Abstract
Here we describe studies in the guinea pig model of genital herpes to evaluate a novel plasmid DNA (pDNA) vaccine encoding the HSV-2 glycoprotein D and UL46 and UL47 genes encoding tegument proteins VP11/12 and VP 13/14 (gD2/UL46/UL47), formulated with a cationic lipid-based adjuvant Vaxfectin®. Prophylactic immunization with Vaxfectin®-gD2/UL46/UL47 significantly reduced viral replication in the genital tract, provided complete protection against both primary and recurrent genital skin disease following intravaginal HSV-2 challenge, and significantly reduced latent HSV-2 DNA in the dorsal root ganglia compared to controls. We also examined the impact of therapeutic immunization of HSV-2 infected animals. Here, Vaxfectin®-gD2/UL46/UL47 immunization significantly reduced both the frequency of recurrent disease and viral shedding into the genital tract compared to controls. This novel adjuvanted pDNA vaccine has demonstrated both prophylactic and therapeutic efficacy in the guinea pig model of genital herpes and warrants further development.
Keywords: Herpes simplex virus type 2, DNA vaccine, Vaxfectin®, therapeutic vaccine
1. Introduction
Genital herpes is a sexually transmitted infection of global importance [1, 2]. Initial infection can be asymptomatic or can result in painful skin or mucosal lesions [3]. It is accompanied by virus invasion of ganglia and the establishment of a lifelong latent infection [4]. Periodic reactivation of latent virus results in recurrent lesions or more frequently asymptomatic genital tract shedding. Asymptomatically shed virus is a major source of virus transmission [5]. Genital herpes infection also increases the risk for HIV acquisition [6]. Thus, it represents a worldwide public health problem with significant attendant economic burden [7]. Vaccines to prevent disease or reduce recurrent disease and viral shedding in those already infected would be significant achievements in helping to control genital herpes.
Unfortunately, there are no licensed vaccines against genital herpes. Recent clinical trials with subunit vaccines containing viral glycoproteins B and/or D have proven disappointing; including the recent failure of a Herpes simplex virus type 2 (HSV-2) glycoprotein D (gD2) vaccine developed by GlaxoSmithKline [8]. There is an increasing belief that successful vaccines must generate a robust T-cell response in addition to the humoral immunity elicited to viral glycoproteins [1, 7].
Plasmid DNA (pDNA) vaccines can elicit both cellular and humoral immune responses [9–12]. We have previously shown that a non-adjuvanted gD2 pDNA vaccine provided partial protection against genital herpes in the guinea pig model [13]. The cationic lipid-based adjuvant Vaxfectin® increased the immunity of pDNA vaccines in Phase 1 clinical trials [14–16]. Recently, we showed that Vaxfectin® improved protection provided by a codon-optimized gD2 pDNA vaccine in a murine model of genital herpes [17]. While we were interested in evaluating the protective efficacy of the Vaxfectin®-gD2 pDNA vaccine in the guinea pig model, we also believe that a successful genital herpes vaccine will require additional antigens and will need to elicit cell-mediated as well as humoral immunity. Accordingly, we included pDNAs containing HSV-2 UL46 and UL47 genes encoding the VP11/12 and VP13/14 tegument proteins, respectively, both known to be potent inducers of CD8+ T-cells [18, 19] in our lead vaccine candidate, Vaxfectin®-gD2/UL46/UL47.
Here we describe studies in the guinea pig showing that prophylactic immunization with both Vaxfectin®-gD2 and Vaxfectin®-gD2/UL46/UL47 provided protection against primary and recurrent disease, with Vaxfectin®-gD2/UL46/UL47 being superior in reducing latent viral load. Further, we explored the efficacy of Vaxfectin®-gD2/UL46/UL47 as a therapeutic vaccine in infected animals and showed that it was able to reduce both recurrent disease and viral shedding into the genital tract.
2. Methods
2.1. Guinea Pigs
Female Hartley guinea pigs (Charles River Breeding Laboratories, Wilmington, MA) were housed in AAALC-approved facilities. All animal studies were approved by the UTMB IACUC.
2.2. Plasmid DNAs and Vaccine Formulation
HSV-2 genes were codon-optimized using proprietary algorithms (Vical; San Diego, CA) and DNA synthesized by GeneArt (Regensburg, Germany). Sequences coding full length gD2 and genes UL46 and UL47 were individually sub-cloned into plasmid VR1012 containing the hCMV immediate early promoter as described previously [18], creating plasmids VR2149, VR2144 and VR2145. pDNAs were formulated with Vaxfectin® adjuvant as described previously [17]. Briefly, on the day of immunization, vials containing Vaxfectin® were reconstituted with 1 mL 0.9% saline. At the same time, pDNA was prepared in 0.9% saline, 20 mM sodium phosphate, pH 7.2. The Vaxfectin® was then streamed into the pDNA at a 1:1 volume dilution yielding 1 mg/mL pDNA and 1.09 mg/mL Vaxfectin® with a final pDNA nucleotide: cationic lipid molar ratio of 4:1 [17].
2.3. Guinea Pig Model of Genital HSV-2 Infection
For all studies, guinea pigs were inoculated intravaginally with 6.0 log10 plaque forming units (pfu) HSV-2 strain MS as described previously [20]. The animals were evaluated daily and primary genital skin disease quantified. The resultant cumulative lesion score was used to measure primary genital skin disease severity [20]. Following resolution of primary disease, animals were examined daily for spontaneous recurrent lesions and the number of lesion days was used to determine the frequency of recurrent disease [20].
2.4. Prophylactic Vaccination Studies
Forty-five guinea pigs were assigned to three groups (n=15/group). Animals were immunized intramuscularly in each rear leg. Group 1 received 300 μg Vaxfectin®-gD2 in one leg and 150 μg each of Vaxfectin®-UL46 and Vaxfectin®-UL47 in the other. Group 2 received 300 μg Vaxfectin®-gD2 in one leg and 300 μg of Vaxfectin®-VR1012 in the other. Group 3 received saline in both legs. The animals were immunized three times at two week intervals. Serum was collected after the second and third immunizations. Two weeks after the final immunization, animals were virus challenged as described above. Vaginal swabs were collected on days 1, 2, 3 and 5 and stored (−80°C) to determine viral load by quantitative real-time PCR (qPCR) [21, 22]. After recovering from primary infection, animals were monitored from days 15–63 for recurrent herpetic lesions. Vaginal swab samples were collected from days 21–41 and stored (−80°C) to determine viral shedding into the genital tract by qPCR. At the conclusion of the study, animals were humanely sacrificed, the dorsal root ganglia (DRG) harvested, and the magnitude of latent viral infection determined by qPCR.
2.5. Therapeutic Vaccination Studies
Guinea pigs were inoculated with HSV-2 and primary genital skin disease quantified as described above. After recovery from primary infection, animals that had experienced symptomatic disease and with genital skin that could be evaluated for recurrent disease were randomized to groups based on primary genital skin scores. Animals were immunized on days 15, 29 and 43 post-challenge intramuscularly in the rear legs. In Study 1, Group 1 (n=18) received 300 μg Vaxfectin®-gD2 in one leg and 150 μg each of Vaxfectin®-UL46 and Vaxfectin®-UL47 in the other. Group 2 (n=18) received 300 μg Vaxfectin®-VR1012 in one leg and 150 μg each of Vaxfectin®-UL46 and Vaxfectin®-UL47 in the other. Group 3 (n=18) were saline controls. In Study 2, Group 1 (n=17) were immunized as for Study 1 while Group 2 (n=16) were saline controls. In both studies, animals were monitored from days 15–63 for recurrent disease. Daily vaginal swabs were taken and stored (−80°C) to determine viral shedding into the genital tract by qPCR. Over the course of the experimental period for Study 2, several animals developed secondary infections of the perineum and could not be evaluated for recurrent disease. As such, these animals were not included in subsequent viral shedding comparisons. This resulted in final group numbers for Study 2 of: Group 1 (n=14) and Group 2 (n=14).
2.6. Neutralization Assays
Neutralizing antibody titers were determined by a modification of our previous methods [22]. Briefly, serum from all animals in each group was pooled and heat inactivated (15 minutes, 56°C). Serial two-fold dilutions were prepared using titration medium (Dulbecco’s modified Eagle medium, 2% newborn calf serum, 2% penicillin/streptomycin and 2% amphotericin B) and Low Tox M rabbit complement (CedarLane, Ontario, Canada). A known titer HSV-2 strain MS stock was added to each dilution and the serum/virus mixture incubated (37°C, 60 minutes). Aliquots of each sample dilution were plated in duplicate on Vero cell monolayers and incubated (3 days, 37°C). Plates were stained with crystal violet and viral plaques enumerated. Counts were compared to those from the control group. The neutralizing titer was defined at the log10 of the final serum dilution that reduced plaque number by 50% [22].
2.7. HSV-2 DNA Isolation and Quantification
DNA was isolated from swab samples (DNeasy 96 Blood and Tissue Kit; Qiagen, Valencia, CA) and from DRG using an automated sample disruption system (TissueLyser II; Qiagen) followed by spin-column extraction (DNeasy Blood and Tissue Kit; Qiagen) as previously described [22]. All extracted DNA was eluted in carbon-free water and stored at −20°C until analyzed.
qPCR utilized the CFX optical platform (Bio-Rad, Hercules, CA) and associated chemistries. HSV-2 DNA was quantified in 25 μl reactions as described previously [22]. qPCR mixtures for all samples contained 1x iQ Supermix (Bio-Rad), 5 pmol each forward and reverse primers, 2.5 pmol gB HSV-2 specific TaqMan probe, and 5 μl template DNA (2.5% of total DNA sample). A second, parallel qPCR was conducted using guinea pig housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) to ensure sample integrity and extraction efficiency. This second PCR run controlled for cellular load in each PCR and was used to normalize HSV-2 copies for each sample to more accurately compare results. Cloned amplimers were included on each plate as a 10-fold dilution series as quantitation standards. Negative template controls and water-only samples were included on each run to ensure integrity. PCR run efficiencies were between 80–120% with correlation coefficients >0.96. Assay sensitivity for these reactions allowed for 100 genome equivalents (GE) per reaction to be detected 100% of the time.
2.8. Statistical Analysis
Comparisons between two groups were analyzed by Student’s t-test. Analysis of primary viral replication during the prophylactic immunization study was conducted using a mixed model ANOVA on group by day. Comparisons among multiple groups in the therapeutic vaccination studies were made by one-way analysis of variance with Bonferroni correction. Incidence data was compared using Fisher’s exact test. All comparisons were two-tailed.
3. Results
3.1. Prophylactic Immunization Study
3.1.1. Vaccine Immunogenicity
Both Vaxfectin®-gD2 and Vaxfectin®-gD2/UL46/UL47 elicited detectable neutralizing antibodies after the second immunization (titers 2560 and 1280, respectively). Titers increased following the third immunization (Vaxfectin®-gD2 10240; Vaxfectin®-gD2/UL46/UL47 5120) indicating that both vaccines elicited robust and comparable functional humoral immune responses.
Genital Tract Infection and Initial Vaginal HSV-2 Replication
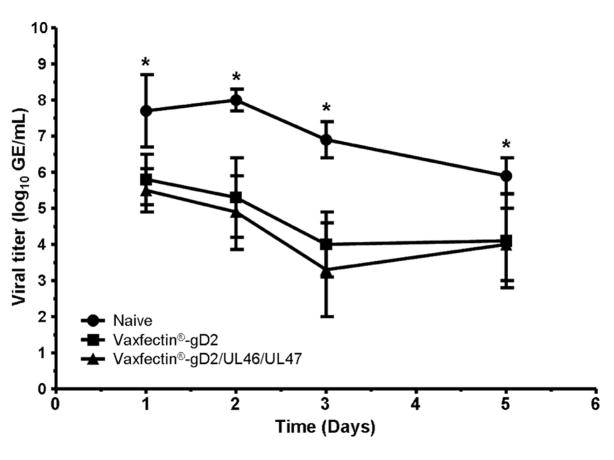
Vaginal HSV-2 DNA was detected in all animals in each group on multiple days after viral challenge, indicating that neither vaccine prevented vaginal virus infection. Virus load in both vaccine groups was significantly lower than controls on all days (p<0.0001, mixed model ANOVA on group by day; Figure 1). Although the viral load was consistently lower in animals immunized with Vaxfectin®-gD2/UL46/UL47 than those receiving Vaxfectin®-gD2 on all days the differences did not reach significance.
Figure 1. Effect of prophylactic vaccination on primary vaginal HSV-2 titers.
Vaginal virus titers were determined by qPCR on days 1–5 after intravaginal HSV-2 challenge in guinea pigs immunized three times with Vaxfectin®-gD2 , Vaxfectin®-gD2/UL46/UL47 or saline controls. Titer values are mean ± SD genome equivalents/ml. Effect of vaccination was compared using a mixed model ANOVA on group by day. * indicates p<0.0001 compared to saline controls for both vaccinated groups.
3.1.2. Genital Skin Disease
Table 1 shows that all control animals developed primary vesiculo-ulcerative disease and subsequently 10/11 control animals experienced spontaneous recurrent disease (4 control animals were lost to analysis of recurrent disease due to the severity of primary infection). In contrast, both vaccine formulations provided complete protection against both primary (p<0.001 each, Fisher’s exact test) and recurrent (p<0.001 each, Fisher’s exact test) genital skin disease.
Table 1.
Prophylactic vaccination with Vaxfectin® pDNA prevents primary and recurrent disease in the guinea pig model.
| Group | Primary disease
|
Recurrent disease
|
||
|---|---|---|---|---|
| Incidence | Severitya | Incidence | Frequencyb | |
| Naïve | 15/15 | 9.3 ± 4.7 | 10/11 | 5.5 ± 3.9 |
| Vaxfectin®-gD2 | 0/15c | 0 ± 0 | 0/15c | 0 ± 0 |
| Vaxfectin®-gD2/UL46/UL47 | 0/15c | 0 ± 0 | 0/15c | 0 ± 0 |
Primary disease severity defined as cumulative daily lesion score, mean ± SD.
Recurrent disease frequency defined as recurrent lesion days between days 15–63 post-inoculation, mean ± SD.
p<0.001 compared to control, Fisher’s exact test
3.1.3. qPCR Determination of Virus Shedding and Latent Viral Load
To further evaluate the protection provided by the two vaccines, vaginal swabs collected from days 21–41 post-challenge were evaluated by qPCR to determine the number of animals shedding HSV-2. Immunization with either Vaxfectin®-gD2 or Vaxfectin®-gD2/UL46/UL47 reduced the number of animals that shed virus, with 6/15 in each vaccine group, compared to 9/11 control animals (p=0.05 for both vaccine groups, Fisher’s exact test).
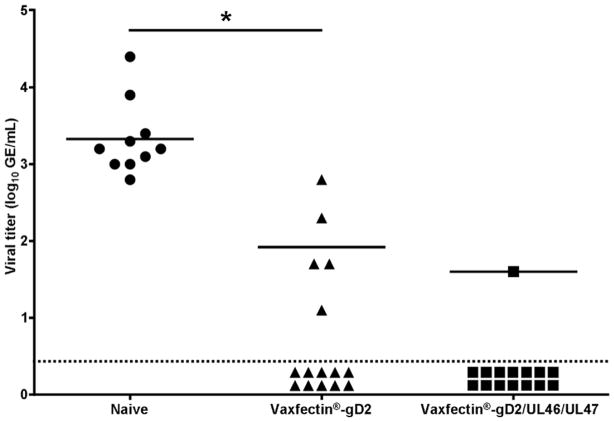
At the conclusion of the study, DRG were harvested and latent viral DNA load determined. It should be noted that one animal in the control group died as a result of unrelated complications prior to DRG harvest, resulting in 10 animals for this group. Figure 2 shows that HSV-2 DNA was detected in all control animals (mean 3.3±0.5 log10 GE/mL). In contrast, immunization with Vaxfectin®-gD2 reduced both the number of animals in which viral DNA was detected (5/15 vs 10/10; p<0.001, Fisher’s exact test) and the amount of virus in those animals (mean 1.9±0.6 log10 GE/mL; p<0.001, Student’s t-test). The impact of immunization with Vaxfectin®-gD2/UL46/UL47 was even greater with HSV-2 DNA being detected in only one animal (p<0.0001, Fisher’s exact test; viral load 1.6 log10 GE/mL); the remaining 14 animals were below the limit of detection of our assay.
Figure 2. Effect of prophylactic vaccination on latent HSV-2 burden in the dorsal root ganglia.
Following determination of the effect of vaccination on recurrent disease, dorsal root ganglia were harvested from guinea pigs vaccinated with Vaxfectin®-gD2 , Vaxfectin®-gD2/UL46/UL47 or saline controls. Dorsal root ganglia were collected from surviving animals (10 control and 15 in each vaccine group) on Day 67 and stored −80°C until the latent HSV-2 burden was determined by qPCR. Viral load data for individual animals is shown. Horizontal lines indicate group averages. Animals below the dotted line were below the level of detection for our assay and were not included in group means for HSV-2 DNA load. As only one animal in the Vaxfectin®-gD2/UL46/UL47 had a detectable level of HSV-2 DNA in the DRG, no statistical comparison could be made between this group and the Vaxfectin®-gD2 or saline controls. * indicates p<0.001 compared to saline controls, Student’s t-test
3.2. Therapeutic Vaccine Studies
3.2.1. Study 1
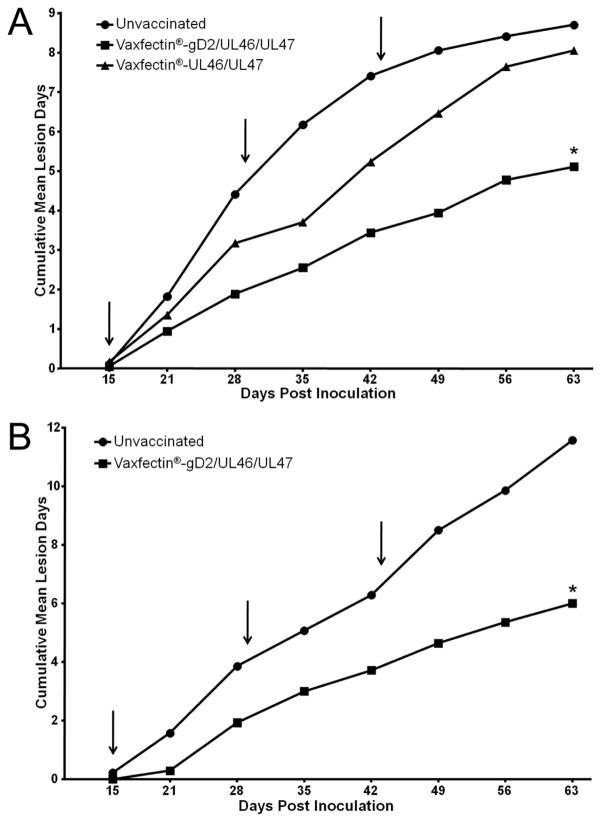
We first examined the ability of Vaxfectin®-gD2/UL46/UL47 and Vaxfectin®-UL46/UL47 to impact recurrent disease when administered following resolution of primary disease. Figure 3a shows that the frequency of recurrent disease in animals immunized with Vaxfectin®-UL46/UL47 was comparable to controls (8.7±4.6 vs 8.1±4.7 mean ± SD recurrent lesion days) between days 15–63 post viral challenge. However, animals immunized with Vaxfectin®-gD2/UL46/UL47 experienced significantly less recurrent disease (5.1±4.6 recurrent lesion days; p<0.05, ANOVA with Bonferroni correction).
Figure 3. Effect of therapeutic vaccination on the frequency of recurrent genital skin disease.
Results are cumulative frequency of recurrent genital skin lesions over time. Arrows indicate days of vaccination relative to intravaginal HSV-2 challenge. A) Study 1: animals were vaccinated with Vaxfectin®-gD2/UL46/UL47, Vaxfectin®-UL46/UL47 or were saline controls. Statistical comparisons were made using the cumulative mean lesion day values at Day 63 for each of the three tested groups using ANOVA with Bonferroni correction. * indicates p<0.05 compared to saline controls B) Study 2: animals were vaccinated with Vaxfectin®-gD2/UL46/UL47 or were saline controls. * indicates p<0.05 compared to saline controls, Student’s t-test
3.2.2. Study 2
A second study was undertaken to confirm the therapeutic efficacy of Vaxfectin®-gD2/UL46/UL47 on recurrent disease. Figure 3b shows that the vaccine again significantly reduced recurrent disease compared to controls (6.07±4.7 vs 11.4±6.6 recurrent lesion days, p<0.05, ANOVA with Bonferroni correction). In addition, we examined the impact of therapeutic immunization on vaginal viral shedding between days 46–59 post challenge by qPCR (Table 2). As with the impact on recurrent disease, immunization significantly reduced the number of days on which shed virus was detected compared to controls (p<0.05, Student’s t-test), but not the amount of virus shed per episode.
Table 2.
Therapeutic vaccination with Vaxfectin®-pDNA vaccine decreases days of viral shedding.
| Group | Animals shedding virus | Days of virus sheddinga | Amount of virus shed (log10 GE/mL)b |
|---|---|---|---|
| Naïve | 12/14 | 2.29 ± 0.41 | 2.91 ± 0.19 |
| jVaxfectin®-gD2/UL46/UL47 | 9/14 | 1.07 ± 0.27c | 2.45 ± 0.20 |
Mean (± SE) number of shedding days/animal over the 14 day period.
Mean (± SE) HSV-2 genome copies per shedding event.
p<0.05 compared to controls, Student’s t-test.
4. Discussion
Recent studies showed that addition of Vaxfectin® to a full length codon-optimized gD2 pDNA vaccine (Vaxfectin®-gD2) increased survival in a mouse genital herpes model compared to a gD2 pDNA vaccine alone. Further, Vaxfectin®-gD2 immunized mice had reduced vaginal viral replication and surviving animals had less latent viral DNA in the DRG than animals immunized with gD2 pDNA only [17]. Further, we found that addition of the adjuvant Vaxfectin® to our vaccine formulation (Vaxfectin®-gD2) was sufficient to completely abrogate HSV-2 disease during primary HSV-2 infection in the guinea pig model of genital herpes. This contrasts with a study by Strasser et al where a gD2 pDNA vaccine was not able to completely protect animals from acute HSV-2 disease [23]. It should be noted that while both the gD2 construct and the amount of pDNA used for immunization in the studies of Strasser et al differed from our studies, we believe that our data shows that the adjuvant Vaxfectin® will be an important tool for developing more effective herpes vaccines.
We and others have shown that prophylactic immunization with HSV-2 gD pDNA vaccines without adjuvants provides some protection against genital herpes in the guinea pig model [13, 23]. Given the results in murine studies with Vaxfectin®-adjuvanted pDNA, we examined the protection afforded by prophylactic immunization with Vaxfectin®-gD2 in the guinea pig, which allows the impact of immunization on spontaneous recurrent disease to be evaluated. Because optimal protection against genital herpes will likely require a vaccine containing multiple antigens, we included a Vaxfectin®-gD2 vaccine with plasmid DNAs for genes UL46 and UL47 that encode viral tegument proteins VP11/12 and VP13/14, respectively [18, 19]. Both proteins contain epitopes important in CD4+ and CD8+ T-cell responses in humans [24], making them good candidates for a more broadly immunogenic vaccine. It should be mentioned that VP11/12 can activate Lck signaling in T-cells [25]. This activation does not globally alter all Lck-dependent signaling events and the HSV-induced signaling blockade of the T-cell receptor does not require this protein [25], therefore incorporation of the UL46 plasmid should not affect the immunogenicity of this vaccine. In our study, neither vaccine prevented genital infection following a high titer viral challenge. It is worth noting that a number of other prophylactic vaccines evaluated in the guinea pig have also failed to elicit sterilizing immunity [13, 26]. Further, guinea pigs latently infected with HSV-2 and rechallenged intravaginally are not protected against vaginal viral replication [27]. Together, these data indicate that it is extremely difficult to elicit immune responses that provide complete protection of the genital mucosa against large viral inocula.
Although neither vaccine provided sterilizing immunity, both significantly reduced viral replication in the genital tract during primary infection. While the vaginal viral load in animals immunized with the two vaccines did not differ significantly, it was consistently lower in Vaxfectin®-gD2/UL46/UL47 than Vaxfectin®-gD2 animals.
All controls developed primary genital skin disease and subsequently all but one of the animals that could be evaluated developed spontaneous recurrences. In contrast, both vaccines provided complete protection against primary and recurrent genital disease. We also examined the impact of immunization on the number of animals in which vaginal viral shedding was detected. The incidence of shedding was high among controls (9/11) and both vaccines produced a comparable reduction (6/15 each; p=0.05, Fisher’s exact test). At the conclusion of the study we examined the latent viral load in the DRG. HSV-2 DNA was detected in all controls. Immunization with Vaxfectin®-gD2 significantly reduced both the number of animals with detectable HSV-2 DNA and the viral load in those animals. Immunization with Vaxfectin®-gD2/UL46/UL47 produced an even greater effect, reducing the burden of latent virus below the limit of detection of the qPCR assay in all but one animal.
While the development of an effective prophylactic vaccine to control genital herpes is important, it will not benefit those already infected with HSV-2. A number of therapeutic vaccine strategies have been evaluated in the guinea pig model. In early studies, glycoprotein-based vaccines reduced recurrent disease; however, results were variable and highly dependent on both the antigen and adjuvant selected [23, 28]. Interestingly, in one study therapeutic immunization also reduced vaginal HSV-2 shedding measured by the number of days that infectious virus was recovered by culture from vaginal swabs [29]. The efficacy of glycoprotein vaccines used therapeutically in these studies resulted in a number of clinical efficacy trials. In the first of these, two immunizations with a vaccine containing 100 μg gD2 protein with alum reduced recurrent disease [30]. However, a second study using 4 immunizations with a vaccine containing 10 μg each of gD2 and gB2 proteins with MF59 failed to significantly reduce recurrent disease resulting in discontinuation of this candidate [31]. Recently there has been a resurgence of interest in therapeutic immunization for genital herpes due in part to the potential not only to reduce recurrent disease, but also asymptomatic shedding, a major source of virus transmission [19, 32]. Both short-term clinical trials with suppressive antiviral therapy and mathematical modeling for prophylactic vaccines strongly suggest that decreased shedding is critical in reducing genital herpes transmission [33–35]. One advantage of therapeutic immunization over suppressive antiviral therapy in this regard would be that it would not be dependent on long-term compliance to a daily treatment regimen.
Consequently, we examined the impact of therapeutic immunization with Vaxfectin®-gD2/UL46/UL47 beginning after primary genital skin disease resolution. Because CD8+ T-cell responses are known to be important in the control of recurrent lesions in humans [36], we also included a group of animals immunized with Vaxfectin®-UL46/UL47 alone. Vaxfectin®-gD2/UL46/UL47, but not Vaxfectin®-UL46/UL47, significantly reduced recurrent disease. The different activities of the two vaccine formulations in this therapeutic setting strongly suggests that the efficacy of Vaxfectin®-gD2/UL46/UL47 resulted from the combined antigen formulation rather than non-specific pDNA effects. To confirm the observed therapeutic immunization impact, a second study was undertaken with Vaxfectin®-gD2/UL46/UL47. Here, immunization again significantly reduced recurrent disease with the impact in both studies being similar (41% and 47%). The reduction in recurrent disease seen with Vaxfectin®-gD2/UL46/UL47 contrasts results reported for bupivacaine-formulated gD2 pDNA which failed to significantly reduce recurrent disease [22]. This strongly suggests that the combination of Vaxfectin® adjuvant and the addition of UL46/UL47 pDNAs to the vaccine are important for its therapeutic efficacy. Further, in the second study we examined the impact of immunization on vaginal virus shedding over 14 days and showed that the number of shedding events in immunized animals was reduced by >53% compared to that in controls (p<0.05, Student’s t-test). The amount of viral DNA detected during shedding events was comparable in immunized and control animals. To our knowledge this is the first demonstration that a therapeutic vaccine can impact the frequency of shedding into the genital tract as well as the frequency of recurrent disease. Studies to further the development of the therapeutic vaccine are currently in the planning stages. These studies will include additional controls, including irrelevant plasmid formulated with Vaxfectin.
These studies show that prophylactic immunization with Vaxfectin®-gD2/UL46/UL47 afforded excellent protection against genital herpes disease. Importantly, the vaccine also reduced recurrent disease when used therapeutically. An intervention that could be used periodically to provide an extended period of reduced recurrent disease would be an important addition to treatment options for genital herpes. Further, the observation that therapeutic immunization with Vaxfectin®-gD2/UL46/UL47 significantly reduced the frequency of virus shedding has public health implications given that this is thought to be a major source of transmission. It is important to note that while immunization reduced the potential for transmission of the virus due to a reduction in the frequency of genital tract shedding, the potential for infection by exposure during a shedding event is not decreased. However, these promising results strongly suggest that Vaxfectin®-gD2/UL46/UL47 warrants further development as a therapeutic vaccine.
If the results of these studies in the guinea pig model can be replicated in clinical trials, the vaccine would provide an excellent alternative to suppressive antiviral therapy that would not require adherence to a strict daily treatment regimen and would not have associated concerns about the possibility of development of antiviral resistance.
Highlights.
Vaxfectin-gD2/UL46/UL47 DNA vaccine effective against genital herpes in guinea pigs
Prophylactic immunization prevents primary and recurrent skin disease
Prophylactic immunization greatly reduces latent HSV-2 DNA in dorsal root ganglia
Therapeutic immunization reduces recurrent genital disease and days of viral shedding
Acknowledgments
We thank Megan Sikes and Rachael Stegall for excellent technical assistance on this project. We also thank Dr Kristofer Jennings for all of his advice and assistance with our statistical analyses.
Financial support: This work was supported by the National Institutes of Health National Institute of Allergy and Infectious Diseases [grant number 5R42AI065015].
Footnotes
Potential conflicts of interest: M.S., Q.W. and S.S. are all employees of Vical, Inc. All other authors: No reported conflicts.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Koelle DM, Corey L. Herpes simplex: insights on pathogenesis and possible vaccines. Annu Rev Med. 2008;59:381–95. doi: 10.1146/annurev.med.59.061606.095540. [DOI] [PubMed] [Google Scholar]
- 2.Xu F, Sternberg MR, Gottlieb SL, Berman SM, Markowitz LE, Forhan SE, et al. Seroprevalence of herpes simplex virus type 2 among persons aged 14–49 years--United States, 2005–2008. MMWR Morb Mortal Wkly Rep. 2010 Apr 23;59(15):456–9. [PubMed] [Google Scholar]
- 3.Whitley RJ, Roizman B. Herpes simplex virus infections. Lancet. 2001 May 12;357(9267):1513–8. doi: 10.1016/S0140-6736(00)04638-9. [DOI] [PubMed] [Google Scholar]
- 4.Brugha R, Keersmaekers K, Renton A, Meheus A. Genital herpes infection: a review. Int J Epidemiol. 1997 Aug;26(4):698–709. doi: 10.1093/ije/26.4.698. [DOI] [PubMed] [Google Scholar]
- 5.Tronstein E, Johnston C, Huang ML, Selke S, Magaret A, Warren T, et al. Genital shedding of herpes simplex virus among symptomatic and asymptomatic persons with HSV-2 infection. JAMA. 2011 Apr 13;305(14):1441–9. doi: 10.1001/jama.2011.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoshino Y, Dalai SK, Wang K, Pesnicak L, Lau TY, Knipe DM, et al. Comparative efficacy and immunogenicity of replication-defective, recombinant glycoprotein, and DNA vaccines for herpes simplex virus 2 infections in mice and guinea pigs. J Virol. 2005 Jan;79(1):410–8. doi: 10.1128/JVI.79.1.410-418.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunningham AL, Mikloska Z. The Holy Grail: immune control of human herpes simplex virus infection and disease. Herpes. 2001 Mar;8( Suppl 1):6A–10A. [PubMed] [Google Scholar]
- 8.Belshe RB, Leone PA, Bernstein DI, Wald A, Levin MJ, Stapleton JT, et al. Efficacy Results of a Trial of a Herpes Simplex Vaccine. N Engl J Med. 2012 Jan 5;366(1):34–43. doi: 10.1056/NEJMoa1103151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cattamanchi A, Posavad CM, Wald A, Baine Y, Moses J, Higgins TJ, et al. Phase I study of a herpes simplex virus type 2 (HSV-2) DNA vaccine administered to healthy, HSV-2-seronegative adults by a needle-free injection system. Clin Vaccine Immunol. 2008 Nov;15(11):1638–43. doi: 10.1128/CVI.00167-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montgomery DL, Ulmer JB, Donnelly JJ, Liu MA. DNA vaccines. Pharmacol Ther. 1997;74(2):195–205. doi: 10.1016/s0163-7258(97)82003-7. [DOI] [PubMed] [Google Scholar]
- 11.Higgins TJ, Herold KM, Arnold RL, McElhiney SP, Shroff KE, Pachuk CJ. Plasmid DNA-expressed secreted and nonsecreted forms of herpes simplex virus glycoprotein D2 induce different types of immune responses. J Infect Dis. 2000 Nov;182(5):1311–20. doi: 10.1086/315879. [DOI] [PubMed] [Google Scholar]
- 12.Sasaki S, Inamura K, Okuda K. Genes that induce immunity--DNA vaccines. Microbiol Immunol. 1999;43(3):191–200. doi: 10.1111/j.1348-0421.1999.tb02393.x. [DOI] [PubMed] [Google Scholar]
- 13.Bourne N, Stanberry LR, Bernstein DI, Lew D. DNA immunization against experimental genital herpes simplex virus infection. J Infect Dis. 1996 Apr;173(4):800–7. doi: 10.1093/infdis/173.4.800. [DOI] [PubMed] [Google Scholar]
- 14.Hartikka J, Bozoukova V, Ferrari M, Sukhu L, Enas J, Sawdey M, et al. Vaxfectin enhances the humoral immune response to plasmid DNA-encoded antigens. Vaccine. 2001 Feb 28;19(15–16):1911–23. doi: 10.1016/s0264-410x(00)00445-x. [DOI] [PubMed] [Google Scholar]
- 15.Reyes L, Hartikka J, Bozoukova V, Sukhu L, Nishioka W, Singh G, et al. Vaxfectin enhances antigen specific antibody titers and maintains Th1 type immune responses to plasmid DNA immunization. Vaccine. 2001 Jun 14;19(27):3778–86. doi: 10.1016/s0264-410x(01)00090-1. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan SM, Doukas J, Hartikka J, Smith L, Rolland A. Vaxfectin: a versatile adjuvant for plasmid DNA- and protein-based vaccines. Expert Opin Drug Deliv. 2010 Dec;7(12):1433–46. doi: 10.1517/17425247.2010.538047. [DOI] [PubMed] [Google Scholar]
- 17.Shlapobersky M, Marshak JO, Dong L, Huang ML, Wei Q, Chu A, et al. Vaxfectin(R)-adjuvanted plasmid DNA vaccine improves protection and immunogenicity in a murine model of genital herpes infection. J Gen Virol. 2012 Mar 7; doi: 10.1099/vir.0.040055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muller WJ, Dong L, Vilalta A, Byrd B, Wilhelm KM, McClurkan CL, et al. Herpes simplex virus type 2 tegument proteins contain subdominant T-cell epitopes detectable in BALB/c mice after DNA immunization and infection. J Gen Virol. 2009 May;90(Pt 5):1153–63. doi: 10.1099/vir.0.008771-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koelle DM, Liu Z, McClurkan CL, Cevallos RC, Vieira J, Hosken NA, et al. Immunodominance among herpes simplex virus-specific CD8 T cells expressing a tissue-specific homing receptor. Proc Natl Acad Sci U S A. 2003 Oct 28;100(22):12899–904. doi: 10.1073/pnas.2131705100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bourne N, Bravo FJ, Francotte M, Bernstein DI, Myers MG, Slaoui M, et al. Herpes simplex virus (HSV) type 2 glycoprotein D subunit vaccines and protection against genital HSV-1 or HSV-2 disease in guinea pigs. J Infect Dis. 2003 Feb 15;187(4):542–9. doi: 10.1086/374002. [DOI] [PubMed] [Google Scholar]
- 21.Corey L, Huang ML, Selke S, Wald A. Differentiation of herpes simplex virus types 1 and 2 in clinical samples by a real-time taqman PCR assay. J Med Virol. 2005 Jul;76(3):350–5. doi: 10.1002/jmv.20365. [DOI] [PubMed] [Google Scholar]
- 22.Bourne N, Milligan GN, Stanberry LR, Stegall R, Pyles RB. Impact of immunization with glycoprotein D2/AS04 on herpes simplex virus type 2 shedding into the genital tract in guinea pigs that become infected. J Infect Dis. 2005 Dec 15;192(12):2117–23. doi: 10.1086/498247. [DOI] [PubMed] [Google Scholar]
- 23.Strasser JE, Arnold RL, Pachuk C, Higgins TJ, Bernstein DI. Herpes simplex virus DNA vaccine efficacy: effect of glycoprotein D plasmid constructs. J Infect Dis. 2000 Nov;182(5):1304–10. doi: 10.1086/315878. [DOI] [PubMed] [Google Scholar]
- 24.Hosken N, McGowan P, Meier A, Koelle DM, Sleath P, Wagener F, et al. Diversity of the CD8+ T-cell response to herpes simplex virus type 2 proteins among persons with genital herpes. J Virol. 2006 Jun;80(11):5509–15. doi: 10.1128/JVI.02659-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wagner MJ, Smiley JR. Herpes simplex virus requires VP11/12 to induce phosphorylation of the activation loop tyrosine (Y394) of the Src family kinase Lck in T lymphocytes. J Virol. 2009 Dec;83(23):12452–61. doi: 10.1128/JVI.01364-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stanberry LR, Myers MG, Stephanopoulos DE, Burke RL. Preinfection prophylaxis with herpes simplex virus glycoprotein immunogens: factors influencing efficacy. J Gen Virol. 1989 Dec;70( Pt 12):3177–85. doi: 10.1099/0022-1317-70-12-3177. [DOI] [PubMed] [Google Scholar]
- 27.Stanberry LR, Bernstein DI, Kit S, Myers MG. Genital reinfection after recovery from initial genital infection with herpes simplex virus type 2 in guinea pigs. J Infect Dis. 1986 Jun;153(6):1055–61. doi: 10.1093/infdis/153.6.1055. [DOI] [PubMed] [Google Scholar]
- 28.Burke RL, Goldbeck C, Ng P, Stanberry L, Ott G, Van Nest G. The influence of adjuvant on the therapeutic efficacy of a recombinant genital herpes vaccine. J Infect Dis. 1994 Nov;170(5):1110–9. doi: 10.1093/infdis/170.5.1110. [DOI] [PubMed] [Google Scholar]
- 29.Myers MG, Bernstein DI, Harrison CJ, Stanberry LR. Herpes simplex virus glycoprotein treatment of recurrent genital herpes reduces cervicovaginal virus shedding in guinea pigs. Antiviral Res. 1988 Nov;10(1–3):83–8. doi: 10.1016/0166-3542(88)90016-2. [DOI] [PubMed] [Google Scholar]
- 30.Straus SE, Corey L, Burke RL, Savarese B, Barnum G, Krause PR, et al. Placebo-controlled trial of vaccination with recombinant glycoprotein D of herpes simplex virus type 2 for immunotherapy of genital herpes. Lancet. 1994 Jun 11;343(8911):1460–3. doi: 10.1016/s0140-6736(94)92581-x. [DOI] [PubMed] [Google Scholar]
- 31.Straus SE, Wald A, Kost RG, McKenzie R, Langenberg AG, Hohman P, et al. Immunotherapy of recurrent genital herpes with recombinant herpes simplex virus type 2 glycoproteins D and B: results of a placebo-controlled vaccine trial. J Infect Dis. 1997 Nov;176(5):1129–34. doi: 10.1086/514103. [DOI] [PubMed] [Google Scholar]
- 32.Wald A. Herpes simplex virus type 2 transmission: risk factors and virus shedding. Herpes. 2004 Aug;11( Suppl 3):130A–7A. [PubMed] [Google Scholar]
- 33.Alsallaq RA, Schiffer JT, Longini IM, Jr, Wald A, Corey L, Abu-Raddad LJ. Population level impact of an imperfect prophylactic vaccine for herpes simplex virus-2. Sex Transm Dis. 2010 May;37(5):290–7. doi: 10.1097/OLQ.0b013e3181d3d023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corey L, Wald A, Patel R, Sacks SL, Tyring SK, Warren T, et al. Once-daily valacyclovir to reduce the risk of transmission of genital herpes. N Engl J Med. 2004 Jan 1;350(1):11–20. doi: 10.1056/NEJMoa035144. [DOI] [PubMed] [Google Scholar]
- 35.Garnett GP, Dubin G, Slaoui M, Darcis T. The potential epidemiological impact of a genital herpes vaccine for women. Sex Transm Infect. 2004 Feb;80(1):24–9. doi: 10.1136/sti.2002.003848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koelle DM, Posavad CM, Barnum GR, Johnson ML, Frank JM, Corey L. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J Clin Invest. 1998 Apr 1;101(7):1500–8. doi: 10.1172/JCI1758. [DOI] [PMC free article] [PubMed] [Google Scholar]