SUMMARY
Background
Standard of practice involves using transarterial therapy for multifocal hepatocellular carcinoma (HCC) alone and sorafenib only for more advanced HCC, but the sorafenib and transarterial therapy combination may provide greater efficacy.
Aim
To evaluate the safety and efficacy of concurrent sorafenib and transarterial therapy in HCC.
Methods
Consecutive cases of HCC were treated with sorafenib and transarterial therapy, receiving sorafenib 2 to 4 weeks before transarterial therapy. Baseline clinical parameters, adverse events (AEs) and survival were collected.
Results
A total of 47 patients received sorafenib and transarterial therapy. The majority of the patients were male (70%) with HCV (60%), median age of 60 years, good performance status (0–1), stable cirrhosis (Child: A 72%; B 28%), unresectable turnour (stage: B 81%; C 19%) and median AFP of 24 ng/mL. Median follow-up was 12 months and median time on sorafenib was 6 months. LC Bead TACE was used with a median frequency of 3. The majority of the patients (89%) experienced AEs. The most common AEs were fatigue (51%), hand-foot skin reaction (51%) and diarrhoea (43%). Grade 3 and 4 AEs included fatigue (13%) and hand-foot skin reaction (26%). Most patients required a dose reduction (66%). The main AE related to transarterial therapy was post-TACE syndrome (23%). The disease control rate was 68% at 6 months. Overall median survival rate was 18.5 months (95% CI 16.1–20.9 months).
Conclusion
Concurrent sorafenib and transarterial therapy is overall safe with no unexpected side effects and encouraging efficacy that warrants further study.
INTRODUCTION
While the incidence of most cancers is declining, that of hepatocellular caricinoma (HCC) continues to increase, accounting for over 600 000 deaths world-wide each year.1, 2 HCC is the third leading cause of cancer-related death and the leading cause of death among patients with cirrhosis in Europe and the United States (US).3, 4 The overriding risk factor in 80–90% of HCC is the presence of the preneoplastic cirrhotic liver.5 The projected increase in HCC over the next two decades is mainly related to the epidemic of chronic hepatitis C (HCV) infection. Furthermore, the increasing incidence in the US of obesity and diabetes, which have also been independently linked to the development of cirrhosis and HCC, is likely to further augment the number of people afflicted with HCC.6
The management of HCC is complex. The co-existence of the oncogenic cirrhotic liver and the HCC in the same organ is unique. As implied in the Barcelona Clinic Liver Cancer Staging (BCLC) system, both the status of the cirrhosis and the stage of the HCC have substantial influence on the choice of therapy and survival.7 Most patients with HCC have poor outcomes as they present with significant tumour burden when treatment with the best potential for cure with liver transplantation, resection and/or ablative therapies are not options.8
The current standard of practice is to treat multifocal (intermediate BCLC stage B) HCC with transarterial chemoembolisation (TACE) alone and more advanced (BCLC stage C) HCC with sorafenib (S) only.9 These treatments have only modest survival benefit when used alone with an average increase in survival of 3 months, highlighting the urgent need for novel treatment approaches. Moreover, TACE is a potent stimulator of neo-angiogenesis. The observed angiogenesis is related to up regulation of local angiogenic factors which in turn promote tumour regrowth, increasing the risk of metastases and worsening outcome.10 By contrast, sorafenib down regulates angiogenesis and induces apoptosis.11 As a result, combining TACE and sorafenib has the potential to increase efficacy of treatment. Our centre has established an institutional treatment paradigm that combines sorafenib and transarterial therapy (T+S) for synergy.12 The objectives of this study were to evaluate the safety, tolerability and efficacy of T+S in patients with intermediate or advanced stage HCC that reflect our institutional protocol.
MATERIALS AND METHODS
Study population
We prospectively treated forty-seven patients with unresectable HCC (n = 47) per our institutional protocol with concomitant T+S between 2007 and 2010. The treatment modality each patient received was decided by consensus in a multidisciplinary HCC conference. HCC was diagnosed radiographically according to accepted guidelines.13, l4 The study was approved by the institutional review board. The inclusion criteria included ECOG (Eastern cooperative oncology group performance status) 0–1, total bilirubin <3.0 mg/dL, albumin >3.0, Child-Pugh A-B (≤8) and BCLC B to C. Patients with portal vein thrombosis and extrahepatic disease were not excluded. Similarly, patients with a history of encephalopathy and ascites were also not excluded but sequela needed to be medically controlled. All consecutive patients who met criteria were included except if they had the following: active cardiac disease particularly with recent interventions; not agreeable to close clinical follow-up and the potential daily side effects related to sorafenib treatment, active healing wounds; and patients with uncontrolled hypertension. All patients were started on S 2 to 4 weeks before undergoing T and remained on S during T. Data collected included: age, gender, race, aetiology of cirrhosis, Child-Pugh status, modified end stage liver disease score (MELD), bilirubin, albumin, INR, creatinine, AST, ALT, alkaline phosphatase, haemoglobin, WBC count, AFP; BCLC stage, presence or absence of oesophageal varices, adverse events (AEs) related to T+S and tumour response at 6 months using the conventional Response Evaluation Criteria in Solid Tumours (RECIST) and the modified RECIST (mRECIST) criteria.l5, l6
Medical treatment
All patients received S 400 mg twice daily with close follow-up during and after discontinuation of treatment with a general interval of every 2 to 6 weeks for assessment of drug tolerance, compliance and AEs. Safety and tolerance evaluation involved documented history and physical examinations, laboratory tests and grading of AEs was performed using the National Cancer Institute-Common Terminology Criteria version 3.0. The follow-up period for each patient was the time from the start of S to death or close of study in December 2010. Dose reductions and temporary drug interruptions were used to manage drug related toxicities (dermatologic reactions, diarrhoea, hypertension and fatigue). Treatment was continued until there was an AE requiring permanent discontinuation, radiologic and/or symptomatic progression or death. Patients with dose discontinuation because of AEs during the study were not excluded when performing survival analysis.
Transarterial treatment
Transcatheter therapy was performed by Interventional Radiology per our institutional protocol. Transarterial therapies included drug eluting beads loaded with doxorubicin (LC bead TACE; Biocompatibles, Farnham, UK) and transarterial injection of yttrium-90 microspheres (SIR-Sphere, Sirtex Medical, Wilmington, MA, USA). Sirtex was reserved for cases with portal vein involvement. During LC Bead TACE doses of doxorubicin (ranging from 75 to 150 mg) were given. The number of treatments and the dose of doxorubicin administered were based on tumour burden and underlying liver disease. Feeding vessels were taken to near stasis. Pre-treatment angiography and technetium-99 m macroaggregated albumin scanning were performed to assess for nontarget shunting. Dynamic imaging with a four-phase CT was performed one to 2 months after T treatment to assess tumour response. Further T was on demand based on the tumour response on imaging and decided by consensus in conference. Patients were admitted to the in-patient gastroenterology service for 24 h observation and AEs immediately after therapy were recorded.
Statistical analysis
Descriptive statistics was used for demographic, clinical and laboratory data. Continuous variables were summarised as sample size, mean, median, and range. Categorical variables were summarised as frequencies and percentages. The Kaplan–Meier method was used to estimate survival and the duration of survival was derived from the survival curves. Survival was calculated from the time S started. A patient was censored if he/she was alive at the end of the study period. Log rank test was used to compare survival and difference was held significant at a P value of less than 0.05. Statistical analysis was performed with the SPSS version 17.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Forty seven patients received the T+S combination. The majority of the patients were Caucasian males with a mean age of 61 years (median 60 years) and HCV as the most common underlying aetiology of their liver disease (Table 1). Patients were mostly BCLC stage B (81%) and nearly 20% had stage C disease. Out of the BCLC stage C patients (n = 9), 11% had intra-abdominal nodal metastasis, 22% had portal vein invasion and 67% had distant metastasis. Most patients had well compensated cirrhosis compatible with Child-Pugh A while close to 30% of the patients had Child-Pugh B cirrhosis. On the staging imaging study, 19% of the patients had mild ascites. The ECOG performance status was 0–1 in all patients. Additional baseline characteristics included a low mean MELD of 8 (2–17) and high mean AFP of 2639 ng/mL (median 24 ng/mL). The study population also included 13 patients (28%) who had received previously failed treatments (TACE and surgical resection).
Table 1.
Baseline clinical features of study group (N = 47)
| Factor | Category | N (%) or mean (range) | ||||
|---|---|---|---|---|---|---|
| Age | 61.21 (46–72) | |||||
|
| ||||||
| Gender | M | 33 (70.2%) | ||||
|
| ||||||
| F | 14 (29.8%) | |||||
|
| ||||||
| Race | Caucasian | 36 (76.6%) | ||||
|
| ||||||
| African American | 9 (19.1%) | |||||
|
| ||||||
| Hispanic | 1 (2.1%) | |||||
|
| ||||||
| Other | 1 (2.1%) | |||||
|
| ||||||
| Aetiology | HCV | 15 (31.9%) | Cryptogenic | 7 (14.9%) | ||
|
| ||||||
| HBV | 3 (6.3%) | ETOH | 2 (4.3%) | |||
|
| ||||||
| HCV/ETOH | 10 (21.3%) | HH** | 3 (6.4%) | |||
|
| ||||||
| HCV/HBV | 2 (4.3%) | NAFLD/NASH | 3 (6.4%) | |||
|
| ||||||
| HCV/HH | 1 (2.1%) | AIATD¶ | 1 (2.1%) | |||
|
| ||||||
| HCV | Positive | 28 (59.6%) | ||||
|
| ||||||
| Negative | 19 (40.4%) | |||||
|
| ||||||
| BCLC stage* | B | 38 (81%) | ||||
|
| ||||||
| C | 9 (19%) | |||||
|
| ||||||
| Child-Pugh class | A | 34 (72.3%) | ||||
|
| ||||||
| B | 13 (27.7%) | |||||
|
| ||||||
| ECOG performance status† | 0 | 35 (74.5%) | ||||
|
| ||||||
| 1 | 12 (25.5%) | |||||
|
| ||||||
| Ascites‡ | Present | 9 (19%) | ||||
|
| ||||||
| Absent | 38 (81%) | |||||
|
| ||||||
| Hepatic Encephalopathy | Absent | 37 (78.7%) | ||||
|
| ||||||
| Mild | 10 (21.3%) | |||||
|
| ||||||
| Oesophageal varices | Absent | 34 (72.3%) | ||||
|
| ||||||
| Small | 11 (23.4%) | |||||
|
| ||||||
| Large | 2 (4.26%) | |||||
|
| ||||||
| AFP | <400 | 36 (76.6%) | ||||
|
| ||||||
| >400 | 11 (23.4%) | |||||
|
| ||||||
| MELD Score§ | <15 | 46 (97.9%) | ||||
|
| ||||||
| >15 | 1 (2.1%) | |||||
|
| ||||||
| Creatinine | 0.91 (0.58-1.58) | |||||
|
| ||||||
| Sodium | 137.5 (128-141) | |||||
|
| ||||||
| Haemoglobin (mg/dL) | 13.2 (9.5-17) | |||||
|
| ||||||
| WBC count | 5.7 (1.3-11) | |||||
|
| ||||||
| Platelet count | 168.3 (57-414) | |||||
|
| ||||||
| Albumin (g/dL) | 3.75 (2.2-4.7) | |||||
|
| ||||||
| T. Bilirubin (mg/dL) | 1.13 (0.3-3.7) | |||||
|
| ||||||
| AST | 71.6 (20-201) | |||||
|
| ||||||
| ALT | 58.5 (15-212) | |||||
|
| ||||||
| ALK | 146.2 (50-801) | |||||
|
| ||||||
| INR | 1.12 (0.9-1.4) | |||||
|
| ||||||
| Prior therapies | Resection | 2 (4.3%) | ||||
| Tace | 11 (23.4%) | |||||
Barcelona clinic liver cancer staging.
Eastern Cooperative oncology group performance status.
Mild documented per CT imaging.
Model for end stage liver disease.
Alpha-1 antitrypsin deficiency.
Haemochromatosis.
In terms of tumour burden, the majority of the patients had multifocal (81%) and bilobar (60%) disease with a mean cumulative size of 9.7 cm (Table S1). The median follow-up was 12 months (range, 2–46 months) and the median time on sorafenib was 6 months (0.8–26 months). While LC bead TACE (85%) was the most common transarterial treatment used, some patients also received LC Bead and sirtex (13%) and sirtex alone (2.1%) (Table S2). The median number of transarterial treatments was 3 (1–13). At study initiation, close to 80% of the patients began full dose sorafenib (400 mg b.d.), while the remaining patients began with a lower dose of sorafenib (23%) because of baseline clinical characteristics.
A large number of patients (89%) experienced AEs during the study (Table 2). The most common AEs were fatigue (51%), hand-foot skin reaction (51%) and diarrhoea (43%). At least one grade 3/4 adverse effect was seen in 43% of the patients. Most of the grade 3 and 4 AEs included fatigue (13%) and hand-foot skin reaction (26%) out of which grade 4 adverse events were <1%. Overall, close to 25% of the patients developed new onset ascites and 11% experienced worsening of previously controlled ascites. By contrast, new onset encephalopathy was seen in a small number of patients (9%) and worsening of previously controlled encephalopathy was seen in only 2%. Among the changes in liver function parameters that include albumin, transaminases and INR, elevations were minimal and increases in total bilirubin were seen in 13% of the patients (grade 3 and 4: 6.4%). Nearly all of the AEs were manageable using dose reduction of sorafenib (66%) (Table 3). A number of patients needed drug interruption (40%) and permanent dose discontinuation (43%) because of multiple AEs. In this study, S was discontinued permanently because of AEs in 38% and because of disease progression with symptoms in 2% of the patients. The overall incidence of AEs in Child-Pugh A vs. B cirrhosis was also similar (61% vs. 64%; not significant – N.S.).
Table 2.
Adverse events of the study group
| System | Side effect | Grade 1/2 | Grade 3/4 | Total |
|---|---|---|---|---|
| Overall | 42 (89%) | |||
|
| ||||
| Constitutional | Fatigue | 18 (38.3%) | 6 (12.8%) | 24 (51.1%) |
|
| ||||
| Weight loss | 3 (6.4%) | 3 (6.4%) | ||
|
| ||||
| Anorexia | 3 (6.4%) | 1 (2.1%) | 4 (8.5%) | |
|
| ||||
| Gastrointestinal | Dysguesia | 2 (4.3%) | 2 (4.3%) | |
|
| ||||
| Nausea | 7 (14.9%) | 7 (14.9%) | ||
|
| ||||
| Vomiting | 1 (2.1%) | 1 (2.1%) | ||
|
| ||||
| Diarrhoea | 17 (36.2%) | 3 (6.4%) | 20 (42.6%) | |
|
| ||||
| Variceal bleeding | 2 (4.3%) | 2 (4.3%) | ||
|
| ||||
| Lower extremity oedema | 1 (2.1%) | 1 (2.1%) | ||
|
| ||||
| Abdominal pain | 8 (17%) | 3 (6.4%) | 11 (23.4%) | |
|
| ||||
| Nonvariceal bleeding | 2 (4.3%) | 2 (4.1% | 4 (8.5%) | |
|
| ||||
| Hypoalbuminaemia | 1 (2.1%) | 1 (2.1%) | 2 (4.3%) | |
|
| ||||
| Pancreatitis | 2 (4.3%) | 2 (4.3%) | ||
|
| ||||
| Hepatobiliary (Cirrhosis Sequelae) | Ascites (new) | 7 (14.9%) | 4 (8.5%) | 11 (23%) |
|
| ||||
| Ascites (worsening) | 3 (6.4%) | 2 (4.3%) | 5 (10.6%) | |
|
| ||||
| Encephalopathy (new) | 3 (6.4%) | 1 (2.1%) | 4 (8.5%) | |
|
| ||||
| Encephalopathy (worsening) | 1 (2.1%) | 1 (2.1%) | ||
|
| ||||
| Lower extremity oedema | 1 (2.1%) | 1 (2.1%) | ||
|
| ||||
| Transamnitis (over baseline) | 1 (2.1%) | 1 (2.1%) | ||
|
| ||||
| INR elevation (over baseline) | 1 (2.1%) | 1 (2.1%) | ||
|
| ||||
| Increase in bilirubin | 3 (6.4%) | 3 (6.4%) | 6 (12.8%) | |
|
| ||||
| Hypoalbuminaemia | 1 (2.1%) | 1 (2.1%) | 2 (4.3%) | |
|
| ||||
| Dermatologic | Hand-foot reaction | 12 (25.5%) | 12 (25.5%) | 24 (51.1%) |
|
| ||||
| Truncal rash | 7 (14.9%) | 7 (14.9%) | ||
|
| ||||
| Facial rash | 1 (2.1%) | 1 (2.1%) | ||
|
| ||||
| Scalp rash | 2 (4.3%) | 2 (4.3%) | ||
|
| ||||
| Alopecia | 2 (4.3%) | 2 (4.3%) | ||
|
| ||||
| Cardiovascular | Hypertension | 9 (19.3%) | 9 (19.2%) | |
|
| ||||
| Hypotension | 3 (6.4%) | 3 (6.4%) | ||
|
| ||||
| Haematological | Leucopenia | 1 (2.1%) | 4 (8.5%) | 5 (10.6%) |
|
| ||||
| Electrolyte abnormalities | Hyponatraemia | 1 (2.1%) | 1 (2.1%) | |
|
| ||||
| Hypokalaemia | 1 (2.1%) | 1 (2.1%) | ||
|
| ||||
| Hypophosphataemia | 1 (2.1%) | 1 (2.1%) | ||
|
| ||||
| Hypomagnesaemia | 2 (4.3%) | 2 (4.3%) | ||
Table 3.
Tolerance profile for sorafenib
| Event | N (%) |
|---|---|
| Overall adverse affects | 42 (89.4%) |
| Median length on medication, months (range) | 5.5 (0.8-26) |
| Number of patients with at least one grade 3/4 adverse affect | 21 (46.8%) |
| Dose reduction | 31 (66%) |
| Temporary drug discontinuation | 19 (40.4%) |
| Permanent drug discontinuation because of adverse effect | 18 (38.3%) |
| Permanent drug discontinuation because of progression of disease and symptomatic progression | 2 (2.1%) |
| Self-discontinuation of medication | 1 (2.1%) |
The main AE related to transarterial therapy was post-TACE syndrome (23%) that did not require prolonged hospitalisation beyond 24 h post-treatment observation (Table 4). Additional AEs occurring within 2 weeks after the TACE treatment included new onset ascites (4.3%), ascites with hydrothorax (2.1%), persistent hiccups (2.1%) and cholecystitis (2.1%). One patient developed haemorrhage into the tumour (2.1%) during treatment that was immediately addressed in a transarterial fashion.
Table 4.
Adverse events developing within 2 weeks after TACE
| Event | N (%) |
|---|---|
| Post-TACE syndrome | 11 (23.4) |
| New ascites | 2 (4.3) |
| Ascites with hydrothorax | 1 (2.1) |
| Active haemorrhage into tumour | 1 (2.1) |
| Cholecystitis | 1 (2.1) |
| Hiccups | 1 (2.1) |
At the time of study analysis, 41 patients had follow-up imaging available to assess tumour response at 6 months. Using the modified RECIST criteria, progressive disease was seen in 32%, stable disease in 12%, partial response in 29% and complete response in 27% (Table 5). The objective response rate was 56% and the disease control rate was 68%. The overall median survival for the study group receiving T+S (Figure 1) was 18.5 months (95% CI 16.1–20.9). When sub-stratifying by BCLC stage, median survival for stage B was 18.5 months and for stage C was 17.0 months (N.S.). Similarly, the median survival for Child-Pugh A was 20.9 months and for Child-Pugh B cirrhosis was 17.6 months (N.S.).
Table 5.
Tumour response at 6 months
| Amended RECIST at 6 months | N (%) |
|---|---|
| Complete response | 11 (26.8) |
| Partial response | 12 (29.3) |
| Stable disease | 5 (12.2) |
| Progression of disease | 13 (31.7) |
| Disease control rate* | 28 (68.2) |
| Objective response rate† | 23 (56.1) |
Calculated as complete response plus partial response plus stable disease.
Calculated as complete response plus partial response.
Figure 1.
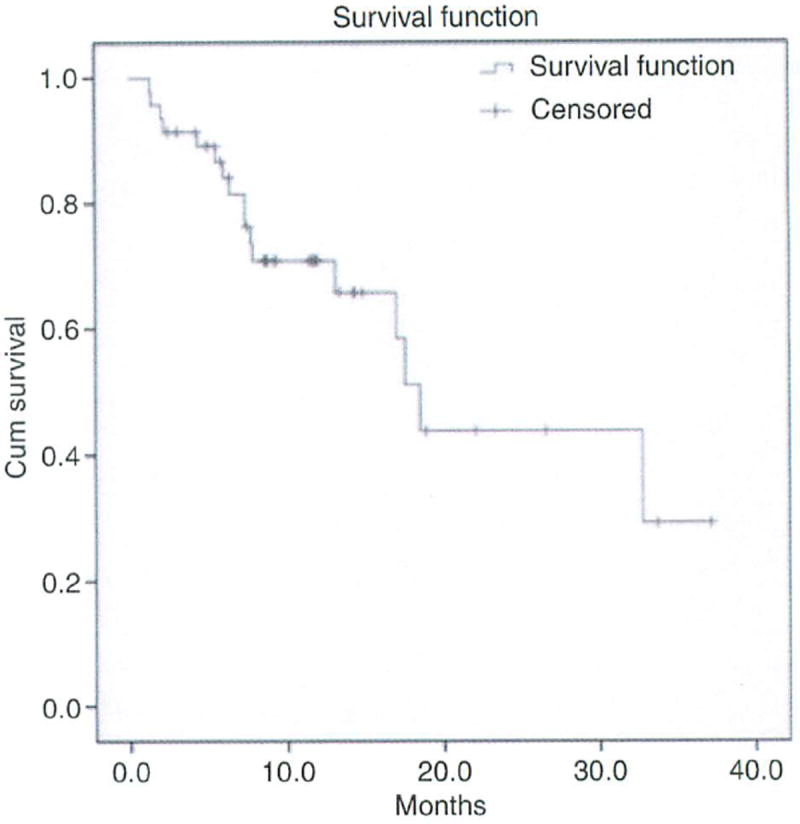
The overall median survival of study group (N = 47) receiving concurrent sorafenib and transarterial treatment was 18.5 months.
DISCUSSION
The current standard of practice is to treat multifocal HCC with TACE alone and more advanced HCC with only sorafenib.17-20 In most centres patients receive these treatments alone. These current treatments have a modest survival advantage but all patients eventually progress highlighting the need for new treatment strategies. Our centre uses both treatments concurrently for synergy to complement each other. In this prospective study, we evaluate the safety, tolerability and efficacy of concurrent TACE and sorafenib therapy in patients with incurable HCC representing intermediate and advanced stage. In this series, we find that concurrent TACE and sorafenib treatment is associated with expected side effects mostly related to drug treatment. While the treatment approach had no unexpected side effects, the majority of patients experienced AEs stressing the importance of close monitoring of the patients during treatment. In addition, the combination did not appear to lead to worse AEs that affected our ability to perform TACE or use sorafenib. Furthermore, this initial clinical experience with combination therapy yielded encouraging efficacy results with a disease control rate of 68% and overall median survival of 18.5 months (95% CI 16.1–20.9 months) particularly in patients with more advanced disease. Our study population included a significant portion of patients with more advanced cirrhosis with nearly 30% patients representing Child class B (median survival 17.6 months) and HCC with close to 20% having stage C HCC (median survival 17.0 months). The possible survival benefit in these subsets of patient can have substantial clinical impact over the current standard of practice. However, these findings require additional evaluation using appropriately powered, randomised studies.
Presently, because standard of practice involves either TACE or sorafenib alone, few studies document the simultaneous use of TACE and sorafenib. Our study shows that the combination is associated with the types of AEs similar to the characteristics of sorafenib alone. However, some patients experienced a greater number and increased intensity of AEs. The principal toxicities observed that represent established AEs related to sorafenib included fatigue, hand-foot skin reaction and diarrhoea. These toxicities were leading causes of dose reductions and interruptions. While the toxicity profile was similar to the SHARP registration trial, the toxicities occurred with a higher incidence.17 Despite the higher intensity of AEs, sorafenib was discontinued with a frequency similar to the registration trials of sorafenib alone. This study used LC bead TACE as the dominant transarterial technique. The majority of patients tolerated the TACE well. The incidence of post-TACE syndrome in our study (23%) was similar to prior reports and the more recent PRECISION V study (24.7%).21,22 Overall the incidence of AE related to the technique of TACE (34%) was also similar to published data.
The increases in AEs are likely due to our study population. The increase in incidence and intensity of AEs may be related to severity of underlying cirrhosis. This study uniquely included patients with Child-Pugh B cirrhosis. In particular, nearly 30% of the patients in our study had Child-Pugh B cirrhosis. Moreover, 20% had advanced, stage C HCC. Patients with Child-Pugh B cirrhosis were excluded from the pivotal clinical trials which had only Child-Pugh A cirrhosis. Moreover, our study had a much smaller sample size which can skew results and findings. In addition, the combination of T+S can make sorafenib harder to tolerate. However, as patients received combination therapy and we lack a comparator arm, it is difficult to conclude that the AEs were completely related to the combination of T+S. Furthermore, the higher incidence of sequelae from cirrhosis progression make this interpretation more challenging as it can represent natural progression of cirrhosis and/or HCC and not necessarily to treatment-related toxicity. The current Phase IV GIDEON study23 evaluating safety and tolerance profile in patients seen in real clinical settings across different disease subclasses and stages could provide very useful information to help clarify these issues. Lastly, it is important to note that although there was a relatively high level of AEs reported that were likely related to S, there was no reported interruption in scheduled TACE events. Thus, using S at our centre did not negatively impact candidacy for appropriate TACE intervention.
A strong rationale exists for combining T+S therapy – two treatments with proven survival and established safety profiles that can complement each other.24, 25 Combining the antiangiogenic agent sorafenib to target the observed up regulation of post-TACE angiogenic factors can potentially enhance the efficacy of TACE. While the combination approach appears promising randomised control studies are required to illustrate the clinical benefit of the combination approach. Ideally we want to see randomised studies and so we look forward to efficacy and safety results of the randomised, controlled phase II trial – sorafenib or placebo in combination with TACE for intermediate-stage HCC (SPACE study). However, if a clinical benefit is not found with the T+S combination, a number of questions will remain to be addressed. Primarily, was the study design optimal to assess the clinical benefit of the combination? Potentially, transient use of sorafenib for a short period only around the time on transarterial intervention may be all that is needed to blunt the rise of pro-tumour promoting angiogenic factors. This treatment approach can lower overall cost of treatments and dramatically limit the frequency and severity of AEs observed with the combination treatment.
To date, this study represents the largest clinical experience of combined therapy using T+S in HCC. Our cohort study largely affirms the potential efficacy of the combination of sorafenib and TACE in patients with HCC and liver dysfunction, but highlights tolerability issues. The concurrent use of sorafenib and TACE is associated with expected AEs similar to sorafenib alone but with higher frequency. However, no unexpected or serious adverse events occurred in the cohort. In summary, this study is largest centre experience with sorafenib in combination with transarterial treatment outside of a clinical trial. The findings suggest that the combination appears promising particularly in patients with more advanced liver disease that are typically excluded from controlled trials. However, the clinical benefit of the combination approach requires further study.
Supplementary Material
Acknowledgments
Declaration of personal interests: Roniel Cabrera and James Caridi have served as speakers for Bayer Healthcare and Onyx Pharmaceutical. Roniel Cabrera and David R. Nelson have served as consultants and advisory board members for Bayer Healthcare and Onyx Pharmaceutical. Roniel Cabrera and David R. Nelson have received research funding from Bayer Healthcare and Onyx Pharmaceutical. Declaration of funding interests: This study was supported by the Office of the Director National Institute of Health (NIH) KL2RR029888 award and the Florida Department of Health Bankhead-Coley new investigator cancer research award 09BN-03 (Roniel Cabrera). Writing support was provided by Jane Yellowlees Douglas of the University of Florida and funded by the NIH KL2RR029888 award.
Footnotes
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article:
Table S1. Baseline turnour characteristics and distribution.
Table S2. Treatment profile.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–6. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Murray T, Ward E, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 3.Benvegnù L, Gios M, Boccato S, Alberti A. Natural history of compensated viral cirrhosis: a prospective study on the incidence and hierarchy of major complications. Gut. 2004;53:744–9. doi: 10.1136/gut.2003.020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sangiovanni A, Prati GM, Fasani P, et al. The natural history of compensated cirrhosis due to hepatitis C virus: a 17-year cohort study of 214 patients. Hepatology. 2006;43:1303–10. doi: 10.1002/hep.21176. [DOI] [PubMed] [Google Scholar]
- 5.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogeneds. Gastroenterology. 2007;132:2557–76. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 6.Veldt BJ, Chen W, Heathcote EJ, et al. Increased risk of hepatocellular carcinoma among patients with hepatitis C cirrhosis and diabetes mellitus. Hepatology. 2008;47:1856–62. doi: 10.1002/hep.22251. [DOI] [PubMed] [Google Scholar]
- 7.Cabrera R, Nelson DR. Review article: the management of hepatocellular carcinoma. Aliment Pharmacol Ther. 2010;31:461–76. doi: 10.1111/j.1365-2036.2009.04200.x. [DOI] [PubMed] [Google Scholar]
- 8.Llovet J, Burroughs AK, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1901–7. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 9.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Hepatology. 2011;53:1020–2. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sergio A, Cristofori C, Cardin R, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103:914–21. doi: 10.1111/j.1572-0241.2007.01712.x. [DOI] [PubMed] [Google Scholar]
- 11.Wilhelm SM, Adnane L, Newell P, et al. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7:3129–40. doi: 10.1158/1535-7163.MCT-08-0013. [DOI] [PubMed] [Google Scholar]
- 12.Cabrera R, Caridi J, George T, et al. Safety of sorafenib alone or in combination with locoregional therapy in patients with advanced HCC and decompensated cirrhosis. American Society of Clinical Oncology (ASCO) Gastrointestinal Cancer Symposium. 2008 Jan 25; Abstract 147. [Google Scholar]
- 13.Bruix J, Sherman M. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–36. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 14.Bruix J, Sherman M, LLovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL Conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–30. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 15.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 16.Lencioni R, Llovet JM. Modified RECIST (rnRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 17.Llovet J, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429–42. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 18.Llovet J, Ricci S, Mazzafero V, et al. Sorafenib versus placebo in advanced hepatocellular carcinoma. N Eng1 J. 2008;359:378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 19.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 20.Llovet JM, Di Bisceglie AM, Bruix J, et al. Panel of experts in HCC-design clinical trials. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 21.Poggi G, Pozzi E, Tonini S, et al. Complications of Image guided transcatheter hepatic chemoembolisation of primary and secondary tuomours of the Liver. Anti Cancer Research. 2010;30:5159–64. [PubMed] [Google Scholar]
- 22.Lammer J, et al. Prospective randomized study of doxorubicin-eluting-bead embolisations in the treatment of hepatocellular carcinoma: results of PRECISION V Study. Cardiovasc Intervent Radio. 2010;33:41–52. doi: 10.1007/s00270-009-9711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lencioni R, Marrero J, Venook A, et al. Design and reationale for the tnon interventional global Investigation of therapeutic decisions in hepatocellular carcinoma and if uts treatment with sorafenib (GIDEON) study. Int J Clin Pract. 2010;64:1034–41. doi: 10.1111/j.1742-1241.2010.02414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaparro M, González Moreno L, Trapero-Marugán M, et al. Review article: pharmacological therapy for hepatocellular carcinoma with sorafenib and other oral agents. Aliment Pharmacol Ther. 2008;28:1269–77. doi: 10.1111/j.1365-2036.2008.03857.x. [DOI] [PubMed] [Google Scholar]
- 25.Huang YH, Wu JC, Chen SC, et al. Survival benefit of transcatheter arterial chemoembolization in patients with hepatocellular carcinoma larger than 10 cm in diameter. Aliment Pharmacol Ther. 2006;23:129–35. doi: 10.1111/j.1365-2036.2006.02704.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


