Abstract
Air pollution exposure is associated with cardiovascular morbidity and mortality, yet the role of individual pollutants remains unclear. In particular, there is uncertainty regarding the acute effect of ozone exposure on cardiovascular disease. In these studies, we aimed to determine the effect of ozone exposure on vascular function, fibrinolysis, and the autonomic regulation of the heart. Thirty-six healthy men were exposed to ozone (300 ppb) and filtered air for 75min on two occasions in randomized double-blind crossover studies. Bilateral forearm blood flow (FBF) was measured using forearm venous occlusion plethysmography before and during intra-arterial infusions of vasodilators 2–4 and 6–8h after each exposure. Heart rhythm and heart rate variability (HRV) were monitored during and 24h after exposure. Compared with filtered air, ozone exposure did not alter heart rate, blood pressure, or resting FBF at either 2 or 6h. There was a dose-dependent increase in FBF with all vasodilators that was similar after both exposures at 2–4h. Ozone exposure did not impair vasomotor or fibrinolytic function at 6–8h but rather increased vasodilatation to acetylcholine (p = .015) and sodium nitroprusside (p = .005). Ozone did not affect measures of HRV during or after the exposure. Our findings do not support a direct rapid effect of ozone on vascular function or cardiac autonomic control although we cannot exclude an effect of chronic exposure or an interaction between ozone and alternative air pollutants that may be responsible for the adverse cardiovascular health effects attributed to ozone.
Key Words: air pollution, blood flow, endothelium, fibrinolysis, oxidative stress, ozone.
Exposure to air pollution is strongly associated with respiratory and cardiovascular morbidity and mortality. These associations are well established for exposure to fine particulate matter (PM) air pollution primarily from the combustion of fossil fuels (Brunekreef and Holgate, 2002; Miller et al., 2007).
Ozone (O3) is a strongly oxidative secondary air pollutant, and epidemiological studies have shown that ozone levels also correlate with respiratory and cardiovascular morbidity and mortality (Bell et al., 2004, 2007; Brook et al., 2010; Ito et al., 2005; Peters et al., 2004). The effects of ozone on health have been difficult to disentangle from the effects of PM in observational studies as these pollutants are often highly correlated. The difficulty in resolving the independent effects of ozone on cardiovascular mortality is illustrated by the divergent results published from the American Cancer Society Cancer Prevention Study cohort (Jerrett et al., 2009; Smith et al., 2009). Although Smith et al. (2009) report an independent association between ozone and cardiovascular death, Jerrett et al. (2009) found that ozone was associated only with respiratory mortality after adjustment for PM2.5. These discordant findings are not necessarily contradictory but may reflect differences in the analyses performed. Although a number of reports suggest an independent effect of ozone on mortality (de Almeida et al., 2011; Gryparis et al., 2004; Halonen et al., 2010; Ren et al., 2008; Stafoggia et al., 2010), acute myocardial infarction (Halonen et al., 2010; Ruidavets et al., 2005), and cardiac arrest (Ensor et al., 2013), it remains unclear how ozone exposure may trigger acute nonfatal and fatal cardiovascular events.
Human chamber studies have defined the effects of short-term exposure to ozone on the respiratory tract indicating an airway inflammatory response along with an alteration in antioxidant defences (Behndig et al., 2009; Blomberg et al., 1999; Frampton et al., 1999; Stenfors et al., 2002). The evidence for a direct cardiovascular effect of ozone, however, is more limited. Exposure to ozone in combination with concentrated ambient particles increases peripheral arterial tone and blood pressure (Brook et al., 2002; Urch et al., 2005) although subsequent studies demonstrate that this may be an effect of ambient particles rather than ozone (Brook et al., 2009), and indeed ozone has been shown to reduce blood pressure in a diabetic population at the same time as particulate air pollution increased blood pressure (Hoffmann et al., 2012). Ozone exposure may influence autonomic regulation of the heart rate and has been shown to alter circulating inflammatory and fibrinolytic markers in healthy persons (Chuang et al., 2007; Devlin et al., 2012).
Exposure to dilute diesel exhaust has been consistently associated with important effects on vascular function, systemic hemodynamics, fibrinolysis, and thrombosis (Cosselman et al., 2012; Lucking et al., 2008; Mills et al., 2005, 2007; Törnqvist et al., 2007). Furthermore, these adverse effects can be abolished by filtration to remove ultrafine particles, suggesting that gaseous copollutants are not primarily responsible for the cardiovascular effects of air pollution (Lucking et al., 2011; Mills et al., 2011). The effect of exposure to ozone in isolation on microvascular endothelial and fibrinolytic function has not been explored.
We hypothesize that short-term exposure to ozone would cause acute microvasomotor and fibrinolytic dysfunction in healthy men.
MATERIALS AND METHODS
Subjects.
Thirty-six healthy, nonsmoking male volunteers of mean age 26 (range: 21–31) years with normal lung function were recruited (Table 1). All subjects reported no ongoing medical conditions, took no regular medication (prescribed or over the counter), and were free of the symptoms of respiratory tract infection for at least 6 weeks prior to enrollment. All subjects had a normal baseline 12-lead electrocardiogram (ECG). The local ethical review board approved the study, and written informed consent was obtained. The study was performed in accordance with Declaration of Helsinki.
TABLE 1.
Baseline Characteristics of Healthy Volunteers
| 2–4h, n = 18 | 6–8h, n = 18 | |
|---|---|---|
| Age, years | 26±1 | 26±1 |
| Height, cm | 183±3 | 180±1 |
| Weight, kg | 77±3 | 79±2 |
| BMI, m2/kg | 23±1 | 24±1 |
| FVC, l | 5.7±0.2 | 5.8±0.2 |
| FEV1, l | 4.3±0.1 | 4.2±0.1 |
Note. Values are reported as mean ± SEM; BMI, body mass index; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s.
Study design.
All subjects were exposed to 300 ppb of ozone or filtered air in a whole-body exposure chamber of approximately 15 m3 for 75min on two occasions at least two weeks apart in a randomized double-blind crossover study (Supplementary data). During exposures, the subjects alternated moderate exercise on a bicycle ergometer to achieve an average minute ventilation of 20 l/min/m2 body surface area, with rest at 15-min intervals (starting with an exercise period).
Venous occlusion forearm plethysmography before and during intra-arterial infusion of endothelium-dependent and endothelium-independent vasodilators was performed 2–4h (n = 18) and 6–8h (n = 18) after all exposures as previously described (Lucking et al. 2011; Mills et al., 2005, 2007, 2011; Törnqvist et al., 2007). A 3-lead continuous ECG was recorded from each subject during the exposure and subsequent 24-h period using a Holter monitor (model 90217, Spacelabs Healthcare, UK).
Ozone exposure.
The exposures were performed in an exposure chamber described in detail elsewhere and in the Supplementary data (Blomberg et al., 1999).
Vascular studies.
Forearm blood flow (FBF) was determined during unilateral brachial artery infusion of endothelium-dependent and -independent vasodilators using venous occlusion plethysmography with mercury-in-silicone elastomer strain gauges, as described previously (Lucking et al. 2011; Mills et al., 2005, 2007, 2011; Törnqvist et al., 2007). Briefly, the brachial artery of one arm was cannulated using a 27-standard wire gauge steel needle, and 17 gauge venous cannulae were inserted in large veins in the antecubital fossa of both arms. Following a 30-min baseline saline infusion, acetylcholine at 5, 10, and 20 µg/min (endothelium-dependent vasodilator that does not release tissue plasminogen activator [t-PA]), bradykinin at 100, 300, and 1000 pmol/min (endothelium-dependent vasodilator that releases t-PA), and sodium nitroprusside (SNP) at 2, 4, and 8 µg/min (endothelium-independent vasodilator that does not release t-PA) were infused for 6min at each dose with FBF measured for the last 3min of each infusion. The infusions of the three vasodilators were given in a random order and separated by 20-min saline infusions. Due to its prolonged action, verapamil at 10, 30, and 100 µg/min (endothelium- and nitric oxide–independent vasodilator that does not release t-PA) was infused at the end of the study protocol. Blood pressure and heart rate were measured during the forearm study using a validated semiautomated oscillometric sphygmanometer.
Heart rate variability.
ECG recordings were analyzed by an experienced operator, blinded to exposure and the subject characteristics, using the Reynolds Medical Pathfinder Digital 700 Series Analysis System (Delmar Reynolds) (Supplementary data).
Peripheral blood analyses.
Peripheral blood was sampled before and 2 and 6h after each exposure for analyses of differential cell counts and fibrinolytic and inflammatory markers with samples obtained during the infusion of each dose of bradykinin for fibrinolytic markers.
Data analysis and statistics.
FBF, t-PA release, and measures of heart rate variability (HRV) were analyzed as previously (Lucking et al., 2011; Mills et al., 2005, 2007, 2011; Törnqvist et al., 2007) using two-way ANOVA with repeated measures or two-tailed Student’s t-tests, where appropriate, using GraphPad Prism (GraphPad Software, Version 4 for Macintosh) and SPSS (SPSS inc., Chicago, IL, version 15). Statistical significance was defined as p < .05.
RESULTS
There were no differences in heart rate, blood pressure, or resting FBF 2 or 6h after exposure to ozone compared with filtered air (Table 2). Systemic inflammatory mediators were not affected by exposure to ozone or filtered air (Supplementary table S1).
TABLE 2.
Hemodynamic Variables Before Forearm Vascular Study, 2 or 6h Postexposure to Ozone
| Air | Ozone | p Value | |
|---|---|---|---|
| 2–4h, n = 18 | |||
| Heart rate, bpm | 54±1 | 54±1 | .94 |
| Systolic blood pressure, mmHg | 129±2 | 130±2 | .64 |
| Diastolic blood pressure, mmHg | 67±2 | 67±2 | .35 |
| Infused FBF, ml/100ml tissue/min | 2.2±0.1 | 2.0±0.1 | .14 |
| Noninfused FBF, ml/100ml tissue/min | 1.9±0.1 | 1.8±0.1 | .51 |
| 6–8h, n = 18 | |||
| Heart rate, bpm | 63±2 | 62±2 | .30 |
| Systolic blood pressure, mmHg | 142±3 | 139±4 | .26 |
| Diastolic blood pressure, mmHg | 68±2 | 67±2 | .45 |
| Infused FBF, ml/100ml tissue/min | 3.2±0.3 | 3.4±0.4 | .55 |
| Noninfused FBF, ml/100ml tissue/min | 2.6±0.2 | 2.7±0.2 | .86 |
Note. Values are reported as mean ± SEM. p Values from Student’s paired t-tests.
Vascular Studies
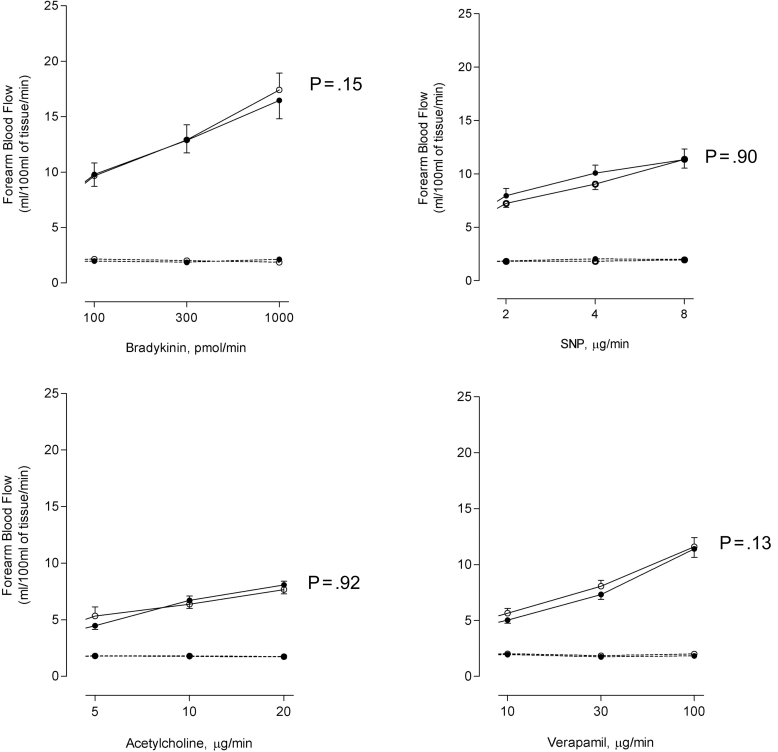
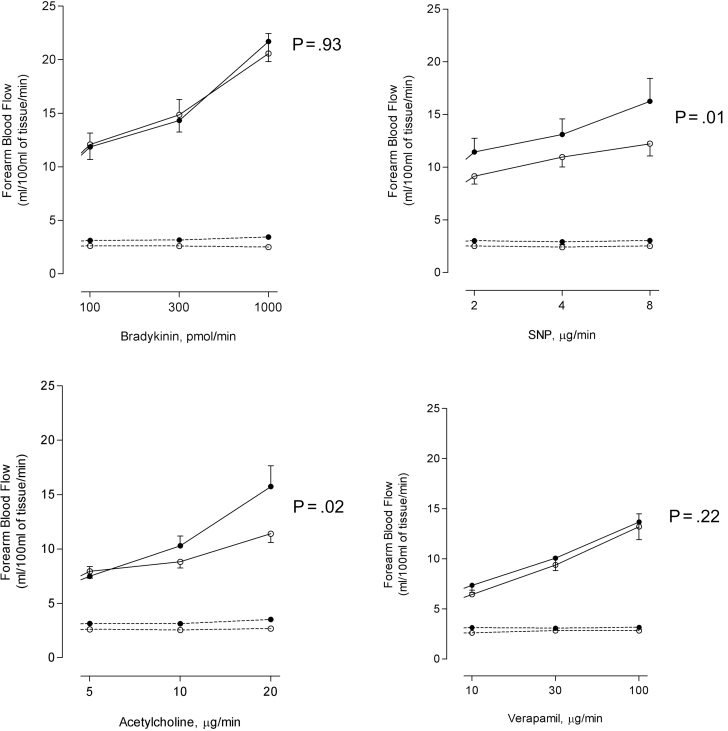
Endothelial-dependent and -independent vasodilators caused dose-dependent increases in FBF (p < .001). There were no significant differences in forearm arm blood flow 2–4h following exposure to ozone or filtered air (Fig. 1). At 6–8h, FBF response to acetylcholine and sodium nitroprusside was not impaired but rather increased after exposure to ozone compared with filtered air (p = .02 and p = .01, respectively). FBF responses to bradykinin and verapamil were similar after both exposures (p > .05) (Fig. 2).
Fig. 1.
FBF in subjects 2–4h after ozone (closed circles) and filtered air (open circles) exposure during unilateral intrabrachial infusion of bradykinin, acetylcholine, SNP, and verapamil. Infused arm (solid line); noninfused arm (dotted line).
Fig. 2.
FBF in subjects 6–8h after ozone (closed circles) and filtered air (open circles) exposure during unilateral intrabrachial infusion of bradykinin, acetylcholine, SNP, and verapamil. Infused arm (solid line); noninfused arm (dotted line).
There were no differences in plasma t-PA or plasminogen activator inhibitor-1 antigen concentrations at baseline, 2h, or 6h following exposure to ozone compared with filtered air (Supplementary table S2). The net release of t-PA antigen following bradykinin infusion was unaffected 6–8h after exposure to ozone compared with filtered air (Table 3).
TABLE 3.
Plasma t-PA Antigen Concentrations Following Ozone and Filtered Air Exposures
| 6–8 h | Air | Ozone | p Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Bradykinin, pmol/min | 0 | 100 | 300 | 1000 | 0 | 100 | 300 | 1000 | |
| t-PA antigen, ng/ml | |||||||||
| Infused arm | 4.5±0.3 | 6.3±0.6 | 8.7±0.8 | 13.7±1.3 | 5.0±1.6 | 6.0±1.8 | 8.1±0.9 | 11.7±1.3 | .16 |
| Noninfused arm | 5.0±0.4 | 4.5±0.4 | 4.9±0.4 | 6.1±0.4 | 5.2±0.6 | 4.8±0.4 | 4.8±0.4 | 6.1±0.5 | .96 |
| Net t-PA release, ng/100ml of tissue/min | −1.4±0.5 | 12.1±2.9 | 34.7±5.1 | 104.1±14.4 | −0.2±1.1 | 8.2±3.0 | 31.1±5.0 | 79.6±17.4 | .31 |
Note. Values are reported as mean ± SEM; two-way ANOVA with repeat measures.
HRV
Subjects did not experience any significant rhythm disturbance during the 24-h study period although subjects’ heart rate was generally higher through the ozone exposure day (at baseline and through the study) (Table 4). Exposure to ozone did not affect time or frequency domain measures of HRV over the 24-h period (Supplementary table S3). Measures of HRV averaged over a 5-min interval immediately prior to exposure were similar and were not altered at 2 and 6h following exposure to ozone or filtered air (Table 4).
TABLE 4.
HRV at Baseline, 2h, and 6h Following Exposure to Ozone or Filtered Air
| Air | Ozone | Two-way ANOVA | |||||
|---|---|---|---|---|---|---|---|
| Baseline | 2 h | 6 h | Baseline | 2 h | 6 h | p Value | |
| Heart rate, bpm | 69±2 | 65±2 | 65±2 | 72±2 | 70±3 | 66±2 | .04 |
| SDNN, ms | 91±8 | 79±5 | 67±4 | 90±9 | 76±6 | 65±5 | .60 |
| RMSSD, ms | 54±5 | 58±5 | 48±4 | 52±6 | 49±5 | 48±5 | .20 |
| PNN50, % | 27±4 | 33±4 | 26±4 | 22±4 | 28±5 | 27±4 | .18 |
| Triangular Ix | 19±1 | 17±1 | 14±1 | 17±1 | 17±1 | 15±1 | .36 |
| HFn, ms | 22±2 | 29±3 | 28±3 | 13±2 | 24±3 | 31±3 | .07 |
| LFn, ms | 76±3 | 68±3 | 70±3 | 86±2 | 72±3 | 66±3 | .10 |
| HF/LF ratio | 0.3±0.0 | 0.5±0.1 | 0.4±0.1 | 0.2±0.0 | 0.4±0.0 | 0.5±0.1 | .86 |
Note. All variables are presented as mean ± SEM; two-way ANOVA filtered air versus ozone for effect of exposure; SDNN, standard deviation of successive RR intervals; RMSSD, root mean square difference of successive RR intervals; HFn, high-frequency power component of HRV expressed in normalized units; LFn, low-frequency power component of HRV expressed in normalized units; HF/LF, ratio of high frequency to low frequency components of HRV; PNN50, percentage of successive RR intervals that differ by > 50ms.
DISCUSSION
Brief exposure to ozone at a high ambient concentration does not impair vascular vasomotor or fibrinolytic function or affect HRV in young healthy subjects. Ozone exposure does not have a direct and rapid effect on the cardiovascular system, and although we cannot exclude an effect of chronic exposure, an interaction of ozone and other air pollutants increasing the toxicity of these pollutants, or an effect in more susceptible patient groups, we suggest that the association between ozone and acute cardiovascular morbidity and mortality are due to other atmospheric air pollutants rather than exposure to ozone per se.
Although ozone is an irritant gas capable of inducing oxidative stress and inflammation in the airways (Behndig et al., 2009; Blomberg et al., 1999; Frampton et al., 1999; Stenfors et al., 2002), the evidence for cardiovascular effects is less clear. Ozone rapidly reacts with low molecular weight antioxidants in the lower respiratory tract lining fluid (Behndig et al., 2009; Mudway and Kelly, 2000) and is therefore unlikely to penetrate the airway epithelium or directly cause systemic oxidant and inflammatory effects. Consistent with this we did not observe any change in circulating blood cells, inflammatory cytokines, or markers of platelet and endothelial activation. Devlin et al. (2012) found a marked fivefold increase in airway neutrophils following exposure to ozone at the same concentration consistent with a localized effect in the airways and reported a transient increase in systemic concentrations of interleukin-8 1h following exposure, consistent with our own findings there were no effects on circulating interleukin-6, tumor necrosis factor, or white blood cells.
Although ozone cannot penetrate the pulmonary epithelium, it may activate receptors in the airways that can trigger an autonomic reflex to influence regulation of heart rate and vascular tone (Lai and Kou, 1998). In murine models, pulmonary C-fibers are activated by ozone, thus decreasing heart rate and cardiac output while resulting in bronchial vasodilatation (Lee and Pisarri, 2001; Taylor-Clark and Undem, 2010). Indeed, a change in cardiac autonomic control is one of the central mechanistic pathways that have been proposed to explain the cardiovascular effects of inhaled air pollutants (Brook et al., 2010). Reduced HRV reflects either an increase in sympathetic drive and/or a reduction in parasympathetic tone and has been associated with increased risk of cardiovascular morbidity and mortality (Tsuji et al., 1996). Chuang et al. (2007) found a decrease in standard deviation of successive RR intervals, root mean square difference of successive RR intervals, low-frequency power component of HRV, and high-frequency power component of HRV (HF) associated with increased ambient ozone concentrations averaged over 1–3 days in healthy students residing in a heavily polluted urban area in Taiwan. Of note, the PM10 concentrations during the study period were in excess of the World Health Organization’s limits of 50 µg/m3, which may be a confounding factor. There have been two recent controlled exposure studies to evaluate the effect of ozone on HRV (Brook et al., 2009; Devlin et al., 2012). Devlin et al. (2012) found no effect of exposure to 300 ppb of ozone on any measure of HRV although they report a 51% reduction in the 1h postexposure/pre-exposure HF power ratio of uncertain significance. Brook et al. (2009) similarly found no effect of exposure to ozone either in isolation or in combination with concentrated ambient particles on HRV. We found no effect of exposure to ozone on any measure of HRV and suggest that any effect of ozone beyond the airways is not mediated by autonomic dysfunction.
It remains possible that ozone may indirectly alter vascular function through the generation of secondary oxidized lipoproteins in the lung that are able to enter the systemic circulation (Mudway and Kelly, 2000). We assessed vascular function in the forearm using a sensitive and repeatable measure of vasomotor and fibrinolytic function. In contrast to previous studies evaluating the effects of dilute diesel exhaust on vascular function (Mills et al., 2005, 2007; Törnqvist et al., 2007), we found no impairment in vasodilatation to either endothelial-dependent or -independent vasodilators following exposure to ozone in two separate studies. Nor did ozone affect other important aspects of vascular function with the stimulated release of t-PA antigen from the vascular endothelium unchanged following exposure. Although it is possible that we have missed an important effect of ozone beyond 8h (and we acknowledge the findings of Devlin et al., 2012 who demonstrated small late increases in plasma IL-1 and CRP concentrations 24h after exposure to ozone), this seems unlikely given the rapid onset of most oxidation processes. If anything, there was an apparent increase in vasodilatation in response to acetylcholine and sodium nitroprusside following ozone, perhaps suggesting enhanced nitric oxide bioavailability in the vasculature. Experimental studies have suggested that ip oxygen/ozone can upregulate endothelial nitric oxide synthase, reduce nitrotyrosine concentrations, and prevent ischaemic injury in an ischaemia-reperfusion model (Di Fillipo et al., 2008, 2010), adding plausibility to our observation. Previous studies have demonstrated that exposure to ozone and concentrated ambient particles together caused arterial vasoconstriction and increased blood pressure (Brook et al., 2002; Urch et al., 2005); however, subsequent studies have established that these effects are mediated by exposure to ambient particles with no effects on arterial tone observed following exposure to ozone in isolation (Brook et al., 2009).
Even though epidemiological studies have suggested an association between exposure to ozone and cardiovascular morbidity and mortality (Bell et al., 2004, 2007; Ito et al., 2005; Katsouyanni et al., 1993; Levy et al., 2005), this relationship is complex and difficult to disentangle from the effects of other major air pollutants (Bhaskaran et al., 2009). Indeed, ozone has a complex relationship with PM air pollution, and in some previous analyses, the effect of ozone on cardiovascular mortality is no longer apparent following adjustment for PM2.5 concentration (Smith et al., 2009). There may also be a seasonal bias in some observational studies as ozone and PM are positively correlated in the warm summer months but have a negative association in the cold winter months (Langrish et al., 2010). Furthermore, ozone can react with other pollutants, and therefore, increased levels may reflect changes in the concentrations of other pollutants.
There are some important limitations to this study. Our study population included only healthy men, and an effect of ozone on vascular function in susceptible populations with cardiovascular disease cannot be excluded. Furthermore, we cannot exclude an effect of chronic exposure to high levels of ozone. We have investigated the effects of ozone exposure in isolation and cannot exclude the possibility that ozone may have important adverse health effects by interacting with and potentially enhancing the toxicity of other ambient air pollutants (Bosson et al., 2008). Further research to evaluate the effect of ozone and diesel exhaust particles on the cardiovascular system is warranted.
CONCLUSIONS
This study indicates that short-term exposure to ozone does not cause acute impairment in vascular function or alter the autonomic regulation of the heart in healthy men. Our findings do not support the view that ozone in isolation is an important determinant of cardiovascular health. Although we cannot exclude an effect of chronic exposure or an interaction between ozone and other air pollutants, we suggest that alternative pollutants in the ambient mixture may be responsible for the adverse cardiovascular health effects attributed to ozone in some epidemiologic studies.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
Swedish Heart Lung Foundation; Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (FORMAS); The Västerbotten County Counsel, Sweden; The Swedish National Air Pollution Programme; British Heart Foundation (BHF) Clinical Research Scholarship (SS/CH/09/002); BHF Intermediate Fellowship (FS/10/024/28266); BHF Chair Award (CH/09/002) and Programme Grant (RG/10/9/28286).
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank research nurses Annika Johansson and Frida Holmström, Department of Medicine, Division of Respiratory Medicine and Allergy, University Hospital, Umeå, and laboratory staff Jamshid Pourazar and Ann-Britt Lundström, Department of Public Health and Clinical Medicine, Division of Medicine, Umeå University, Umeå, Sweden. All authors declare they have no financial interests to declare.
REFERENCES
- Behndig A. F., Blomberg A., Helleday R., Duggan S. T., Kelly F. J., Mudway I. S. (2009). Antioxidant responses to acute ozone challenge in the healthy human airway. Inhal. Toxicol. 21, 933–942 [DOI] [PubMed] [Google Scholar]
- Bell M. L., Kim J. Y., Dominici F. (2007). Potential confounding of particulate matter on the short-term association between ozone and mortality in multisite time-series studies. Environ. Health Perspect. 115, 1591–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell M. L., McDermott A., Zeger S. L., Samet J. M., Dominici F. (2004). Ozone and short-term mortality in 95 US urban communities, 1987-2000. JAMA 292, 2372–2378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskaran K., Hajat S., Haines A., Herrett E., Wilkinson P., Smeeth L. (2009). Effects of air pollution on the incidence of myocardial infarction. Heart 95, 1746–1759 [DOI] [PubMed] [Google Scholar]
- Blomberg A., Mudway I. S., Nordenhäll C., Hedenström H., Kelly F. J., Frew A. J., Holgate S. T., Sandström T. (1999). Ozone-induced lung function decrements do not correlate with early airway inflammatory or antioxidant responses. Eur. Respir. J. 13, 1418–1428 [DOI] [PubMed] [Google Scholar]
- Bosson J., Barath S., Pourazar J., Behndig A. F., Sandström T., Blomberg A., Adelroth E. (2008). Diesel exhaust exposure enhances the ozone-induced airway inflammation in healthy humans. Eur. Respir. J. 31, 1234–1240 [DOI] [PubMed] [Google Scholar]
- Brook R. D., Brook J. R., Urch B., Vincent R., Rajagopalan S., Silverman F. (2002). Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults. Circulation 105, 1534–1536 [DOI] [PubMed] [Google Scholar]
- Brook R. D., Rajagopalan S., Pope C. A., 3rd, Brook J. R., Bhatnagar A., Diez-Roux A. V., Holguin F., Hong Y., Luepker R. V., Mittleman M. A., et al. ; American Heart Association Council on Epidemiology and Prevention, Council on the Kidney in Cardiovascular Disease, and Council on Nutrition, Physical Activity and Metabolism (2010). Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 121, 2331–2378 [DOI] [PubMed] [Google Scholar]
- Brook R. D., Urch B., Dvonch J. T., Bard R. L., Speck M., Keeler G., Morishita M., Marsik F. J., Kamal A. S., Kaciroti N., et al. (2009). Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertension 54, 659–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunekreef B., Holgate S. T. (2002). Air pollution and health. Lancet 360, 1233–1242 [DOI] [PubMed] [Google Scholar]
- Chuang K. J., Chan C. C., Su T. C., Lee C. T., Tang C. S. (2007). The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. Am. J. Respir. Crit. Care Med. 176, 370–376 [DOI] [PubMed] [Google Scholar]
- Cosselman K. E., Krishnan R. M., Oron A. P., Jansen K., Peretz A., Sullivan J. H., Larson T. V., Kaufman J. D. (2012). Blood pressure response to controlled diesel exhaust exposure in human subjects. Hypertension 59, 943–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida S. P., Casimiro E., Calheiros J. (2011). Short-term association between exposure to ozone and mortality in Oporto, Portugal. Environ. Res. 111, 406–410 [DOI] [PubMed] [Google Scholar]
- Devlin R. B., Duncan K. E., Jardim M., Schmitt M. T., Rappold A. G., Diaz-Sanchez D. (2012). Controlled exposure of healthy young volunteers to ozone causes cardiovascular effects. Circulation 126, 104–111 [DOI] [PubMed] [Google Scholar]
- Di Filippo C., Luongo M., Marfella R., Ferraraccio F., Lettieri B., Capuano A., Rossi F., D’Amico M. (2010). Oxygen/ozone protects the heart from acute myocardial infarction through local increase of eNOS activity and endothelial progenitor cells recruitment. Naunyn Schmiedebergs Arch. Pharmacol. 382, 287–291 [DOI] [PubMed] [Google Scholar]
- Di Filippo C., Marfella R., Capodanno P., Ferraraccio F., Coppola L., Luongo M., Mascolo L., Luongo C., Capuano A., Rossi F., et al. (2008). Acute oxygen-ozone administration to rats protects the heart from ischemia reperfusion infarct. Inflamm. Res. 57, 445–449 [DOI] [PubMed] [Google Scholar]
- Ensor K. B., Raun L. H., Persse D. (2013). A case-crossover analysis of out-of-hospital cardiac arrest and air pollution. Circulation 127, 1192–1199 [DOI] [PubMed] [Google Scholar]
- Frampton M. W., Pryor W. A., Cueto R., Cox C., Morrow P. E., Utell M. J. (1999). Ozone exposure increases aldehydes in epithelial lining fluid in human lung. Am. J. Respir. Crit. Care Med. 159(4 Pt 1), 1134–1137 [DOI] [PubMed] [Google Scholar]
- Gryparis A., Forsberg B., Katsouyanni K., Analitis A., Touloumi G., Schwartz J., Samoli E., Medina S., Anderson H. R., Niciu E. M., et al. (2004). Acute effects of ozone on mortality from the “air pollution and health: A European approach” project. Am. J. Respir. Crit. Care Med. 170, 1080–1087 [DOI] [PubMed] [Google Scholar]
- Halonen J. I., Lanki T., Tiittanen P., Niemi J. V., Loh M., Pekkanen J. (2010). Ozone and cause-specific cardiorespiratory morbidity and mortality. J. Epidemiol. Community Health 64, 814–820 [DOI] [PubMed] [Google Scholar]
- Hoffmann B., Luttmann-Gibson H., Cohen A., Zanobetti A., de Souza C., Foley C., Suh H. H., Coull B. A., Schwartz J., Mittleman M., et al. (2012). Opposing effects of particle pollution, ozone, and ambient temperature on arterial blood pressure. Environ. Health Perspect. 120, 241–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., De Leon S. F., Lippmann M. (2005). Associations between ozone and daily mortality: Analysis and meta-analysis. Epidemiology 16, 446–457 [DOI] [PubMed] [Google Scholar]
- Jerrett M., Burnett R. T., Pope C. A., 3rd, Ito K., Thurston G., Krewski D., Shi Y., Calle E., Thun M. (2009). Long-term ozone exposure and mortality. N. Engl. J. Med. 360, 1085–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsouyanni K., Pantazopoulou A., Touloumi G., Tselepidaki I., Moustris K., Asimakopoulos D., Poulopoulou G., Trichopoulos D. (1993). Evidence for interaction between air pollution and high temperature in the causation of excess mortality. Arch. Environ. Health 48, 235–242 [DOI] [PubMed] [Google Scholar]
- Lai C. J., Kou Y. R. (1998). Stimulation of vagal pulmonary C fibers by inhaled wood smoke in rats. J. Appl. Physiol. 84, 30–36 [DOI] [PubMed] [Google Scholar]
- Langrish J. P., Mills N. L., Donaldson K., Newby D. E. (2010). Response to Peter Joseph. Heart 96, 472–47320299417 [Google Scholar]
- Lee L. Y., Pisarri T. E. (2001). Afferent properties and reflex functions of bronchopulmonary C-fibers. Respir. Physiol. 125, 47–65 [DOI] [PubMed] [Google Scholar]
- Levy J. I., Chemerynski S. M., Sarnat J. A. (2005). Ozone exposure and mortality: An empiric bayes metaregression analysis. Epidemiology 16, 458–468 [DOI] [PubMed] [Google Scholar]
- Lucking A. J., Lundbäck M., Barath S. L., Mills N. L., Sidhu M. K., Langrish J. P., Boon N. A., Pourazar J., Badimon J. J., Gerlofs-Nijland M. E., et al. (2011). Particle traps prevent adverse vascular and prothrombotic effects of diesel engine exhaust inhalation in men. Circulation 123, 1721–1728 [DOI] [PubMed] [Google Scholar]
- Lucking A. J., Lundback M., Mills N. L., Faratian D., Barath S. L., Pourazar J., Cassee F. R., Donaldson K., Boon N. A., Badimon J. J., et al. (2008). Diesel exhaust inhalation increases thrombus formation in man. Eur. Heart J. 29, 3043–3051 [DOI] [PubMed] [Google Scholar]
- Miller K. A., Siscovick D. S., Sheppard L., Shepherd K., Sullivan J. H., Anderson G. L., Kaufman J. D. (2007). Long-term exposure to air pollution and incidence of cardiovascular events in women. N. Engl. J. Med. 356, 447–458 [DOI] [PubMed] [Google Scholar]
- Mills N. L., Miller M. R., Lucking A. J., Beveridge J., Flint L., Boere A. J., Fokkens P. H., Boon N. A., Sandstrom T., Blomberg A., et al. (2011). Combustion-derived nanoparticulate induces the adverse vascular effects of diesel exhaust inhalation. Eur. Heart J. 32, 2660–2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills N. L., Törnqvist H., Gonzalez M. C., Vink E., Robinson S. D., Söderberg S., Boon N. A., Donaldson K., Sandström T., Blomberg A., et al. (2007). Ischemic and thrombotic effects of dilute diesel-exhaust inhalation in men with coronary heart disease. N. Engl. J. Med. 357, 1075–1082 [DOI] [PubMed] [Google Scholar]
- Mills N. L., Törnqvist H., Robinson S. D., Gonzalez M., Darnley K., MacNee W., Boon N. A., Donaldson K., Blomberg A., Sandstrom T., et al. (2005). Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation 112, 3930–3936 [DOI] [PubMed] [Google Scholar]
- Mudway I. S., Kelly F. J. (2000). Ozone and the lung: A sensitive issue. Mol. Aspects Med. 21, 1–48 [DOI] [PubMed] [Google Scholar]
- Peters A. von Klot S. Heier M. Trentinaglia I. Hörmann A. Wichmann H. E. Löwel H.; Cooperative Health Research in the Region of Augsburg Study Group (2004). Exposure to traffic and the onset of myocardial infarction. N. Engl. J. Med. 351, 1721–1730 [DOI] [PubMed] [Google Scholar]
- Ren C., Williams G. M., Morawska L., Mengersen K., Tong S. (2008). Ozone modifies associations between temperature and cardiovascular mortality: Analysis of the NMMAPS data. Occup. Environ. Med. 65, 255–260 [DOI] [PubMed] [Google Scholar]
- Ruidavets J. B., Cournot M., Cassadou S., Giroux M., Meybeck M., Ferrières J. (2005). Ozone air pollution is associated with acute myocardial infarction. Circulation 111, 563–569 [DOI] [PubMed] [Google Scholar]
- Smith K. R., Jerrett M., Anderson H. R., Burnett R. T., Stone V., Derwent R., Atkinson R. W., Cohen A., Shonkoff S. B., Krewski D., et al. (2009). Public health benefits of strategies to reduce greenhouse-gas emissions: Health implications of short-lived greenhouse pollutants. Lancet 374, 2091–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafoggia M., Forastiere F., Faustini A., Biggeri A., Bisanti L., Cadum E., Cernigliaro A., Mallone S., Pandolfi P., Serinelli M., et al. ; EpiAir Group (2010). Susceptibility factors to ozone-related mortality: A population-based case-crossover analysis. Am. J. Respir. Crit. Care Med. 182, 376–384 [DOI] [PubMed] [Google Scholar]
- Stenfors N., Pourazar J., Blomberg A., Krishna M. T., Mudway I., Helleday R., Kelly F. J., Frew A. J., Sandström T. (2002). Effect of ozone on bronchial mucosal inflammation in asthmatic and healthy subjects. Respir. Med. 96, 352–358 [DOI] [PubMed] [Google Scholar]
- Taylor-Clark T. E., Undem B. J. (2010). Ozone activates airway nerves via the selective stimulation of TRPA1 ion channels. J. Physiol. (Lond.) 588(Pt 3), 423–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Törnqvist H., Mills N. L., Gonzalez M., Miller M. R., Robinson S. D., Megson I. L., Macnee W., Donaldson K., Söderberg S., Newby D. E., et al. (2007). Persistent endothelial dysfunction in humans after diesel exhaust inhalation. Am. J. Respir. Crit. Care Med. 176, 395–400 [DOI] [PubMed] [Google Scholar]
- Tsuji H., Larson M. G., Venditti F. J., Jr, Manders E. S., Evans J. C., Feldman C. L., Levy D. (1996). Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation 94, 2850–2855 [DOI] [PubMed] [Google Scholar]
- Urch B., Silverman F., Corey P., Brook J. R., Lukic K. Z., Rajagopalan S., Brook R. D. (2005). Acute blood pressure responses in healthy adults during controlled air pollution exposures. Environ. Health Perspect. 113, 1052–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.