Abstract
Background
Sepsis is a lethal syndrome annually affecting ~900,000 patients in the United States alone. Despite their benefit in rheumatoid disease, selective anti-tumor necrosis factor (anti-TNF) agents failed to improve outcome in early sepsis trials in the 1990’s. However, data from additional sepsis trials testing these agents is now available.
Purpose
To determine the effect on survival of selective anti-TNF agents in randomized clinical sepsis trials.
Data Sources
PubMed, Scopus, EMBASE, and Web of Science.
Study Selection
Randomized human sepsis trials of selective anti-TNF agents reporting survival rates.
Data Synthesis
Two investigators independently collected relevant data on study characteristics, treatment interventions, and patient from each study.
Results
Anti-TNF agents in 15 sepsis trials (n=8,896 patients) meeting inclusion criteria had similar effects (I2=0, p=0.84) and compared to controls (placebo in 14 trials or a lower dose in 1 trial) overall decreased the relative risk (RR) of death (95% CI) [0.93 (0.88, 0.98), p=0.01]. In subgroup analysis, TNF monoclonal antibodies (10 trials, n=6,818) alone produced a significant survival benefit [0.93 (0.87, 0.99), p=0.02] (I2=0, p=0.83). TNF polyclonal antibodies (2 trials, n=151) and low molecular weight soluble receptor (2 trials, n=1,786) had similar beneficial effects to anti-TNF agents overall [0.82(0.49, 1.37), p=0.45; 0.93(0.81, 1.08), p=0.33, respectively]. The effect of TNF high molecular weight soluble receptor (1 trial, n=141) was not significantly different from other agents but was on the side of harm [1.50 (0.86, 2.61), p=0.16].
Limitations
Limited secondary end-point data.
Conclusion
Anti-TNF agents produced a modest but significant decrease in the risk of dying with sepsis. Prior individual trials failed to demonstrate benefit, likely because they were underpowered. A definitive trial demonstrating the potential benefit of such agents might require 10,000 or more septic patients.
Keywords: Sepsis, septic shock, tumor necrosis factor, treatment
Introduction
Selective inhibitors of tumor necrosis factor-α (TNF) to limit inflammation and joint injury have become a mainstay for managing rheumatoid arthritis(1). Early in their development, similar anti-TNF agents were also tested in patients with severe infection causing sepsis and septic shock(2-10). Preclinical sepsis studies had shown that TNF levels were elevated and selective inhibition of TNF prevented injury and death(11). This and other data led to the hypothesis that excessive TNF release during infection caused an injurious host inflammatory response central to the pathogenesis of sepsis(11, 12). Application of selective anti-TNF agents for sepsis was viewed as a therapeutic breakthrough for a syndrome which may affect up to 900,000 patients annually in the United States alone with a mortality rate of 15 to 45%, depending on disease severity(13, 14). Despite high expectations, however, anti-TNF agents failed during the 1990’s to improve survival in clinical sepsis trials.
This early clinical experience with anti-TNF agents in sepsis was a setback to the biotechnology and pharmaceutical industries, which had made substantial investments in the research effort. More fundamentally, the experience raised questions regarding the role of TNF in the pathogenesis of sepsis(15). However interest in the potential for anti-TNF therapy for sepsis, although dampened, has continued. Since the results from trials with anti-TNF agents in sepsis were last systematically examined almost a decade ago (16, 17), survival data from additional randomized trials testing similar agents and including an additional 2,758 patients have been published (18-20). Based on the continued interest in such therapies and with the increased power provided by more recent trials and patients, we have performed a meta-analysis examining whether selective anti-TNF agents improve survival in septic patients.
Methods
Data Sources and Searches
PubMed, Scopus, EMBASE, and Web of Knowledge (Web of Science, Biological Abstracts and MEDLINE) data bases were searched using the terms tumor necrosis factor inhibitor, sepsis and human clinical trial to identify clinical trials of anti-TNF therapies in sepsis (last searched August of 2011). To maximize our ability to find studies, the specific MESH and EMTREE controlled vocabulary terms were adapted (JW) to the unique searching features of each database (Table E1 in supplemental material). Searches were not limited by date, language or publication status.
Study Selection
Studies meeting the following criteria were included: randomized trial design; enrollment of adult patients (>18 y/o) with sepsis or septic shock; similar treatment for all study groups with the exception of a predetermined anti-TNF regimen; and comparison of survival rates between patients randomized to receive an anti-TNF agent or either placebo or a very low dose of anti-TNF agent. Criteria for sepsis or septic shock needed to be consistent with the American College of Chest Physicians and Society of Critical Care Medicine Consensus Conference sepsis definition (21).
Data Extraction and Quality Assessment
Two investigators knowledgeable of critical care medicine (PQ and PQE) independently reviewed the included studies using a standardized data collection protocol. A third author evaluated and resolved discrepancies (CN). Data was collected on study characteristics, treatment interventions, and patient outcomes (Tables 1 and 2; Figure 1). The Jadad score was used to compare the quality of included trials (Table E2 in supplemental material) (22). Financial relationships between manufacturers and authors were recorded to examine sources of potential bias.
Table 1.
Summary of clinical trials
| Author,Y [Ref] | Treatment | Years Enrolled | Blinding | Jadad Score | Inclusion Criteria | Reported Mortality | ||
|---|---|---|---|---|---|---|---|---|
| Anti-TNF Agent | Preparation | Manufacturer | ||||||
| Abraham E et al., 1995[2] | mAb | BAYx1351 | Bayer/Miles | NR | Double Blinded | 5 | Severe Sepsis * | 28d # |
| Cohen J et al., 1996[3] | mAb | BAYx1351 | Bayer/Miles | 05/’91-07/’93 | Double Blinded | 5 | Severe Sepsis | 28d # |
| Abraham E et al., 1998[4] | mAb | BAYx1351 | Bayer/Miles | NR | Double Blinded | 4 | Severe Sepsis ** | 28d # |
| Fisher CJ Jr et al., 1993[5] | mAb | CB006 | Celltech | NR | Open Label | 0 | Severe Sepsis | 28d |
| Reinhart K et al., 1996[6] | mAb | MAK195F | Knoll | NR | Open Label | 1 | Severe Sepsis | 28d |
| Gallagher J et al., 2001[23] | mAb | MAK195F | Knoll | NR | Open Label | 1 | Severe Sepsis | 28d |
| Reinhart K et al., 2001[24] | mAb | MAK195F | Knoll | 08/’95-04/’97 | Double Blinded | 3 | Severe Sepsis ** | 28d # |
| Panacek EA et al., 2004[19] | mAb | Afelimomab## | Abbott | 01/’96-06/’99 | Double Blinded | 5 | Severe Sepsis | 28d # |
| Dhainaut JF et al., 1995[7] | mAb | CDP571 | Celltech | 09/’92-05/’93 | Blinded | 1 | Severe Sepsis ** | 28d |
| Clark MA et al., 1998[8] | mAb | cA2 | Centacor | 05/’93-07/’95 | Double Blinded | 3 | Severe Sepsis | 40d |
| Rice TW et al., 2006[20] | pAb | CytoFab | Protherics | 09/’97-07/’98 | Double Blinded | 4 | Severe Sepsis | 28d # |
| Morris PE et al., 2012[18] | pAb | AZD9773 | AztraZeneca | NR | Double Blinded | 2 | Severe Sepsis | 28d |
| Abraham E et al., 1997[9] | p55 | p55-IgG | Hoffman | NR | Double Blinded | 3 | Severe Sepsis | 28d # |
| Abraham E et al., 2001[25] | p55 | TNFsr-p55 | Hoffman | NR | Double Blinded | 3 | Severe Sepsis | 28d # |
| Fisher CJ Jr et al., 1996[10] | p75 | TNFsr-p75 | Immunex | NR | Double Blinded | 3 | Severe Sepsis ** | 28d # |
NR=not reported, Y=Yes; mAb=monoclonal antibody; pAb=polyclonal antibody;
Severe sepsis-According to ACCP/SCCM Consensus Conference Criteria21;
Required that patients meeting ACCP/SCCM criteria also have prespecified decreases in blood pressure;
28d all cause mortality rate was reported as a primary endpoint;
Same preparation as MAK195F.
Table 2.
Summary of treatment regimens
| Author [Ref] | Anti-TNF Agent | Regimen
|
#Nonsurvivors/Total | |||
|---|---|---|---|---|---|---|
| Dose | Duration (min) | Timing (h)* | Volume (mL) | |||
| Abraham E et al., [2] | mAb | Placebo:0.25%HSA | 30±10 | 4 | 100 | 108/326 |
| 7.5mg/kg; | 95/322 | |||||
| 15mg/kg | 101/323 | |||||
|
| ||||||
| Cohen J et al., [3] | mAb | Placebo:0.25% HSA | 30 | NR | 100 | 66/167 |
| 3mg/kg; | 57/181 | |||||
| 15mg/kg | 87/205 | |||||
|
| ||||||
| Abraham E et al., [4] | mAb | Placebo:0.25% HSA | 30±10 | 4 | 100 | 398/930 |
| 7.5mg/kg | 382/948 | |||||
|
| ||||||
| Fisher CJ Jr et al., [5] | mAb | 0.1mg/kg; | 5~30 | NR | NR | 6/19 |
| 1.0mg/kg; | 8/19 | |||||
| 10mg/kg; | 9/20 | |||||
| 1.0mg/kg qdX2 | 10/22 | |||||
|
| ||||||
| Reinhart K et al., [6] | mAb | Placebo: NS | 15 | NR | 25 | 12/29 |
| 0.1mg/kg q8hrsX9 | 19/34 | |||||
| 0.3mg/kg q8hrsX9 | 14/30 | |||||
| 1.0mg/kg q8hrsX9 | 11/29 | |||||
|
| ||||||
| Gallagher J et al., [25] | mAb | Placebo: (NR) | 20 | NR | NR | 5/9 |
| 0.3mg/kg q8hrsX9 | 3/9 | |||||
| 1.0mg/kg q8hrsX9 | 2/9 | |||||
| 3.0mg/kg q8hrsX9 | 2/9 | |||||
|
| ||||||
| Reinhart K et al., [26] | mAb | Placebo: (NR) | 15 | NR | NR | 128/222 |
| 1mg/kg q 8hrsX9 | 121/224 | |||||
|
| ||||||
| Panacek EA et al., [18] | mAb | Placebo: 1.5mg/kg HSA | 15 | 24 | NR | 477/1329 |
| 1mg/kg q 8hrsX9 | 421/1305 | |||||
|
| ||||||
| Dhainaut JF et al., [7] | mAb | Placebo: (NR) | NR | NR | NR | 6/10 |
| 0.1mg/kg; | 3/6 | |||||
| 0.3mg/kg; | 6/6 | |||||
| 1.0mg/kg; | 6/10 | |||||
| 3.0mg/kg | 5/10 | |||||
|
| ||||||
| Clark MA et al., [8] | mAb | Placebo: PBS | 60 | 12 | 250 | 11/28 |
| 300mg | 10/28 | |||||
|
| ||||||
| Rice TW et al., [19] | pAb | Placebo: 5mg/kg HSA | NR | NR | 100 | 14/38 |
| 250U/kg load+50U/kg q12hrsX9 | 11/43 | |||||
|
| ||||||
| Morris PE et al., [20] | pAb | Placebo: (NR) | NR | NR | NR | 6/23 |

|
13/47** | |||||
|
| ||||||
| Abraham E et al., [9] | p55 | Placebo: (NR) | ≤30 | 12 | 20 | 20/49# |
| 0.008mg/kg | 31/54 | |||||
| Placebo: (NR) | 54/140 | |||||
| 0.042mg/kg; | 53/145 | |||||
| 0.083mg/kg; | 52/159 | |||||
|
| ||||||
| Abraham E et al., [27] | p55 | Placebo: (NR) | 5 to 7 | 12 | NR | 192/680 |
| 0.125mg/kg | 177/662 | |||||
|
| ||||||
| Fisher CJ Jr et al., [10] | p75 | Placebo: (NR) | 30 | NR | 100 | 10/33 |
| 0.15mg/kg; | 9/30 | |||||
| 0.45mg/kg; | 14/29 | |||||
| 1.5mg/kg | 26/49 | |||||
NR=not reported; mAb=monoclonal antibody; pAb=polyclonal antibody; HAS= human serum albumin; NS= normal saline; PBS= phosphate buffer saline;
Time following either randomization or fulfilling enrolment criteria;
All doses combined;
subgroup of the overall placebo patients (i.e. 86 survivors of 140 total patients).
Figure 1.
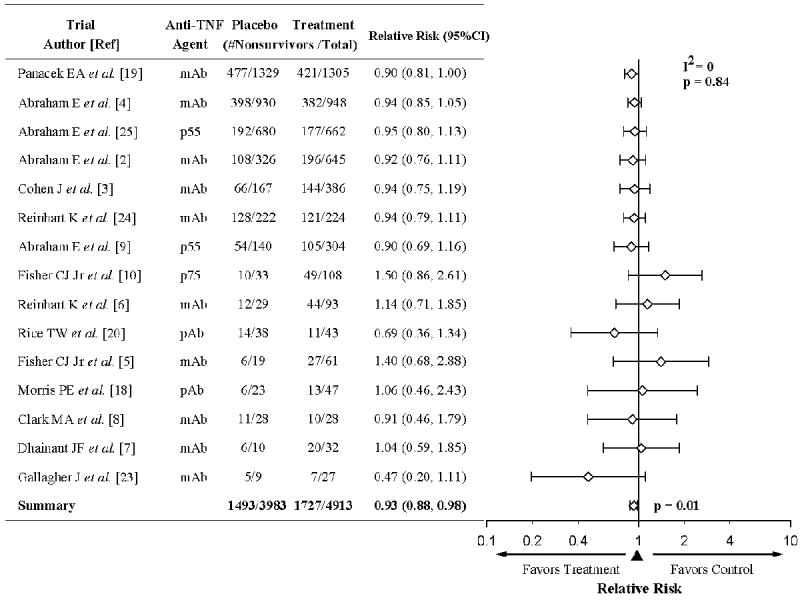
Effects of anti-TNF agents on survival in randomized controlled trials. The level of consistency among the trials (I2 value) and the relative risk (RR) of death and 95% CI with anti-TNF therapy are shown. Nine of the 15 trials tested multiple doses of anti-TNF agents (Table 2). In eight trials, the RR with different anti-TNF therapy doses were similar (p=ns) so we averaged over dose to increase our ability to find significant effects.(2, 3, 5-7, 10, 18, 23) In one trial [Abraham et al., 1997, (9)] enrollment was stopped early for the lowest dose of anti-TNF agents studied (TNFsr-p55). This low dose had a survival effect on the side of harm, while the two higher doses studied had effects on the side of benefit (p=0.02 for the difference) (9). Because this low dose subgroup was not included in our primary analysis in the original trial report, it was not included in the analysis presented in the Figure 1 here (However, see our sensitivity analysis results where we show the results if it is included). As shown, overall anti-TNF agents had highly consistent effects and were associated with a significant decrease in the relative risk of death. In all seven trials enrolling more than 400 patients, anti-TNF agents had effects on the side of benefit.
Sensitivity Analysis. Including the low dose TNFsr-p55 subgroup from the trial noted above [Abraham et al., 1997 (9)] in the meta-analysis did not change the overall results (p=ns). The effects of anti-TNF agents were still consistent across the 15 trials (I2 = 0%) and the RR still significant [0.94 (0.89, 0.99), p =0.02]. Also, removing the trial in which the lowest dose of treatment was employed as control [Fisher et al., 1993 (5)] did not change the overall results (p=ns); the findings were still consistent for the 14 remaining trials (I2 = 0%) and the RR was still significant [0.93 (0.88, 0.98), p=0.01].
Data Synthesis and Analysis
In trials testing more than one dose of an anti-TNF agent, we assessed the effect of dose (treated as continuous) on survival rate across those studies testing the same type of agent. Random-effect logistic regression models were used to account for the correlation of subgroups within a study using SAS PROC GLIMMIX (SAS version 9.2, Cary, NC). This is the only analysis where we used odds ratio (OR). In all other analyses, the treatment effects were compared using the relative risk (RR) of death. All treatment doses within each study were pooled when appropriate. Random-effect models were used to compare treatment effects. For one study examining four doses of an agent but not a placebo, the lowest treatment dose was employed as control in analysis(5). Sensitivity analysis was conducted with this study excluded. Heterogeneity among studies was assessed using the Q statistic and I2 value. Studies were only combined when I2 <30%. In subgroup analysis, the influence of type of anti-TNF agent and study size was examined. The effects of therapy were also examined in trials comparing similar subgroups of patients based on the presence or absence of shock, cytokine levels (TNF and IL-6), type of underlying bacterial infection and severity of illness. Potential publication bias and its influence on the treatment effect across studies were evaluated with funnel plot and Eggers regression. Numbers needed to treat (NNT) were calculated as the inverse of risk difference, which was itself calculated based on the estimated overall RR and the median control group mortality rate of the studies included in this meta-analysis. This calculation also generated the treatment and control group mortality rates that were used for the power calculation. All analyses were done using R package meta version 1.6-1 (http://cran.r-project.org/web/packages/meta/index.html) and metafor version 1.6-0 (http://www.metafor-project.org/) unless noted otherwise.
Results
Overview of Included Studies
Four hundred and fifty-two studies were identified by our literature searches; fifteen met inclusion criteria (2-10, 18-20, 23-25) (Figure E1 in supplemental materials). These studies encompassed 8,896 septic patients and investigated four anti-TNF agents. In 12 trials, two types of anti-TNF antibodies were studied; 10 trials (6,818 patients) testing anti-TNF monoclonal antibodies (TNFmAb)(2-8, 19, 23, 24) and two trials (151 patients) testing anti-TNF polyclonal antibodies (TNFpAb)(18, 20). In three trials, two types of soluble TNF receptors were studied; two trials (1,786 patients) testing a low molecular weight TNF soluble receptor (TNFsr-p55)(9, 25) and one trial (141 patients) testing a higher molecular weight TNFsr (TNFsr-p75)(10). The median control mortality rate in these 15 trials was 38.6% (intra-quartile range 32.4 to 42.1%) (range of 26.1 to 60.0%). Other trial characteristics are summarized in Tables 1 and 2.
Effect of Anti-TNF Agents on Mortality
For the 15 trials combined, there were 1,493 deaths in 3,983 control patients (37.5%) and 1,727 deaths in 4,913 patients receiving anti-TNF agents (35.2%). The effects on mortality were not significantly different across these trials (I2=0%, p=0.84), and overall, anti-TNF agents produced a significant decrease in the relative risk of death [RR (95% CI)] [0.93 (0.88, 0.98), p=0.01] (Figure 1). Notably, in all seven trials enrolling more than 400 patients, anti-TNF agents produced consistent nominal improvements in survival rates, a pattern unlikely to have occurred by chance (p=0.02, based on a geometric distribution). Based on these trial data, with a median control mortality rate of 38.6%, the number of septic patients needed to treat (NNT) with anti-TNF therapy to save one life would be 38.
Subgroup Analysis Comparing Anti-TNF Trials
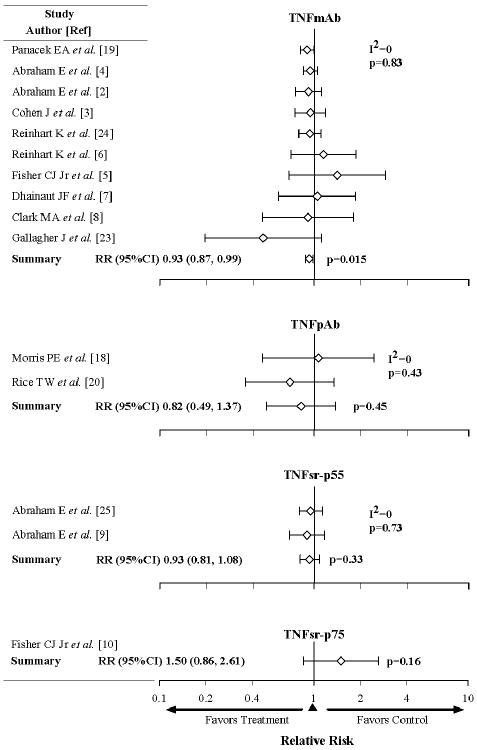
Trials testing each type of anti-TNF agent were examined separately. Across 10 trials, TNF-mAb had effects on the RR of death that were consistent (I2=0%, p=0.83) and significantly beneficial [0.93 (0.87, 0.99), p=0.015] (Figure 2) in a pattern similar to anti-TNF agents overall (Figure 1). For the two TNF-mAb agents investigated in more than a single trial (afeliomab, n=4 and Bay 1351, n=3) the effects of therapy were consistent (I2=9.6%, p=0.35 and I2=0%, p=0.97 respectively) and both on the side of benefit [0.91 (0.82, 1.01), p=0.08 and 0.94 (0.86, 1.02) p=0.14 respectively]. TNFpAb and TNFsr-p55 had effects on the RR of death, which also did not differ across the trials testing each agent (I2=0%, p=0.43 and 0.73, respectively). However, these survival effects while similar to that of anti-TNF agents overall, did not reach statistical significance [0.82 (0.49, 1.37), p=0.45 and 0.93 (0.81, 1.08), p=0.33, respectively], possibly because far fewer patients were studied. TNFsr-p75 was studied in only one trial. Its effect on the RR of death was not significantly different from other anti-TNF agents (Figure 2) but was on the side of harm [1.50 (0.86, 2.61), p=0.16].
Figure 2.
Effects of the four different types of anti-TNF agents, including TNF monoclonal antibodies (TNFmAb), TNF polyclonal antibodies (TNFpAb), p55 TNF soluble receptor (TNFsr-p55) and p75 TNFsr (TNFsr-p75) in randomized controlled trials. The level of consistency among the trials (I2 value) and relative risk (RR) of death and 95% CI among the trials for each type of agent are shown. The effects of each type of anti-TNF agent were consistent across the trials testing them. TNFmAb, the most frequently studied (10 trials), was itself alone associated with a significant increase in the RR (95%CI) (p=0.015).
Trials Comparing the Effects of Anti-TNF agents in Similar Subgroups of Septic Patients
We also examined the results of trials comparing the effects of anti-TNF agents in similar patient subgroups. Four trials compared the effects of anti-TNF agents in septic patients with shock versus without shock at enrollment (2, 3, 9, 25) (Figure E2 in supplemental material). The effects of anti-TNF agents on the RR of death were not significantly different across trials in these two subgroup categories (I2=0; p=0.81 and 0.72). Overall, anti-TNF agents had no significant effect on the RR of death in septic patients without shock [RR 0.99 (0.83, 1.19); p=0.93] but did produce a beneficial trend in those with shock [RR 0.90 (0.80, 1.02); p=0.10]. However, there was no significant difference comparing the overall survival effects of anti-TNF agents in patients with versus without shock (p=0.41).
Five and three trials compared the effects anti-TNF agents in patients with high versus low blood IL-6 [4, 6, 25, 18, 9] or TNF levels (4, 5, 9) respectively and four trials compared these effects in patients with evidence of either Gram-positive or Gram-negative infection at enrollment(3, 4, 9, 25). Across trials, the effects of treatment on mortality were variable in subgroups with either high IL-6 levels (I2= 59%; p=0.05), low TNF levels (I2= 47%; p=0.15) or Gram-negative infections (I2= 45%; p=0.14). Therefore, these trials were not combined and no overall inferences were made.
Finally, at enrollment acute physiology and chronic health evaluation 2 scores (APACHE 2) were reported in one trial(3) and simplified acute physiology scores (SAPS) were in another(25). Anti-TNF agents did not affect the RR of death significantly in patients with either high or low APACHE 2 scores [1.08 (0.81, 1.44), p=0.60 and 0.83 (0.56, 1.24), p=0.36 respectively]. However, treatment did increase the RR of death significantly in those with high [0.79 (0.65, 0.96), p=0.02], but not low SAPS scores [1.25 (0.91, 1.73), p=0.17].
Other Outcome Measures Examined
Eight trials provided data regarding the effect of anti-TNF agents on resolution of shock (six trials)(2-4, 8, 9, 20) and/or organ injury (five trials)(2-4, 19, 24). However, these trials presented either differing measures of these outcomes or only p-values without analyzable data (Table E3 in supplemental material). Thus it was not possible to combine this data and formulate overall conclusions. However only two of these eight trials noted significant beneficial effects; one on speed to shock reversal and on time to development of new organ failure (3) and another on the reduction in organ injury scores over time(19).
Seven trials presented data regarding the effect of anti-TNF agents on blood TNF (four trials) (3, 18-20) and/or IL-6 cytokine levels (five trials)(Table 3)(6, 8, 19, 20, 24). Treatment was reported to significantly lower one or both of these cytokine levels compared to controls in six of the seven trials [TNF in four trials (3, 18-20) ; IL-6 in four trials(6, 19, 20, 24)].
Table 3.
Effect of anti-TNF agents on blood TNF and IL-6 Levels
| Author, [Ref] | Anti-TNF Agent | Time Following Treatment (h) | Effect of Treatment on Blood Cytokine Levels | Reported p value |
|---|---|---|---|---|
|
TNF levels
| ||||
| Cohen J et al, [3] | mAb | 1 | ↓ | 0.0001 |
|
| ||||
| Panacek EA et al, [19] | mAb | 72 | ↓ | <0.001 |
|
| ||||
| Rice TW et al, [20] | pAb | 168 | ↓↑* | 0.001 |
|
| ||||
| Morris PE et al, [18] | pAb | 2 | ↓** | <0.02 |
|
| ||||
|
IL-6 Levels
| ||||
| Reinhart K et al, [6] | mAb | 24 | ↓ | <0.01 |
|
| ||||
| Reinhart K et al, [24] | mAb | 8 | ↓ | 0.001 |
| 72 | ↓ | 0.047 | ||
|
| ||||
| Panacek EA et al, [19] | mAb | 8 | ↓ | <0.001 |
|
| ||||
| Clark MA et al, [8] | mAb | 96 | ↔ | 0.24 |
|
| ||||
| Rice TW et al, [20] | pAb | 24 to 96 | ↓ | 0.002 |
Decreased early and increased later
TNF level were reported to be significantly decreased in the 3 higher dose cohorts (see Table 2)
Serious Adverse Events, Secondary Infections, and Development of Antibodies
In the seven trials reporting data regarding the effects of anti TNF agents on the incidence of serious adverse events (SAE), these did not differ significantly across trials (I2 = 0, p=0.70) (Figure 3)(2, 4, 9, 18, 19, 24, 25). Overall, the RR of having an SAE tended to be decreased with anti-TNF agents compared to controls (RR 0.955 [0.914, 0.999], p=0.046). In one trial reporting only the incidence of adverse events, there was no significant difference comparing treatment and control groups [121 of 386 (31.3%) vs. 48 of 167 (28.7%), respectively; p=0.54](3). Consistent with these findings, six additional trials not providing numerical data, did report that there were no significant differences in adverse or serious events comparing treatment to controls.
Figure 3.
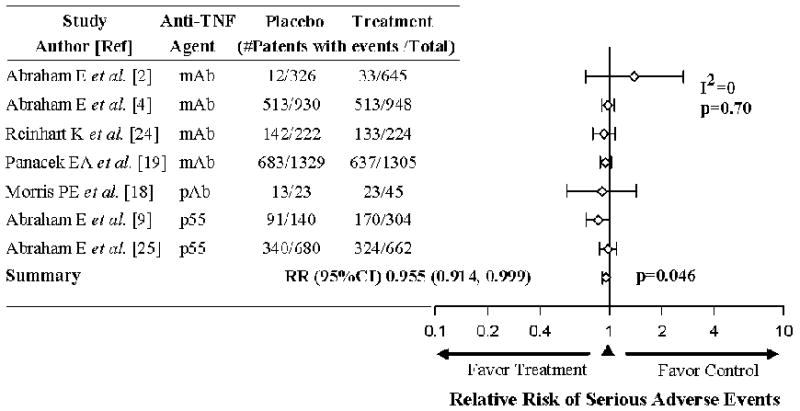
Effects of anti-TNF therapies on the relative risk (95% CI) of increasing the number of serious of adverse events in the seven trials reporting such data. The level of consistency among the trials (I2 value) and relative risk (RR) of serious adverse events and 95% CI among the trials for are shown. Serious adverse events were not increased with treatment and actually showed a small but significant reduction.
In eight trials reporting on secondary infections (2-4, 7, 9, 18, 19, 25), only two provided analyzable data to compare the number of patients with secondary infection in treatment versus control groups. In both trials, the incidence of secondary infection was not increased with treatment compared to control [493 of 1305 patients (37.8%) vs. 525 of 1329 (39.5%) in one trial and 19 of 47 (40.4%) vs. 10 of 23 (43.5%) in the other](18, 19). In the remaining 6 trials, rates of infection were not reported increased with treatment.
Seven trials testing TNFmAb reported that from 16 to 98% of patients developed human anti-mouse antibodies [median (IQR); 51% (32 – 88%)](2, 3, 5, 6, 19, 23, 24). In two trials testing TNFpAb, 41% of treatment patients in one trial and 15% in the other developed anti-ovine antibodies(18, 20). Finally, in the one trial testing TNFsr-p75, 15% of patients developed antibodies to treatment(10).
Potential Sources of Bias
A funnel plot of the 15 trials (Figure E3 in supplemental material) and formal testing using Egger’s regression methods (p=0.50) did not suggest publication bias. Fourteen of the trials analyzed were funded by the manufacturer of the anti-TNF agent tested and for each at least one contributing author reported receiving financial support from the manufacturer (Table E4 in supplemental material)(2-7, 9, 10, 18-20, 25). Five trials presented the number of treated patients lost to follow-up (Table E4 in supplemental material)(2, 4, 8, 18, 25). When missing placebo and anti-TNF treatment patients were considered as survivors or non-survivors respectively, the effect of anti-TNF agents on the relative risk of death across all 15 trials remained significant [0.94 (0.89, 0.99), p=0.02]. The number of patients initially receiving appropriate antibiotics for a documented source of infection(2-4, 10, 24) and the number having surgery(2, 3, 6, 10, 19, 20, 24) were only recorded in five and seven studies respectively (data not shown). Across these studies, the RR of receiving either appropriate antibiotics or surgery in patients receiving anti-TNF agents did not differ significantly compared to control [0.99 (0.96,1.02), p=0.63 and 1.01 (0.94,1.08), p=0.83 respectively].
Discussion
Across 15 clinical trials in sepsis, selective anti-TNF agents had effects on survival which did not differ significantly and which overall decreased the RR (95% CI) of death significantly [0.93 (0.88, 0.98); p=0.01]. In subgroup analysis, trials with greater enrollment (>400 septic patients, n=7) demonstrated significant overall beneficial treatment effects. Anti-TNF agents also appeared to have minimal risk since the number of serious adverse events or infectious complications were not increased across trials reporting such data (n=7 and 8 respectively). Six other trials, while not providing data, reported that adverse events were also not increased with treatment. These findings suggest that agents selectively inhibiting TNF have a favorable benefit-to-risk ratio in septic patients and large trials are necessary to capture this beneficial effect. Consistent with this, TNFmAb which was studied in more trials and patients than other anti-TNF agents analyzed (10 trials and 6,818 patients) and in several of the largest trials, by itself demonstrated a significant overall survival benefit.
Based on these findings, failure of anti-TNF agents to show benefit in any of the 15 individual trials appears largely related to insufficient patient sample sizes. A power analysis shows that for a similar agent in a septic population with a mortality rate like the one studied (38%), 10,000 patients would be required to show a significant survival benefit at a 0.05-level (two sided) with an 80% power. If as suggested by a recent large survey conducted by the Surviving Sepsis Campaign (25,000 patients), the mortality rate in sepsis is now closer to 32%, then as many as 14,000 patients might be required for study (26). Yet the largest trial investigating these anti-TNF agents included only 2634 patients(19).
The finding that trials testing these types of anti-TNF agents may require 10,000 or more septic patients to demonstrate significant efficacy raises several concerns. Enrolling such a large number of patients would be made easier by limiting trial exclusion criteria. Although anti-TNF agents did not have significantly harmful effects in any subgroup described, such analysis was limited. Whether anti-TNF therapy might prove harmful in an as yet unidentified subgroup if investigated in a larger number of less stringently enrolled septic patients is unknown. Also, a study enrolling 10,000 or more septic patients may require prolonged enrollment across many centers, which could confound results. Reassuringly, of the 15 trials analyzed, the one which enrolled patients over the longest period (3.5 y) and across multiple centers (157), demonstrated benefit with treatment approaching significance(19). It is also notable that the recent Surviving Sepsis Campaign survey reported enrolling more than 25,000 septic patients over only 5 years (January 2005 to January 2010), or a little over 5,000 patients per year (26). If only half of these patients were eligible for a treatment study, then for a class of agents such as anti-TNF ones, with what appear to be limited adverse effects but wide applicability, it might have only required four to five years to enroll 10,000 to 12,500 patients in a treatment study. Ideally this would be a randomized controlled trial. Logistically such a study would be difficult and safety monitoring would be critical. Funding might also require aid from governmental agencies with an interest in public health as has been the case for other sepsis therapies like fluid and vasopressor support (27). However, such a study appears feasible and could be done in a reasonable period of time.
Whether enrollment in future trials of selective anti-TNF agents could be limited by targeting treatment to more responsive subgroups is unclear from this analysis. For example, while evidence has suggested that both mediator selective and nonselective anti-inflammatory agents have greater efficacy in septic patients with a higher risk of death(28-30), in this meta-analysis anti-TNF agents had similar effects across trials with a relatively wide range of risk (i.e. control mortality rates from 26 to 60%). However, even a control mortality rate of 26% represents a relatively high risk of death. Prior analysis has suggested that anti-inflammatory agents may only lose their beneficial effects or show harm when mortality rates are less than 20 to 22% (28, 30, 31). Thus, if studies with a lower mortality rates had been available, it is possible a relationship between risk of death and treatment effects might have been noted. Although only two trials provided data regarding the effects of therapy based on a predicted risk of death score, anti-TNF therapy had significant benefit with a higher but not lower risk of death in one of these. Also, in the four trials stratifying patients based on the presence or absence of shock at enrollment, anti-TNF agents had an effect on the side of benefit in those with shock but not in those without shock, although this did not reach significance. Variability in the effect of therapy in subgroups based on blood TNF and IL-6 levels and type of bacterial infection precluded analysis regarding a patient subgroup which anti-TNF agents might be targeted against. Certainly, if a large prospective study of anti-TNF agents were to be undertaken, examining the effects of therapy based on prospective measures such as risk prediction scores, the presence or absence of shock or organ injury, and cytokine or other biomarker levels would be important.
Despite the size of a single trial necessary to confirm the potential benefit of anti-TNF agents in sepsis, such a trial appears warranted. The overall treatment effect noted with anti-TNF agents in this analysis in combination with the observed overall control mortality rate (38%) indicates that if 37 septic patients were treated, one death would be prevented. Conservatively then, if 400,000 patients developed severe sepsis or septic shock annually in the US with a median mortality rate of 38%, approximately 4,000 of these patients would be saved with anti-TNF therapy. Even if the mortality rate from sepsis has decreased over time to the level suggested by the recent SSC survey (32%), then based on the NNT at this lower rate (i.e. 45), approximately 3,000 of the 400,000 patients described above would be saved with anti-TNF therapy. However, broad use of anti-TNF agents would depend in part on the therapy’s cost.
The basis for the survival benefit noted with anti-TNF agents is unclear. Consistent with their recognized anti-inflammatory effects, there was evidence in this meta-analysis that treatment reduced blood TNF and IL-6 cytokine levels. In six of the seven trials reporting such data (see Table 3), anti-TNF therapies were noted to significantly reduce one or both of these cytokines. In three of the four studies showing reductions in TNF levels, anti-TNF therapies had effects on the side of benefit. Also, in the largest of the 15 trials [Panacek et al. (19)] - the one showing benefit closest to significance - TNF and IL-6 levels were lower with treatment. However for other secondary endpoint data, although limited, it was not evident that anti-TNF agents resulted in either greater resolution of shock or organ injury. Notably, while administration of an anti-inflammatory agent in septic patients might suppress host defenses and increase risk, the incidence of serious adverse events was not increased with anti-TNF agents. Also, secondary infection was not reported significantly increased. However, the methods employed to track infectious complications were not provided in most trials and insufficient methodology may have confounded these findings. Use of selective anti-TNF agents in patients with arthritis treated over longer periods than in these sepsis trials, has been associated with increased risk of infection(1). Increased risk related to the primary or a secondary infection must still be a concern if anti-TNF agents are considered for sepsis.
When compared to septic controls, the three doses of TNFsr-p75 studied in the only trial testing this agent were reported to produce a significant dose dependent increase in mortality (p=0.02)(10). However, as also noted in the original publication, when these three TNFsr-p75 doses were either compared to each other or combined and compared to control, significant differences were not noted. Nonetheless, the significant dose dependent effect noted is concerning, and in comparison with the other types of anti-TNF agents investigated, raises the possibility that this particular one may be associated with increased risk. It is notable however that when examining results of 15 trials it is not unexpected that by chance one of the smaller trials (141 patients) could fall on the side of harm and even reach statistical significance. Regardless, TNFsr-p75 has a known infection risk when used for other inflammatory conditions and should not be tested in septic patients until the result of this single sepsis trial is better understood.
This study has limitations. There was limited data from published reports on relevant secondary endpoints to elucidate the nature of the survival benefit with anti-TNF therapy. Likewise, the methods to record serious adverse events, most importantly secondary infection, were missing or incomplete. Thus, any assessment of risk with treatment across trials cannot be made with assurance. Four trials reported losing patients to follow-up. However, even after accounting for these lost patients, anti-TNF agents still had an overall significant survival benefit. Fourteen of the trials analyzed received support from the manufacturer of the anti-TNF agent tested and for each of these one or more authors had financial relationships with the manufacturer. Despite this potential for bias, none of these trials individually reported a significant survival benefit. Finally, in a recent Phase 2b trial, treatment with a polyclonal antibody to TNF (AZD9773) was reported in the news media to not show significant improvement in survival compared to placebo(32). Although this trial was designed to only enroll 300 patients, efforts to obtain additional information regarding enrollment and results were unsuccessful(33).
In conclusion, selective anti-TNF agents as a class produced a significant overall survival benefit in septic patients and these agents have continued to undergo investigation in sepsis. Based on this meta-analysis however, unless such trials are large enough, it is unlikely that they will demonstrate significant survival benefits. However, the potential for benefit with selective anti-TNF agents suggests that conducting sufficiently large sepsis trials testing them, while logistically complex, might be warranted.
Acknowledgments
Funding/Support: The Intramural Research Program of the National Institutes of Health and the NIH Clinical Center, Bethesda, Maryland provided support for this study.
Study Funding:
The Clinical Center at the National Institutes of Health, Bethesda, Maryland, provided intramural funds for this study. The funding source played no role in the design, conduct, or reporting of the study, nor in the decision to submit the manuscript for publication.
Footnotes
Author Contributions:
Drs. Qiu and Eichacker had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis
Study concept and design: Qiu, Cui, Sun, Natanson, Eichacker
Acquisition of data: Qiu, Cui, Welsh, Natanson, Eichacker
Analysis and interpretation of data: Qiu, Cui, Natanson, Eichacker
Critical revision of the manuscript for important intellectual content: Qiu, Cui, Sun, Welsh, Natanson, Eichacker
Statistical analysis: Qiu, Cui, Sun, Natanson, Eichacker
Administrative, technical, or administrative support: Qiu, Cui, Sun, Welsh, Eichacker
Study supervision: Eichacker
Appendix
Figure E1. Study selection flow diagram.
Figure E2. Effects of anti-TNF therapies in trials that stratified patients based on the presence or absence of shock at enrollment.
Figure E3. A funnel plot of precision (standard error) versus the log odds ratio of survival for the 15 anti-TNF trials.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. No authors reported any financial disclosures.
References
- 1.Thalayasingam N, Isaacs JD. Anti-TNF therapy. Best Pract Res Clin Rheumatol. 2011;25(4):549–567. doi: 10.1016/j.berh.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Abraham E, Wunderink R, Silverman H, et al. Efficacy and safety of monoclonal antibody to human tumor necrosis factor alpha in patients with sepsis syndrome. A randomized, controlled, double-blind, multicenter clinical trial. TNF-alpha MAb Sepsis Study Group. JAMA. 1995;273(12):934–941. [PubMed] [Google Scholar]
- 3.Cohen J, Carlet J. INTERSEPT: an international, multicenter, placebo-controlled trial of monoclonal antibody to human tumor necrosis factor-alpha in patients with sepsis. International Sepsis Trial Study Group. Crit Care Med. 1996;24(9):1431–1440. doi: 10.1097/00003246-199609000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Abraham E, Anzueto A, Gutierrez G, et al. Double-blind randomised controlled trial of monoclonal antibody to human tumour necrosis factor in treatment of septic shock. NORASEPT II Study Group. Lancet. 1998;351(9107):929–933. [PubMed] [Google Scholar]
- 5.Fisher CJ, Jr, Opal SM, Dhainaut JF, et al. Influence of an anti-tumor necrosis factor monoclonal antibody on cytokine levels in patients with sepsis. The CB0006 Sepsis Syndrome Study Group. Crit Care Med. 1993;21(3):318–327. doi: 10.1097/00003246-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Reinhart K, Wiegand-Lohnert C, Grimminger F, et al. Assessment of the safety and efficacy of the monoclonal anti-tumor necrosis factor antibody-fragment, MAK 195F, in patients with sepsis and septic shock: a multicenter, randomized, placebo-controlled, dose-ranging study. Crit Care Med. 1996;24(5):733–742. doi: 10.1097/00003246-199605000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Dhainaut JF, Vincent JL, Richard C, et al. CDP571, a humanized antibody to human tumor necrosis factor-alpha: safety, pharmacokinetics, immune response, and influence of the antibody on cytokine concentrations in patients with septic shock. CPD571 Sepsis Study Group. Crit Care Med. 1995;23(9):1461–1469. doi: 10.1097/00003246-199509000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Clark MA, Plank LD, Connolly AB, et al. Effect of a chimeric antibody to tumor necrosis factor-alpha on cytokine and physiologic responses in patients with severe sepsis--a randomized, clinical trial. Crit Care Med. 1998;26(10):1650–1659. doi: 10.1097/00003246-199810000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Abraham E, Glauser MP, Butler T, et al. p55 Tumor necrosis factor receptor fusion protein in the treatment of patients with severe sepsis and septic shock. A randomized controlled multicenter trial Ro 45-2081 Study Group. JAMA. 1997;277(19):1531–1538. [PubMed] [Google Scholar]
- 10.Fisher CJ, Jr, Agosti JM, Opal SM, et al. Treatment of septic shock with the tumor necrosis factor receptor:Fc fusion protein. The Soluble TNF Receptor Sepsis Study Group. N Engl J Med. 1996;334(26):1697–1702. doi: 10.1056/NEJM199606273342603. [DOI] [PubMed] [Google Scholar]
- 11.Beutler B, Milsark IW, Cerami AC. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- 12.Qiu P, Cui X, Barochia A, et al. The evolving experience with therapeutic TNF inhibition in sepsis: considering the potential influence of risk of death. Expert Opin Investig Drugs. 2011;20(11):1555–1564. doi: 10.1517/13543784.2011.623125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wenzel RP. Treating sepsis. N Engl J Med. 2002;347(13):966–967. doi: 10.1056/NEJMp020096. [DOI] [PubMed] [Google Scholar]
- 14.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Marshall JC. Such stuff as dreams are made on: mediator-directed therapy in sepsis. Nat Rev Drug Discov. 2003;2(5):391–405. doi: 10.1038/nrd1084. [DOI] [PubMed] [Google Scholar]
- 16.Natanson C, Esposito CJ, Banks SM. The sirens’ songs of confirmatory sepsis trials: selection bias and sampling error. Crit Care Med. 1998;26(12):1927–1931. doi: 10.1097/00003246-199812000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Reinhart K, Karzai W. Anti-tumor necrosis factor therapy in sepsis: update on clinical trials and lessons learned. Crit Care Med. 2001;29(7 Suppl):S121–125. doi: 10.1097/00003246-200107001-00037. [DOI] [PubMed] [Google Scholar]
- 18.Morris PE, Zeno B, Bernard AC, et al. A placebo-controlled, double-blind, dose-escalation study to assess the safety, tolerability and pharmacokinetics/pharmacodynamics of single and multiple intravenous infusions of AZD9773 in patients with severe sepsis and septic shock. Crit Care. 2012;16(1):R31. doi: 10.1186/cc11203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Panacek EA, Marshall JC, Albertson TE, et al. Efficacy and safety of the monoclonal anti-tumor necrosis factor antibody F(ab’)2 fragment afelimomab in patients with severe sepsis and elevated interleukin-6 levels. Crit Care Med. 2004;32(11):2173–2182. doi: 10.1097/01.ccm.0000145229.59014.6c. [DOI] [PubMed] [Google Scholar]
- 20.Rice TW, Wheeler AP, Morris PE, et al. Safety and efficacy of affinity-purified, anti-tumor necrosis factor-alpha, ovine fab for injection (CytoFab) in severe sepsis. Crit Care Med. 2006;34(9):2271–2281. doi: 10.1097/01.CCM.0000230385.82679.34. [DOI] [PubMed] [Google Scholar]
- 21.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee American College of Chest Physicians/Society of Critical Care Medicine 1992. Chest. 2009;136(5 Suppl):e28. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 22.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 23.Gallagher J, Fisher C, Sherman B, et al. A multicenter, open-label, prospective, randomized, dose-ranging pharmacokinetic study of the anti-TNF-alpha antibody afelimomab in patients with sepsis syndrome. Intensive Care Med. 2001;27(7):1169–1178. doi: 10.1007/s001340100973. [DOI] [PubMed] [Google Scholar]
- 24.Reinhart K, Menges T, Gardlund B, et al. Randomized, placebo-controlled trial of the anti-tumor necrosis factor antibody fragment afelimomab in hyperinflammatory response during severe sepsis: The RAMSES Study. Crit Care Med. 2001;29(4):765–769. doi: 10.1097/00003246-200104000-00015. [DOI] [PubMed] [Google Scholar]
- 25.Abraham E, Laterre PF, Garbino J, et al. Lenercept (p55 tumor necrosis factor receptor fusion protein) in severe sepsis and early septic shock: a randomized, double-blind, placebo-controlled, multicenter phase III trial with 1,342 patients. Crit Care Med. 2001;29(3):503–510. doi: 10.1097/00003246-200103000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Levy MM, Artigas A, Phillips GS, et al. Outcomes of the Surviving Sepsis Campaign in intensive care units in the USA and Europe: a prospective cohort study. Lancet Infect Dis. 2012;12(12):919–924. doi: 10.1016/S1473-3099(12)70239-6. [DOI] [PubMed] [Google Scholar]
- 27.Angus D, JA K, Yealy DM. Protocolized Care for Early Septic Shock (ProCESS) [January 14, 2013]; Available at: http://wwwclinicaltrialsgov/ct2/show/NCT00510835?term=ProCESS&rank=1.
- 28.Minneci PC, Deans KJ, Eichacker PQ, et al. The effects of steroids during sepsis depend on dose and severity of illness: an updated meta-analysis. Clin Microbiol Infect. 2009;15(4):308–318. doi: 10.1111/j.1469-0691.2009.02752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lorente JA, Marshall JC. Neutralization of tumor necrosis factor in preclinical models of sepsis. Shock. 2005;24(Suppl 1):107–119. doi: 10.1097/01.shk.0000191343.21228.78. [DOI] [PubMed] [Google Scholar]
- 30.Eichacker PQ, Parent C, Kalil A, et al. Risk and the efficacy of antiinflammatory agents: retrospective and confirmatory studies of sepsis. Am J Respir Crit Care Med. 2002;166(9):1197–1205. doi: 10.1164/rccm.200204-302OC. [DOI] [PubMed] [Google Scholar]
- 31.Knaus WA, Harrell FE, Jr, LaBrecque JF, et al. Use of predicted risk of mortality to evaluate the efficacy of anticytokine therapy in sepsis. The rhIL-1ra Phase III Sepsis Syndrome Study Group. Crit Care Med. 1996;24(1):46–56. doi: 10.1097/00003246-199601000-00010. [DOI] [PubMed] [Google Scholar]
- 32.BTG Kills AZD9773 Development. [January 13, 2013]; Available at: http://wwwdddmagcom/news/2012/08/btg-kills-azd9773-development.
- 33.Bernard G, Botnick W, Lindemann J, et al. A Study to Compare the Efficacy and Safety of 2 Dosing Regimens of IV Infusions of AZD9773 (CytoFab™) With Placebo in Adult Patients With Severe Sepsis and/or Septic Shock. [Januray 13, 2013]; Available at: http://clinicaltrialgov/ct2/show/NCT01145560?term=NCT01145560&rank=1.