Abstract
Since the finding in the 1930s, a large number of polycyclic aromatic hydrocarbons (PAHs) of different structures have been tested for potential tumorigenicity. Structure-activity relationships of halo-PAHs have been investigated to determine the regions of a PAH that may be involved in cancer initiation. From these studies, a number of halo-PAHs were found to be tumorigenic in experimental animals. It was not until the 1980s that halo-PAHs were found to be present in the environment, including municipal incinerator fly ash, urban air, coal combustion, soil, snow, automobile exhausts, and tap water. Due to their widespread presence in the environment and their genotoxic activities, including carcinogenicity, many of these compounds may pose a health risk to humans. Although the biological activities, including metabolism, mutagenicity, and carcinogenicity, of halo-PAHs have been studied their phototoxicity and photo-induced biological activity have not been well examined. In this study, we study the photoirradiation of a series of structure-related halo-PAHs by UVA light in the presence of a lipid, methyl linoleate, and determine as to whether or not these compounds can induce lipid peroxidation. The halo-PAHs chosen for study include 2-bromonaphthalene, 1-chloroanthracene, 9,10-dibromoanthracene, 9-chlorophenanthrene, 9-bromophenanthrene, 7-chlorobenz[a]anthracene, 7-bromobenz[a]anthracene, 7-bromo-5-methylbenz[a]anthracene, 6-chlorobenzo[a]pyrene, and 6-bromobenzo[a]pyrene. The results indicate that upon photoirradiation by UVA all these compounds induced lipid peroxidation at different levels. These results suggest that halo-PAHs may be harmful to human health.
Introduction
Halogenated- polycyclic aromatic hydrocarbons (halo- PAHs) are a class of compounds with one or more halogen groups attached to the aromatic rings of a polycyclic aromatic hydrocarbon (PAH) [1, 2]. The interest in halo- PAH compounds stems from their chemistry, including synthesis, reactions, properties, and utilization [1–6]. Since the finding in the 1930s that many PAHs are carcinogenic and are present in the environment, a large number of PAHs of different structures have been tested for potential tumorigenicity [1–3]. Structure-activity relationships of halo-PAHs have been investigated to determine the regions of a PAH that may be involved in cancer initiation [1, 2]. From these studies, a number of halo-PAHs were found to be tumorigenic in experimental animals. It was not until the 1980s that halo-PAHs were found to be present in the environment, including municipal incinerator fly ash, urban air, coal combustion, soil, snow, automobile exhausts, and tap water [1–3, 6]. Due to their widespread presence in the environment and their genotoxic activities, including carcinogenicity, many of these compounds may pose a health risk to humans. To date, the biological activities, including metabolism, mutagenicity, and carcinogenicity, of a large number of halo-PAHs have been studied [1–3, 7–15]. While people exposed to the environmental halo-PAHs on the skin are unavoidably exposed to sunlight, it is not known whether contact of halo-PAHs with concomitant exposure to sunlight would result in any deleterious effects. Consequently we report in this study the photoirradiation of a series of representative halo-PAHs that have been detected in the environment by UVA light in the presence of a lipid, methyl linoleate, and determine whether these halo-PAHs can induce lipid peroxidation. The structures, names, abbreviations, and the numberings of the halo-PAHs employed in this study are given in Figure 1, which include: 2-bromonaphthalene (2-Br-Naph), 1-chloroanthracene (1-Cl-A), 9,10-dibromoanthracene (9,10-DiBr-A), 9-chlorophenanthrene (9-Cl-Ph), 9-bromophenanthrene (9-Br-Ph), 7-chlorobenz[a]anthracene (7-Cl-BA), 7-bromobenz[a]anthracene (7-Br-BA), 7-bromo-5-methylbenz[a]anthracene (7-Br-5MBA), 6-chlorobenzo[a]pyrene (6-Cl-BaP), and 6-bromobenzo[a]pyrene (6-Br-BaP).
Figure 1.
Names and structures of the halo-PAHs used for study.
Materials and Methods
Materials
2-Bromohaphthalene, 1-Chloroanthracene, 9-chlorophenanthrene, 9-bromophenanthrene, 9,10-dibromoanthracene, anthracene, pheanthrene, benz[a]anthracene, and benzo[a]pyrene were purchased from Aldrich Chemical Co. (Milwaukee, WI). All other reagents were obtained through commercial sources and were the highest quality available. All solvents used were HPLC grade.
Synthesis of Halo-PAHs
Electrophilic aromatic substitution halogenation generally occurs by reacting PAHs with molecular halogen in the presence of Lewis acid as a catalyst, such as FeCl3, AlCl3, and Tl(OAc)3[1, 4, 5]. Because PAHs contain two or more aromatic rings, which can facilitate electrophilic reactions, halogenation of PAHs can sometimes proceed without the presence of a catalyst. Under mild conditions, substitution occurs at the most reactive carbon position of a PAH, producing the kinetically controlled geometric isomer as the predominant product [1, 4, 5]. The most reactive carbon positions of benz[a]anthracene and benzo[a]pyrene are the C7 and C6 positions, respectively. Thus, chlorination of benz[a]anthracene and benzo[a]pyrene with N-chlorosuccinimide under mild conditions yields 7-chlorobenz[a]anthracene and 6-chlorobenzo[a]pyrene, respectively. Similarly, 7-bromobenz[a]anthracene, 7-bromo-5-methylbenz[a]anthracene, and 6-bromomethylbenzo[a]pyrene were prepared by reaction of benz[a]anthracene, 5-methylbenz[a]anthracene, and 6-benzo[a]pyrene with N-bromosuccinimide, respectively.
Light Source
The UVA light box was custom made with a 4-lamp unit using UVA lamps (National Biologics). The irradiance of light was determined using an Optronics OL754 Spectroradiometer (Optronics Laboratories, Orlando, FL), and the light dose was routinely measured using a Solar Light PMA-2110 UVA detector (Solar Light Inc., Philadelphia, PA). The maximum emission of the UVA is between 340–355 nm [16]. The light intensities at wavelengths below 320 nm (UVB light) and above 400 nm (visible light) are about two orders of magnitude lower than the maximum at 340–355 nm.
Peroxidation of Methyl Linoleate Initiated by Photoirradiation of Halogenated Pahs and their Parent Pahs with UVA Light
Experiments were conducted with a solution of 100 mM methyl linoleate and 1.0 mM substrate in methanol. Samples were placed in a UV-transparent cuvette and irradiated with 0, 7, and 21 J/cm2 of UVA light. After irradiation, the methyl linoleate hydroperoxide products were separated by HPLC using a Prodigy 5 m ODS column (4.6 × 250 mm, Phenomenex, Torrance, CA) eluted isocratically with 10% water in methanol (v/v) at 1 mL/min. The levels of lipid peroxidation were measured by calculation of the amount of methyl linoleate hydroperoxides from the HPLC peak area by monitoring the elution at 235 nm following the method of Tokita [17].
Results and Discussion
Photoirradiation of Representative Halo-PAHs
Ten representative parent halo-PAHs were selected for the study of photoirradiation with UVA in the presence of a lipid, methyl linoleate. As shown in Figure 1, these halo-PAHs include: 2-bromonaphthalene (2-Br-Naph), 1-chloroanthracene (1-Cl-A), 9,10-dibromoanthracene (9,10-DiBr-A), 9-chlorophenanthrene (9-Cl-Ph), 9-bromophenanthrene (9-Br-Ph), 7-chlorobenz[a]anthracene (7-Cl-BA), 7-bromobenz[a]anthracene (7-Br-BA), 7-bromo-5-methylbenz[a]anthracene (7-Br-5MBA), 6-chlorobenzo[a]pyrene (6-Cl-BaP), and 6-bromobenzo[a]pyrene (6-Br-BaP). The halo-PAHs possess different sizes, ranging from two to five benzo-rings, and exhibit various carcinogenic potencies [2] (Table 1). For comparison, their parent PAHs, anthracene, phenanthrene, benz[a]anthracene (BA), 5-methylbenz[a]anthracene (5-MBA), and benzo[a]pyrene (BaP) are included for study. Each of the halo-PAHs and parent PAHs received two light doses, 7 and 21 J/cm2, respectively.
Table 1.
Induction lipid peroxidation by halo-PAHs with concomitant exposure to UVA light irradiationa
| Compound | 0 J/cm2 | 7 J/cm2 | 21 J/cm2 |
|---|---|---|---|
| Methyl linoleate (ML) | 145 | 212 ± 34 | 267 ± 47 |
| 2-Br-Naph | 137 | 402 ± 49 | 1135 ± 93 |
| Anthracene (A) | 138 | 1350 ± 66 | 2459 ± 197 |
| 1-Cl-A | 122 | 1976 ± 100 | 3759 ± 218 |
| 9,10-DiBr-A | 137 | 2260 ± 150 | 5144 ± 330 |
| Phenanthrene (Ph) | 144 | 321 ± 13 | 803 ± 37 |
| 9-Cl-Ph | 138 | 645 ± 22 | 1301 ± 100 |
| 9-Br-Ph | 134 | 537 ± 27 | 721 ± 58 |
| Benz[a]anthracene (BA) | 141 | 2719 ± 133 | 3917 ± 403 |
| 7-Cl-BA | 119 | 2540 ± 179 | 3640 ± 225 |
| 7-Br-BA | 137 | 2494 ± 301 | 5309 ± 638 |
| 5MBA | 142 | 1582 ± 58 | 1885 ± 236 |
| 7-Br-5MBA | 115 | 2296 ± 112 | 3769 ± 216 |
| Benzo[a]pyrene (BaP) | 100 | 2467 ± 217 | 5819 ± 425 |
| 6-Cl-BaP | 153 | 2404 ± 29 | 4840 ± 137 |
| 6-Br-BaP | 135 | 2639 ± 224 | 5201 ± 291 |
Average of three Experiments/standard deviation
The resulting photoirradiation products were separated and analyzed by reversed HPLC. Following the method of Tokita [17], the levels of lipid peroxidation from each experiment were measured by calculation of the amount of methyl linoleate hydroperoxides from the HPLC peak area by monitoring the elution at 235 nm. Upon calculations of the HPLC peak areas, the levels of induction of lipid peroxidation of the halo-PAHs and PAHs were identified (Table 1). In general, each of the halo-PAHs and PAHs received two light doses, 7 and 21 J/cm2, respectively, and the resulting lipid peroxidation was found to be dose- dependent (Table 1). Among the tested PAHs, phenanthrene was the only PAH that did not induce lipid peroxidation in considerable amount. The levels of lipid peroxidation induction by these PAH are in the order: BaP > BA > anthracene > 5-MBA > phenanthrene. Compared with their reported tumorigenicity potency [2], there is no correlation between the photo-induced lipid peroxidation and tumorgenicity.
Comparison of Level of Lipid Peroxidation Formation
Anthracene Series
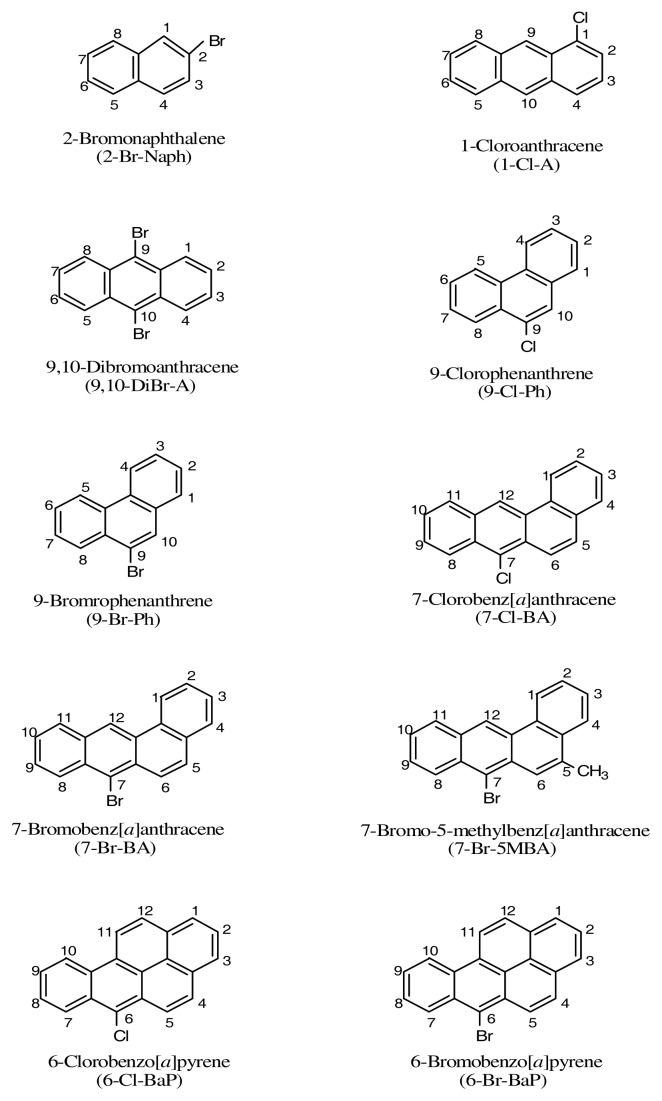
Upon photoirradiation, anthracene, 1-chloroanthracene, and 9,10-dimethylanthracene all induced lipid peroxidation, with 9,10-dimethylanthracene most effectively (Figure 2).
Figure 2.
Peroxidation of methyl linoleate initiated by anthracene, 1-Cl-anthracene and 9,10-DiBr-anthracene with, 0, 7, and 21 J/cm2 of UVA light.
Phenenthrene Series
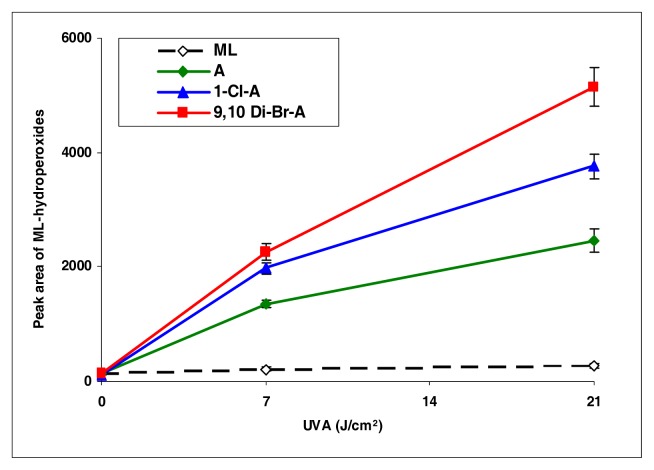
This series is different from the anthracene series. Among phenanthrenes (9-chlorophenanthrene and 9-bromophenanthrene), 9-chlorophenanthrene exhibited strong induction of lipid peroxidation, while the other compounds were weak inducers (Figure 3).
Figure 3.
Peroxidation of methyl linoleate initiated by phenanthrene, 9-Cl-phenanthrene, 9-Br-phenanthrene, with, 0, 7, and 21 J/cm2 of UVA light.
Benz[a]anthracene Series
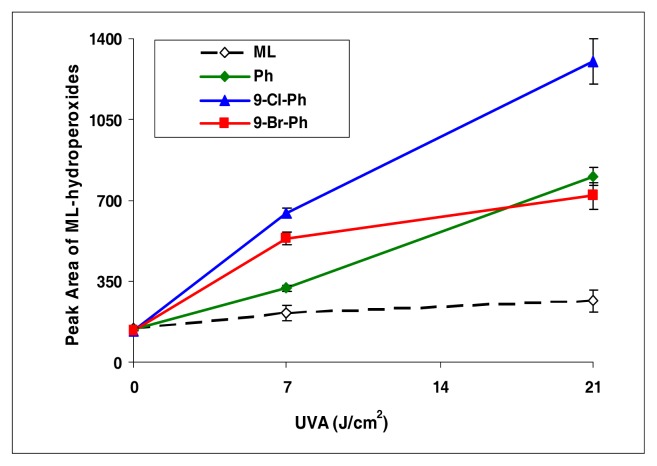
BA, 7-Cl-BA, and 7-Br-BA are strong inducers (Figure 4). 5-Methylbenz[a]anthracene, with a methyl group attached to the BA molecule, induced lipid peroxidation as about half of that by BA (Figure 5). However, with an additional bromo group at the C7 position, the resulting compound 7-Br-5-MBA induced lipid peroxidation at a level similar to that by BA.
Figure 4.
Peroxidation of methyl linoleate initiated by benzo[a]pyrene, 7-Cl-benzo[a]pyrene, 7-Br-benzo[a]pyrene, with, 0, 7, and 21 J/cm2 of UVA light.
Figure 5.
Peroxidation of methyl linoleate initiated by 5-methylbenz[a]anthracene and 7-bromo-5-methylbenz[a]anthracene with, 0, 7, and 21 J/cm2 of UVA light.
Benzo[a]pyrene Series
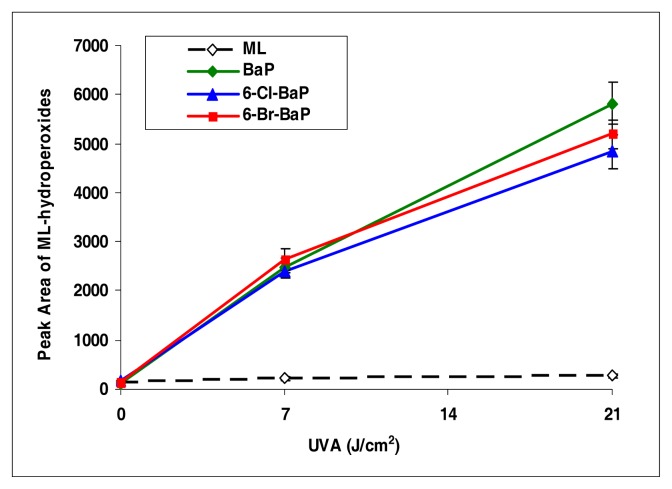
Among all the halo-PAHs and PAHs used in this study, BaP, 6-Cl-BaP, and 6-Br-BaP induced lipid peroxidation at the highest levels (Figure 6). However, there were no significant differences among these three compounds in induction of lipid peroxidation.
Figure 6.
Peroxidation of methyl linoleate initiated by benzo[a]pyrene, 6-Cl-benzo[a]pyrene, 6-Br-benzo[a]pyrene, with, 0, 7, and 21 J/cm2 of UVA light.
Conclusion
Upon photoirration with UVA light, all the halo-PAHs used in this study induced lipid peroxidation, but with different induction potencies.
The levels of lipid peroxidation by each halo-PAHs and PAHs were dose (light) dependent.
In general, the addition of a halogen atom to the parent PAH resulted in lipid peroxidation either increase, decrease or no effect on the capability of induction of lipid peroxidation.
There was no correlation between the level of lipid peroxidation and the tumorigenic potency of the compounds studied.
Halo-PAHs are environmental contaminants and, as shown in our study, can induce lipid peroxidation, these compounds may pose adverse health effect in humans.
References
- 1.Fu P. P., Von Tungeln L. S., Chiu L.-H., Own Z. Y. Halogenateo-polycyclic aromatic hydrocarbons: a class of genotoxic environmental pollutants. Environ. Carcinogen. Ecotoxicol. Rev. 1999;C17(2):71–109. [Google Scholar]
- 2.Dipple A., Moschel R. C., Bigger C. A. H. In: Chemical Carcinogens. Searle C. E., editor. American Chemical Society; Washington, DC: 1984. p. 41. Monograph 182. [Google Scholar]
- 3.Hartwell J. L. Survey of Compounds Which Have Been Tested for Carcinogenic Activity. Washington, D.C: 1951. U.S. Public Health Service Publication No. 149. [Google Scholar]
- 4.Clar E. Polycyclic Hydrocarbons. 1 and 2 Academic Press; New York, NY: 1964. [Google Scholar]
- 5.Harvey R. G. Polycyclic Aromatic Hydrocarbons. Wiley-VCH, Inc; 1997. [Google Scholar]
- 6.Thompson, J. I. & Company. Survey of Compounds Which Have Been Tested for Carcinogenic Activity. Washington, D.C: 1968–1969. U.S. Public Health Service Publication No. 149. [Google Scholar]
- 7.Fu P. P., Yang S. K. Stereoselective metabolism of 7-bromobenz[a]anthracene by rat liver microsomes: absolute configuration of trans-dihydrodiol metabolites. Carcinogenesis. 1983;4:979–984. doi: 10.1093/carcin/4.8.979. [DOI] [PubMed] [Google Scholar]
- 8.Chiu P.-L., Fu P. P., Yang S. K. Stereoselectivity of rat liver microsomal enzymes in the metabolism of 7-fluorobenz[a]anthracene and mutagenicity of metabolites. Cancer Res. 1984;44:562–570. [PubMed] [Google Scholar]
- 9.Fu P. P., Von Tungeln L. S., Unruh L. E., Ni Y-C., Chou M. W. Comparative regioselective and stereoselective metabolism of 7-chlorobenz[a]anthracene and 7-bromobenz[a]anthracene by mouse and rat liver microsomes. Carcinogenesis. 1991;12:371–378. doi: 10.1093/carcin/12.3.371. [DOI] [PubMed] [Google Scholar]
- 10.Xiao Y., Von Tungeln L. S., Chou M. W., Hart R. W., Fu P. P. Effect of caloric restriction on the metabolism of 7-bromobenz[a]anthracene and 7-fluorobenz[a]anthracene by male B6C3F1mouse liver microsomes: Reduction of metabolic activation pathway. Age. 1993;16(4):160–165. [Google Scholar]
- 11.Xiao Y., Von Tungeln L. S., Chou M. W., Hart R. W., Fu P. P. Effect of caloric restriction on the stereoselective metabolism of 7-chlorobenz[a]anthracene by male B6C3F1mouse liver microsomes. Age. 1993;16(4):166–172. [Google Scholar]
- 12.Own Z. Y., Chiu L-H., Von Tungeln L. S., Deck S. J., Vingiello F. A., Fu P. P. Synthesis and rat liver microsomal metabolism of 2-chlorodibenz[a,l]pyrene and 10-chlorodibenz[a,l]pyrene. Polycyclic Aromat. Cpds. 1996;11:333–340. [Google Scholar]
- 13.Fu P.P., Von Tungeln L. S., Zhan D-J., Bucci T. Potent tumorigenicity of 7-chlorobenz[a]anthracene and 7-bromobenz[a]anthracene in the neonatal B6C3F1 male mouse. Cancer Lett. 1996;101:37–42. doi: 10.1016/0304-3835(96)04111-0. [DOI] [PubMed] [Google Scholar]
- 14.Xia Q., Yi P., Zhan D.-J., Von Tungeln L. S., Hart R. W., Heflich R. H., Fu P. P. Liver tumors induced in B6C3F1mice by 7-chlorobenz[a]anthracene and 7-bromobenz[a]anthracene contain K-ras oncogene mutations. Cancer Letters. 1998;123:21–25. doi: 10.1016/s0304-3835(97)00366-2. [DOI] [PubMed] [Google Scholar]
- 15.Hong F., Von Tungeln L. S., Fu P. P., Watson F. Stereoselective metabolism of anthracene, 9-methylanthracene, 9,10-dimethylanthracene, 9-chloroanthracene, and 9-nitroanthracene by liver microsomes of 15-day-old male B6C3F1 mice. Polycyclic Aromat. Cpd. 1999;16:235–244. [Google Scholar]
- 16.Cherng S., Xia H. Q., Blankenship L. R., Freeman J. P., Wamer W. G., Howard P. C., Fu P. P. Photodecomposition of retinyl palmitate in ethanol by UVA light - formation of photodecomposition products, reactive oxygen species, and lipid peroxides. Chem. Res. Toxicol. 2005;18:129–138. doi: 10.1021/tx049807l. [DOI] [PubMed] [Google Scholar]
- 17.Totika M. Identification of new geometric isomers of methyl linoleate hydroperoxide and their chromatographic behavior. Biosci. Biotechnol. Biochem. 2000;64:1044–1056. doi: 10.1271/bbb.64.1044. [DOI] [PubMed] [Google Scholar]