Abstract
Objective
Corticotropin releasing hormone (CRH)-mediated hypercortisolemia has been demonstrated in anorexia nervosa (anorexia), a psychiatric disorder characterized by food restriction despite low body weight. While CRH is anorexigenic, downstream cortisol stimulates hunger. Using a food-related fMRI paradigm, we have demonstrated hypoactivation of brain regions involved in food motivation in women with anorexia, even after weight-recovery. The relationship between hypothalamic-pituitary-adrenal (HPA) axis dysregulation and appetite, and the association with food motivation neurocircuitry hypoactivation is unknown in anorexia. We investigated the relationship between HPA activity, appetite and food motivation neurocircuitry hypoactivation in anorexia.
Design
Cross-sectional study of 36 women [13 anorexia (AN), 10 weight-recovered AN (ANWR), 13 healthy controls (HC)].
Methods
Peripheral cortisol and ACTH levels were measured fasting and 30, 60, and 120min after a standardized mixed meal. The Visual Analogue Scale was used to assess homeostatic and hedonic appetite. fMRI was performed during visual processing of food and non-food stimuli to measure brain activation pre- and post-meal.
Results
In each group, serum cortisol levels decreased following the meal. Mean fasting, 120min post-meal, and nadir cortisol levels were high in AN vs. HC. Mean postprandial ACTH levels were high in ANWR compared to HC and AN. Cortisol levels were associated with lower fasting homeostatic and hedonic appetite, independent of BMI and depressive symptoms. Cortisol levels were also associated with between-group variance in activation in food-motivation brain regions (e.g., hypothalamus, amygdala, hippocampus, OFC and insula).
Conclusions
HPA activation may contribute to the maintenance of anorexia by suppression of appetitive drive.
Keywords: Anorexia nervosa, cortisol, fMRI, appetite, neuroimaging
Introduction
Anorexia nervosa is a psychiatric disorder characterized by food restriction despite extremely low weight. Corticotrophin releasing hormone (CRH)-mediated hypercortisolemia has been described in these patients, presumably due to the stress of chronic starvation 1-7. However, hypothalamic-pituitary-adrenal (HPA) dysregulation may persist in anorexia nervosa after weight gain, suggesting that this pathway may be involved in disease pathogenesis 8, 9. While CRH is anorexigenic, signaling satiety, cortisol excess, such as in Cushing’s disease or exogenous glucocorticoid exposure, results in increased appetite and weight gain 10-12. This may be related to a direct cortisol effect on appetite-regulating regions of the brain, or indirect effects through inhibition of the anorexigenic hypothalamic hormone CRH or modulation of other appetite-regulating hormones 11, 13, 14. We have previously shown that nocturnal cortisol levels are associated with the severity of disordered eating psychopathology in women across the weight spectrum, independent of body mass index (BMI) 15. Using a food-related fMRI paradigm, we have also reported hypoactivation of food motivation neurocircuitry in women with anorexia nervosa compared to healthy women, even after recovery 16. In the aforementioned study, women with anorexia nervosa reported lower subjective appetite levels than healthy women 16. Whether HPA dysregulation is associated with differences in appetite or hypoactivation of brain circuits involved in food motivation in anorexia nervosa in unknown.
In this study, we use a previously validated fMRI paradigm with food and non-food related visual stimuli and endocrine assessment fasting and following a standardized mixed meal to examine the link between HPA secretory abnormalities and appetite and food motivation brain circuitry deficits in anorexia nervosa 16. Comorbid psychiatric disorders, including depression, are common in anorexia nervosa, and there is overlap between brain regions (e.g. hypothalamus, amygdala, hippocampus, insula) involving stress and food motivation pathways. Importantly, modulation of appetite and feeding behavior by HPA hormones is specifically potentiated through dense expression of receptors (CRF 1, CRF 2, GR) in these same limbic and paralimbic regions: hypothalamic nuclei (ventromedial hypothalamus; paraventricular nucleus), amygdala, hippocampus, nucleus accumbens, and insula 17-21. We hypothesized that the relationships between HPA hormone levels and measures of appetite and brain activation would be independent of depressive symptoms.
Method
Subjects
We studied 36 women between 18 and 28 years of age: 13 with anorexia nervosa (AN), 10 who had recovered from anorexia nervosa (ANWR) and 13 normal-weight in good health (HC). Subject demographics, validation of the fMRI paradigm, and cortisol levels have been previously published 16, 22, 23. In this paper, we investigated the relationship between hypothalamic-pituitary-adrenal activation and appetite and food motivation neurocircuitry. All study participants were recruited from the community through advertisements and referrals from healthcare providers.
Subjects were excluded if they had any contraindication to magnetic resonance imaging such as an implanted medical device, significant orthopedic hardware or severe claustrophobia. Additional exclusion criteria included active abuse of drugs or alcohol, use of hormones or medications known to affect hormone levels (including estrogen) within eight weeks of the study visit, use of depot medroxyprogesterone within six months, diabetes mellitus, history of gastrointestinal tract surgery, pregnancy or breastfeeding within eight weeks of the study, and hematocrit less than 30% or hemoglobin less than 10 g/dl.
Subjects met diagnostic criteria for AN with the Structured Clinical Interview for DSM Disorders-IV (SCID), including intense fear of gaining weight, evidence of body image disturbance, significantly low body weight [operationalized as less than 85% of ideal body weight (IBW) as determined by the 1983 Metropolitan Life tables], and amenorrhea for at least three consecutive months 24. AN subjects who reported more than one binge and one purge episode per month in the three months preceding the study were excluded. Subjects with a history of psychosis by SCID were also excluded.
ANWR were between 90-110% IBW and were required to have regular menstrual cycles and stable weight for at least six months prior to the study. ANWR met a diagnosis of AN by DSM-IV criteria other than amenorrhea, as assessed by SCID, in the past. The recovered subjects had not exercised more than 10 hours per week and had not run more than 25 miles per week in the three months preceding the study.
HC were between 90 and 110% of IBW and reported regular menstrual cycles. HC had no history of amenorrhea, no acute or chronic illnesses, and no history of a psychiatric disorder (including an eating disorder) as assessed by SCID. HC were excluded if they had exercised more than 10 hours per week or ran more than 25 miles per week in the three months preceding the study.
Methods
This study was approved by the Partners Human Research Committee. Written informed consent was obtained from all subjects prior to conducting any procedures. All subjects were admitted to the Massachusetts General Hospital (MGH) Clinical Research Center for an outpatient screening visit and to the MGH Clinical Research Center and Athinoula A. Martinos Imaging Center for a morning, outpatient main visit.
At the screening visit, height, weight and elbow breadth were measured by research dietitians, blood was drawn for screening laboratory tests, and a comprehensive history and physical exam was performed. Exercise patterns and alcohol intake were assessed. Percent IBW was calculated as above. Body mass index (BMI) was obtained by dividing the weight in kilograms by the square of height in meters. Frame size was determined by comparing elbow breadth to race-specific norms derived from the US Health and Nutritional Examination Survey-I 25. The Mood Episode, Psychotic and Associated Symptoms, Mood Disorder, Anxiety, Somatoform, Substance Abuse and Disordered Eating modules of the Structured Clinical Interview for DSM Disorders-IV (SCID) were administered in person during the screening visit or over the telephone before the main visit by a trained psychiatric nurse practitioner or psychologist 24.
At the main visit, %IBW and BMI were reevaluated. A brief medical history was performed. HC and ANWR presented during the follicular phase of the menstrual cycle (day 1-10). Subjects were asked to fast for 12 hours prior to the visit. Subjects were given a 400 kcal mixed breakfast meal standardized for micro- and macro-nutrient content (approximately 20% calories from protein, 20% from fat, and 60% from carbohydrates) at 9:00 AM. The participants selected one of the following options of similar macro- and micronutrient content, provided by the Clinical Research Center bionutritionists: cereal, lowfat milk, yogurt and wheat germ; or minibagel with peanut butter, nonfat lactaid milk, craisins. Participants were asked to eat the entire meal over a 15 minute interval. Upon completion of the meal, bionutrition staff weighed the meal to determine exact caloric intake. Blood was drawn at four different time points throughout the morning for hormone levels: a fasting blood draw obtained immediately before the standardized mixed meal, and blood draws at 30, 60, and 120 min after the meal. Subjective appetite was assessed using the Visual Analogue Scale. Depressive symptoms were assessed using the Beck Depression Inventory-2 (BDI-2). Functional MRI using a food-related paradigm was performed before and after the meal (see below).
Biochemical analysis
Plasma samples were immediately placed on ice. Serum and plasma samples were stored at −80° C until analysis. Serum cortisol levels were measured using a chemiluminescent immunoassay from Beckman-Coulter (Fullerton, CA). The intra-assay CV was 4.4-6.7%, the inter-assay CV was 6.4-7.9%, and the sensitivity was 0.4 mcg/dl. Plasma ACTH levels were measured using an IRMA assay from DiaSorin, Inc. (Stillwater, MN). The intra-assay CV was 3.5-4.8%, the inter-assay CV was 3.2-5.7% and the lowest reportable value was 1.5 pg/mL. Area under the curve was calculated using the trapezoidal method.
Assessment of appetite
Visual analogue scales, a reliable and widely used method to assess appetite 26, were administered fasting and following the mixed meal. Subjects were asked to answer questions about appetite by making a mark on a line with extremes on either end indicating how they felt at that moment. For example, in response to the question, “How hungry are you?,” they marked their degree of current hunger between the two extremes, “I am not hungry at all,” on the left and “I have never been more hungry,” on the right. Scores were calculated by measuring the distance from the left side of the line.
Data analysis
JMP Statistical Discoveries (version 9.0; SAS Institute, Inc., Cary, NC) was used for statistical analyses. Hormone levels were not normally distributed, and were log-transformed prior to analysis. Clinical characteristics, hormone levels, and Visual Analogue scores were compared using overall Analysis of Variance; variables that were significantly different were then compared by Fisher’s Least Significant Difference Test. Within group comparisons of hormone levels and appetite at different timepoints were made using the two-sided Paired T-test. Linear regression analyses were used to investigate the relationships between cortisol levels and subjective appetite measures. Multivariate least-square analyses were constructed to control for potential confounders. Statistical significance was defined as a two-tailed p-value < 0.05. Data are reported as mean ± SEM.
fMRI procedures
fMRI procedures have been previously validated in this population 16. Briefly, fMRI scanning was performed while subjects viewed high-calorie food stimuli, low-calorie food stimuli, non-food stimuli, and low-level baseline stimuli in a block design while participants underwent standard gradient-echo EPI imaging on a Siemens 3T Trio (Malvern, PA).
fMRI data analysis
fMRI data were analyzed as previously described (16). Data were preprocessed using Statistical Parametric Mapping (SPM8) (Wellcome Trust Centre for Neuroimaging at University College London, 2008) and custom routines in MATLAB (Mathworks, Inc., 2000; Natick, MA). Standard preprocessing steps included realignment and geometric unwarping of EPI images using magnetic fieldmaps, correction for bulk-head motion, nonlinear volume-based spatial normalization using the standard Montreal Neurological Institute (Montreal, Canada) brain template, spatial smoothing with a Gaussian filter (6mm full-width at half-maximum), and outlier detection and exclusion 27. Following preprocessing, statistical analysis was performed at the single-subject level. Specific comparisons of interest (high-calorie foods versus objects, separately for pre-meal and post-meal) were tested using linear contrasts, and SPM maps were created based on these contrasts. Results from the single-subject level were submitted to a second-level random effects analysis. Independent sample t-tests were used to compare the size of a particular effect between groups. Clusters were identified within our regions of interest (hypothalamus, amygdala, hippocampus, orbitofrontal cortex (OFC), and anterior insula) in between-group contrasts, significant at p<0.05 (uncorrected) and p<0.1 [corrected for multiple comparisons within the search volume using voxel-level family-wise error (FWE) correction].
Anatomic overlays were used on each subject’s statistical maps to acquire signal change values across regions of interest. Values indicated the degree of change in magnetic resonance signal detected between the high-calorie food and object conditions. Average percent signal change values (beta weights averaged across all voxels within an anatomical region) were obtained using the REX toolbox for SPM8 (17) and used for brain-hormone GLM analyses. Using PROC MIXED model approach in SAS (version 9.2; SAS Institute, Inc., Cary, NC), the effect of cortisol on the association between group status and brain activity was assessed. The percent change in the estimate for case status when the model was adjusted for the hormone was calculated as the estimate for case status in the univariate model (b1) minus the estimate for case status in the model adjusted for the hormone (b2) together over the univariate estimate (b1), [(b1 - b2)/b1]. Due to our interest in the mediating effect of cortisol on the case status effect on brain activity, decreases in percent change were of interest, indicating the percent of the case group’s effect on brain activity accounted for by the hormone. To examine whether the effects of cortisol on between-group differences in brain activity were independent of depressive symptoms, BDI-2 scores were entered into the model with cortisol. The percent change in the estimate for case status in the model adjusted for both cortisol and the potential confounder (BDI-2) (b3) was compared to the estimate for the case status adjusted solely for the hormone (b2). Again, decreases in percent change (b3 > b2) were of interest, indicating the percent of the case group’s effect on brain activity was not confounded by BDI-2 scores.
Results
Subject characteristics
The mean age of subjects was 22.3±0.4 years and did not differ between groups. As per study design, BMI and %IBW were lower in AN (17.7±0.3 kg/m2 and 80.6±1.3%, respectively) than ANWR (21.9±0.7 kg/m2 and 97.7±3.4%) and HC (22.5±0.4 kg/m2 and 97.2±1.7%) (p<0.0001). Mean time since last menstrual period was 50.2±11.1 months for AN. For ANWR, time since weight recovery was 41.5±11.1 months, and time since restoration of menstrual cycles was 42.4±15.4 months. All ANWR reported weight stability for at least 12 months and regular menstrual cycles for at least 14 months. Duration of illness did not significantly differ between groups (AN 52.7±11.2 vs. ANWR 43.2±9.0 months). Three AN and four ANWR reported a remote (at least 14 months prior to the study) history of purging activity, but none were actively binging or purging. Five AN were taking psychotropic medications: two were taking venlafaxine, one was taking fluoxetine, one was taking a low dose of amphetamine/dextroamphetamine (5 mg 24 hours prior to the scan), and one was taking escitalopram and aripiprazole. Two ANWR were taking psychotropic medications: one was taking fluoxetine, and one was taking bupropion and lorazepam. BDI-2 scores were higher in AN compared to ANWR and HC (16.8±3.3 vs. 7.7±2.2 and 0.8±0.4, p<0.02), indicating greater severity of depressive symptoms. No subjects smoked cigarettes on the morning of the study or consumed caffeine within 12 hours of the study. Hours of sleep the prior night, time since last p.o. intake, and calories consumed at breakfast did not differ between the groups.
Hormone levels
Hormone levels are shown in Table 1. Mean fasting, 120 min post-meal, and nadir cortisol levels were higher in AN than HC. In contrast, mean ACTH levels were higher in ANWR than AN and HC at 60 min post-meal and HC at 120 min post-meal. In each group, cortisol levels decreased after the meal. However, the post-meal decrease in ACTH levels was significant only in HC, not in AN or ANWR.
Table 1. Hormone levels.
| Mean±SEM (pg/ml) | P-values AN vs. ANWR |
||||||
|---|---|---|---|---|---|---|---|
| AN | ANWR | HC | AN vs. HC | ANWR vs. HC |
Overall | ||
| Serum Cortisol | |||||||
| T 0 Fasting | 15.9±1.4 | 12.9±1.0 | 11.7±1.1 | NS | 0.013 | NS | 0.041 |
| T 30 | 15.1±1.6 | 11.3±1.08* | 11.7±0.8 | - | - | - | 0.089 |
| T 60 | 13.3±1.5* | 11.5±0.7 | 10.9±0.8 | - | - | - | NS |
| T120 | 12.9±1.3* | 11.1±0.8 | 8.8±0.7* | NS | 0.006 | 0.067 | 0.019 |
| Nadira | 11.7±1.1* | 9.9±0.7* | 8.4±0.6* | NS | 0.008 | NS | 0.028 |
| AUC | 1679±159 | 1383±83 | 1280±73 | - | - | - | 0.084 |
| Plasma ACTH | |||||||
| T 0 Fasting | 27.4±2.3 | 26.0±2.1 | 21.3±1.3 | - | - | - | NS |
| T 30 | 23.6±3.0 | 25.6±1.9 | 22.4±1.4 | - | - | - | NS |
| T 60 | 21.8±1.7 | 29.8±3.1 | 21.9±1.2 | 0.017 | NS | 0.024 | 0.033 |
| T 120 | 23.2±1.7 | 26.2±1.7 | 19.6±1.2 | 0.098 | - | 0.006 | 0.023 |
| Nadira | 20.9±1.9 | 24.2±1.6 | 19.0±1.2* | - | - | - | 0.074 |
| AUC | 2689±262 | 3286±247 | 2563±135 | - | - | - | 0.096 |
p<0.05 compared to T0
nadir, minimum post-meal level
Fasting and post-prandial appetite ratings
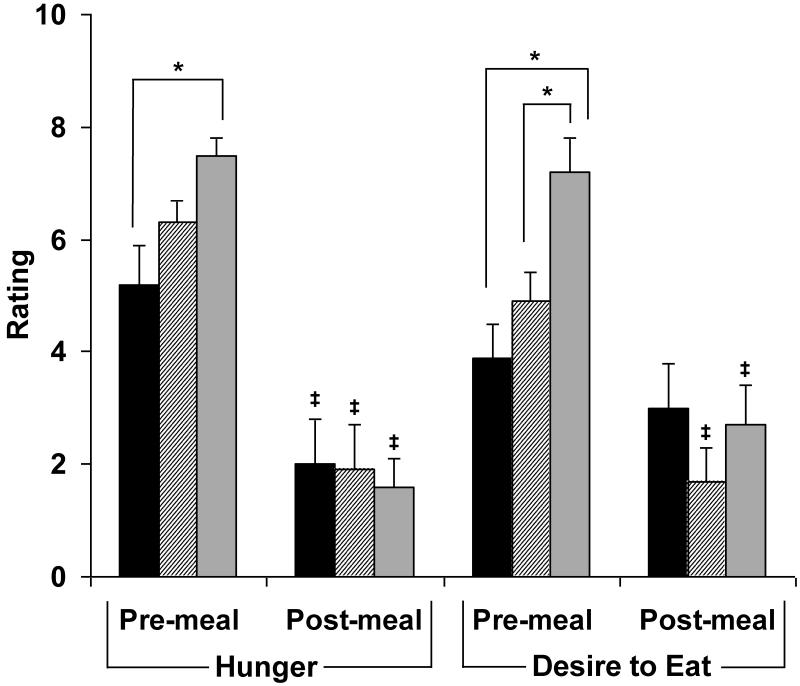
Subjective ratings of homeostatic (i.e., hunger) and hedonic (i.e., desire to eat favorite food) appetite using the visual analogue scale are reported in Figure 1. AN reported less hunger and lower desire to eat favorite foods and ANWR reported lower desire to eat favorite foods than HC in the fasting state (p<0.04). In all groups, subjective hunger decreased after the meal. In ANWR and HC, but not AN, desire to eat favorite foods decreased after the meal. Post-prandial appetite ratings did not differ between groups.
Figure 1.
Pre- and post-meal appetite scores. Pre-meal hunger ratings were lower in AN than the other groups, and desire to eat was lower in AN and ANWR than HC. All groups reported significant decreases in hunger, and all but AN reported a reduction in desire to eat after the meal. Post-meal there were no significant between-group differences in hunger or desire to eat. *, p<0.04 compared to HC. ‡, p<0.05 vs. premeal. Black, AN (active anorexia nervosa); striped, ANWR (weight-recovered anorexia nervosa); grey, HC (healthy control).
Relationship between HPA activation and appetite
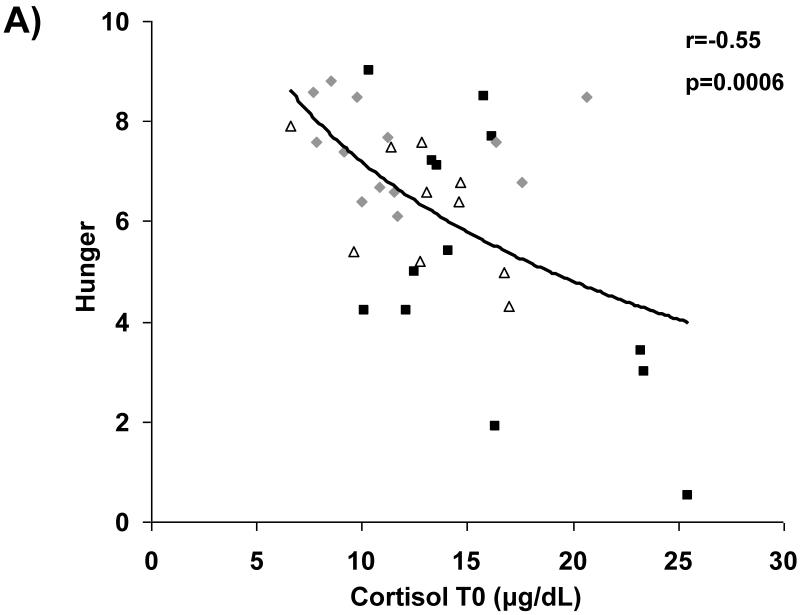
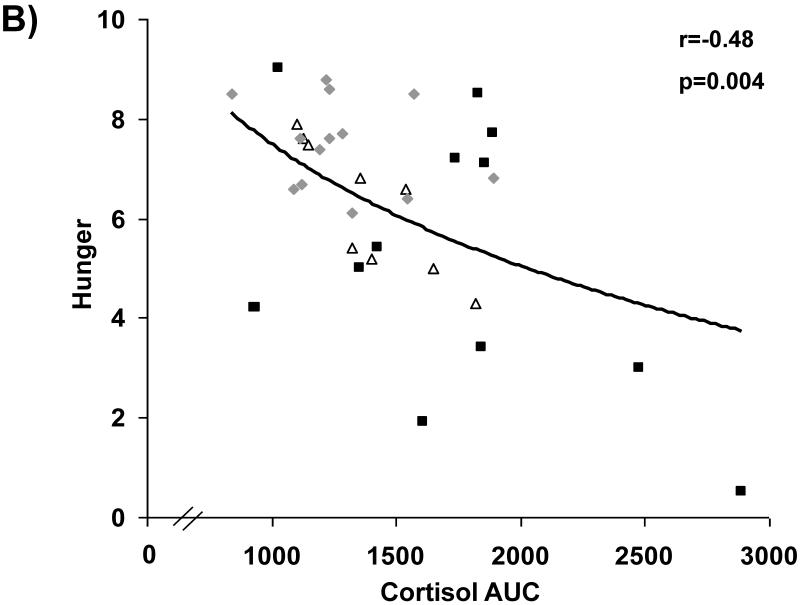
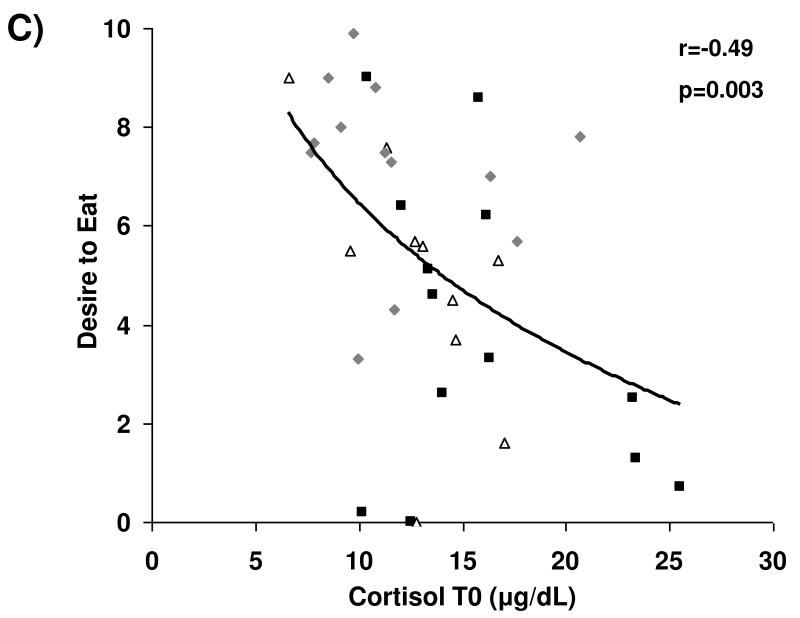
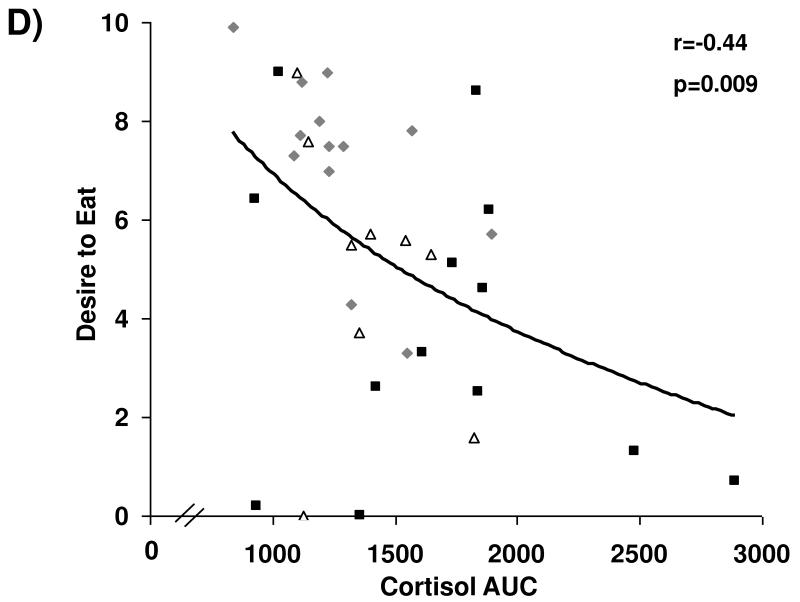
Associations between cortisol levels and subjective appetite are presented in Figure 2. Across groups, fasting serum cortisol and cortisol AUC were negatively associated with subjective assessment of homeostatic (i.e., hunger) and hedonic (i.e., desire to eat favorite food) appetite in the fasting state. After controlling for BMI and depressive symptoms as assessed by BDI-2 scores, these relationships remained significant.
Figure 2.
Cortisol levels and appetite. Fasting and AUC cortisol levels are inversely associated with fasting assessments of hunger (A and B, respectively) and desire to eat favorite foods (C and D, respectively). Squares, active anorexia nervosa; trianges, weight-recovered anorexia nervosa; diamonds, healthy controls.
Relationship between cortisol levels and neurocircuitry involved in appetite and food motivation
Table 2 shows how much of the variance in group differences in signal changes in brain activity associated with our significant food motivation brain regions is related to cortisol levels, as measured by T0 and AUC. We previously demonstrated pre-meal hypoactivation in AN (vs. HC) in the hypothalamus, amygdala, hippocampus, OFC, and insula, and in ANWR (vs. HC) in the hypothalamus, amygdala, and insula. We now demonstrate that cortisol levels, as assessed by T0 and AUC, are associated with variance in activation in the hypothalamus (16-26%), amygdala (24-45%), hippocampus (20-23%), OFC (19-46%), and insula (11-12%) in AN vs. HC, and the hypothalamus (10-18%), amygdala (31-42%), and insula (10-12%) in ANWR vs. HC. After the meal, we reported decreased activation in AN (vs. HC) in the amygdala and insula. Cortisol was associated with 13-16% of the between-group difference in amygdalar activation. Finally, we previously found that post-meal activation in AN (vs. ANWR) was increased in the amygdala and decreased in the insula. Cortisol was associated with 9-12% of between-group differences in brain activation in the insula. Controlling for BDI-2 did not alter results for AN vs. HC, indicating cortisol-brain relationships for this between-group contrast are independent of depressive symptoms in the AN group.
Table 2. Mediation of group effects at selected brain regions by Cortisol TO and Cortisol AUC.
| Estimate, Adjusted for Cortisol T0 |
%Change in Estimate, Adjusted for Cortisol T0 |
Estimate, Adjusted for Cortisol AUC |
%Change in Estimate, Adjusted for Cortisol AUC |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Session | Group contrast |
Region | Hemisphere | MNI coordinates (x, y, z) | Estimate | ||||
| Pre-Meal | |||||||||
| AN vs. HC | |||||||||
| Hypothalamus | L | −3, −7, −5 | −0.92 | −0.69 | 25.5 | −0.77 | 15.8 | ||
| Amygdala | L | −21, −10, −11 | −0.61 | −0.34 | 44.8 | −0.47 | 23.8 | ||
| Hippocampus | L | −9, −40, 1 | −2.27 | −1.73 | 23.8 | −1.80 | 20.5 | ||
| OFC | R | 36, 23, −11 | −1.12 | −0.61 | 45.7 | −0.90 | 19.5 | ||
| Insula | R | 33, 8, 4 | −1.39 | −1.23 | 11.5 | −1.26 | - | ||
| L | −30, 17, 7 | −1.16 | −1.04 | 10.8 | −1.13 | - | |||
| ANWR vs. HC | |||||||||
| Hypothalamus | R | 9, −7, −5 | −0.66 | −0.57 | 14.1 | −0.59 | 10.2 | ||
| L | −6, −10, −5 | −0.90 | −0.79 | 11.8 | −0.74 | 17.5 | |||
| Amygdala | L | −24, −10, −11 | −0.68 | −0.40 | 41.5 | −0.47 | 31.4 | ||
| Insula | R | 30, 26, −11 | −0.80 | −0.71 | 11.8 | −0.71 | 11.5 | ||
| R | 39, 26,−8 | −1.14 | −1.03 | 9.7 | −1.11 | - | |||
|
| |||||||||
| Post-Meal | |||||||||
| AN vs. HC | |||||||||
| Amygdala | L | −24, −10, −14 | −0.37 | −0.32 | 13.2 | −0.31 | 16.0 | ||
| L | −30, −1, −20 | −0.67 | −0.64 | − | −0.64 | - | |||
| Insula | L | −33, 5, −5 | −0.63 | −0.76 | − | −0.77 | - | ||
| L | −39, −7, 4 | −0.99 | −1.11 | − | −1.13 | - | |||
| AN vs. ANWR | |||||||||
| Amygdala | R | 15, −1, −17 | 0.61 | 0.66 | − | 0.64 | - | ||
| Insula | R | 36, −10, 13 | −0.99 | −0.90 | 8.9 | −1.06 | - | ||
| L | −39, −7, 4 | −1.49 | −1.31 | 12.0 | −1.53 | - | |||
Discussion
Using a novel approach combining neuroendocrine, subjective appetite and brain imaging assessments fasting and in response to food, we show for the first time that HPA dysregulation in women with active and weight-recovered anorexia nervosa is associated with altered subjective appetite and food motivation brain circuits. Importantly, these findings are independent of depressive symptoms. These data suggest that abnormalities in HPA secretory patterns are associated with altered perception of appetite, increasing the maintenance of anorexia nervosa symptoms, and, given findings in weight-recovered cases, represent a trait of AN illness.
We found that cortisol levels were increased in women with active anorexia nervosa compared to healthy women fasting and in response to a meal. Although postprandial cortisol levels have been studied in healthy individuals, with mixed results of increased, unchanged or decreased levels following food intake 28-31, there are little data on cortisol and ACTH patterns following a meal in anorexia nervosa. Gastric infusion of liquid caloric mixtures resulted in an increase in ACTH and cortisol in 15 women with anorexia nervosa compared to no change in 15 healthy women 31. To our knowledge, however, the effect of eating a meal on HPA hormones has not been reported in women with anorexia nervosa. In our study, cortisol levels decreased after the meal in all groups, while ACTH decreased in the healthy women only, suggesting excessive postprandial ACTH secretion in active and weight-recovered anorexia nervosa. The relatively higher cortisol levels that we report in women with active anorexia nervosa before and after a meal is consistent with the known HPA hyperactivation in this disorder 1-7. We also found evidence of increased HPA drive in weight-recovered women with anorexia nervosa, who had higher postprandial ACTH levels compared to women with active anorexia nervosa and healthy women despite comparable cortisol levels. This is in line with prior reports of persistent dysregulated HPA function in anorexia nervosa following weight gain 8, 9.
Reports of appetite are abnormal in anorexia nervosa 32, 33. We report lower levels of subjective homeostatic (i.e., hunger) and hedonic (i.e., desire to eat favorite foods) appetite in women with active anorexia nervosa and lower levels of hedonic appetite in weight-recovered women with anorexia nervosa compared to healthy women. We now show that cortisol levels, as assessed by fasting cortisol or postprandial cortisol AUC, are negatively associated with self-reported homeostatic and hedonic appetite levels in anorexia nervosa, independent of BMI or depressive symptoms. Our data raise the question of whether HPA dysregulation, presumably driven by anorexigenic CRH, may promote altered perception of appetite in anorexia nervosa.
Our previous work demonstrated hypoactivation of numerous regions of the brain involved in food motivation, including the hypothalamus (a key control center for appetitive signaling), amygdala (a region important for learning satiety cues and assessing the reward value of food), hippocampus (implicated in processing food-related memories), OFC (involved in integration of emotion and reward expectation) and insula (integrates visceral, homeostatic and emotional signals) in women with anorexia nervosa compared to healthy women 16. Recent human neuroimaging studies offer additional insight into the role of these regions, particularly in the interaction between appetite and stress in healthy populations. For example, acute stress has been shown to elicit variable responses to rewarding food stimuli in the amygdala and hippocampus, depending on appetite level and BMI classification 34, 35, with a significant association between basal cortisol and amygdala activation in response to palatable food 35. Moreover, the OFC appears to modulate the long-term effects of this interaction, with activation positively related to BMI only during stress 35. These findings demonstrate the dynamic interplay between HPA activation and food intake in these regions, although the effect of chronic stress on these systems has yet to be investigated.
Using a food-related fMRI paradigm, we previously showed that in response to viewing high-calorie foods compared to objects, premeal brain activation was decreased in women with active anorexia nervosa compared to healthy women in the hypothalamus, amygdala, hippocampus, OFC and insula 16. We now show that postprandial cortisol levels are associated with 16-46% of these between-group differences (HC vs. AN) in activation in the hypothalamus, amygdala, hippocampus, OFC, and insula. Premeal activation was decreased in women with weight-recovered anorexia nervosa in the hypothalamus, amygdala and insula 16. Cortisol levels are associated with 10-42% of between-group differences (HC vs. ANWR) in activation in the hypothalamus, amygdala and insula. After the meal, activation was decreased in women with active anorexia nervosa in the amygdala compared to healthy women and compared to healthy and weight-recovered women in insula 16. Cortisol levels were associated with 4-16% of between-group differences in activation of the amygdala and 9-12% of between-group differences in insular activation. Importantly, these associations were independent of depressive symptoms as measured by the BDI-2.
Overall, these results are consistent with findings relating acute HPA activation and response to food stimuli in the amygdala, hippocampus, and OFC. Importantly, they extend these previous results suggesting HPA disruption of appetitive signals to the effect of chronic stress and hypercortisolemia as a function of state (i.e., AN) as well as trait (ANWR). Given the density of CRF2 receptors, which (as opposed to CRF1 receptors) are particularly involved in appetitive signaling of CRF and downstream hormones 18, in these limbic and paralimbic regions 17, in combination with evidence of disruption of HPA hormone levels in AN and ANWR, it is likely that the anorexigenic actions of CRH and orexigenic effects of cortisol influence activation of regions outside the hypothalamus and pituitary to influence hedonic and homeostatic perception of appetite in AN and ANWR.
Limitations of this study include small sample size, which may have reduced the power to detect between-group differences in endpoints, as well as correlations between HPA secretory patterns and appetite and brain activation. However, even with a relatively small sample size, our findings are robust. This is a cross-sectional study and causality cannot be established. Further research will be important to explore the effect of HPA dysregulation on appetite pathways in anorexia nervosa.
In summary, we provide evidence that dysregulation of HPA signaling in anorexia nervosa may be involved in disease pathogenesis. Fasting and postprandial cortisol levels are higher in women with active anorexia nervosa compared to healthy women, while postprandial ACTH levels are higher in women with weight-recovered anorexia nervosa compared to healthy women despite no significant difference in cortisol levels. Cortisol levels are negatively associated with homeostatic and hedonic measures of subjective appetite, independent of BMI and depressive symptoms. In addition, cortisol levels are associated with between-group differences in activation of brain regions involved in food motivation, independent of depressive symptoms. Together, these data suggest that HPA dysregulation is associated with the maintenance of anorexia nervosa symptoms, particularly given findings in weight-recovered cases, through altered perception of appetite.
Acknowledgements
The authors thank the nurses and bionutritionist in the Massachusetts General Hospital Clinical Research Center and the subjects who participated in this study.
Funding: This work was supported by the NIH (Awards: UL1 RR025758, K12 HD051959, and K23 MH092560).
Footnotes
Declaration of interest: There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- 1.Misra M, Miller KK, Almazan C, Ramaswamy K, Lapcharoensap W, Worley M, Neubauer G, Herzog DB, Klibanski A. Alterations in cortisol secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. J Clin Endocrinol Metab. 2004;89:4972–2. doi: 10.1210/jc.2004-0723. [DOI] [PubMed] [Google Scholar]
- 2.Putignano P, Dubini A, Toja P, Invitti C, Bonfanti S, Redaelli G, Zappulli D, Cavagnini F. Salivary cortisol measurement in normal-weight, obese and anorexic women: comparison with plasma cortisol. Eur J Endocrinol. 2001;145:165–171. doi: 10.1530/eje.0.1450165. [DOI] [PubMed] [Google Scholar]
- 3.dos Santos E, dos Santos JE, Ribeiro RP, Rosa ESAC, Moreira AC, Silva de Sa MF. Absence of circadian salivary cortisol rhythm in women with anorexia nervosa. J Pediatr Adolesc Gynecol. 2007;20:13–18. doi: 10.1016/j.jpag.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Gold PW, Gwirtsman H, Avgerinos PC, Nieman LK, Gallucci WT, Kaye W, Jimerson D, Ebert M, Rittmaster R, Loriaux DL, et al. Abnormal hypothalamic-pituitary-adrenal function in anorexia nervosa. Pathophysiologic mechanisms in underweight and weight-corrected patients. N Engl J Med. 1986;314:1335–1342. doi: 10.1056/NEJM198605223142102. [DOI] [PubMed] [Google Scholar]
- 5.Hotta M, Shibasaki T, Masuda A, Imaki T, Demura H, Ling N, Shizume K. The responses of plasma adrenocorticotropin and cortisol to corticotropin-releasing hormone (CRH) and cerebrospinal fluid immunoreactive CRH in anorexia nervosa patients. J Clin Endocrinol Metab. 1986;62:319–324. doi: 10.1210/jcem-62-2-319. [DOI] [PubMed] [Google Scholar]
- 6.Lawson EA, Donoho D, Miller KK, Misra M, Meenaghan E, Lydecker J, Wexler T, Herzog DB, Klibanski A. Hypercortisolemia is associated with severity of bone loss and depression in hypothalamic amenorrhea and anorexia nervosa. J Clin Endocrinol Metab. 2009;94:4710–4716. doi: 10.1210/jc.2009-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawson EA, Misra M, Meenaghan E, Rosenblum L, Donoho DA, Herzog D, Klibanski A, Miller KK. Adrenal glucocorticoid and androgen precursor dissociation in anorexia nervosa. J Clin Endocrinol Metab. 2009;94:1367–1371. doi: 10.1210/jc.2008-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayer L, Walsh BT, Pierson RN, Jr., Heymsfield SB, Gallagher D, Wang J, Parides MK, Leibel RL, Warren MP, Killory E, Glasofer D. Body fat redistribution after weight gain in women with anorexia nervosa. Am J Clin Nutr. 2005;81:1286–1291. doi: 10.1093/ajcn/81.6.1286. [DOI] [PubMed] [Google Scholar]
- 9.Grinspoon S, Thomas L, Miller K, Pitts S, Herzog D, Klibanski A. Changes in regional fat redistribution and the effects of estrogen during spontaneous weight gain in women with anorexia nervosa. Am J Clin Nutr. 2001;73:865–869. doi: 10.1093/ajcn/73.5.865. [DOI] [PubMed] [Google Scholar]
- 10.Britton DR, Koob GF, Rivier J, Vale W. Intraventricular corticotropin-releasing factor enhances behavioral effects of novelty. Life Sci. 1982;31:363–367. doi: 10.1016/0024-3205(82)90416-7. [DOI] [PubMed] [Google Scholar]
- 11.Cavagnini F, Croci M, Putignano P, Petroni ML, Invitti C. Glucocorticoids and neuroendocrine function. Int J Obes Relat Metab Disord. 2000;24(Suppl 2):S77–79. doi: 10.1038/sj.ijo.0801284. [DOI] [PubMed] [Google Scholar]
- 12.Heinrichs SC, Menzaghi F, Pich EM, Hauger RL, Koob GF. Corticotropin-releasing factor in the paraventricular nucleus modulates feeding induced by neuropeptide Y. Brain Res. 1993;611:18–24. doi: 10.1016/0006-8993(93)91771-j. [DOI] [PubMed] [Google Scholar]
- 13.Tataranni PA, Larson DE, Snitker S, Young JB, Flatt JP, Ravussin E. Effects of glucocorticoids on energy metabolism and food intake in humans. Am J Physiol. 1996;271:E317–325. doi: 10.1152/ajpendo.1996.271.2.E317. [DOI] [PubMed] [Google Scholar]
- 14.Tempel DL, Leibowitz SF. Adrenal steroid receptors: interactions with brain neuropeptide systems in relation to nutrient intake and metabolism. J Neuroendocrinol. 1994;6:479–501. doi: 10.1111/j.1365-2826.1994.tb00611.x. [DOI] [PubMed] [Google Scholar]
- 15.Lawson EA, Eddy KT, Donoho D, Misra M, Miller KK, Meenaghan E, Lydecker J, Herzog D, Klibanski A. Appetite-regulating hormones cortisol and peptide YY are associated with disordered eating psychopathology, independent of body mass index. Eur J Endocrinol. 2011;164:253–261. doi: 10.1530/EJE-10-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holsen LM, Lawson EA, Blum J, Ko E, Makris N, Fazeli PK, Klibanski A, Goldstein JM. Food Motivation Circuitry Hypoactivation Related to Hedonic and Non-Hedonic Aspects of Hunger and Satiety in Women with Active and Weight-Restored Anorexia Nervosa. Journal of Psychiatry and Neuroscience. 2012;37 doi: 10.1503/jpn.110156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chalmers DT, Lovenberg TW, Grigoriadis DE, Behan DP, De Souza EB. Corticotrophin-releasing factor receptors: from molecular biology to drug design. Trends Pharmacol Sci. 1996;17:166–172. doi: 10.1016/0165-6147(96)81594-x. [DOI] [PubMed] [Google Scholar]
- 19.McEwen BS. Glucocorticoid receptors in the brain. Hosp Pract (Off Ed) 1988;23:107–111. 114, 119–121. doi: 10.1080/21548331.1988.11703523. [DOI] [PubMed] [Google Scholar]
- 20.McEwen BS, De Kloet ER, Rostene W. Adrenal steroid receptors and actions in the nervous system. Physiol Rev. 1986;66:1121–1188. doi: 10.1152/physrev.1986.66.4.1121. [DOI] [PubMed] [Google Scholar]
- 21.McEwen BS, Weiss JM, Schwartz LS. Uptake of corticosterone by rat brain and its concentration by certain limbic structures. Brain Res. 1969;16:227–241. doi: 10.1016/0006-8993(69)90096-1. [DOI] [PubMed] [Google Scholar]
- 22.Lawson EA, Holsen LM, Santin M, Meenaghan E, Eddy KT, Becker AE, Herzog DB, Goldstein JM, Klibanski A. Oxytocin Secretion Is Associated with Severity of Disordered Eating Psychopathology and Insular Cortex Hypoactivation in Anorexia Nervosa. J Clin Endocrinol Metab. 2012;97:E1898–1908. doi: 10.1210/jc.2012-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawson EA, Holsen LM, Santin M, DeSanti R, Meenaghan E, Eddy KT, Herzog DB, Goldstein JM. Postprandial oxytocin secretion is associated with severity of anxiety and depressive symptoms in anorexia nervosa. Journal of Clinical Psychiatry. 2013 doi: 10.4088/JCP.12m08154. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diagnostic and statistical manual of mental disorders. DSM-IV-TR. 4th ed. American Psychiatric Association; Washington, D.C.: 2000. [Google Scholar]
- 25.Frisancho AR, Flegel PN. Elbow breadth as a measure of frame size for US males and females. Am J Clin Nutr. 1983;37:311–314. doi: 10.1093/ajcn/37.2.311. [DOI] [PubMed] [Google Scholar]
- 26.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24:38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 27.Friston K, Ashburner J, Kiebel S, Nichols T, Penny W, editors. Statistical parameter mapping: The analysis of functional brain images. Academic Press; Boston, MA: 2007. [Google Scholar]
- 28.Martens EA, Lemmens SG, Adam TC, Westerterp-Plantenga MS. Sex differences in HPA axis activity in response to a meal. Physiol Behav. 2012;106:272–277. doi: 10.1016/j.physbeh.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 29.Van Cauter E, Shapiro ET, Tillil H, Polonsky KS. Circadian modulation of glucose and insulin responses to meals: relationship to cortisol rhythm. Am J Physiol. 1992;262:E467–475. doi: 10.1152/ajpendo.1992.262.4.E467. [DOI] [PubMed] [Google Scholar]
- 30.Alleman RJ, Jr., Bloomer RJ. Hormonal response to lipid and carbohydrate meals during the acute postprandial period. J Int Soc Sports Nutr. 2011;8:19. doi: 10.1186/1550-2783-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rigaud D, Verges B, Colas-Linhart N, Petiet A, Moukkaddem M, Van Wymelbeke V, Brondel L. Hormonal and psychological factors linked to the increased thermic effect of food in malnourished fasting anorexia nervosa. J Clin Endocrinol Metab. 2007;92:1623–1629. doi: 10.1210/jc.2006-1319. [DOI] [PubMed] [Google Scholar]
- 32.Halmi KA, Sunday SR. Temporal patterns of hunger and fullness ratings and related cognitions in anorexia and bulimia. Appetite. 1991;16:219–237. doi: 10.1016/0195-6663(91)90060-6. [DOI] [PubMed] [Google Scholar]
- 33.Hetherington MM, Rolls BJ. Eating behavior in eating disorders: response to preloads. Physiol Behav. 1991;50:101–108. doi: 10.1016/0031-9384(91)90505-i. [DOI] [PubMed] [Google Scholar]
- 34.Born JM, Lemmens SG, Rutters F, Nieuwenhuizen AG, Formisano E, Goebel R, Westerterp-Plantenga MS. Acute stress and food-related reward activation in the brain during food choice during eating in the absence of hunger. Int J Obes (Lond) 2010;34:172–181. doi: 10.1038/ijo.2009.221. [DOI] [PubMed] [Google Scholar]
- 35.Rudenga KJ, Sinha R, Small DM. Acute stress potentiates brain response to milkshake as a function of body weight and chronic stress. Int J Obes (Lond) 2012 doi: 10.1038/ijo.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]