Abstract
Many low molecular weight compounds undergo biotransformation to chemically reactive metabolites (CRMs) that covalently modify cellular proteins. However, the mechanisms by which this covalent binding leads to cytotoxicity are not understood. Prior analyses of lists of target proteins sorted by functional categories or hit frequency have not proven informative. In an attempt to move beyond covalent binding, we hypothesized that xenobiotic posttranslational modification of proteins might disrupt important protein-protein interactions (PPIs) and thereby direct cells from homeostasis into cell death pathways. To test this hypothesis, we analyzed a list of 302 proteins (66% rat, 26% mouse, 5% human) known to be targeted by 41 different cytotoxic CRMs. Human orthologs of rodent proteins were found by blast sequence alignment, and their interacting partners were found using the Human Protein Reference Database. The combined set of target orthologs and partners was sorted into KEGG pathways and Gene Ontology categories. Those most highly ranked based on sorting statistics and toxicological relevance were heavily involved with intracellular signaling pathways, protein folding, unfolded protein response, and regulation of apoptosis. Detailed examination revealed that many of the categories were flagged primarily by partner proteins rather than target proteins and that a majority of these partners interacted with just a small number of proteins in the CRM target set. A similar analysis performed without the partner proteins flagged very few categories as significant. These results support the hypothesis that disruption of important PPIs may be a major mechanism contributing to CRM-induced acute cytotoxicity.
Key Words: reactive metabolites, protein covalent binding, protein-protein interaction, cytotoxicity.
Posttranslational modification (PTM) is a well-known mechanism for regulating the activity of cellular proteins and the metabolic and signaling pathways they comprise (Krishna and Wold, 1998; Reinders and Sickmann, 2007). In contrast, xenobiotic PTMs stemming from reactive chemicals or chemically reactive metabolites (CRMs) are frequently associated with toxic consequences for the cell (Evans et al., 2004; Liebler, 2006; Liebler and Guengerich, 2005; Park et al., 2005). The enzymes that convert relatively innocuous chemicals into CRMs, and the covalent modification of cellular proteins by the latter, have been studied intensively for more than 40 years (Hanzlik et al., 2009; Park et al., 2011; Snyder, 2011). Early interest focused primarily on the small molecule precursors, the enzymes that bioactivated them, and the structures and reactivity of the CRMs formed. Later, improvements in protein separation technology facilitated the isolation and identification of individual CRM target proteins. Initially, the quest to identify target protein identification was driven by the hope that knowing these targets would provide mechanistic understanding of CRM cytotoxicity. Ironically, many of the early identified targets were enzymes (e.g., aldehyde dehydrogenase, cytochromes P450 2B1, 2C11, and 2E1, liver carboxylesterase, dipeptidyl peptidase IV, alpha-ketoglutarate dehydrogenase, and others), but none appeared to be critical for cell survival in the context of acute cytotoxicity (Hanzlik et al., 2009).
As increasing numbers of target proteins became known, it was necessary to take a broader view and not look just for a few potentially “critical” targets. For example, grouping targets according to their function, as shown in Table 1 for 62 targets of thiobenzamide metabolites (Ikehata et al., 2008), showed that some categories were more highly populated than others, but the categories were too broad to reveal mechanisms or even suggest plausible mechanistic hypotheses. Another approach to identifying potentially important target proteins is to rank them according to how frequently they are reported as targets of various CRMs. Such a compilation is listed in Table 2. This listing again contains some enzymes but also a number of structural proteins (e.g., albumin, hemoglobin, actin, tropomyosin, and tubulin) that would not immediately appear to be important for direct acute cytotoxicity. Finally, bioinformatic approaches such as Ingenuity Pathway Analysis (Druckova et al., 2007) and similar ad hoc approaches based on sorting target proteins according to KEGG pathways or the Gene Ontology (GO) classification schemes (Fang et al., 2009) have also been tried, but these approaches too have not been particularly helpful in illuminating mechanisms of toxicity or deciding which target proteins may be more important than others. Thus, the hope of understanding mechanisms of cytotoxicity by analyzing target proteins remains unfulfilled.
Table 1.
Functional Classification of Cytosolic and Microsomal Protein Targets of Thiobenzamide Metabolites
| Functional class | No. of cyta | No. of mica |
|---|---|---|
| Binding/Carrier proteins | 6 | 7 |
| Cytoskeleton/Structural proteins | 1 | 1 |
| Receptors/Signal transduction | 0 | 1 |
| Intermediary metabolism | 18 | 0 |
| Xenobiotic metabolism | 2 | 3 |
| Redox regulation | 5 | 2 |
| Protein folding, heat shock, and stress response | 1 | 10 |
| Protein degradation | 2 | 0 |
| Nucleic acid metabolism | 3 | 0 |
| Other | 1 | 1 |
Note.aNumber of cytosolic (cyt) and microsomal (mic) target proteins found in each functional class. The data are compiled from a listing by Ikehata et al. (2008).
Table 2.
Reactive Metabolite Target Proteins Ranked by Number of CRMs That Target thema
| Entry | No. of hits | Target protein name(s) (rat, mouse) |
|---|---|---|
| 1 | 12 | Protein disulfide isomerase, PDI, Erp59 |
| 2 | 12 | Protein disulfide isomerase, PDI A3, ER-60 |
| 3 | 9 | Heat shock 60 KD protein 1 (chaperonin), HSP-60 |
| 4 | 9 | Albumin |
| 5 | 8 | Selenium-binding protein 2, 56kDa acetaminophen-binding protein |
| 6 | 8 | Tropomyosin 3, tropomyosin gamma, tropomyosin alpha-3 chain |
| 8 | 7 | GAPDH, glyceraldehyde-3-phosphate dehydrogenase |
| 9 | 7 | Hemoglobin beta chain |
| 10 | 7 | Mortalin, GRP 75 |
| 11 | 7 | Triosephosphate isomerase, TIM |
| 12 | 6 | Actin, cytoplasmic 2; gamma-actin |
| 13 | 6 | Alpha-enolase, Enolase 1 |
| 14 | 6 | Alpha-tubulin |
| 15 | 6 | Heat shock 70kDa protein 8, Hsc 70 |
| 16 | 6 | Peroxiredoxin 6 |
| 17 | 5 | Aldehyde dehydrogenase, mitochondrial |
| 18 | 5 | ATP synthase subunit beta |
| 19 | 5 | Carbonic anhydrase 3, CA-III |
| 20 | 5 | Beta-diketonase, Fumarylacetoacetate hydrolase |
| 21 | 5 | Hemoglobin alpha chain |
| 22 | 4 | Alpha-1 protease inhibitor 1, Alpha-1-antitrypsin 1-1 |
| 23 | 4 | ATP synthase subunit alpha (mitochondrial) |
| 24 | 4 | Calreticulin |
| 25 | 4 | Cytochrome P450 2B1 |
| 26 | 4 | Elongation factor 1-alpha 1 |
| 27 | 4 | Fatty acid–binding protein 1 |
Note.aData taken from reactive metabolite target protein database, http://tpdb.medchem.ku.edu:8080/protein_database/
In this article, we report the application of a new approach to winnowing the large number of known target proteins in the Reactive Metabolite Target Protein Database (TPDB, 2013) to find those whose covalent modification by CRMs is most strongly related to cytotoxic outcomes. It is based on the hypothesis (Fang et al., 2009) that the detrimental consequences of protein modification by CRMs may stem not from the partial loss of enzymatic activities, but rather from the disruption or inadvertent mimicry of important protein-protein interactions (PPIs). This new approach, therefore, involves consideration of not just target proteins but also of their immediate partner proteins. Using this approach leads to a “ranking of target proteins” significantly different from that produced by considering target proteins alone (Table 2). We suggest that ranking target proteins this way may provide a better perspective on their relevance to toxicity and a better guide for formulating testable mechanistic hypotheses about their potential role in CRM cytotoxicity.
MATERIALS AND METHODS
The basic method of our analysis is outlined schematically in Figure 1. As in several previous analyses, the KEGG (www.genome.jp/kegg/) and GO (www.geneontology.org/) classification schemes were used to sort proteins objectively and obtain a statistical sense of the extent to which any particular pathway or category is significantly enriched in proteins from the list being sorted. A key difference in this analysis is that proteins reported to interact directly (i.e., physically) with any of the CRM target proteins are also included in the overall sorting operation. The partner proteins were included for two reasons. First, earlier analyses based solely on target proteins were not very fruitful. Second, the partner proteins are needed to represent fully the PPIs in which the target proteins participate.
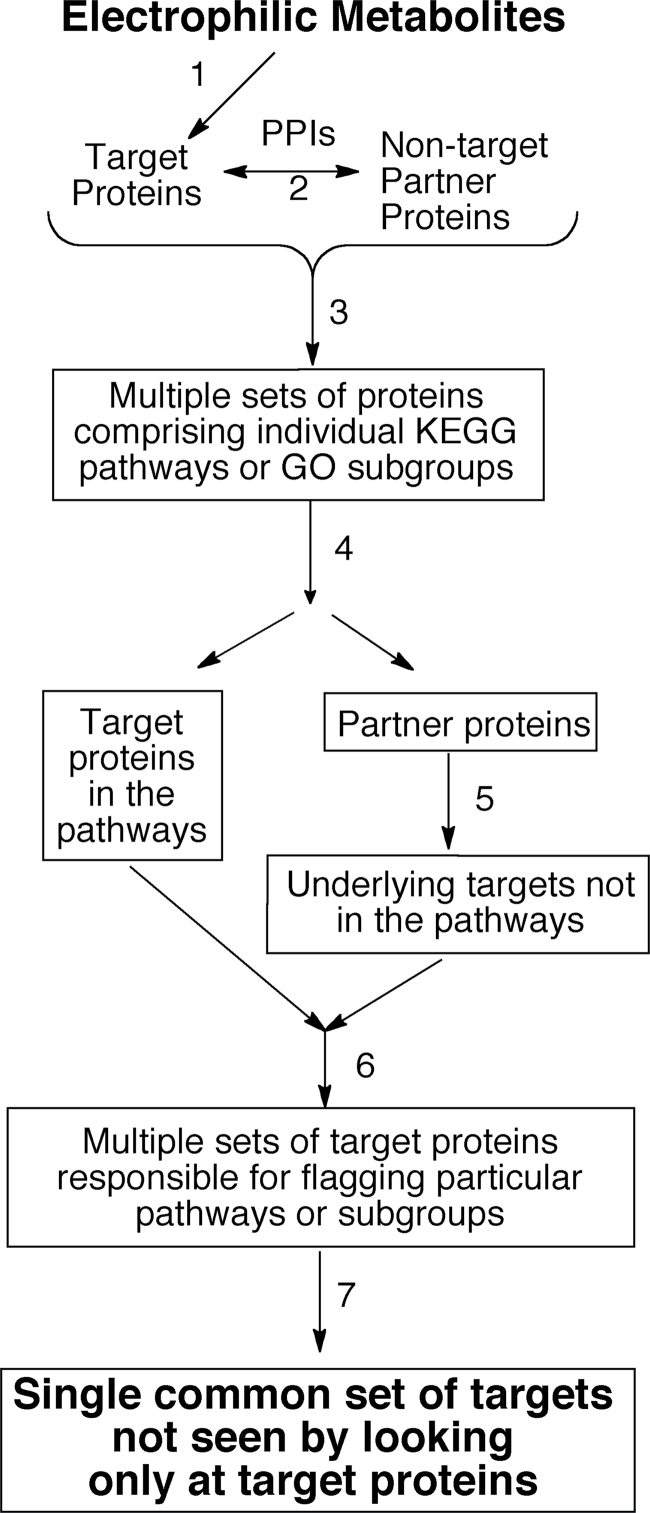
Fig. 1.
Strategy for identification of important target proteins among others. The strategy is based on the idea that covalent modification of proteins by electrophilic metabolites (step 1) mimics or interferes with PPIs. After identification of a large number of target proteins, their interacting partners are found through PPI databases such as the HPRD (step 2). Target proteins and their partners are then combined, and the overall set is sorted into bins corresponding to KEGG pathways or GO categories (step 3). This yields a large number of bins that are evaluated for statistical significance and likely relevance to cytotoxicity. Some bins contain many partners and few or no targets. For each bin deemed significant and relevant, the proteins that sorted into it are then parsed (step 4) into a target protein set or a partner protein set. Step 5 is a key step. In this step, the residual partner proteins are then analyzed to determine which target proteins invoked them in the first place. For each bin, the underlying targets are combined with the targets found in step 4. The result is many sets of target proteins containing much redundancy among them. This redundancy gives insight into the ability of a given target protein to influence, through PPIs, multiple pathways of importance to the cell. Compressing the redundancy in the lists into numbers of occurrences for each protein (step 7) gives a list of target proteins, rank ordered by likely importance to toxicity, as judged by the number of PPIs that may be affected by their covalent modification. In this way, a list of 302 proteins in the TPDB was reduced to a short list; the top 10 or 15 of which have a very broad “reach” into cellular pathways and may therefore be more important than many other known target proteins.
For this analysis, a list of 302 proteins known to be adducted by one or more of 41 different CRMs was taken from the Reactive Metabolite Target Protein Database (TPDB, 2013). Sixty-six percent of all proteins in the TPDB were from rat, 26% were from mouse, and 5% were from human. By tissue, the distribution of target proteins was 57% liver, 16% lung, 13% kidney, 8% nervous, and 3% blood cells. Although 27 of these proteins were targeted by ≥ 4 different CRMs (Table 2), a great many were reported as targets of only one or two CRMs (TPDB “rank by hits” function). Considering only those proteins targeted by two or more CRMs reduced the total number of targets to 70. From these, we eliminated hemoglobin and one EST of unknown function giving a total of 68 target proteins of interest, mostly from rat or mouse or in some cases both. To eliminate interspecies redundancy and enable searching for partner proteins, human orthologs of the rat and mouse proteins were found by searching target proteins against human proteins using BLAST sequence alignment (Hanzlik et al., 2009). This gave a list of 52 nonredundant human orthologs (Table 3) whose interacting partners were identified using the Human Protein Reference Database (www.hprd.org). Of the 52 human protein orthologs, 16 had no reported partner proteins, whereas 36 others had a total of 327 known partners. A spreadsheet was made listing all the partners for each target (Supplementary table S1), and a copy was then re-sorted (inverted) to list the target protein(s) that invoked each individual partner protein (Supplementary table S2). The ability to look up which target invoked a particular partner protein, and conversely, becomes important later in the analysis.
Table 3.
Human Orthologs of CRM Target Proteins Listed in the Reactive Metabolite Target Protein Database
| Entry | Protein name | Gene name | Target proteins having known partners (n = 36) |
|---|---|---|---|
| 1 | P63261 | ACTG1 | Actin, cytoplasmic 2; gamma-actin |
| 2 | P00326 | ADH1C | Alcohol dehydrogenase 1C |
| 3 | P42330 | AKR1C3 | Aldo keto reductase family 1, member C3 |
| 4 | P02768 | ALB | Albumin |
| 5 | P05091 | ALDH2 | Aldehyde dehydrogenase, mitochondrial |
| 6 | P05062 | ALDOB | Aldolase B, fructose-bisphosphate |
| 7 | P05089 | ARG1 | Arginase |
| 8 | A5A6H5 | ATP5A1 | ATP synthase, alpha |
| 9 | P06576 | ATP5B | ATP synthase, beta |
| 10 | P07451 | CA3 | Carbonic anhydrase III |
| 11 | P27797 | CALR | Calreticulin |
| 12 | P04040 | CAT | Catalase |
| 13 | P27487 | DPP4 | Dipeptidyl peptidase IV |
| 14 | P30040 | ERP29 | Endoplasmic reticulum protein 29 |
| 15 | Q05CP7 | FABP1 | Fatty acid–binding protein 1 |
| 16 | P08263 | GSTA1 | Glutathione S-transferase alpha 1 |
| 17 | P09488 | GSTM1 | Glutathione S-transferase Mu-1 |
| 18 | P09211 | GSTP1 | Glutathione S-transferase Pi |
| 19 | Q6PK50 | HSP90AB1 | HSP90B, HSP 84 |
| 20 | Q96GW1 | HSP90B1 | GRP94, tumor rejection antigen 1 |
| 21 | P11021 | HSPA5 | BIP, GRP 78, HSPA5 |
| 22 | P11142 | HSPA8 | Heat shock 70kDa protein 8, Hsc 70 |
| 23 | P10809 | HSPD1 | Heat shock 60 KD protein 1 (chaperonin), HSP-60 |
| 24 | O75874 | IDH1 | Isocitrate dehydrogenase 1 |
| 25 | P07237 | P4HB | Protein disulfide isomerase A1, PDI, Erp59 |
| 26 | P30101 | PDIA3 | Protein disulfide isomerase A3, PDI A3, ER-60 |
| 27 | Q15084 | PDIA6 | Protein disulfide isomerase A6 |
| 28 | P30086 | PEBP1 | Phosphatidylethanolamine-binding protein 1, Raf kinase inhibitor protein |
| 29 | P00558 | PGK1 | Phosphoglycerate kinase 1 |
| 30 | P32119 | PRDX2 | Peroxiredoxin 2 |
| 31 | P30041 | PRDX6 | Peroxiredoxin 6 |
| 32 | Q13228 | SELENBP1 | Selenium-binding protein 1 |
| 33 | P01009 | SERPINA1 | Protease inhibitor 1, Alpha-1-antitrypsin 1-1 |
| 34 | P09493 | TPM1 | Tropomyosin alpha 1 chain |
| 35 | P02766 | TTR | Transthyretin |
| 36 | P10599 | TXN | Thioredoxin |
| Target proteins having no reported partners (n = 16) | |||
| 1 | P49189 | ALDH9A1 | 4-trimethylaminobutyraldehyde dehydrogenase |
| 2 | P60709 | ACTB | Actin, cytoplasmic 1; beta-actin |
| 3 | P06733 | enol | Alpha-enolase |
| 4 | Q9UBR1 | UPB1 | Beta-alanine synthase, ureidopropionase |
| 5 | Q8TDZ9 | CEShBr2 | Brain carboxylesterase hBr2 |
| 6 | P20813 | CYP2B6 | Cytochrome P450 2B6 |
| 7 | P30046 | ddt | D-dopachrome decarboxylase |
| 8 | P68104 | EEF1A1 | Elongation factor 1-alpha 1 |
| 9 | P07099 | ephX1 | Epoxide hydrolase 1 |
| 10 | P16930 | FAH | Beta-diketonase, Fumarylacetoacetase |
| 11 | Q14353 | Gamt | Guanidinoacetate N-methyltransferase |
| 12 | P28330 | acadl | Long-chain-specific acyl-CoA dehydrogenase, mitochondrial |
| 13 | Q02252 | Aldh6a1 | Methylmalonate-semialdehyde dehydrogenase [acylating], mitochondrial |
| 14 | P52758 | Hrsp12 | 14.5kDa translational inhibitor protein, Ribonuclease UK114 |
| 15 | Q00266 | mat1a | S-adenosylmethionine synthase isoform type-1 |
| 16 | Q53HE2 | TPI1 | Triosephosphate isomerase, TIM |
Once all the known partner proteins were found, the 52 targets and 327 partners were combined into a single set of 379 proteins. This set was then sorted into “bins” in the form of GO categories and KEGG pathways using The Database for Annotation, Visualization and Integrated Discovery (DAVID) (Huang et al., 2009). To identify bins of potential interest in relation to mechanisms of cytotoxicity, we initially used low stringency sorting criteria, namely, that there be at least two target and/or partner proteins in the bin and that p < 0.1. Because of the hierarchical nature of the classification schemes, the total number of bins found was much greater than the total number of proteins (see Results section and Supplementary tables S3, S4, and S5). To reduce the total number of pathways and categories populated, we examined the statistics of their filling including count, p value, and fold enrichment. Count gives the number of proteins that sorted into any given bin. A high count might indicate greater biological relevance of that bin (and the proteins in it) to CRM-induced cytotoxicity. A very low p value indicates the improbability of the proteins in a bin arriving there by chance. Finally, the fold enrichment value for a particular bin is the ratio of the fraction of the sorted set appearing in that bin versus the fraction of all classified proteins that sorts into that bin. A high fold enrichment could suggest a higher relevance of that bin and its proteins to CRM-induced cytotoxicity. To simplify the consideration of these statistical parameters, we created a composite index (CI), defined as CI = (fold enrichment) × (−log p), and used it to rank the various KEGG pathways and GO categories. The full rank ordered but unedited lists of all categories (bins) from the KEGG and GO sorting operations can be found in Supplementary tables S3, S4, and S5.
The ranking process ordered the categories but did not reduce their number, which remained much larger than the number of proteins entered to start with (see Results section). Furthermore, the quantitative indices of category rank formed a more or less continual distribution with no pronounced gaps or breaks. Therefore, to reduce the redundancy and shift the focus toward the more important proteins, the final selection of categories for further detailed analysis was based on their likely relevance to cytotoxicity. This step was not undertaken lightly because it runs the risk of introducing bias. Nevertheless, we found impossible to proceed further based purely on mathematical or statistical considerations. We therefore excluded pathways and categories that seemed unlikely to be related to or contribute to acute cytotoxicity. For example, we eliminated pathways and categories devoted to metabolism of plant natural products, bacterial virulence factors, central nervous system disorders, Alzheimer’s disease, and cancer, as these were judged to be of little or no direct relevance for acute CRM-induced cytotoxicity in mammalian cells. We also excluded pathways related to blood clotting because CRM-induced cytotoxicity is well known to occur under cell culture conditions in the absence of blood and clotting factors. Finally, we eliminated categories related to metabolism of DNA, RNA, and complex structural lipids, as these processes generally occur on time scales much slower than acute cytotoxic responses to CRMs. In this way, we were able to come up with a more reasonable subset of flagged bins and proceed with their analysis toward the objective of determining which target proteins were most strongly connected to cytotoxic outcomes.
In addition to the statistical parameters associated with each bin, the sorting program DAVID also furnished a list of the gene names of all the target and partner proteins that had sorted into each bin. Using the spreadsheet lists of targets versus partners and partners versus targets mentioned above (Supplementary tables S1 and S2), it was easy to subdivide the proteins in each bin into a target subset and a partner subset, and for each partner protein to find the target protein(s) that originally invoked it. This last step was vital as some bins were found to contain few or even no known target proteins (see Tables 4 and 5 and Supplementary table S3 for examples). Although most target proteins have several or more partner proteins, only a few pairs of target proteins interact directly with each other. These observations emphasize the value and importance of including partner proteins in the analysis. It may also explain why earlier analyses using only target proteins gave no strong clues as to mechanisms or pathways of cytotoxicity and why they failed to distinguish potentially important targets from other targets. Finally, by analyzing a large number of KEGG and GO bins selected for high fold enrichment, low p value, and likely relevance to cytotoxicity, it became clear that a relatively small number of target proteins were consistently responsible for invoking a majority of the partner proteins that sorted into bins relevant for cytotoxicity.
Table 4.
Ten Top-Ranking KEGG Pathways and Their Statistical Parameters
| Subcategory groups | Count | p value | Fold enrichment | CIa |
|---|---|---|---|---|
| Group 1. Signaling pathways | ||||
| hsa04620:Toll-like receptor signaling pathway | 20 | 1.59E−07 | 4.15 | 28.23 |
| hsa04920:Adipocytokine signaling pathway | 13 | 1.21E−04 | 3.77 | 14.77 |
| hsa04010:MAPK signaling pathway | 26 | 3.75E−04 | 2.13 | 7.28 |
| hsa04012:ErbB signaling pathway | 11 | 6.00E−03 | 2.74 | 6.09 |
| hsa04310:Wnt signaling pathway | 14 | 2.40E−02 | 1.96 | 3.18 |
| hsa04370:VEGF signaling pathway | 8 | 4.76E−02 | 2.39 | 3.16 |
| Group 2. Antigen processing | ||||
| hsa04612:Antigen processing and presentation | 14 | 7.14E−05 | 3.71 | 15.37 |
| Group 3. Apoptosis related | ||||
| hsa04210:Apoptosis | 13 | 4.73E−04 | 3.28 | 10.90 |
| Group 4. Xenobiotic metabolism | 6 | 3.42E−02 | 3.26 | 4.78 |
| hsa00480:Glutathione metabolism | 7 | 9.82E−02 | 2.18 | 2.20 |
| hsa00980:Metabolism of xenobiotics by cyt P450 | 18 | 3.61E−07 | 4.38 | 28.23 |
Note. For the gene names associated with each subcategory, see Table 5.
aComposite index defined as (fold enrichment) × (−log p).
Table 5.
Gene Names of Proteins in Ten Top-Ranked KEGG Pathways Listed in Table 4
| hsa04620: Toll-like receptor signaling pathway | hsa04920: Adipocytokine signaling pathway | hsa04010: MAPK signaling pathway | hsa04012: ErbB signaling pathway | hsa04310: Wnt signaling pathway | hsa04370: VEGF signaling pathway | hsa04612: Antigen processing and presentation | hsa04210: Apoptosis | hsa00480: Glutathione metabolism | hsa00980: Metabolism of xenobiotics by cyt P450 |
|---|---|---|---|---|---|---|---|---|---|
| AKT1 | AKT1 | AKT1 | ABL2 | CSNK2A1 | AKT1 | B2M | AKT1 | GSTA1 | ADH1C |
| CCL5 | CHUK | ATF4 | AKT1 | CSNK2A2 | CASP9 | CALR | CASP3 | GSTA2 | AKR1C3 |
| CD40 | IKBKB | CASP3 | ERBB2 | JUN | MAP2K1 | CANX | CASP7 | GSTM1 | GSTA1 |
| CHUK | IKBKG | CHUK | JUN | MAP3K7 | MAPK1 | CD4 | CASP9 | GSTM2 | GSTA2 |
| CXCL10 | JAK2 | IKBKB | MAP2K1 | MAPK8 | PRKCA | HLA-A | CHUK | GSTP1 | GSTM1 |
| CXCL11 | MAPK8 | IKBKG | MAPK1 | MMP7 | PRKCB1 | HLA-C | CYCS | IDH1 | GSTM2 |
| CXCL9 | NFKB1 | JUN | MAPK8 | PPARD | PRKCG | HSP90AA1 | IKBKB | 6 | GSTP1 |
| IKBKB | PPARA | MAP2K1 | PRKCA | PPP2R1A | RAF1 | HSP90AB1 | IKBKG | 7 | |
| IKBKE | PTPN11 | MAP3K14 | PRKCB1 | PPP2R1B | 8 | HSPA5 | MAP3K14 | ||
| IKBKG | SLC2A1 | MAP3K3 | PRKCG | PRKACA | HSPA8 | NFKB1 | |||
| JUN | SLC2A4 | MAP3K5 | RAF1 | PRKCA | PDIA3 | PRKACA | |||
| MAP2K1 | STAT3 | MAP3K7 | 11 | PRKCB1 | PSME3 | TP53 | |||
| MAP3K7 | TRAF2 | MAPK1 | PRKCG | TAP1 | TRAF2 | ||||
| MAPK1 | 13 | MAPK8 | TP53 | TAPBP | 13 | ||||
| MAPK8 | MAPT | 14 | 14 | ||||||
| NFKB1 | NFKB1 | ||||||||
| TBK1 | PDGFRB | ||||||||
| TLR1 | PRKACA | ||||||||
| TLR2 | PRKCA | ||||||||
| TLR4 | PRKCB1 | ||||||||
| 20 | PRKCG | ||||||||
| RAF1 | |||||||||
| RASA1 | |||||||||
| STMN1 | |||||||||
| TP53 | |||||||||
| TRAF2 | |||||||||
| 26 |
Note. The gene names in boldface italics correspond to target proteins; all others correspond to partner proteins. The numbers at the bottom of each column are the count values for the column; the total for all 10 columns is 132.
RESULTS AND DISCUSSION
Our objective was to analyze a list of CRM target proteins to identify those whose modification by CRMs was most strongly linked to cytotoxicity or in other words to identify which targets among the many were potentially most important to causing cytotoxicity upon modification by CRMs. Directly interacting partner proteins were included in the analysis to represent the presumably important PPIs of the chemically modified target proteins. Sorting the 379 target and partner proteins into KEGG and GO gave the following results: 199 proteins sorted into 46 KEGG pathways; 345 proteins sorted into 466 GO Biological Process (BP) categories; 355 proteins sorted into 119 GO Molecular Function (MF) categories; and 343 proteins sorted into 85 GO Cellular Component categories. We did not analyze the cellular component category further because in many cases target proteins had been identified after subcellular fractionation. Instead, we performed a detailed analysis of those target and partner proteins that sorted into the KEGG, GO-BP, and GO-MF classification schemes. A key part of the analysis was to use partner proteins to highlight the CRM target proteins that invoked them in the first place (see Materials and Methods). Once the three main sortings were the analyzed and the results rank ordered, the results were pooled into a single list containing only target proteins rank ordered for likely relevance to acute cytotoxicity. The analyses are described in the following sections.
KEGG Pathway Analysis of Targets and Partners
After rank ordering the 46 KEGG pathway bins by their CI value (Supplementary table S3), 10 were selected for further detailed analysis. They included six intracellular signaling pathways, two pathways related to metabolism of xenobiotics, and one each related to antigen processing and presentation and apoptosis (Table 4). The sorting process also yielded lists of genes representing each of the proteins in each of the bins. As an example, the 132 genes associated with the 10 pathways in Table 4 are listed in Table 5. Several of these genes appear in more than one pathway, but a more striking observation is that among the six intracellular signaling pathways, none of the genes correspond to actual CRM target proteins. A likely reason for this is that although the target proteins have definite cellular functions, they are not actually members of the signaling pathways, even though they may interact with one or more of the pathway members. The converse issue of why the signaling pathway members are not detected as CRM targets is almost certainly related to analytical sensitivity, and the fact that the cellular concentrations of these signaling proteins are typically very low compared with target proteins detectable by the usual proteomic methods (Koen et al., 2000). In contrast, a majority of the genes in the pathways related to xenobiotic metabolism and antigen processing and presentation do correspond directly to target proteins (Table 5). The absence of genes for CRM targets among the signaling pathway genes naturally raises an important question: with which CRM target proteins do these partner proteins interact, or stated another way, which CRM targets invoked these partners? This would be of considerable interest in view of the hypothesis that perturbing the PPIs of these targets could possibly impact intracellular signaling by their partners and thereby have important toxicological consequences.
To find the targets that invoked the partners comprising the signaling cascades in Table 5, we returned to our original spreadsheet listing the interacting partners of each of the CRM target (Supplementary table S1) and re-sorted it by partner to reveal the target protein(s) associated with each of them (Supplementary table S2). When this was done for the individual KEGG pathways, the corresponding lists of target proteins were observed to display considerable redundancy between them (Table 6A). We suggest that this redundancy gives an important clue to the potential “reach” that the covalent modification of a given target protein may have in terms of its potential to disrupt multiple PPIs and perhaps initiate toxicological outcomes for a cell. When this redundancy is compressed, simply by counting the number of times a given target either appears or is invoked by its partners (Table 6B), it is found that just seven target proteins are responsible for invoking 73 of the proteins listed in Table 5. These seven proteins are GSTP1, Hsp90AB1, HspA5, HspA8, HspD1, PEBP1 (also known as RKIP), and thioredoxin. This information will be combined with comparable information gleaned from similar analyses of the highest ranking GO categories as described below.
Table 6.
Example of Spreadsheet Used for Manual Counting of Target Proteins Associated With Partner Proteins in KEGG Pathways Such As Those in Table 5
| A. Gene names of target proteins whose partners are listed in the KEGG pathways in Tables 4 and 5 | B. Gene names of common denominator target proteins (redundancies removed from part A) | Number of occurrences | ||||||
|---|---|---|---|---|---|---|---|---|
| TLR | MAPK | Antigen processing | Apoptosis | TLR | MAPK | Antigen processing | Apoptosis | |
| DPP4 | GSTM1 | ATP5B | GSTP1 | ATP5B | 1 | |||
| DPP4 | GSTP1 | CALR | HSP90AB1 | CALR | 1 | |||
| DPP4 | GSTP1 | CALR | HSP90AB1 | DPP4 | DPP4 | 2 | ||
| DPP4 | HSP90AB1 | CALR | HSP90AB1 | GSTM1 | 1 | |||
| GSTP1 | HSP90AB1 | CALR | HSP90AB1 | GSTP1 | GSTP1 | GSTP1 | 3 | |
| HSP90AB1 | HSP90AB1 | DPP4 | HSP90AB1 | HSP90AB1 | HSP90AB1 | HSP90AB1 | HSP90AB1 | 4 |
| HSP90AB1 | HSP90AB1 | HSP90AB1 | HSP90AB1 | HSP90B1 | 1 | |||
| HSP90AB1 | HSP90AB1 | HSPA5 | HSPA5 | HSPA5 | HSPA5 | HSPA5 | 3 | |
| HSP90AB1 | HSP90AB1 | HSPA5 | HSPA8 | HSPA8 | HSPA8 | HSPA8 | HSPA8 | 4 |
| HSP90AB1 | HSP90AB1 | HSPA5 | HSPD1 | HSPD1 | HSPD1 | HSPD1 | 3 | |
| HSP90AB1 | HSPA5 | HSPA8 | HSPD1 | PDIA3 | 1 | |||
| HSP90B1 | HSPA8 | HSPA8 | HSPD1 | PEBP1 | PEBP1 | PEBP1 | 3 | |
| HSP90B1 | HSPA8 | PDIA3 | PEBP1 | PRDX2 | 1 | |||
| HSP90B1 | HSPA8 | PDIA3 | PEBP1 | TPM1 | TPM1 | 2 | ||
| HSPA8 | HSPD1 | PDIA3 | PEBP1 | TTR | 1 | |||
| HSPD1 | HSPD1 | PDIA3 | TXN | TXN | TXN | TXN | 3 | |
| PEBP1 | HSPD1 | Total 17 | Total 17 | |||||
| PEBP1 | PEBP1 | |||||||
| PEBP1 | PEBP1 | |||||||
| PEBP1 | PEBP1 | |||||||
| TPM1 | PEBP1 | |||||||
| TXN | PEBP1 | |||||||
| Total 22 | PEBP1 | |||||||
| PEBP1 | ||||||||
| PEBP1 | ||||||||
| PEBP1 | ||||||||
| PRDX2 | ||||||||
| TPM1 | ||||||||
| TTR | ||||||||
| TXN | ||||||||
| TXN | ||||||||
| Total 30 | ||||||||
Note. The left-hand part of this table (A) shows the redundancy among targets in any given bin from Table 5. This redundancy arises from the fact that one target protein may have many partners that sort into different KEGG pathway bins. The total number of target proteins listed at the bottom of each column is slightly larger than the number of partner proteins shown in Table 5 because several partners are invoked by more than one target. After collapsing this redundancy, the right-hand half of the table (B) shows that certain target proteins may be responsible for invoking partners in several different pathways. This redundancy is collapsed by counting the number of pathways in which a target protein may influence partners, even though the target protein may not formally belong to that pathway. In this way, it can be seen that 73 of the nearly 115 target proteins listed in Table 5 are invoked by just the seven proteins highlighted in boldface.
GO-BP Analysis of Targets and Partners
Because of the detailed and nested hierarchical structure of the GO classification system, the number of categories and subcategories is much larger than the number of KEGG pathways, but the approach to evaluating the sorting results is essentially the same. In the GO-BP category, 466 subcategory bins were flagged using low stringency criteria (Supplementary table S4). Ranking the bins by their CI and further culling for relevance to cytotoxicity reduced the number to 46 bins that we arranged into six groups (Table 7). As with the KEGG analysis above, each of these subcategory bins contained a number of proteins represented by a listing of their gene names. The total number of genes across all 46 subcategories was 1,670, but there was a great deal of redundancy among them, due again to the nested hierarchical nature of the GO classification scheme. Three of the largest subgroups in Table 7, namely groups 1, 2, and 6, were selected for further detailed analysis. Group 5 (kinases and signaling pathways), although large, was not analyzed further because of substantial redundancy with the KEGG analysis above. Group 3 (NO and inflammation) and group 4 (oxidative stress), while relevant for cytotoxicity, were not analyzed further because they were very small compared with the other groups.
Table 7.
Top-Ranking GO-BP Subcategories (n = 46) and Their Statistical Parameters, Grouped as Described in the Text
| GO subcategory group | Count | p value | Fold enrichment | CI |
|---|---|---|---|---|
| Group 1. Apoptosis, its regulation, and its effects | ||||
| GO:0051014~actin filament severing | 4 | 4.42E−05 | 44.4 | 193.3 |
| GO:0006915~apoptosis | 66 | 1.67E−20 | 3.7 | 73.9 |
| GO:0012501~programmed cell death | 66 | 2.66E−20 | 3.7 | 72.5 |
| GO:0042981~regulation of apoptosis | 50 | 3.38E−17 | 4.1 | 68.0 |
| GO:0016265~death | 67 | 9.50E−20 | 3.6 | 67.8 |
| GO:0008219~cell death | 67 | 9.50E−20 | 3.6 | 67.8 |
| GO:0043067~regulation of programmed cell death | 50 | 5.29E−17 | 4.1 | 66.4 |
| GO:0008632~apoptotic program | 13 | 2.75E−07 | 7.0 | 46.2 |
| GO:0043066~negative regulation of apoptosis | 24 | 2.22E−09 | 4.6 | 40.1 |
| GO:0043069~negative regulation of programmed cell death | 24 | 2.86E−09 | 4.6 | 39.1 |
| GO:0006916~antiapoptosis | 19 | 4.84E−08 | 5.0 | 36.5 |
| GO:0006917~induction of apoptosis | 17 | 2.60E−05 | 3.5 | 16.2 |
| GO:0012502~induction of programmed cell death | 17 | 2.75E−05 | 3.5 | 16.1 |
| GO:0043065~positive regulation of apoptosis | 19 | 1.81E−05 | 3.3 | 15.7 |
| GO:0043068~positive regulation of programmed cell death | 19 | 2.01E−05 | 3.3 | 15.5 |
| Group 2. ER stress, protein folding, and unfolded protein response | ||||
| GO:0065008~regulation of biological quality | 75 | 1.02E−23 | 3.8 | 86.9 |
| GO:0006983~ER overload response | 3 | 4.81E−03 | 26.6 | 61.7 |
| GO:0006457~protein folding | 31 | 8.42E−13 | 5.0 | 60.2 |
| GO:0030968~unfolded protein response | 5 | 2.09E−04 | 15.9 | 58.3 |
| GO:0006986~response to unfolded protein | 15 | 2.20E−08 | 7.1 | 54.2 |
| Group 3. NO and inflammation | ||||
| GO:0046209~nitric oxide metabolic process | 7 | 1.94E−05 | 12.0 | 56.3 |
| GO:0006809~nitric oxide biosynthetic process | 7 | 1.94E−05 | 12.0 | 56.3 |
| GO:0006954~inflammatory response | 30 | 4.11E−11 | 4.4 | 46.0 |
| Group 4. Oxidative stress | ||||
| GO:0006979~response to oxidative stress | 11 | 5.84E−05 | 5.1 | 21.5 |
| Group 5. Kinases and signaling cascades | ||||
| GO:0043549~regulation of kinase activity | 20 | 1.65E−06 | 3.8 | 21.8 |
| GO:0033673~negative regulation of kinase activity | 8 | 3.05E−04 | 6.1 | 21.5 |
| GO:0006469~negative regulation of protein kinase activity | 8 | 3.05E−04 | 6.1 | 21.5 |
| GO:0033674~positive regulation of kinase activity | 13 | 6.57E−05 | 4.2 | 17.5 |
| GO:0007243~protein kinase cascade | 26 | 3.05E−06 | 2.9 | 16.2 |
| GO:0043405~regulation of MAP kinase activity | 10 | 3.56E−04 | 4.5 | 15.6 |
| GO:0000165~MAPKKK cascade | 13 | 3.18E−04 | 3.5 | 12.4 |
| GO:0007242~intracellular signaling cascade | 63 | 1.43E−06 | 1.9 | 10.9 |
| GO:0007165~signal transduction | 123 | 3.05E−06 | 1.5 | 8.0 |
| GO:0007267~cell-cell signaling | 30 | 4.67E−04 | 2.0 | 6.7 |
| Group 6. Homeostasis, regulation, wounding, and general stress | ||||
| GO:0009611~response to wounding | 39 | 5.24E−13 | 4.0 | 49.3 |
| GO:0042592~homeostatic process | 37 | 4.45E−12 | 3.9 | 44.6 |
| GO:0033554~cellular response to stress | 5 | 4.72E−04 | 13.1 | 43.4 |
| GO:0006952~defense response | 44 | 4.23E−12 | 3.4 | 38.5 |
| GO:0048518~positive regulation of biological process | 64 | 4.95E−12 | 2.6 | 29.0 |
| GO:0048519~negative regulation of biological process | 64 | 7.90E−11 | 2.4 | 24.3 |
| GO:0048522~positive regulation of cellular process | 56 | 4.44E−10 | 2.5 | 23.3 |
| GO:0048523~negative regulation of cellular process | 61 | 3.15E−10 | 2.4 | 22.7 |
| GO:0006968~cellular defense response | 9 | 2.72E−04 | 5.3 | 19.0 |
| GO:0042060~wound healing | 12 | 1.57E−04 | 4.1 | 15.7 |
| GO:0065007~biological regulation | 176 | 1.00E−10 | 1.5 | 14.9 |
In Table 7, group 1 consists of 15 subcategory bins containing a total of 522 genes of target or partner proteins. For convenience in analysis, and in parallel with the KEGG analysis above, these 15 subcategories were recombined into four subgroups according to the similarity (redundancy) of the genes included (data not shown). In the first subgroup, the total number of nonredundant genes was 68, among which only seven correspond to known target proteins, whereas the remaining 61 genes represent partner proteins. Analysis of these partners revealed that just 16 target proteins invoked all 61 partner proteins. Furthermore, as in the KEGG analysis above, these 16 target proteins are not members of these GO subcategories, but rather they interact with some of the members and thus flag the categories through the interacting partners. Analysis of the other three group 1 subgroups produced similar patterns of results regarding numbers of target versus partner proteins and redundancies among them. Pooling all the results for group 1 of Table 5 showed that overall only nine target proteins emerged from all four subgroups while nine more emerged from at least three of the four subgroups. In a similar fashion, analysis of group 2 from Table 5 (five subcategories listing 129 genes) revealed that seven target proteins occurred three or more times, whereas eight proteins occurred two or more times. Likewise for group 6 of Table 5 (12 subcategories listing 650 genes), 13 target proteins emerged from all five subgroups, whereas four more emerged from at least four of the five subgroups. This information was eventually combined with comparable information from the KEGG analysis (above) and the GO-MF analysis as described below.
GO-MF Category Analysis of Targets and Partners
In a parallel fashion, sorting our set of target plus partner proteins into the GO-MF classification initially yielded 119 subcategories (Supplementary table S5), but this number was reduced to 18 (Table 8) by considering CI values and relevance to cytotoxicity. These 18 subcategories contained a total of 158 genes representing target and partner proteins, but the 158 genes corresponded to only 44 nonredundant proteins, among which only 5 were actual CRM target proteins, whereas 39 corresponded to partner proteins. Analysis of these partner protein genes as a single group yielded a list of just 16 targets that were responsible for invoking all 39 partner proteins among the 18 subcategories. Of the 21 target proteins identified by this search, all had already been identified as relevant and potentially important through the KEGG or GO-BP searches described above. Thus, although the GO-MF analysis revealed no new proteins of interest, it clearly reinforced the likely importance of those already identified using the KEGG and GO-BP approaches.
Table 8.
Top-Ranking GO-MF Subcategories (n = 18) and Their Statistical Parameters
| GO-MF category | Count | p value | Fold enrichment | CI |
|---|---|---|---|---|
| GO:0008384-IkappaB kinase activity | 3 | 1.28E−03 | 47.8 | 138 |
| GO:0030911-TPR domain binding | 3 | 1.28E−03 | 47.8 | 138 |
| GO:0003756-protein disulfide isomerase activity | 5 | 3.54E−05 | 23.9 | 106 |
| GO:0030188-chaperone regulator activity | 4 | 2.96E−04 | 27.3 | 96 |
| GO:0030235-nitric-oxide synthase regulator activity | 3 | 2.53E−03 | 35.8 | 93 |
| GO:0030192-Hsp70/Hsc70 protein regulator activity | 3 | 2.53E−03 | 35.8 | 93 |
| GO:0051082-unfolded protein binding | 18 | 2.82E−09 | 6.4 | 55 |
| GO:0008092-cytoskeletal protein binding | 36 | 7.34E−12 | 4.0 | 44 |
| GO:0004879-ligand-dependent nuclear receptor activity | 11 | 1.57E−06 | 7.6 | 44 |
| GO:0003779-actin binding | 28 | 2.19E−10 | 4.4 | 43 |
| GO:0004709-MAP kinase kinase kinase activity | 4 | 5.82E−03 | 10.6 | 24 |
| GO:0019904-protein domain–specific binding | 10 | 4.94E−04 | 4.3 | 14 |
| GO:0031072-heat shock protein binding | 7 | 2.14E−03 | 5.2 | 14 |
| GO:0051087-chaperone binding | 3 | 3.81E−02 | 9.6 | 14 |
| GO:0005200-structural constituent of cytoskeleton | 8 | 2.27E−03 | 4.4 | 12 |
| GO:0004860-protein kinase inhibitor activity | 4 | 3.10E−02 | 5.8 | 9 |
| GO:0042287-MHC protein binding | 3 | 8.24E−02 | 6.2 | 7 |
| GO:0016209-antioxidant activity | 5 | 2.93E−02 | 4.3 | 7 |
PPIs as Targets for CRMs
Examples of the modulation of PPIs by small changes at a single residue is a well-known phenomenon. For example, mutation of the single cysteine residue in murine Hsp25 to alanine interferes with its protective functions by preventing its dimerization (Diaz-Latoud et al., 2005). Covalent modification of Cys49 in the cytosolic domain of microsomal GST-1 by electrophiles activates this protective enzyme and thereby “acts as an antenna for the detection of chemical or oxidative stress” (Busenlehner et al., 2004). In nonstressed cells, the activity of JNK is kept low by its 1:1 complexation to GST-pi (which has four reactive sulfhydryl groups near its active site), but this suppression is relieved by oxidative stress, by the addition of GSH analogs that bind to GST-pi, and by ethacrynic acid (a Michael acceptor that S-alkylates GST-pi) (Townsend and Tew, 2003). These and other examples suggest that covalent modification of proteins by CRMs could affect cellular homeostasis by modulating or interfering with PPIs.
Of course, not all CRM-induced changes in PPIs are necessarily deleterious. The Keap1-Nrf2-ARE pathway provides a clear example (Dinkova-Kostova et al., 2005; Goldring et al., 2004; Kwak et al., 2004; Nguyen et al., 2003). This pathway regulates several protective mechanisms including the upregulation of conjugating and antioxidant enzymes, anti-inflammatory responses, the molecular chaperone/stress-response system, and the ubiquitin/proteasome system. The protein Keap1 has 27 cysteine sulfhydryl groups, many of which are reactive toward electrophiles (Hong et al., 2005). Modification of sulfhydryl groups on Keap1 causes the transcription factor Nrf2 to dissociate, allowing it to migrate to the nucleus and trigger the expression of genes favorable to cell defense that are under the control of the antioxidant response element (ARE, also called the electrophile response element). Although the CRM-induced changes in the activity of the Keap1-Nrf2-ARE pathway appear to be protective, alteration of other PPIs could, in principle, just as easily lead to detrimental effects on the cell.
Summary and Conclusions
The names and frequencies of occurrence of the target proteins emerging from the GO-MF, GO-BP, and KEGG analyses are compiled in Table 9, and a diagram of the interactions of 12 “well-connected” targets with partners is given in Figure 2. It should be noted that the individual frequencies of occurrence of the target proteins listed in Table 9 could have been made to appear higher, and the resulting rank ordering could perhaps have changed slightly if we had included even more subsets of GO and KEGG pathways in our analysis. However, the effect of “stacking the deck” like this would have been to increase redundancy among the bins and decrease stringency from a statistical and a biological relevance perspective. In our view, doing so would have added no further value or precision to the result, which is a small subset of CRM target proteins, winnowed from many, by an objective process that is also mindful of relevance to toxicity. This process explicitly took into account the hypothesis that disruption of PPIs (Fig. 2) by covalent binding contributes significantly to CRM-induced cytotoxicity. The implication is that target proteins near the top of Table 9 may therefore be more important than many other target proteins in generating biological responses to cytotoxic CRMs.
Table 9.
Target Proteins With the Strongest Direct Links to Toxicity
| Target protein name | Gene name | GO-MF | GO-BP | KEGG | Sum | Overall rank | Hit ranka |
|---|---|---|---|---|---|---|---|
| Actin, cytoplasmic 2; gamma-actin | ACTG1 | 41 | 6 | 0 | 47 | 1 | 12 |
| Heat shock 70kDa protein 8, Hsc 70 | HSPA8 | 17 | 8 | 4 | 29 | 2 | 15 |
| HSP90B, HSP 84 | HSP90AB1 | 11 | 8 | 4 | 23 | 3 | — |
| BIP, GRP 78, HSPA5 | HSPA5 | 8 | 8 | 3 | 19 | 4 | — |
| Tropomyosin 1 alpha chain | TPM1 | 10 | 5 | 0 | 15 | 5 | 6 |
| Heat shock 60 KD protein 1 (chaperonin), HSP-60 | HSPD1 | 3 | 8 | 3 | 14 | 6 | 3 |
| Phosphatidylethanolamine-binding protein 1, Raf kinase inhibitor protein | PEBP1 | 4 | 6 | 3 | 13 | 7 | — |
| Thioredoxin | TXN | 2 | 8 | 3 | 13 | 8 | — |
| Calreticulin | CALR | 6 | 6 | 0 | 12 | 9 | 24 |
| Albumin | ALB | 4 | 7 | 0 | 11 | 10 | 4 |
| GRP 94, tumor rejection antigen 1 | HSP90B1 | 1 | 8 | 1 | 10 | 11 | — |
| Dipeptidyl peptidase IV | DPP4 | 2 | 5 | 2 | 9 | 12 | — |
| Glutathione S-transferase Pi | GSTP1 | 0 | 5 | 3 | 8 | 13 | — |
| Protein disulfide isomerase A3, PDI A3, ER-60 | PDIA3 | 2 | 5 | 1 | 8 | 14 | 2 |
| Aldo keto reductase family 1, member C3 | AKR1C3 | 0 | 6 | 0 | 6 | 15 | — |
| Aldehyde dehydrogenase, mitochondrial | ALDH2 | 2 | 4 | 0 | 6 | 16 | — |
| Endoplasmic reticulum protein 29 | ERP29 | 2 | 4 | 0 | 6 | 17 | — |
| Peroxiredoxin 6 | PRDX6 | 3 | 3 | 0 | 6 | 18 | 16 |
| Protease inhibitor 1, Alpha-1-antitrypsin 1-1 | SERPINA1 | 2 | 4 | 0 | 6 | 19 | 22 |
| ATP synthase, alpha | ATP5A1 | 0 | 5 | 0 | 5 | 20 | 23 |
| Protein disulfide isomerase A1, PDI, Erp59 | P4HB | 4 | 1 | 0 | 5 | 21 | 1 |
| ATP synthase, beta | ATP5B | 0 | 4 | 0 | 4 | 22 | 18 |
| Glutathione S-transferase Mu-1 | GSTM1 | 0 | 3 | 1 | 4 | 23 | — |
| Peroxiredoxin 2 | PRDX2 | 0 | 3 | 1 | 4 | 24 | — |
| Transthyretin | TTR | 1 | 2 | 1 | 4 | 25 | — |
| Aldolase B, fructose-bisphosphate | ALDOB | 0 | 3 | 0 | 3 | 26 | — |
| Fatty acid–binding protein 1 | FABP1 | 2 | 1 | 0 | 3 | 27 | 27 |
| Phosphoglycerate kinase 1 | PGK1 | 0 | 3 | 0 | 3 | 28 | — |
Note. The ranking is based on taking PPIs into account as described in the text and in Figure 1. The overall rank is based on the sum of the KEGG, GO-BP, and GO-MF rankings (Tables 4, 7 and 8, respectively). The ranking by number of hits given in Table 2 is repeated here for comparison.
aHit rank scores are from Table 2.
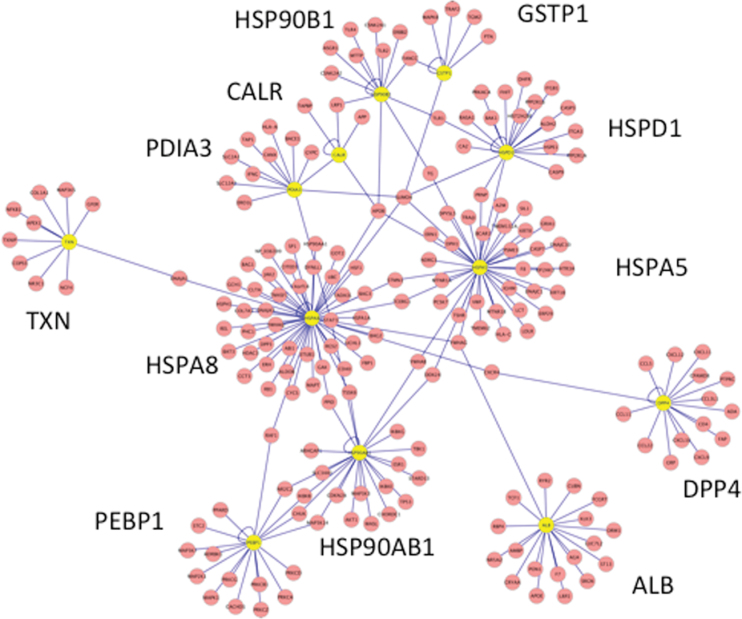
Fig. 2.
PPIs among 12 key CRM target proteins and 194 interacting partner proteins most strongly linked to cytotoxicity. The 12 target proteins (yellow nodes) are among the top 14 proteins listed in Table 9. Only two target proteins interact with each other directly (PDIA3-CALR), whereas 14 partner proteins interact directly with two or more target proteins.
The relative rankings of proteins generated by the method described in this article (Table 9) are obviously considerably different from those given in Table 2, which considered only the number of times a given protein is reported as a target in the literature. It must be noted that the literature on target proteins is actually rather limited, and the data correspondingly sparse, for a number of reasons. For example, low abundance proteins and proteins with low levels of adduction are likely not to be reported because they are missed (i.e., not detected) in autoradiograms of 2D gels or because they fail to be detected as identifiable peptides in digests of excised spots (Koen et al., 2007). Another major reason that the literature on CRM targets is incomplete is the general lack of systematic searching for adducts of a given drug or chemical across multiple species, or tissues or cell lines. The vast majority of the 46 chemicals for which target proteins have been identified have been studied in only one species or one type of cell. Consequently, many of the proteins listed in the TPDB are reported only in a single study involving a single chemical and a single biological system. This can easily be seen by using the “commonality matrix” and “rank by hits” functions built into the TPDB (2013).
The comprehensive bioinformatic analysis described in this report is an attempt to transcend deficiencies in the CRM-target literature and differences in experimental design choices among the numerous published studies and to distill out a small number of proteins that may have the highest relevance to CRM-dependent cytotoxicity. The result is a rather different view of which target proteins might be most important for CRM-induced cytotoxicity compared with any previous analysis. For example, only 7 of the top 15 proteins from Table 2 are included among the top 15 proteins in Table 9, and 1 of those 7, calreticulin, was ranked quite low in Table 2. One possible reason that calreticulin is ranked relatively low in Table 2 is that being a relatively acidic protein (pI = 4.29) it is often missed in 2DGE analyses of target proteins. On the other hand, its relatively higher listing in Table 9 stems in part from the fact that it has at least 34 known partner proteins and many of them sort into KEGG pathways and GO categories that relate in a direct way to cytotoxicity. Thus, calreticulin can be considered to have a high potential “reach” into a number of biochemical pathways. Conversely, the two highest ranking proteins in Table 2, protein disulfide isomerase A1 and A3, are ranked somewhat lower in Table 9, probably because they have many fewer known partners that also happen to sort into KEGG pathways and GO categories of intrinsically lower relevance to acute cytotoxicity.
In conclusion, we suggest that taking partner proteins into account when examining collections of CRM target proteins is useful and may prove to be important in facilitating mechanistic understanding of reactive metabolite cytotoxicity. Our results also support the hypothesis that disruption of PPIs can be at least a major contributor if not a major mechanism of CRM-induced acute cytotoxicity. Additional experimental work will obviously be required to examine in detail the roles of individual proteins highlighted through this bioinformatic approach.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
U.S. National Institutes of Health (GM-21784 to R.P.H.).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr Jeffrey Staudinger, Dr John Karanicolas, and Dr Todd Williams for helpful discussions during the writing of this manuscript.
REFERENCES
- Busenlehner L. S., Codreanu S. G., Holm P. J., Bhakat P., Hebert H., Morgenstern R., Armstrong R. N. (2004). Stress sensor triggers conformational response of the integral membrane protein microsomal glutathione transferase 1. Biochemistry 43, 11145–11152 [DOI] [PubMed] [Google Scholar]
- Diaz-Latoud C., Buache E., Javouhey E., Arrigo A. P. (2005). Substitution of the unique cysteine residue of murine Hsp25 interferes with the protective activity of this stress protein through inhibition of dimer formation. Antioxid. Redox Signal. 7, 436–445 [DOI] [PubMed] [Google Scholar]
- Dinkova-Kostova A. T., Holtzclaw W. D., Kensler T. W. (2005). The role of Keap1 in cellular protective responses. Chem. Res. Toxicol. 18, 1779–1791 [DOI] [PubMed] [Google Scholar]
- Druckova A., Mernaugh R. L., Ham A. J., Marnett L. J. (2007). Identification of the protein targets of the reactive metabolite of teucrin A in vivo in the rat. Chem. Res. Toxicol. 20, 1393–1408 [DOI] [PubMed] [Google Scholar]
- Evans D. C., Watt A. P., Nicoll-Griffith D. A., Baillie T. A. (2004). Drug-protein adducts: An industry perspective on minimizing the potential for drug bioactivation in drug discovery and development. Chem. Res. Toxicol. 17, 3–16 [DOI] [PubMed] [Google Scholar]
- Fang J., Koen Y. M., Hanzlik R. P. (2009). Bioinformatic analysis of xenobiotic reactive metabolite target proteins and their interacting partners. BMC Chem. Biol. 9, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldring C. E., Kitteringham N. R., Elsby R., Randle L. E., Clement Y. N., Williams D. P., McMahon M., Hayes J. D., Itoh K., Yamamoto M., et al. (2004). Activation of hepatic Nrf2 in vivo by acetaminophen in CD-1 mice. Hepatology 39, 1267–1276 [DOI] [PubMed] [Google Scholar]
- Hanzlik R. P., Fang J., Koen Y. M. (2009). Filling and mining the reactive metabolite target protein database. Chem. Biol. Interact. 179, 38–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong F., Freeman M. L., Liebler D. C. (2005). Identification of sensor cysteines in human Keap1 modified by the cancer chemopreventive agent sulforaphane. Chem. Res. Toxicol. 18, 1917–1926 [DOI] [PubMed] [Google Scholar]
- Huang D. W., Sherman B. T., Lempicki R. A. (2009). Bioinformatics Enrichment Tools: Paths toward the Comprehensive Functional Analysis of Large Gene Lists. Nucleic Acids Res. 37, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikehata K., Duzhak T. G., Galeva N. A., Ji T., Koen Y. M., Hanzlik R. P. (2008). Protein targets of reactive metabolites of thiobenzamide in rat liver in vivo. Chem. Res. Toxicol. 21, 1432–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koen Y. M., Gogichaeva N. V., Alterman M. A., Hanzlik R. P. (2007). A proteomic analysis of bromobenzene reactive metabolite targets in rat liver cytosol in vivo. Chem. Res. Toxicol. 20, 511–519 [DOI] [PubMed] [Google Scholar]
- Koen Y. M., Williams T. D., Hanzlik R. P. (2000). Identification of three protein targets for reactive metabolites of bromobenzene in rat liver cytosol. Chem. Res. Toxicol. 13, 1326–1335 [DOI] [PubMed] [Google Scholar]
- Krishna R. G., Wold , F. (1998) Possttranslational modifications. In Proteins: Analysis and design (Angeletti, R. H., Eds.) pp 121–207
- Kwak M. K., Wakabayashi N., Kensler T. W. (2004). Chemoprevention through the Keap1-Nrf2 signaling pathway by phase 2 enzyme inducers. Mutat. Res. 555, 133–148 [DOI] [PubMed] [Google Scholar]
- Liebler D. C. (2006). The poisons within: Application of toxicity mechanisms to fundamental disease processes. Chem. Res. Toxicol. 19, 610–613 [DOI] [PubMed] [Google Scholar]
- Liebler D. C., Guengerich F. P. (2005). Elucidating mechanisms of drug-induced toxicity. Nat. Rev. Drug Discov. 4, 410–420 [DOI] [PubMed] [Google Scholar]
- Nguyen T., Sherratt P. J., Pickett C. B. (2003). Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu. Rev. Pharmacol. Toxicol. 43, 233–260 [DOI] [PubMed] [Google Scholar]
- Park B. K., Kitteringham N. R., Maggs J. L., Pirmohamed M., Williams D. P. (2005). The role of metabolic activation in drug-induced hepatotoxicity. Annu. Rev. Pharmacol. Toxicol. 45, 177–202 [DOI] [PubMed] [Google Scholar]
- Park B. K., Laverty H., Srivastava A., Antoine D. J., Naisbitt D., Williams D. P. (2011). Drug bioactivation and protein adduct formation in the pathogenesis of drug-induced toxicity. Chem. Biol. Interact. 192, 30–36 [DOI] [PubMed] [Google Scholar]
- Reinders J., Sickmann A. (2007). Modificomics: Posttranslational modifications beyond protein phosphorylation and glycosylation. Biomol. Eng. 24, 169–177 [DOI] [PubMed] [Google Scholar]
- Snyder R. (2011). Origins of the International Symposium on Biological Reactive Intermediates and some thoughts on homeostasis and the biological significance of widespread covalent binding of biological reactive metabolites. Chem. Biol. Interact. 192, 3–7 [DOI] [PubMed] [Google Scholar]
- Townsend D. M., Tew K. D. (2003). The role of glutathione-S-transferase in anti-cancer drug resistance. Oncogene 22, 7369–7375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- TPDB (2013). Reactive Metabolite Target Protein Database Available at: http://tpdb.medchem.ku.edu:8080/protein_database/ Accessed February 2, 2013.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.