Abstract
Background and Purpose
Functional magnetic resonance imaging (fMRI) has proven to be an effective component of pretreatment planning in patients harboring a variety of different brain lesions. Our group has recently reported significant relationships concerning distances between brain tumor border and area of functional activation (Lesion-to-Activation-Distance; LAD) with regard to patient morbidity and mortality. This study further examines the relationship between LAD, focusing on a host of vascular lesions, and pre- and posttreatment morbidity.
Materials and Methods
This study included a sample population (n=106) of patients with vascular lesions, primarily arteriovenous malformations (AVM) and cavernomas. These patients underwent pretreatment fMRI-based motor mapping (n=72) or language mapping (n=84). The impact of LAD and other variables derived from the patient medical record were analyzed with respect to functional deficits in terms of morbidity (weakness and/or aphasia).
Results
In patients with no pretreatment deficits, there was trend for a significant relationship between Wernicke's area LAD and posttreatment language deficits. In patients with or without pretreatment deficits, a trend toward significance was observed between sensorimotor LAD and posttreatment motor deficits. Additionally, lesion type (AVMs or cavernomas) impacted posttreatment deficits with more patients with cavernomas showing posttreatment language deficits than patients with AVMs. This difference was however not observed for posttreatment motor deficits.
Conclusion
These findings suggest that the proximity of a vascular lesion to sensorimotor and language areas is a relevant parameter in estimating patient prognosis in the peri-operative period. Additionally, vascular lesion type and existence of pretreatment deficits play a significant role in outcomes.
INTRODUCTION
Blood oxygen level dependent functional MRI (BOLD-fMRI)11,12 has gained acceptance as an effective, noninvasive method of brain mapping for pretreatment planning.13 In particular, it has been demonstrated to be an effective means to establish language hemisphere dominance2,15,22 and its rendering of functional anatomy has correlated well with direct cortical stimulation mapping.3,8,14,21 For patients about to undergo treatment for various vascular brain lesions, however, fMRI presents unique challenges to the investigator.7,18 Radiologic examination of eloquent cortex utilizing principles of BOLD contrast in the vicinity of a lesion such as an AVM that features abnormal blood flow requires scrutiny of the reliability of activation and overall interpretation of results. Further, given the sensitivity of echo planar imaging to areas of parenchymal hemosiderin staining, vascular brain lesions such as cavernomas with previous hemorrhage may feature accelerated T2 shortening and T2* susceptibility effects that could compromise or render the regional BOLD signal unattainable. Thickbroom et al18 determined that flow related effects were less important than susceptibility artifact from hemosiderin staining, as the latter could contribute to possible underestimation of the extent of true activation. However, Lehericy et al7 suggested that AVMs resulting in severe flow anomalies could potentially compromise the reliability of BOLD signal in a region of interest.
The importance of delineating areas of eloquent cortex remains an important goal for both clinicians and neuroradiologists. The compromise of eloquent areas as determined either by localizing the clinical deficits or based on conventional imaging has significant implications for treatment and prognosis. Specific grading schemes for arteriovenous malformations such as the widely used Spetzler-Martin criteria17 classically take into account AVM nidus measurement, location, and organization of venous drainage (See Table 1). Recently, additional prognostic characteristics of AVMs have been added to this list that have been termed angioarchitectual weakpoints.6 These weakpoints may confer greater propensity for hemorrhage and post-treatment risks including presence of nidal aneurysm, venous stenosis or ectasia, deep venous drainage, isolated or single venous drainage, and posterior fossa location. While some of these features are not specifically included in the classic Spetzler-Martin grading, it is important to consider these variables because of their prognostic value and impact on treatment strategies. For example, nidal aneurysms, particularly those exceeding 5 mm, may be treated with coil-embolization prior to surgical resection of the AVM. Further, identification and preservation of en passant arteries that might supply normal brain as well as an AVM is an important task.
Table 1.
Classic Spetzler-Martin grading of AVM.
| Size | Eloquent* Cortex | Venous drainage |
|---|---|---|
| < 3 cm (1) | No (0) | Superficial (0) (All cortical) |
| 3 - 6 cm (2) | Yes (1) | Deep (1) |
| > 6 cm (3) |
Note. Summation of characteristics of a particular AVM (numbers in parentheses) determines grade and estimates surgical risk.
Eloquent cortex constitutes sensorimotor, language, visual cortex as well as internal capsule, thalamus, hypothalamus, cerebellar peduncles, brainstem, deep cerebellar nuclei.
Currently, the imaging examinations in conjunction with clinical examination provide the diagnostic basis for a vascular lesion such as an AVM or cavernoma. There is a paucity of literature describing the utility of fMRI in routine pretreatment assessment of patients with vascular lesions. fMRI has been shown to be a robust technique for establishing language hemisphere dominance as well as useful for identification of eloquent cortex to be spared during surgery. However, its role in the special context of a vascular lesion is yet to be determined. This situation is further complicated by the fact that vascular lesion patients, including those examined prior to clinical intervention, may have atypical language networks.19
In the present study we investigate fMRI lesion-to-activation distance (LAD) and its relationship to morbidity in patients with vascular brain lesions about to undergo treatment, primarily AVMs and cavernomas. We hypothesize that as the LAD decreases, overall morbidity will increase.
MATERIALS AND METHODS
Subject Characteristics
This study was reviewed and approved by the University of Wisconsin–Madison Health Sciences Institutional Review Board (IRB). The sample population of (n=106) patients underwent pretreatment fMRI between June 1999 and July 2011 for various vascular brain lesions including AVMs and cavernomas. Demographic information is presented in Table 2. Patients gave informed consent in accordance with the study protocol approved by the IRB. Patients’ clinical information was extracted from the electronic medical record. Any record of pretreatment or posttreatment motor weakness (e.g., upper extremity, lower extremity, or facial weakness) or aphasia (e.g., Broca's type, Wernicke's type, conduction, or global aphasia) was included in the analysis. Only gross motor and language deficits were considered and no specific neuropsychological testing was included. Vascular lesions were diagnosed with catheter cerebral angiography, conventional CTA, or MRI/MRA imaging. Lesions were categorized as encroaching on sensorimotor cortex or primary language centers (i.e., Broca's area or Wernicke's area). Functional paradigms were selected depending upon the vascular lesion location in question with the aim of eliciting either primary sensorimotor or language center activation.9 fMRI activations were individually thresholded at the time of the radiologic examination in question and then made available for clinical use and decision-making; these same thresholded maps were used retrospectively in this study for distance measurements to characterize the prognostic utility of measures derived from these clinical fMRI maps. Given the wide variation in appearance and distribution of many vascular lesions, distance to the closest presumed edge of a vascular nidus was utilized. If a compact vascular nidus could not be definitively identified, distance to closest involved component of the vascular lesion was utilized. Distances from vascular lesion edge to the periphery of fMRI activation and distances from vascular lesion edge to the center of maximum primary motor or language fMRI activation were both measured. Distances were then correlated with pre- and posttreatment morbidity information obtained from the electronic medical record.
Table 2.
Subject characteristics of patients with vascular lesions near the primary sensorimotor area, Broca's area, and Wernicke's area, grouped by distance from lesion edge to area of activation.
| Lesion-Activation-Distance (LAD) | ||||
|---|---|---|---|---|
| < 10 mm | 10–20 mm | > 20 mm | p value | |
| Primary sensorimotor area | ||||
| Gender (% male) | 54 | 62 | 40 | 0.19 |
| Age (mean) | 31 | 34 | 38 | 0.38 |
| Handedness (% right) | 54 | 76 | 64 | 0.68 |
| AVM (% yes) | 54 | 50 | 47 | 0.90 |
| Broca's area | ||||
| Gender (% male) | 0 | 46 | 49 | 0.60 |
| Age (mean) | 20 | 37 | 37 | 0.47 |
| Handedness (% right) | 0 | 55 | 65 | 0.47 |
| AVM (% yes) | 100 | 27 | 49 | 0.23 |
| Wernicke's area | ||||
| Gender (% male) | 0 | 25 | 51 | 0.05* |
| Age (mean) | 30 | 31 | 38 | 0.14 |
| Handedness (% right) | 25 | 58 | 62 | 0.75 |
| AVM (% yes) | 25 | 58 | 47 | 0.49 |
fMRI Language Paradigms
The language paradigms used to assess patients are described in more detail in Moritz et al.9 In brief, activation of Broca's area was best seen with word generation fMRI tasks. Two word generation tasks were used: (1) alternating 20-second blocks of antonym word generation and rest, and (2) alternating 20-second blocks of letter word generation task and rest. In the antonym word generation the subject is asked to silently think of the opposite of the word displayed on the screen. In the letter word generation task, the subject is asked to silently think of words starting with the letter displayed on the screen. Wernicke's area was identified with alternating 20-second blocks of text reading and symbols task. In this task, the patient silently read a short paragraph in the text reading block. During the control block, the patient was shown a paragraph of symbols and asked to scan for specific symbols within the paragraph. The control block controlled for eye movements during reading, which helped discriminate visual and eye movement-related activity from the true language areas. Not all patients performed all tasks. Tasks were individualized to the patient so that clinically useful and robust language-related activation could be imaged.
fMRI Acquisition and Processing
Radiologic examination was performed with either a 1.5T or 3T commercial MR scanner (GE Medical Systems; Milwaukee, WI) equipped with high-speed gradients. BOLD-weighted single-shot echo-planar images (EPIs) were obtained continuously for each patient during task performance. Technical parameters for images were the following: FOV: 24cm; matrix: 64x64; TR: 2000ms; TE: 40ms (for 1.5T), 27ms (for 3T); FA: 85 (for 1.5T), 75 (for 3T); 6mm coronal plane sections (for 1.5T), 5mm axial plane sections (for 3T). Spatial coverage was sufficient to provide mapping of the entire cortex. The number of images and length of imaging varied with paradigm. MR examination duration ranged from 3 to 5 minutes. Additional high-resolution anatomic scans, including 3D volumetric T1- and T2-weighted contrast sequences, were acquired as part of the pretreatment assessment. Post-processing of the EPI signal included both spatial and temporal smoothing. 3Dvolreg in AFNI software was used to correct for head motion in the reconstructed time-courses. The EPIs were spatially coregistered with structural images. Activation was determined by cross-correlation of the time-course of the EPI signal at each voxel with a generalized least squares fitting algorithm to a smoothed and temporally delayed boxcar reference function modeling the presumed hemodynamic response. This comparison provided a voxel-wise t statistic with which images were thresholded individually to optimize visualization of motor and language areas and overlaid on the spatially coregistered anatomic brain volume maps. Thresholding was applied to each individual fMRI examination by an expert clinical fMRI technician and verified by a diagnostic neuroradiologist with the intent of optimizing specificity and sensitivity of task response while minimizing artifacts such as spurious correlations due to head motion. For example, in a dataset that exhibited significant task correlated head motion, it may not have been possible to minimize the artifacts while still retaining a sufficient sensitivity to the task-related responses. The task-related response magnitudes were also dependent on factors such as the patient's ability to perform a particular fMRI task or whether the BOLD response was compromised by presence of the vascular lesion in question. Thus, thresholds were individually varied for each fMRI scan by the attending neuroradiologist based on the quality of the data and mapping concerns relevant to neurosurgical planning.
Data Measurements
Both right and left hemisphere lesions were included in the analysis. LAD (Lesion-Activation-Distance, i.e. Distance between lesion border and area of functional activation(centroid – “hot spot”)) was measured. Lesions contralateral to the language or motor center in the dominant hemisphere were considered to have LAD >2 cm by default. The edge of an AVM was defined as the nidus, or the closest most focal network of enhancing flow voids. If a clear nidus was not distinct, the edge of the AVM was defined as the closest abnormal enhancing flow void of the lesion. For cavernomas, the lesion edge was defined as the thin margin of the characteristic hemosiderin ring. For computing lesion volume, measurements on the structural scans were recorded in the transverse (x), anterior/posterior (y), and superior/inferior (z) axes in Picture Archiving System (PACS). Cavernoma volume was calculated using the equation: (x*y*z)/2 where x,y,and z are the maximum dimensions of the lesion along the X,Y,and Z axes respectively10. T2* sequences were not used in measurements to avoid error introduced by confounding blooming artifacts. As a control comparison, 20 second breath-holding fMRI acquisitions were obtained to delineate areas of the brain capable of producing measurable BOLD signal. Examples of typical measurements are depicted in Figure 1.
Figure 1.
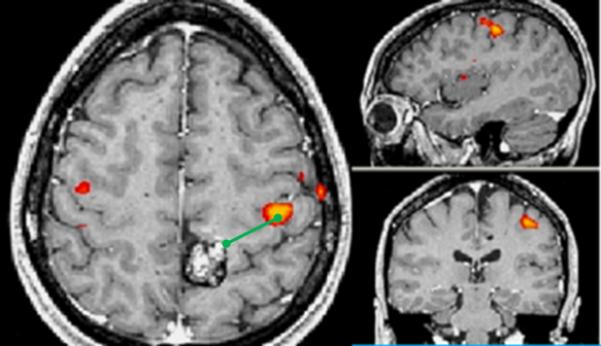
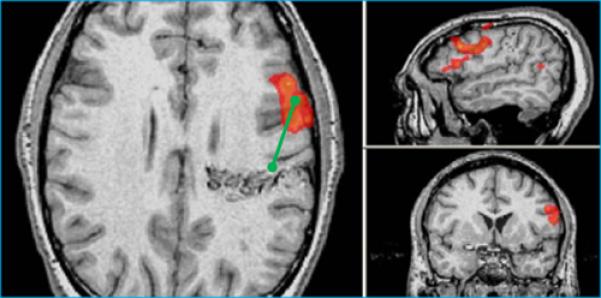
Typical measurements. In each panel, the images are axial (left), sagittal (top), and coronal (bottom), images are shown following radiological convention, with left on right. A. Patient showing left sensorimotor activation (red-orange colors) in close proximity to a posterior left frontal cavernoma. B. Patient with left frontal AVM showing Broca's activation during antonym word generation task. LAD distances (green lines) are shown from lesion edge to activation centroid as measured in PACS(Picture Archiving and Communication System).
Data Analyses
All statistical analyses were performed using SPSS statistical package release 20.0.0 (IBM Corp., Armonk, NY, USA). Categorical variables(gender, handedness) were compared between groups using the chi-square tests. Age, a continuous measure, was compared using the Kruskal–Wallis one-way analysis of variance tests(non-parametric equivalent of one-way ANOVA). LAD was analyzed as a categorical variable(distance between lesion edge and activation centroid <10 mm, between 10 – 20 mm, and > 20 mm). Multiple logistic regression models with LAD as predictor and posttreatment motor and language deficits as outcome measures were tested to investigate if LAD was a significant predictor of posttreatment deficits. Because there was a significant gender difference among the patients when grouped by Wernicke's LAD, gender was included as a predictor in the regression model for Wernicke's LAD. Results were considered as statistically significant if p < .05, and as showing a trend towards significance if between .05 and 0.10. Results across all patients are first described below followed by results from separate analyses for patients with AVMs and cavernomas.
RESULTS
All Patients Demographic variables
Patients harboring vascular lesions were studied with regard to proximity of the lesion to primary motor cortex (n = 72) and primary language areas (n = 84). When grouped by LAD, there were no significant differences based on age or handedness (Table 2). Gender when grouped by Wernicke's LAD was found to have a statistically significant difference (p = 0.05). When grouped by presence or absence of posttreatment sensorimotor deficits, there were no significant differences based on gender, age or handedness (Table 3). Table 4 similarly shows patient characteristics when grouped by posttreatment language deficits.
Table 3.
Subject characteristics of patients (with or without pretreatment deficits) with vascular lesions near the primary sensorimotor area, grouped by presence or absence of posttreatment sensorimotor deficits.
| No Weakness | Weakness | p value | ||
|---|---|---|---|---|
| Gender (% male) | 46 | 48 | 0.56 | |
| Age (mean) | 37 | 37 | 0.64 | |
| Handedness (% right) | 80 | 80 | 0.83 | |
| AVM (%yes) | 60 | 78 | 0.17† | .21 |
| Cavernoma (%yes) | 35 | 17 | .13 | |
p value for main effect of lesion type. Additional p values are when comparing proportion of AVMs (or cavernomas) grouped by presence or absence of posttreatment sensorimotor deficits
Table 4.
Subject characteristics of patients (with or without pretreatment deficits) with vascular lesions near Broca's or Wernicke's area, grouped by presence or absence of posttreatment language deficits.
| No Aphasia | Aphasia | p value | ||
|---|---|---|---|---|
| Gender (% male) | 49 | 38 | .23 | |
| Age (mean) | 37 | 38 | .31 | |
| Handedness (% right) | 79 | 84 | .84 | |
| AVM(%yes) | 65 | 62.5 | .59† | .34 |
| Cavernoma(%yes) | 29 | 37.5 | .81 | |
p value for main effect of lesion type. Additional p values are when comparing proportion of AVMs (or cavernomas) grouped by presence or absence of posttreatment language deficits
Types of Lesions
In this study there were 68 AVMs (64%), 33 cavernomas (31%, with an average size of 2.8 cm3), and 5 other types of lesions (5%, those with an unclear diagnosis such as cavernoma/AVM or intracerebral hemorrhage/hemorrhagic lesion) Table 5 shows the Spetzler-Martin grade of patients grouped by proximity of lesion to either Wernicke's or Broca's area or the sensorimotor cortex. Lesion size and total lesion burden varied from patient to patient, as did history of sentinel hemorrhage, seizure, and pretreatment language or motor deficit. While most cavernoma patients had a single lesion, one patient demonstrated multiple cavernomas and the lesion closest to the area of eloquent cortex in question was used in the analysis. For the 106 patients in the study, 51 (48%) underwent surgical resection, 16 (15%) had radiotherapy, 23 (22%) had embolization, and 16 (15%) conservative treatment . Patients with cavernomas either had surgical resection or conservative management. Patients with AVMs may have received any of the different treatment types as well a combination of invasive and noninvasive treatments.
Table 5.
Spetzler-Martin grade for patients with AVM near Wernicke's or Broca's area or the sensorimotor cortex
| Spetzler-Martin Breakdown for AVMs | Near Wernicke's | Near Broca's | Near Sensorimotor Cortex |
|---|---|---|---|
| Grade 1 | 5% | 13% | 7% |
| Grade 2 | 43% | 39% | 34% |
| Grade 3 | 19% | 13% | 23% |
| Grade 4 | 33% | 35% | 33% |
| Grade 5 | 0% | 0% | 3% |
Nature of deficits: Transient or persistent
Record of pretreatment and posttreatment deficits was made from clinician's notes of follow-up visits, wherever available. Transient deficits were defined as deficits that resolved within 6 months. Of the 24 patients with posttreatment aphasia(with or without prior deficits), 19 patients had transient deficits (< 6 months since date of first treatment) and 5 had persistent deficits (lasting > 6 months since date of first treatment). Of the 23 patients with posttreatment weakness and/or motor impairments (with or without prior deficits), 19 patients had transient deficits and 4 had persistent deficits. Details regarding pre- and posttreatment deficits in patients(with or without prior deficits) with vascular lesions in proximity to sensorimotor or language areas are shown in Table 6. Only 2 patients presented with no prior language deficit but had posttreatment deficit; one patient's deficit resolved within 6 months whereas the second patient's deficits persisted even at 12 months follow-up visit. Both the patients with persistent deficits had cavernomas. One AVM patient presented with no motor deficit pretreatment, but showed posttreatment deficit and his deficits persisted at 6 months follow-up.
Table 6.
Posttreatment deficits in patients (with or without pretreatment deficits)) with vascular lesions in proximity to sensorimotor or language areas
| LAD < 10 mm | 10mm<LAD < 20 mm | LAD > 20mm | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |||||||||
| Deficit | No deficit | Deficit | No deficit | Deficit | No deficit | Deficit | No deficit | Deficit | No deficit | Deficit | No deficit | • p value | p value | |
| Motor (N=87) | 1 | 12 | 1 | 12 | 12 | 9 | 8 | 13 | 15 | 38 | 14 | 39 | .78 | .06‡ |
| Broca's (N=97) | 1 | 0 | 1 | 0 | 2 | 9 | 2 | 9 | 22 | 63 | 18 | 67 | .10 | .21†† |
| Wernicke's (N=89) | 2 | 2 | 1 | 3 | 7 | 5 | 5 | 7 | 16 | 57 | 15 | 58 | .077 | .47†† |
Note.
p values shown are for patients who have posttreatment deficits but no pretreatment deficits when grouped by LAD (Lesion activation distance)
p values shown are for the logistic regression analysis with LAD as predictor for patients aged > 18 years and posttreatment weakness as outcome
p values shown are for the logistic regression analysis with LAD and gender as predictor and posttreatment aphasia as outcome.
All Patients with no pretreatment deficits
For patients without any pretreatment motor deficits, when grouped by SMC (sensorimotor cortex) LAD there were no significant differences (p = .78). Only 1 patient presented with no motor deficit at pretreatment, and had posttreatment deficit, and his deficits persisted at 6 months+ follow-up. When grouped by Broca's LAD, there were no significant differences (χ2(2,N=72)=2.64, p = .10). When grouped by Wernicke's LAD, there was a trend towards significance (χ2(2,N=64)=5.12, p = .077) with patients in the <10 mm and the 10-20 mm category more likely to show greater posttreatment language deficits in comparison to LAD > 20mm.
Patients with AVMs (N = 68): Demographic variables
When grouped by distance from lesion edge to area of activation, there were no significant differences based on gender for SMC, p =.40, Broca's, p = .43, and a trend towards significance for Wernicke's p = .09. There were no significant differences based on handedness (SMC, p =.13; Broca's, p = .48; Wernickes p = .90). Additionally, there were no significant differences based on age for SMC, p =.49 and Broca's, p=.15 but a trend towards significance for Wernicke's p =.09.
AVMs with no pretreatment deficits
As seen in Table 7, of the 50 patients with AVMs free of pretreatment language deficits, none had any posttreatment language deficits. Of the 49 patients with AVMs free of pretreatment motor deficits, 1 patient had posttreatment motor deficit.
Table 7.
Posttreatment deficits in patients with no pretreatment deficits by lesion type
| Lesion Type (patients with no pretreatment deficits) | Posttreatment deficit | |||||
|---|---|---|---|---|---|---|
| Motor(n) | p value | Language(n) | p value | |||
| No | Yes | No | Yes | |||
| AVMs | 48 | 1 | 0.47 | 50 | 0 | 0.03 |
| Cavernomas | 25 | 0 | 21 | 2 | ||
| Other* | 1 | 0 | 2 | 0 | ||
unclear diagnosis (cavernoma /AVM or intracerebral hemorrhage / hemorrhagic lesion)
Patients with Cavernomas (N=33): Demographic variables
When grouped by distance from lesion edge to area of activation, there were no significant differences based on gender (SMC, p =.25; Broca's, p = .26; Wernicke's p = .32, ), handedness (SMC, p =.81; Broca's, p = .15; Wernicke's p = .82) and age(SMC, p =.17; Broca's, p=.48;Wernicke's p =.59).
Cavernomas with no pretreatment deficits
As seen in Table 7, of the 23 patients with cavernomas free of pretreatment language deficits , 2 had posttreatment deficits. Of the 25 patients free of pretreatment motor deficits, none had any posttreatment deficit.
AVMs vs. Cavernomas
For patients with no pretreatment deficits, Chi-square test showed that the difference in posttreatment language deficit by type of lesion, whether AVM or cavernomas was statistically significant – more patients with cavernomas had posttreatment language deficits than patients with AVMs (χ2(1,N=73)=4.47, p = .03). The difference in posttreatment motor deficit by type of lesion, whether AVM or cavernomas was not statistically significant (p = .47)(see Table 7).
Multivariate analysis
All Patients with and without pretreatment deficits
Multiple logistic regression showed that distance from lesion edge to sensorimotor cortex was not a significant predictor of posttreatment deficits (p = 0.11). In a subset of patients aged 18 years and greater, there was a trend towards a significant association between distance from vascular lesion to motor activation and the existence of weakness or paresis (p = 0.06). Similar analysis was done with Broca's and Wernicke's area which showed that distance from lesion edge to these language areas was not a significant predictor of posttreatment deficits (p = 0.21 and p=.47 respectively) (Table 6). A model combining distance and gender also demonstrated that these were not significant predictors of posttreatment aphasia.
Patients with AVMs
Because age and gender showed a trend towards significance for patients with AVMs, a multiple logistic regression with LAD as a predictor and age and gender as covariates was tested. LAD was not a significant predictor of posttreatment motor or language deficits (p-values for SMC, Broca's and Wernicke's were 0.36, 0.26, and 0.35 respectively).
Patients with Cavernomas
A similar analysis was not performed for cavernomas given the smaller N.
DISCUSSION
FMRI is increasingly used for neurosurgical planning due to its ability to provide valuable information on the spatial relationships between intracranial lesions and functionally eloquent areas. The validity of these imaging studies has been demonstrated to be sensitive and specific for mapping language and motor functions; for example, Bizzi et al found that fMRI exhibited high sensitivity (83%) and specificity (83%) for mapping functional cortical areas, with motor function mapping more sensitive and specific than language function mapping. While there are many studies exploring the value of its use in brain tumor patients, there is a paucity of literature exploring the use of fMRI in AVM and cavernoma patients.5 To date, there are no studies reporting an association between vascular lesion LAD and clinical outcomes. This information may be useful both to inform patients about their prognosis and to aid in surgical planning.
The present study investigated the clinical outcomes associated with BOLD fMRI parameters measured in vascular lesion patients. We searched for possible confounds between vascular lesion proximity and demographic characteristics and found no statistically significant relationships. The relationships between LAD and weakness or paresis were investigated. While we found a weak significant relationship between clinical deficits and lesion proximity to functionally eloquent cortex, it did not exist for all cortical areas. Considering both Broca and Wernicke language centers together, the area of closest language center LAD was not significant. However, the proximity of a vascular lesion to the primary sensorimotor area or Wernicke's area was found to be significantly associated with neurological deficits. Non-parametric tests showed that when grouped by Wernicke's LAD there was a trend towards significance with patients without pretreatment deficits in the <10 mm and the 10 – 20 mm category more likely to show posttreatment language deficits than in the > 20mm category. Additionally, regression analysis showed in patients with and without pretreatment deficits, there was a trend towards a significant association between distance from vascular lesion to motor activation and posttreatment motor deficits. These findings are in part consistent with previous literature in brain tumor patients. For example, Wood et al. studied primary and metastatic brain tumor patients and reported a distinctly increasing linear prevalence of motor deficits as LAD decreased20. However, they reported that the prevalence of all aphasias decreased dramatically once the language areas LAD was greater than 1cm and leveled off. In contrast to our study, Wood et al. found that the aphasia prevalence trend was only significant for Broca's area LAD and not for Wernicke's area LAD. They hypothesized that proximity to compact eloquent cortical areas was the most important factor, but that white matter involvement could be contributing and confounding the relationship. Indeed, language dysfunction through white matter tract damage has been studied in other pathologies but not in AVMs or cavernomas to date.4 The extent to which these differences reflect differences in the organization of primary sensorimotor areas compared to language areas is not known. The high variability of anatomical-functional correspondence of language areas has been well reported in the literature in comparison to motor and sensory areas, which have been demonstrated to exhibit greater structural-functional correspondence.
Since a significant relationship between LAD and Broca's aphasia was not observed, it raises questions of language center organization in the setting of complex vascular lesions. For example, Alkadhi et al.1 found somatotopic differences in organization of primary motor cortex in close proximity to an AVM. These differences were classified as either (1) functionally displaced independent of direct, structural distortion, (2) activation of motor cortex ipsilateral to the moving limb, in the unaffected hemisphere, or (3) activation in non-primary motor areas such as SMA(supplementary motor area), pre-motor, cingulate, or parietal regions. Sailor et al. 16 reported similar findings of shifted SMA activity contralateral to the unaffected hemisphere in patients with AVMs. For example, a patient with a right frontal AVM that overlapped anatomically with the right supplemental motor area featured left SMA activity during a left finger-tapping paradigm. In the present study, lesion type impacted posttreatment deficits. Specifically, patients with cavernomas and not AVMs showed significant posttreatment language deficits who had been deficit free before treatment. This may have been due to the wide range of treatments available for AVM patients in comparison to Cavernoma patients in our study. It is also possible that the number of AVM patients in this study was twice that of cavernoma patients and the unequal sample size may have biased the results (Table 8).
This study has several notable limitations. Individualized thresholds were used to counteract the intersubject variability of activation, reduce spurious artifact, and maximize sensitivity and specificity. The same technologist(CM) assessed all images. While retrospectively using the individualized threshold maps most closely modeled how fMRI is used in clinical practice today, this nevertheless may have introduced a confounding element of subjectivity in the study. Another limitation is the minimal consideration of white matter tracts, including the arcuate fasciculus or superior longitudinal fasciculus. Lastly, we used all available patient data that satisfied inclusion criteria and it is possible that because our sample size was not determined by a power analysis, the study could potentially be underpowered and thus insufficiently sensitive to detect some significant differences in outcomes especially in multivariate analyses. Additionally in terms of the range of outcomes, we categorized patients in terms of deficit versus no deficits, but did not look at the severity of deficits which may also have limited our ability to characterize the impact of all of the predictor variables on outcomes.
This study provides valuable information on the prevalence of clinical deficits as a function of LAD to eloquent cortex in patients with AVMs and cavernomas. Patients can to some degree be evaluated before cortical stimulation mapping to provide additional prognostic and management data.
CONCLUSION
In the present study we investigated fMRI lesion-to-activation- distance (LAD) and its relationship to morbidity in patients with vascular brain lesions about to undergo treatment, primarily AVMs and cavernomas. There was a significant trend between increasing clinical deficits and shorter LAD for sensorimotor and language areas. Additionally, this study also demonstrates the importance of examining deficits by lesion type to obtain a clearer picture about the nature of posttreatment deficits.
Acknowledgements
The project described was supported by the University of Wisconsin – Madison, Department of Radiology, Shapiro Program; University of Wisconsin Institute for Clinical and Translational Research NIH/UL1RR025011 Pilot Grant and KL2 Scholar Award; UWSMPH Medical Scientist Training Program; AHA Midwest Postdoctoral, Medical Student, and Undergraduate Grants; RC1MH090912-01 NIH NIMH Challenge Grant. We would also like to thank Dr. Hanefi Yildirim for his help during this project and especially in identifying the Spetzler-Martin grade of the AVMs for several of the patients in this study.
Abbreviation Key
- BOLD
blood oxygen level-dependent
REFERENCES
- 1.Alkadhi H, Kollias SS, Crelier GR, Golay X, Hepp-Reymond MC, Valavanis A. Plasticity of the human motor cortex in patients with arteriovenous malformations: a functional MR imaging study. AJNR Am J Neuroradiol. 2000;21:1423–1433. [PMC free article] [PubMed] [Google Scholar]
- 2.Binder JR, Swanson SJ, Hammeke TA, Morris GL, Mueller WM, Fischer M, et al. Determination of language dominance using functional MRI: a comparison with the Wada test. Neurology. 1996;46:978–984. doi: 10.1212/wnl.46.4.978. [DOI] [PubMed] [Google Scholar]
- 3.Bizzi A, Blasi V, Falini A, Ferroli P, Cadioli M, Danesi U, et al. Presurgical functional MR imaging of language and motor functions: validation with intraoperative electrocortical mapping. Radiology. 2008;248:579–589. doi: 10.1148/radiol.2482071214. [DOI] [PubMed] [Google Scholar]
- 4.Breier JI, Hasan KM, Zhang W, Men D, Papanicolaou AC. Language dysfunction after stroke and damage to white matter tracts evaluated using diffusion tensor imaging. AJNR Am J Neuroradiol. 2008;29:483–487. doi: 10.3174/ajnr.A0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cannestra AF, Pouratian N, Forage J, Bookheimer SY, Martin NA, Toga AW. Functional magnetic resonance imaging and optical imaging for dominant-hemisphere perisylvian arteriovenous malformations. Neurosurgery. 2004;55:804–812. doi: 10.1227/01.neu.0000137654.27826.71. discussion 812-804. [DOI] [PubMed] [Google Scholar]
- 6.Geibprasert S, Pongpech S, Jiarakongmun P, Shroff MM, Armstrong DC, Krings T. Radiologic assessment of brain arteriovenous malformations: what clinicians need to know. Radiographics. 2010;30:483–501. doi: 10.1148/rg.302095728. [DOI] [PubMed] [Google Scholar]
- 7.Lehericy S, Biondi A, Sourour N, Vlaicu M, du Montcel ST, Cohen L, et al. Arteriovenous brain malformations: is functional MR imaging reliable for studying language reorganization in patients? Initial observations. Radiology. 2002;223:672–682. doi: 10.1148/radiol.2233010792. [DOI] [PubMed] [Google Scholar]
- 8.Lehericy S, Duffau H, Cornu P, Capelle L, Pidoux B, Carpentier A, et al. Correspondence between functional magnetic resonance imaging somatotopy and individual brain anatomy of the central region: comparison with intraoperative stimulation in patients with brain tumors. J Neurosurg. 2000;92:589–598. doi: 10.3171/jns.2000.92.4.0589. [DOI] [PubMed] [Google Scholar]
- 9.Moritz C, Haughton V. Functional MR imaging: paradigms for clinical preoperative mapping. Magn Reson Imaging Clin N Am. 2003;11:529–542. v. doi: 10.1016/s1064-9689(03)00062-x. [DOI] [PubMed] [Google Scholar]
- 10.Newman G. Clarification of the abc/2 rule for the ICH volume. Stroke. 2007;38:862. doi: 10.1161/01.STR.0000257309.50643.0a. [DOI] [PubMed] [Google Scholar]
- 11.Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogawa S, Lee TM, Nayak AS, Glynn P. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med. 1990;14:68–78. doi: 10.1002/mrm.1910140108. [DOI] [PubMed] [Google Scholar]
- 13.Pillai JJ. The evolution of clinical functional imaging during the past 2 decades and its current impact on neurosurgical planning. AJNR Am J Neuroradiol. 2010;31:219–225. doi: 10.3174/ajnr.A1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roessler K, Donat M, Lanzenberger R, Novak K, Geissler A, Gartus A, et al. Evaluation of preoperative high magnetic field motor functional MRI (3 Tesla) in glioma patients by navigated electrocortical stimulation and postoperative outcome. J Neurol Neurosurg Psychiatry. 2005;76:1152–1157. doi: 10.1136/jnnp.2004.050286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sabbah P, Chassoux F, Leveque C, Landre E, Baudoin-Chial S, Devaux B, et al. Functional MR imaging in assessment of language dominance in epileptic patients. Neuroimage. 2003;18:460–467. doi: 10.1016/s1053-8119(03)00025-9. [DOI] [PubMed] [Google Scholar]
- 16.Sailor J, Meyerand ME, Moritz CH, Fine J, Nelson L, Badie B, et al. Supplementary motor area activation in patients with frontal lobe tumors and arteriovenous malformations. AJNR Am J Neuroradiol. 2003;24:1837–1842. [PMC free article] [PubMed] [Google Scholar]
- 17.Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg. 1986;65:476–483. doi: 10.3171/jns.1986.65.4.0476. [DOI] [PubMed] [Google Scholar]
- 18.Thickbroom GW, Byrnes ML, Morris IT, Fallon MJ, Knuckey NW, Mastaglia FL. Functional MRI near vascular anomalies: comparison of cavernoma and arteriovenous malformation. J Clin Neurosci. 2004;11:845–848. doi: 10.1016/j.jocn.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 19.Vikingstad EM, Cao Y, Thomas AJ, Johnson AF, Malik GM, Welch KM. Language hemispheric dominance in patients with congenital lesions of eloquent brain. Neurosurgery. 2000;47:562–570. doi: 10.1097/00006123-200009000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Wood JM, Kundu B, Utter A, Gallagher TA, Voss J, Nair VA, et al. Impact of Brain Tumor Location on Morbidity and Mortality: A Retrospective Functional MR Imaging Study. AJNR Am J Neuroradiol. 2011;32:1420–1425. doi: 10.3174/ajnr.A2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yetkin FZ, Mueller WM, Morris GL, McAuliffe TL, Ulmer JL, Cox RW, et al. Functional MR activation correlated with intraoperative cortical mapping. AJNR Am J Neuroradiol. 1997;18:1311–1315. [PMC free article] [PubMed] [Google Scholar]
- 22.Yetkin FZ, Swanson S, Fischer M, Akansel G, Morris G, Mueller W, et al. Functional MR of frontal lobe activation: comparison with Wada language results. AJNR Am J Neuroradiol. 1998;19:1095–1098. [PMC free article] [PubMed] [Google Scholar]


