Abstract
Blockade of N-methyl-D-aspartate receptors (NMDARs) produces behavior in healthy people that is similar to the psychotic symptoms and cognitive deficits of schizophrenia and can exacerbate symptoms in people with schizophrenia. However, an endogenous brain disruption of NMDARs has not been clearly established in schizophrenia. We measured mRNA transcripts for five NMDAR subunit mRNAs and protein for the NR1 subunit in the dorsolateral prefrontal cortex (DLPFC) of schizophrenia and control (n=74) brains. Five NMDAR single-nucleotide polymorphisms (SNPs) previously associated with schizophrenia were tested for association with NMDAR mRNAs in postmortem brain and for association with cognitive ability in an antemortem cohort of 101 healthy controls and 48 people with schizophrenia. The NR1 subunit (mRNA and protein) and NR2C mRNA were decreased in postmortem brain from people with schizophrenia (P=0.004, P=0.01 and P=0.01, respectively). In the antemortem cohort, the minor allele of NR2B rs1805502 (T5988C) was associated with significantly lower reasoning ability in schizophrenia. In the postmortem brain, the NR2B rs1805502 (T5988C) C allele was associated with reduced expression of NR1 mRNA and protein in schizophrenia. Reduction in NR1 and NR2C in the DLPFC of people with schizophrenia may lead to altered NMDAR stoichiometry and provides compelling evidence for an endogenous NMDAR deficit in schizophrenia. Genetic variation in the NR2B gene predicts reduced levels of the obligatory NR1 subunit, suggesting a novel mechanism by which the NR2B SNP may negatively influence other NMDAR subunit expression and reasoning ability in schizophrenia.
Keywords: cognition, expression, NMDA receptor, prefrontal cortex, schizophrenia, SNP
INTRODUCTION
Schizophrenia is characterized by hallucinations, delusions, disorganization and lack of motivation. People with schizophrenia commonly display executive function/reasoning deficits related to dorsolateral prefrontal cortex (DLPFC) and possible glutamate dysfunction.1, 2 Blockade of N-methyl-D-aspartate (NMDA)-type glutamate receptors (NMDARs) in healthy people produces behavior that resembles the symptoms and cognitive deficits of schizophrenia3, 4, 5, 6 and administration of NMDAR antagonists exacerbates symptoms in schizophrenia.7, 8, 9 Additional evidence from blood, brain tissue and structural imaging genomics suggests that NMDAR function can be attenuated in schizophrenia due to reduction in an endogenous ligand for NMDAR, D-serine.10, 11, 12 As postmortem studies have failed to find a clear and simple molecular basis for NMDAR hypofunction, some leaders in the field are suggesting that the endogenous failure in NMDAR function must lie in interacting partners or downstream effectors of NMDARs.13 However, before prematurely concluding that the NMDAR itself is not abnormal, further studies examining this receptor in the brains of people with schizophrenia are needed.
The NMDAR is composed of four subunits: two obligatory NR1 subunits, and two NR2 (NR2A, NR2B, NR2C and NR2D) and/or NR3 (NR3A and NR3B) subunits. Each subunit is encoded by a distinct gene and differential assembly of NR2/3 subunits endows the receptor with different properties.14 Genetic polymorphisms in NMDARs, particularly NR1 (GRIN1), NR2A (GRIN2A) and NR2B (GRIN2B), are associated with schizophrenia15, 16, 17, 18, 19, 20, 21, 22 and genetic variation in GRIN promoter regions impact transcript levels in vitro.15, 18 The extent to which NMDAR subunit mRNAs are altered in the brains of people with schizophrenia is controversial, with decreases,23, 24, 25, 26 increases27, 28 and no change29 found in schizophrenia. The lack of consistency in the postmortem findings hinders progress into the mechanism of NMDAR pathophysiology in the disease.
NMDARs have a key role in behavior and cognition as NR1 mutant mice show metabolic reductions in brain, social withdrawal and working memory and attention deficits.30, 31, 32, 33, 34, 35 Elimination of interneuronal NR1 results in cortical disinhibition, hyperlocomotion, increased anhedonia and anxiety, and memory impairment.36 In rodents, increased NR2B levels are associated with enhanced neuronal plasticity and spatial learning,37 whereas mice lacking the NR2B/NR1 gene have impaired memory.38, 39, 40, 41, 42 Human studies have shown a relationship between variation in the NR2B gene and short-term memory in dyslexia43 and between the NR2A gene and attention deficit hyperactivity disorder,44 suggesting that NMDAR polymorphisms may influence human cognition.
In the present study, we determined whether people with schizophrenia had altered levels of NMDAR mRNAs and NR1 protein in the prefrontal cortex using the largest postmortem sample to date to address this question. We also tested whether putative schizophrenia genetic risk polymorphisms in NMDAR genes were associated with mRNA or protein levels in the postmortem cohort or with cognition in antemortem samples of people with schizophrenia and healthy adults. Our hypotheses were that (1) NMDAR would be reduced in people with schizophrenia, (2) polymorphisms linked to decreases in cortical NMDAR levels would impair cognition, and (3) changes in mRNA and/or protein would be related to genetic polymorphisms associated with schizophrenia.
Methods and materials
Human postmortem brain samples
DLPFC tissue from people with schizophrenia or schizoaffective disorder (n=37) and controls (n=37) was obtained (New South Wales Tissue Resource Centre, Sydney, Australia) and matched according to tissue pH, postmortem interval, RNA integrity number (RIN) and age (Table 1a), see Weickert et al.45 Only one fresh-frozen hemisphere (randomly left or right) was available for each case, with the opposing hemisphere fixed, although overall both right and left hemispheres were assessed in patients and controls (Table 1a). This study was approved by the Human Research Ethics Committee of the University of New South Wales (HREC 07261).
Table 1. Summary of demographics for control and schizophrenia groups in the postmortem (a) and antemortem (b) cohorts.
|
a. Postmortem cohort |
|
|
|
|
|
|
|---|---|---|---|---|---|---|
| Control group (n=37) | Schizophrenia group (n=37) | df | P | |||
| Age (years) | 51.1 (14.6) | 51.3 (14.1) | t=−0.06 | 72 | 0.96 | |
| Gender | 7 F, 30 M | 13 F, 24 M | χ2=2.47 | 1 | 0.12 | |
| Caucasian | 36 | 36 | ||||
| Asian | 1 | 1 | ||||
| Hemisphere | 23 R, 14 L | 17 R, 20 L | χ2=1.96 | 1 | 0.16 | |
| pH | 6.66 (0.3) | 6.61 (0.3) | t=0.64 | 72 | 0.52 | |
| PMI (h) | 24.8 (11.0) | 28.8 (14.1) | t=−1.26 | 72 | 0.21 | |
| RIN | 7.3 (0.6) | 7.3 (0.6) | t=0.24 | 72 | 0.81 | |
| Age of onset (years) | — | 23.7 (0.1) | ||||
| DOI (days) | — | 27.6 (2.3) | ||||
| Manner of death | 36 Natural, 1 accidental | 27 Natural, 8 suicide, 1 accidental, 1 undetermined | ||||
| Antipsychotics | — | 30 Predominantly typicals, 6 predominantly atypicals, 1 typical and atypical | ||||
| Antidepressant history | — | 19 Yes, 18 no | ||||
| Subclass | — | 16 Paranoid, 7 undifferentiated, 5 disorganized, 4 schizoaffective depressed, 3 schizoaffective bipolar, 2 residual | ||||
|
b. Antemortem cohort | ||||||
| Control group (n=101) | Schizophrenia group (n=48) | |
df |
P |
||
| Age (years) | 26.1 (7.5) | 34.4 (7.8) | t=−6.26 | 147 | <0.0001 | |
| Gender | 52 F, 49 M | 17 F, 31 M | χ2=3.38 | 1 | 0.07 | |
| Caucasian | 63 | 47 | ||||
| Asian | 35 | 1 | ||||
| Hispanic | 1 | 0 | ||||
| African | 2 | 0 | ||||
| Education (years) | 15.1 (2.0) | 12.7 (2.3) | t=6.3 | 147 | <0.0001 | |
| WAIS-III | ||||||
| LNS SS | 12.2 (3.2) | 8.2 (3.0) | t=7.4 | 147 | <0.0001 | |
| Picture completion SS | 21.0 (2.4) | 18.3 (3.3) | t=5.67 | 147 | <0.0001 | |
| DSST SS | 12.3 (3.1) | 6.8 (2.3) | t=10.84 | 147 | <0.0001 | |
| Similarities SS | 12.0 (2.8) | 9.8 (3.0) | t=4.5 | 147 | <0.0001 | |
| Arithmetic SS | 12.7 (2.5) | 7.9 (3.4) | t=9.68 | 147 | <0.0001 | |
| FSIQ | 113.4 (13.7) | 91.6 (13.9) | t=9.06 | 147 | <0.0001 | |
| WTAR reading | 108.2 (8.3) | 104.9 (9.0) | t=2.27 | 147 | <0.0001 | |
| Age of onset (years) | — | 27.4 (20.1) | ||||
| DOI | — | 14.0 (14.6) | ||||
| BMI | — | 31.3 (14.3) | ||||
| Antidepressants | 0 | 19 | ||||
| CPZ | — | 661.1 (531.9) | ||||
| Subtype | — | 25 Paranoid, 6 undifferentiated, 3 disorganized, 6 schizoaffective depressed, 6 schizoaffective bipolar, 2 residual | ||||
| PANSS | ||||||
| Positive score | — | 15.9 (4.8) | ||||
| Negative score | — | 14.6 (6.8) | ||||
| General score | — | 33.5 (10.0) | ||||
| Total score | — | 64.0 (18.7) | ||||
Abbreviations: BMI, body mass index; CPZ, chlorpromazine equivalent dose; DOI, duration of illness; DSST, digit symbol substitution test; FSIQ, full-scale IQ; LNS, letter number sequencing; L/R, left/right; PANSS, positive and negative syndrome scale; M/F, male/female; PMI, postmortem interval; RIN, RNA integrity number; SS, standard score; WAIS-III, Wechsler Adult Intelligence Scale, 3rd edition; WTAR, Wechsler Test of Adult Reading.
Quantitative reverse transcription-PCR and western blot analyses
Quantitative reverse transcription-PCR and western blot analyses of DLPFC homogenate were performed with TaqMan gene expression assays (Applied Biosystems, Mulgrave, VIC, Australia) and antibodies listed in Supplementary Table S1. These standard procedures are described in detail in Supplementary material. Supplementary Figures S1 and S2 show the location of TaqMan assay binding to known transcripts of the NR1 and NR2A mRNA, respectively, in the present study relative to previous studies.
Cognitive assessments of healthy adults and people with schizophrenia
One hundred and one healthy adults were recruited via advertisements. Forty-eight people meeting diagnostic criteria for schizophrenia or schizoaffective disorder based on structured clinical interview for DSM-IV-TR axis I disorders (SCID-1) interview by trained clinicians also participated (see Table 1b for demographic characteristics of the groups). All people with schizophrenia were chronically ill and were receiving second-generation antipsychotic medication at the time of testing. The Wechsler Adult Intelligence Scale, 3rd edition (WAIS-III),46 letter number sequencing test was administered to all participants as a measure of working memory function. The Wechsler Test of Adult Reading47 used as an estimate of premorbid IQ in schizophrenia, along with four subtests from the WAIS-III, including arithmetic, similarities, picture completion and digit symbol coding, which was used as current estimate of full-scale IQ were also administered to all participants. These assays provided measures of reasoning, language/verbal comprehension, perceptual organization and processing speed, respectively. All volunteers provided written informed consent and this study was approved by the South Eastern Sydney and Illawarra Area Health Service and UNSW HRECs (numbers 07/259, 07121, 10155).
Polymorphism genotyping of brain and blood
DNA was isolated from DLPFC tissue using a PUREGENE DNA purification kit (QIAGEN, Doncaster, VIC, Australia) from 20 mg of tissue or from whole blood using slightly modified versions of the manufacturer's protocol (described in Supplementary Material). The NR2B single-nucleotide polymorphisms (SNPs) rs1805502 (T5988C 3′UTR), rs1805247 (T4197C), rs7301328 (C366G exon 2) and rs1019385 (T200G) and NR1 SNP rs11146020 (G1001C) genotypes were determined using TaqMan SNP genotyping assays (Applied Biosystems) (see Supplementary Table S2), with a call rate of 97.9%. A no template control was run for each assay and no signal was detected. Allele frequencies were calculated (see Supplementary Table S2) and χ2 demonstrated that the SNPs were all in Hardy–Weinberg equilibrium. Heterozygotes were grouped with individuals homozygous for the rare allele to allow statistical comparisons where minor allele frequency was <0.3. The number of dinucleotide repeats (GT)n in the promotor of the NR2A gene15 was amplified by Prevention Genetics (Wisconsin, USA) using forward primer 5′-AGGAAGCATGTGGGAAATGCAG-3′ and reverse primer 5′-GCTGGGTACAGTTATCCCCCT-3′.
Statistical analyses: postmortem sample
Gene expression data were normalized to the geometric mean of the four housekeeping genes and group outliers (±2 s.d. from mean) were removed. Gene and protein expression data for all NMDAR subunit genes assayed exhibited a normal distribution (Kolmogorov–Smirnov (K–S) d=0.97–0.147, P>0.20) with the exception of NR1 and NR3A mRNAs, which were skewed to the right for the schizophrenia group (K–S d=0.165–0.195, P<0.20). Normalized gene expression data for these two mRNAs were, therefore, transformed by taking the logarithm of gene expression values yielding a normal distribution (K–S d=0.69–0.114, P>0.20). Pearson's correlations were performed to determine whether sample characteristics (age, pH, postmortem interval and RIN) correlated with our measures in controls and/or people with schizophrenia. Analysis of covariance testing for diagnosis effects on mRNA and protein expression were performed and covaried for factors that correlated with mRNA and protein expression. Pearson's correlations were also performed between gene/protein expression and clinical variables, including medication dosage converted to chlorpromazine equivalents (CPZ) dose for people with schizophrenia. Where gene expression differed by hemisphere or gender, a diagnosis x hemisphere/gender factorial analysis of variance was performed. Planned post-hoc testing was performed in the postmortem cohort to explore the association of significant NMDAR SNPs with mRNA and protein expression in the brain.
Statistical analyses: antemortem sample
The effects of genotype on cognition (WAIS-III arithmetic, similarities, picture completion, digit symbol coding, letter number sequencing and Wechsler test of adult reading) in people with schizophrenia and healthy adults was assessed using a series of analysis of variances in which diagnosis (schizophrenia versus control) and genotype were used as independent grouping variables and cognitive score was used as the dependent variable. For cognitive tests from the WAIS-III, age-scaled scores derived from the WAIS-III manual were used in all analyses to adjust for the significant age difference between people with schizophrenia and controls. All statistical tests were performed with Statistica software (version 7.1, Tulsa, OK, USA).
Results
Detection of NMDAR subunit mRNAs in DLPFC
We found that NR1, NR2A, NR2B, NR2C and NR3A mRNAs were reliably detected in adult human DLPFC, whereas NR2D and NR3B mRNAs were not. Several NMDAR subunit mRNAs were significantly correlated with age, tissue pH and RIN (see Supplementary Table S3).
Expression of NR1 mRNA and protein are reduced in the DLPFC of people with schizophrenia
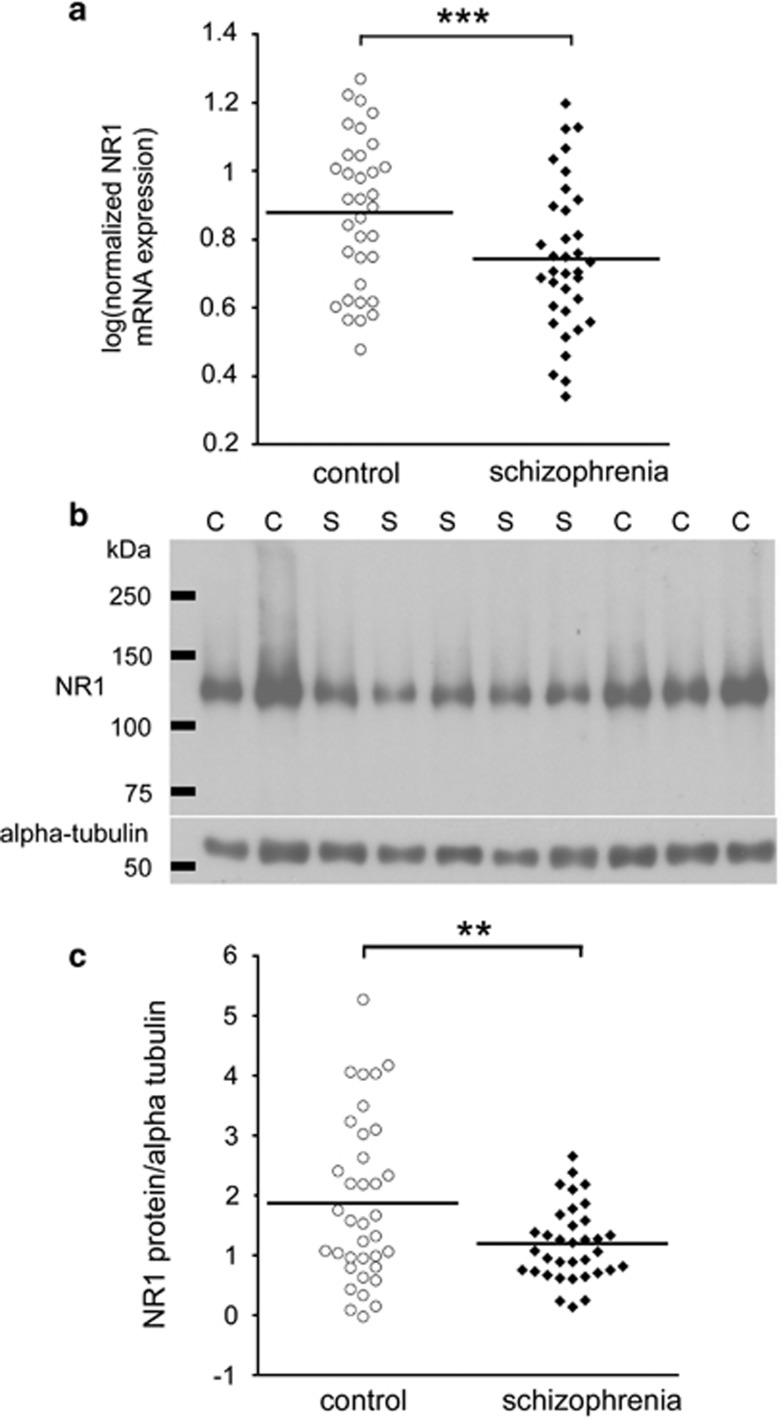
NR1 mRNA was significantly reduced in people with schizophrenia by 22% (F(1,65)=8.91, P=0.004) after co-varying for pH and RIN (Figure 1a). NR1 mRNA expression was greater in the right hemisphere DLPFC compared with the left hemisphere (t(67)=−2.24, P=0.028); however, this effect was no longer significant when we covaried for pH and RIN (F(1,65)=2.059, P=0.156). There was no effect of gender on NR1 mRNA expression. We found that the schizophrenia group had 36% less NMDA NR1 subunit protein than the control group (t(67)=6.95, P=0.010) after co-varying for pH and postmortem interval (Figure 1b and c). We observed no difference in NR1 protein levels between left and right hemisphere in the whole cohort. There was also no effect of gender on NR1 protein in the total cohort.
Figure 1.
NR1 expression is reduced in people with schizophrenia. Dorsolateral prefrontal cortex (DLPFC) NR1 mRNA expression, measured by quantitative reverse transcription-PCR (qRT-PCR) and normalized to the geometric mean of four housekeeping genes, was reduced in people with schizophrenia relative to normal controls (a). NR1 protein expression, normalized to alpha-tubulin, was quantified by western blot (representative blot shown in (b). A single band for NR1 was present at the predicted size (∼120 kDa). NR1 protein was reduced in the DLPFC of people with schizophrenia compared with matched controls (c). **P⩽0.01, ***P<0.005. C, control; S, schizophrenia case.
Expression of NR2C mRNA was reduced in people with schizophrenia
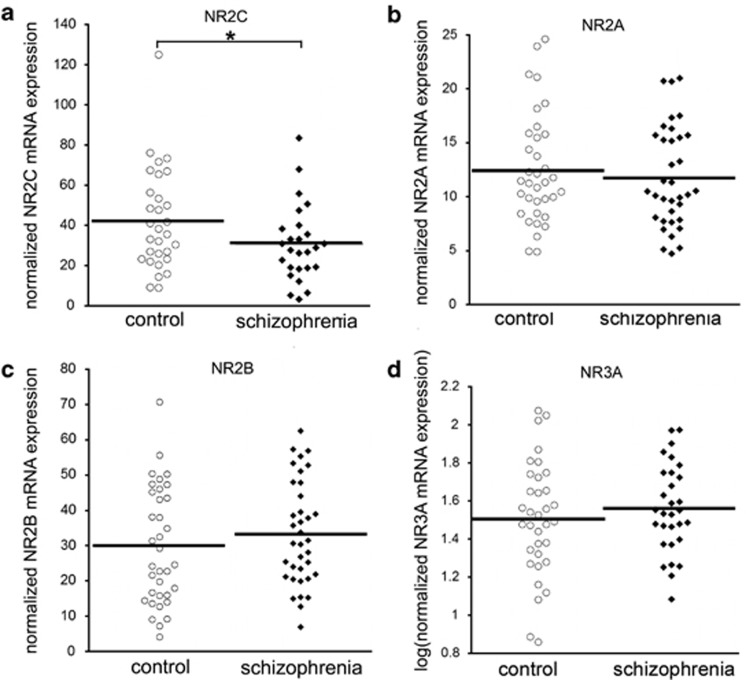
NR2C mRNA was also significantly reduced, by 28%, in people with schizophrenia (F(1,51)=6.446, P=0.014, after co-varying for pH, RIN and age) (Figure 2a). The other NMDAR subunit mRNAs, NR2A, NR2B and NR3A, did not significantly differ according to diagnosis (NR2A: F(1,66)=0.303, P=0.584; NR2B: F(1,68)=1.228, P=0.272; NR3A: (F(1,59)=1.255, P=0.267, respectively) (Figures 2b–d).
Figure 2.
NR2C, NR2A, NR2B and NR3A mRNA expression in people with schizophrenia and matched controls. Expression of N-methyl-D-aspartate (NMDA) receptor subunit mRNAs was measured in the dorsolateral prefrontal cortex (DLPFC) of people with schizophrenia and matched controls by quantitative reverse transcription-PCR (qRT-PCR). Quantity means of transcripts were normalized to the geometric mean of four housekeeping genes. NR2C mRNA (a) was reduced in people with schizophrenia, whereas NR2A (b), NR2B (c) and NR3A (d) were unaltered. *P<0.05.
Lateralization and gender effects on NR2B subunit mRNA expression
NR2B mRNA expression was 39% higher in the left versus right DLPFC in the whole cohort, (t(69)=2.938, P=0.004). There was no significant diagnosis x hemisphere interaction for NR2B mRNA expression (F(1,66)=1.590, P=0.212). NR2B mRNA expression was 33% greater in DLPFC in males relative to females (t(69)=2.107, P=0.039); however, this effect was no longer statistically significant after we covaried for age at death (F(1,68)=3.286, P=0.074).
Correlations with disease characteristics and medication estimates
We correlated NMDAR subunit mRNA expression and NR1 protein with age of illness onset, duration of illness, daily mean CPZ dose, last CPZ dose, and lifetime CPZ dose (see Supplementary Table S4) in the postmortem cohort. We found significant negative correlations between NR3A mRNA expression and duration of illness and lifetime CPZ dose; however, neither significant correlation was preserved after a partial correlation for age was performed (duration of illness: r=−0.166, P=0.39; lifetime CPZ: r=−0.078, P=0.69).
NMDAR SNPs and cognitive function
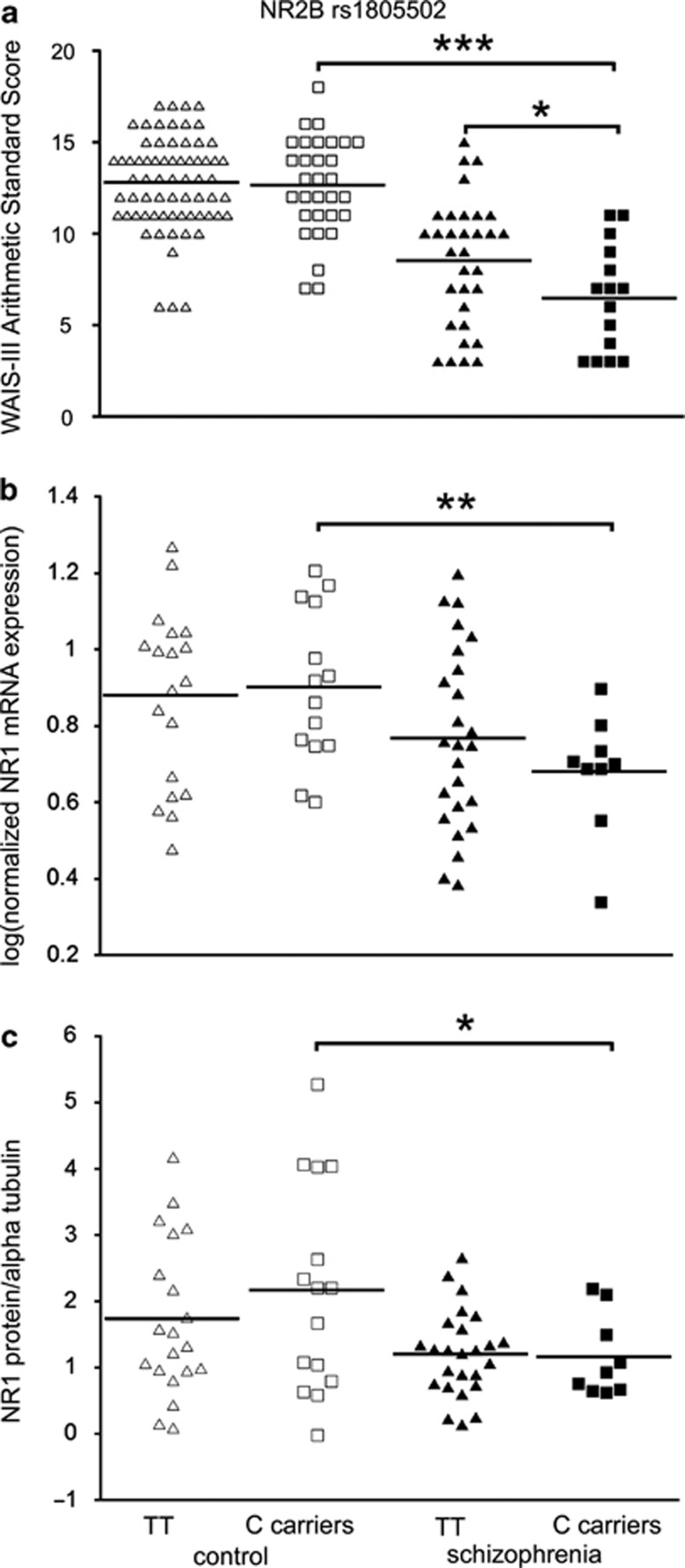
In general, people with schizophrenia scored significantly lower than controls on the basis of all cognitive variables assessed (see Table 1b). Two-way analysis of variances showed a significant main effect of diagnostic group such that people with schizophrenia had significantly lower reasoning performance than controls as measured by WAIS-III Arithmetic (F(142)=94.9, P<0.001). We found a significant main effect of NMDAR genotype such that carriers of the minor allele (C) of the NR2B gene SNP rs1805502 had significantly reduced reasoning performance relative to homozygotes (TT) (Arithmetic: F(1, 142)=4.3, P=0.04) and post hoc least significant difference (LSD) analysis showed that Arithmetic scores were significantly reduced in people with schizophrenia carrying the C allele of this SNP compared with homozygous (TT) people with schizophrenia, P=0.02, and control C carriers, P<.0001 (Figure 3a). We did not detect a significant difference in letter number sequencing or other subtests of the WAIS-III, total FSIQ or Wechsler test of adult reading scores in relation to the NR2B rs1805502 genotype.
Figure 3.
Effect of NR2B SNP rs1805502 on reasoning ability, NR1 mRNA and protein in controls and schizophrenia. NR2B rs1805502 genotype is related to reasoning ability (WAIS-III Arithmetic scores) in people with schizophrenia (a). Schizophrenia carriers of the C allele of rs1805502 display a significantly worse performance than homozygous (TT genotype) people with schizophrenia. Minor allele carriers with schizophrenia also perform worse than control carriers of the minor allele. NR1 mRNA expression (b) and protein levels (c) are significantly lower in individuals with schizophrenia who were C allele carriers than in control individuals. *P<0.05, **P<0.01 and ***P<0.0001.
Effect of genotype on NMDAR subunit mRNA and protein expression
We tested whether the NR2B rs1805502 SNP17, 19, 20 altered expression of NMDAR subunits whose mRNA or protein levels were altered in the DLPFC in schizophrenia. We found that the expression of the NR1 subunit mRNA was significantly reduced in individuals with schizophrenia who were C carriers of SNP rs1805502 relative to heterozygous controls (P=0.008; Figure 3b). Similarly, we found the level of NR1 protein to be significantly reduced in C carriers with schizophrenia relative to C carrier controls (P=0.025; Figure 3c). In contrast, we found no difference in NR2C mRNA levels according to NR2B rs1805502 genotype in either controls or people with schizophrenia (P>0.05).
We also examined whether the length of the (GT)n repeat in the promoter of the NR2A gene15, 16, 21 was related to mRNA expression of NR1 or NR2C. No significant correlations between NR2A repeat length and NR1 or NR2C mRNA expression were found in controls or people with schizophrenia, and there were no significant differences in average repeat length between controls (44.3 bases) and people with schizophrenia (44.7 bases) on the basis of NR2A genotype.
Discussion
Our findings, from the largest postmortem sample assessing NMDAR mRNA, NR1 protein and NMDAR genetic polymorphisms in schizophrenia brain to date, provide clear evidence of a decrease in the obligatory NR1 subunit at both the mRNA and protein level in schizophrenia. This supports the theory that hypofunction of NMDARs exists endogenously in prefrontal cortex in schizophrenia and strongly supports that the hypofunction can reside within the NMDAR. Thus, the next step for the field would be to determine the mechanism by which NMDAR subunits are reduced rather than to only test for diagnostic changes in NMDAR interacting proteins. We also, for the first time, show that the NR2B gene polymorphism (rs1805502) predicts reasoning deficits in people with schizophrenia and suggest that reduced prefrontal NR1 mRNA and protein levels are relevant to this cognitive deficit.
Previous studies of mRNA for the obligatory NR1 subunit in the prefrontal cortex of people with schizophrenia are at odds, reporting no change,29 an increase in elderly patients,27 or reduction in schizophrenia23, 26 (with group n=15–26). Our study (with group n=37) is now the third of five studies showing NR1 mRNA reductions in the prefrontal cortex of people with schizophrenia. Two previous studies of NR1 protein did not observe significant changes in schizophrenia patients,48, 49 whereas we found a robust decrease, possibly due to our larger sample size. The decrease in NR1 protein may limit assembly of the functional NMDAR complex and, thus, we would predict that reduced NMDAR binding should also exist. However, other studies on NMDAR binding have reported an increase,50, 51, 52 or no change52, 53, 54, 55, 56 in NMDAR binding in the brains of people with schizophrenia. The reasons for lack of the expected decrease in NMDAR binding in these other studies are unknown, but may be a result of technical differences between the assays, smaller sample sizes, the ion channel open/closed state57 or receptor internalization.
In addition to the reduction in NR1 mRNA, we also provide further support for a reduction in NR2C mRNA expression in people with schizophrenia compared with controls.23, 29 Knockout of the NR2C subunit gene in mice leads to deficits in associative and working memory,58 demonstrating the functional role for this subunit and the potential for a deficit in this subunit to influence cognitive processes in schizophrenia. NR2C expression has been shown in parvalbumin-positive interneurons in the prefrontal cortex,59 and thus reductions in NR2C could be a consequence of altered inhibitory neuronal health.60 If an NR2C deficit in interneurons was primary, then this would be expected to result in dysregulated glutamatergic transmission in interneurons, which could impact on inhibitory control of excitatory cortical circuits. We and others60, 61, 62, 63, 64, 65, 66, 67 have demonstrated that there is a deficit in parvalbumin and somatostatin expressing interneurons in schizophrenia, which may be linked to reduced NR2C expression in the disease.
Genetic variation in NR2B has been associated with human cognitive dysfunction, for example in dyslexia.43 Given that glutamate has been associated with prefrontal cortex function and schizophrenia, there is a surprising paucity of studies examining the relationship between genetic polymorphisms in NMDAR genes and cognition in schizophrenia. Interestingly, we demonstrate that a genetic polymorphism in the NR2B gene, SNP rs1805502, predicts impaired DLPFC-dependent reasoning ability in schizophrenia. Impaired performance on WAIS-III Arithmetic suggests that reasoning ability is particularly compromised in those people with schizophrenia carrying the minor allele in the 3′UTR of the NR2B gene. The WAIS-III Arithmetic subtest is a sensitive assay of the construct of reasoning, and although working memory and reasoning are interrelated, there is abundant evidence showing that these two constructs are distinct.68, 69, 70, 71, 72, 73 Given that our antemortem patient sample size is equivalent to other studies reporting genetic effects on cognition, the effect in the present study should be reliable; however, this finding should be replicated in a larger, independent sample.
Our findings suggest that genetic variation in the human NR2B gene is associated with changes in reasoning ability in schizophrenia putatively via altering NR1 mRNA and protein levels. In support of this, Humphries et al.25 reported that lower postmortem NR1 mRNA expression in the temporal cortex of people with schizophrenia was strongly correlated with worse premorbid IQ and cognitive function. Although we did not find a relationship between the rs11146020 SNP in NR1 that has previously been associated with schizophrenia22 and NR1 mRNA, this NR1 SNP has been shown to interact with the NR2B SNP rs1805502 in people with schizophrenia.20 Although it is unclear how such genetic variations in NR2B would impact the gene expression of other NMDAR subunits, there is evidence that variation in the schizophrenia genes can impact mRNA levels of binding partners.74 Similarly, a SNP in the schizophrenia susceptibility gene neuregulin has been shown to impact NR2C mRNA levels in the cerebellum in schizophrenia.28 Although the mechanistic pathway by which NMDAR hypofunction may lead to cognitive deficits remains speculative, other recent work suggests that altered NMDAR function can be related to cortical thickness, suggesting a potential neurobiological substrate.12
We find that the C allele carriers of NR2B SNP rs1805502 relates to worse cognition in people with schizophrenia relative to controls with the same genotype. Although this may seem surprising, diagnosis-specific polymorphic effects on cognition can be found in other studies. For example, Jablensky et al.75 have reported another NR2B gene SNP (rs220599) is associated with altered verbal memory in cognitively impaired people with schizophrenia, but not in healthy controls. Further, this group found that two SNPs also in glutamate signaling genes (GRIM3 and PRKCA), both had significant, but opposite effects on verbal memory in healthy controls and people with schizophrenia.75 We suggest that genetic changes that may contribute to beneficial effects of glutamate in controls and typically serve to improve cognitive performance (that is, plasticity and synaptic strengthening) could also enhance deleterious effects (that is, neurotoxic effects) of glutamate in schizophrenia leading to worse cognition.
In summary, we have shown that NR1 mRNA and protein is reduced in the DLPFC of people with schizophrenia. Given that NR1 is the obligatory subunit of the NMDAR, a reduction in overall NMDAR function may be expected in schizophrenia; thus, knowledge of this risk allele may help to identify those who may benefit from future NMDAR agonist treatment studies in schizophrenia. We also report that the NR2B SNP rs1805502 C allele carriers display significant reasoning deficits in people with schizophrenia. We find that people with schizophrenia also have reduced NR2C mRNA in their frontal cortex that appears to be independent of genotype. To our knowledge, this represents the first study to link NMDAR genotypes, mRNA transcript expression, NR1 protein and cognition in people with schizophrenia. These findings provide support for a multifaceted model of NMDAR dysfunction in schizophrenia and for a role of NMDAR hypofunction in the pathophysiology of cognitive dysfunction in schizophrenia. Our results support that the augmentation of NMDAR signaling may represent a neurobiological convergence point for potential new treatment approaches for cognitive impairment in schizophrenia.
Acknowledgments
We thank Shan Yuan Tsai, Dominique S Derminio, Alice Rothwell and Duncan Sinclair for technical support. This work was supported by the University of New South Wales School of Psychiatry, the National Health and Medical Research Council (NHMRC) of Australia Project Grant no. 568807, Neuroscience Research Australia, the Schizophrenia Research Institute utilizing infrastructure funding from NSW Ministry of Health and the Macquarie Group Foundation, and the Australian Schizophrenia Research Bank which is supported by the NHMRC of Australia, the Pratt Foundation, Ramsay Health Care, and the Viertel Charitable Foundation. Tissue samples were received from the Australian Brain Donor Programs NSW Tissue Resource Centre, which is supported by The University of Sydney, the NHMRC of Australia, Schizophrenia Research Institute, National Institute of Alcohol Abuse and Alcoholism, National Institutes of Health, USA and NSW Health.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
Supplementary Material
References
- Weickert TW, Goldberg TE, Gold JM, Bigelow LB, Egan MF, Weinberger DR. Cognitive impairments in patients with schizophrenia displaying preserved and compromised intellect. Arch Gen Psychiatry. 2000;57:907–913. doi: 10.1001/archpsyc.57.9.907. [DOI] [PubMed] [Google Scholar]
- Catts V, Catts S.The psychotomimetic effects of PCP, LSD and Ecstacy: Pharmacological models of schizophreniaIn: Sachdev P, Keshavan M (eds).Secondary Schizophrenia Cambridge University Press: Cambridge; 2010 [Google Scholar]
- Gilmour G, Dix S, Fellini L, Gastambide F, Plath N, Steckler T, et al. NMDA receptors, cognition and schizophrenia—testing the validity of the NMDA receptor hypofunction hypothesis. Neuropharmacology. 2012;63:1401–1412. doi: 10.1016/j.neuropharm.2011.03.015. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Kantrowitz JT, Javitt DC. Thinking glutamatergically: changing concepts of schizophrenia based upon changing neurochemical models. Clin Schizophr Relat Psychoses. 2010;4:189–200. doi: 10.3371/CSRP.4.3.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Koffel B, LaPorte D, Tamminga CA. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology. 1995;13:9–19. doi: 10.1016/0893-133X(94)00131-I. [DOI] [PubMed] [Google Scholar]
- Luby ED, Cohen BD, Rosenbaum G, Gottlieb JS, Kelley R. Study of a new schizophrenomimetic drug; sernyl. AMA Arch Neurol Psychiatry. 1959;81:363–369. doi: 10.1001/archneurpsyc.1959.02340150095011. [DOI] [PubMed] [Google Scholar]
- Malhotra AK, Pinals DA, Adler CM, Elman I, Clifton A, Pickar D, et al. Ketamine-induced exacerbation of psychotic symptoms and cognitive impairment in neuroleptic-free schizophrenics. Neuropsychopharmacology. 1997;17:141–150. doi: 10.1016/S0893-133X(97)00036-5. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Fukushima T, Shimizu E, Komatsu N, Watanabe H, Shinoda N, et al. Decreased serum levels of D-serine in patients with schizophrenia: evidence in support of the N-methyl-D-aspartate receptor hypofunction hypothesis of schizophrenia. Arch Gen Psychiatry. 2003;60:572–576. doi: 10.1001/archpsyc.60.6.572. [DOI] [PubMed] [Google Scholar]
- Madeira C, Freitas ME, Vargas-Lopes C, Wolosker H, Panizzutti R. Increased brain D-amino acid oxidase (DAAO) activity in schizophrenia. Schizophr Res. 2008;101:76–83. doi: 10.1016/j.schres.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Schultz CC, Nenadic I, Koch K, Wagner G, Roebel M, Schachtzabel C, et al. Reduced cortical thickness is associated with the glutamatergic regulatory gene risk variant DAOA Arg30Lys in schizophrenia. Neuropsychopharmacology. 2011;36:1747–1753. doi: 10.1038/npp.2011.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 2012;37:4–15. doi: 10.1038/npp.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, et al. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itokawa M, Yamada K, Yoshitsugu K, Toyota T, Suga T, Ohba H, et al. A microsatellite repeat in the promoter of the N-methyl-D-aspartate receptor 2A subunit (GRIN2A) gene suppresses transcriptional activity and correlates with chronic outcome in schizophrenia. Pharmacogenetics. 2003;13:271–278. doi: 10.1097/00008571-200305000-00006. [DOI] [PubMed] [Google Scholar]
- Iwayama-Shigeno Y, Yamada K, Itokawa M, Toyota T, Meerabux JM, Minabe Y, et al. Extended analyses support the association of a functional (GT)n polymorphism in the GRIN2A promoter with Japanese schizophrenia. Neurosci Lett. 2005;378:102–105. doi: 10.1016/j.neulet.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Martucci L, Wong AH, De Luca V, Likhodi O, Wong GW, King N, et al. N-methyl-D-aspartate receptor NR2B subunit gene GRIN2B in schizophrenia and bipolar disorder: Polymorphisms and mRNA levels. Schizophr Res. 2006;84:214–221. doi: 10.1016/j.schres.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Miyatake R, Furukawa A, Suwaki H. Identification of a novel variant of the human NR2B gene promoter region and its possible association with schizophrenia. Mol Psychiatry. 2002;7:1101–1106. doi: 10.1038/sj.mp.4001152. [DOI] [PubMed] [Google Scholar]
- Ohtsuki T, Sakurai K, Dou H, Toru M, Yamakawa-Kobayashi K, Arinami T. Mutation analysis of the NMDAR2B (GRIN2B) gene in schizophrenia. Mol Psychiatry. 2001;6:211–216. doi: 10.1038/sj.mp.4000808. [DOI] [PubMed] [Google Scholar]
- Qin S, Zhao X, Pan Y, Liu J, Feng G, Fu J, et al. An association study of the N-methyl-D-aspartate receptor NR1 subunit gene (GRIN1) and NR2B subunit gene (GRIN2B) in schizophrenia with universal DNA microarray. Eur J Hum Genet. 2005;13:807–814. doi: 10.1038/sj.ejhg.5201418. [DOI] [PubMed] [Google Scholar]
- Tang J, Chen X, Xu X, Wu R, Zhao J, Hu Z, et al. Significant linkage and association between a functional (GT)n polymorphism in promoter of the N-methyl-D-aspartate receptor subunit gene (GRIN2A) and schizophrenia. Neurosci Lett. 2006;409:80–82. doi: 10.1016/j.neulet.2006.09.022. [DOI] [PubMed] [Google Scholar]
- Zhao X, Li H, Shi Y, Tang R, Chen W, Liu J, et al. Significant association between the genetic variations in the 5' end of the N-methyl-D-aspartate receptor subunit gene GRIN1 and schizophrenia. Biol Psychiatry. 2006;59:747–753. doi: 10.1016/j.biopsych.2005.10.023. [DOI] [PubMed] [Google Scholar]
- Beneyto M, Meador-Woodruff JH. Lamina-specific abnormalities of NMDA receptor-associated postsynaptic protein transcripts in the prefrontal cortex in schizophrenia and bipolar disorder. Neuropsychopharmacology. 2008;33:2175–2186. doi: 10.1038/sj.npp.1301604. [DOI] [PubMed] [Google Scholar]
- Law AJ, Deakin JF. Asymmetrical reductions of hippocampal NMDAR1 glutamate receptor mRNA in the psychoses. Neuroreport. 2001;12:2971–2974. doi: 10.1097/00001756-200109170-00043. [DOI] [PubMed] [Google Scholar]
- Humphries C, Mortimer A, Hirsch S, de Belleroche J. NMDA receptor mRNA correlation with antemortem cognitive impairment in schizophrenia. Neuroreport. 1996;7:2051–2055. doi: 10.1097/00001756-199608120-00040. [DOI] [PubMed] [Google Scholar]
- Sokolov BP. Expression of NMDAR1, GluR1, GluR7, and KA1 glutamate receptor mRNAs is decreased in frontal cortex of ‘neuroleptic-free' schizophrenics: evidence on reversible up-regulation by typical neuroleptics. J Neurochem. 1998;71:2454–2464. doi: 10.1046/j.1471-4159.1998.71062454.x. [DOI] [PubMed] [Google Scholar]
- Dracheva S, Marras SA, Elhakem SL, Kramer FR, Davis KL, Haroutunian V. N-methyl-D-aspartic acid receptor expression in the dorsolateral prefrontal cortex of elderly patients with schizophrenia. Am J Psychiatry. 2001;158:1400–1410. doi: 10.1176/appi.ajp.158.9.1400. [DOI] [PubMed] [Google Scholar]
- Schmitt A, Koschel J, Zink M, Bauer M, Sommer C, Frank J, et al. Gene expression of NMDA receptor subunits in the cerebellum of elderly patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2010;260:101–111. doi: 10.1007/s00406-009-0017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbarian S, Sucher NJ, Bradley D, Tafazzoli A, Trinh D, Hetrick WP, et al. Selective alterations in gene expression for NMDA receptor subunits in prefrontal cortex of schizophrenics. J Neurosci. 1996;16:19–30. doi: 10.1523/JNEUROSCI.16-01-00019.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel S, Lipp HP, Umbricht D. Impaired attentional modulation of auditory evoked potentials in N-methyl-D-aspartate NR1 hypomorphic mice. Genes Brain Behav. 2007;6:558–568. doi: 10.1111/j.1601-183X.2006.00283.x. [DOI] [PubMed] [Google Scholar]
- Duncan G, Miyamoto S, Gu H, Lieberman J, Koller B, Snouwaert J. Alterations in regional brain metabolism in genetic and pharmacological models of reduced NMDA receptor function. Brain Res. 2002;951:166–176. doi: 10.1016/s0006-8993(02)03156-6. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Moy SS, Perez A, Eddy DM, Zinzow WM, Lieberman JA, et al. Deficits in sensorimotor gating and tests of social behavior in a genetic model of reduced NMDA receptor function. Behav Brain Res. 2004;153:507–519. doi: 10.1016/j.bbr.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Dzirasa K, Ramsey AJ, Takahashi DY, Stapleton J, Potes JM, Williams JK, et al. Hyperdopaminergia and NMDA receptor hypofunction disrupt neural phase signaling. J Neurosci. 2009;29:8215–8224. doi: 10.1523/JNEUROSCI.1773-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halene TB, Ehrlichman RS, Liang Y, Christian EP, Jonak GJ, Gur TL, et al. Assessment of NMDA receptor NR1 subunit hypofunction in mice as a model for schizophrenia. Genes Brain Behav. 2009;8:661–675. doi: 10.1111/j.1601-183X.2009.00504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohn AR, Gainetdinov RR, Caron MG, Koller BH. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell. 1999;98:427–436. doi: 10.1016/s0092-8674(00)81972-8. [DOI] [PubMed] [Google Scholar]
- Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, et al. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13:76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawasli AH, Benavides DR, Nguyen C, Kansy JW, Hayashi K, Chambon P, et al. Cyclin-dependent kinase 5 governs learning and synaptic plasticity via control of NMDAR degradation. Nat Neurosci. 2007;10:880–886. doi: 10.1038/nn1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewoehner B, Single FN, Hvalby O, Jensen V, Meyer zum Alten Borgloh S, Seeburg PH, et al. Impaired spatial working memory but spared spatial reference memory following functional loss of NMDA receptors in the dentate gyrus. Eur J Neurosci. 2007;25:837–846. doi: 10.1111/j.1460-9568.2007.05312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampon C, Tang YP, Goodhouse J, Shimizu E, Kyin M, Tsien JZ. Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1-knockout mice. Nat Neurosci. 2000;3:238–244. doi: 10.1038/72945. [DOI] [PubMed] [Google Scholar]
- Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87:1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- von Engelhardt J, Doganci B, Jensen V, Hvalby O, Gongrich C, Taylor A, et al. Contribution of hippocampal and extra-hippocampal NR2B-containing NMDA receptors to performance on spatial learning tasks. Neuron. 2008;60:846–860. doi: 10.1016/j.neuron.2008.09.039. [DOI] [PubMed] [Google Scholar]
- Zhao MG, Toyoda H, Lee YS, Wu LJ, Ko SW, Zhang XH, et al. Roles of NMDA NR2B subtype receptor in prefrontal long-term potentiation and contextual fear memory. Neuron. 2005;47:859–872. doi: 10.1016/j.neuron.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Ludwig KU, Roeske D, Herms S, Schumacher J, Warnke A, Plume E, et al. Variation in GRIN2B contributes to weak performance in verbal short-term memory in children with dyslexia. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:503–511. doi: 10.1002/ajmg.b.31007. [DOI] [PubMed] [Google Scholar]
- Turic D, Langley K, Mills S, Stephens M, Lawson D, Govan C, et al. Follow-up of genetic linkage findings on chromosome 16p13: evidence of association of N-methyl-D aspartate glutamate receptor 2A gene polymorphism with ADHD. Mol Psychiatry. 2004;9:169–173. doi: 10.1038/sj.mp.4001387. [DOI] [PubMed] [Google Scholar]
- Weickert CS, Sheedy D, Rothmond DA, Dedova I, Fung S, Garrick T, et al. Selection of reference gene expression in a schizophrenia brain cohort. Aust N Z J Psychiatry. 2010;44:59–70. doi: 10.3109/00048670903393662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. The Psyhological Corporation: San Antonio, TX; 1997. [Google Scholar]
- Wechsler D. Wechsler Test of Adult Reading. The Psychological Corporation: San Antonio, TX; 2001. [Google Scholar]
- Kristiansen LV, Bakir B, Haroutunian V, Meador-Woodruff JH. Expression of the NR2B-NMDA receptor trafficking complex in prefrontal cortex from a group of elderly patients with schizophrenia. Schizophr Res. 2010;119:198–209. doi: 10.1016/j.schres.2010.02.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen LV, Beneyto M, Haroutunian V, Meador-Woodruff JH. Changes in NMDA receptor subunits and interacting PSD proteins in dorsolateral prefrontal and anterior cingulate cortex indicate abnormal regional expression in schizophrenia. Mol Psychiatry. 2006;11:737–747. doi: 10.1038/sj.mp.4001844. [DOI] [PubMed] [Google Scholar]
- Kornhuber J, Mack-Burkhardt F, Riederer P, Hebenstreit GF, Reynolds GP, Andrews HB, et al. [3H]MK-801 binding sites in postmortem brain regions of schizophrenic patients. J Neural Transm. 1989;77:231–236. doi: 10.1007/BF01248936. [DOI] [PubMed] [Google Scholar]
- Simpson MD, Slater P, Royston MC, Deakin JF. Alterations in phencyclidine and sigma binding sites in schizophrenic brains. Effects of disease process and neuroleptic medication. Schizophr Res. 1991;6:41–48. doi: 10.1016/0920-9964(91)90019-n. [DOI] [PubMed] [Google Scholar]
- Nudmamud S, Reynolds GP. Increased density of glutamate/N-methyl-D-aspartate receptors in superior temporal cortex in schizophrenia. Neurosci Lett. 2001;304:9–12. doi: 10.1016/s0304-3940(01)01727-x. [DOI] [PubMed] [Google Scholar]
- Dean B, Hussain T, Hayes W, Scarr E, Kitsoulis S, Hill C, et al. Changes in serotonin2A and GABA(A) receptors in schizophrenia: studies on the human dorsolateral prefrontal cortex. J Neurochem. 1999;72:1593–1599. doi: 10.1046/j.1471-4159.1999.721593.x. [DOI] [PubMed] [Google Scholar]
- Dean B, Pavey G, McLeod M, Opeskin K, Keks N, Copolov D. A change in the density of [(3)H]flumazenil, but not [(3)H]muscimol binding, in Brodmann's Area 9 from subjects with bipolar disorder. J Affect Disord. 2001;66:147–158. doi: 10.1016/s0165-0327(00)00294-9. [DOI] [PubMed] [Google Scholar]
- Noga JT, Hyde TM, Herman MM, Spurney CF, Bigelow LB, Weinberger DR, et al. Glutamate receptors in the postmortem striatum of schizophrenic, suicide, and control brains. Synapse. 1997;27:168–176. doi: 10.1002/(SICI)1098-2396(199711)27:3<168::AID-SYN2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Scarr E, Beneyto M, Meador-Woodruff JH, Dean B. Cortical glutamatergic markers in schizophrenia. Neuropsychopharmacology. 2005;30:1521–1531. doi: 10.1038/sj.npp.1300758. [DOI] [PubMed] [Google Scholar]
- Huettner J, Bean B. Block of N-methyl-D-aspartate-activated current by the anticonvulsant MK-801: selective binding to open channels. Proc Natl Acad Sci USA. 1988;85:1307–1311. doi: 10.1073/pnas.85.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman BG, Gupta SC, Stairs DJ, Buonanno A, Dravid SM. Behavioral analysis of NR2C knockout mouse reveals deficit in acquisition of conditioned fear and working memory. Neurobiol Learn Mem. 2011;95:404–414. doi: 10.1016/j.nlm.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi D, Keeler B, Zhang W, Houle JD, Gao WJ. NMDA receptor subunit expression in GABAergic interneurons in the prefrontal cortex: application of laser microdissection technique. J Neurosci Methods. 2009;176:172–181. doi: 10.1016/j.jneumeth.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung SJ, Webster MJ, Sivagnanasundaram S, Duncan C, Elashoff M, Weickert CS. Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. Am J Psychiatry. 2010;167:1479–1488. doi: 10.1176/appi.ajp.2010.09060784. [DOI] [PubMed] [Google Scholar]
- Beasley CL, Reynolds GP. Parvalbumin-immunoreactive neurons are reduced in the prefrontal cortex of schizophrenics. Schizophr Res. 1997;24:349–355. doi: 10.1016/s0920-9964(96)00122-3. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, Lewis DA. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am J Psychiatry. 2008;165:479–489. doi: 10.1176/appi.ajp.2007.07081223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Cruz DA, Melchitzky DS, Pierri JN. Lamina-specific deficits in parvalbumin-immunoreactive varicosities in the prefrontal cortex of subjects with schizophrenia: evidence for fewer projections from the thalamus. Am J Psychiatry. 2001;158:1411–1422. doi: 10.1176/appi.ajp.158.9.1411. [DOI] [PubMed] [Google Scholar]
- Morris HM, Hashimoto T, Lewis DA. Alterations in somatostatin mRNA expression in the dorsolateral prefrontal cortex of subjects with schizophrenia or schizoaffective disorder. Cereb Cortex. 2008;18:1575–1587. doi: 10.1093/cercor/bhm186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds GP, Zhang ZJ, Beasley CL. Neurochemical correlates of cortical GABAergic deficits in schizophrenia: selective losses of calcium binding protein immunoreactivity. Brain Res Bull. 2001;55:579–584. doi: 10.1016/s0361-9230(01)00526-3. [DOI] [PubMed] [Google Scholar]
- Zhang ZJ, Reynolds GP. A selective decrease in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia. Schizophr Res. 2002;55:1–10. doi: 10.1016/s0920-9964(01)00188-8. [DOI] [PubMed] [Google Scholar]
- Carlson R, Khoo B, Yaure R, Schneider W. Acquisition of a problem-solving skill: levels of organization and use of working memory. J Exp Psychol. 1990;119:193–214. [Google Scholar]
- Colom R, Quiroga M, Shih P, Martinez K, Burgaleta M, Martinez-Molina A, et al. Improvement in working memory is not related to increased intelligence scores. Intelligence. 2010;38:497–505. [Google Scholar]
- de Jong P, de Jong P. Working memory, intelligence, and reading ability in children. Pers Indiv Differ. 1996;21:1007–1020. [Google Scholar]
- Kyllonen P, Christal R. Reasoning ability is (little more than) working-memory capacity?! Intelligence. 1990;14:389–433. [Google Scholar]
- Unsworth N. On the division of working memory and long-term memory and their relation to intelligence: A latent variable approach. Acta Psychol. 2010;134:16–28. doi: 10.1016/j.actpsy.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Unsworth N, Spillers G. Working memory capacity: Attention control, secondary memory or both? A direct test of the dual component model. J Mem Lang. 2010;62:392–406. [Google Scholar]
- Lipska BK, Peters T, Hyde TM, Halim N, Horowitz C, Mitkus S, et al. Expression of DISC1 binding partners is reduced in schizophrenia and associated with DISC1 SNPs. Hum Mol Genet. 2006;15:1245–1258. doi: 10.1093/hmg/ddl040. [DOI] [PubMed] [Google Scholar]
- Jablensky A, Morar B, Wiltshire S, Carter K, Dragovic M, Badcock JC, et al. Polymorphisms associated with normal memory variation also affect memory impairment in schizophrenia. Genes Brain Behav. 2011;10:410–417. doi: 10.1111/j.1601-183X.2011.00679.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.