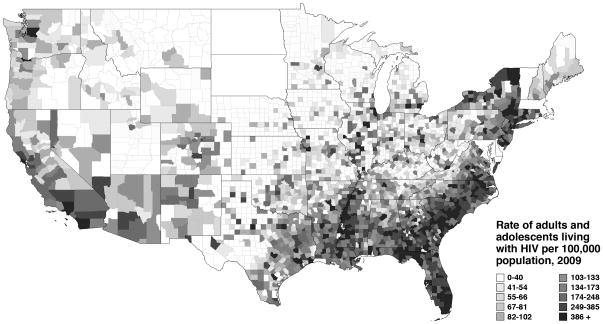
One need only glance at a map of HIV prevalence in the United States to realize that the Southeast is disproportionately impacted (Figure). Though this region contains a little over one third of the national population, more than half of all newly diagnosed cases of HIV came from the South in 2010.1 Inequity in the social determinants of health has served to concentrate a variety of communicable and non-communicable diseases among the South’s poor and disadvantaged, of whom Blacks make up a outsized proportion.2, 3 Recent estimates suggest that 1 in every 5 Southern Black men who have sex with men (MSM) are living with HIV infection, a number nearly 5 times that of Southern White MSM.4 In the past several years, the epidemic has begun to affect young MSM more greatly. In 2011, the Centers for Disease Control and Prevention (CDC) reported that young, Black MSM aged 13–29 experienced a 48% increase in HIV incidence from 2006–2009.5 Unfortunately, such statistics are no longer surprising for those of us living in the South and providing care for the scores of young, Black MSM being diagnosed with HIV each year. The real challenge for the future lies in identifying and addressing the root causes of this disparity, in order to effect meaningful reductions in HIV incidence among those at greatest risk.
Figure 1. Prevalence of HIV infection in the United States, 2009.
Source: AIDSVu (http://www.aidsvu.org). Emory University, Rollins School of Public Health. [Accessed December 10, 2012.] Data are not included for Delaware, Maryland, Massachusetts, North Dakota, South Dakota, Vermont, and the District of Columbia.
The two-year investigation of an HIV outbreak among young, Black MSM in and around Jackson, Mississippi yields significant insights into this challenge. The study, coordinated by the CDC and the Mississippi State Department of Health (MSDH), was modeled on a 2004 investigation of multiple HIV diagnoses among Black MSM attending colleges and universities in central North Carolina.6, 7 In five previous papers8–12 and their article in this issue of Sexually Transmitted Diseases,13 Oster and her colleagues from the CDC and MSDH provide us with a nuanced picture of the state of the HIV epidemic among Black MSM in the Deep South and a glimpse of how similar outbreak investigations could take place in the future.
In their work, Oster et al. present a portrait of the epidemic that is heavily influenced by stigma surrounding homosexuality and HIV in the Black community.14–16 Young, Black MSM, ostracized by their families and support systems,12 migrate to a handful of “safe” venues located in the more urban area of Jackson to socialize and meet new sex partners.11–13 HIV-infected and uninfected men are both drawn to (or forced toward) the same few venues, characterized in the current paper11 using an “affiliation” network.17 This co-localization creates an overlapping social and sexual network with an enriched HIV prevalence – a phenomenon similar to recent observations in North Carolina, as well.18 Black MSM in the resulting tight-knit system find safety in numbers, but may also have the mistaken impression that HIV prevalence is low among people with demographics like their own.19 Evidence for this comes from two sources: over half of HIV-infected men thought they were “unlikely” or “very unlikely” to ever acquire HIV,8 and participants reported less consistent condom usage with casual partners, compared to main partners.13 Among Black MSM, intense stigmatization of persons living with HIV12 further complicates efforts to have open, honest discussion about serostatus with sexual partners. When young men with less education about HIV and STI prevention10 enter the sexual network and have sex with older partners, among whom the prevalence of HIV is greater, their odds of HIV infection are markedly increased.9, 10, 20 Taken together, it becomes clearer why HIV infections are more frequent among young, Black MSM, despite no demonstrable differences in their risk behavior, compared to MSM of other racial/ethnic groups.21 In a sexual network with high HIV prevalence, even a single lapse in preventive behavior has a much higher probability of resulting in a transmission event.18
In-depth investigations like the one conducted by CDC and MSDH are relatively rare in the HIV literature, given the enormous commitment of time and resources needed to successfully bring the data from field notes to published manuscripts. Perhaps the most unique aspect of the Jackson investigation was its use of molecular epidemiological methods in order to corroborate and extend the findings from “traditional” case-control,9, 10 qualitative,12 and network analyses (social and affiliation).13 The study team analyzed genotypic sequence data from nearly 800 individuals diagnosed with HIV in Mississippi between 2005–2008, collected from CDC’s Variant, Atypical, and Resistant HIV Surveillance (VARHS) system and local surveillance specimens from Black MSM aged 16–25 diagnosed in early 2008.11 Using sophisticated computational methods, they reconstructed phylogenetic trees depicting the genetic relatedness of the viruses, and then analyzed the characteristics of individuals whose viruses appeared in groups or “clusters” of highly similar sequences. Such clusters represent groups of individuals who likely share transmission of the same virus. It is important to note that only nucleotide sequences are included in phylogenetic analyses; tree structure is “solved” without considering the demographic or behavioral characteristics of individuals who supplied the viral specimens. Therefore, this method provides an additional way to understand transmission, independent of participant-reported history. Additional information can be gleaned by looking for patterns of shared characteristics among individuals identified in these clusters. Sequences from young Black MSM in Mississippi appeared in 21 out of 82 clusters, though these men contributed only 16% of the overall viruses studied. The members of these 21 clusters were all remarkably homogenous; of the 69 individuals in these clusters, all were men, 96% were Black, 88% were MSM, and 70% were under age 25. When examining the geographic locations from which the individuals came, most clusters involving young, Black MSM contained residents from two or more regions of Mississippi. Together, these findings strongly and independently support the conceptual framework of the epidemic among Southern Black MSM, as outlined above.
Phylogenetic analyses can complement traditional methods in other ways, as well. In the present paper,13 Oster and colleagues were only able to obtain very limited identifying information concerning sex partners, such that the resulting sexual network was fractured into 20 separate components. Given the findings of the phylogenetic study, it seems likely this network would coalesce into fewer, larger groupings if the genetic relatedness of the participants’ viruses could be evaluated. Phylogenetic data can also shed light on broader patterns of HIV transmission, if large numbers of sequences are analyzed in conjunction with clinical and demographic data. Two recent studies, one from North Carolina22 and another from sites around the United States23 both found homogeneity among Blacks within clusters of related sequences, suggesting the same assortative mixing or homophily seen among Black MSM in the Jackson investigation is true across the country. Phylogenetic analyses are not without their limitations, however. Individuals with closely related sequences could have directly transmitted HIV to one another – or they could have a common shared partner or multiple intermediaries. Neither the directionality nor exact date of transmission can be determined using sequences alone, but phylogenetic data can be used to support or refute information collected from putative partners.24–26
Molecular epidemiology’s role in assisting public health efforts and improving our understanding of HIV transmission in the United States undoubtedly will increase over time. HIV nucleotide sequence data from “baseline” or pre-treatment resistance testing are widely available, since such testing is recommended for all individuals entering care.27 Phylogenetic analytical methods and computational speed have advanced markedly in the past decade, allowing large datasets to be analyzed more rapidly than ever before. As elegantly shown in the Jackson investigation, integrating these new methods with traditional, time-tested epidemiological approaches can deepen our understanding of the complex transmission dynamics of HIV in the United States.
Though the Jackson outbreak investigation has revealed much about HIV’s spread in young, Black MSM, we still must parlay these findings into more effective strategies for both primary and secondary prevention. Testing campaigns and sexual health messages ought to be directed at the “virtual” and physical venues frequented by these men, but we also need to address stigmatizing forces in their home communities through direct engagement and education. HIV treatment resources need to be available in rural and urban communities, to ensure access to the treatment we know will sustain individual health and reduce infectiousness to others. We must better understand why Black MSM living with HIV are shunned by their peers, and empower men to make discussions of serostatus more common. This will require bringing together an unlikely group of stakeholders for frank discussions about HIV and what can be done to prevent it. Urban and rural community and religious leaders, business and website owners, at-risk uninfected MSM, and men living with HIV all need to be a part of the effort. The stakes are as high, as are the barriers to success – but we really have no choice at this point but to rise to the challenge.
Acknowledgments
Sources of Support
CBH and AMD are supported by the National Center for Advancing Translational Sciences at the National Institutes of Health (8KL2TR000084-05).
Footnotes
Conflicts of Interest
Neither author has any conflicts of interest to report.
References
- 1.Southern HIV/AIDS Strategy Initiative. SASI update: the continuing HIV crisis in the US South: Duke Center for Health Policy and Inequalities Research (CHPIR) Duke University; 2012. [Accessed December 7, 2012.]. http://southernaids.files.wordpress.com/2012/11/sasi-update-the-continuing-hiv-crisis-in-the-us-south.pdf. [Google Scholar]
- 2.Aral SO, O’Leary A, Baker C. Sexually transmitted infections and HIV in the southern United States: an overview. Sex Transm Dis. 2006 Jul;33(7 Suppl):S1–5. doi: 10.1097/01.olq.0000223249.04456.76. [DOI] [PubMed] [Google Scholar]
- 3.Gant Z, Lomotey M, Hall HI, et al. A county-level examination of the relationship between HIV and social determinants of health: 40 states, 2006–2008. Open AIDS J. 2012;6:1–7. doi: 10.2174/1874613601206010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lieb S, Prejean J, Thompson DR, et al. HIV prevalence rates among men who have sex with men in the southern United States: population-based estimates by race/ethnicity. AIDS Behav. 2011 Apr;15(3):596–606. doi: 10.1007/s10461-010-9820-y. [DOI] [PubMed] [Google Scholar]
- 5.Prejean J, Song R, Hernandez A, et al. Estimated HIV Incidence in the United States, 2006–2009. PLoS ONE. 2011;6(8):e17502. doi: 10.1371/journal.pone.0017502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CDC. HIV transmission among black college student and non-student men who have sex with men--North Carolina, 2003. MMWR Morbidity and mortality weekly report. 2004 Aug 20;53(32):731–734. [PubMed] [Google Scholar]
- 7.Hightow LB, MacDonald PD, Pilcher CD, et al. The unexpected movement of the HIV epidemic in the Southeastern United States: transmission among college students. J Acquir Immune Defic Syndr. 2005 Apr 15;38(5):531–537. doi: 10.1097/01.qai.0000155037.10628.cb. [DOI] [PubMed] [Google Scholar]
- 8.CDC. HIV infection among young black men who have sex with men--Jackson, Mississippi, 2006–2008. MMWR Morbidity and mortality weekly report. 2009 Feb 6;58(4):77–81. [PubMed] [Google Scholar]
- 9.Oster AM, Dorell CG, Mena LA, et al. HIV risk among young African American men who have sex with men: a case-control study in Mississippi. Am J Public Health. 2011 Jan;101(1):137–143. doi: 10.2105/AJPH.2009.185850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dorell CG, Sutton MY, Oster AM, et al. Missed opportunities for HIV testing in health care settings among young African American men who have sex with men: implications for the HIV epidemic. AIDS Patient Care STDS. 2011 Nov;25(11):657–664. doi: 10.1089/apc.2011.0203. [DOI] [PubMed] [Google Scholar]
- 11.Oster AM, Pieniazek D, Zhang X, et al. Demographic but not geographic insularity in HIV transmission among young black MSM. AIDS. 2011 Nov 13;25(17):2157–2165. doi: 10.1097/QAD.0b013e32834bfde9. [DOI] [PubMed] [Google Scholar]
- 12.Balaji AB, Oster AM, Viall AH, et al. Role flexing: how community, religion, and family shape the experiences of young black men who have sex with men. AIDS Patient Care STDS. 2012 Dec;26(12):730–737. doi: 10.1089/apc.2012.0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oster AM, Wejnert C, Mena LA, et al. Network analysis among HIV-infected young black men who have sex with men demonstrates high connectedness around few venues. Sexually transmitted diseases. 2013 doi: 10.1097/OLQ.0b013e3182840373. TBA(TBA):TBA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darrow WW, Montanea JE, Gladwin H. AIDS-related stigma among Black and Hispanic young adults. AIDS Behav. 2009 Dec;13(6):1178–1188. doi: 10.1007/s10461-009-9601-7. [DOI] [PubMed] [Google Scholar]
- 15.Glick SN, Golden MR. Persistence of racial differences in attitudes toward homosexuality in the United States. Journal of acquired immune deficiency syndromes. 2010 Dec;55(4):516–523. doi: 10.1097/QAI.0b013e3181f275e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wohl AR, Galvan FH, Myers HF, et al. Do social support, stress, disclosure and stigma influence retention in HIV care for Latino and African American men who have sex with men and women? AIDS Behav. Aug. 2011;15(6):1098–1110. doi: 10.1007/s10461-010-9833-6. [DOI] [PubMed] [Google Scholar]
- 17.Frost SD. Using sexual affiliation networks to describe the sexual structure of a population. Sex Transm Infect. 2007 Aug;83( Suppl 1):i37–42. doi: 10.1136/sti.2006.023580. [DOI] [PubMed] [Google Scholar]
- 18.Hurt CB, Beagle S, Leone PA, et al. Investigating a sexual network of Black men who have sex with men: implications for transmission and prevention of HIV infection in the United States. Journal of Acquired Immune Deficiency Syndromes. 2012 Dec 1;61(4):515–521. doi: 10.1097/QAI.0b013e31827076a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Millett GA, Ding H, Marks G, et al. Mistaken assumptions and missed opportunities: correlates of undiagnosed HIV infection among black and Latino men who have sex with men. Journal of acquired immune deficiency syndromes. 2011 Sep 1;58(1):64–71. doi: 10.1097/QAI.0b013e31822542ad. [DOI] [PubMed] [Google Scholar]
- 20.Hurt CB, Matthews DD, Calabria MS, et al. Sex with older partners is associated with primary HIV infection among men who have sex with men in North Carolina. J Acquir Immune Defic Syndr. 2010 Jun;54(2):185–190. doi: 10.1097/QAI.0b013e3181c99114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Millett GA, Peterson JL, Flores SA, et al. Comparisons of disparities and risks of HIV infection in black and other men who have sex with men in Canada, UK, and USA: a meta-analysis. Lancet. 2012 Jul 28;380(9839):341–348. doi: 10.1016/S0140-6736(12)60899-X. [DOI] [PubMed] [Google Scholar]
- 22.Dennis AM, Hue S, Hurt CB, et al. Phylogenetic insights into regional HIV transmission. AIDS. 2012 Sep 10;26(14):1813–1822. doi: 10.1097/QAD.0b013e3283573244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aldous JL, Pond SK, Poon A, et al. Characterizing HIV transmission networks across the United States. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012 Oct;55(8):1135–1143. doi: 10.1093/cid/cis612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ou CY, Ciesielski CA, Myers G, et al. Molecular epidemiology of HIV transmission in a dental practice. Science. 1992 May 22;256(5060):1165–1171. doi: 10.1126/science.256.5060.1165. [DOI] [PubMed] [Google Scholar]
- 25.Lemey P, Van Dooren S, Van Laethem K, et al. Molecular testing of multiple HIV-1 transmissions in a criminal case. AIDS. 2005 Oct 14;19(15):1649–1658. doi: 10.1097/01.aids.0000187904.02261.1a. [DOI] [PubMed] [Google Scholar]
- 26.Eshleman SH, Hudelson SE, Redd AD, et al. Analysis of genetic linkage of HIV from couples enrolled in the HIV Prevention Trials Network 052 trial. J Infect Dis. 2011 Dec 15;204(12):1918–1926. doi: 10.1093/infdis/jir651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents. US Department of Health and Human Services; Apr 6, 2012. [Google Scholar]