Abstract
The adult liver is wrapped in a connective tissue sheet called the liver capsule, which consists of collagen fibrils and fibroblasts. In this study, we set out to construct a liver organoid tissue that would be comparable to the endogenous liver, using a bioreactor. In vitro liver organoid tissue was generated by combining collagen fibrils, fibroblasts, and primary murine hepatocytes or Hep G2 on a mesh of poly-lactic acid fabric using a bioreactor. Then, the suitability of this liver organoid tissue for transplantation was tested by implanting the constructs into partially hepatectomized BALB/cA-nu/nu mice. As determined by using scanning and transmission electron microscopes, the liver organoid tissues were composed of densely packed collagen fibrils with fibroblasts and aggregates of oval or spherical hepatocytes. Angiogenesis was induced after the transplantation, and blood vessels connected the liver organoid tissue with the surrounding tissue. Thus, a novel approach was applied to generate transplantable liver organoid tissue within a condensed collagen fibril matrix. These results suggested that a dense collagen network populated with fibroblasts can hold a layer of concentrated hepatocytes, providing a three-dimensional microenvrionment suitable for the reestablishment of cell–cell and cell–extracellular matrix (ECM) interactions, and resulting in the maintenance of their liver-specific functions. This liver organoid tissue may be useful for the study of intrahepatic functions of various cells, cytokines, and ECMs, and may fulfill the fundamental requirements of a donor tissue.
Introduction
Extracellular matrices (ECMs) provide structural support for cells and perform various important functions. Collagens, a family of fibrous proteins, are the most abundant proteins in the ECM.1 The collagens are secreted by a variety of cell types, especially by connective tissue cells.
The adult liver is wrapped in a connective tissue sheet named the liver capsule, which consists of collagen fibrils and fibroblasts.2 A great deal of research has been focused on the maintenance of hepatocyte functions in vitro. Primary hepatocytes have been cultured on biomaterials in vitro, for example, collagen gels,3–6 Engelbreth–Holm–Swarm gels,7–9 and other materials.10–13 There are also some recent studies using collagen sandwich hepatocyte cultures that have achieved long culture periods and the maintenance of hepatic functions.14–16 However, no studies have described the reconstruction of highly concentrated connective tissue in vitro with properties similar to endogenous liver tissue. In the liver, type I collagen fibrils serve as a primary scaffold upon which are deposited microfibrils and filaments of collagen types III, V, and VI.17,18 To construct useful in vitro liver models, it is very important to form collagen fibrils in the connective tissue. Recently, it has become possible to stably construct a fibroblast-embedded condensed collagen fibril layer using a closed loop system composed of three major parts: a reservoir bottle, a diaphragm pump, and a bioreactor chamber.19 Because fibroblasts are embedded in the network collagen fibrils of this artificial tissue, it is useful for reconstructing the hepatic interstitial structure. Here, we constructed a liver organoid tissue using an originally designed bioreactor system, and implanted this tissue into the nude mouse.
Materials and Methods
Animals
Male C57BL/6JJcl mice, 6−8 weeks old (20–25 g; CLEA Japan, Tokyo, Japan), were used for hepatocyte isolation. Pregnant female ICR mice at 13 days postcoitus (CLEA Japan) were used for embryonic fibroblast isolation. Female BALB/cAJcl-nu/nu mice, 6 weeks old (17–18 g; CLEA Japan), were used as reconstructive hepatic transplant recipients. The animal protocols were approved by the Animal Experimentation Committee of Tokyo Institute of Technology.
Cell lines
The human hepatocellular carcinoma cell line, Hep G2, was provided by the RIKEN Bio-Resource Center (Tsukuba, Japan). Human diploid fibroblast, HFO, was provided by Dr. Satoshi Amano (Shiseido Institute, Shiseido, Tokyo, Japan). Both cell types were cultured in the Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Tokyo, Japan) containing 10% fetal bovine serum (FBS; Nichirei Biosciences, Tokyo, Japan) under 5% CO2 at 37°C. These cells were subcultured by treatment with 0.05% trypsin (Invitrogen) and 20 μM ethylenediaminetetraacetic acid (EDTA; Nacalai Tesque, Kyoto, Japan).
Isolation of murine hepatocytes
Hepatocytes were prepared from anesthetized BALB/cA mice by a two-step in situ collagenase perfusion method,20 with slight modifications. Briefly, murine liver was preperfused in situ with Hank's balanced salt solution (HBSS) containing 0.5 mM ethylene glycol tetraacetic acid (EGTA). Next, the liver was perfused with 0.015% collagenase in HBSS. Then, the liver was removed, and the cells were dispersed in ice-cold HBSS without EGTA. The resulting cells were filtered through a 100-μm-pore mesh nylon cell strainer (BD Biosciences, MA) and centrifuged twice for 2 min at 500 g to remove nonparenchymal cells. The remaining cells were centrifuged for 2 min at 500 g, and then subjected to a 40% Percoll density gradient centrifugation for 10 min at 1200 g. At this stage, cell viability as measured by trypan blue was >90%. The isolated hepatocytes were plated at a density of 1.2×106 cells per well in collagen-coated six-well plates. Cells were grown in the high-glucose (25 mM) DMEM (Invitrogen) containing 10% (v/v) heat-inactivated FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen) at 37°C in a humidified incubator with 5% CO2. The medium was changed after the first 4 h of incubation, and was replaced daily thereafter.
Preparation of murine embryonic fibroblasts
A pregnant female ICR at 13.5 days postcoitum was sacrificed by cervical dislocation, and embryos were removed. The limbs of the embryos were minced and treated with 0.25% trypsin (Invitrogen)+1 mM EDTA (∼2 mL per embryo) and incubated with gentle stirring at 37°C for 10–15 min. The recovered cells were subsequently cultured in the DMEM containing 10% (v/v) FBS.
Establishment of DsRed-expressing Hep G2
Hep G2 cells were cultured in the DMEM supplemented with penicillin/streptomycin and 10% (v/v) FBS. The CAG promoter-driven DsRed2 expression vector was constructed by subcloning the 1.7-kb Sal I-CAG promoter-EcoR I fragment from pCAGGS into the corresponding site of the pDsRed2-1 vector in the sense orientation.21 Twenty-five micrograms of CAG-DsRed2-1/EcoR I were transfected into human hepatocellular carcinoma cells by electroporation (1×107 cells; 230 V; 500 μF; 0.4 cm electrode gap). After electroporation, the cells were plated in 100-mm dishes. Selection was initiated the next day by adding 3 mg/mL G-418 to the culture medium. Clones of human hepatocellular carcinoma cells transfected with CAG-DsRed2-1/EcoR I were selected and maintained, and DsRed2 expression in these cells was assessed by flow cytometry. The highly fluorescent clones were isolated as Hep G2Red cells and used in transplantation experiments.
Generation of structural liver organoid tissue
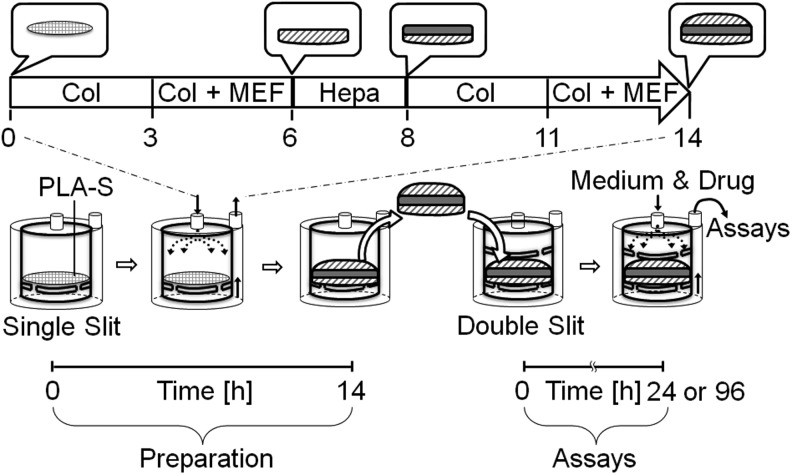
As can be seen in Figure 1, structural liver organoid tissue was generated by combining collagen fibrils, primary murine embryonic fibroblasts, and primary murine hepatocytes or Hep G2Red cells in vitro using a closed-loop system within a bioreactor chamber (diameter 17 mm×height 20 mm) developed by our group.19 We circulated 42.5 mL of 10% FBS/DMEM, supplemented with 7.5 mL of 50 mg/mL type I collagen prepared from calf skin by pepsin treatment (Koken Collagen, Tokyo, Japan), through the closed-loop system for 3 h. Then, we used a syringe to inject primary murine embryonic fibroblasts (5.0×106 cells suspended in 2 mL 10% FBS/DMEM, high glucose) into the system upstream of the bioreactor chamber. The mixed solution flowed through the closed-loop system at a predetermined flow rate (1–5 mL/min) for 6 h. Subsequently, 50 mL of 10% FBS/DMEM was circulated through the closed-loop system, and primary murine hepatocytes (1.0×107 cells suspended in 2 mL 10% FBS/DMEM) were injected into the system upstream of the bioreactor chamber. After 2 h, 42.5 mL of 10% FBS/DMEM, supplemented with 7.5 mL of 50 mg/mL type I collagen prepared from calf skin by pepsin treatment, was circulated through the closed-loop system for 3 h, and primary murine embryonic fibroblasts (5.0×106 cells suspended in 2 mL 10% FBS/DMEM) were injected into the system upstream of the bioreactor chamber.
FIG. 1.
A schematic illustration showing the strategy of hepatic constructs generated in a bioreactor.
Morphological analyses
The liver organoid tissue was fixed with Zamboni's fixative for light microscopy, scanning and transmission electron microscopy. For light microscopy, the samples were dehydrated with an ethanol series and embedded in paraffin. The sections were stained with hematoxylin and eosin and examined with a light microscope. The samples were postfixed with 2% osmium tetroxide in 0.1 M phosphate buffer, processed routinely, and ultimately examined with a scanning electron microscope (JSM 6360; JEOL Co., Ltd., Tokyo, Japan) or a transmission electron microscope (H8100; Hitachi Co., Ltd., Tokyo, Japan).
Hepatic function assays in liver organoid tissue
Urea production in the conditioned medium, 24 h after the addition of 2 mM ammonium chloride (NH4Cl; Sigma-Aldrich Japan, Tokyo, Japan) was quantified using a urea assay kit (Bioassay Systems, Hayward, CA). Albumin production in the conditioned medium was quantified using the Albuwell M albumin EIA kit (Exocell, Philadelphia, PA).
Testosterone metabolites in the conditioned medium were quantified by high performance liquid chromatography (HPLC) analysis.22 The liver organoid tissue was incubated with a fresh medium containing 0.25 mM testosterone for 24 h. Next, the conditioned medium was collected and mixed with 5 mL of ethyl acetate and 1 μL of 2.5 mM 11α-hydroxy-progesterone/dimethyl sulfoxide. After centrifugation, the organic layer was evaporated to prepare the HPLC sample. HPLC analysis was performed using LC-10ADVP (Shimadzu, Kyoto, Japan) with Cadenza columns (Cadenza CD-C18; Imtakt, Kyoto, Japan) and SPD-10A VP (Shimadzu). Testosterone hydroxylation was assessed using the C-R8A software (Shimadzu).
Transplantation of liver organoid tissue
While under pentobarbital anesthesia, the mice were subjected to an upper abdominal incision, and then the right portal vein branch was exposed and ligated, and the right lobes of the liver (30%) were hepatectomized. Then, the liver organoid tissue was transplanted into the mice. Two weeks later, the intraperitoneal liver organoid tissue was removed for histological analysis of the vascular network or transplanted hepatocytes at the same location.
Statistical evaluation
Results of multiple experiments (n=3–4) are reported as the mean±standard error (SE). Statistical comparisons were made using a Student's t-test.
Results
Three-dimensional reconstruction of hepatic tissue using a bioreactor
A solution of type I collagen and primary murine embryonic fibroblasts was introduced into a bioreactor via a closed-loop system, followed by the sequential addition of primary murine hepatocytes and a second round of collagen and primary murine embryonic fibroblasts. These manipulations produced a hepatic aggregate consisting of a layer of primary hepatocytes sandwiched between two layers of embryonic fibroblasts and deposited collagen fibrils on a sheet of poly-lactic acid (PLA). Glossy aggregates had accumulated on the PLA sheet after 14 h of circulation through the bioreactor (Fig. 1). The dissolved oxygen content was 5.16±0.07 mg/L at the outlet of the circulating medium.
The liver organoid tissue was 1.5 mm in thickness and 17 mm in diameter (Fig. 2A, B). The average weight of the organoid tissue was ∼0.4 g. Further, the density of the collagen layer was 34±2.5 mg/cm2, which is almost same as that of endogenous murine connective tissue.
FIG. 2.
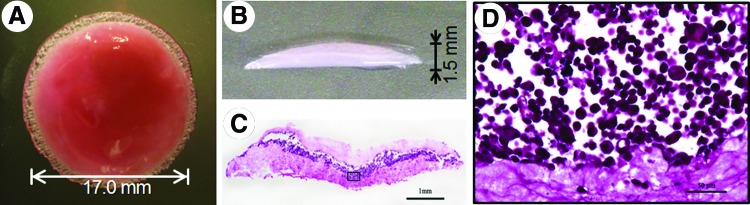
Micrographic observations of liver organoid tissue. (A) Top view of a whole liver organoid tissue construct. The glossy and reddish organoid tissue is discoidal. (B) Cross-sectional profile of an organoid tissue construct. (C, D) Cross sections were stained with hematoxylin and eosin. (C) A hepatic cell mass is sandwiched between two layers of collagen fibrils populated with fibroblasts. (D) Light micrograph showing an inscribed area in (C). Primary hepatocytes are round and ∼20 μm in diameter. The scale bars correspond to 1 mm (C) and 50 μm (D). Color images available online at www.liebertpub.com/tea
Morphological analysis of hepatocytes in the liver organoid tissue
Cross-sectional profiles of the liver organoid tissue were stained with hematoxylin and eosin (Fig. 2C). Clusters of hepatocytes were sandwiched between two layers of collagen fibrils populated with murine fibroblasts. We could clearly see a layer of primary hepatocytes ∼200–500 μm thick, and two layers of collagen fibrils populated with embryonic fibroblasts, ∼300–500 μm thick. Upon close inspection, round or spherical hepatocytes, ∼20 μm in diameter, were seen on collagen fibers, while fibroblasts tended to be bipolar or stellate in shape within the layers of collagen fibrils (Fig. 2D).
The liver organoid tissue was examined by scanning electron microscopy to investigate the extracellular microenvironment of the hepatocytes. The collagen layers were composed of densely packed collagen fibrils running parallel to the plane of the PLA sheet in the three-dimensional (3D) culture. Primary hepatocytes were oval or spherical in shape and formed clusters. By contrast, hepatocytes cultured in two dimensions (2D) on collagen-coated dishes were generally flat, and displayed lamellipodia after 3 days (Fig. 3A). These results suggest that the layers of collagen fibrils played an important role in establishing or maintaining the globular morphology of hepatocytes in the 3D microenvironment (Fig. 3B).
FIG. 3.
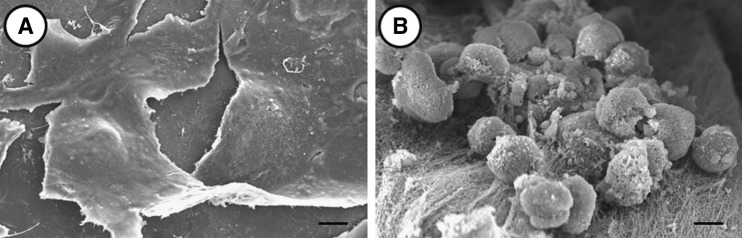
Scanning electron micrographic observations of primary hepatocytes on a collagen-coated dish (two dimensional [2D]) or in the liver organoid tissue (three dimensional [3D]). Hepatocytes cultured on collagen-coated dishes (A) and in liver organoid tissue (B). The scale bar corresponds to 10 μm.
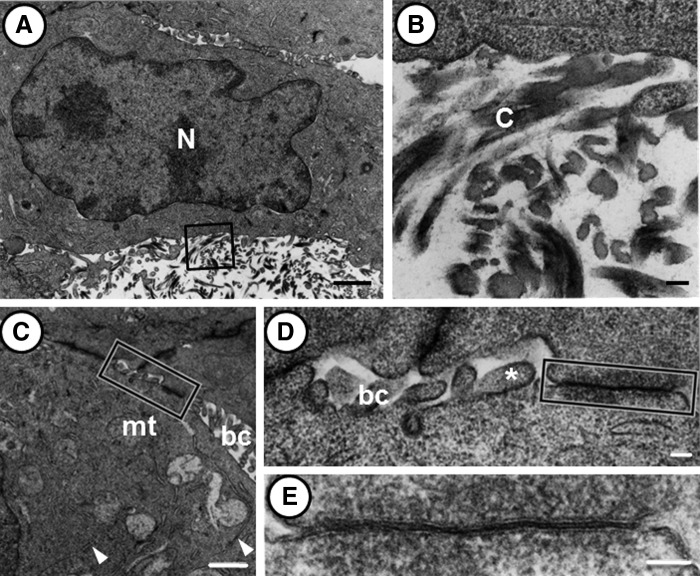
Under a transmission electron microscope, we could observe clusters of primary hepatocytes (Fig. 4). They had a round nucleus characterized by an irregular contour (Fig. 4A). In the hepatocyte cytoplasm, we could identify several mitochondria with cristae, 0.2–0.6 μm in diameter, and a small Golgi apparatus composed of five to seven flattened cisternae. We also encountered flattened endoplasmic reticula in the peripheral region of primary hepatocytes. A limited number of collagen fibrils approached the primary hepatocytes and appeared to attach to their surface (Fig. 4B). In the cluster of hepatocytes, numerous channels resembling bile canaliculi could be observed between neighboring cells (Fig. 4C–E). The size of these channels varied from 0.2 to 0.5 μm wide. They had short microvilli, 0.1–0.2 μm long, protruding into the lumen. Frequently, the cell membranes were more closely apposed than usual. Adherent junctions and gap junctions were commonly observed in the vicinity of bile canaliculi, but tight junctions were rarely seen.
FIG. 4.
Transmission electron micrographs showing primary hepatocytes in liver organoid tissue. (A) The cells are ∼15 μm wide, spherical in shape, and have a prominent cytoplasm containing mitochondria and endoplasmic reticulum. (B) Higher magnification of the inscribed area in (A). Collagen fibrils appear to be attached to the cell surface. (C–E) Transmission electron micrographs showing the cytoplasm (C), bile canaliculi (C, D), and a gap junction (E) between the neighboring cells. (C) In the cytoplasm, mitochondria with cristae and endoplasmic reticulum (arrowheads) can be observed. Tubular bile canaliculi are frequently observed between the cells. (D) Higher magnification of the inscribed area in (C). Short microvilli (asterisk) protrude into the lumen of the bile canaliculi. (E) Higher magnification of the inscribed area in (D). The cell membranes are closely apposed to form a gap junction. The scale bars correspond to 1 μm (A), 100 nm (B), 1 μm (C), and 100 nm (D, E). Abbreviations: mt, mitochondria; bc, bile canaliculi; C, collagen fibril; N, nuclei.
Structural liver organoid tissue exhibits multiple liver-specific functions
Several liver-specific functions, for example, the production of urea and albumin and drug metabolism activity, were analyzed in the liver organoid tissue. Ammonia, which is toxic to the central nervous system, may be detoxified into urea through the coordinated actions of the urea cycle in hepatocytes. Therefore, we investigated urea production as a liver-specific function that is mediated in the mitochondria and cytoplasm of hepatocytes. To investigate the potential for dynamic urea synthesis, the urea concentrations were measured in liver organoid tissue conditioned media after the addition of NH4Cl (Fig. 5). Urea production was observed in the liver organoid tissue after the addition of the ammonium ion. We compared the functional activity of the liver organoid tissue to that in 2D hepatocyte cultures, and observed that urea production in the former was significantly higher at 72 h than in the latter, suggesting that liver organoid tissue culture can maintain hepatic functions that are lost or impaired in 2D cultures.
FIG. 5.
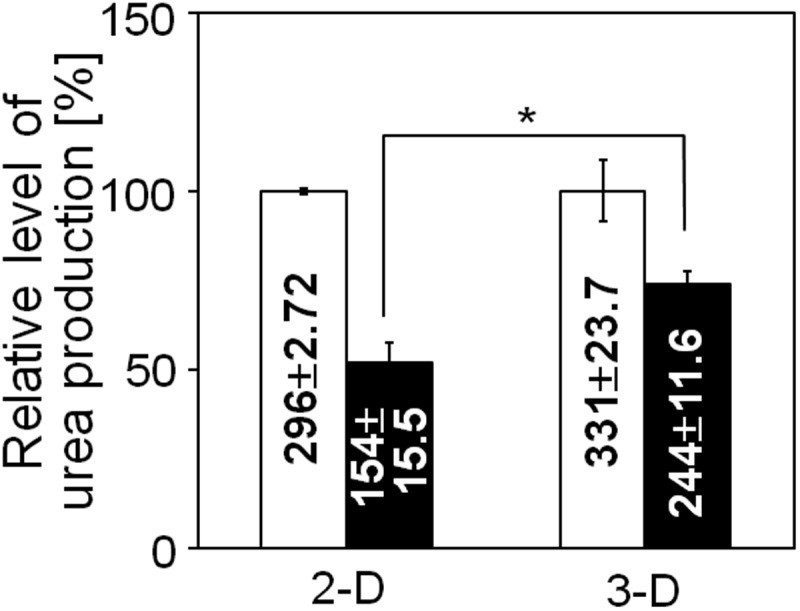
Urea concentrations in the conditioned medium of 2D (collagen-coated dishes) and 3D (liver organoid tissue) hepatocyte cultures. The relative amount of urea in the conditioned media after 72 h was calculated by setting the values obtained at 24 h to 100%. The absolute values (μg/106 cells) are noted in the corresponding columns. Mean±standard error (SE), n=3; *p<0.01.
We also measured the levels of albumin secretions in the conditioned media from the liver organoid tissues or 2D hepatocyte cultures. A measurable amount of albumin was synthesized in the organoid tissues. The level of albumin production in the organoid tissue was well maintained; specifically, the levels on day 3 were ∼85% of those observed on day 1. The values obtained in the 2D cultures indicated a steeper drop in albumin production over time for the cells cultured on collagen alone (Fig. 6).
FIG. 6.
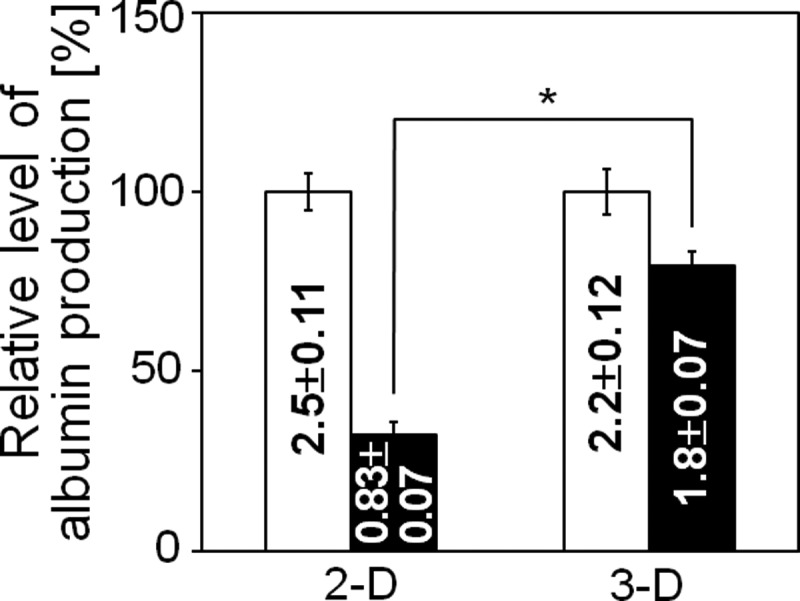
Albumin concentrations in the conditioned medium of 2D (collagen-coated dishes) and 3D (liver organoid tissue) hepatocyte cultures. The relative amount of albumin in the conditioned media after 72 h was calculated by setting the values obtained at 24 h to 100%. The absolute values (μg/106 cells) are noted in the corresponding columns. Mean±SE, n=3; * p<0.01.
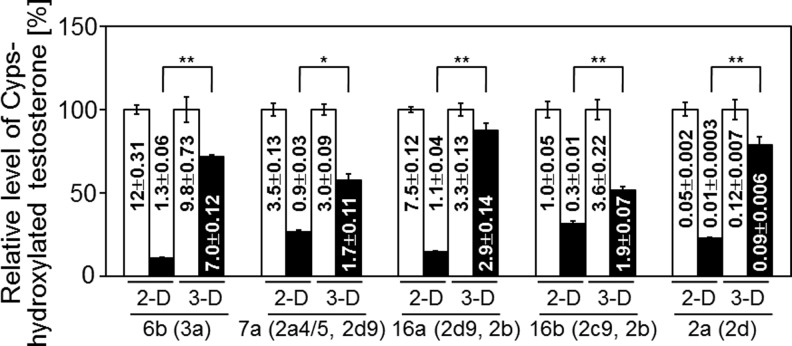
Many poisonous compounds in the blood enter hepatocytes through various mechanisms, including endocytosis and passive diffusion. These compounds may be metabolized in the microsomal system, which includes the cytochrome P450 (CYP450) enzymes. We tested the activities of the CYP450 enzymes in the liver organoid tissue and in 2D hepatocyte cultures. Specifically, we measured the testosterone oxidation patterns in the organoid tissue-conditioned media using high-performance liquid chromatography. We quantified the concentration of each hydroxylated testosterone: 15α-OHT, 6β-OHT, 7α-OHT, 16α-OHT, 16β-OHT, 2α-OHT, and 2β-OHT, corresponding to oxidation by Cyp2a4/5, Cyp3a, Cyp2a4/5 and 2d9, Cyp2d9 and 2b, Cyp2c29 and 2b, and Cyp2d, respectively. The concentrations of hydroxylated testosterones, for example, 6β-OHT, 7α-OHT, 16α-OHT, and 16β-OHT, in the organoid tissue conditioned media on day 3 were ∼50%–70% of those observed on day 1. These results indicate that the organoid tissues maintained 50%–70% of their cytochrome P450 enzyme activity after 3 days in culture. By contrast, the concentrations of hydroxylated testosterones on day 3 of the 2D culture were <25% of those recorded on day 1 (Fig. 7).
FIG. 7.
Testosterone hydroxylation in 2D (collagen-coated dishes) and 3D (liver organoid tissue) hepatocyte cultures. The relative amount of each hydroxylated testosterone was calculated by setting the values obtained at 24 h to 100%. The absolute values (nmol/106 cells) are noted in the corresponding columns. Mean±SE, n=3; *p<0.05; **p<0.01.
Hep G2/HFO liver organoid tissue was successfully engrafted in a partially hepatectomized nude mouse
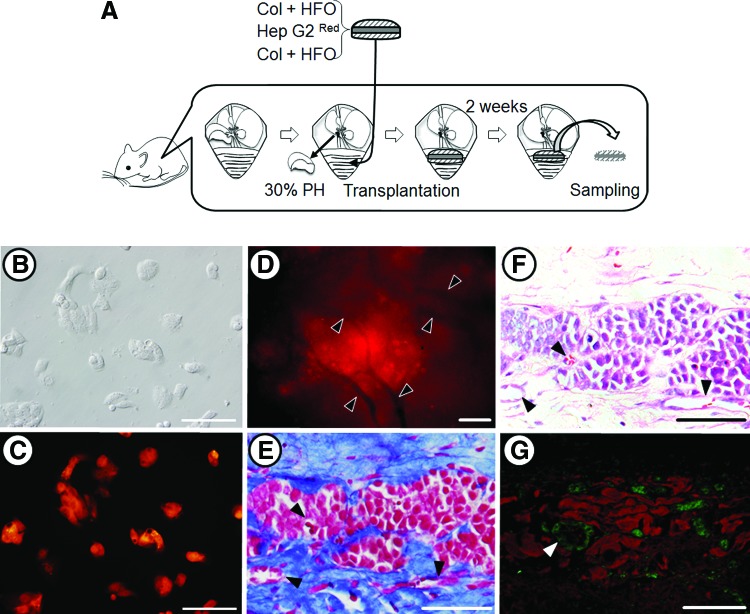
In addition to the experiments using primary murine hepatocytes, we also prepared liver organoid tissue using Hep G2, a human hepatocellular carcinoma cell line, for use in transplantation experiments (Fig. 8A). To easily discriminate the donor cells from the recipient cells in the engrafted area, a DsRed2 expression vector was introduced into the Hep G2 cells and the vector integrated into the genomic DNA. Seven clones that consistently expressed high levels of DsRed2, as determined by flow cytometry, were obtained. A clone that had 100% DsRed2+ progeny was designated Hep G2Red (Fig. 8B, C) and used for the following experiments. A liver organoid tissue was generated consisting of Hep G2Red, in place of primary hepatocytes, and HFO, a human fibroblast cell line, in place of primary fibroblasts. The Hep G2Red/HFO liver organoid tissue was ∼1.6 mm in thickness and 17 mm in diameter. This liver organoid tissue was ectopically transplanted into the peritoneal cavity of a female BALB/cA-nu/nu mouse after a partial hepatectomy (Fig. 8A). The graft could be observed in the peritoneal cavity 2 weeks after transplantation. Microvascular networks could be observed throughout this engrafted tissue (Fig. 8D). We prepared 4-μm sections of the graft treated with Zamboni's fixation. The AZAN staining of this specimen showed that collagen remained abundant in the graft, that fibroblasts existed within the collagen, and that vessel-like tube formation could be observed in both the collagen and the Hep G2Red areas (Fig. 4E). Next, hematoxylin–eosin staining and immunohistochemical examination with anti-albumin and anti-CD31/PECAM-1 antibodies were performed (Fig. 8F, G, respectively). Endothelial cells, CD31+ cells, formed a tube-like structure in the albumin-positive region of the graft (Fig. 8G). These results indicate that the Hep G2Red/HFO liver organoid tissue was successfully engrafted and vascularized in the partially hepatectomized nude mouse.
FIG. 8.
Transplanted liver organoid tissues in a partially hepatectomized mouse model. (A) A schematic illustration showing the transplantation of liver organoid tissue. Light micrograph (B) and fluorescent micrograph (C) images of transplanted DsRed2-expressing Hep G2 cells. (D–G) Micrographs of liver organoid tissue. (D) Fluorescent image. The red fluorescence indicates surviving DsRed-expressing Hep G2Red cells. Arrowheads indicate new blood vessels, which appear black. (E, F) Histological analyses: AZAN and hematoxylin–eosin staining, respectively, of liver organoid tissue sections after transplantation. Vascularization was detected at the condensed collagen fibril matrices. Arrowheads indicate new blood vessels. (G) Immunohistochemical analysis of the transplanted hepatic construct indicting albumin-positive hepatic cells and CD31/PECAM-1-positive endothelial cells using anti-albumin (red) and anti-CD31/PECAM-1 (green) antibodies. Arrowheads indicate new blood vessels. The scale bar corresponds to 200 μm. The animal transplantation experiments were carried out four times. Color images available online at www.liebertpub.com/tea
Discussion
Primary cultured hepatocytes have been extensively used as a model system for pharmacological, toxicological, and metabolic studies; however, the metabolism and gene expression patterns of primary cultured cells are frequently altered during culture in a 2D system, which in turn is influenced by changes in cellular morphology, intercellular signal transduction, and other extracellular environmental cues.23,24 Cells in tissues and organs exist in a 3D environment surrounded by other cells. The cuboidal cell shape, distinct polarity, and 3D cellular communication are known to be crucial for key metabolic pathways and tissue-specific phenotypes. Novel in vitro culture systems that more authentically represent the cellular environment are required for advancing our understanding of complex biological phenomena.
Ten million hepatocytes were entrapped between two layers of collagen fibrils populated with fibroblasts using a bioreactor that we designed. Hepatocytes cultured as liver organoid tissue maintained urea and albumin synthesis and the CYP450 activity significantly better than hepatocytes cultured on collagen-coated dishes. Morphologically, hepatocytes in the liver organoid tissue were oval or spherical in shape and ∼15 μm in diameter. Hepatocytes in the liver organoid tissue formed clusters that were surrounded by a network of densely packed collagen fibrils. A limited number of collagen fibrils approached the hepatocytes and appeared to be anchored to their surface. Therefore, these hepatocytes could interact with collagen fibrils through integrins. Collagen fibrils may offer scaffolds for hepatocytes and may alter their behavior, including their growth, viability, and liver-specific functions.
There are several reports describing collagen sandwich hepatocyte monolayer cultures that demonstrate the maintenance of hepatic functions and long culture periods (8 days). Dunn et al. showed that the hepatocyte morphology under collagen-sandwich culture conditions was normal.5 In this report, we showed that after 3 days in culture, the hepatocytes still had a round shape, resembling their endogenous counterparts (Fig. 3). It is considered to be structurally impossible to have a bile canaliculi in the context of a 2D culture; by contrast, we observed a bile canaliculi in our 3D system (Fig. 4C, D). Thus, three dimensions are needed to form a bile canaliculi.
Hepatocytes in 3D cultures had oval nuclei with irregular contours and larger amounts of cytoplasm as compared to those in 2D cultures. This observation suggests that hepatocytes in the organoid tissue contained more organelles, for example, mitochondria, Golgi apparatus, and endoplasmic reticula, than those on culture dishes. Between the hepatocytes, we could frequently observe bile canaliculi and cell–cell junctions, resembling adherent and gap junctions. The bile canaliculi in the liver organoid tissue were 0.5–1.0 μm wide, and short microvilli protruded into their lumen. The formation of bile canaliculi in artificial liver tissues has also been reported elsewhere.3,25 Bile acid excretion is considered to be one of the primary detoxification mechanisms in the liver, because the accumulated bile acids in hepatocytes may provide detergent effects on the cell membrane. Based on these morphological findings, primary hepatocytes entrapped between the collagen networks can restore the polarization of hepatocytes in vivo.
Many reports suggest that liver-specific functions could be maintained by a 3D organization, cell density,17,26 and interaction with ECM.4,5 The liver organoid tissue generated in our bioreactor could serve as artificial liver tissue demonstrating strong hepatic differentiated functions, for example, the expression of albumin, tyrosine amino transferase, transthyretin, and tryptophan 2, 3-dioxygenase (data not shown). We also confirmed that urea synthesis could be maintained in liver organoid tissues, probably because stacked hepatocytes possessed a large amount of cytoplasm and mitochondria.
Testosterone is metabolized in a region-selective manner by different P450 enzymes, and can be used as a multienzymatic substrate to simultaneously investigate the activities of multiple enzymes. The testosterone hydroxylation system is localized in the endoplasmic reticulum. In this study, we noted that the testosterone hydroxylation activity was maintained in the liver organoid tissue hepatocytes after 3 days in culture. It is conceivable that oval or spherical hepatocytes in the liver organoid tissues could maintain a considerable amount of endoplasmic reticulum in their cytoplasm, because the average volume of oval hepatocytes should be greater compared with flattened cells on culture dishes.
One of the major objectives in using liver organoid tissue for hepatocyte transplantation was to achieve sufficient cell engraftment and survival. With respect to treating liver-based inherited metabolic deficiencies and liver failures, liver transplantation is well established as an effective final option.27 However, the progressive demand for transplantable livers far outweighs the donated organ supply.28 Because this donor shortage issue will likely never be resolved, investigators have been prompted to search for alternate treatment options, including the creation of new cell-based therapies using hepatocytes. Researchers have transplanted hepatocytes into several different extrahepatic sites, including the intraperitoneal cavity, the pancreas, the mesenteric leaves, the lung parenchyma, under the kidney capsule, and in the subcutaneous space. It has been shown that providing ECMs to heterotopically transplanted hepatocytes affords significantly greater hepatocyte survival.29 In the liver, hepatic cells are surrounded by the ECM that is important for functional and structural maintenance through cell–cell and cell–ECM interactions.30,31 The intact liver is enwrapped within a capsule that is mainly composed of collagen fibrils and fibroblasts. Taking account of this liver architecture, we have generated a liver organoid tissue with a collagen fibril matrix, using a bioreactor to generate an artificial tissue in vitro. The histological structure of this construct is close to that of the liver itself. The liver organoid tissue has been experimentally investigated by transplantation into extrahepatic sites. To easily visualize the transplanted hepatocytes, Hep G2Red cells, constitutively expressing DsRed were used during the preparation of the liver organoid tissue for transplantation. The transplanted liver organoid tissue revealed the formation of microvascular networks throughout the tissue constructs, indicating that integration with the host animal had occurred. This liver organoid tissue will be useful for applications related to transplantation.
The transplantation of the liver organoid tissue to the mesenteric vessels inside the intraperitoneal cavity has several advantages: it permits the transplantation of a cell number that is equivalent to an intact liver, the transplantation of genetically altered cells, and the engraftment of transplanted cells. The present study demonstrated that providing a condensed collagen fibril matrix in the transplantation contributed to increased hepatic cell engraftment, and to the stable survival of hepatic cells. These hepatic aggregates had a collagen density approaching endogenous tissue levels, and are mechanically suitable for in vivo implantation. Based on the lack of sufficient vascular support for the transplanted hepatocytes (not shown), we expected that establishing a local vascular network at the transplantation site would allow for nutrient and gas exchange with the grafts and that this would reduce graft loss.
In conclusion, by considering the 3D interactions between hepatocytes and the ECM, we made progress toward developing a liver model. The advantage of our system is that it consists of an artificial hepatic construct, which is structurally similar to the anatomical structures that occur naturally in the liver. The organoid tissue can be generated in a bioreactor within 24 h, and could serve as a model tissue to study the intrahepatic functions of various cells, cytokines, and ECMs. By mimicking the structure of the natural liver, our system effectively maintains multiple functions of liver tissue.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research (B) (No. 21300178 and 22300167) from the Japan Society for the Promotion of Science (JSPS); and a Grant-in-Aid for Scientific Research on Innovative Areas (No. 23119003) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan. Miho Tamai was supported by a Research Fellowship from JSPS.
Disclosure Statement
The authors who have taken part in this study declared that they do not have anything to disclose regarding funding or conflict of interests with respect to this article.
References
- 1.Di Lullo G.A. Sweeney S.M. Korkko J. Ala-Kokko L. San Antonio J.D. Mapping the ligand-binding sites and disease-associated mutations on the most abundant protein in the human, type I collagen. J Biol Chem. 2002;277:4223. doi: 10.1074/jbc.M110709200. [DOI] [PubMed] [Google Scholar]
- 2.Wood R.L. Kelly D.E. Bailey F.R. Copenhaver W.M. Bailey's Textbook of Histology. Williams & Wilkins; Baltimore, MD: 1978. [Google Scholar]
- 3.Marion T.L. Leslie E.M. Brouwer K.L. Use of sandwich-cultured hepatocytes to evaluate impaired bile acid transport as a mechanism of drug-induced hepatotoxicity. Mol Pharmacol. 2007;4:911. doi: 10.1021/mp0700357. [DOI] [PubMed] [Google Scholar]
- 4.Berthiaume F. Moghe P.V. Toner M. Yarmush M.L. Effect of extracellular matrix topology on cell structure, function, and physiological responsiveness: hepatocytes cultured in a sandwich configuration. FASEB J. 1996;10:1471. doi: 10.1096/fasebj.10.13.8940293. [DOI] [PubMed] [Google Scholar]
- 5.Dunn J.C. Yarmush M.L. Koebe H.G. Tompkins R.G. Hepatocyte function and extracellular matrix geometry: long-term culture in a sandwich configuration. FASEB J. 1989;3:174. doi: 10.1096/fasebj.3.2.2914628. [DOI] [PubMed] [Google Scholar]
- 6.Michalopoulos G. Sattler C.A. Sattler G.L. Pitot H.C. Cytochrome P-450 induction by phenobarbital and 3-methylcholanthrene in primary cultures of hepatocytes. Science. 1976;193:907. doi: 10.1126/science.948753. [DOI] [PubMed] [Google Scholar]
- 7.Toyoda Y. Tamai M. Kashikura K. Kobayashi S. Fujiyama Y. Soga T. Tagawa Y. Acetaminophen-induced hepatotoxicity in a liver tissue model consisting of primary hepatocytes assembling around an endothelial cell network. Drug Metab Dispos. 2012;40:169. doi: 10.1124/dmd.111.041137. [DOI] [PubMed] [Google Scholar]
- 8.Ben-Ze'ev A. Robinson G.S. Bucher N.L. Farmer S.R. Cell-cell and cell-matrix interactions differentially regulate the expression of hepatic and cytoskeletal genes in primary cultures of rat hepatocytes. Proc Natl Acad Sci U S A. 1988;85:2161. doi: 10.1073/pnas.85.7.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trautwein C. Davies M. Elias E. Strain A. Manns M.P. Extracellular matrix proteins modulate cytochrome P450 2D6 expression in human hepatocytes. J Hepatol. 1995;22:50. doi: 10.1016/0168-8278(95)80259-2. [DOI] [PubMed] [Google Scholar]
- 10.Skardal A. Smith L. Bharadwaj S. Atala A. Soker S. Zhang Y. Tissue specific synthetic ECM hydrogels for 3-D in vitro maintenance of hepatocyte function. Biomaterials. 2012;33:4565. doi: 10.1016/j.biomaterials.2012.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim Y. Rajagopalan P. 3D hepatic cultures simultaneously maintain primary hepatocyte and liver sinusoidal endothelial cell phenotypes. PLoS One. 2010;5:15456. doi: 10.1371/journal.pone.0015456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim M. Lee J.Y. Jones C.N. Revzin A. Tae G. Heparin-based hydrogel as a matrix for encapsulation and cultivation of primary hepatocytes. Biomaterials. 2010;31:3596. doi: 10.1016/j.biomaterials.2010.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ananthanarayanan A. Narmada B.C. Mo X. McMillian M. Yu H. Purpose-driven biomaterials research in liver-tissue engineering. Trends Biotechnol. 2011;29:110. doi: 10.1016/j.tibtech.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Kim Y. Lasher C.D. Milford L.M. Murali T.M. Rajagopalan P. A comparative study of genome-wide transcriptional profiles of primary hepatocytes in collagen sandwich and monolayer cultures. Tissue Eng Part C Methods. 2010;16:1449. doi: 10.1089/ten.tec.2010.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathijs K. Kienhuis A.S. Brauers K.J. Jennen D.G. Lahoz A. Kleinjans J.C. van Delft J.H. Assessing the metabolic competence of sandwich-cultured mouse primary hepatocytes. Drug Metab Dispos. 2009;37:1305. doi: 10.1124/dmd.108.025775. [DOI] [PubMed] [Google Scholar]
- 16.Giri S. Acikgoz A. Pathak P. Gutschker S. Kursten A. Nieber K. Bader A. Three dimensional cultures of rat liver cells using a natural self-assembling nanoscaffold in a clinically relevant bioreactor for bioartificial liver construction. J Cell Physiol. 2012;227:313. doi: 10.1002/jcp.22738. [DOI] [PubMed] [Google Scholar]
- 17.Sudo R. Mitaka T. Ikeda M. Tanishita K. Reconstruction of 3D stacked-up structures by rat small hepatocytes on microporous membranes. FASEB J. 2005;19:1695. doi: 10.1096/fj.04-3269fje. [DOI] [PubMed] [Google Scholar]
- 18.Martinez-Hernandez A. Amenta P.S. The hepatic extracellular matrix. I. Components and distribution in normal liver. Virchows Arch A Pathol Anat Histopathol. 1993;423:1. doi: 10.1007/BF01606425. [DOI] [PubMed] [Google Scholar]
- 19.Iwashiro H. Hosoya S. Hirai K. Mima T. Ohashi S. Aihara T. Ito S. Ohara S. Adachi E. Characterization of dense artificial connective tissues generated in a newly designed bioreactor. Connect Tissue Res. 2011;52:340. doi: 10.3109/03008207.2010.531801. [DOI] [PubMed] [Google Scholar]
- 20.Seglen P.O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- 21.Ryu J.Y. Siswanto A. Harimoto K. Tagawa Y.I. Chimeric analysis of EGFP and DsRed2 transgenic mice demonstrates polyclonal maintenance of pancreatic acini. Transgenic Res. 2012 doi: 10.1007/s11248-012-9661-8. [DOI] [PubMed] [Google Scholar]
- 22.Tsutsui M. Ogawa S. Inada Y. Tomioka E. Kamiyoshi A. Tanaka S. Kishida T. Nishiyama M. Murakami M. Kuroda J. Hashikura Y. Miyagawa S. Satoh F. Shibata N. Tagawa Y. Characterization of cytochrome P450 expression in murine embryonic stem cell-derived hepatic tissue system. Drug Metab Dispos. 2006;34:696. doi: 10.1124/dmd.105.007674. [DOI] [PubMed] [Google Scholar]
- 23.Griffith L.G. Naughton G. Tissue engineering—current challenges and expanding opportunities. Science. 2002;295:1009. doi: 10.1126/science.1069210. [DOI] [PubMed] [Google Scholar]
- 24.Shin H. Jo S. Mikos A.G. Biomimetic materials for tissue engineering. Biomaterials. 2003;24:4353. doi: 10.1016/s0142-9612(03)00339-9. [DOI] [PubMed] [Google Scholar]
- 25.Gerlach J.C. Mutig K. Sauer I.M. Schrade P. Efimova E. Mieder T. Naumann G. Grunwald A. Pless G. Mas A. Bachmann S. Neuhaus P. Zeilinger K. Use of primary human liver cells originating from discarded grafts in a bioreactor for liver support therapy and the prospects of culturing adult liver stem cells in bioreactors: a morphologic study. Transplantation. 2003;76:781. doi: 10.1097/01.TP.0000083319.36931.32. [DOI] [PubMed] [Google Scholar]
- 26.Hamilton G.A. Jolley S.L. Gilbert D. Coon D.J. Barros S. LeCluyse E.L. Regulation of cell morphology and cytochrome P450 expression in human hepatocytes by extracellular matrix and cell-cell interactions. Cell Tissue Res. 2001;306:85. doi: 10.1007/s004410100429. [DOI] [PubMed] [Google Scholar]
- 27.Crespo G. Marino Z. Navasa M. Forns X. Viral hepatitis in liver transplantation. Gastroenterology. 2012;142:1373. doi: 10.1053/j.gastro.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 28.Ogawa S. Miyagawa S. Potentials of regenerative medicine for liver disease. Surg Today. 2009;39:1019. doi: 10.1007/s00595-009-4056-z. [DOI] [PubMed] [Google Scholar]
- 29.Ohashi K. Yokoyama T. Yamato M. Kuge H. Kanehiro H. Tsutsumi M. Amanuma T. Iwata H. Yang J. Okano T. Nakajima Y. Engineering functional two- and three-dimensional liver systems in vivo using hepatic tissue sheets. Nat Med. 2007;13:880. doi: 10.1038/nm1576. [DOI] [PubMed] [Google Scholar]
- 30.Bhatia S.N. Balis U.J. Yarmush M.L. Toner M. Effect of cell-cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal cells. FASEB J. 1999;13:1883. doi: 10.1096/fasebj.13.14.1883. [DOI] [PubMed] [Google Scholar]
- 31.Liu L. Yannam G.R. Nishikawa T. Yamamoto T. Basma H. Ito R. Nagaya M. Dutta-Moscato J. Stolz D.B. Duan F. Kaestner K.H. Vodovotz Y. Soto-Gutierrez A. Fox I.J. The microenvironment in hepatocyte regeneration and function in rats with advanced cirrhosis. Hepatology. 2012;55:1529. doi: 10.1002/hep.24815. [DOI] [PMC free article] [PubMed] [Google Scholar]