Abstract
Immune tolerance is defined as nonresponsiveness of the adaptive immune system to antigens. Immune mechanisms preventing inappropriate immune reactivity to innocuous antigens include deletion of reactive lymphocytes and generation of regulatory T (Treg) cells. The normal response to food antigens is the generation of antigen-specific Treg cells. In patients with food allergy, the dominant immune response is a TH2-skewed T-cell response and the generation of food-specific IgE antibodies from B cells. It is not known whether a failure of the Treg cell response is behind this inappropriate immune response, but interventions that boost the Treg cell response, such as mucosal immunotherapy, might lead to a restoration of immune tolerance to foods. Tolerance has been notoriously difficult to restore in animal disease models, but limited data from human trials suggest that tolerance (sustained nonresponsiveness) can be re-established in a subset of patients. Furthermore, studies on the natural history of food allergy indicate that spontaneous development of tolerance to foods over time is not uncommon. The current challenge is to understand the mechanisms responsible for restoration of natural or induced tolerance so that interventions can be developed to more successfully induce tolerance in the majority of patients with food allergy.
Keywords: Oral tolerance, food allergy, mucosal immunology, immunotherapy, regulatory T
Immune tolerance is defined as a nonresponsiveness of the adaptive immune system to an antigen and can be mediated either by deletion or inactivation of antigen-specific lymphocytes or deviation of antigen-specific T lymphocytes into regulatory T (Treg) cells. Immune tolerance is the basis of nonresponsiveness to self-antigens, and disruption of normal tolerance pathways leads to autoimmunity. In addition to discriminating self-antigens from non–self-antigens, the immune system must discriminate harmful non–self-antigens from innocuous antigens, such as those derived from food or the commensal flora. There is some overlap between immune mechanisms responsible for tolerance to self-antigens and innocuous non–self-antigens, which can also be mediated by deletion, anergy, or generation of antigen-specific Treg cells.
Removal of autoreactive lymphocytes is a process that occurs in the thymus and bone marrow and is known as central tolerance. Receiving a strong signal through the lymphocyte receptor at this early stage of lymphocyte development leads to apoptosis of the cell. The thymus has a specialized population of medullary epithelial cells that express a wide range of peripheral tissue antigens under the control of the transcription factor autoimmune regulator (AIRE).1 Mutations in AIRE lead to autoimmune polyendocrinopathy–candidiasis–ectodermal dystrophy (APECED) in human subjects, showing the importance of this pathway in tolerance to self-antigens.2,3 The thymus is also the origin of a population of Treg cells that express the transcription factor forkhead box protein 3 (FoxP3) and are termed natural regulatory T (nTreg) cells. These are distinct from another population of regulatory CD4+ T cells that are induced in the periphery and also express FoxP3 termed induced regulatory T (iTreg) cells. iTreg cells will be discussed at a later point in this review. Deletion of autoreactive T cells during development in the thymus is incomplete, and nTreg cells are involved in the suppression of autoreactive effector T cells in the periphery. Human subjects and mice lacking Treg cells caused by mutations in the FoxP3 gene have severe autoimmunity, which is known as immunodysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) in human subjects. Ablation of FoxP3+ Treg cells, even in adulthood, leads to rapid onset of autoimmunity in mice, showing that continued presence of FoxP3+ Treg cells is necessary for maintenance of self-tolerance.4
The paradigm of deletion of antigen-specific lymphocytes and generation of Treg cells also applies to tolerance induced in mature lymphocytes outside the thymus or bone marrow, and this process is known as peripheral tolerance. Exposure of naive T cells to antigens presented in the absence of costimulatory signals results in inactivation or anergy of the responder cell. In the absence of activation of the innate immune system by microbial signals (pathogen-associated molecular patterns) or damage signals (damage-associated molecular patterns), presentation of self-antigens or environmental antigens does not generate an effector T-cell response but rather deletion or anergy.
The site of antigen presentation also plays a significant role in determining the nature of the T-cell response. We know that antigen presentation in the gastrointestinal tract under homeostatic conditions results in the generation of an active regulatory response, which is termed oral tolerance, that is mediated by the generation of antigen-specific Treg cells. The preferential induction of T cells with regulatory activity is provided by tissue-specific factors, suggesting that the route of antigen exposure might be a critical factor in the development of immune tolerance.
TOLEROGENIC CAPACITY OF THE GASTROINTESTINAL MUCOSA
The phenomenon of oral tolerance was first described by Wells and Osborne in 1911.5,6 They used guinea pigs to show that inclusion of egg white, purified egg allergens, or oats in the diet rendered the animals hyporesponsive to sensitization and anaphylaxis to those proteins. Six decades later, a number of research groups showed that antigen feeding led to the development of suppressor T cells first in the gastrointestinal lymphoid tissue (Peyer patches and mesenteric lymph nodes) and at later time points in the spleen.7-9 These suppressor cells, when transferred to naive animals, could inhibit IgE responses or delayed-type hypersensitivity responses in the recipient mice. IgE production is highly sensitive to oral tolerance, and feeding of antigen has been shown to prevent symptoms in experimental models of asthma10,11 and food allergy or anaphylaxis.12-15
Weiner and colleagues initially showed that oral tolerance to myelin basic protein could be mediated by either CD4 or CD8 T cells,16,17 and subsequent work from the group focused on a subset of Treg cells that they termed TH3 cells.18 TH3 cells produce TGF-β and variable levels of IL-4 and IL-10 and mediate their suppression in a TGF-b–dependent manner.19 These cells are induced in both human subjects18 and mice20 after antigen feeding, and in mice they suppress the clinical severity of experimental autoimmune encephalitis (a model of multiple sclerosis). Regulatory cells other than TH3 cells have been shown to be involved in oral tolerance. Similar to the early findings that CD8 T cells could transfer tolerance, feeding of mice with an MHC class I epitope of ovalbumin induced oral tolerance to ovalbumin in mice in a CD8-dependent manner.21 Interestingly, these CD8+ Treg cells could suppress TH1 and TH17 responses but not TH2 responses. Thymus-derived nTreg cells have been shown to be dispensable for oral tolerance induction,10 but in contrast, iTreg cells (CD4+CD25+FoxP3+ cells) are required for tolerance induction. This was shown by ablation of FoxP3+ cells by using a transgenic mouse expressing the diphtheria toxin receptor under the control of the FoxP3 promoter (the DEREG mouse).13,22 Injection of diphtheria toxin into the mice abolishes all FoxP3+ Treg cells, including those induced after antigen feeding. After allowing the global Treg cell population to rebound, mice were immunized. Transient ablation of the Treg cell population resulted in a loss of oral tolerance.13 TH3 and iTreg cells might not be mutually exclusive in their function because TH3 cells can promote the development of FoxP3+ Treg cells.23 A number of investigators have shown that TGF-β is necessary for the induction of tolerance through the oral mucosa.10,19,20,24 In contrast, there are conflicting data about the role of IL-10 in oral tolerance.10,14,22,24 In addition to effects on other T cells mediated by secretion of cytokines, Treg cells induced by antigen feeding can affect other T cells indirectly by acting through a common antigen-presenting cell.25
In addition to tolerance mediated by the generation of regulatory cells, antigen feeding can also result in deletion of antigen-specific effector T cells.26-28 This phenomenon of deletion was initially described by using mice transgenic for a T-cell receptor against a peptide from ovalbumin, and they described that a high dose of antigen administered orally could induce deletion of these antigen-specific CD4+ T cells, whereas low doses led to expansion of cells with a regulatory phenotype.26 However, there are several reports of the induction of regulatory CD4+ T cells in response to high doses of antigen administered orally,13,29 suggesting that this paradigm of deletion at a high antigen dose and regulatory induction at a low antigen dose might not always hold true. Feeding of hapten before induction of hapten-induced contact hypersensitivity has shown that deletion of cells (CD8+ T effector cells) and induction of CD4+ Treg cells can be coexisting mechanisms promoting the development of immune tolerance.27,28 Mice that have a defect in the gene related to anergy in lymphocytes (GRAIL) in their T cells cannot be orally tolerized.30 Anergy is defined as nonresponsiveness of the T cells without having suppressive or tolerogenic activity, and the lack of tolerance to fed antigens in GRAIL-deficient mice suggests an additional role for anergic T cells in peripheral tolerance. It is likely that all 3 mechanisms of deletion, anergy, and active regulation play a role in maintaining immune tolerance to fed antigens.
The selective induction of Treg cells in response to antigen delivered to the gastrointestinal mucosa is mediated by a specialized subset of gastrointestinal dendritic cells (DCs). There are 2 developmentally distinct lineages of mononuclear phagocytes expressing CD11c within the intestinal lamina propria: those that express the surface marker CD103 and those that express the chemokine receptor CX3CR1.31,32 CX3CR1 mononuclear phagocytes can extend dendrites between epithelial cells and sample antigen directly from the lumen.33,34 However, they are thought to be nonmigratory and are not able to transmit these antigens to the mesenteric lymph nodes for the induction of an adaptive immune response.32 Recent evidence suggests that these CD103− mononuclear phagocytes are transcriptionally closer to macrophages than DCs.35 CD103+ DCs are migratory and constitutively traffic to the mesenteric lymph nodes.32 CD103+ DCs were recently found to acquire antigen through intestinal goblet cells that functioned as a conduit for delivery of antigens from the intestinal lumen.36 Under homeostatic conditions, these CD103+ DCs selectively induce the development of iTreg cells through mechanisms dependent on TGF-β, retinoic acid, the enzyme indoleamine 2,3-deoxygenase, and the cosignaling molecule 4-1BB.37-40 In addition to imprinting regulatory function on naive T cells, these CD103+ DCs also imprint gut homing in a retinoic acid–dependent manner41 and promote the generation of gut-homing, IgA-secreting B cells.42 Surgical ablation of the mesenteric lymph nodes abolishes oral tolerance,43 whereas Peyer patches have been shown to be dispensable for tolerance.44,45
Is the gastrointestinal mucosa uniquely tolerogenic? Although the gastrointestinal tract has specialized mechanisms to suppress immune responsiveness to the gut flora, such as high constitutive levels of IL-10 from intestinal macrophages46 and high levels of retinoic acid generated by stromal cells,47 Treg cells can be initiated at other sites. Tolerance to antigen through the respiratory tract is well established,48-50 and, as in the gut, regulatory responses to antigen in the lung are mediated by distinct airway DC subsets.51 Immune tolerance has also been described in response to antigen applied through the mouth mucosa52,53 and the skin.54,55 As in the gut, a population of skin-draining DCs was found to express high levels of the enzyme retinaldehyde dehydrogenase, which is necessary for retinoic acid production, and facilitate the development of FoxP3+ Treg cells.56 Therefore it is likely that immune tolerance can be induced at multiple sites in the body.
IS FOOD ALLERGY ASSOCIATED WITH A DEFECTIVE Treg CELL RESPONSE?
The adaptive immune response to food antigens in patients with food allergy is characterized by food-specific IgE production from B cells and a TH2-skewed T-cell response that drives the IgE class-switching. By definition, this is a failure of immune tolerance, but the Treg cell response to foods has been difficult to study in human subjects. Food antigen–specific T-cell lines grown from PBMCs of subjects with food allergy were found to be primarily of a TH2 phenotype, secreting IL-4 and IL-13 but little IFN-γ.57-59 There has been mixed success in growing food-specific T-cell lines from control subjects,58,60,61 but studies that have grown T-cell lines from control subjects have reported that they have a TH1 or TH0 profile in comparison with allergic subjects, who have a TH2 profile.61 Short-term stimulation of PBMCs and analysis of the T-cell cytokine phenotype in proliferating cells by using flow cytometry has also indicated that healthy control subjects have detectable peanut-specific T cells that are primarily of a TH1 phenotype compared with peanut-reactive T cells from subjects with peanut allergy who have a TH2 profile.62 The use of more specific detection methods of allergen-specific T cells, such as tetramers, or detection of CD154+ T cells after short-term stimulation with peanut antigen (6 hours) has demonstrated that there is a considerably lower frequency of peanut-specific T cells in healthy control subjects compared with that seen in subjects with peanut allergy,63,64 and although levels of IFN-γ are similar between groups, healthy control subjects have a noted lack of TH2 cytokine production.63 The Treg cell response in food allergy has been addressed by using 2 approaches: depletion of CD25+ T cells (including Treg cells) before in vitro restimulation with food allergen and detection of dividing Treg cells (CD4+CD25high) in PBMCs cultured with food allergen for 7 days in the presence of IL-2. The latter approach was used to show that subjects with milk allergy who were tolerant to heated milk had higher levels of milk-responsive Treg cells than subjects who were reactive to heated milk or those who were tolerant to all forms of milk.65 These subjects who are tolerant to heated milk are thought to be in the process of outgrowing their milk allergy, and therefore it was hypothesized that this Treg cell expansion was involved in the development of tolerance. However, there was no difference observed in the frequency of milk-specific Treg cells when comparing subjects with milk allergy with control subjects, suggesting that a Treg cell defect might not underlie the development of food allergy. Using the approach of Treg cell depletion to look at the effect on effector T-cell proliferation in milk-restimulated cultures, it has been found that there was detectable Treg cell activity in children who have outgrown their milk allergy.66,67 There is little evidence in these latter studies for a significant milk-specific Treg cell population in healthy control subjects, although this might be difficult to observe if there are few milk-specific effector T cells present to proliferate after Treg cells have been depleted. Studies are needed that directly address the frequency of food allergen–specific Treg cells in healthy subjects and subjects with food allergy to determine whether baseline clinical tolerance to foods is associated with an active food-specific Treg cell response.
In animal models the default response to an antigen delivered through the oral route is one of active immune tolerance, and therefore adjuvants must be used to elicit allergic sensitization. Commonly used adjuvants include cholera toxin (CT) and staphylococcal enterotoxin B. Oral administration of CT drives an increase in the migration of the normally tolerogenic CD103+ DCs from the lamina propria to the draining lymph nodes and induces a TH2 response from naive T cells through the costimulatory molecule OX40L.68 It is not known whether the antigen-specific Treg cell responses are also suppressed by CT. Mice sensitized with staphylococcal enterotoxin B as an adjuvant were found to have reduced levels of TGF-β and FoxP3 expression in antigen-restimulated splenocytes,69 suggesting a suppressive effect of this adjuvant on regulatory pathways. Van Wijk et al70 inhibited the regulatory molecule cytotoxic T lymphocyte–associated antigen 4 (CTLA-4) during exposure to peanut extract. They observed that when CTLA-4 was blocked during exposure to peanut in the presence of CT, there was an enhancement of sensitization and symptoms on allergen challenge. When CTLA-4 was blocked during feeding of peanut without adjuvant, there was no induction of IgE and there were no symptoms induced on allergen challenge.70
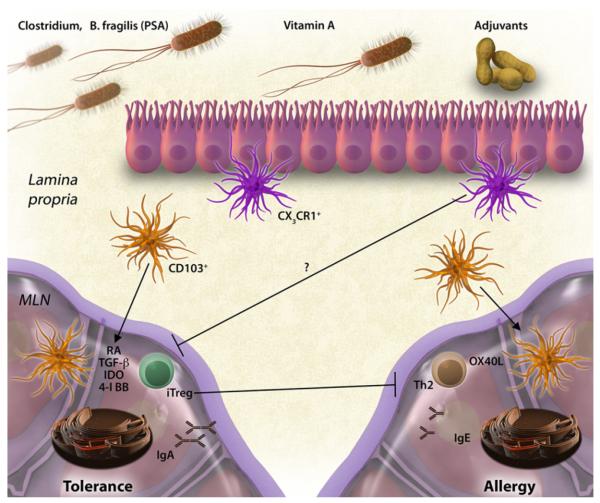
CTLA-4 is only 1 potential immunomodulatory mechanism used by Treg cells. It was recently described that mice deficient in iTreg cells (but with normal levels of thymic-derived nTreg cells) have spontaneous TH2-skewed inflammation in the gastrointestinal tract and antibodies against both gastrointestinal autoantigens and antigens derived from the mouse chow.71 The latter data suggest that iTreg cells have a constitutive role in the suppression of allergic sensitization to dietary antigens. Fig 1 summarizes de novo mechanisms of tolerance and sensitization in the gastrointestinal tract.
FIG 1.
Tolerance and sensitization in the gastrointestinal tract. Under homeostatic conditions, antigens are acquired in the lamina propria and presented in the mesenteric lymph node (MLN) by CD103+ DCs. Through mechanisms involving retinoic acid (RA), TGF-β, indoleamine 2,3-dioxygenase (IDO), and 4-1BB, DCs induce the production of gut-homing iTreg cells and IgA-producing plasma cells. Dietary factors (vitamin A) and microbial factors (Clostridium species and Bacteroides fragilis polysaccharide A [PSA]) promote the generation of Treg cells. Under sensitizing conditions that are induced in mice with adjuvants, TH2 cells are generated through mechanisms that involve OX40 ligand (OX40L), and IgE production is induced. Treg cells actively suppress allergic sensitization to foods. These mechanisms have been described in the naive state; it remains to be determined how antigen delivered as OIT in a sensitized subject would be presented by gastrointestinal DCs and how that would modify the adaptive immune response.
Why is it important to know whether food allergy is associated with a defective Treg cell response? This might have important implications for the response to immunotherapy. A defective allergen-specific Treg cell response might indicate that providing allergen alone as immunotherapy might not be sufficient to induce a robust Treg cell response in some subjects and that providing a protolerogenic adjuvant might be required for the induction of immune tolerance mediated by Treg cells. Genetic factors play an important role in the susceptibility to atopic disease, including food allergy. Studies in patients with inflammatory bowel disease and their first-degree relatives suggest that genetic factors can also contribute to defects in the generation of oral tolerance to fed antigens.72,73
CAN TOLERANCE BE INDUCED THERAPEUTICALLY IN SUBJECTS WITH FOOD ALLERGY?
Immune tolerance is defined as the absence of an antigen-specific adaptive immune response or, alternatively, as the presence of an active Treg cell response. When we refer to the induction of tolerance in food allergy, we define this as a sustained clinical nonresponsiveness to food allergen after discontinuation of therapy. This is distinct from desensitization, which is clinical nonresponsiveness while antigen-specific immunotherapy is maintained. Desensitization to food allergens through oral immunotherapy (OIT) remains experimental, and the literature to date does not support the routine use of OIT for desensitization.74,75
The focus of this review will specifically be on the establishment of tolerance. Tolerance that is generated in a sensitized subject might or might not be mediated by immune mechanisms similar to those involved in experimental oral tolerance. We know from studies on the natural history of food allergy that clinical tolerance can develop spontaneously after allergic sensitization has occurred, and in fact, this occurs in the majority of young children who are allergic to milk or egg. For children sensitized to allergens including peanut, tree nuts, fish, and shellfish, the occurrence of clinical tolerance is much lower but not rare (approximately 20% of patients with peanut allergy and 10% of patients with tree nut allergy were found to outgrow their allergy76,77). The immune mechanisms responsible for this development of clinical tolerance are not well understood but, as mentioned above, might involve a transient expansion of Treg cells65,66 in addition to waning allergen-specific IgE levels. The loss of sensitization in early childhood might represent a maturation of the mucosal immune system and the development of a regulatory tone, potentially through changes in microbial colonization.
For those with persistent food allergy, the question remains whether tolerance can be induced by therapeutic interventions. In the case of anaphylaxis induced by insect stings, subcutaneous immunotherapy (SCIT) leads to complete protection from sting-induced anaphylaxis in the majority (>75%) of subjects.78 A prolonged duration of SCIT (4-5 years) is associated with sustained clinical protection and a continued waning of allergenspecific IgE levels off therapy.79 Venom allergy and food allergy are comparable in their clinical manifestations (anaphylaxis) and infrequent allergen exposure. Despite these similarities, SCIT for peanut allergy was attempted but abandoned as a therapeutic approach because of the unacceptable rate of reactions to the therapy.80,81 The differences in response to immunotherapy of these 2 allergic disorders can tell us something about the unique pathways involved in sensitization and tolerance.
There are reports of successful desensitization to food allergens through the oral route throughout the last century.82 Renewed interest has led to a number of trials of OIT, demonstrating that the majority of subjects undergoing OIT with peanut, egg, or milk tolerate the immunotherapy and become desensitized, such that they can tolerate a food challenge while receiving daily allergen immunotherapy.83-87 The question remains whether this approach is disease modifying and whether true tolerance (sustained nonresponsiveness after a period of time off therapy) develops. This question has been addressed in a small number of trials to date.
Children receiving OIT for egg or milk allergy for a median period of 21 months had a tolerance rate of 36% during a double-blind, placebo-controlled food challenge (DBPCFC) performed 2 months after OIT discontinuation.88 However, the tolerance rate in the control untreated group was surprisingly high at 35%, indicating a lack of efficacy of OIT in the development of immune tolerance. Buchanan et al86 performed a 2-year uncontrolled OIT trial for egg allergy, in which 4 of 7 patients passed a DBPCFC at 24 months, and 2 of these 4 patients passed a second DBPCFC 3 months after discontinuation of OIT. This promising but relatively low success rate of tolerance induction was improved in a follow-up trial by Vickery et al89 that used an OIT dosing regimen in which the maintenance dose was increased stepwise until the egg-specific IgE levels decreased to less than 2 kU/L. At that point, patients underwent a DBPCFC and a second DBPCFC 1 month after OIT discontinuation to determine tolerance development. Six of 6 patients who passed the first DBPCFC also passed the second DBPCFC. Keet et al90 initially treated patients with milk sublingual immunotherapy (SLIT) before randomization to continue receiving SLITor receiving OITat one of 2 maintenance doses for a total of 80 weeks. When a tolerance challenge was performed 6 weeks after completion of immunotherapy, 1 of 10 patients receiving SLIT (7-mg daily maintenance dose) were tolerant, 3 of 10 patients receiving 1000 mg of milk as a maintenance OIT dose were tolerant, and 5 of 10 patients receiving 2000 mg of milk as a maintenance OIT dose were tolerant. The numbers of patients in these trials are small, and the trials are not placebo controlled but provide preliminary data supporting the hypothesis that higher doses and longer duration of immunotherapy can promote sustained nonresponsiveness or tolerance. These data suggest that tolerance might be dose dependent, but this needs to be systematically tested with placebo-controlled trials powered to test significant differences. A recent placebo-controlled trial of OIT for egg allergy with 40 children in the OIT group and 15 in the placebo group demonstrated a desensitization rate of 75% after 22 months of OIT and a tolerance rate of 28% at 24 months, as determined by using a DBPCFC performed 2 months after discontinuation of OIT.91 No placebo-treated children passed the desensitization challenge at 10 months, but they were not rechallenged at 22 or 24 months except in the case of one subject with an IgE level of less than 2 kU/L (who did not pass the challenge). Children in the OIT group who passed the tolerance challenge added egg to their diet ad libitum and did not report any adverse reactions at 30 or 36 months’ follow-up. This result suggests that approximately one quarter of children with egg allergy can achieve tolerance after a 2-year period of OIT, although the lack of challenge data in the placebo group at 22 to 24 months is a concern in this interpretation, particularly given the high rate of spontaneous tolerance observed in the placebo group of the trial discussed earlier.88
With the caveats discussed above, the data from these studies show that a subset of treated patients achieve sustained nonresponsiveness to foods. Unfortunately, this occurs for only a minority of subjects undergoing this prolonged immunotherapy. The challenge ahead of us is to study these patients to understand how tolerance does occur from an immunologic perspective, so that we can design more rational therapies to facilitate those immune changes in subjects with persistent food allergy. As reviewed in earlier sections and summarized in Fig 1, primary immune tolerance in mice is dependent on the induction of allergenspecific Treg cells that block the generation of allergen-specific IgE. There are very limited data to determine whether the same Treg cell mechanisms are at play in tolerance induced in patients with food allergy after immunotherapy. OIT has been reported to be associated with changes in various immune parameters, including a boosting of levels of IgG4 and IgA, which function as blocking antibodies; reduction in basophil and mast cell reactivity; and changes in Treg cell or T effector cell numbers (measured based on TH2 cytokine release from antigen-stimulated PBMCs).83,85,86,92,93 There is a rationale for all of these mechanisms to play a role in desensitization, and if these changes are maintained after discontinuation of therapy, they might play a role in tolerance. With the exception of the increase in IgG4 levels, not all findings are consistent between different trials. Changes in the T-cell response (either induction of Treg cells or anergy or deletion of TH2 effector cells) have not yet been addressed in tolerance compared with desensitization. In the recent placebo-controlled egg OIT trial, immune markers that were significantly different between those who were tolerized versus desensitized to egg were egg-specific IgG4 levels after 10 months of treatment (but this difference was no longer apparent after 22 months of treatment) and wheal size after skin prick testing after 22 months of treatment.91 The relationship between antigen-specific Treg cells and clinical tolerance needs to be carefully explored. The induction of Treg cells can prevent the generation of an IgE response by preventing the TH2 or T follicular helper response needed for IgE class-switching. When IgE has already been generated in a patient with food allergy, it is not clear how much the generation of an antigen-specific Treg cell response modifies the effector arm of the allergic response, although direct suppression of mast cell activation by Treg cells has been described.94,95 It remains to be understood whether clinical tolerance is primarily due to an induction of a Treg cell response or a waning of allergic sensitization.
Further studies are needed to determine the relationship between immune markers, such as basophil reactivity, antigen-specific antibody levels or affinity, antigen-specific Treg cells or T effector cells, and clinical reactivity. Performing these immune studies at the time of desensitization and tolerance challenges might be particularly informative for determining the relationship between these immune parameters and clinical tolerance and will help us to understand whether these are biomarkers or potential mechanisms of tolerance. In addition, experimental approaches that are not limited by our current hypotheses of tolerance are needed, such as functional genomic profiling to identify pathways selectively activated in those subjects achieving tolerance. Such an approach is also warranted for the study of immune mechanisms responsible for the natural outgrowth of food allergy, which might be significantly different from the outgrowth induced by immunotherapy. Alternatively, immunotherapy may hasten the outgrowth of food allergy in a subset of patients already predisposed to the development of tolerance. We can begin to address these important questions through advanced immune profiling of subjects who achieve tolerance compared with those with persistent food allergy refractory to immunotherapy.
FUTURE DIRECTIONS OF IMMUNOTHERAPY
Relatively few preclinical studies have addressed immunotherapy from a therapeutic rather than a preventative approach. Feeding of antigen to naive mice efficiently shuts down food-induced allergic responses through the induction of Treg cells that prevent IgE production.13 In contrast, mice that were orally sensitized to egg white proteins and then subsequently received a course of conventional egg OIT had desensitization but not immune tolerance,96 which is similar to the response reported for the majority of human subjects. In contrast to these findings, immunotherapy administered through the intraperitoneal route to tree nut–sensitized mice led to an abrogation of anaphylactic symptoms when mice were challenged several weeks after immunotherapy discontinuation.97 As mentioned previously, immunotherapy through the subcutaneous route was abandoned as a therapeutic approach because of safety concerns. However, modification of allergens to abrogate their IgE binding yet retain their capacity for presentation to T cells can allow for systemic or subcutaneous delivery of doses high enough to promote tolerance. Supporting this concept, pepsin digestion of cashew allergen decreased the allergenicity of cashew extract in vivo, yet when administered systemically as immunotherapy to cashew-sensitized mice, the digested extract was able to abrogate anaphylactic responses to a similar degree as the native protein.98 Modification of allergen by means of mannosylation14 or delivery within mannosylated liposomes99 can effectively prevent the development of food-induced allergic symptoms through a mechanism involving specific ICAM3 grabbing nonintegrin-related 1 (SIGNR1) on the DC, and induction of IL-10–producing Treg cells. This approach has been shown to be effective, even when administered after sensitization.99
In addition to altering or encapsulating the antigen, other approaches to improve safety include providing antigen by alternative routes. Topical delivery of peanut extract as immunotherapy (epicutaneous immunotherapy) has been shown in preclinical studies to modify clinical reactivity to peanut in mice; this is seen as a reduction in peanut-induced allergic airway inflammation100 or peanut-induced eosinophilic inflammation of the gastrointestinal tract.101 Epicutaneous immunotherapy has not yet been tested in food-induced anaphylaxis models to determine its efficacy, but human trials are underway with this novel approach.
In addition to modifying the antigen or route of delivery to have a safer method of immunotherapy, another approach has been to provide adjuvants to promote tolerance or skew the adaptive immune response from a TH2-dominated response. Avaccine was constructed from Escherichia coli bearing modified peanut allergens Ara h 1, Ara h 2, and Ara h 3 that had the IgE-binding epitopes modified. The E coli was heat killed and administered through the intrarectal route to peanut-sensitized mice. A sustained reversal of clinical reactivity to peanut was observed in mice treated with this vaccine,102 leading to the initiation of a current phase I clinical trial in human subjects. Another recent approach used intravenous administration of peanut antigen coupled to syngeneic spleen cells.103 This approach could provide appropriate self-antigens from the apoptotic cells that can function as a tolerogenic adjuvant. This strategy was safe in that it did not induce anaphylactic responses in peanut-sensitized mice, was a very effective prophylactic approach when given to naive mice, and had modest effects as a therapeutic approach to suppress peanut-induced anaphylaxis in peanut-sensitized mice. The administration of antigen-coupled syngeneic cells is effective in preclinical models of autoimmunity104 and is currently being tested in human trials for multiple sclerosis.
Provision of antigen with a defined microbial ligand has also been used in preclinical studies for the treatment of food allergy. A fusion protein of flagellin from Listeria monocytogenes and ovalbumin was prepared and administered as an intraperitoneal injection before or after sensitization and oral challenge of mice with ovalbumin. Treatment with flagellin-OVA but not flagellin or OVA alone resulted in a significant reduction (but not abrogation) in gastrointestinal symptoms when administered as either a preventative or therapeutic approach.105 Other potential protolerogenic adjuvants include polysaccharide A from Bacteroides fragilis, which promotes the development of IL-10–producing CD4+ Treg cells in a Toll-like receptor 2–dependent manner.106,107 Colonization with strains of Clostridium species also markedly induces IL-10–producing Treg cells in the intestine and prevents the generation of an IgE response after systemic immunization of mice.108 Manipulation of the gut flora has not been tested in conjunction with immunotherapy, but theoretically, factors that promote the development of Treg cells can be useful adjuvants for the induction of immune tolerance. Fig 2 shows some of the novel approaches to allergen-specific immunotherapy that have been tested in preclinical studies.
FIG 2.
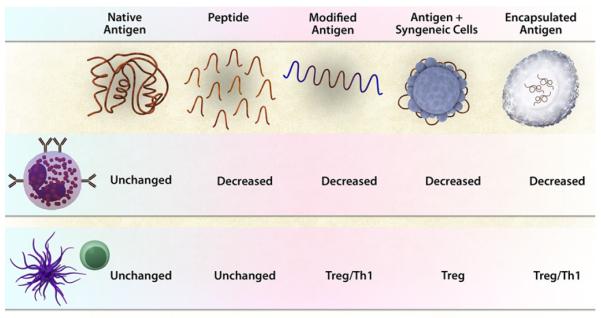
Approaches to maximize the safety and efficacy of immunotherapy with food allergens. Immunotherapy with native antigen results in side effects caused by activation of allergic effector cells and a modification of the adaptive T-cell response to enhance Treg cells and suppress TH2 cells. Approaches to increase safety by reducing the activation of allergic effector cells includes using peptides that cannot cross-link IgE, modifying the allergens (by heating or mannosylation), binding to particles like syngeneic leukocytes, and encapsulating in nanoparticles or within microbial carriers. Approaches to boost the immunomodulatory effects on the adaptive immune response include modifying the antigen to provide adjuvants that act on antigen-presenting cells (mannosylation and Toll-like receptor ligands), binding to syngeneic leukocytes (providing tolerogenic cues), or encapsulating the antigen together with microbial adjuvants. Studies are needed to test the effect of adding novel immunomodulatory agents (tolerogenic adjuvants or neutralizing antibodies that target antigen presenting cells) to immunotherapy protocols.
CONCLUSIONS
The natural history of food allergy indicates that such allergy can be outgrown and therefore shows that it is possible to acquire tolerance after sensitization has occurred. Unfortunately, natural tolerance is infrequent for antigens such as peanut, tree nuts, fish, or shellfish. Two placebo-controlled trials have been performed that directly address tolerance in response to OIT, one showing no beneficial effect of OIT on tolerance88 and the other showing tolerance induction in a minority of subjects with egg allergy.91 Although the latter study shows promise, these findings need to be verified through repetition and expanded to other food allergens. The data do not yet support the use of OIT as a therapy to induce immune tolerance. However, by carefully profiling immune tolerance when it has been successfully established either through natural outgrowth or experimental intervention, we expect to identify means of establishing tolerance in the remaining majority of patients with food allergy.
Acknowledgments
Supported by National Institute of Allergy and Infectious Diseases grant AI044236.
GLOSSARY
- CD11c
Also known as p150, CD11c is an integrin expressed on DCs (much less on macrophages) and is involved in leukocyte adhesion through ligands, such as intercellular adhesion molecule 1.
- CD25
CD25 is the α chain of the IL-2 receptor and is expressed on activated T cells and Treg cells. Daclizumab, a humanized anti-CD25 antibody, has been used in the treatment of allograft rejection and adult T-cell leukemia.
- CD103
Also known as integrin αE, CD103 binds to β7 to form αEβ7 on intraepithelial T cells that are retained in the intestinal mucosa (by binding to E cadherin).
- CD154
CD154 is also known as CD40 ligand (CD40L), is expressed on activated T cells, and is required for isotype switching. Mutations in CD40L can cause X-linked hyper-IgM syndrome.
- CYTOTOXIC T LYMPHOCYTE–ASSOCIATED ANTIGEN 4 (CTLA-4)
Also known as CD152, CTLA-4 is upregulated by activation of T cells and is constitutively expressed by Treg cells. CTLA-4 is a member of the immunoglobulin superfamily and contains an immunoreceptor tyrosine-based inhibitory motif (ITIM). CTLA-4 binds to CD80 and CD86 on the antigen-presenting cell and counteracts activation delivered by the T cell receptor and CD28.
- CX3CR1
Part of the chemokine receptor family, all chemokine receptors are 7-transmembrane G protein–coupled receptors. CX3CR1 binds fractalkine (CX3CL1), a membrane-bound chemokine.
- FORKHEAD BOX PROTEIN 3 (FoxP3)
FoxP3 is expressed in some Treg cells. Congenital absence of Foxp3 Treg cells causes immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome, an immunodeficiency associated with polyorgan autoimmunity.
- IL-10
IL-10 is associated with dampening immune responses by working through DCs and macrophages (decreased class II expression, costimulatory molecule expression, and costimulatory cytokine levels) and is produced by Treg cells (TR1 cells).
- OX40 LIGAND (OX40L)
OX40L is a second signal molecule that is involved in multiple aspects of TH2 inflammation, including eosinophilic inflammation.
- REGULATORY T (Treg) CELLS
Some Treg cells can be CD4+CD25+FoxP3+ and function to dampen the immune response to both allergenic and autoimmune antigens.
- RETINOIC ACID
In the intestinal tract retinoic acid production promotes the development of FoxP3+ Treg cells by inducing CD103.
- SIGNR1
SIGNR1 is a C-type lectin that is expressed on DCs (DC-SIGN homologue), binds intercellular adhesion molecules 2 and 3, is a receptor for nonendosomal/nonlysosomal-mediated uptake, and is involved in T cell–mediated primary immune responses.
- TETRAMERS
MHC peptide tetramers are used to stain antigen-specific T cells for flow cytometric analysis. Tetramers are multimers of peptide–MHC II molecules that can bind to the antigen-specific T-cell receptor.
- TGF-β
TGF-β is a pleiotropic growth factor produced by epithelial cells and inflammatory cells, including eosinophils, mast cells, and T cells. TGF-β1 can have profibrotic effects, be a switch factor for IgA, and be a very immunosuppressant cytokine. TGF-β1 can also be produced by Treg cells.
- TH17
TH17 cells are CD4+ T cells that are defined by the production of IL-17A, IL-17F, IL-21, and IL-22. TH17 cells are involved in autoimmunity and defense against bacteria, stimulated to produce IL-17 by IL-23, and maintained by the transcription factor retinoic acid–related orphan receptor γt.
The Editors wish to acknowledge Seema Aceves, MD, PhD, for preparing this glossary.
Abbreviations used
- CT
Cholera toxin
- CTLA-4
Cytotoxic T lymphocyte–associated antigen 4
- DBPCFC
Double-blind, placebo-controlled food challenge
- DC
Dendritic cell
- FoxP3
Forkhead box protein 3
- iTreg
Induced regulatory T
- nTreg
Natural regulatory T
- OIT
Oral immunotherapy
- SCIT
Subcutaneous immunotherapy
- SLIT
Sublingual immunotherapy
- Treg
Regulatory T
Footnotes
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 2.An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat Genet. 1997;17:399–403. doi: 10.1038/ng1297-399. [DOI] [PubMed] [Google Scholar]
- 3.Nagamine K, Peterson P, Scott HS, Kudoh J, Minoshima S, Heino M, et al. Positional cloning of the APECED gene. Nat Genet. 1997;17:393–8. doi: 10.1038/ng1297-393. [DOI] [PubMed] [Google Scholar]
- 4.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–7. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 5.Wells HG. Studies on the chemistry of anaphylaxis (III) Experiments with isolated proteins, especially those of the hen’s egg. J Infect Dis. 1911;9:24. [Google Scholar]
- 6.Wells HG, Osborne TB. The biological reactions of the vegetable proteins. I. Anaphylaxis. J Infect Dis. 1911;8:66–124. [Google Scholar]
- 7.Ngan J, Kind LS. Suppressor T cells for IgE and IgG in Peyer’s patches of mice made tolerant by the oral administration of ovalbumin. J Immunol. 1978;120:861–5. [PubMed] [Google Scholar]
- 8.Mattingly JA, Waksman BH. Immunologic suppression after oral administration of antigen. I. Specific suppressor cells formed in rat Peyer’s patches after oral administration of sheep erythrocytes and their systemic migration. J Immunol. 1978;121:1878–83. [PubMed] [Google Scholar]
- 9.Kagnoff MF. Effects of antigen-feeding on intestinal and systemic immune responses. II. Suppression of delayed-type hypersensitivity reactions. J Immunol. 1978;120:1509–13. [PubMed] [Google Scholar]
- 10.Mucida D, Kutchukhidze N, Erazo A, Russo M, Lafaille JJ, Curotto de Lafaille MA. Oral tolerance in the absence of naturally occurring Tregs. J Clin Invest. 2005;115:1923–33. doi: 10.1172/JCI24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russo M, Nahori MA, Lefort J, Gomes E, de Castro Keller A, Rodriguez D, et al. Suppression of asthma-like responses in different mouse strains by oral tolerance. Am J Respir Cell Mol Biol. 2001;24:518–26. doi: 10.1165/ajrcmb.24.5.4320. [DOI] [PubMed] [Google Scholar]
- 12.Frossard CP, Tropia L, Hauser C, Eigenmann PA. Lymphocytes in Peyer patches regulate clinical tolerance in a murine model of food allergy. J Allergy Clin Immunol. 2004;113:958–64. doi: 10.1016/j.jaci.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 13.Hadis U, Wahl B, Schulz O, Hardtke-Wolenski M, Schippers A, Wagner N, et al. Intestinal tolerance requires gut homing and expansion of Foxp3+ regulatory T cells in the lamina propria. Immunity. 2011;34:237–46. doi: 10.1016/j.immuni.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Y, Kawasaki H, Hsu SC, Lee RT, Yao X, Plunkett B, et al. Oral tolerance to food-induced systemic anaphylaxis mediated by the C-type lectin SIGNR1. Nat Med. 2010;16:1128–33. doi: 10.1038/nm.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tunis MC, Dawicki W, Carson KR, Wang J, Marshall JS. Mast cells and IgE activation do not alter the development of oral tolerance in a murine model. J Allergy Clin Immunol. 2012;130:705–15.e1. doi: 10.1016/j.jaci.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Inobe J, Weiner HL. Induction of oral tolerance to myelin basic protein in CD8-depleted mice: both CD4+ and CD8+ cells mediate active suppression. J Immunol. 1995;155:910–6. [PubMed] [Google Scholar]
- 17.Lider O, Santos LM, Lee CS, Higgins PJ, Weiner HL. Suppression of experimental autoimmune encephalomyelitis by oral administration of myelin basic protein. II. Suppression of disease and in vitro immune responses is mediated by antigen-specific CD8+ T lymphocytes. J Immunol. 1989;142:748–52. [PubMed] [Google Scholar]
- 18.Fukaura H, Kent SC, Pietrusewicz MJ, Khoury SJ, Weiner HL, Hafler DA. Induction of circulating myelin basic protein and proteolipid protein-specific transforming growth factor-beta1-secreting Th3 T cells by oral administration of myelin in multiple sclerosis patients. J Clin Invest. 1996;98:70–7. doi: 10.1172/JCI118779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–40. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 20.Miller A, Lider O, Roberts AB, Sporn MB, Weiner HL. Suppressor T cells generated by oral tolerization to myelin basic protein suppress both in vitro and in vivo immune responses by the release of transforming growth factor beta after antigen-specific triggering. Proc Natl Acad Sci U S A. 1992;89:421–5. doi: 10.1073/pnas.89.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arnaboldi PM, Roth-Walter F, Mayer L. Suppression of Th1 and Th17, but not Th2, responses in a CD8(+) T cell-mediated model of oral tolerance. Mucosal Immunol. 2009;2:427–38. doi: 10.1038/mi.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cassani B, Villablanca EJ, Quintana FJ, Love PE, Lacy-Hulbert A, Blaner WS, et al. Gut-tropic T cells that express integrin alpha4beta7 and CCR9 are required for induction of oral immune tolerance in mice. Gastroenterology. 2011;141:2109–18. doi: 10.1053/j.gastro.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carrier Y, Yuan J, Kuchroo VK, Weiner HL. Th3 cells in peripheral tolerance. I. Induction of Foxp3-positive regulatory T cells by Th3 cells derived from TGF-beta T cell-transgenic mice. J Immunol. 2007;178:179–85. doi: 10.4049/jimmunol.178.1.179. [DOI] [PubMed] [Google Scholar]
- 24.Verhasselt V, Milcent V, Cazareth J, Kanda A, Fleury S, Dombrowicz D, et al. Breast milk-mediated transfer of an antigen induces tolerance and protection from allergic asthma. Nat Med. 2008;14:170–5. doi: 10.1038/nm1718. [DOI] [PubMed] [Google Scholar]
- 25.Alpan O, Bachelder E, Isil E, Arnheiter H, Matzinger P. ‘Educated’ dendritic cells act as messengers from memory to naive T helper cells. Nat Immunol. 2004;5:615–22. doi: 10.1038/ni1077. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y, Inobe J, Marks R, Gonnella P, Kuchroo VK, Weiner HL. Peripheral deletion of antigen-reactive T cells in oral tolerance. Nature. 1995;376:177–80. doi: 10.1038/376177a0. [DOI] [PubMed] [Google Scholar]
- 27.Dubois B, Joubert G, Gomez de Aguero M, Gouanvic M, Goubier A, Kaiserlian D. Sequential role of plasmacytoid dendritic cells and regulatory T cells in oral tolerance. Gastroenterology. 2009;137:1019–28. doi: 10.1053/j.gastro.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 28.Goubier A, Dubois B, Gheit H, Joubert G, Villard-Truc F, Asselin-Paturel C, et al. Plasmacytoid dendritic cells mediate oral tolerance. Immunity. 2008;29:464–75. doi: 10.1016/j.immuni.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gilbert RS, Kobayashi R, Sekine S, Fujihashi K. Functional transforming growth factor-beta receptor type II expression by CD4+ T cells in Peyer’s patches is essential for oral tolerance induction. PLoS One. 2011;6:e27501. doi: 10.1371/journal.pone.0027501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kriegel MA, Rathinam C, Flavell RA. E3 ubiquitin ligase GRAIL controls primary T cell activation and oral tolerance. Proc Natl Acad Sci U S A. 2009;106:16770–5. doi: 10.1073/pnas.0908957106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M, et al. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513–25. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schulz O, Jaensson E, Persson EK, Liu X, Worbs T, Agace WW, et al. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med. 2009;206:3101–14. doi: 10.1084/jem.20091925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial mono-layers to sample bacteria. Nat Immunol. 2001;2:361–7. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 34.Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–8. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 35.Miller JC, Brown BD, Shay T, Gautier EL, Jojic V, Cohain A, et al. Deciphering the transcriptional network of the dendritic cell lineage. Nat Immunol. 2012;13:888–99. doi: 10.1038/ni.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDole JR, Wheeler LW, McDonald KG, Wang B, Konjufca V, Knoop KA, et al. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature. 2012;483:345–9. doi: 10.1038/nature10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–64. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaensson E, Uronen-Hansson H, Pabst O, Eksteen B, Tian J, Coombes JL, et al. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J Exp Med. 2008;205:2139–49. doi: 10.1084/jem.20080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matteoli G, Mazzini E, Iliev ID, Mileti E, Fallarino F, Puccetti P, et al. Gut CD103+ dendritic cells express indoleamine 2,3-dioxygenase which influences T regulatory/T effector cell balance and oral tolerance induction. Gut. 2010;59:595–604. doi: 10.1136/gut.2009.185108. [DOI] [PubMed] [Google Scholar]
- 40.Lee SW, Park Y, Eun SY, Madireddi S, Cheroutre H, Croft M. Cutting edge: 4-1BB controls regulatory activity in dendritic cells through promoting optimal expression of retinal dehydrogenase. J Immunol. 2012;189:2697–701. doi: 10.4049/jimmunol.1201248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johansson-Lindbom B, Svensson M, Pabst O, Palmqvist C, Marquez G, Forster R, et al. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J Exp Med. 2005;202:1063–73. doi: 10.1084/jem.20051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–60. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 43.Worbs T, Bode U, Yan S, Hoffmann MW, Hintzen G, Bernhardt G, et al. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J Exp Med. 2006;203:519–27. doi: 10.1084/jem.20052016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kraus TA, Brimnes J, Muong C, Liu JH, Moran TM, Tappenden KA, et al. Induction of mucosal tolerance in Peyer’s patch-deficient, ligated small bowel loops. J Clin Invest. 2005;115:2234–43. doi: 10.1172/JCI19102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spahn TW, Weiner HL, Rennert PD, Lugering N, Fontana A, Domschke W, et al. Mesenteric lymph nodes are critical for the induction of high-dose oral tolerance in the absence of Peyer’s patches. Eur J Immunol. 2002;32:1109–13. doi: 10.1002/1521-4141(200204)32:4<1109::AID-IMMU1109>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 46.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–94. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 47.Hammerschmidt SI, Ahrendt M, Bode U, Wahl B, Kremmer E, Forster R, et al. Stromal mesenteric lymph node cells are essential for the generation of gut-homing T cells in vivo. J Exp Med. 2008;205:2483–90. doi: 10.1084/jem.20080039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Heer HJ, Hammad H, Soullie T, Hijdra D, Vos N, Willart MA, et al. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med. 2004;200:89–98. doi: 10.1084/jem.20040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Akbari O, Freeman GJ, Meyer EH, Greenfield EA, Chang TT, Sharpe AH, et al. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat Med. 2002;8:1024–32. doi: 10.1038/nm745. [DOI] [PubMed] [Google Scholar]
- 50.Alvarez D, Swirski FK, Yang TC, Fattouh R, Croitoru K, Bramson JL, et al. Inhalation tolerance is induced selectively in thoracic lymph nodes but executed pervasively at distant mucosal and nonmucosal tissues. J Immunol. 2006;176:2568–80. doi: 10.4049/jimmunol.176.4.2568. [DOI] [PubMed] [Google Scholar]
- 51.Lambrecht BN, Hammad H. Lung dendritic cells in respiratory viral infection and asthma: from protection to immunopathology. Annu Rev Immunol. 2012;30:243–70. doi: 10.1146/annurev-immunol-020711-075021. [DOI] [PubMed] [Google Scholar]
- 52.Mascarell L, Lombardi V, Louise A, Saint-Lu N, Chabre H, Moussu H, et al. Oral dendritic cells mediate antigen-specific tolerance by stimulating TH1 and regulatory CD4+ T cells. J Allergy Clin Immunol. 2008;122:603–9.e5. doi: 10.1016/j.jaci.2008.06.034. [DOI] [PubMed] [Google Scholar]
- 53.Mascarell L, Saint-Lu N, Moussu H, Zimmer A, Louise A, Lone Y, et al. Oral macrophage-like cells play a key role in tolerance induction following sublingual immunotherapy of asthmatic mice. Mucosal Immunol. 2011;4:638–47. doi: 10.1038/mi.2011.28. [DOI] [PubMed] [Google Scholar]
- 54.Gomez de Aguero M, Vocanson M, Hacini-Rachinel F, Taillardet M, Sparwasser T, Kissenpfennig A, et al. Langerhans cells protect from allergic contact dermatitis in mice by tolerizing CD8(+) T cells and activating Foxp3(+) regulatory T cells. J Clin Invest. 2012;122:1700–11. doi: 10.1172/JCI59725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li W, Zhang Z, Saxon A, Zhang K. Prevention of oral food allergy sensitization via skin application of food allergen in a mouse model. Allergy. 2012;67:622–9. doi: 10.1111/j.1398-9995.2012.02798.x. [DOI] [PubMed] [Google Scholar]
- 56.Guilliams M, Crozat K, Henri S, Tamoutounour S, Grenot P, Devilard E, et al. Skin-draining lymph nodes contain dermis-derived CD103(−) dendritic cells that constitutively produce retinoic acid and induce Foxp3(+) regulatory T cells. Blood. 2010;115:1958–68. doi: 10.1182/blood-2009-09-245274. [DOI] [PubMed] [Google Scholar]
- 57.Eigenmann PA, Huang SK, Sampson HA. Characterization of ovomucoid-specific T-cell lines and clones from egg-allergic subjects. Pediatr Allergy Immunol. 1996;7:12–21. doi: 10.1111/j.1399-3038.1996.tb00100.x. [DOI] [PubMed] [Google Scholar]
- 58.Flinterman AE, Pasmans SG, den Hartog Jager CF, Hoekstra MO, Bruijnzeel-Koomen CA, Knol EF, et al. T cell responses to major peanut allergens in children with and without peanut allergy. Clin Exp Allergy. 2010;40:590–7. doi: 10.1111/j.1365-2222.2009.03431.x. [DOI] [PubMed] [Google Scholar]
- 59.Schulten V, Radakovics A, Hartz C, Mari A, Vazquez-Cortes S, Fernandez-Rivas M, et al. Characterization of the allergic T-cell response to Pru p 3, the nonspecific lipid transfer protein in peach. J Allergy Clin Immunol. 2009;124:100–7. doi: 10.1016/j.jaci.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 60.Beyer K, Castro R, Birnbaum A, Benkov K, Pittman N, Sampson HA. Human milk-specific mucosal lymphocytes of the gastrointestinal tract display a TH2 cytokine profile. J Allergy Clin Immunol. 2002;109:707–13. doi: 10.1067/mai.2002.122503. [DOI] [PubMed] [Google Scholar]
- 61.Tordesillas L, Cuesta-Herranz J, Gonzalez-Munoz M, Pacios LF, Compes E, Garcia-Carrasco B, et al. T-cell epitopes of the major peach allergen, Pru p 3: identification and differential T-cell response of peach-allergic and non-allergic subjects. Mol Immunol. 2009;46:722–8. doi: 10.1016/j.molimm.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 62.Turcanu V, Maleki SJ, Lack G. Characterization of lymphocyte responses to peanuts in normal children, peanut-allergic children, and allergic children who acquired tolerance to peanuts. J Clin Invest. 2003;111:1065–72. doi: 10.1172/JCI16142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Prussin C, Lee J, Foster B. Eosinophilic gastrointestinal disease and peanut allergy are alternatively associated with IL-5+ and IL-5(−) T(H)2 responses. J Allergy Clin Immunol. 2009;124:1326–32. doi: 10.1016/j.jaci.2009.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.DeLong JH, Simpson KH, Wambre E, James EA, Robinson D, Kwok WW. Ara h 1-reactive T cells in individuals with peanut allergy. J Allergy Clin Immunol. 2011;127:1211–8.e3. doi: 10.1016/j.jaci.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shreffler WG, Wanich N, Moloney M, Nowak-Wegrzyn A, Sampson HA. Association of allergen-specific regulatory T cells with the onset of clinical tolerance to milk protein. J Allergy Clin Immunol. 2009;123:43–52.e7. doi: 10.1016/j.jaci.2008.09.051. [DOI] [PubMed] [Google Scholar]
- 66.Karlsson MR, Rugtveit J, Brandtzaeg P. Allergen-responsive CD4+CD25+ regulatory T cells in children who have outgrown cow’s milk allergy. J Exp Med. 2004;199:1679–88. doi: 10.1084/jem.20032121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sletten GB, Halvorsen R, Egaas E, Halstensen TS. Memory T cell proliferation in cow’s milk allergy after CD25+ regulatory T cell removal suggests a role for casein-specific cellular immunity in IgE-mediated but not in non-IgE-mediated cow’s milk allergy. Int Arch Allergy Immunol. 2007;142:190–8. doi: 10.1159/000097021. [DOI] [PubMed] [Google Scholar]
- 68.Blazquez AB, Berin MC. Gastrointestinal dendritic cells promote Th2 skewing via OX40L. J Immunol. 2008;180:4441–50. doi: 10.4049/jimmunol.180.7.4441. [DOI] [PubMed] [Google Scholar]
- 69.Ganeshan K, Neilsen CV, Hadsaitong A, Schleimer RP, Luo X, Bryce PJ. Impairing oral tolerance promotes allergy and anaphylaxis: a new murine food allergy model. J Allergy Clin Immunol. 2009;123:231–8.e4. doi: 10.1016/j.jaci.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Wijk F, Hoeks S, Nierkens S, Koppelman SJ, van Kooten P, Boon L, et al. CTLA-4 signaling regulates the intensity of hypersensitivity responses to food antigens, but is not decisive in the induction of sensitization. J Immunol. 2005;174:174–9. doi: 10.4049/jimmunol.174.1.174. [DOI] [PubMed] [Google Scholar]
- 71.Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, et al. Extra-thymically generated regulatory T cells control mucosal TH2 inflammation. Nature. 2012;482:395–9. doi: 10.1038/nature10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kraus TA, Toy L, Chan L, Childs J, Mayer L. Failure to induce oral tolerance to a soluble protein in patients with inflammatory bowel disease. Gastroenterology. 2004;126:1771–8. doi: 10.1053/j.gastro.2004.03.076. [DOI] [PubMed] [Google Scholar]
- 73.Kraus TA, Cheifetz A, Toy L, Meddings JB, Mayer L. Evidence for a genetic defect in oral tolerance induction in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:82–8. doi: 10.1097/01.MIB.0000200343.61707.52. discussion 81. [DOI] [PubMed] [Google Scholar]
- 74.Nurmatov U, Venderbosch I, Devereux G, Simons FE, Sheikh A. Allergen-specific oral immunotherapy for peanut allergy. Cochrane Database Syst Rev. 2012;9:CD009014. doi: 10.1002/14651858.CD009014.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sheikh A, Nurmatov U, Venderbosch I, Bischoff E. Oral immunotherapy for the treatment of peanut allergy: systematic review of six case series studies. Prim Care Respir J. 2012;21:41–9. doi: 10.4104/pcrj.2011.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fleischer DM, Conover-Walker MK, Matsui EC, Wood RA. The natural history of tree nut allergy. J Allergy Clin Immunol. 2005;116:1087–93. doi: 10.1016/j.jaci.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 77.Skolnick HS, Conover-Walker MK, Koerner CB, Sampson HA, Burks W, Wood RA. The natural history of peanut allergy. J Allergy Clin Immunol. 2001;107:367–74. doi: 10.1067/mai.2001.112129. [DOI] [PubMed] [Google Scholar]
- 78.Golden DB. Long-term outcome after venom immunotherapy. Curr Opin Allergy Clin Immunol. 2010;10:337–41. doi: 10.1097/ACI.0b013e32833bc0ba. [DOI] [PubMed] [Google Scholar]
- 79.Golden DB, Kagey-Sobotka A, Lichtenstein LM. Survey of patients after discontinuing venom immunotherapy. J Allergy Clin Immunol. 2000;105:385–90. doi: 10.1016/s0091-6749(00)90092-7. [DOI] [PubMed] [Google Scholar]
- 80.Nelson HS, Lahr J, Rule R, Bock A, Leung D. Treatment of anaphylactic sensitivity to peanuts by immunotherapy with injections of aqueous peanut extract. J Allergy Clin Immunol. 1997;99:744–51. doi: 10.1016/s0091-6749(97)80006-1. [DOI] [PubMed] [Google Scholar]
- 81.Oppenheimer JJ, Nelson HS, Bock SA, Christensen F, Leung DY. Treatment of peanut allergy with rush immunotherapy. J Allergy Clin Immunol. 1992;90:256–62. doi: 10.1016/0091-6749(92)90080-l. [DOI] [PubMed] [Google Scholar]
- 82.Edwards HE. Oral desensitization in food allergy. CMAJ. 1940;43:234–6. [PMC free article] [PubMed] [Google Scholar]
- 83.Skripak JM, Nash SD, Rowley H, Brereton NH, Oh S, Hamilton RG, et al. A randomized, double-blind, placebo-controlled study of milk oral immunotherapy for cow’s milk allergy. J Allergy Clin Immunol. 2008;122:1154–60. doi: 10.1016/j.jaci.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Varshney P, Jones SM, Pons L, Kulis M, Steele PH, Kemper AR, et al. Oral immunotherapy (OIT) induces clinical tolerance in peanut-allergic children. J Allergy Clin Immunol. 2009;123:665. [Google Scholar]
- 85.Varshney P, Jones SM, Scurlock AM, Perry TT, Kemper A, Steele P, et al. A randomized controlled study of peanut oral immunotherapy: clinical desensitization and modulation of the allergic response. J Allergy Clin Immunol. 2011;127:654–60. doi: 10.1016/j.jaci.2010.12.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Buchanan AD, Green TD, Jones SM, Scurlock AM, Christie L, Althage KA, et al. Egg oral immunotherapy in nonanaphylactic children with egg allergy. J Allergy Clin Immunol. 2007;119:199–205. doi: 10.1016/j.jaci.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 87.Staden U, Blumchen K, Blankenstein N, Dannenberg N, Ulbricht H, Dobberstein K, et al. Rush oral immunotherapy in children with persistent cow’s milk allergy. J Allergy Clin Immunol. 2008;122:418–9. doi: 10.1016/j.jaci.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 88.Staden U, Rolinck-Werninghaus C, Brewe F, Wahn U, Niggemann B, Beyer K. Specific oral tolerance induction in food allergy in children: efficacy and clinical patterns of reaction. Allergy. 2007;62:1261–9. doi: 10.1111/j.1398-9995.2007.01501.x. [DOI] [PubMed] [Google Scholar]
- 89.Vickery BP, Pons L, Kulis M, Steele P, Jones SM, Burks AW. Individualized IgE-based dosing of egg oral immunotherapy and the development of tolerance. Ann Allergy Asthma Immunol. 2010;105:444–50. doi: 10.1016/j.anai.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Keet CA, Frischmeyer-Guerrerio PA, Thyagarajan A, Schroeder JT, Hamilton RG, Boden S, et al. The safety and efficacy of sublingual and oral immunotherapy for milk allergy. J Allergy Clin Immunol. 2012;129:448–55. e1–5. doi: 10.1016/j.jaci.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Burks AW, Jones SM, Wood RA, Fleischer DM, Sicherer SH, Lindblad RW, et al. Oral immunotherapy for treatment of egg allergy in children. N Engl J Med. 2012;367:233–43. doi: 10.1056/NEJMoa1200435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Patriarca G, Nucera E, Roncallo C, Pollastrini E, Bartolozzi F, De Pasquale T, et al. Oral desensitizing treatment in food allergy: clinical and immunological results. Aliment Pharmacol Ther. 2003;17:459–65. doi: 10.1046/j.1365-2036.2003.01468.x. [DOI] [PubMed] [Google Scholar]
- 93.Jones SM, Pons L, Roberts JL, Scurlock AM, Perry TT, Kulis M, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. 2009;124:292–300. doi: 10.1016/j.jaci.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kashyap M, Thornton AM, Norton SK, Barnstein B, Macey M, Brenzovich J, et al. Cutting edge: CD4 T cell-mast cell interactions alter IgE receptor expression and signaling. J Immunol. 2008;180:2039–43. doi: 10.4049/jimmunol.180.4.2039. [DOI] [PubMed] [Google Scholar]
- 95.Ganeshan K, Bryce PJ. Regulatory T cells enhance mast cell production of IL-6 via surface-bound TGF-beta. J Immunol. 2012;188:594–603. doi: 10.4049/jimmunol.1102389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Leonard SA, Martos G, Wang W, Nowak-Wegrzyn A, Berin MC. Oral immuno-therapy induces local protective mechanisms in the gastrointestinal mucosa. J Allergy Clin Immunol. 2012;129:1579–87.e1. doi: 10.1016/j.jaci.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kulis M, Li Y, Lane H, Pons L, Burks W. Single-tree nut immunotherapy attenuates allergic reactions in mice with hypersensitivity to multiple tree nuts. J Allergy Clin Immunol. 2011;127:81–8. doi: 10.1016/j.jaci.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 98.Kulis M, Macqueen I, Li Y, Guo R, Zhong XP, Burks AW. Pepsinized cashew proteins are hypoallergenic and immunogenic and provide effective immunotherapy in mice with cashew allergy. J Allergy Clin Immunol. 2012;130:716–23. doi: 10.1016/j.jaci.2012.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kawakita A, Shirasaki H, Yasutomi M, Tokuriki S, Mayumi M, Naiki H, et al. Immunotherapy with oligomannose-coated liposomes ameliorates allergic symptoms in a murine food allergy model. Allergy. 2012;67:371–9. doi: 10.1111/j.1398-9995.2011.02777.x. [DOI] [PubMed] [Google Scholar]
- 100.Mondoulet L, Dioszeghy V, Vanoirbeek JA, Nemery B, Dupont C, Benhamou PH. Epicutaneous immunotherapy using a new epicutaneous delivery system in mice sensitized to peanuts. Int Arch Allergy Immunol. 2010;154:299–309. doi: 10.1159/000321822. [DOI] [PubMed] [Google Scholar]
- 101.Mondoulet L, Dioszeghy V, Larcher T, Ligouis M, Dhelft V, Puteaux E, et al. Epicutaneous immunotherapy (EPIT) blocks the allergic esophago-gastroenteropathy induced by sustained oral exposure to peanuts in sensitized mice. PLoS One. 2012;7:e31967. doi: 10.1371/journal.pone.0031967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li XM, Srivastava K, Grishin A, Huang CK, Schofield B, Burks W, et al. Persistent protective effect of heat-killed Escherichia coli producing “engineered,” recombinant peanut proteins in a murine model of peanut allergy. J Allergy Clin Immunol. 2003;112:159–67. doi: 10.1067/mai.2003.1622. [DOI] [PubMed] [Google Scholar]
- 103.Smarr CB, Hsu CL, Byrne AJ, Miller SD, Bryce PJ. Antigen-fixed leukocytes tolerize Th2 responses in mouse models of allergy. J Immunol. 2011;187:5090–8. doi: 10.4049/jimmunol.1100608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Miller SD, Turley DM, Podojil JR. Antigen-specific tolerance strategies for the prevention and treatment of autoimmune disease. Nat Rev Immunol. 2007;7:665–77. doi: 10.1038/nri2153. [DOI] [PubMed] [Google Scholar]
- 105.Schulke S, Burggraf M, Waibler Z, Wangorsch A, Wolfheimer S, Kalinke U, et al. A fusion protein of flagellin and ovalbumin suppresses the TH2 response and prevents murine intestinal allergy. J Allergy Clin Immunol. 2011;128:1340–8.e12. doi: 10.1016/j.jaci.2011.07.036. [DOI] [PubMed] [Google Scholar]
- 106.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–5. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 107.Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, et al. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011;332:974–7. doi: 10.1126/science.1206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–41. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]