Abstract
Background
Relevant preclinical models are necessary for further mechanistic and translational studies of c-kit+ cardiac stem cells (CSCs). The present study was undertaken to determine whether intracoronary CSCs are beneficial in a porcine model of chronic ischemic cardiomyopathy.
Methods and Results
Pigs underwent a 90-min coronary occlusion followed by reperfusion. Three months later, autologous CSCs (n=11) or vehicle (n=10) were infused into the infarct-related artery. At this time, all indices of LV function were similar in control and CSC-treated pigs, indicating that the damage inflicted by the infarct in the two groups was similar; one month later, however, CSC-treated pigs exhibited significantly greater LV ejection fraction (echocardiography) (51.7 ± 2.0% vs. 42.9 ± 2.3 %, P<0.01), systolic thickening fraction in the infarcted LV wall, and max LV dP/dt, as well as lower LVEDP. Confocal microscopy showed clusters of small α-sarcomeric actin positive cells expressing Ki67 in the scar of treated pigs, consistent with cardiac regeneration. The origin of these cycling myocytes from the injected cells was confirmed in four pigs that received EGFP-labeled CSCs, which were positive for the cardiac markers troponin I, troponin T, myosin heavy chain, and connexin-43. Some engrafted CSCs also formed vascular structures and expressed α-smooth muscle actin.
Conclusions
Intracoronary infusion of autologous CSCs improves regional and global LV function and promotes cardiac and vascular regeneration in pigs with old MI (scar). The results mimic those recently reported in humans (SCIPIO trial) and establish this porcine model of ischemic cardiomyopathy as a useful and clinically-relevant model for studying CSCs.
Keywords: Stem cells, Heart failure, Myocardial infarction, Myogenesis, Angiogenesis
INTRODUCTION
Over the last decade, numerous animal studies and clinical trials have established the ability of various stem cell populations to improve cardiac function and attenuate left ventricular (LV) remodeling in heart failure (HF) 1–11. Among the cells tested, resident cardiac stem cells (CSCs) appear particularly promising. Based on the expression of the surface receptor tyrosine kinase c-kit, in 2003, Beltrami et al. 12 isolated a distinct population of resident CSCs (c-kit+ CSCs) in adult rat hearts that are self-renewing, clonogenic, and multipotent - i.e., they differentiate into all three major cardiac lineages (myocytes, vascular smooth muscle cells, and endothelial cells) 5, 12–15. The practical utility of c-kit+ CSCs is supported by the fact that these cells can be isolated from small fragments of cardiac tissue and expanded for subsequent autologous administration 4, 12, 16.
Prior work in our laboratory using autologous or syngeneic c-kit+ CSCs has shown that transplantation of these cells attenuates LV remodeling and improves LV function in the settings of both acute and chronic myocardial infarction (MI) in rodents 4, 5, 12–15. We have recently obtained similar results in SCIPIO (Stem Cell Infusion in Patients with Ischemic CardiOmyopathy; NCT00474461), the first clinical trial of CSCs 10. SCIPIO is a Phase I, open label, randomized study designed to investigate the safety and feasibility of autologous CSC infusion in patients with severe HF resulting from ischemic heart disease 10, 17. However, despite these encouraging results, many questions pertaining to the therapeutic efficacy of CSCs remain unanswered (e.g., what is the optimal time for CSC administration? Is the intracoronary route the optimal modality of administration? What is the optimal protocol for intracoronary infusion?). Many of these questions cannot be realistically or safely examined in clinical trials, nor can they be addressed in a rodent model where cell delivery with an intracoronary catheter (similar to the human procedure) is impossible. Answering these important questions will require the use of large and clinically-relevant animal models.
Accordingly, the goal of the present investigation was to develop a porcine model of chronic ischemic cardiomyopathy caused by an old MI (scar) and to determine whether intracoronary delivery of autologous CSCs (similar to the protocol used in the SCIPIO trial) recapitulates the results obtained clinically in SCIPIO.
METHODS
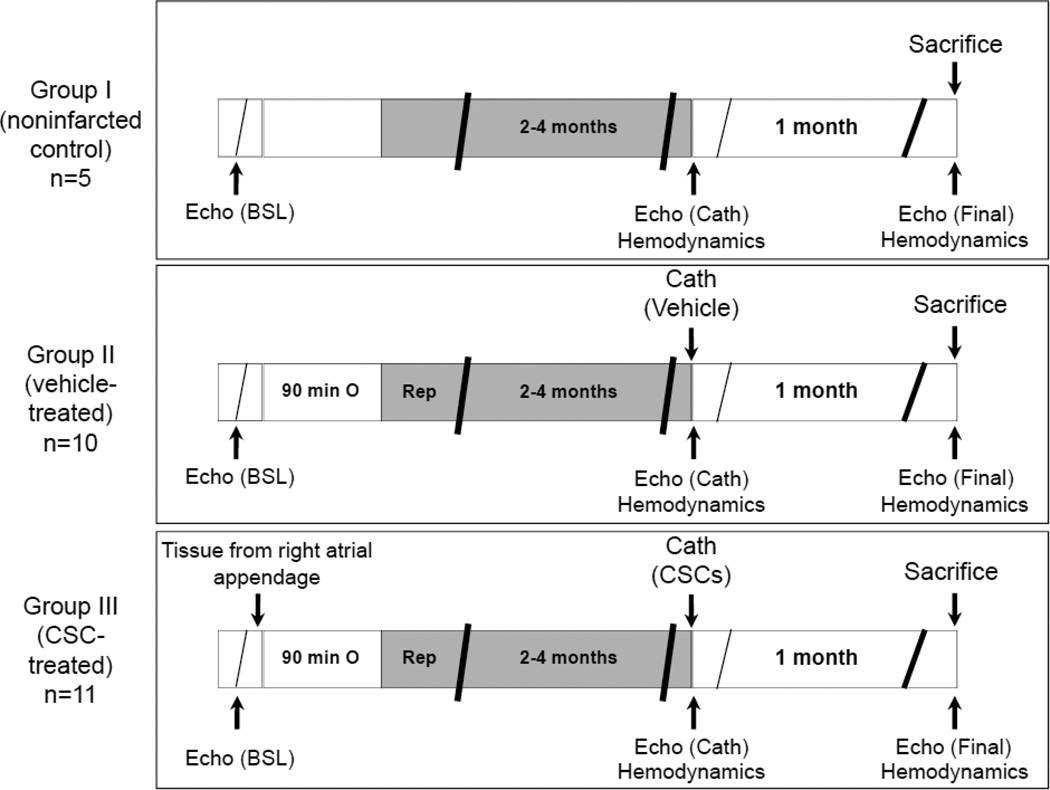
This study was performed in accordance with the guidelines of the Animal Care and Use Committee of the University of Louisville (KY) School of Medicine and following the guidelines set forth by the 1996 Guide for the Care and Use of Laboratory Animals. The experimental protocol of the study is illustrated in Figure 1.
Figure 1.
Experimental protocol. Three groups of pigs were studied (groups I–III). Four days after a baseline echocardiogram, pigs underwent a 90-min coronary occlusion followed by reperfusion or sham surgery. At three to four months after MI (97 ± 12 d in vehicle-treated and 96 ± 6 d in CSC-treated groups), pigs received intracoronary infusion of vehicle (group II), or autologous CSCs into the infarct-related artery using a balloon catheter (group III). Group I served as noninfarcted controls. Echocardiographic and hemodynamic assessment of cardiac function was performed before treatment and at the time of sacrifice. At 31 days after vehicle/CSC therapy, pigs were euthanized for morphometric and histological studies.
Induction of myocardial infarction and tissue harvest
Male Yorkshire pigs (13.5 ± 0.8 kg, age 8–10 weeks) were anesthetized and the heart was exposed by a median sternotomy. The right atrial appendage was cross-clamped and the tip (1–2 g) resected for isolation of CSCs. The harvested atrial samples were rinsed in PBS, cut into small (1–2 mg) pieces, and snap frozen in a freezing medium composed of the growth culture medium pre-mixed with DMSO (9:1 vol/vol). The growth medium consisted of Ham’s F12 (BioWhittaker), 10% fetal bovine serum (Gibco) and penicillin/streptomycin (BioWhittaker). The left anterior descending (LAD) coronary artery was occluded for 90 min distal to the origin of the second diagonal branch and then reperfused. A group of non-operated pigs was studied as normal (noninfarcted) controls. These animals did not undergo any surgical procedure and were monitored for a period of time equivalent to the infarcted animals.
Isolation and culture of CSCs
The frozen atrial specimens were thawed, cut into thinner slices, and plated on uncoated dishes containing growth medium. One week after tissue seeding, outgrowth of CSCs was apparent and documented by microscopic examination. After an additional week, a cluster of ~5,000 cells surrounded each tissue fragment. The growth medium was removed and cells were detached with 3–4 ml of 0.25% trypsin (Sigma) per dish. Cells were sorted for c-kit with Miltenyi immunomagnetic beads (Miltenyi Biotech), and c-kit+ cells were plated in growth medium for expansion. The characteristics of c-kit+ cells were analyzed by immunocytochemistry and fluorescence activated cell sorting (FACS) using antibodies against c-kit and against markers of cardiac lineage or vascular commitment (GATA-4, MEF2C, α-sarcomeric actin, myosin heavy chain [MHC], von Willebrand factor, and smooth muscle actin).
In preparation for the infusion, CSCs were transferred to a vial filled with growth medium. The final autologous CSC product was prepared by centrifuging the cells at 400 g. The supernatant was removed and the cellular pellet re-suspended in sterile Plasma-Lyte A solution (Baxter Healthcare Corporation) to obtain a CSC concentration of 100,000 CSCs/ml. Vehicle consisted of similar volumes of Plasma-Lyte A solution.
Intracoronary CSC delivery
Three to four months after MI (97 ± 12 d in vehicle-treated and 96 ± 6 d in CSC-treated), pigs were anesthetized as described above for the open-chest procedure. The right carotid artery was cannulated; under fluoroscopic guidance, a 6F guiding catheter (Cordis) was used to engage the ostium of the left coronary artery and a Maverick (2.5 × 9 mm or 2.0 × 9 mm) angioplasty balloon catheter (Boston Scientific) was advanced over a guidewire (BMW, Abbott Vascular) and positioned at the level of the mid LAD. A 2-min balloon inflation was performed once to verify cessation of coronary flow distal to the balloon catheter (using contrast medium injection) and to increase microvascular permeability. A sequence of three 3-min balloon inflations (4–6 atmospheres) interspersed with 3-min deflation periods was then performed. The CSC solution (~500,000 cells in 5 ml of sterile Plasma-Lyte A solution, divided into 3 injections) or vehicle (5 ml of sterile Plasma-Lyte A solution) was injected manually at a constant rate through the central port of the angioplasty balloon catheter during the 3-min balloon inflation time.
Blood samples for serial measurement of cardiac markers were obtained before and at serial times after catheterization. Details are provided in the Online Supplement. Pigs were followed for 31 ± 1 d after intracoronary vehicle or CSC delivery. Aspirin (325 mg/d) was administered orally starting 2 d before catheterization until euthanasia.
Echocardiographic and hemodynamic studies
Echocardiograms and hemodynamic measurements were obtained at baseline (before CSC delivery) and 31 d after CSC delivery (just before sacrifice). A detailed description is provided in the Online Supplement.
Morphometry and histology
At the completion of the echocardiographic and hemodynamic measurements, the thorax was reopened. Pigs received an i.v. bolus of heparin (100 IU/kg) followed by an i.v. bolus of CdCl2 (100 mM) and KCl (1 M) to arrest the heart in diastole. The aortic root was perfused with 10% buffered formalin at a pressure adjusted to match the mean arterial pressure. The perfusion-fixed heart was weighed and cut into serial slices perpendicular to its longitudinal axis and the slices were embedded in paraffin. Samples were harvested also from brain, lung, liver, spleen, and kidney and kept in formalin.
Immunohistochemistry was performed on formalin-fixed 4 µm thick sections by using various antibodies. CSCs were identified with the c-kit antibody; myocytes with α-sarcomeric actin, TnI, TnT, cardiac MHC and connexin-43 (Cnx43) antibodies; and smooth muscle cells with α-smooth muscle actin antibodies. Scar tissue was detected with a mixture of collagen type III and type I antibodies. Cycling cells were detected with Ki67 antibodies. Colocalization of cell-specific markers with enhanced green fluorescent protein (EGFP) was used to identify cells that originated from CSCs. Nuclei were identified with propidium iodide (PI) 12, 14, 18, 19.
Statistical analysis
Data are reported as mean ± SEM. Comparisons of serial measurements in 2 or 3 groups were performed with two-way repeated measures ANOVA (time and group). Post-treatment LVEDP (and dP/dtmax) were compared using analysis of covariance to take into account the pre-treatment values. Post- vs. pre-treatment and/or vehicle vs. CSC-treated comparisons were performed using paired and unpaired Student’s t tests, respectively, with the Bonferroni correction, in which the correction factor was the number of comparisons made for each variable. The statistical software packages used are SigmaStat 2.0 for Windows and R (http://www.r-project.org/). P values < 0.05 were considered statistically significant.
RESULTS
Exclusions
A total of 34 pigs were used in this study (5 non-operated and 29 infarcted pigs). Of the 29 pigs subjected to MI, 8 were excluded before treatment (7 pigs died [1 due to ventricular fibrillation during LAD occlusion, 4 due to ventricular fibrillation within 24 h after reperfusion, 1 at 2 months after infarction, and 1 during cardiac catheterization] and 1 pig was excluded because of a complication unrelated to CSCs [bladder extrusion]). Of the remaining 21 pigs, 10 were assigned to the vehicle-treated group and 11 to the CSC-treated group. Among these, 11 pigs (5 control and 6 CSC-treated) developed ventricular fibrillation during LAD occlusion or within 2 h of reperfusion and were successfully cardioverted by internal defibrillation (5–20 joules).
General characteristics
The interval from MI to treatment (intracoronary infusion of vehicle or CSCs) was 97 ± 12 d in vehicle-treated and 96 ± 6 d in CSC-treated pigs. There was no significant inter- or intragroup difference in heart rate, mean arterial pressure, serum electrolytes, or hemoglobin levels between the vehicle- and the CSC-treated groups during surgery or during cardiac catheterization (Supplemental Table). Arterial blood gases were within normal limits in both groups (data not shown). Body weight was similar among the three groups throughout the protocol (Supplemental Table).
Cardiac enzymes
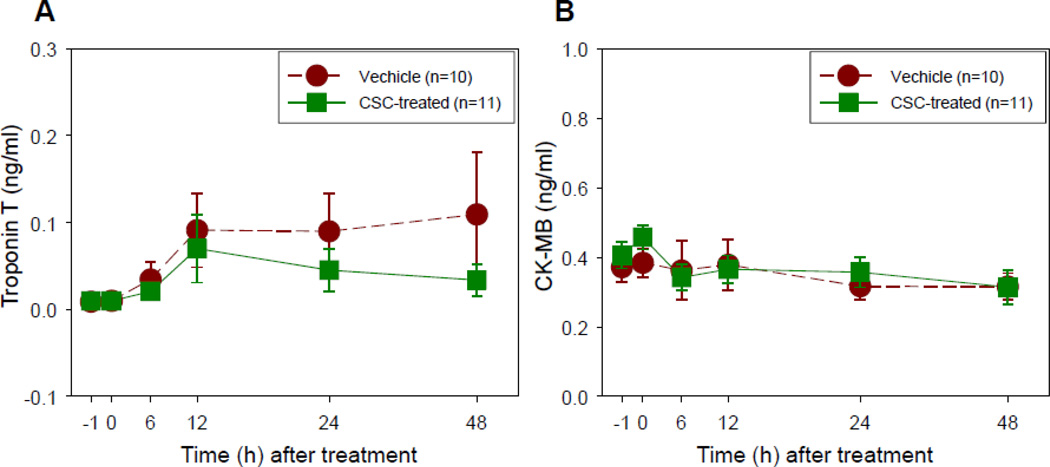
Before intracoronary infusion, CK and LDH levels (which are nonspecific markers of muscle injury) were elevated to a similar extent in vehicle-treated and CSC-treated pigs (Table 1), likely as a result of the neck dissection required for carotid arterial access; however, in the subset of pigs in which they were measured, TnT and CK-MB levels (specific markers of myocardial injury) were normal (Figure 2). After balloon inflation, LDH, total CK, and myoglobin continued to rise and peaked at 6–12 h after the procedure (Table 1) - probably a delayed result of the neck dissection; in contrast, TnT and CK-MB levels exhibited minimal or no elevation (Figure 2). Importantly, neither peak nor cumulative enzyme levels differed significantly between vehicle-treated and CSC-treated groups (Table 1 and Figure 2), indicating that CSC delivery was not associated with more frequent or more extensive myocardial injury.
Table 1.
Cardiac enzymes during and after cardiac catheterization
| Before intracoronary infusion |
Peak levels after catheterization |
Cumulative levels over 48 h post catheterization |
|
|---|---|---|---|
|
Vehicle-treated (n=10) |
|||
| LDH, U/L | 798 ± 83 | 4506 ± 1473* | 50958 ± 10273 |
| CK, U/L | 844 ± 256 | 10241 ± 2769* | 129659 ± 40278 |
| TnT, ng/mL | 0.009 ± 0.001 | 0.034 ± 0.018 | 1.584 ± 1.106 |
| CKMB, ng/mL | 0.37 ± 0.04 | 0.30 ± 0.04 | 8.51 ± 2.18 |
| CSC-treated (n=11) | |||
| LDH, U/L | 865 ± 102 | 4730 ± 1241* | 53441 ± 13284 |
| CK, U/L | 925 ± 278 | 14672 ± 6462* | 162734 ± 59527 |
| TnT, ng/mL | 0.010 ± 0.000 | 0.044 ± 0.026 | 0.944 ± 0.521 |
| CKMB, ng/mL | 0.40 ± 0.04 | 0.34 ± 0.04 | 5.83 ± 0.76 |
Values are means±SEM.
P<0.05 vs. pre-infusion value (paired t test).
Figure 2.
Plasma troponin T (A) and CKMB (B) levels at baseline (−1), immediately after i.c. cell/vehicle delivery (0), and 6, 12, 24 and 48 h after delivery. Data are means ± SEM.
Hemodynamic data
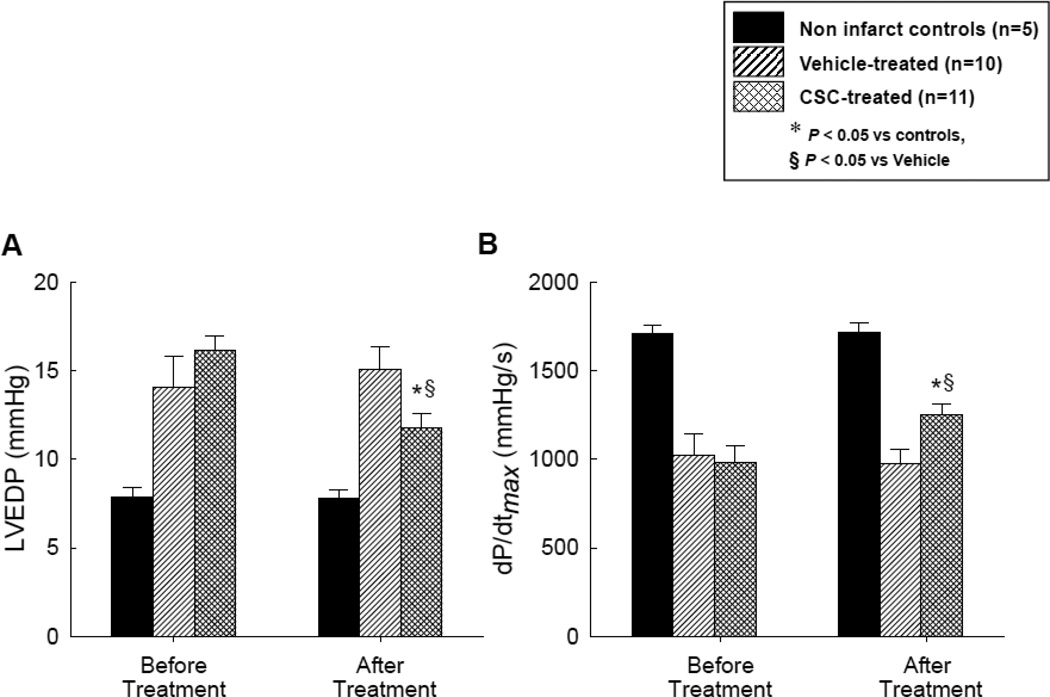
As shown in Table 2 and Figures 3A and 3B, at the time of catheterization (before intracoronary infusion of vehicle or CSCs), there was no significant difference between vehicle- and CSC-treated groups with respect to heart rate, left ventricular (LV) systolic pressure (LVSP), LV end-diastolic pressure (LVEDP), LV dP/dtmax, or LV dP/dtmin (all variables were measured with a Millar catheter). In both groups, heart rate increased significantly at the 31 d follow-up (from 80.3 ± 3.6 to 87.7 ± 4.1 bpm in vehicle-treated group and from 82.6 ± 4.2 to 89.4 ± 2.9 bpm in the CSC-treated group, P<0.05 in both). In vehicle-treated pigs, LVSP, LVEDP, LV dP/dtmax, and LV dP/dtmin did not change significantly. In contrast, in CSC-treated pigs the LVEDP decreased (from 15.9 ± 0.8 mmHg at catheterization to 11.8 ± 1.0 mmHg at the 31 d follow-up, P<0.05) and the LV dP/dtmax increased (from 967 ± 91 mmHg/s at catheterization to 1251 ± 74 mmHg at the 31 d follow-up, P<0.05) (Table 2, Figures 3A and 3B).
Table 2.
Hemodynamic data before treatment and before euthanasia
| Vehicle-treated (n=10) |
CSC-treated (n=11) |
||
|---|---|---|---|
| Heart rate (bpm) | Before treatment | 80 ± 4 | 83 ± 4 |
| 31-day follow-up | 88 ± 4* | 89 ± 3* | |
| LVSP (mmHg) | Before treatment | 85 ± 6 | 82 ± 4 |
| 31-day follow-up | 82 ± 4 | 83 ± 2 | |
| dP/dtmin(mmHg/s) | Before treatment | −1328 ± 95 | −1375 ± 101 |
| 31-day follow-up | −1233 ± 116 | −1388 ± 97 |
Values are means±SEM. LVSP, left ventricular systolic pressure.
P<0.05 vs. before treatment (paired t test),
P<0.05 vs. vehicle-treated pigs (unpaired t test).
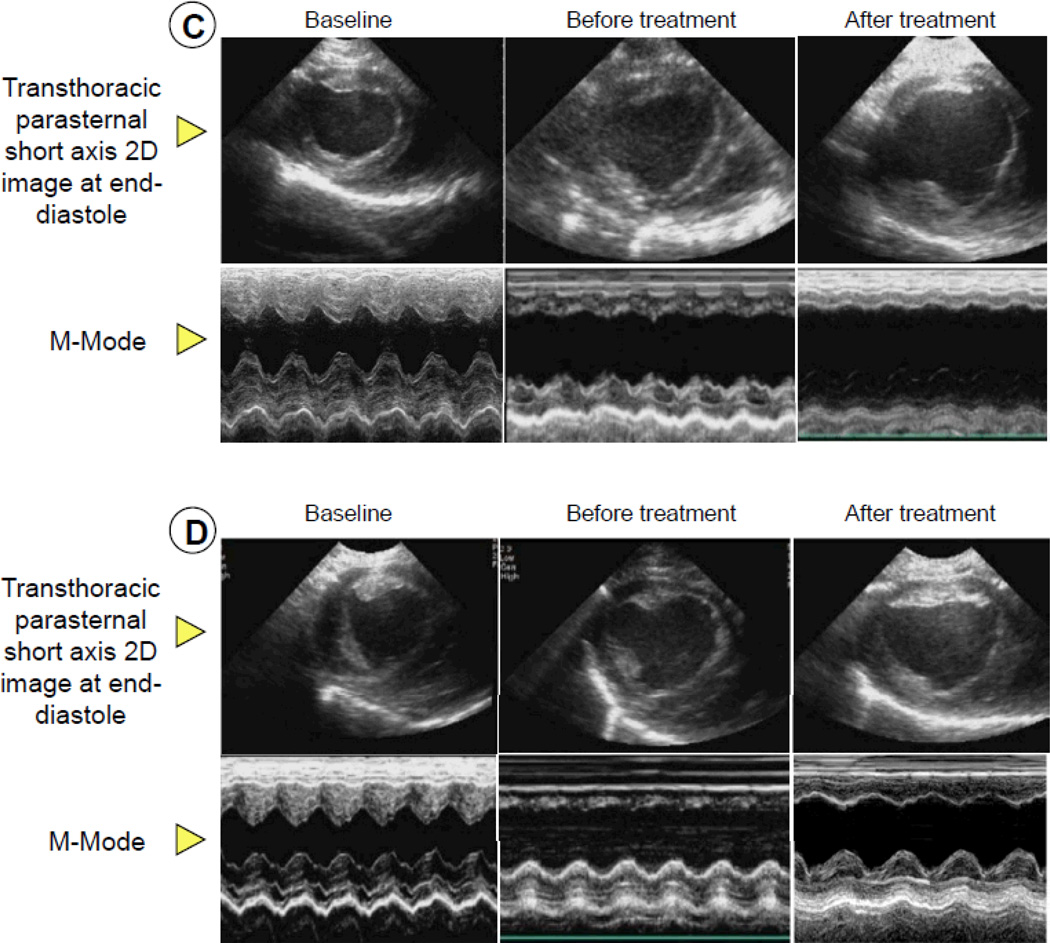
Figure 3.
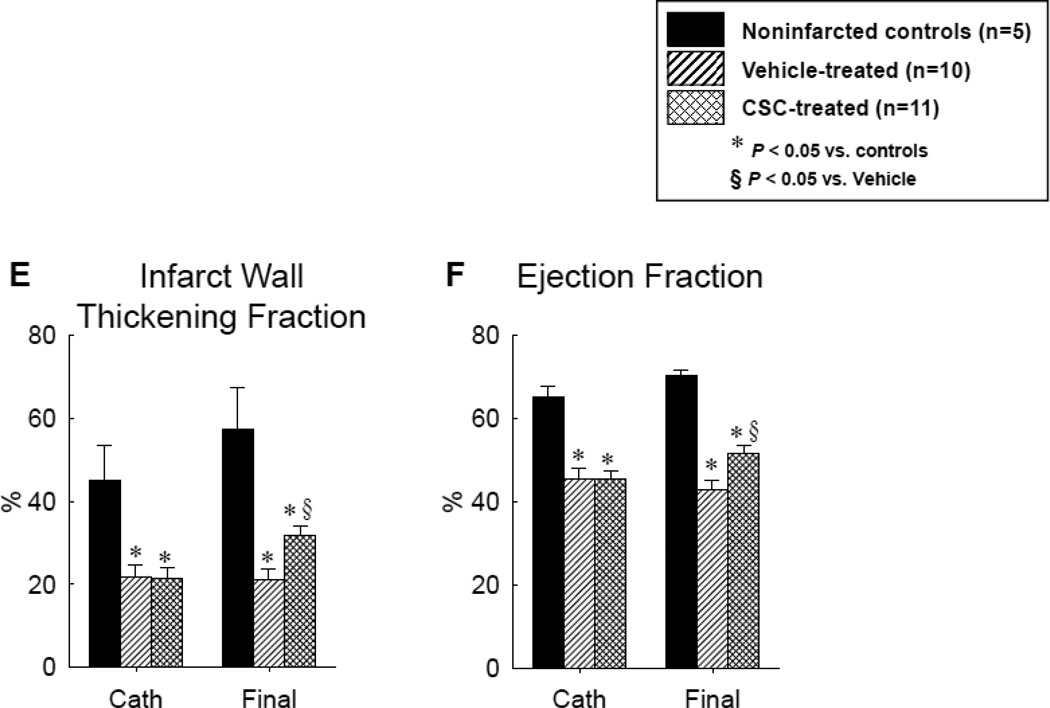
Assessment of LV function before and after vehicle or CSC therapy: hemodynamic variables [LV end-diastolic pressure (A) and LV dP/dtmax (B)], representative M-mode echocardiographic images at 30 d after treatment in pigs that were given vehicle (C) and CSCs (D), and quantitative echocardiographic analysis of LV function (IW thickening fraction and infarcted wall thickening fraction) (E and F). Compared with the vehicle-treated pig, the CSC-treated animal exhibited a smaller LV cavity, a thicker infarcted wall, and improved motion of the infarcted wall (C and D), Quantitative echocardiographic analysis shows improvement in LV functional parameters at 30 d after CSC treatment (E and F). Data are means ± SEM. *, P<0.05 versus noninfarcted controls and §, P<0.05 vs. vehicle-treated pigs (unpaired t test).
Echocardiographic data
Before cardiac catheterization, echocardiographic parameters of LV structure and function were similar in vehicle-treated and CSC-treated infarcted pigs (although they differed from noninfarcted control animals) (Table 3, Figures 3C–3F). At 31 d of follow-up, these variables had not changed significantly in noninfarcted control animals (Table 3, Figures 3C–3F). In vehicle-treated pigs, the LV end-diastolic diameter and volume (calculated by the Teichholz formula) increased by 11.3 ± 8.1% and 38.7 ± 24.3%, respectively, over the ensuing 31 d, but the differences did not reach statistical significance (Table 3). In contrast, these variables remained virtually unchanged in CSC-treated pigs (Table 3). During the 31 d following vehicle infusion, the diastolic thickness of the infarcted LV wall (as assessed by M-mode echocardiography) decreased by 0.57 ± 0.49 mm (5.5 ± 4.9%) in control animals but increased by 1.55 ± 0.64 mm (21.8 ± 9.0% [P=0.029]) in CSC-treated animals (Table 3).
Table 3.
Echocardiographic data before treatment and before euthanasia
| Noninfarcted control (n=5) |
Vehicle-treated (n=10) |
CSC-treated (n=11) |
||
|---|---|---|---|---|
| IWTd (mm) | Before Cath | 9.84 ± 0.34 | 9.75 ± 0.43 | 8.50 ± 0.42 |
| Final | 9.48 ± 0.73 | 9.19 ± 0.57 | 10.05 ± 0.38* | |
| IWTs (mm) | Before Cath | 15.22 ± 0.79 | 12.15 ± 0.65 | 10.47 ± 0.59 |
| Final | 15.28 ± 1.04 | 11.01 ± 0.66 | 13.28 ± 0.43* | |
| PWTd (mm) | Before Cath | 10.69 ± 0.59 | 10.20 ± 0.54 | 10.07 ± 0.55 |
| Final | 10.41 ± 0.71 | 9.57 ± 0.39 | 10.56 ± 0.52 | |
| PWTs (mm) | Before Cath | 15.20 ± 1.16 | 14.03 ± 0.83 | 13.83 ± 0.67 |
| Final | 15.18 ± 1.04 | 13.69 ± 0.74 | 14.88 ± 0.56 | |
| PW ThF | Before Cath | 42.02 ± 6.57 | 37.44 ± 3.06 | 37.92 ± 2.68 |
| (%) | Final | 47.11 ± 8.6 | 40.51 ± 2.66 | 42.32 ± 3.25 |
| LVEDD | Before Cath | 33.44 ± 2.13 | 34.78 ± 1.63 | 35.42 ± 1.56 |
| (mm) | Final | 36.53 ± 1.68 | 38.06 ± 2.36 | 35.73 ± 1.45 |
| LVESD | Before Cath | 21.34 ± 1.55 | 25.63 ± 1.49 | 25.63 ± 1.31 |
| (mm) | Final | 20.23 ± 1.55 | 28.76 ± 2.11 | 24.90 ± 1.38 |
| FS (%) | Before Cath | 36.24 ± 2.12 | 26.5 ± 1.75 | 27.15 ± 1.53 |
| Final | 44.44 ± 2.77 | 24.73 ± 1.51 | 30.68 ± 1.47* | |
| FAC (%) | Before Cath | 48.83± 5.24 | 42.25 ± 2.09 | 45.18 ± 1.51 |
| Final | 44.6 ± 2.97 | 40.55 ± 1.89 | 46.64 ± 2.07* | |
| EDV (ml) | Before Cath | 46.74 ± 7.23 | 51.66 ± 5.26 | 53.92 ± 5.53 |
| Final | 50.67 ± 2.17 | 65.16 ± 9.61 | 54.72 ± 5.38 | |
Values are means±SEM. EDV, end-diastolic volume; FAC, fractional area change; FS, fractional shortening; IWTd, infarct wall thickness in diastole; IWTs, infarct wall thickness in systole; PW ThF, posterior wall thickening fraction; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; PWTs, posterior wall thickness in systole; PWTd, posterior wall thickness in diastole.
P<0.05 vs. vehicle-treated group (unpaired t test).
At the time of treatment, the systolic thickening fraction (ThF) in the infarcted wall was depressed to a similar extent in the two groups (Table 3, Figure. 3E). In vehicle-treated pigs, this variable did not change over the ensuing 31 d, whereas in CSC-treated pigs, it increased from 22.7 ± 2.5% to 32.7 ± 2.7% (P<0.05 vs. pre-catheterization values in CSC-treated pigs and P<0.05 vs. final values in vehicle-treated pigs) (Table 3, Figure 3E), indicating improved regional systolic function. In the noninfarcted LV wall, neither the diastolic thickness nor the systolic thickening fraction (ThF) changed appreciably over time, and neither variable exhibited a significant difference between the two groups (Table 3).
LV fractional shortening and EF were similarly depressed in the two groups at the time of treatment; these variables did not change in vehicle-treated pigs but increased significantly in CSC-treated animals (Table 3, Figure 3F). In vehicle-treated pigs, the EF was 45.6 ± 2.5 % at catheterization and 42.9 ± 2.3% 31 d later, whereas in CSC-treated pigs it increased from 45.4 ± 2.0% to 51.7 ± 2.0 % (P<0.05 vs. pre-catheterization values in CSC-treated pigs and P<0.01 vs. final values in vehicle-treated pigs, Figure 3F), indicating improved global LV systolic function.
Gross pathology and histopathology of the heart
As expected, gross inspection of the heart revealed the presence of scars in the anteroseptal and anterior LV walls (Figures 4 and 5). Histologic and immunohistochemical examination of the heart showed the presence of confluent areas of collagen accumulation in the scarred areas. In treated pigs, islands of viable myocardium were observed within the scar (Figure 5). At higher magnification, these consisted of clusters of small (average cross sectional area, ~50 µm2) fetal-neonatal-like myocytes, as identified by the presence of α-sarcomeric actin. These cells were absent in vehicle-treated hearts and some of them expressed Ki67 in the nucleus (2.36 ± 0.84% of the infarcted area, n=5) (Figure 6), suggesting that at 4 months after infarction, these CSC-derived myocytes had not reached terminal differentiation and growth arrest but rather possessed a residual capacity to divide. The percentage of Ki67 positive nuclei in the remote region with no infarction (postero-lateral LV wall) was similar in the two groups (0.17 ± 0.08% in controls [n=8] vs. 0.19 ± 0.06% in the treated pigs [n=6]). More mature, dispersed cardiomyocytes were also detected in a scattered fashion within the scar; these myocytes were both mononucleated and binucleated.
Figure 4.
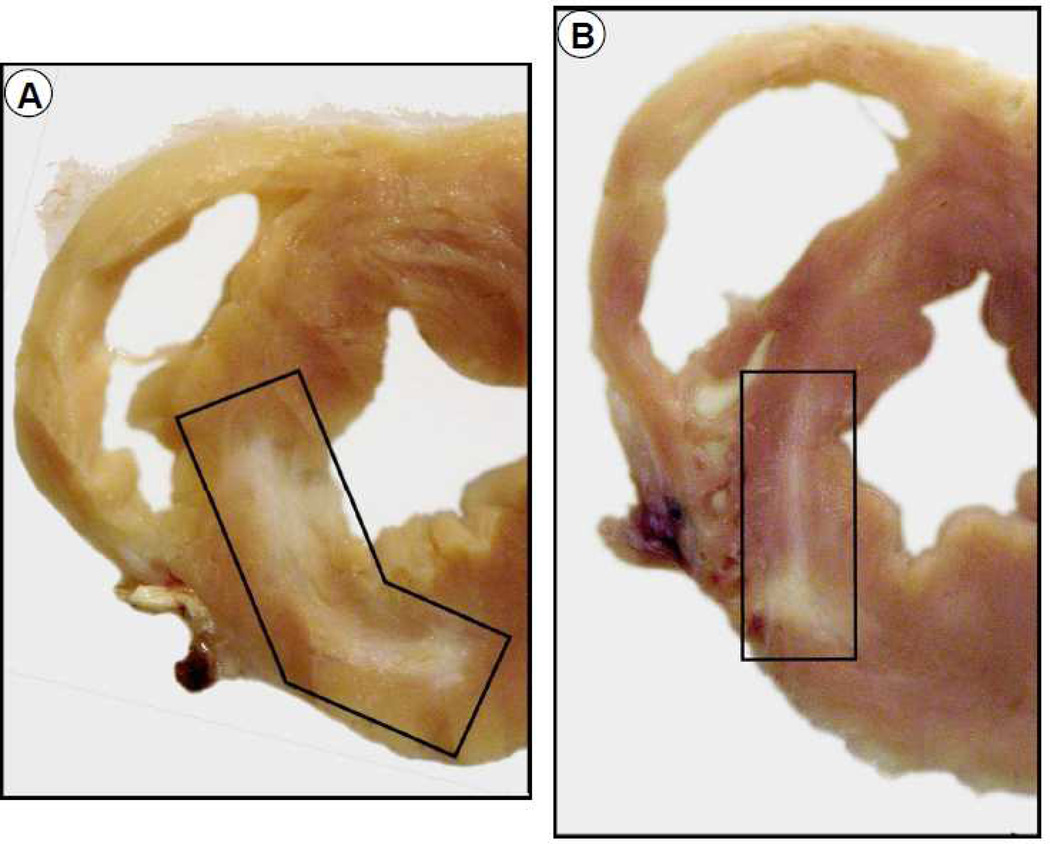
Impact of CSC therapy on LV anatomy. Representative transverse sections of hearts from a vehicle-treated (A) and a CSC-treated (B) pig after 30 d of follow-up. Scar tissue (whitish patch) is highlighted in both the sections. Note that the scar area is smaller and the infarct wall thicker in the CSC-treated heart.
Figure 5.
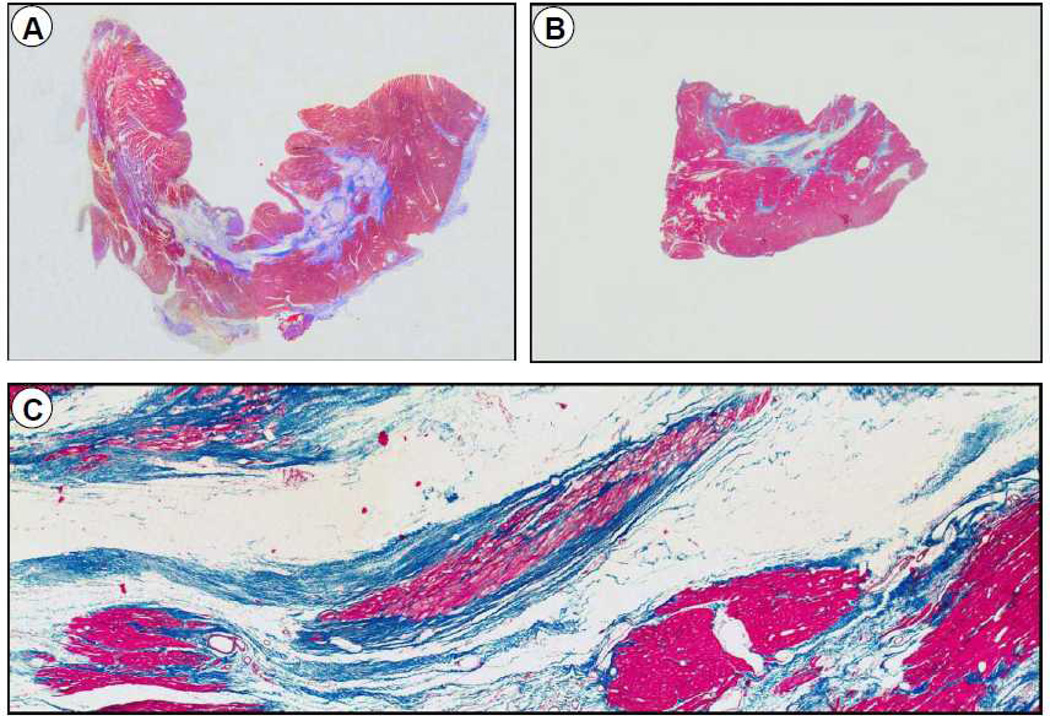
Representative slides of transmural blocks from the core infarct zone from a vehicle-treated (A) and a CSC-treated (B) pig after 30 d of follow-up (hematoxylin and eosin stain). The lower panel (C) shows a higher magnification of the section from a CSC-treated pig. A, Dense transmural fibrosis in a vehicle-treated pig with a homogeneous pattern of scar with less viable tissue; B and C, Mid wall fibrosis surrounded by thick viable myocyte bundles in a pig treated with CSCs.
Figure 6.
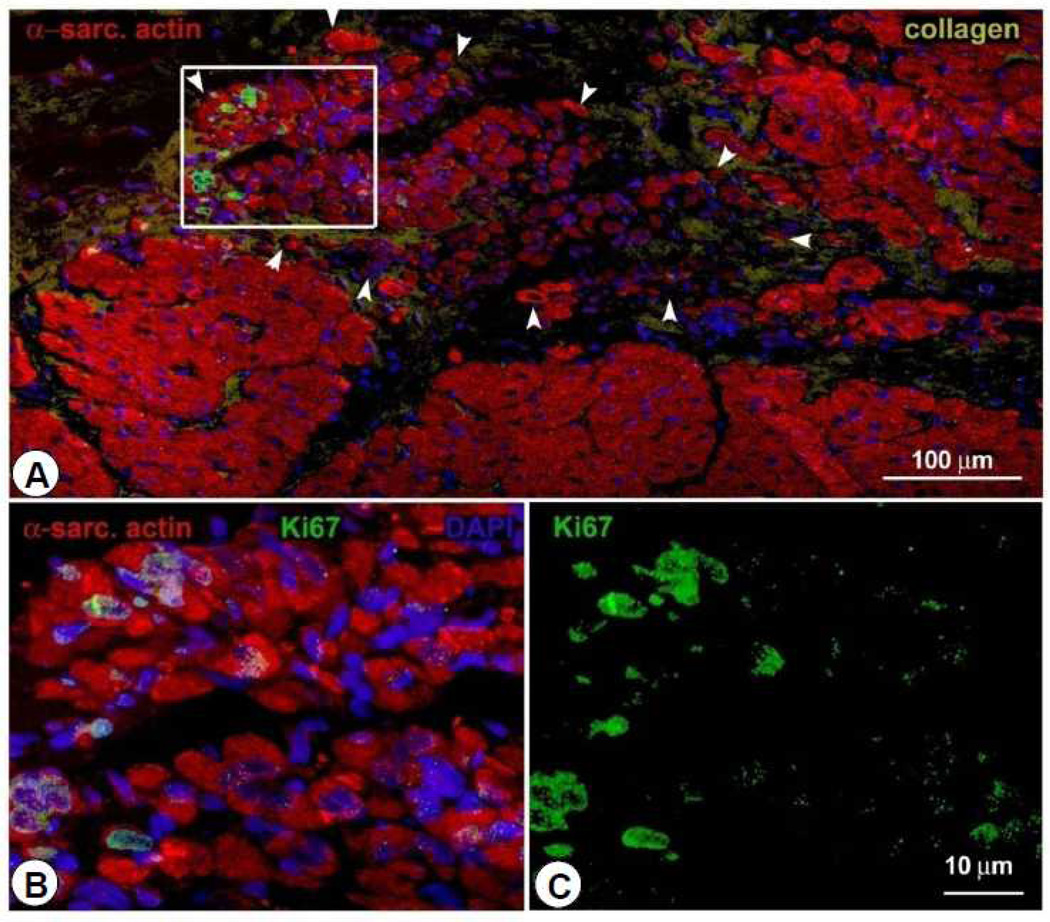
Representative confocal microscopic image from a CSC-treated pig showing small cycling Ki67-positive myocytes in the infarcted region at 30 d after CSC infusion. Positivity for α-sarcomeric actin (red) identifies cardiomyocytes.
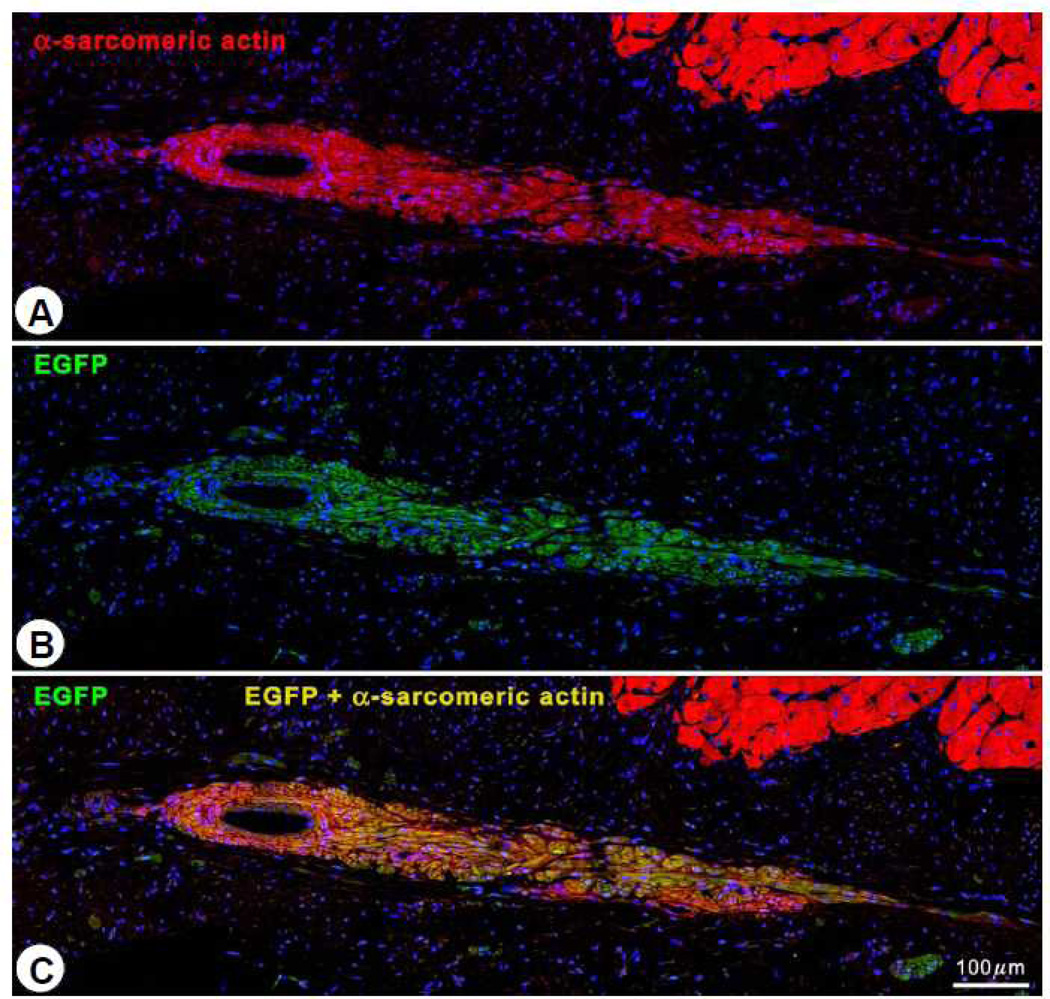
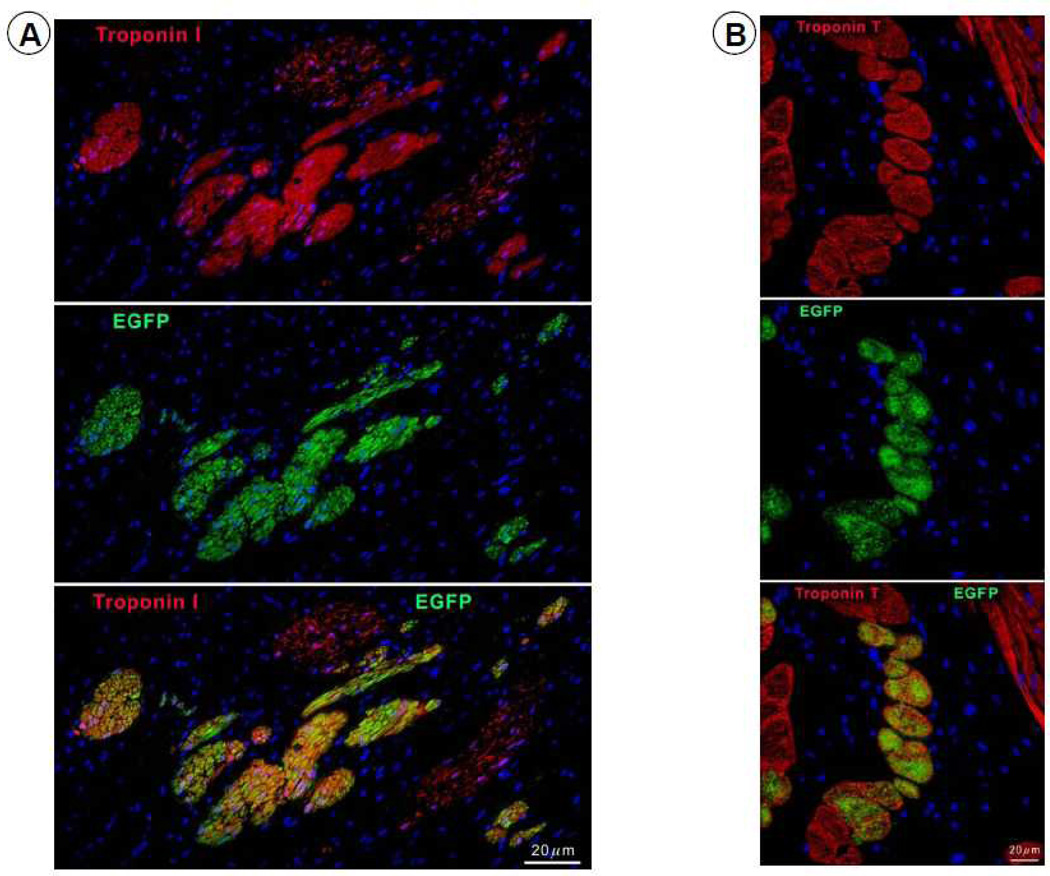
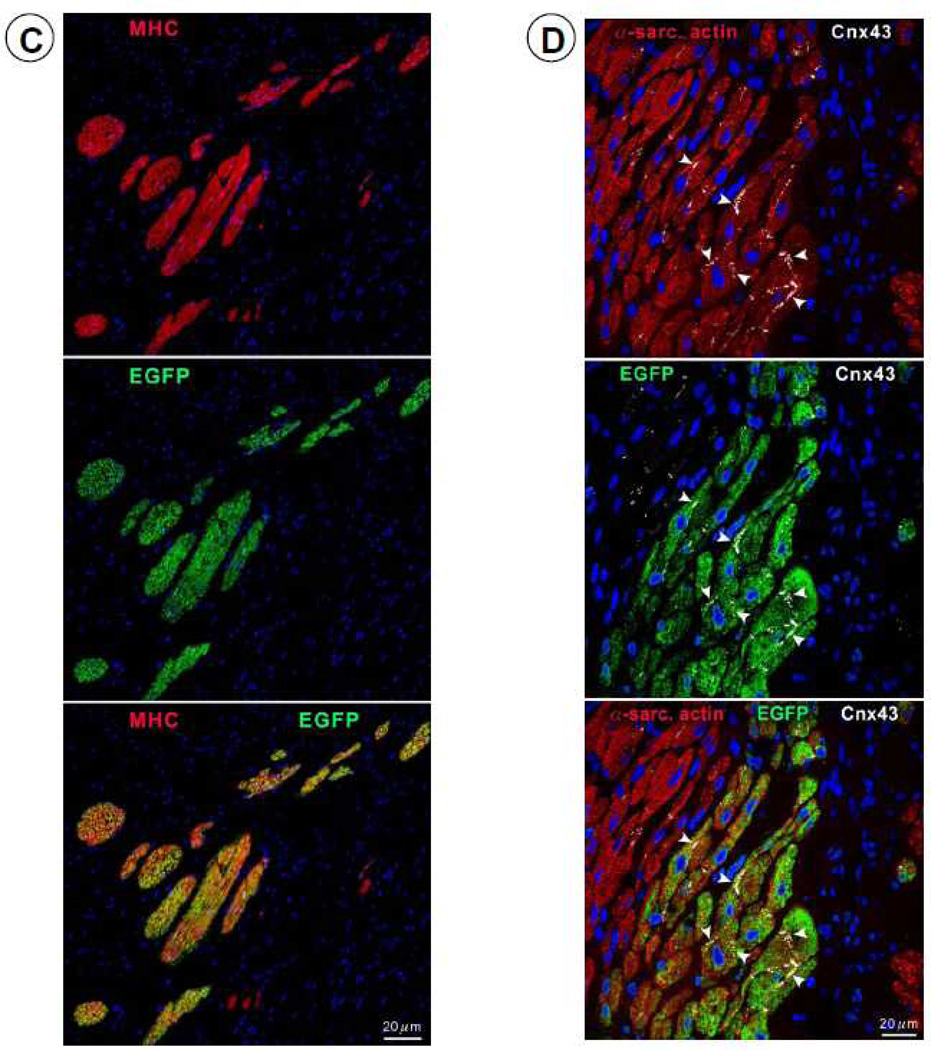
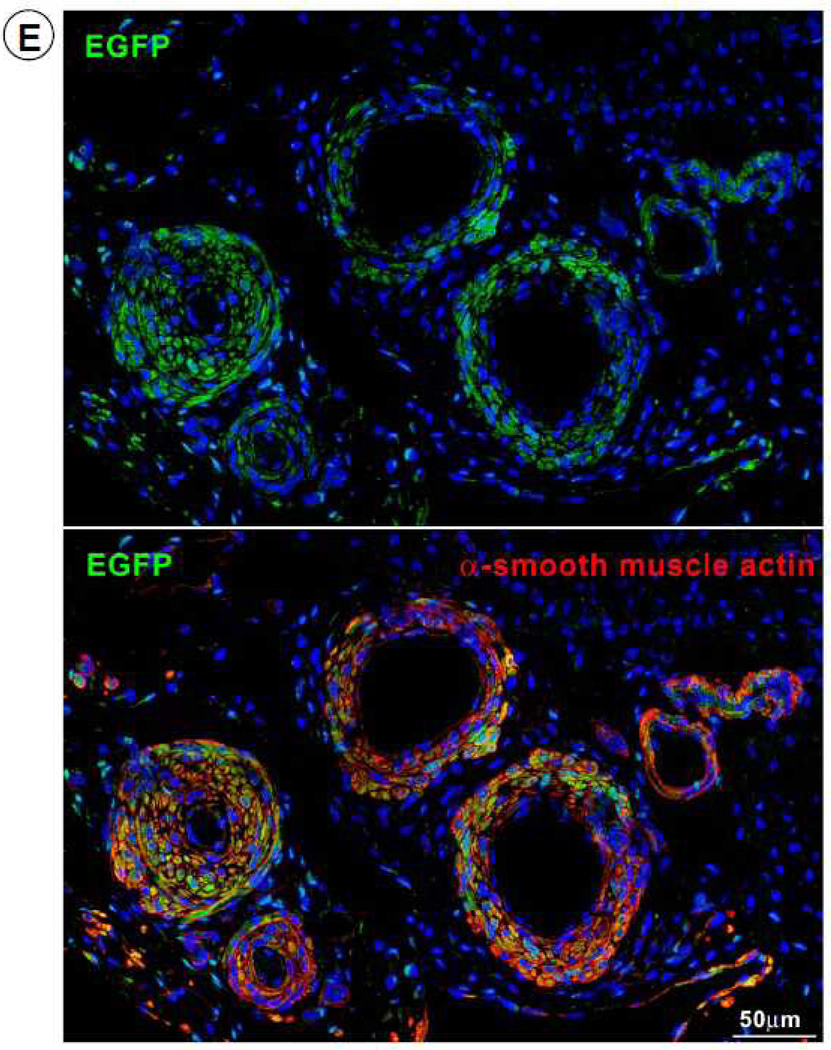
To investigate the origin of the myocytes observed in the scar, four pigs received EGFP-labeled CSCs. In these animals, EGFP-positive cells (0.42 ± 0.09%, n=4) were observed in the infarcted region (Figure 7). The co-expression of α-sarcomeric actin, TnI (Figure 8A), TnT (Figure 8B), and MHC (Figure 8C) in these cells indicates that they were newly-formed myocytes derived from the autologous CSCs. Newly formed myocytes expressed Cnx43 (Figure 8D) suggesting functional integration with adjacent myocytes. EGFP-positive small arterioles (green vessel density: 132 ± 12/mm2) and capillaries (green vessel density: 59.9 ± 8.9/mm2) were also observed in the scarred region, suggesting vascular regeneration (Figure 8E).
Figure 7.
Intracoronary administration of CSCs promotes myocardial regeneration. Regenerated EGFP-positive myocytes in the infarcted region in a CSC-treated heart are labeled with α-sarcomeric actin (red) (A) and EGFP (green) (B). Panel (C) shows the combination of EGFP and α-sarcomeric actin (yellow-green).
Figure 8.
Expression of cardiac-specific TnI, TnT, MHC, Cnx43, and vascular smooth muscle protein (α-smooth muscle actin) in EGFP-positive cells. Representative confocal microscopic images showing colocalization of EGFP with TnI (A), TnT (B), MHC (C), Cnx43 (D), and α-smooth muscle actin (E) in the infarct zone of a CSC-treated pig. Positivity for α-sarcomeric actin (red) identifies cardiomyocytes. In Figure 8E, the structures illustrated are most likely represent arterioles.
Histopathological examination of other organs
There was no gross evidence of tumors or organ damage in the liver, lung, spleen, kidney, and brain of CSC-treated animals (data not shown). Detailed qualitative histopathological analysis of tissue sections from these organs showed no differences between the two groups, and specifically no macro- or microinfarcts, tumors, or increased inflammation in CSC-treated as compared with vehicle-treated pigs. Furthermore, the number of c-kit+ cells in the lungs was not significantly different between vehicle-treated (120 ± 16 cells/mm2 [n=4]) and CSC-treated groups (85 ± 14 cells/mm2 [n=6], P=NS). Thus, there was no evidence of systemic complications following intracoronary infusion of CSCs.
DISCUSSION
We have previously demonstrated in rats that administration of autologous c-kit+ CSCs is effective in regenerating cardiac tissue and alleviating postinfarction LV remodeling and dysfunction when these cells are infused via the intracoronary route in the setting of an old MI 5. More recently, we have performed the first clinical trial of CSCs (SCIPIO), in which we found that intracoronary infusion of autologous CSCs improves LV function, quality of life, and NYHA functional class, reduces scar size, and increases viable myocardium in patients with ischemic cardiomyopathy 10, 17. However, many questions regarding the optimal use of CSCs remain to be addressed. Answers to these questions will require the use of a large, clinically-relevant animal model to study intracoronary infusion of c-kit+ cells in the setting of scarred myocardium (in which the expression of growth factors and adhesion molecules is markedly diminished or even absent). The present study was undertaken to fill this gap and develop such a model. We used a transient coronary occlusion followed by reperfusion (as opposed to a permanent coronary occlusion) because, in current practice, most patients with acute MI receive reperfusion therapy. A model of a 3 month-old reperfused MI was selected because, by this time, the acute inflammatory response in the pig has resolved and the formation of the scar is complete - a setting analogous to that of patients with chronic ischemic cardiomyopathy.
The salient results of the present study can be summarized as follows: (i) in this porcine model of chronic ischemic cardiomyopathy, intracoronary infusion of 5×105 autologous CSCs is well tolerated, with no rise in cardiac enzymes or evidence of microembolization; (ii) intracoronary infusion of 5×105 CSCs results in an improvement in both regional function in the infarcted region and global LV function, as demonstrated by two independent methods (echocardiography and hemodynamic studies), as well as in attenuation of LV wall thinning in the infarcted region; (iii) these salubrious effects are associated with formation of new cardiomyocytes and vascular structures that are derived from transplanted cells. To our knowledge, this is the first time that CSCs have been isolated and utilized for therapeutic studies in a porcine model. The clinical relevance of these preclinical observations is underscored by the fact that the beneficial effects of CSCs were observed in the setting of transient ischemia followed by reperfusion, which is relevant to the majority of patients with MI, and using a route of CSC administration (intracoronary infusion) that is easily applicable to patients and has already been used clinically in the SCIPIO trial 10, 17. Thus, this porcine model should be useful to further study the utility of CSCs in treating ischemic cardiomyopathy. Taken together, the present results demonstrate that transplantation of CSCs exerts important salutary effects on post-MI LV dysfunction even after the healing process is completed.
Potential mechanisms
In CSC-treated pigs, the infarcted region was thicker and exhibited greater wall thickening than in controls (Table 3 and Figure 3E), which may reflect the increased content of viable myocardium consisting mainly of small myocytes (Figure 5).
To determine whether the small myocytes that we observed (Figures 6, 7, and 8) represented dividing amplifying cells, we evaluated Ki67, a nuclear protein that is expressed in cycling cells in late G1, S, G2 and early mitosis 20, 21. Positivity for Ki67 provides a quantitative estimate of the fraction of cells in the cell cycle at the time of euthanasia. As shown in Figure 6, in treated pigs there was an increase in Ki67+ myocytes in the infarcted region, suggesting myocyte regeneration. Dividing myocytes were small, with partially aligned myofibrils, resembling late fetal/neonatal cells. To gain further insights into the efficacy and mechanism of the beneficial effects of CSCs, the fate of transplanted CSCs was determined in four pigs by labeling them with EGFP and assessing proteins specific for myocytes and smooth muscle cells. We found EGFP+ cells that expressed the cardiac specific markers α-sarcomeric actin, TnI, TnT, and MHC (Figures 7 and 8A–8C), suggesting differentiation of transplanted cells into cardiac myocytes. Overall, in the infarcted region, all myocytes positive for Ki67 expressed EGFP. To characterize further the properties of these new cells, we determined the expression of Cnx43, and found it to be present at the surface of closely aligned differentiating cells, between new myocytes and preexisting and regenerated myocytes (Figure 8D). This result suggests onset of functional competence in the regenerating heart muscle. We also found EGFP expressing cells in the vessel wall; these cells were positive for α-smooth muscle actin, documenting the differentiation of CSCs into smooth muscle cells (Figure 8E). This finding is consistent with previous studies in which transplantation of CSCs into ischemic myocardium induced angiogenesis 22.
Taken together, these observations are consistent with the concept that adoptive transfer of CSCs resulted in their proliferation and differentiation into cardiac lineages.
Previous studies
Prior studies of bone marrow-derived mesenchymal stem cells in pigs have demonstrated an increase in endogenous c-kit+ CSCs 23 as well as mobilization of c-kit+ bone marrow progenitor cells 24, with improvement in LV function after MI. The only previous study of cardiac-derived cells in a porcine model of chronic ischemic cardiomyopathy was performed with intracoronary infusion of autologous cardiosphere-derived cells (CDCs), produced from endomyocardial biopsy samples, in pigs with a 4 week-old MI 6. The authors claimed that CDC infusion resulted in engraftment, formation of mature cardiac cells, reduction in “relative” infarct size, and improvement in LV remodeling and hemodynamic function 8 weeks later. The evidence provided in support of these claims, however, was largely inadequate. Early engraftment, myocardial damage, and long-term engraftment were assessed in only 2 pigs for a given dose of CDCs. The only evidence of “engraftment” provided in that study was one photograph (Figure 4) purporting to show few X-gal+ “cardiomyocytes” which, however, cannot be clearly recognized as such because of the quality of the image. Infarct (scar) size did not change after CDCs (11.0 g before CDCs vs. 10.6 g after CDCs). The concept of a decrease in “relative infarct size” (i.e., infarct size expressed as % of LV mass) has uncertain significance; in that study 6, it reflected simply an increase in total LV mass (possibly due to hypertrophy), not a decrease in scar size. In addition, CDCs produced no significant change in LV end-diastolic or end-systolic volume, LV EF, or LV end-diastolic pressure 6. Thus, the author’s conclusion that CDC delivery “results in formation of new cardiac tissue, reduces relative infarct size, and attenuates adverse remodeling” is not supported by the data.
Intracoronary infusion is an attractive method for cell delivery to the heart because it can disseminate cells relatively uniformly to the entire region infused 25, it is widely available clinically, it is less invasive than intramyocardial injection, and it has been used in numerous clinical trials 26–33. Other adult stem cells, such as mesenchymal stem cells (MSCs) 34–36 and CDCs 6, have been shown to produce microvascular occlusion after intracoronary delivery, raising concern over the use of this approach in patients. This is not surprising, as the diameter of MSCs and CDCs is ~20 µm, which may exceed the diameter of some resistance arterioles 37. In contrast, CSCs are ~10 µm in diameter, providing an advantage over other adult stem cells for intracoronary delivery.
Study limitations
The present study has a number of limitations. First, CSCs were compared with vehicle. We did not examine the effects of a control cell population (i.e., a population of nonprogenitor cells). Second, although the improvement in cardiac function and the finding of cardiac-specific markers in Ki67+ and EGFP+ cells suggests regeneration, we did not measure the actual targets of regenerative therapy - scar mass and viable myocardial mass – because of the unavailability of MRI. Nevertheless, the functional improvement afforded by CSCs was impressive, and would be clinically significant regardless of regeneration. Moreover, the results were similar to those that we have obtained In the SCIPIO trial 10, 17, supporting the utility of this porcine model. Finally, we wish to emphasize that the present work describes a promising new cell therapy strategy but only begins to explore the mechanisms of benefit. The functional improvement and histological evidence of regeneration in CSC-treated hearts now serve to motivate studies aimed at establishing how much of the observed benefit is attributable to regeneration by exogenous vs. endogenous CSCs or to other factors, such as paracrine effects and enhanced angiogenesis.
Conclusions
This is the first study to provide evidence that intracoronary infusion of c-kit+ CSCs promotes myocardial and vascular regeneration and improves cardiac function in a large animal model of chronic ischemic cardiomyopathy. This is also the first preclinical study to demonstrate that intracoronary delivery of CSCs exerts beneficial effects even when implemented as late as three months after MI, when the infarcted tissue has been replaced by a mature scar. From a clinical perspective, CSCs are an attractive candidate for cardiac repair because of the large preclinical evidence for their efficacy 4, 5, 14, because of the striking results obtained in SCIPIO 10, 17, and because these cells can be isolated from endomyocardial biopsies 16, expanded, and administered back to patients, avoiding rejection and other complications associated with nonautologous transplantation. The results reported herein provide a clinically-relevant model of chronic ischemic cardiomyopathy that should be useful for further investigation of the efficacy and mechanism of action of CSCs.
Supplementary Material
Acknowledgments
Funding Sources: This study was supported in part by National Institutes of Health grants R01-HL-68088, HL-70897, HL-76794, HL-78825, HL-55757, HL-74351, and HL-91202. Federico Mosna was supported by a Medical Research Council (MRC) grant G0300395.
We gratefully acknowledge Qiuli Bi for expert technical assistance during the course of these studies and Maiying Kong for expert consultation on statistical analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: None.
References
- 1.Farahmand P, Lai TY, Weisel RD, Fazel S, Yau T, Menasche P, Li RK. Skeletal myoblasts preserve remote matrix architecture and global function when implanted early or late after coronary ligation into infarcted or remote myocardium. Circulation. 2008;118:S130–S137. doi: 10.1161/CIRCULATIONAHA.107.757617. [DOI] [PubMed] [Google Scholar]
- 2.Waksman R, Fournadjiev J, Baffour R, Pakala R, Hellinga D, Leborgne L, Yazdi H, Cheneau E, Wolfram R, Seabron R, Horton K, Kolodgie F, Virmani R, Rivera E. Transepicardial autologous bone marrow-derived mononuclear cell therapy in a porcine model of chronically infarcted myocardium. Cardiovasc Radiat Med. 2004;5:125–131. doi: 10.1016/j.carrad.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Schuleri KH, Feigenbaum GS, Centola M, Weiss ES, Zimmet JM, Turney J, Kellner J, Zviman MM, Hatzistergos KE, Detrick B, Conte JV, McNiece I, Steenbergen C, Lardo AC, Hare JM. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. Eur Heart J. 2009;30:2722–2732. doi: 10.1093/eurheartj/ehp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rota M, Padin-Iruegas ME, Misao Y, De Angelis A, Maestroni S, Ferreira-Martins J, Fiumana E, Rastaldo R, Arcarese ML, Mitchell TS, Boni A, Bolli R, Urbanek K, Hosoda T, Anversa P, Leri A, Kajstura J. Local activation or implantation of cardiac progenitor cells rescues scarred infarcted myocardium improving cardiac function. Circ Res. 2008;103:107–116. doi: 10.1161/CIRCRESAHA.108.178525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang XL, Rokosh G, Sanganalmath SK, Yuan F, Sato H, Mu J, Dai S, Li C, Chen N, Peng Y, Dawn B, Hunt G, Leri A, Kajstura J, Tiwari S, Shirk G, Anversa P, Bolli R. Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30-day-old infarction. Circulation. 2010;121:293–305. doi: 10.1161/CIRCULATIONAHA.109.871905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnston PV, Sasano T, Mills K, Evers R, Lee ST, Smith RR, Lardo AC, Lai S, Steenbergen C, Gerstenblith G, Lange R, Marban E. Engraftment, differentiation, and functional benefits of autologous cardiosphere-derived cells in porcine ischemic cardiomyopathy. Circulation. 2009;120:1075–1083. doi: 10.1161/CIRCULATIONAHA.108.816058. 1077 p following 1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menasche P, Alfieri O, Janssens S, McKenna W, Reichenspurner H, Trinquart L, Vilquin JT, Marolleau JP, Seymour B, Larghero J, Lake S, Chatellier G, Solomon S, Desnos M, Hagege AA. The myoblast autologous grafting in ischemic cardiomyopathy (magic) trial: First randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117:1189–1200. doi: 10.1161/CIRCULATIONAHA.107.734103. [DOI] [PubMed] [Google Scholar]
- 8.Perin EC, Silva GV, Henry TD, Cabreira-Hansen MG, Moore WH, Coulter SA, Herlihy JP, Fernandes MR, Cheong BY, Flamm SD, Traverse JH, Zheng Y, Smith D, Shaw S, Westbrook L, Olson R, Patel D, Gahremanpour A, Canales J, Vaughn WK, Willerson JT. A randomized study of transendocardial injection of autologous bone marrow mononuclear cells and cell function analysis in ischemic heart failure (focus-hf) Am Heart J. 2011;161:1078–1087. doi: 10.1016/j.ahj.2011.01.028. e1073. [DOI] [PubMed] [Google Scholar]
- 9.Assmus B, Honold J, Schachinger V, Britten MB, Fischer-Rasokat U, Lehmann R, Teupe C, Pistorius K, Martin H, Abolmaali ND, Tonn T, Dimmeler S, Zeiher AM. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 10.Bolli R, Chugh AR, D'Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J, Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (scipio): Initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marban L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marban E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (caduceus): A prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 13.Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N, Cascapera S, Beltrami AP, D'Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, Anversa P. Human cardiac stem cells. Proc Natl Acad Sci U S A. 2007;104:14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dawn B, Stein AB, Urbanek K, Rota M, Whang B, Rastaldo R, Torella D, Tang XL, Rezazadeh A, Kajstura J, Leri A, Hunt G, Varma J, Prabhu SD, Anversa P, Bolli R. Cardiac stem cells delivered intravascularly traverse the vessel barrier, regenerate infarcted myocardium, and improve cardiac function. Proc Natl Acad Sci U S A. 2005;102:3766–3771. doi: 10.1073/pnas.0405957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer KM, Cottage CT, Wu W, Din S, Gude NA, Avitabile D, Quijada P, Collins BL, Fransioli J, Sussman MA. Enhancement of myocardial regeneration through genetic engineering of cardiac progenitor cells expressing pim-1 kinase. Circulation. 2009;120:2077–2087. doi: 10.1161/CIRCULATIONAHA.109.884403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D'Amario D, Fiorini C, Campbell PM, Goichberg P, Sanada F, Zheng H, Hosoda T, Rota M, Connell JM, Gallegos RP, Welt FG, Givertz MM, Mitchell RN, Leri A, Kajstura J, Pfeffer MA, Anversa P. Functionally competent cardiac stem cells can be isolated from endomyocardial biopsies of patients with advanced cardiomyopathies. Circ Res. 2011;108:857–861. doi: 10.1161/CIRCRESAHA.111.241380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chugh AR, Beache GM, Loughran JH, Mewton N, Elmore JB, Kajstura J, Pappas P, Tatooles A, Stoddard MF, Lima JA, Slaughter MS, Anversa P, Bolli R. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: The scipio trial: Surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation. 2012;126:S54–S64. doi: 10.1161/CIRCULATIONAHA.112.092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quaini F, Urbanek K, Beltrami AP, Finato N, Beltrami CA, Nadal-Ginard B, Kajstura J, Leri A, Anversa P. Chimerism of the transplanted heart. N Engl J Med. 2002;346:5–15. doi: 10.1056/NEJMoa012081. [DOI] [PubMed] [Google Scholar]
- 19.Urbanek K, Quaini F, Tasca G, Torella D, Castaldo C, Nadal-Ginard B, Leri A, Kajstura J, Quaini E, Anversa P. Intense myocyte formation from cardiac stem cells in human cardiac hypertrophy. Proc Natl Acad Sci U S A. 2003;100:10440–10445. doi: 10.1073/pnas.1832855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scholzen T, Gerdes J. The ki-67 protein: From the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 21.Jordan CT, Yamasaki G, Minamoto D. High-resolution cell cycle analysis of defined phenotypic subsets within primitive human hematopoietic cell populations. Exp Hematol. 1996;24:1347–1355. [PubMed] [Google Scholar]
- 22.Tillmanns J, Rota M, Hosoda T, Misao Y, Esposito G, Gonzalez A, Vitale S, Parolin C, Yasuzawa-Amano S, Muraski J, De Angelis A, Lecapitaine N, Siggins RW, Loredo M, Bearzi C, Bolli R, Urbanek K, Leri A, Kajstura J, Anversa P. Formation of large coronary arteries by cardiac progenitor cells. Proc Natl Acad Sci U S A. 2008;105:1668–1673. doi: 10.1073/pnas.0706315105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, Mazhari R, Boyle AJ, Zambrano JP, Rodriguez JE, Dulce R, Pattany PM, Valdes D, Revilla C, Heldman AW, McNiece I, Hare JM. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki G, Iyer V, Lee TC, Canty JM., Jr Autologous mesenchymal stem cells mobilize ckit+ and cd133+ bone marrow progenitor cells and improve regional function in hibernating myocardium. Circ Res. 2011;109:1044–1054. doi: 10.1161/CIRCRESAHA.111.245969. [DOI] [PubMed] [Google Scholar]
- 25.Li Q, Guo Y, Ou Q, Chen N, Wu WJ, Yuan F, O'Brien E, Wang T, Luo L, Hunt GN, Zhu X, Bolli R. Intracoronary administration of cardiac stem cells in mice: A new, improved technique for cell therapy in murine models. Basic Res Cardiol. 2011;106:849–864. doi: 10.1007/s00395-011-0180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Assmus B, Schachinger V, Teupe C, Britten M, Lehmann R, Dobert N, Grunwald F, Aicher A, Urbich C, Martin H, Hoelzer D, Dimmeler S, Zeiher AM. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (topcare-ami) Circulation. 2002;106:3009–3017. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 27.Fernandez-Aviles F, San Roman JA, Garcia-Frade J, Fernandez ME, Penarrubia MJ, de la Fuente L, Gomez-Bueno M, Cantalapiedra A, Fernandez J, Gutierrez O, Sanchez PL, Hernandez C, Sanz R, Garcia-Sancho J, Sanchez A. Experimental and clinical regenerative capability of human bone marrow cells after myocardial infarction. Circ Res. 2004;95:742–748. doi: 10.1161/01.RES.0000144798.54040.ed. [DOI] [PubMed] [Google Scholar]
- 28.Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, Fichtner S, Korte T, Hornig B, Messinger D, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: The boost randomised controlled clinical trial. Lancet. 2004;364:141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 29.Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, Kalantzi M, Herbots L, Sinnaeve P, Dens J, Maertens J, Rademakers F, Dymarkowski S, Gheysens O, Van Cleemput J, Bormans G, Nuyts J, Belmans A, Mortelmans L, Boogaerts M, Van de Werf F. Autologous bone marrow-derived stem-cell transfer in patients with st-segment elevation myocardial infarction: Double-blind, randomised controlled trial. Lancet. 2006;367:113–121. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 30.Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, Endresen K, Ilebekk A, Mangschau A, Fjeld JG, Smith HJ, Taraldsrud E, Grogaard HK, Bjornerheim R, Brekke M, Muller C, Hopp E, Ragnarsson A, Brinchmann JE, Forfang K. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199–1209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 31.Erbs S, Linke A, Schuler G, Hambrecht R. Intracoronary administration of circulating blood-derived progenitor cells after recanalization of chronic coronary artery occlusion improves endothelial function. Circ Res. 2006;98:48. doi: 10.1161/01.RES.0000214407.58341.c8. [DOI] [PubMed] [Google Scholar]
- 32.Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, Zuba-Surma EK, Al-Mallah M, Dawn B. Adult bone marrow-derived cells for cardiac repair: A systematic review and meta-analysis. Arch Intern Med. 2007;167:989–997. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 33.Meyer GP, Wollert KC, Lotz J, Steffens J, Lippolt P, Fichtner S, Hecker H, Schaefer A, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary bone marrow cell transfer after myocardial infarction: Eighteen months' follow-up data from the randomized, controlled boost (bone marrow transfer to enhance st-elevation infarct regeneration) trial. Circulation. 2006;113:1287–1294. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- 34.Freyman T, Polin G, Osman H, Crary J, Lu M, Cheng L, Palasis M, Wilensky RL. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur Heart J. 2006;27:1114–1122. doi: 10.1093/eurheartj/ehi818. [DOI] [PubMed] [Google Scholar]
- 35.Perin EC, Silva GV, Assad JA, Vela D, Buja LM, Sousa AL, Litovsky S, Lin J, Vaughn WK, Coulter S, Fernandes MR, Willerson JT. Comparison of intracoronary and transendocardial delivery of allogeneic mesenchymal cells in a canine model of acute myocardial infarction. J Mol Cell Cardiol. 2008;44:486–495. doi: 10.1016/j.yjmcc.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 36.Vulliet PR, Greeley M, Halloran SM, MacDonald KA, Kittleson MD. Intra-coronary arterial injection of mesenchymal stromal cells and microinfarction in dogs. Lancet. 2004;363:783–784. doi: 10.1016/S0140-6736(04)15695-X. [DOI] [PubMed] [Google Scholar]
- 37.Kassab GS, Fung YC. Topology and dimensions of pig coronary capillary network. Am J Physiol. 1994;267:H319–H325. doi: 10.1152/ajpheart.1994.267.1.H319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.