RP, 13-ethoxy-14-hydroxy-RP is mediated through a free radical chain reaction, and that the formation of AR is through the ionic photodissociation reaction [7].
The photoirradiation of RP with UVB light in the presence of a lipid (methyl linoleate) resulted in the formation of lipid peroxidation products (methyl linoleate hydroperoxides). The formation of the lipid hydroperoxides was inhibited by the free radical scavenger sodium azide.
We have demonstrated that the photodecomposition products formed following irradiation of RP with UVB light are very similar to those formed following photoirradiation of RP with UVA light. In addition, our results strongly suggest that RP, photoexcited by a high output UVB light source (53.6 % UVB light and 46% UVA light), reacts with molecular oxygen to generate both superoxide radical anion and singlet oxygen. Analogously, we have reported that photoexcitation of RP using a high output UVA light source (98.9% UVA light) results in the generation of the same ROS. These results are important for the design and interpretation of long-term biological testing of RP. A variety of light sources are used to perform photocarcinogenicity and photoaging studies. These studies frequently use light sources whose emissions resemble those described for our high output UVA light souces and high output UVB light sources. Our results suggest that, independent of light source, qualitatively and quantitatively similar photodecomposition products and ROS will be formed.
The biological effects caused by photoexcitation of RP are not well understood. Based on our present results and previously reported in vitro studies on photoexcited RP, we propose a mechanism for product formation and biological consequences following irradiation of RP (Figure 7). We have previously reported that UVA irradiation of RP, AR and 5,6-epoxy-RP induces DNA damage and cytotoxicity [23]. We have also demonstrated that treatment of mouse lymphoma cells with RP and subsequent exposure to UVA light resulted in mutations and loss of heterozygosity (LOH), suggesting that photoirradiated RP is phototoxic and photoclastogenic [24]. These in vitro studies suggest that exposure of RP to sunlight is a potential health risk. However, one must be cautious in using these results for evaluation of risks associated with exposure to topically applied RP-containing cosmetics and sunlight. Additional factors, such as rates of percutaneous absorption and potential sunscreen effects, must be considered when assessing the effects of topically applied RP on light-induced toxicity. Indeed, Antille et al. [25] have shown that RP, topically applied to the skin of human volunteers, can reduce acute sensitivity to UV light measured as minimal erythema dose and formation of thymine dimers. Long-term biological tests are warranted to investigate the relative importance of RP’s photoprotective activity and of photodecomposition products and ROS formed after photoexcitation of RP.
Figure 7.
The proposed mechanistic pathways initiated by photoirradiation of RP with UVB light leading to generation of RP photodecomposition products, ROS, lipid peroxidation, and DNA damage.
Acknowledgement
This research was supported in part by an Interagency Agreement #2143-0001 between the Food and Drug Administration/National Center for Toxicological Research (FDA/NCTR) and the National Institute for Environmental Health Sciences/National Toxicology Program (NIEHS/NTP). Through this agreement, this research was supported by an appointment (S.C.) to the Postgraduate Research Program at the NCTR administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the FDA.
References
- 1.(IARC) International Agency for Research on Cancer. Handbooks of Cancer Prevention: Vitamin A. Vol. 3 Lyon, France: 1998. [Google Scholar]
- 2.Tee E. S. Carotenoids and retinoids in human nutrition. Crit. Rev. Food Sci. Nutr. 1992;31:103–163. doi: 10.1080/10408399209527563. [DOI] [PubMed] [Google Scholar]
- 3.Idson B. Vitamins in cosmetics, an update I. Overview and Vitamin A. Drug Cosmet. Ind. 1990;146:26–91. [Google Scholar]
- 4.Data from the U.S. Food and Drug Administration’s Voluntary Cosmetics Registration Program, 2004
- 5.Fu P. P., Howard P. C., Culp S., Xia Q., Webb P. J., Blankenship L., Wamer W. G., Bucher J. R. Do topically applied skin creams containing retinyl palmitate affect the photocarcinogenicity of simulated solar light? J. Food Drug Anal. 2002;10:262–268. [Google Scholar]
- 6.Ihara H., Hashizume N., Hirase N., Suzue R. Esterification makes retinol more labile to photolysis. J. Nutr. Sci. Vitaminol. 1999;45:353–358. doi: 10.3177/jnsv.45.353. [DOI] [PubMed] [Google Scholar]
- 7.Cherng S.H., Xia Q., Blankenship L. R., Freeman J. P., Wamer W. G., Howard P. C., Fu P. P. Photodecomposition of retinyl palmitate in ethanol by UVA light - formation of photodecomposition products, reactive oxygen species, and lipid peroxides. Chem. Res. Toxicol. 2005;18:129–138. doi: 10.1021/tx049807l. [DOI] [PubMed] [Google Scholar]
- 8.Xia Q., Yin J. J., Cherng S.-H., Wamer W. G., Boudreau M., Howard P.C. P. P. Fu. UVA Photodecomposition of retinyl palmitate – formation of singlet oxygen and superoxide, and their role in induction of lipid peroxidation. Toxicol. Lett. 2006 doi: 10.1016/j.toxlet.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Mukhtar H., editor. Skin Cancer: Mechanisms and Human Relevance. CRC Press; Boca Raton: 1995. [Google Scholar]
- 10.Ahmad H., Mukhtar H. Toxicology of the skin: new and emerging concepts. Toxicol Applied Pharm. 2004;195:265–266. [Google Scholar]
- 11.Sarasin A. The molecular pathways of ultraviolet-induced carcinogenesis. Mutation Res. 1999;428:5–10. doi: 10.1016/s1383-5742(99)00025-3. [DOI] [PubMed] [Google Scholar]
- 12.Tokita M., Morita M. Identification of new geometric isomers of methyl linoleate hydroperoxide and their chromatographic behavior. Biosci. Biotechnol. Biochem. 2000;64:1044–1056. doi: 10.1271/bbb.64.1044. [DOI] [PubMed] [Google Scholar]
- 13.Stadtman E. R., Berlett B. S. Reactive oxygen-mediated protein oxidation in aging and disease. Chem. Res. Toxicol. 1997;10:485–494. doi: 10.1021/tx960133r. [DOI] [PubMed] [Google Scholar]
- 14.Feig D. I., Reid T. M., Loeb L. A. Reactive oxygen species in tumorigenesis. Cancer Res. 1994;54:1890–1894. [PubMed] [Google Scholar]
- 15.de Zwart L. L., Meerman J. H., Commandeur J. N., Vermeulen N. P. Biomarkers of free radical damage applications in experimental animals and in humans. Free Radic. Biol. Med. 1999;26:202–206. doi: 10.1016/s0891-5849(98)00196-8. [DOI] [PubMed] [Google Scholar]
- 16.Burkle A. Mechansim of Ageing. Eye. 2001;15(part 3):371–375. [Google Scholar]
- 17.Floyd R. A., West M., Hensley K. Oxidative biochemical markers: clues to understanding aging in long-lived species. Exp. Gerontol. 2001;36:619–640. doi: 10.1016/s0531-5565(00)00231-x. [DOI] [PubMed] [Google Scholar]
- 18.Loft S., Poulsen H. E. Cancer risk and oxidative DNA damage in man. J. Mol. Med. 1996;74(6):297–312. doi: 10.1007/BF00207507. [DOI] [PubMed] [Google Scholar]
- 19.Poli G., Leonarduzzi G., Biasi F., Chiarpotto E. Oxidative stress and cell signaling. Curr. Med. Chem. 2004;11(9):1163–1182. doi: 10.2174/0929867043365323. [DOI] [PubMed] [Google Scholar]
- 20.Trosko J. E., Upham B. L. The emperor wears no clothes in the field of carcinogen risk assessment: ignored concepts in cancer risk assessment. Mutagenesis. 2005;20(2):81–92. doi: 10.1093/mutage/gei017. [DOI] [PubMed] [Google Scholar]
- 21.Nath R. G., Chung F.-L. Detection of exocyclic 1,N2-propanodeoxyguanosine adducts as common DNA lesions in rodents and humans. Proc. Natl. Acad. Sci. USA. 1994;91:7491–7495. doi: 10.1073/pnas.91.16.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ni Y-C., Kadlubar F. F., Fu P. P. Formation of malondialdehyde-modified 2′-deoxyguanosyl adduct from metabolism of chloral hydrate by mouse liver microsomes. Biochem Biophys Res Commun. 1995;216:1110–1117. doi: 10.1006/bbrc.1995.2735. [DOI] [PubMed] [Google Scholar]
- 23.Yan J., Xia Q., Cherng S-H., Wamer W. G., Howard P. C., Yu H., Fu P. Photo-induced DNA damage and phototoxicity of retinyl palmitate and its photodecomposition products. Toxicol. Ind. Health. 2005;12:167–175. doi: 10.1191/0748233705th225oa. [DOI] [PubMed] [Google Scholar]
- 24.Mei N., Xia Q., Chen L., Moore M. M., Fu P. P., Chen T. Photomutagenicity of retinyl palmitate by ultraviolet A irradiation in mouse lymphoma cells. Toxicological Sciences. 2005;88:142–149. doi: 10.1093/toxsci/kfi291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antille C., Tran C., Sorg O., Carraux P., Didierjean L., Saurat J. H. Vitamin A exerts a photoprotective action in skin by absorbing ultraviolet B radiation. J. Invest. Dermatol. 2003;121:1163–1167. doi: 10.1046/j.1523-1747.2003.12519.x. [DOI] [PubMed] [Google Scholar]