Abstract
Background
Living kidney donors (LKD) allow for increased access to lifesaving organs for transplantation. There is a relative paucity of African American (AA) live kidney donors. The prevalence of medical disease in LKD candidates has not been well studied. We examined the medical limitations to living kidney donation in a large Mid-Western transplant center.
Methods
A total of 2,519 adults (age ≥ 18 years) evaluated as potential LKD between January 1, 1996 and June 30, 2006 were prospectively followed until evaluation outcome (completed live donation, medical exclusion from live donation, non-medical exclusion from live donation). Logistic regression was used to examine the effect of age on donor exclusion and Chi square tests were used to compare the likelihood of donor exclusions between racial and gender groups.
Results
Sixty percent of PD were female (n=1300) and 86% were Caucasian (CA) (n=1862). Overall, 48.7% of PD who underwent evaluation became LKD. The odds of donation were 52% lower in AA compared to CA (OR 0.48 p< 0.001). Among PD excluded from donation, the most common medical diagnoses were HTN (24.7%), inadequate creatinine clearance, (10.6%) and a positive final crossmatch (10.5%). The rate of PD exclusion for obesity was two-fold higher in AA compared to CA (12.8% vs. 5.8%, p < 0.001).
Conclusions
Hypertension in PD is equally significant barrier to living kidney donation in AA and CA whereas obesity is a greater barrier in AA.
Keywords: Donation, access, contraindications, hypertension, obesity
Introduction
Kidney transplantation is the renal replacement therapy of choice for end-stage renal disease (ESRD). Over the last 20 years, the number of waitlisted candidates has increased substantially, with a relatively small increase in the deceased donor pool1. As a result, there has been increasing need for living kidney donors. Access to living kidney donors has varied among ethnic groups in part due to differences in waitlist representation. African-Americans (AA) are overrepresented in the ESRD and kidney transplant waitlist populations (29% and 34%, respectively)1, 2, relative to their percentage within the U.S. population (13%)3. However, over 70% of deceased kidney donors are Caucasian (CA). Differences in the distribution of human leukocyte (HLA) antigens, antibody sensitization and ABO blood types, these demographic differences often lead to prolonged deceased donor waiting times for AA as opposed to other ethnic groups1.
Despite the growing need, there is a disproportionately lower rate of live kidney donation among AA1. Previous literature has focused primarily on ethnic differences in living donor willingness, trust in the health care system and completion of donor evaluations as reasons for the lower rates of live kidney donation in AA4–9. Little attention has been paid to the possibility that medical contraindications may be a significant barrier to live donation among AA10 despite the fact that AA have a relatively high prevalence of hypertension (HTN), diabetes mellitus (DM) and obesity11, 12, which are disease states that conventionally preclude living donation. To evaluate the impact of medical disease on the likelihood of successful living kidney donation in AA, we examined the outcomes of a large cohort of living donor candidates evaluated at a large academic medical center in the Midwestern region of the United States.
Subjects and methods
Study design and patient population
After obtaining University of Michigan Institutional Review Board approval, we prospectively collected data on all adults (age ≥ 18 years) who underwent a first-time evaluation as potential living kidney donors at the University of Michigan Transplant Center between January 1, 1995 and June 30, 2006. We stratified subjects by evaluation outcome and evaluated and compared characteristics of medically acceptable and excluded donor candidates.
Data sources
Data was obtained from electronic patient records kept in the Organ Transplant Information System (OTIS) database as well as the general University of Michigan Hospital patient care database. Potential donors were classified according to three primary outcomes: (1) occurrence of live kidney donation; (2) medical exclusion from donation; and (3) non-medical exclusion from donation. In instances where more than one potential medical exclusion diagnosis was documented the primary investigator reviewed the medical records, including interview notes, labs and evaluation meeting minutes to establish the primary reason for donor exclusion. In the case of multiple exclusion diagnoses, priority was given to inadequate kidney function, followed by HTN and DM (e.g. a candidate with both HTN and obesity would have a primary exclusion diagnosis of HTN).
Demographic and clinical data of potential donors included: Serum creatinine, urea nitrogen, potassium, calcium, phosphorus, glucose, albumin, alanine aminotransferase, aspartate aminotransferase, bilirubin, hemoglobin and hematocrit, platelet count, spot urinalysis and microalbumin/creatinine ratio, ABO blood type, height, weight, age at evaluation, race (AA, CA or other), gender, body mass index (BMI) and relation to intended recipient. Disease states and medical contraindications discovered during the donor evaluation process were collapsed into following categories: Hypertension (defined as systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg, use of anti-hypertensive medications or 24-hour ambulatory blood pressure monitor results with > 25% of readings above 135/85 mmHg in daytime and/or 120/80 in nighttime), diabetes mellitus (abnormal glucose/abnormal glucose tolerance testing/anti-diabetic medication use), obesity (operationally a BMI of ≥ 40 kg/m2 or a BMI 30–39 kg/m2 with additional medical problems), cardiac abnormalities, viral hepatitis (B and C), kidney abnormalities (proteinuria, hematuria, nephrolithiasis), inadequate creatinine clearance (by Cockroft-Gault equation with iothalamate confirmation as appropriate), positive final crossmatch, abnormal imaging study (CT scan or ultrasound) and “other”.
Donor evaluation process
In our center, potential donor candidates either call in by phone or volunteer themselves in person if they accompany the potential recipient to an evaluation visit. The donor candidates are asked a series of screening questions to uncover absolute and relative contraindications to live kidney donation (Table 1). Donors without apparent contraindications to donation have initial ABO blood typing and tissue typing (cytotoxic (CDC-AHG) or flow crossmatch in repeat transplant and sensitized recipient candidates) performed. Compatible donors are scheduled for and in-person comprehensive evaluation visit. All evaluated candidates are ultimately discussed with our transplant group in a meeting consisting of nephrologists, surgeons, and social workers; transplant coordinators, HLA lab representatives and financial coordinators. Donors may be excluded at the initial meeting (or a future meeting if decision requires additional studies) and a primary reason for exclusion is listed in the chart along with other contributing medical conditions. The primary exclusion reason is decided by consensus with the evaluating team.
Table 1.
Donor Candidate Screening
| Absolute Contraindications |
| Active: |
| Kidney disease |
| Elevated blood pressures or diagnosis of HTN |
| Use of anti-hypertensive medication |
| Diabetes mellitus |
| Cardiac problems |
| Cancers |
| Body mass index > 40 kg/m2 |
| Nephrotoxic medication use |
| Relative Contraindications |
| Historic: |
| Gestational HTN, preeclampsia or eclampsia |
| Gestational diabetes |
| Cardiac problems or cancers |
| Other medical problems that may be exacerbated by donation |
Study endpoints and statistical analysis
There are three outcomes of the donor candidate evaluation: (a) live kidney donation; (b) medical exclusion from donation; (c) non-medical exclusion from donation. Logistic regression was used to examine the effect of age, race and gender on the likelihood of donor exclusion (medical or non-medical). The effects of age, race and gender on the BMI and creatinine clearance values were studied with gamma regression due to the positively skewed distribution of the observations. Chi square estimates were made comparing donation exclusion rates between racial and gender groups. Due to small numbers, candidates with self identified races other than AA and CA were excluded from analysis. In addition, candidates with no race recorded in the medical record were excluded from analysis. For all analyses, the statistical significance was set at P value less than 0.05. All analyses were performed using SAS statistical package, version 9.1 (Institute Inc., Cary, NC, USA).
Results
Screening survey results for 2006 for donor candidates excluded prior to in-person evaluation were examined as they represented the most complete and well-documented records during the study period. During this period a total of 93 donors were screened for 85 recipient candidates. The intended recipients were primarily CA (67%), followed by AA (25.9%) and other races (7.1%). The mean BMI of donors to CA and AA were similar (29.3 vs. 29.4 kg/m2, respectively). There were diverse screening exclusions. Hypertension accounted for exclusion of 14% of intended donors to CA and 22.7% of donors to AA. There were no exclusions for DM and obesity accounted for exclusion of 7% and 13.6% of donors to CA and AA respectively.
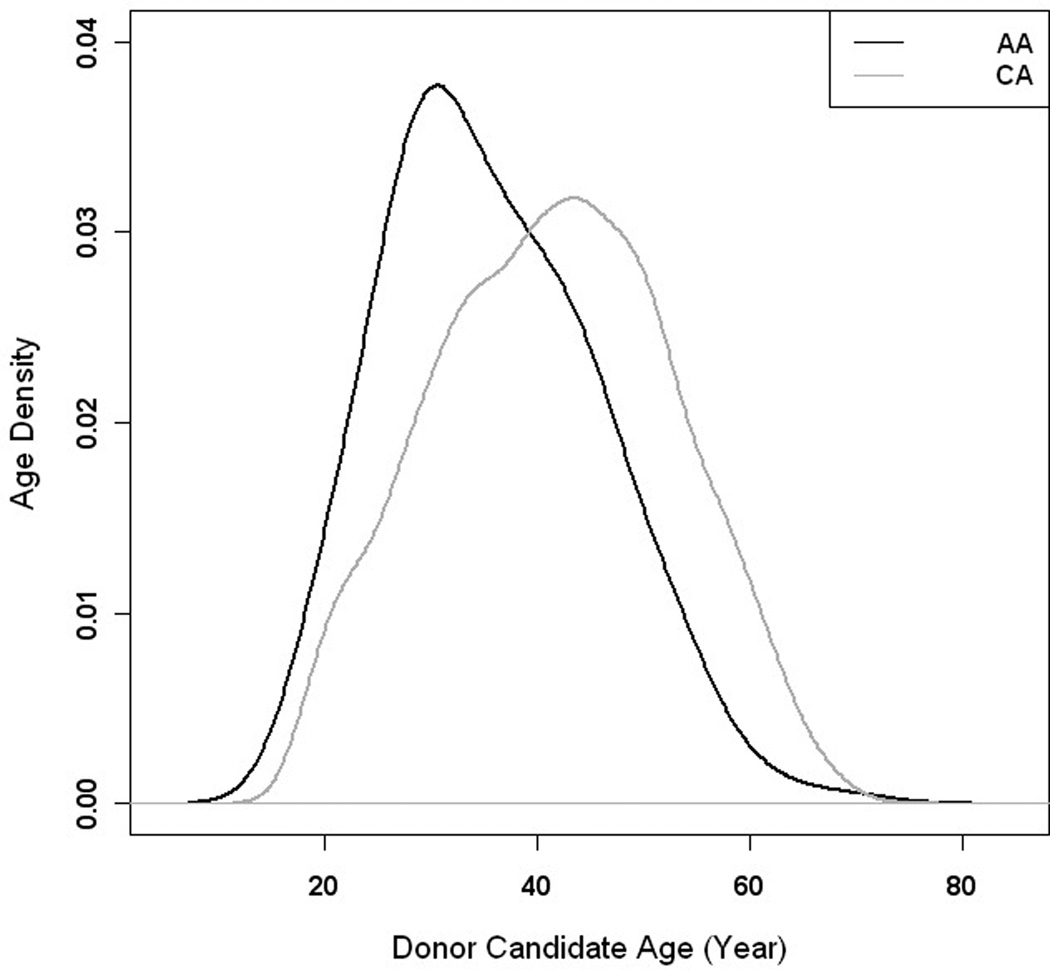
There were 2,519 individuals who passed the initial screening survey of medical status and then presented to the medical center for in-person donor evaluation. Among all donor candidates evaluated, 2165 were suitable for analysis (Table 2). Evaluated donors excluded from analysis included 300 with no race documented in the medical record, 35 Hispanic, 15 Asian, 3 Native American and 1 Bi-racial potential donor. In addition, thirteen potential donors had been tentatively approved as donors, but had not completed donation (0.5%). The majority of analyzed potential donors were of female gender and CA race. The overall mean age of donor candidates was 40.7 (±11.2) years. African American donors were over 5 years younger on average than CA donors (p < 0.001) (Figure 1). The mean BMI of donor candidates was 27.9 kg/m2 (±5.54) and ranged from 16.5–56.0 kg/m2. Three BMI outliers (56.0, 54.5, 50.2 kg/m2) were excluded from the mean calculations to give a more accurate representation of the overall cohort. Donor BMI was higher in AA vs. CA candidates (1.05, p< 0.001) and the distribution of weights was different between AA and CA candidates (Table 2) but gender was not significantly associated with BMI (p = 0.226) in this study population.
Table 2.
Evaluated Donor Candidate Demographics
| Characteristic | N (Percent) |
p-value | ||
|---|---|---|---|---|
| Total Donor Candidates | 2519 | |||
| No Race Documented | 300 | |||
| Hispanic Ethnicity | 35 | |||
| Asian | 15 | |||
| Native American | 3 | |||
| Bi-Racial | 1 | |||
| Analyzed Donor Candidates | 2165 | |||
| Female | 1300 (60) | |||
| Caucasian | 1862 (86) | |||
| African American | 303 (14) | |||
| Age, mean, years ± sd | 40.7± 11.2 | |||
| Caucasian | 41.5± 11.1 | |||
| African-American (ref. CA) | 35.8± 9.9 | < 0.001 | ||
| BMI, mean, kg/m2 ± sd | 27.9 ± 5.54 | |||
| Caucasian | 27.7 | |||
| African-American (ref. CA) | 29.1 | < 0.001 | ||
| BMI Distribution, mean, percent | ||||
| kg/m2 | Overall | CA | AA | |
| < 20 | 11.7 | 11.4 | 13.5 | 0.281 |
| 20–24.9 | 26.8 | 27.8 | 20.5 | 0.007 |
| 25–29.9 | 33.9 | 34.2 | 31.7 | 0.389 |
| ≥ 30 | 27.7 | 26.6 | 34.2 | 0.005 |
| Age Categories (AA and CA Only) | ||||
| 18–44 | 1410 (65.1) | |||
| 45–64 | 739 (34.1) | |||
| 65+ | 16 (0.8) | |||
BMI = Body Mass Index
Figure 1.
Density of age distribution by race. African-American (black line) and Caucasian (grey line)
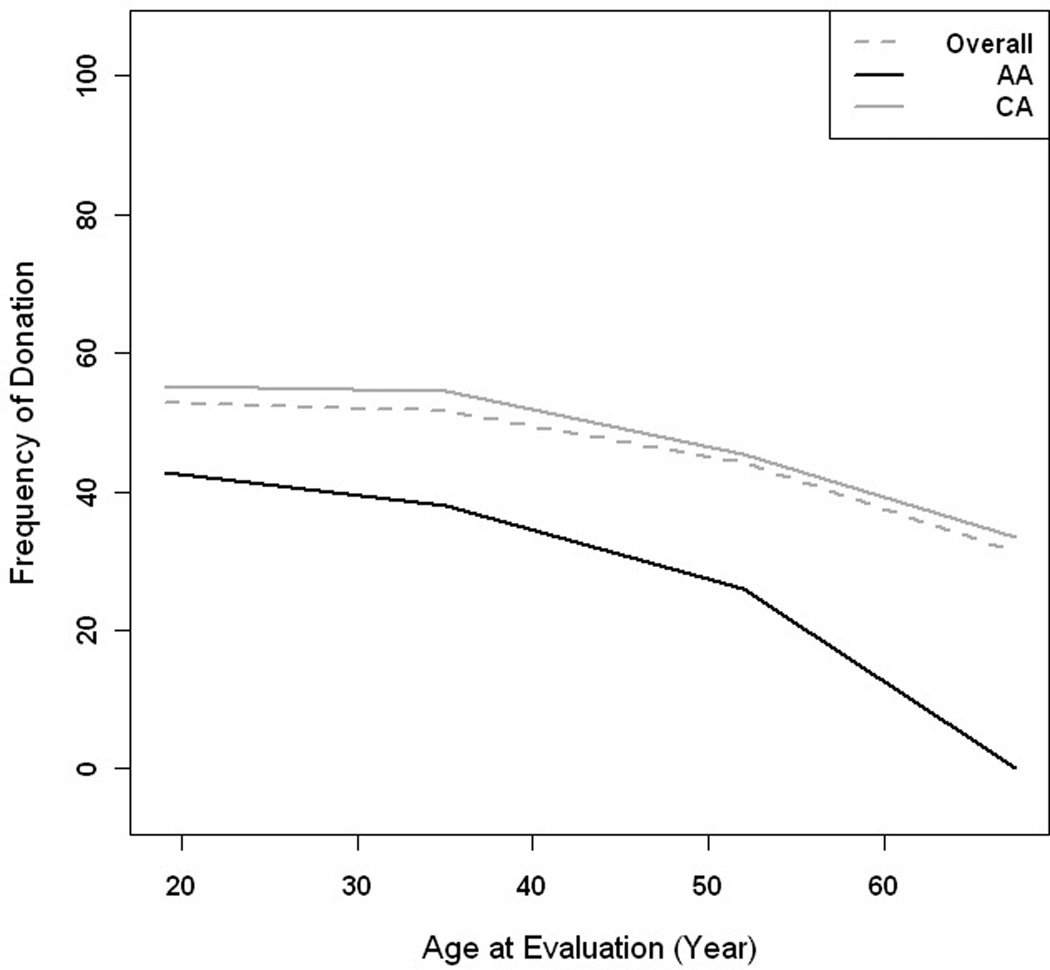
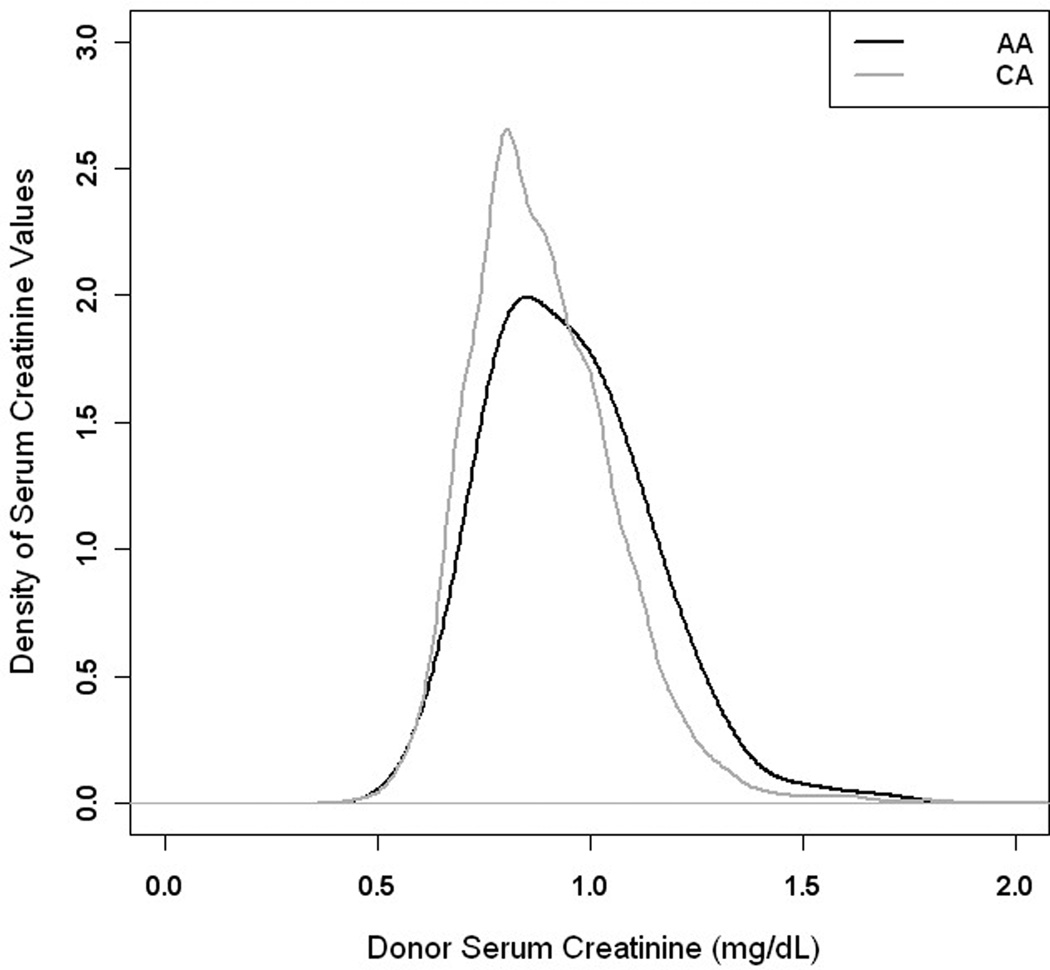
Overall, 48.7% of donor candidates were successful (Table 3). Male and female candidates were equally successful as donors (p = 0.112) but AA were only half as likely as CA to become donors (p< 0.001). The odds of medical donor exclusion increased by 2% with each year of increasing age (Figure 2). Estimated creatinine clearance did not differ by race (p = 0.623), but was 9% lower in female (0.91 compared to male, p < 0.001) donor candidates. The mean serum creatinine values in AA donor candidates were 5% higher than that of CA donor candidates (p < 0.001) (Figure 3).
Table 3.
Evaluated Donor Candidate Outcomes
| Characteristic | N | OR (Point estimate) | OR (95% CI) |
p-value |
|---|---|---|---|---|
| Excluded from Donation | 1350 (53.6) | |||
| Completed Donation | 1156 (45.9) | |||
| Incomplete Evaluations | 13 (0.8) | |||
| Odds of completed donation | ||||
| Female (reference =Male) | 0.87 | (0.73, 1.03) | 0.112 | |
| AA (reference CA) | 0.48 | (0.37, 0.62) | < 0.001 | |
| Donation by increasing year of age | 0.98 | (0.97, 0.99) | < 0.001 | |
| Age Categories (reference 18–44) | ||||
| 45–64 | 0.68 | (0.58, 0.82) | < 0.001 | |
| 65+ | 0.38 | (0.12, 1.05) | < 0.072 | |
| Estimated Creatinine Clearance | ||||
| AA (reference CA) | 0.993 | (0.96,1.02) | 0.623 | |
| Female (reference =Male) | 0.91 | (0.89,0.93) | < 0.001 | |
| Serum Creatinine | ||||
| AA (reference CA) | 1.072 | (1.05,1.10) | < 0.001 | |
Figure 2.
Plot of frequency of donation vs. age by race. African American (black line), Caucasian (grey) and overall (dashed)
Figure 3.
Density of distribution of serum creatinine for African American (black line) and Caucasian (grey line) donor candidates
Among donor candidates excluded for medical reasons, the most common diagnoses overall were HTN (24.7%), followed by inadequate creatinine clearance, (10.6%) and a positive final crossmatch (10.5%) (Table 4). HTN was equally prevalent in excluded AA and CA donors (22.6% and 25.1% respectively, p= 0.434), however, in excluded AA donors, the second most frequent exclusion diagnosis was obesity (12.8%), which was two-fold higher than the rate of exclusion for obesity among CA (5.8%). Donor candidates excluded for obesity had higher mean BMI values than the overall cohort (40.1 kg/m2 and 41.4 kg/m2 in AA and CA respectively). There was some overlap in categories, with 9.3% of candidates excluded for HTN also having an obese BMI. Overall, AA had a higher frequency of non-medical exclusion than CA candidates (Table 4). The most frequent reason donor candidates were excluded for a non-medical reason was the intended recipient received a deceased donor transplant (27.1%). The second and third most frequent non-medical exclusion reasons were recipient death (18.6%) and donors being lost to follow up (15%).
Table 4.
Frequency of Major Donor Exclusions
| Medical Donor Exclusion (%) | AA | CA | p-value |
|---|---|---|---|
| HTN | 22.6 | 25.1 | 0.43 |
| Diabetes Mellitus | 1.96 | 3.77 | 0.36 |
| Obesity | 12.75 | 5.75 | 0.01 |
| Cardiac Abnormalities | 0.98 | 3.95 | 0.13 |
| Viral Hepatitis (B and C) | 6.86 | 4.13 | 0.22 |
| Kidney Abnormalities | 6.86 | 10.05 | 0.31 |
| Inadequate Renal Function | 10.78 | 10.59 | 0.95 |
| Final Crossmatch Positive | 9.80 | 10.59 | 0.81 |
| Abnormal Imaging Study | 1.96 | 6.28 | 0.08 |
| Other | 25.45 | 19.79 | 0.12 |
| Non-Medical Donor Exclusion (%) | |||
| Overall Frequency of Exclusion | 46.88 | 38.59 | 0.003 |
| IR Received DD Txp | 28 | 23.3 | 0.37 |
| IR Received LD Txp | 4 | 2.2 | 0.42 |
| IR Death | 19.7 | 14.4 | 0.25 |
| DC Lost to Follow Up | 13.4 | 21.1 | 0.07 |
| DC Changed Mind | 8.9 | 13.3 | 0.20 |
| Other | 26.29 | 26.67 | 0.942 |
We examined era effects for the most common donor exclusions comparing donors evaluated January 1, 1995 through December 31, 2000 (era 1) with those evaluated January 1, 2001 through June 30, 2006 (era 2)(Table 5). The overall rate of exclusion increased from 44.4% in era 1 to 56.7% in the most recent era (p < 0.001). Among donor candidates excluded for medical reasons, we found that the rates of exclusion for HTN decreased between eras from 32.6% to 20.6% (p < 0.001). The frequency of exclusion for inadequate kidney function increased (4.9% to 13.7%, p < 0.001) and there were no significant differences in exclusion for positive crossmatch and obesity between eras. The overall serum creatinine values declined slightly between eras (0.92 to 0.89 mg/dL, p < 0.001) but were significant only in CA. The mean Cockroft-Gault creatinine clearance was slightly higher in the current era (116.5 vs. 112.3 mL/min, p= 0.008), and was increased for both AA and CA candidates (Table 5). Iothalamate GFR determinations were made in 10 candidates who successfully donated (1 AA) and 3 candidates who were ultimately excluded for low GFR (1 AA). All iothalamate GFR’s were measured in the current era.
Table 5.
Donor Outcome by Era of Evaluation
| Donor Exclusion Frequency (%) |
Era | p-value | |
|---|---|---|---|
| 1995–2000 | 2001–2006 | ||
| Overall | 44.4 | 56.7 | < 0.001 |
| HTN | 32.6 | 20.6 | < 0.001 |
| Inadequate Renal Function | 4.9 | 13.7 | < 0.001 |
| Final Crossmatch Positive | 8.4 | 11.6 | 0.20 |
| Obesity | 6.2 | 7.2 | 0.63 |
| Serum Creatinine, mean, mg/dL +/− sd | |||
| 1995–2000 | 2001–2006 | ||
| Overall | 0.92 +/− 0.197 | 0.89 +/− 0.173 | < 0.001 |
| CA | 0.91 +/− 0.196 | 0.88 +/− 0.170 | < 0.001 |
| AA | 0.96 +/− 0.198 | 0.94 +/− 0.182 | 0.415 |
| Creatinine Clearance, mean, mL/min +/− sd | |||
| (Cockroft-Gault estimate) | 1995–2000 | 2001–2006 | |
| Overall | 112.3 +/− 32.50 | 116.5 +/− 33.15 | 0.008 |
| CA | 112.3 +/− 32.40 | 115.8 +/− 32.57 | < 0.001 |
| AA | 110.8 +/− 33.21 | 121.5 +/− 36.52 | 0.025 |
Discussion
In 2001, the number of live kidney donors surpassed deceased donors in the U.S.1. Live kidney donation increased 73% in the period from 1997 through 2004, with only a small decline since that time. Currently, the largest growth of live kidney donation is from biologically unrelated donors who account for 36% of all live donors1. However most of the growth in live kidney donation has been limited to CA donors even though AA and other racial minorities are disproportionately over-represented among ESRD patients awaiting kidney transplantation. Since access to living kidney donation is not governed by an allocation system, the ability to receive a live donor kidney transplant is determined by the transplant candidates being able to find relatives and non-relatives who could step forward as potential donor candidates. According to U.S. census data, the average family size and number of sibships is larger for AA and Hispanic individuals compared to CA13. At the same time, AA and Hispanic individuals have a higher prevalence of medical conditions that often preclude them from being donor candidates such as HTN, obesity and DM11. Thus, the impetus for the current study was to determine whether these medical contraindications disproportionately affect potential AA donors who have passed an initial medical screening and have presented for a more comprehensive in-center donor evaluation. Consistent with national data, our donor candidates were primarily female and Caucasian1. We found that AA donor candidates who entered the in-center donor evaluation process were only 48% as likely as CA to become donors. We also found that AA donor candidates had a younger age distribution than CA candidates, perhaps reflecting the younger median age of ESRD in the AA population2, and the tendency of most donors to be immediate relatives, spouses and peers. Our findings have significant implications for access to living kidney donation as it shows that unwillingness to come forward as donors may not be the most important limiting factor for live donor transplant AA recipient candidates.
Despite a prior telephone screening with specific questions about HTN, DM, obesity and other commonly accepted medical contraindications to live donation, our study showed that a new diagnosis of HTN was established at an alarming 25% of evaluated donors, which is similar to the prevalence of HTN (29%) in the general adult population11, 12, 14. The finding is particularly concerning as a number of hypertensive individuals were excluded by the telephone screening. Because AA in the general population have a higher prevalence of HTN compared to CA (41% vs. 28%)11, 15 and that AA also have an earlier onset of HTN16, 17, we would have expected that new diagnosed HTN would be more common in AA potential donors compared to CA. To the contrary, we found a similar rate of newly diagnosed HTN in both AA and CA (22.6% and 25.1% respectively). However, although the frequency of HTN was similar, AA donor candidates were significantly younger than CA, consistent with the earlier onset of HTN often seen in AA. Our screening data did suggest increased HTN exclusions among screened AA potential donors, but the small numbers, lack of complete screening information and lack of direct knowledge of donor race limited our conclusions. The increasing incidence and earlier age of onset of HTN in the general population portends even a greater limitation for the living kidney donor pool in the U.S. As AA have a disproportionate prevalence of HTN in the general population, the impact on living kidney donation may be especially detrimental to this group.
We found that AA were more likely to be excluded for obesity compared to CA. Our finding is consistent with the higher prevalence of obesity in AA compared to CA individuals11, but it also reflects that donor candidates did not recognize themselves as obese since the initial pre-evaluation screening included questions to about weight and obesity which these potential donors were required to answer negatively before an in-center donor evaluation was scheduled. Among evaluated donors eventually excluded for obesity, 50% of CA and 38% of AA donors ideally should have been excluded by telephone screening for BMI > 40 kg/m2. With better screening exclusion we would have seen an even larger tendency for AA exclusion for obesity than we documented. Our findings appear to reflect underreporting on the part of screened candidates. We cannot determine if the underreporting was deliberate or unintentional. Our findings are consistent with the documented tendency for individuals to underestimate their weight and overestimate their height when surveyed18. In a positive light, the high rate of obesity among evaluated potential donors may reflect a high degree of motivation to become donors despite being overweight.
As a practice, we have evaluated donor candidates as they are at the time of the evaluation. We specifically have not requested weight loss for the purpose of donation nor do we contract with donors for them to maintain a weight after donation. We are aware of the high recidivism rate in individuals who lose weight19 and feel that using such donors at what may be an artificial nadir of their weight is not in their best interest. Consistent with this practice, for donors who report historic obesity, we usually expect to see an acceptable weight maintained for a least one year prior to the evaluation. It is not clear if our approach has any implications on donor acceptability by race, but our practice may result in overall more exclusion for obesity than in other centers.
The issue of overweight and obese donor candidates is of particular importance given the negative metabolic impact of obesity. Increasing BMI has been consistently shown to increase the risk of HTN,20 DM, and hyperlipidemia, overall increasing the risk of cardiovascular death in overweight individuals.21 Interestingly, increased BMI has not been consistently found to directly contribute to increased risk of CKD.22 However, increasing waist-to-hip ratios have been positively associated with increased CKD risk and cardiovascular death.23, 24 Waist circumference (WC) and waist-to-hip ratios (WHR) have not been well studied in living donor candidates, but appear to differ among races and be better predictors for metabolic abnormalities than BMI alone.25, 26 In addition, the weight of live donor candidates considered acceptable for donation varies widely by transplant center.25–27 There is evidence from the general population that young age, small WC and AA race are associated with decreased likelihood of metabolic abnormalities being associated with an obese BMI.28 As a result, simple BMI measurements are unlikely to fully describe our candidates metabolic risk profiles and with a more comprehensive description, perhaps some of the excluded AA candidates would be found acceptable for donation. In our center, we historically have not measured waist-to-hip ratios in our donor candidates, but WHR and other such anthropomorphic measures as percent body fat, assessment of visceral adipose and abdominal subcutaneous tissue and weight-height ratios will be important for future stratification of metabolic risk in live donor candidates.
Age was a significant factor in candidate appropriateness for donation, with the overall likelihood of donor exclusion increasing by 2% per year of donor age. Our finding is particularly important given the demographics of the ESRD and recipient candidate population. Individuals over age 64 make up the fastest growing group of ESRD patients and constitute 17% of currently waitlisted recipient candidates.1 The majority of live donors are spouses, siblings and parents of recipients. As donors and recipients age, there may be a significant decrease in access to live kidney donors due to medical exclusion. We may see increased pressure on the deceased donor system for recipient candidates with decreased live kidney donor access. Interestingly, age seemed to have a bigger impact on AA than CA likelihood of successful donation. The difference is unexpected and may be an artifact of having a number of young African American donor candidates.
Low estimated renal function was a cause for medical exclusion in over 10% of donor candidates. The frequencies were similar in AA and CA candidates. Our findings potentially highlight and reflect the lack of awareness of kidney disease and kidney disease risk in the U.S. population. In our study, AA candidates had higher serum creatinine values but clinically equivalent creatinine clearance. The AA mean creatinine clearance values appeared to increase over the course of the study, but not by a clinically meaningful amount. Throughout the study period, we utilized Cockroft-Gault calculations of creatinine clearance as our primary estimator of renal function. We used iothalamate measures rarely during the study, and only in the current era. In our center, the use of isotopic measures has historically been used to rule in otherwise acceptable candidates with low calculated clearance (usually slim females) rather than to rule out candidates. There is a potential for overestimation of renal function in our donor candidates such that we may be accepting higher risk candidates inadvertently. Inadequate donor kidney function poses a challenge for the future, as increasing age, HTN and obesity play a role in renal dysfunction, and more routine use of isotopic measures may be necessary.12, 29, 30
The analysis of era effects on likelihood of live donation revealed an overall significant increase in the likelihood of a donor candidate being excluded for medical reasons in the most recent era studied. Although the increased donor exclusions could be attributed to changes in the health of donor candidates in general, the results suggest somewhat different explanations. Interestingly, the likelihood of exclusion for HTN actually decreased in the recent era compared to earlier evaluations. Less HTN exclusions are probably not the result of healthier donors (as HTN prevalence has increased between the eras analyzed), but rather the result of more effective donor candidate screening prior to evaluation.
The largest increase in donor exclusions between the two eras studied for both AA and CA was among candidates with inadequate renal function. Our exclusion criteria (estimated creatinine clearance ≥ 80 ml/min) did not change during the eras. However, we have increasingly utilized more accurate measures of renal function (i.e. iothalamate) in our donor candidates, so we may be excluding candidates who previously would have been allowed to donate. The effect may have been more pronounced in AA given the higher BMI’s and the resultant increase in creatinine clearance when using the Cockroft-Gault equation. We have not used the modification of diet in renal disease (MDRD) calculation in our donor candidates, as the MDRD tends to underestimate true kidney function in individuals without kidney disease. We cannot exclude the possibility that as the need for living donors has increased that we have been seeing candidates with increasingly marginal kidney function. In the future, consistently utilizing iothalamate or a similar method will enhance our ability to determine the true trends in donor candidate kidney function.
The rate of donor exclusion for both positive final crossmatch and obesity did not increase over the study period. We know that during the study period that the U.S. population as a whole has had a significant increase in obesity prevalence. Our donor candidates however remain a selected, relatively healthy population with seemingly low obesity rates. A small number of our candidates were obese, but had a more prominent medical condition (HTN), which was their primary cause of exclusion. Certainly, the frequency of donor exclusion underestimates the prevalence of obesity in our donor population. At the same time, the concern that increasingly obese donor candidates were being accepted for donation is not strongly supported by our data.
During the study period, there was a trend towards more CA candidates than AA being lost to follow up. Our results are in contrast to the findings of both Lunsford31 and Reeves-Daniel32, who found AA more likely to be lost to follow up. African-Americans showing a greater interest in live donation than CA would directly contradict much of the literature suggesting a lack of interest among AA to donate. Loss to follow up was not a primary focus of our study and the overall number of donor candidates in our study limits our conclusions. In addition there are many socioeconomic and cultural issues not addressed by our study that could impact the likelihood of follow through with donor evaluation. In the future deliberate evaluation of patient, provider or center factors that may improve donor follow through in general and AA donation specifically could be important to expanding the donor pool.
Our transplant center is located in the southeastern part of Michigan. Fourteen percent of donors evaluated over the study period were AA. Our AA potential donor population was consistent the U.S. population representation of 13% (and the Michigan population of 14%)3, although falling short of the waiting list (34%) representation. The significant numbers of AA individuals volunteering as donor candidates highlights the willingness on the part of AA to donate, although the proportion of donors does not meet the recipient candidate need. Our study suggests that a significant limiting factor to living kidney donation is donor medical disease, and supports the data generated by Lunsford and colleagues31. From a policy standpoint, our findings indicate a need to focus efforts not just on encouraging donation, but on educating the population on medical contraindications to donation to help select the most appropriate cohort of donor candidates. Our study also highlights the complex nature of the shortage of donor organs, as previously discussed by Young and colleagues33. Furthermore, the results of these study points to high reservoir of unrecognized potentially serious medical illnesses in a relatively young cohort of candidates who considered themselves “healthy” and it shows that educational awareness about kidney disease, HTN and obesity has not penetrated to all relevant segments of the society.
We found a high frequency of donor exclusion resulting from the intended recipient receiving a deceased donor transplant. Although initially counter-intuitive, such a finding accurately reflects our patient population. Many if not most of our candidates do not have a live donor candidate at the time of recipient evaluation. A number of our waitlisted candidates look for years before having a potential candidate who volunteers and makes it through the initial screening. In addition, many of our candidates are reluctant to accept a live donor initially and only consider one after significant time on dialysis or after suffering medical complications. We see this not uncommonly, particularly when the potential donors are likely to be a recipient’s children.
In our program HTN, DM, CrCl < 80ml/min, current pregnancy, ischemic heart disease, chronic NSAID therapy, active infection and active malignancy are and were absolute contraindications to donation throughout the study period. We have increasingly utilized iothalamate measurements in selected donors (usually thin females) who were thought to likely be acceptable, usually finding true renal function that is above our cutoff. Over the study period understanding of HTN and consensus HTN recommendations (JNC-7) have likely lowered our threshold for exclusion on that basis. In addition, the increased availability of 24-hour ambulatory blood pressure monitoring has added to our accuracy in detecting sub-clinical HTN.
One of the most surprising findings was that 300 individuals had no race data available. In our screened candidates, we expect this as we do not ask for race. However, in those who appear for evaluation, the lack of race data reveals multiple factors at work. We looked for race data among the patient demographics captured at the time of patient registration, among the nephrology, surgery and social work notes generated as part of the evaluation and in the minutes of the evaluation team meetings. To have no race data means that information was absent in all five places. The finding is most interesting because a number of these individuals successfully donated. Perhaps excess political correctness led us to not dictate race in our notes. At the same time, the donor candidates themselves had to choose to leave race off of their registration forms. This was allowable as many of the data elements, including race and religion are optional. The absence of this information perhaps exposes a discomfort among both patients and providers with race information that is worthy of additional examination.
This was a registry study, so we were limited to information in the database. Small numbers of older donors and other ethnicities limit interpretation. Our donor population may have changed over the study period in ways not appreciated by our analysis that may influence our results. We did not measure waist-to hip ratios or other anthropomorphic measures that would help to better describe the metabolic risk profiles of our donor candidates. In addition, we were unable to report on the demographic and medical disease distribution of all volunteer donor candidates. Our donor screening forms do not capture race data. In addition, as our donor screening forms were developed for clinical not research purposes, historically there has not been accurate tracking of donors excluded by screening. Our screening data presented is consistent with our more recent experience, but nevertheless the lack of complete data over the full study period does leave the question of screening exclusion frequency incompletely answered.
Conclusion
A plethora of treatable medical disorders go unrecognized in a significant proportion of Americans in the middle age and older age groups. Hypertension and obesity (major impediments to live kidney donation) shrink the potential living kidney donor pool by more than one-third of all U.S. adults. These medical barriers to donation have a disproportionately greater impact on limiting access of AA to living donor kidney transplantation.
Acknowledgments
Funding Source: No external funding
Footnotes
Transparency Declaration
This paper has not been published previously in whole or in part, except in abstract form
| Silas P. Norman, M.D. | None to declare |
| Peter XK. Song, Ph.D. | None to declare |
| Youna Hu, M.S. | None to declare |
| Akinlolu O. Ojo, M.D., Ph.D. | None to declare |
Literature Cited
- 1.Rockville, MD: Health Resources and Services Administration HSB, Division of Transplantation; 2007 Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1996–2006. [Google Scholar]
- 2.U.S. Renal Data System. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2007. USRDS 2007 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United, States. [Google Scholar]
- 3.Profiles of General Demographic Characteristics. Census US, editor. 2000, Census of Population and Housing. 2001
- 4.Monet T, Pullen-Smith B, Haisch C, Ensley D, Royal R. Living renal donation in the African-American community. Transplant Proc. 1997 Dec;29(8):3649–3650. doi: 10.1016/s0041-1345(97)01058-0. [DOI] [PubMed] [Google Scholar]
- 5.Hidalgo G, Tejani C, Clayton R, et al. Factors limiting the rate of living-related kidney donation to children in an inner city setting. Pediatr Transplant. 2001 Dec;5(6):419–424. doi: 10.1034/j.1399-3046.2001.t01-2-00033.x. [DOI] [PubMed] [Google Scholar]
- 6.Shilling LM, Norman ML, Chavin KD, et al. Healthcare professionals' perceptions of the barriers to living donor kidney transplantation among African Americans. J Natl Med Assoc. 2006;Jun;98(6):834–840. [PMC free article] [PubMed] [Google Scholar]
- 7.Trollinger J, Flores J, Corkill JK, Ryan R, Light JA. Increasing living kidney donation in African Americans. Transplant Proc. 1997 Dec;29(8):3748–3750. doi: 10.1016/s0041-1345(97)01097-x. [DOI] [PubMed] [Google Scholar]
- 8.Rodrigue JR, Cornell DL, Lin JK, Kaplan B, Howard RJ. Increasing live donor kidney transplantation: a randomized controlled trial of a home-based educational intervention. Am J Transplant. 2007 Feb;7(2):394–401. doi: 10.1111/j.1600-6143.2006.01623.x. [DOI] [PubMed] [Google Scholar]
- 9.Boulware LE, Ratner LE, Sosa JA, Cooper LA, LaVeist TA, Powe NR. Determinants of willingness to donate living related and cadaveric organs: identifying opportunities for intervention. Transplantation. 2002 May 27;73(10):1683–1691. doi: 10.1097/00007890-200205270-00029. [DOI] [PubMed] [Google Scholar]
- 10.Tankersley MR, Gaston RS, Curtis JJ, et al. The living donor process in kidney transplantation: influence of race and comorbidity. Transplant Proc. 1997 Dec;29(8):3722–3723. doi: 10.1016/s0041-1345(97)01086-5. [DOI] [PubMed] [Google Scholar]
- 11.National Center for Health Statistics. Hyattsville, MD: U.S. Government Printing Office; 2007. Health, United States, 2007 With Chartbook on Trends in the Health of Americans. 2007. [PubMed] [Google Scholar]
- 12.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003 Dec;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 13.Bureau USC. Current Population Survey Reports, Families and Living Arangements. [Accessed April 23, 2008];2006 Mar; Http://www.census.gov, 2006.
- 14.Calhoun DA, Jones D, Textor S, et al. Resistant Hypertension: Diagnosis, Evaluation, and Treatment. A Scientific Statement From the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008 Apr 7; doi: 10.1161/HYPERTENSIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 15.Ostchega Y, Yoon SS, Hughes J, Louis T. National Center for Health Statistics Data Brief No. 3. Hyattsville, MD: National Center for Health Statistics; 2008. Hypertension Awareness, Treatment and Control-Continued Disparities in Adults: United States, 2005–2006. [PubMed] [Google Scholar]
- 16.Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. Jama. 2003 Jul 9;290(2):199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- 17.Wilhelmsen L, Rosengren A, Eriksson H, Lappas G. Heart failure in the general population of men--morbidity, risk factors and prognosis. J Intern Med. 2001 Mar;249(3):253–261. doi: 10.1046/j.1365-2796.2001.00801.x. [DOI] [PubMed] [Google Scholar]
- 18.Johnson WD, Bouchard C, Newton RL, Jr, Ryan DH, Katzmarzyk PT. Ethnic differences in self-reported and measured obesity. Obesity (Silver Spring) 2009 Mar;17(3):571–577. doi: 10.1038/oby.2008.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr. 2005 Jul;82(1 Suppl):222S–225S. doi: 10.1093/ajcn/82.1.222S. [DOI] [PubMed] [Google Scholar]
- 20.Gelber RP, Gaziano JM, Manson JE, Buring JE, Sesso HD. A prospective study of body mass index and the risk of developing hypertension in men. Am J Hypertens. 2007 Apr;20(4):370–377. doi: 10.1016/j.amjhyper.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caprio S, Daniels SR, Drewnowski A, et al. Influence of race, ethnicity, and culture on childhood obesity: implications for prevention and treatment: a consensus statement of Shaping America's Health and the Obesity Society. Diabetes Care. 2008 Nov;31(11):2211–2221. doi: 10.2337/dc08-9024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foster MC, Hwang SJ, Larson MG, et al. Overweight, obesity, and the development of stage 3 CKD: the Framingham Heart Study. Am J Kidney Dis. 2008 Jul;52(1):39–48. doi: 10.1053/j.ajkd.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elsayed EF, Sarnak MJ, Tighiouart H, et al. Waist-to-hip ratio, body mass index, and subsequent kidney disease and death. Am J Kidney Dis. 2008 Jul;52(1):29–38. doi: 10.1053/j.ajkd.2008.02.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elsayed EF, Tighiouart H, Weiner DE, et al. Waist-to-hip ratio and body mass index as risk factors for cardiovascular events in CKD. Am J Kidney Dis. 2008 Jul;52(1):49–57. doi: 10.1053/j.ajkd.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perry A, Wang X, Kuo YT. Anthropometric correlates of metabolic syndrome components in a diverse sample of overweight/obese women. Ethn Dis. 2008 Spring;18(2):163–168. [PubMed] [Google Scholar]
- 26.Lee S, Kuk JL, Hannon TS, Arslanian SA. Race and gender differences in the relationships between anthropometrics and abdominal fat in youth. Obesity (Silver Spring) 2008 May;16(5):1066–1071. doi: 10.1038/oby.2008.13. [DOI] [PubMed] [Google Scholar]
- 27.Davis CL, Delmonico FL. Living-donor kidney transplantation: a review of the current practices for the live donor. J Am Soc Nephrol. 2005 Jul;16(7):2098–2110. doi: 10.1681/ASN.2004100824. [DOI] [PubMed] [Google Scholar]
- 28.Wildman RP, Muntner P, Reynolds K, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004) Arch Intern Med. 2008 Aug 11;168(15):1617–1624. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 29.Kambham N, Markowitz GS, Valeri AM, Lin J, D'Agati VD. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int. 2001 Apr;59(4):1498–1509. doi: 10.1046/j.1523-1755.2001.0590041498.x. [DOI] [PubMed] [Google Scholar]
- 30.Molenaar EA, Hwang SJ, Vasan RS, et al. Burden and Rates of Treatment and Control of Cardiovascular Disease Risk Factors in Obesity: The Framingham Heart Study. Diabetes Care. 2008 Mar 28; doi: 10.2337/dc07-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lunsford SL, Simpson KS, Chavin KD, et al. Racial disparities in living kidney donation: is there a lack of willing donors or an excess of medically unsuitable candidates? Transplantation. 2006 Oct 15;82(7):876–881. doi: 10.1097/01.tp.0000232693.69773.42. [DOI] [PubMed] [Google Scholar]
- 32.Reeves-Daniel A, Adams PL, Daniel K, et al. Impact of race and gender on live kidney donation. Clin Transplant. 2008 Sep 11; doi: 10.1111/j.1399-0012.2008.00898.x. [DOI] [PubMed] [Google Scholar]
- 33.Young CJ, Gaston RS. Renal transplantation in black Americans. N Engl J Med. 2000 Nov 23;343(21):1545–1552. doi: 10.1056/NEJM200011233432107. [DOI] [PubMed] [Google Scholar]