Abstract
Aims
To compare prevalences of self-reported comorbid headaches, neck, back, and joint pains in respondents with temporomandibular joint and muscle disorder (TMJMD)-type pain in the 2000–2005 US National Health Interview Survey (NHIS), and to analyze these self-reported pains by gender and age for Non-Hispanic (NH) Whites (Caucasians), Hispanics and NH Blacks (African Americans).
Methods
Data from the 2000–2005 NHIS included information on gender, age, race, ethnicity, education, different common types of pain (specifically: TMJMD-type, severe headache/migraine, neck, and low back pains), changes in health status; and health care utilization. Estimates and test statistics (i.e. Pearson correlations, regressions and logistic models) were conducted using SAS survey analysis and SUDAAN software that take into account the complex sample design.
Results
A total of 189,977 people: 52% female and 48% males; 73% NH Whites, 12% Hispanic, 11% NH Blacks and 4% “Other” were included. A total of 4.6% reported TMJMD-type pain and only 0.77% overall reported it without any comorbid headache/migraine, neck, or low back pains; also 59% of the TMJMD-type pain (N = 8,964) reported ≥two comorbid pain. Females reported more comorbid pain than males (odds ratio (OR) = 1.41, p <0.001), Hispanic and NH Blacks reported more than NH Whites (OR = 1.56, p <0.001; OR = 1.38, p <0.001, respectively). In addition, 53% of those with TMJMD-type pain had severe headache/migraines; 54% had neck pain, 64% low back pain and 62% joint pain. Differences in gender, race by age patterns were detected. For females, headache/migraine pain with TMJMD-type pain peaked around age 40 and decreased thereafter regardless of race/ethnicity. Neck pain continued to increase up to about age 60, with higher prevalence for Hispanic women at younger ages, and more pronounced in males, being the highest in the non-Whites. Low back pain was higher in Black and Hispanic females across the age span and higher among non-White males after age 60. Joint pain demonstrated similar patterns by race/ethnicity, which higher rates for Black females, and increased with age regardless of gender.
Conclusions
TMJMD-type pain was most often associated with other common pains, and seldom existed alone. Two or more comorbid pains were common. Gender, race, and age patterns for pains with TMJMD-type pain resembled the specific underlying comorbid pain.
Keywords: age, back pain, chronic pain, headache/migraine, neck pain, prevalence, race/ethnicity, sample survey, self-report, temporomandibular joint and muscle disorders
Introduction
Earlier studies by the authors reported racial differences in temporomandibular joint and muscle disorders (TMJMDs) of young women, with Blacks reporting lower prevalence and incidence than Whites (1, 2). To further investigate the racial/ethnic differences, the US National Health Interview Survey (NHIS) data pooled over a 6-year period of 2000–2005 (3) was analyzed. The NHIS is a nationally representative survey of health of non-institutionalized US population. (4) Findings from this study replicated the earlier results on racial differences in young women; at younger ages, White females reported higher rates of TMJMD pain than Black females. (5) Additionally, it was found, however, that patterns of differences in these groups shifted across the adult age span so that in older adults, Hispanic females had higher TMJMD-type pain rates compared to Whites and Blacks. (3) Racial/ethnic differences by age were noted for other types of pain, such as neck and back pain and, to some degree, headaches. (3)
TMJMDs have been associated with other chronic pains including headaches, neck back, and joint pain (6–18) causing significant physical and psychological disability (19–21) and tremendous health care costs. (22, 23) A longitudinal study showed that co-morbid pain, such as musculoskeletal pain and headaches, contributed significantly to the onset, persistence and severity of TMJMD-type pain. (24) Since the NHIS also included data on severe headaches and migraine, neck pain, and low back pain, the present study investigated how comorbidity of TMJMD-type pain with each pain or the number of pains may differ based on age and race/ethnicity. The rationale for including 4 years of data on joint pain was based on recent findings demonstrating that pain in the TMJs is very commonly associated with other types of joint pain. (16) Increased joint pain with age (e.g. due to arthritis) (16, 25, 26) may be related to the observed TMJMD-type pain increasing with age, which may affect non-Whites differently. The large NHIS sample size with oversampling non-Whites enabled a more careful exploration of the age effects on minority populations, including Hispanics. Therefore, the aims of this study were to compare prevalences of self-reported comorbid headaches, neck, back, and joint pains in respondents with (TMJMD)-type pain in the 2000–2005 NHIS and to analyze these self-reported pains by gender and age for non-Hispanic Whites (Caucasians), Hispanics, and non-Hispanic Blacks (African Americans).
Materials and Methods
Data Source
The 2000–2005 NHIS data were pooled for these analyses. The NHIS is an ongoing nationwide household survey designed to obtain information on the demographic characteristics, health status, and health care use patterns of the US civilian non-institutionalized population. The survey has three modules: a basic module; a periodic module; and a topical module. The basic module contains three components: the family core, the sample adult core, and the sample child core. The variables utilized for the present analyses were taken from the sample adult core. Pooling data from 2000–2005 resulted in an adequate sample size to allow detailed analyses by gender and race/ethnicity across the adult age span. The response rates for the sample adult questionnaire ranged from 69% in 2005 to 74% in 2002 and 2004.
Variable Construction
The sample adult core included information on sociodemographic characteristics, health conditions and limitations, and health care utilization.
Common Pain Variables
Adult NHIS participants were asked a series of questions assessing experiences of pains in the past 3 months. Participants were asked to “report pain that lasted a whole day or more and not to report fleeting or minor aches or pains”. Four pains were assessed using the following stem: “During the past 3 months, did you have…” The TMJMD-type pain question ended with “facial ache or pain in the jaw muscles or the joint in front of the ear?” This question is very similar to questions asked in other studies and examined for validity. (27, 28) The other three assessed pains were neck pain, low back pain, and severe headache or migraine. Joint pain was assessed with a different question: “During the past 30 days have you had any symptoms of pain, aching, or stiffness in or around a joint?” Joint pain was included in the assessment of pains for the sample from years 2002–2005, when it was included in the NHIS sample adult questionnaire. Thus the time duration for TMJMD-type pain, headaches, and neck and low back pain was 3 months, and the time frame for joint pain was 1 month. Persons responding “yes” or “no” to questions were included in these analyses, excluding those with missing and “don’t know” responses (less than 1% of the sample).
While respondents reported information about both their racial background and their ethnic background, these analyses followed the common convention describing the three major racial/ethnic category variables available from the National Center for Health Statistics (NCHS) surveillance surveys: non-Hispanic White (referred to in the text as White), non-Hispanic Black (referred to in the text as Black), and Hispanic adults. The NCHS gender and age variables were also used.
A summed variable of the number of the three comorbid pains that were reported (headaches, neck pain, and low back pain, range = 0 – 3) was created. To assess pain burden, a four-level variable categorizing TMJMD and number of comorbid pains was created; 1 = TMJMD only; 2 = TMJMD and 1 comorbid pain; 3 = TMJMD and 2 comorbid pains; and 4 = TMJMD and 3 comorbid pains was created. This variable was used to examine the level of comorbid pain with TMJMD by gender and race/ethnicity. To test for differences within subgroups between those with a lesser degree of comorbidity compared to those with a greater degree, the sum score was thendichotomized: 0 or 1 comorbid pain versus 2 or 3 comorbid pains. To assess prevalence of reported specific comorbid pains with TMJMD, variables representing the comorbid status of TMJMD with each of the following: headache/migraines; neck pain; low back pain; and joint pain were created. The variable assessing joint pain was available in the data sets for years 2002–2005 only, thus it was not used in analyses of summed variables, but was included in specific single analyses.
Data Analysis
Estimates presented here (prevalences) used sampling weights to reflect national population totals. The weights, provided by NCHS estimated the inverse of the sampling probability for each respondent, adjusted for nonresponse. Following NCHS technical report (4) recommendations, the weights were divided by six (or four for joint pain analyses from 2002–2005), so the results still pertained to the national population total. Estimates and test statistics were derived using SAS survey analysis procedures (version 9.1.3, SAS Institute) and SUDAAN (version 9, RTI, RTI) software that took into account the complex sample design of the survey, including household and intrafamilial clustering of sample observations (29). The results are presented in tabular and graphical form.
First, the estimates are reported for TMJMD-type pain and the summed number of comorbid pains for the total sample and for the TMJMD-type pain subsample. All subsequent analyses in the study pertained specifically to the subsample of those reporting TMJMD-type pain. The summed estimates were presented for gender and race/ethnicity groups.
Second, prevalences for the specific comorbid pains were presented: headaches/migraines, neck pain, low back pain, and joint pain for the total sample and by TMJMD-type pain status. Pearson correlations to test the degree of association between reported TMJMD-type pain and the four comorbid pains were conducted. Survey logistic models to determine odds ratios (OR) and 95% confidence intervals (CI) for the association of TMJMD-type pain with each of the four pains were fitted.
Third, analyses were conducted to examine the racial/ethnic and age patterns of reported TMJMD-type pain and comorbidities separately by gender for the dichotomized summed comorbidity variables and the specific comorbid pains. To maintain consistency with the authors’ recent study reporting estimated prevalences for TMJMD-type, headache/migraine, neck, and low back pains (5), the regression models included linear and quadratic (age2) effects to estimate the race/ethnicity-specific age curves. While an “Other” categorywas included for the overall estimates of comorbidities by race/ethnicity, they were excluded in models as they represented a wide range of racial/ethnic characteristics, not characteristic of any particular group. Model results are presented in graphical form.
Results
Our analyses included 189,977 adults, at least 18-years-old, pooled over six years. Overall, 48% percent were males, 52% females; 73% White, 12% Hispanic, 11% Black, and 4% Other. The results in Table 1 show the prevalence of reported TMJMD-type pain with and without the comorbidities of the other three types of pain. A total of 8,964 or 4.6% of the sample reported TMJMD-type pain and only 0.77% overall reported TMJMD without comorbid headache/migraine, neck, or low back pain. Among the reported TMJMD-type pain subsample (N = 8,964) 16.9%reported no comorbid pain. Thus, the vast majority of those who reported TMJMD-type pain also reported one or more comorbid pains. Almost 59% of the TMJMD-type pain sample reported two or more comorbid pains. Females were more likely to report two or more comorbid pains than males (OR = 1.41, p<0.001). Hispanics and Blacks were more likely to report two or more comorbid pains than Whites (OR = 1.56, p<0.001; OR = 1.38, p<0.001, respectively).
Table 1.
Prevalence (percent) of reported TMJMD-type pain and Co-morbid Pains; Odds Ratios (95% Confidence Intervals) comparing reported TMJMD-type Pain with 2–3 Co-morbid Conditions by Sex and Race/Ethnicity
| TMJMD with Number of Pains | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 0 | 1 | 0 or 1 | 2 | 3 | 2 or 3 | Odds Ratio(95% CI) | |
| Total Sample(n=189,977) | 0.77 | 1.1 | 1.87 | 1.36 | 1.31 | 2.67 | |
|
| |||||||
| TMJMD-type pain sample(n=8,964) | 16.9 | 24.5 | 41.4 | 29.8 | 28.8 | 58.6 | |
|
| |||||||
| Gender | |||||||
| Female | 15.1 | 23.8 | 38.9 | 30.4 | 30.7 | 61.1 | 1.41*(1.25–1.59) |
| Malea | 20.9 | 26.3 | 47.2 | 28.4 | 24.4 | 52.8 | |
|
| |||||||
| Race/ethnicity | |||||||
| Whitea | 17.8 | 25.1 | 42.9 | 29.5 | 27.6 | 57.1 | |
| Hispanic | 11.9 | 20.6 | 32.5 | 33.6 | 33.9 | 67.5 | 1.56*(1.34–1.81) |
| Black | 12.5 | 22.7 | 35.2 | 31.6 | 33.2 | 64.8 | 1.38*(1.17–1.63) |
| Other | 20.7 | 26.9 | 47.6 | 21.0 | 31.4 | 52.4 | --- |
referent group
p<0.001
Table 2 presents the prevalences of reported TMJMD-type pain with specific co-morbidities. Among those who reported TMJMD-type pain, 52.5% reported severe headaches/migraines versus 13.6% among those who did not report TMJMD-type pain, (OR = 7.0, p<0.001). Almost 55% of those who reported TMJMD-type pain also reported neck pain versus 13% of those who did not report TMJMD-type pain (OR =7.9, p<0.001). Approximately 64% of those who reported TMJMD reported low back pain versus 26.3% of those without TMJMD-type pain (OR = 5.0, p<0.001). Finally, 62.4% of those who reported TMJMD-type pain had joint pain, compared to 29.4% of those who did notreport TMJMD-type pain, (OR = 4.0, p<0.001). Correlations with TMJMD-type pain ranged from 0.15 for comorbid joint pain to 0.25 for comorbid neck pain (all p<0.001). Neck pain was most strongly associated with TMJMD-type pain, followed by headache/migraine pain, low back pain, and then joint pain.
Table 2.
Prevalence of Migraine/Headache pain, Neck pain, Low back pain, and Joint pain for total sample and by reported TMJMD-type pain status: Prevalences, Odds Ratios (95% Confidence Intervals); and Correlation (r) between reported TMJMD-type pain and each-co-morbid pain.
| Headache/Migraine pain | Neck pain | Low back pain | Joint pain | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| % | OR(CI) | r | % | OR(CI) | r | % | OR(CI) | r | % | OR(CI) | r | |
| Total sample | 15.4 | 0.23* | 14.9 | 0.25* | 28.0 | 0.18* | 30.9 | 0.15* | ||||
|
| ||||||||||||
| TMJMD | ||||||||||||
| yes | 52.5 | 7.0 | 54.2 | 7.9 | 63.9 | 5.0 | 62.4 | 4.0 | ||||
| noa | 13.6 | (6.6–7.5)* | 13.0 | (7.5–8.4)* | 26.3 | (4.7–5.2)* | 29.4 | (3.7–4.2)* | ||||
referent group;
p≤0.001
The graphs in Figure 1 illustrate differences by age and race/ethnicity for females and males among those with two to three comorbid pains accompanying TMJMD-type pain. The patterns among females indicated that prevalence of two to three comorbid pains peaked around age 50 and then declined for all racial/ethnic groups. For Hispanic and Black females, the prevalence was significantly higher regardless of age (p<0.001). Interestingly, for males at younger ages, the lowest prevalence was for Black males, and Hispanic and White males had similar prevalences, although this difference was not significant. After ages 40 to 60, prevalences declined for White males, but after age 60 were significantly higher for Hispanic (p<0.05) and Black males (p<0.01).
Figure 1.
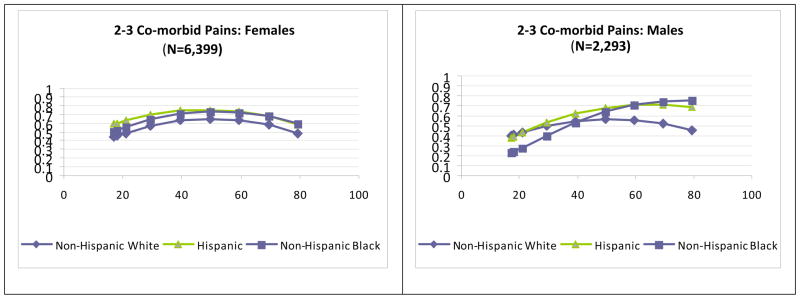
Estimates of Prevalences for reported TMJMD-type Pain Variables based on number of co-morbid pains for Females (N = 6,399) and Males (N = 2,293)
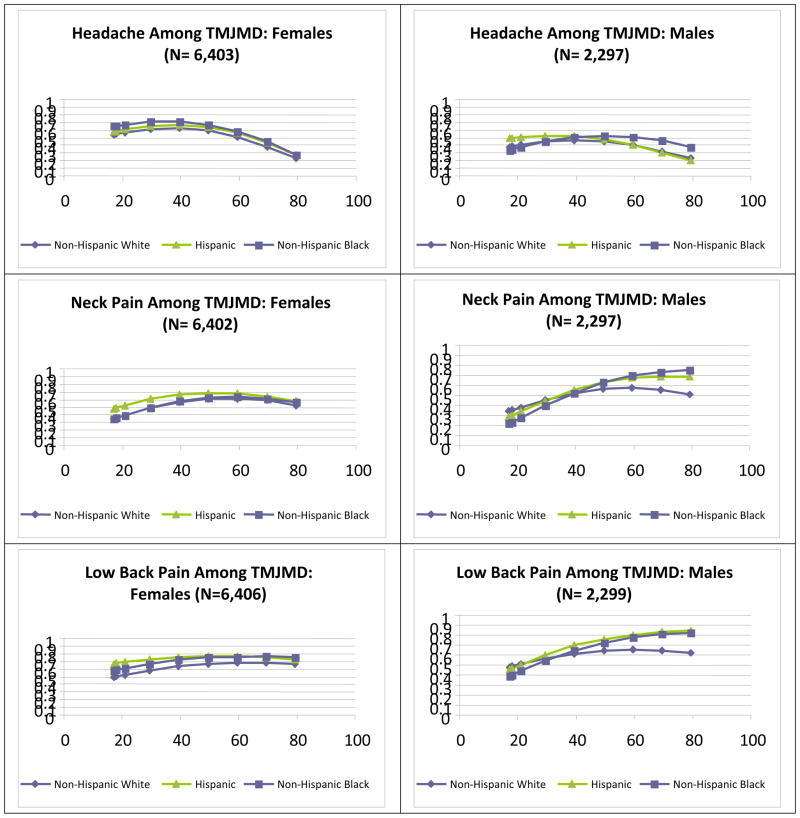
The graphs in Figure 2 illustrate differences by age of specific pain comorbidities for females and males. The top graphs show the patterns for severe headaches/migraine among those who reported TMJMD-type pain. For females, regardless of race/ethnicity, the pattern was similar to that of migraine/headache alone (3), peaking around age 40 and decreasing thereafter. Black and Hispanic females had significantly higher comorbid headaches/migraine across the age span (p<0.001 and p<0.05, respectively). For males of a younger age, Hispanics reported more pain, but the difference was not significant. Blacks reported significantly more headache/migraine pain than Whites starting at age 60, (p<0.05). The next graphs show the neck pain prevalences among those who reported TMJMD-type pain for females and males by race/ethnicity and age. Again, the pattern was more similar to that of the neck pain alone (3). Compared to severe migraine/headache comorbidity, the neck pain comorbidity for females tended to increase with age up to about 60 for all the groups. The largest racial/ethnic differences were at younger ages; Hispanic women reported significantly higher levels of comorbid neck pain than White women (p<0.001) up to age 40. For males the increase with age was more pronounced compared to women; Blacks and Hispanics reported higher comorbid neck pain than White males after age 60, but the difference was significant only for Blacks (p<0.05). Comorbid low back pain among females was fairly consistent across ages, but Hispanics and Blacks had significantly higher rates (both p<0.001). However, for males the pattern was very similar to that of neck pain, with significantly higher rates among Black and Hispanic males after age 60 (both p<0.05). Joint pain, on the other hand, demonstrated gender and racial/ethnic similarities in pattern; however Black females reported significantly more comorbid joint pain (p<0.01) than White females across the age span. Differences among males were not significant, indicating that comorbid joint pain in males increase with age regardless of gender.
Figure 2.
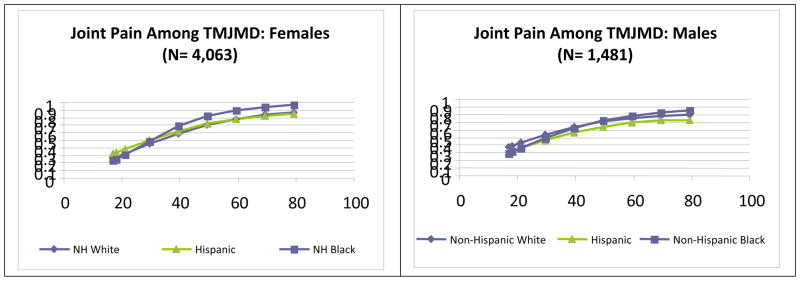
Estimates of Prevalences for severe migraine/headaches, neck, back and joint pains among the reported TMJMD-type Pain groups for Females and Males
Discussion
This is one of the first studies to investigate common comorbid pains in a nationally representative sample of respondents who have reported TMJMD-type pain, based on gender, age, and race/ethnicity. TMJMD-type pain was seldom reported alone: usually (83%) this pain coexisted with other common body pains (headache/migraine, neck, back, and joint pain), confirming previous reports (6–16, 18). Furthermore, the results present several new findings: (1) differences among racial/ethnic groups varied across the adult life span and (2) females, Blacks, and Hispanics displayed a greater burden of comorbidity with TMJMD-type pain than males and Whites. These unique findings may offer a new insight into the biopsychosocial model as it relates to factors involved in the progression of human pain across the adult lifespan.
In the general population the percentage of people with TMJMD-type pain alone without the presence of any other body pain may be even lower than found in the present study (i.e. 0.77%) or less than 17% of those reported TMJMD-type pain with no comorbidity. Other possible comorbid pains not assessed in the NHIS, such as abdominal pain (17), may further reduce the percentage of people who would report only TMJMD-type pain. Furthermore, over 60% of those who reported TMJMD-type pain also reported two or more other pains. There would be no reason to believe that these comorbid pains would be less frequent in a clinical population. Therefore, the natural history of TMJMD-type pain with fluctuation in pain (remission or progression) should be considered in the context of its frequent coexistence with other body pains.
The present results confirmed previous reports on comorbidity of TMJMD-type pain with common pains such as headache/migraine (6–11), neck (12–14) back (15, 18), or joint pain (16), showing the highest association with neck pain and headache/migraine pain, followed by low back pain and joint pain. Furthermore, the finding that women reported a greater number of comorbid pains confirms previous research that women have higher prevalence of certain types of pain than men (30, 31).
To the authors’ knowledge, this is the first study to investigate gender and racial/ethnic differences in co-occurrence of common pains with reported TMJMD-type pain across the adult age span in a representative US sample. While earlier research (1, 3, 5) revealed that minority young adults reported lower prevalences of TMJMDs-type pain than White adults, the current results indicate that among those who reported TMJMD-type pain, both Black and Hispanic adults reported a greater number of co-existing pains. Black and Hispanic females had consistently higher prevelances across ages, and minority males had higher prevalences with steeper increases in prevalences after age 60. Thus, the presence of TMJMD-type pain may be a marker of increased vulnerability to multiple pains not apparent in other types of pain.
An increase in number of body pains has a great impact on general health and suffering (19, 20, 32, 33). The higher number of co-morbid pains in Blacks and Hispanics who reported TMJMD-type pain may be directly or indirectly related to greater deterioration in general health at older age (33). Studies show that persistent life stresses as measured by allostatic load is associated with decreased general health ratings (34). The cumulative effect of psychosocial stress across the life span that may affect Blacks and Hispanics more than Whites may in part contribute to increased deterioration in general health and increased pain reports (33–35).
Interestingly, when patterns of reported TMJMD-type pain prevalence were examined with specific comorbidities based on gender, race/ethnicity, and age, clear differences based on the type of coexisting pain were found. For example, regardless of race/ethnicity, the age and gender patterns of reported TMJMD-type pain with headaches were similar to those of headaches alone (i.e. higher in women, decreasing after reproductive ages) (3). The decline in the prevalence of reported TMJMD-type pain with headaches after reproductive ages and little or no racial/ethnic differences suggest a common biological mechanism, such as hormonal mediation, that may affect all racial/ethnic groups similarly. A recent study reported the highest comorbidity of TMJMD with migraine or mixed migraine with tension-type headache (TTH), but not TTH alone as previously thought (11). The revised International Headache Society classification (36) included within the migraine group a subgroup termed “pure menstrual” or “menstrually-related” migraine. Future studies should determine whether this particular subgroup of menstrually-related migraine displays a stronger association with TMJMD-type pain, thus partly explaining age/gender patterns. On the other hand, the prevalence of reported TMJMD-type pain with neck, joint, and low back pain (mostly in males), increased with age, even more among Blacks and Hispanics. High co-morbidity between reported TMJMD-type and other joint pains lends support to previous clinical research findings showing that counts of painful nonarticular and articular sites predicted jaw pain(16). These authors hypothesized that TMJMD-type pain may be a manifestation of a more general, global pain increase, due to problems related to neurotransmitters (16).
The present data provide a more global view on manifestations of different coexisting pains along the life span. Based on such results, one may ask whether the decrease in prevalence of reported TMJMDs with headaches at older age may indicate less pain or an increased chance for manifesting other pains such as those observed to increase with age (i.e. musculoskeletal/joint pains). A previous longitudinal study of young women showed that the onset of headaches usually precedes the onset of TMJMDs, with headaches developing earlier than TMJMDs and back pain (1). Thus, headaches could play an important role in the early stages of neuroplastic changes involved in the pain sensitization process. Studies show that sex steroids affect serotonergic, catecholaminergic, glutaminergic, GABAergic, and opioidergic systems, all postulated to play a role in migraine (37,38). Electrophysiological studies have also reported extensive convergent input from afferent sources, such as vascular and meningeal tissues, TMJ, jaw muscles, and neck muscles (39–41), implicated in the process underlying headaches, TMJMD, neck pain, and referred pain (39, 42,43). In addition to the biological processes, emotional and psychosocial factors also play important roles in pain persistence (35,44). Racial differences for neck, back, and joint pains tending to increase more in the minority populations with age may indicate that psychosocial factors such as chronic stress (35), perhaps related to hard labor and work dissatisfaction, affect racial/ethnic groups differently, playing a more cumulative role at an older age. However, longitudinal studies are needed to provide better scientific and clinical insights into the progression of different chronic body pain manifestations along the human life span.
The present results support the overwhelming evidence that TMJMD–type pain should not be considered as a single entity in isolation but rather part of a “continuum” or inter-related entity of a more global pain manifestation. Of course, local trauma, including surgical or microtrauma due to bruxism, should not be ignored as a cause for acute orofacial pain. However, pain persistence is of great clinical significance (45).
While this study has much strength, it also has limitations. As with all cross-sectional studies, the age differences noted may not reflect developmental or maturational patterns exclusively. It is possible that the age effects seen in these data may include cohort or secular trends, eg, respondents who grew up during the Depression or who had traumatic experiences during the war years may have different pain experiences than younger respondents will when they reach those ages. Nevertheless, it is valuable for researchers and clinicians to understand the prevalence of comorbid pain conditions present in the current population, regardless of the reasons explaining them. Furthermore, a lack of standardization of pain assessments, due to different clinical/scientific groups introducing them to the NHIS at different times has been acknowledged as a limitation (4). Prevalence differences in reported pain from a more recent report from the National Health and Nutrition Examination Survey (NHANES) of 10,291 subjects may be explained by methodological differences including differences in pain questionnaires (46). However, the TMJMD-type pain (27, 28) and other pain questionnaires (47) have been used successfully in other studies.
Overall, however, the strengths of the study outweigh the limitations. The availability of the NHIS data allowed for the first time a description of common comorbidities with reported TMJMD-type pain in a large representative national sample and to describe gender, racial/ethnic and age differences. The results shed new light on the presentation and relationship of different common pains along the life span. Future longitudinal studies targeting more specific questions about pain progression should further explore the complex mechanisms involved in chronic human pain.
In conclusion, the present results support existing evidence regarding high pain comorbidity with TMJMD-type pain and provide a new perspective regarding age differences based on gender and race/ethnicity, offering a new glimpse into the complexity of human pain from a global biopsychosocial model. These findings suggest the potential value of further emphasizing the age effects on pain syndromes and of taking a more global view of pain progression. Given the findings of higher pain comorbidity with TMJMD-type pain among Black and Hispanic adults, it may be particularly important to extend these findings to understand the role of psychosocial burden accumulated over the life span within socially disadvantaged groups to further address health disparities in these groups.
Acknowledgments
Supported by US DHHS NIH / NIDCR R03DE018759
Contributor Information
O Plesh, Department of Preventive and Restorative Dental Sciences, University of California, San Francisco, 707 Parnassus Ave, San Francisco, CA 94143-0758.
SH Adams, Division of Adolescent Medicine, Department of Pediatrics, University of California, San Francisco, 3333 California St San Francisco, CA94143-0503.
SA Gansky, Department of Preventive and Restorative Dental Sciences, Center to Address Disparities in Children’s Oral Health, University of California, San Francisco, 3333 California St, San Francisco, CA 94143-1361.
References
- 1.Plesh O, Crawford PB, Gansky SA. Chronic pain in a biracial population of young women. Pain. 2002;99:515–523. doi: 10.1016/S0304-3959(02)00262-2. [DOI] [PubMed] [Google Scholar]
- 2.Gansky SA, Plesh O. Widespread pain and fibromyalgia in a biracial cohort study of young women. J Rheumatol. 2007;34:810–817. [PubMed] [Google Scholar]
- 3.Plesh O, Adams S, Gansky SA. Racial/ethnic and gender prevalences in reported common pains in a national sample. J Orofacial Pain. 2010 In Press. [PMC free article] [PubMed] [Google Scholar]
- 4.NationalCenter for Health Statistics. Data File Documentation, National Health Interview Survey, 2004 (machine readable data file and documentation) National Center for Health Statistics, Centers for Disease Control and Prevention; Hyattsville, Maryland: 2005. [Google Scholar]
- 5.Isong U, Gansky SA, Plesh O. Temporomandibular joint and muscle disorder-type pain in US adults: The National Interview Survey. J Orofacial Pain. 2008;22:317–322. [PMC free article] [PubMed] [Google Scholar]
- 6.Ciancaglini R, Randaelli G. The relationship between headache and symptoms of temporomandibular disorders in the general population. J Dent Res. 2001;29:93–98. doi: 10.1016/s0300-5712(00)00042-7. [DOI] [PubMed] [Google Scholar]
- 7.Glaros AG, Urban D, Locke J. Headache and temporomandibular disorders: Evidence for diagnostic and behavioral overlap. Cephalgia. 2007;27:542–547. doi: 10.1111/j.1468-2982.2007.01325.x. [DOI] [PubMed] [Google Scholar]
- 8.Rantala MA, Ahlberg J, Suvinen TI, Nissien M, Lindholm H. Temporomandibular joint related painless symptoms, orofacial pain, neck pain, headaches, and psychological factors among non-patients. Acta Odontol Scand. 2003;61:217–222. doi: 10.1080/00016350310004089. [DOI] [PubMed] [Google Scholar]
- 9.Ballegaard V, Thede-Schmidt-Hansen P, Svensson P, Jensen R. Are headache and temporomandibular disorders related? A blind study. Chephalgia. 2008;28:832–841. doi: 10.1111/j.1468-2982.2008.01597.x. [DOI] [PubMed] [Google Scholar]
- 10.Goncalves DAG, Bigal ME, Jales LCF, Speciali JG. Headache and symptoms of temporomaddibular disorders: An epidemiological study. Headache. 2009;50:10–15. doi: 10.1111/j.1526-4610.2009.01511.x. [DOI] [PubMed] [Google Scholar]
- 11.Scher AI, Midgette LA, Lipton RB. Risk factors for headache chronification. Headache. 2008;48:16–25. doi: 10.1111/j.1526-4610.2007.00970.x. [DOI] [PubMed] [Google Scholar]
- 12.Wanman A. The relationship between muscle tenderness and craniomandibular disorders: a studyof 35-year-olds from general population. J Orofacial Pain. 1995;9:235–243. [PubMed] [Google Scholar]
- 13.Clark GT, Green EM, Dornan MR, Flack VF. Craniocervical dysfunction levels in a patient sample from a temporomandibular joint clinic. J Am Dent Assoc. 1987;115:251–256. doi: 10.14219/jada.archive.1987.0231. [DOI] [PubMed] [Google Scholar]
- 14.DeLaat A, Meuleman H, Stevens A, Verbeke G. Correlation between cervical spine and temporomandibular disorders. Clinical Oral Investig. 1998;2:54–57. doi: 10.1007/s007840050045. [DOI] [PubMed] [Google Scholar]
- 15.Wiesinger B, Malker H, Englund E, Wanman A. Back pain in relation to musculoskeletal disorders in the jaw-face: a matched case-control study. Pain. 2007:311–319. doi: 10.1016/j.pain.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 16.Wolfe F, Katz RS, Michaud K. Jaw pain: its prevalence and meaning in patients with rheumatoid arthritis, osteoarthritis, and fibromyalgia. J Rheumatol. 2005;32:2421–8. [PubMed] [Google Scholar]
- 17.Aaron LA, Burke MM, Buckwald D. Overlapping conditions among patients with chronic fatigue syndrome, fibromyalgia and temporomandibular disorders. Arch Intern Med. 2000;160:221–227. doi: 10.1001/archinte.160.2.221. [DOI] [PubMed] [Google Scholar]
- 18.Turp JC, Kowalski CJ, O’Leary N, Stohler CS. Pain maps from facial pain patients indicate a broad pain geography. J Dent Res. 1998;77:1462–1472. doi: 10.1177/00220345980770061101. [DOI] [PubMed] [Google Scholar]
- 19.VonKorff M, Omel M, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain. 1992;50:133–149. doi: 10.1016/0304-3959(92)90154-4. [DOI] [PubMed] [Google Scholar]
- 20.Plesh O, Wolfe F, Lane N. The relationship between fibromyalgia and temporomandibular disorders: prevalence and symptom severity. J Rheumatol. 1996;23:1948–1952. [PubMed] [Google Scholar]
- 21.Meisler JG. Chronic pain conditions in women. J Women Health. 1999;8:313–320. doi: 10.1089/jwh.1999.8.313. [DOI] [PubMed] [Google Scholar]
- 22.White BA, Williams LA, Leben JR. Health care utilization and cost among health maintenance organization members with temporomandibular disorders. J Orofacial Pain. 2001;15:158–69. [PubMed] [Google Scholar]
- 23.Stowell AW, Gatchel RJ, Wildenstein L. Cost-effectiveness of treatment for temporomandibular disorders. Biopsychosocial intervention versus treatment as usual. JADA. 2007;138:202–208. doi: 10.14219/jada.archive.2007.0137. [DOI] [PubMed] [Google Scholar]
- 24.Velly AM, Look JO, Schiffman E, Lenton PA, et al. The effect of fibromyalgia and widespread pain on the clinically significant temporomandibular muscle and joint disorders: A prospective 18-month cohort study. J Pain. 2010:1–10. doi: 10.1016/j.jpain.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parsons S, Breen A, Foster NE, Letley L, Pincus T, Vogel S, Underwood M. Prevalence and comparative troublesomeness by age of musculoskeletal pain in different body locations. FamPract. 2007;24:308–316. doi: 10.1093/fampra/cmm027. [DOI] [PubMed] [Google Scholar]
- 26.Johansson A, Unell L, Carlsson GE, Soderfeldt B, Halling A. Risk factors associated with symptoms of temporomandibular disorders in a population of 50 and 60-year-old subjects. J Oral Rehab. 2006;33:473–481. doi: 10.1111/j.1365-2842.2005.01574.x. [DOI] [PubMed] [Google Scholar]
- 27.Nilsson IM, List T, Drangsholt M. The reliability and validity of self-reported temporomandibular disorder pain in adolescents. J Orofacial Pain. 2006;20:138–144. [PubMed] [Google Scholar]
- 28.Pinelli C, de Castro Monteiro Loffredo L. Reproducibility and validity of self-perceived oral health conditions. Clin Oral Investig. 2007;11:431–437. doi: 10.1007/s00784-007-0133-0. [DOI] [PubMed] [Google Scholar]
- 29.Research Triangle Institute. SUDAN Manual Release 9.0. Research Triangle Park: NC Research Triangle Institute; 2004. [Google Scholar]
- 30.Unruh AM. Gender variations in chronic pain experience. Pain. 1996;65:123–167. doi: 10.1016/0304-3959(95)00214-6. [DOI] [PubMed] [Google Scholar]
- 31.Berkley KJ. Sex Differences in pain. Behav Brain Scien. 1997;20:1–10. doi: 10.1017/s0140525x97221485. [DOI] [PubMed] [Google Scholar]
- 32.Green CR, Baker TA, Smith EM, Sato Y. The effect of race in older adults presenting chronic pain management: A comparative study of black and white Americans. J Pain. 2003;4:82–90. doi: 10.1054/jpai.2003.8. [DOI] [PubMed] [Google Scholar]
- 33.Green CR, Anderson K, Baker T, Campbell S, Decker R, Fillingim R, et al. The unique burden of pain: Confronting racial and ethnic disparities in pain. MedBiol Res Nursing. 2005;7:7–15. doi: 10.1046/j.1526-4637.2003.03034.x. [DOI] [PubMed] [Google Scholar]
- 34.Seeman TE, Singer BH, Rowe JB, McEwing B. Price of adaptation-allostatic load and its health consequences. MacArthur studies of successful aging. Arch Inter Med. 1997;157:2259–2268. [PubMed] [Google Scholar]
- 35.McEwens BS, Kalia M. The role of corticosteroids and stress in chronic pain conditions. Metabolism. 2010;59(Suppl 1):s9–15. doi: 10.1016/j.metabol.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 36.Headache Classification Subcommitteee of the International Headache Society. The International Classification of aHeadache Disorders. Cephalgia. (2) 2004;24 (Supl 1):1–60. [Google Scholar]
- 37.Martin VT, Behbebani M. Ovarian hormones and migraine headache: understanding mechanism and pathogenesis: part I. Headache. 2006;46:3–23. doi: 10.1111/j.1526-4610.2006.00309.x. [DOI] [PubMed] [Google Scholar]
- 38.Gupta S, Mehrotras S, Villalon CM, Saxena PR, Massen Van Den Brik A. Potential role of female sex hormones in the pathology of migraine. Pharmacol Therap. 2007;113:32–40. doi: 10.1016/j.pharmthera.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 39.Sessle BJ. Acute and chronic craniofacial pain: brainstem mechanisms of nociceptive transmission and neuroplasticity, and their clinical correlates. Crit Rev Oral Biol Med. 2000;11:57–91. doi: 10.1177/10454411000110010401. [DOI] [PubMed] [Google Scholar]
- 40.Morch CD, Hu JW, Arendt-Nielsen L, Sessle BJ. Convergence of cutaneous, musculoskeletal, dural and visceral afferents onto nociceptive neurons in the first cervical dorsal horn. Europ J Neurosci. 2007;26:142–154. doi: 10.1111/j.1460-9568.2007.05608.x. [DOI] [PubMed] [Google Scholar]
- 41.Sessle BJ. Trigeminal central sensitization. Rev Analg. 2005;8:85–102. [Google Scholar]
- 42.Bevilaqua-Grossi D, Lipton RB, Napchan U, Bigal ME. Temporomandibular disorders and cutaneous allodynia are associated in individuals with migraine. Cephalgia. 2009;3:209–215. doi: 10.1111/j.1468-2982.2009.01928.x. [DOI] [PubMed] [Google Scholar]
- 43.Olesen J, Burstein R, Ashina M, Tfelt-Hansen P. Origin of pain in migraine: Evidence for peripheral sensitization. Lancet Neurol. 2009;8:679–689. doi: 10.1016/S1474-4422(09)70090-0. [DOI] [PubMed] [Google Scholar]
- 44.Rollman GB, Shaheed J, Gillespie JM, Jones KS. Does past pain influence current pain: Biological and psychosocial models. Europ J Pain. 2004;8:427–433. doi: 10.1016/j.ejpain.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 45.Van Korff M, Dunn KM. Chronic pain reconsidered. Pain. 2009;138:267–276. doi: 10.1016/j.pain.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hardt J, Jacobsen C, Goldberg J, Nickel R, Buchwald D. Prevalence of chronic pain in a representative sample in the United States. Pain Med. 2008;9:803–812. doi: 10.1111/j.1526-4637.2008.00425.x. [DOI] [PubMed] [Google Scholar]
- 47.Perreault N, Brisson C, Dionne CE, Montreuil S, Punnett L. Agreement between a self-administered questionnaire on musculoskeletal disorders of the neck-shoulder region and a physical examination. BMC Musculoskelet Disord. 2008;17:9–34. doi: 10.1186/1471-2474-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]