Abstract
Cardiac troponin is a Ca2+- dependent switch for the contraction in heart muscle and a potential target for drugs in the therapy of heart failure. Bepridil is a drug that binds to troponin and increases calcium sensitivity of muscle contraction. Because bepridil has been well studied, it is a good model for analysis by computational and experimental methods. Molecular dynamics (MD) simulations were performed on troponin complexes of different sizes in the presence and absence of bepridil bound within the hydrophobic pocket at the N-terminal domain of troponin C. About 100ns of simulation trajectory data were generated, which were analyzed using cross-correlation analyses, MMPBSA and MMGBSA techniques. The results indicated that bepridil binding within the hydrophobic pocket of cardiac TnC decreases the interaction of TnC with TnI at both the N-domain of TnC and the C-domain of TnC, and decreases the correlations of motions among the segments of the troponin subunits. The estimated calcium binding affinities using MMPBSA showed that bepridil has a sensitizing effect for the isolated system of TnC, but loses this effect for the complex. Our experimental measurements of calcium dissociation rates were consistent with that prediction. We also observed that while bepridil enhanced the troponintropomyosin-actin-activated ATPase activity of myosin S1 at low calcium concentrations it was slightly inhibitory at high calcium concentrations. Bepridil increases the ATPase activity and force generation in muscle fibers, but its effects appear to depend on the concentration of calcium.
Keywords: cardiac troponin, bepridil, calcium binding, ATPase activity
I. Introduction
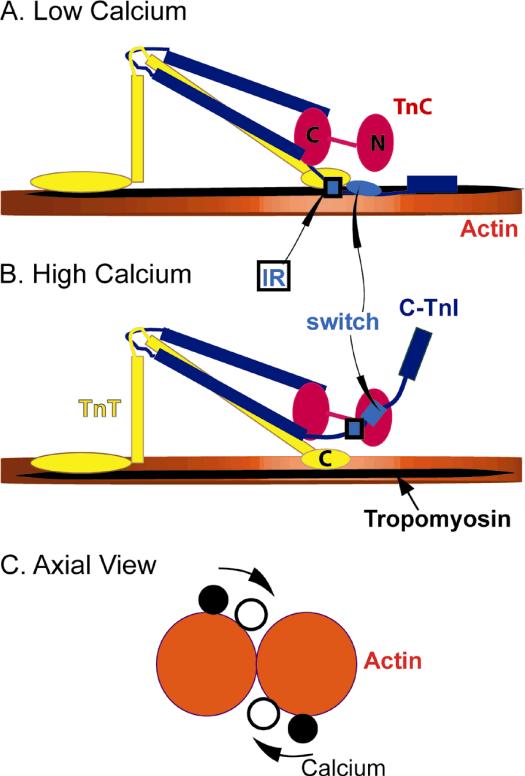
Muscle contraction is driven by the cyclic interaction of myosin thick filaments with actin thin filaments. The regulation of cardiac muscle is highly cooperative as a result of the proteins troponin (Tn), and tropomyosin (Tm) that bind to the actin filaments shown in Figure 1.1 Cardiac Troponin (cTn) is a heterotrimeric complex composed of an inhibitory subunit, troponin I (TnI, blue in Fig. 1), a tropomyosin binding subunit, troponin T (TnT, yellow in Fig. 1) and a calcium binding subunit, troponin C (TnC, red in Fig. 1). Ca2+ binding to the regulatory site at the N-domain of TnC exposes a hydrophobic pocket that acts as a binding site for the switch segment of TnI. The switch segment of TnI is adjacent to an inhibitory segment that binds to actin in the absence of calcium. Binding of TnI to TnC occurs at the expense of the interaction between the inhibitory segment of TnI with actin. These changes allow tropomyosin to move freely toward the helical groove of the actin filament as shown in Figure 1C. Different positions of tropomyosin on actin are associated with different abilities to activate the ATPase activity of myosin. Calcium binding, thus increases the ability of actin to accelerate ATP hydrolysis by myosin.3,4
Figure 1.
Schematic view of the troponin-tropomyosin-actin complex. Actin is in orange, tropomyosin in black, TnI in blue, TnT in yellow and TnC in red. The switch segment of TnI is shown either as a light blue ellipse or rectangle. The inhibitory region of TnI (IR) is shown as a blue and black rectangle. A. Calcium free state13 B. Calcium bound state13 C. Tropomyosin position change with calcium2
The subunit interactions (TnI, TnT and TnC) are mediated via multiple interaction sites. Hence mutations in the different regions of the Tn subunits can result in conformational changes that either increase or decrease activity. So far approximately 60 mutations in troponin have been linked to cardiac disorders such as familial hypertrophic cardiomyopathy.5 Several of these mutations have been shown to change ATPase activities by altering the distribution of states of the actin thin filament.6-8 The cooperative nature of the troponin-tropomyosin-actin complex means that binding of drugs to any of the troponin subunits might help to normalize this distribution.
The regulatory properties of cTnC (cardiac troponin C) make it an attractive target for cardiotonic drugs that can aid in treating diseases by modulating TnC's sensitivity to calcium. Among several potential compounds that have calcium sensitizing properties, bepridil was identified to bind to the N-domain hydrophobic pocket of TnC.9 Bepridil was shown to increase ATPase rates and isometric force in muscle fibres10. Bepridil was further shown to delay calcium release in the isolated TnC.11 However bepridil is not an effective sensitizer of force at high calcium concentrations.10 The mechanism of action of bepridil is thought to result from stabilization of the hydrophobic pocket of TnC. 9,11 When bepridil binds to a complex of TnC and the switch segment of TnI, the switch segment tends to be displaced.12 Thus the effects of bepridil on calcium sensitivity may depend on other members of the regulatory complex.
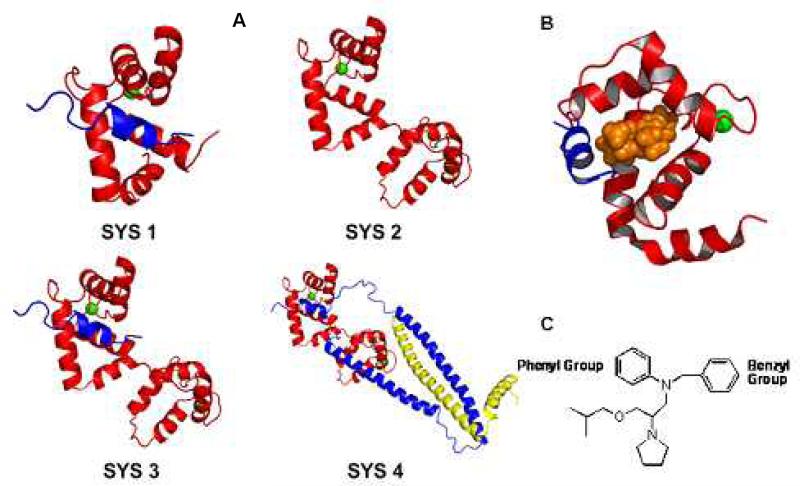
In this work to understand how bepridil affects troponin at an atomic level, four systems of troponin were investigated using MD simulations based on available structures. The four systems, as shown in Figure 2, contain different parts of troponin. Each system was simulated with and without bepridil. Since the hydrophobic pocket which is exposed on TnC by calcium binding is the binding site for bepridil, all structures used in the simulation have calcium. The theoretical aspects of this work were complemented by experimental measurements of effects of bepridil on ATPase activities and the rates of Ca2+ detachment.
Figure 2.
A: Components of cardiac troponin used for 4 systems. TnC is in red, TnI is in blue, and TnT is in yellow, calcium ions are in green sphere. Sys. 1: TnC1-89, TnI147-163, Sys. 2: TnC1-161. Sys. 3: TnC1-161, TnI147-163. Sys. 4: TnC1-161, TnI31-163, TnT183-288. B: The NMR structure of the complex composed of the N-domain of TnC1-89 in red, the switch segment of TnI147-163 in blue and bepridil in orange. (PDB ID:1LXF). C. The structure of bepridil.
II. Methods and Materials
Simulation Systems
The details of the four systems are listed in Table 1. System 1 was based on the complex NMR structure of 1LXF and contains the N-domain of TnC1-89, the switch segment of TnI147-163 and bepridil. (PDB ID:1LXF).12 Bepridil was removed for the simulation in System 1A. The initial structure for System 2 was based on the X-ray structure (PDB ID: 1J1D)13 by removing the TnI and TnT segments from the structure. In order to place the bepridil in the binding pocket of TnC in System 2B, the bepridil complex structure of 1LXF was first aligned to the N-domain of this system and these coordinates were used to dock the ligand in the pocket. System 3 was modeled by using the N and C domains from the System 2 model and aligning to the 1LXF model. The TnI segment (147-163: 1LXF) coordinates were used after the alignment for System 3 and the bepridil coordinates were used for placing the ligand in the pocket for System 3B. The initial structure for System 4 was the complete model of 1J1D with the TnI linker connecting the switch segment with the two large helices built using homology module in Insight II.14,15 Bepridil was placed in the pocket using the alignment to 1LXF for System 4B. All the four systems were neutralized and explicitly solvated using TIP3P waters in a rectangle box with a minimum distance of 14 Å from the protein to the wall of the box. The MD simulation for SYS 4A was carried out previously to investigate the difference from skeletal troponin15. In this work, we analyzed the simulation on cardiac troponin without bepridil again to study the impact of bepridil binding.
Table 1.
Molecular dynamics simulation summary
| System | Components | Ligand | Simulation Time |
|---|---|---|---|
| 1A | TnC(1-89), TnI(147-163) | None | 12.0 ns |
| 1B | TnC(1-89), TnI(147-163) | Bepridil | 12.0 ns |
| 2A | TnC(1-161) | None | 12.0 ns |
| 2B | Tnc(1-161) | Bepridil | 12.0 ns |
| 3A | TnC(1-161), TnI(147-163) | None | 11.9 ns |
| 3B | Tnc(1-161), TnI(147-163) | Bepridil | 9.6 ns |
| 4A | TnC(1-161), TnI(31-163), TnT(183-288) | None | 12.0 ns |
| 4B | TnC(1-161), TnI(31-163), TnT(183-288) | Bepridil | 12.0 ns |
Simulation Details
Computations including the minimization and MD simulations for the systems were performed on a 128-processor Altix 4700 at East Carolina University. Calculations were carried out using AMBER's all-atom force field as implemented in AMBER 9 software.16 The SANDER module of AMBER program was used for the computation. Simulations were carried out under periodic boundary conditions with a cutoff of 10 Å to truncate the VDW interactions. The particle-mesh Ewald method17-20 was used to treat the long-range electrostatic contribution to the force field. The SHAKE algorithm21 was applied to constrain the hydrogen atoms and allow the usage of longer step size of 1.5 fs.
All the systems were energy minimized before each simulation. A restrained minimization was first performed on the solvent while keeping the protein fixed; then the entire system was minimized. The minimized systems were warmed for 20 ps to a final temperature of 300K. Then constant pressure dynamics (NPT) was performed on the system for about 300 ps at 1 atmosphere till the density of the systems was stabilized. Finally, constant volume dynamics (NVT) was performed on each of the systems.
The empirical binding affinities of bepridil within the hydrophobic pocket of TnC and calcium in the binding loop of TnC were analyzed by the Molecular Mechanics-Poisson Boltzmann Surface Area (MM-PBSA) method22-24 integrated in AMBER 9.16
Proteins
Myosin25 and actin26 were isolated from rabbit back muscle. Bovine cardiac troponin27 and tropomyosin28 were prepared from left ventricles. Human cardiac troponin was expressed in E. Coli.26 Human cardiac TnT2 subcloned into psBETA vector was expressed in freshly transformed BL21(DE3)pLysS cells (Carlsbad, CA) and purified as previously described.29 Human cardiac TnI and TnC were subcloned into pET3d vector (gift from K. Jaquet) and were co-expressed in freshly transformed BL21(DE3)pLysS cells and selected with ampicillin and chloramphenicol. Bacteria were harvested and resuspended in 6M Urea, 1mM EDTA, 20mM Tris-HCl pH 8.0, 5% sucrose, 0.2mg/mL lysozyme and protease inhibitor cocktail (Mannheim, Germany) and allowed to incubate overnight at 4°C after an initial 15 minutes at room temperature. The suspension was sonicated and the supernatant was loaded on a SP Sepharose High Performance 16/10 column in 6M Urea, 1mM EDTA, 20mM Tris-HCl pH 8.0. Proteins were eluted with a 0-0.5M NaCl gradient. Non-bound fractions were used for the preparation of TnC. Fractions containing TnI were then passed over a 20mL DEAE sephacel column before going over a SP Sepharose High Resolution column for final purification. Crude TnC from the SP column was loaded on a DEAE sephacel column and eluted with a 0-0.5M NaCl gradient. For final purification TnC fractions were loaded on a phenyl sepharose column in 1M NaCl, 20mM Tris-HCl pH 8.0, 0.5mM CaCl2 and 1mM dithiothreitol and then eluted by replacing the CaCl2 in the buffer with 1mM EDTA.
Human cardiac troponin was reconstituted following the previously described protocol30 with some modifications. TnT2, TnI and TnC were mixed at a 1:1:1.2 molar ratio and a Protein Pak DEAE 15HR column was used for final purification with start buffer of 0.1M NaCl, 20mM Tris-HCl pH 8.0, 5mM MgCl2. Troponin was eluted with a 0.1-1.0M NaCl gradient in the same buffer.
Assays
ATPase activities were measured by the release of 32Pi from γ 32P labeled ATP (GE Healthcare, Boston, MA) using four time points to establish each initial rate.31
The rate of calcium detachment was measured by changes in Quin 2 (Invitrogen, Carlsbad, CA) fluorescence using a DX17.MV/2 sequential mixing stopped flow spectrofluorometer (Applied Photophysics, Leatherhead, UK). The excitation wavelength was 330 nm and the emission was collected through a Schott 370 nm high pass filter (transmission midpoint of 400 nm). The temperature was maintained at 4° or 20° C with a circulating water bath. The basic reaction buffer (100 mM KCl, 20 mM MOPS, pH 7.0, 1 mM dithiothreitol) was passed through a Chelex-100 column (Bio-Rad, Hercules, CA) to lower the free calcium concentration. TnC, the TnC-TnI complex or whole troponin were in the basic reaction buffer containing 30 μM CaCl2. Those protein mixtures were rapidly mixed with equal volumes of the basic buffer containing 60 or 120 μM Quin 2. The increase in fluorescence was generally fit with a biexponential function to obtain apparent rate constants. Control experiments done in the absence of troponin showed that binding of Quin 2 to the free probe was too fast to measure under these conditions. Furthermore, there was no significant photobleaching of the Quin 2 over 0.5 sec. The same concentration of bepridil (Sigma, St. Louis, MO) was present in both syringes to avoid changes resulting from bepridil detachment from troponin. Bepridil was dissolved in 100% ethanol to give a 25 mM stock and then diluted to 5 mM in water prior to adding to the reactions. The concentration of bepridil was determined spectrophotometrically at 296 nm with an extinction coefficient of 3544 cm−1 M−1.32 The concentration of ethanol was held constant at 0.95% in all assays as this concentration of ethanol, by itself, was found to depress the rate of calcium release by 23%. It was equally important to keep the ethanol concentration constant in ATPase assays.
III. Computational Results
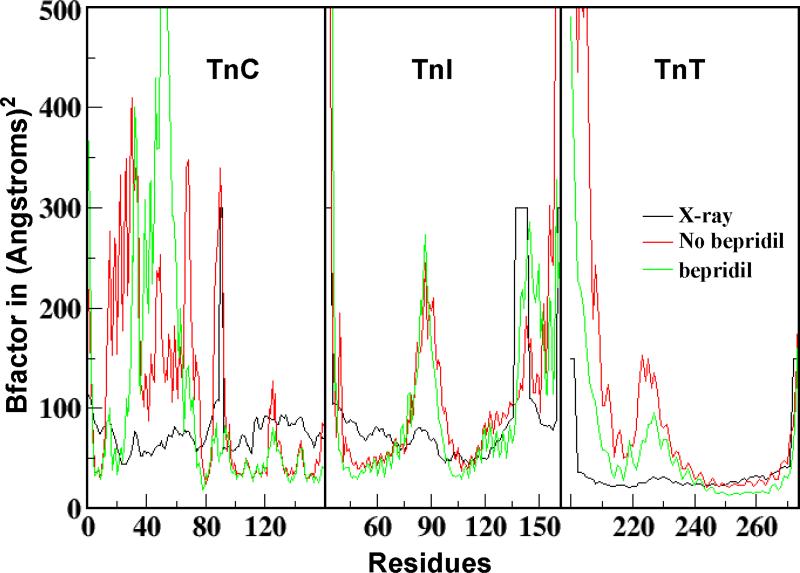
Table 1 shows the NVT simulations performed on the four different systems. All simulations were carried out for at least 10 ns to ensure equilibrium. The stability of each simulation was determined by the stability of root mean square coordinate deviation of main chain atoms and the maintenance of a constant average temperature. The B-factor of each residue in each system with and without bepridil was calculated using “atomicflunct” of “ptraj” in the Amber program based on the equilibrated simulations. Prior to the B-factor calculation using “atomicfluct”, the calculation with “rms” of “ptraj” was performed to remove the rot and tran motions from each step. The B-factors of the backbone atoms of SYS 4A and SYS 4B are shown in Figure 3. The calculated B-factors of SYS 4 were generally consistent with the X-ray B-factors, except for the N-terminus helix (H1, TnT200-222) of the incomplete TnT and the N domain of cTnC (TnC1-89). The calculated high B-factor of the incomplete TnT helix is due to the fact that during simulation the incomplete TnT helix of cTn is very flexible and gradually loses its secondary structure. The calculated high B-factor of the N-domain of cTnC may be due to the fact that this domain is connected with the C-domain of TnC in cTn by a loop linker and is flexible during simulation. The overall consistency of the calculated B-factor and the X-ray B-factor indicated that the molecular dynamics simulations we performed were appropriate. Based on the simulation trajectory data, the effect of bepridil binding was predicted.
Figure 3.
The calculated B-factor of SYS 4 with (green) and without bepridil15 (red), and the X-ray B-factors13 (black).
Bepridil Binding
Early experimental studies have found a 10 fold decrease in bepridil binding constant in going from isolated cTnC to the cTnC·TnI complex. The dissociation constant increased from a KD of 10 μM to 140 μM respectively.10,33 Moreover a structural study of bepridil binding to isolated cTnC found 3 bepridil molecules bound to both the N and C domain pockets of TnC9 while only a single bepridil was bound to the TnC-TnI complex.12
Based on the simulation trajectory data generated for each of the four systems, bepridil binding affinities were estimated using the MMPBSA approach assuming one bepridil was bound per TnC in all cases. The results, shown in Table 2, do not reflect the experimentally observed weakening of bepridil binding in the presence of TnI. The apparent binding affinities for systems 1, 3, 4 are quite similar. In the case of isolated TnC in system 2, the affinity of the specific binding to the hydrophobic pocket is actually predicted to be weaker than in the more complex systems. Although the large complex, SYS 4, does not have the complete TnI subunit, it does include the switch segment of TnI which binds to hydrophobic pocket. Since bepridil binds to the N-domain of TnC, the direct effects of TnI binding to bepridil can be observed with a structure of a system with the switch segment of TnI. Our differences from the experimental values may result from effects resulting from the other 2 bepridil molecules bound to isolated TnC that were not present in our simulations.
Table 2.
Predicted bepridil-binding affinities (kcal/mol) by MMPBSA
| Complex | Eele | EVDW | Esol | ΔG | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | STD | Mean | STD | Mean | STD | Mean | STD | |
| SYS 1B | −4.00 | 1.46 | −40.77 | 3.19 | 12.50 | 2.44 | −32.27 | 3.45 |
| SYS 2B | −1.82 | 1.78 | −36.54 | 3.23 | 9.52 | 2.97 | −28.85 | 3.64 |
| SYS 3B | −2.88 | 1.57 | −46.14 | 2.98 | 14.23 | 2.92 | −34.79 | 3.73 |
| SYS 4B | −0.27 | 1.80 | −49.43 | 3.69 | 13.82 | 3.53 | −35.88 | 3.56 |
The bepridil binding energy change between SYS 1 and SYS 3 is within the error range. Thus, the C-domain of TnC may not have a direct effect on N-domain binding of bepridil. By comparing the bepridil binding energies between SYS 3 and SYS 4, it also appears that TnT and TnI long helices present in the structure do not play a role in the binding of bepridil. Hence, the major changes in binding energies of the different systems occur with the presence of the TnI switch segment (TnI147-163). SYS 2 and 3 only differ structurally with the presence of the switch segment of TnI but binding energy changes with a larger non-bonded contribution from van der Waals forces.
Calcium Binding
Calcium binding to troponin triggers muscle contraction. Calcium-binding affinities were estimated using the MMPBSA approach for the different systems as shown in Table 3. The low affinity site (site II) has been reported to bind calcium on the order of 106 M−1 in isolated TnC and shown to increase by an order of magnitude once in the troponin complex.34 Our results in Table 3 also show an increase in binding affinity when comparing the site II binding energies of the isolated TnC (SYS 2A, −67.69 kcal/mol) and the complex (SYS 4A, −115.76 kcal/mol).
Table 3.
Estimated Calcium-binding affinities (kcal/mol) by MMPBSA
| Complex | Ligand | Site II | |
|---|---|---|---|
| Mean | STD | ||
| SYS 1A | None | −113.2 | 10.83 |
| SYS 1B | Bepridil | −118.26 | 15.61 |
| SYS 2A | None | −67.69 | 5.83 |
| SYS 2B | Bepridil | −108.95 | 20.13 |
| SYS 3A | None | −133.65 | 15.49 |
| SYS 3B | Bepridil | −125.16 | 11.18 |
| SYS 4A | None | −115.76 | 9.43 |
| SYS 4B | Bepridil | −108.86 | 20.96 |
Bepridil has been reported to enhance calcium binding. Bepridil was shown to delay the rate of calcium release from the low affinity calcium specific site in the N-domain of TnC in the isolated system.11 Simulations of SYS 2 with the isolated TnC showed a similar increase in the calcium sensitivity of N-domain site upon bepridil binding (Table 3). Interestingly, calcium binding energies for the N-domain site in the 3-subunit complex in SYS 4 did not show a significant change with the addition of bepridil. This suggested a difference in the effects of bepridil in the complex. Although experiments demonstrated increased force and calcium sensitization with bepridil in muscle fibres, the effect of bepridil was lower at higher concentrations of calcium10. Since the structure used in SYS 4 is based on the calcium saturated X-ray structure of cardiac troponin, bepridil's calcium sensitizing activity may be lowered as seen in the simulation.
In addition, we noticed a decrease in affinity for the C-domain sites in TnC with the addition of bepridil to the N-domain of SYS 4. This suggested that bepridil may have long range effects that involve changing interactions in the C-domain of TnC.
Our results can be explained more elaborately by considering the distribution of actin-tropomyosin-troponin among the 3 possible regulatory states. The degree of activity increases as the fraction of actin-tropomyosin-troponin in the active state increases. Since regulated actin is highly cooperative, troponin should have three structures that correspond to the three states of actin. Changes in ligand binding to TnC (TnI or calcium) will affect the structure of troponin and change the distribution of states. The change in state distributions should be reflected in a change in calcium affinity. However lack of structural data prevented us from identifying the exact structure of the intermediate or active states of troponin. In SYS 2A (isolated TnC) it is known that in cardiac troponin, the lack of TnI keeps the hydrophobic pocket in the closed conformation12. That may indicate that in the absence of TnI a greater fraction of TnC is in the inactive state even at saturating calcium. With bepridil present, the calcium affinities of N and C-domains increase suggesting a shift to the intermediate or active state. That is, because calcium stabilizes the intermediate state, that state must have the highest calcium affinity. Adding TnI to TnC (comparing SYS 2A to 3A or 2A to 4A) increases calcium affinities of both N and C domain. This shows that the troponin complex is shifting to a more intermediate or active state.
We believe that the SYS 4A structure is not that of the fully active state. That is, Ca2+ alone gives a distribution of states dominated by an intermediate state.35 Full activation, in solution, requires additional binding of rigor type myosin.36,37 With bepridil it may shift to an even lesser active form (intermediate or inactive) that does not change calcium affinities in the N-domain but decreases calcium affinities in the C-domain. Bepridil seems to be stabilizing the transition from the inactive to intermediate or active to intermediate state.
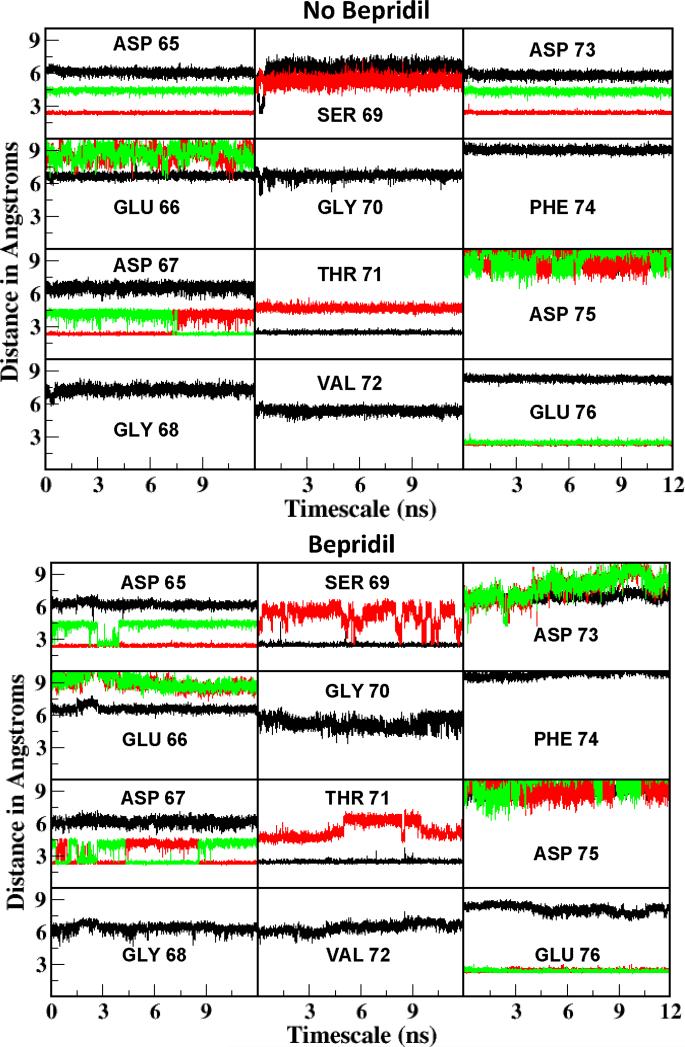
The calcium coordination at site II was studied to learn more about how bepridil may affect Ca2+ affinity. The distances between each oxygen around the binding loop of site II and calcium were analyzed for SYS 4B (Fig. 4B) and compared with that of SYS 4 A (Fig. 4A) 15. Calcium oxygen distance below 3 Å were considered as coordinating distances. In the system without bepridil (4A) and with bepridil (4B), 6 oxygens from the protein ligands were coordinated with calcium with additional oxygen from water (not shown but observed from analysis). The main difference in the ligands is that the no bepridil system (4A) had ASP 73 OD1 oxygen coordinating with calcium, but this coordination was lost in the bepridil system (4B). Similarly SER 69 coordinated in SYS 4B but was not seen in the coordination sphere of SYS 4A. In other words, with bepridil the calcium loses coordination with ASP 73 oxygen but gains coordination with SER 69. This may be a reason why we do not see a difference in the binding energies calculated from MM-PBSA for site II as one ligand is being replaced by another.
Figure 4.
Calcium coordination distances between two TnC complex systems of no bepridil (SYS 4A)15 and bepridil (SYS 4B) at site II. The backbone oxygens are shown in black, whereas the side chain oxygens are shown in red (OG/OD1/OE1) and green (OD2/OE2).
TnC-TnI Interaction
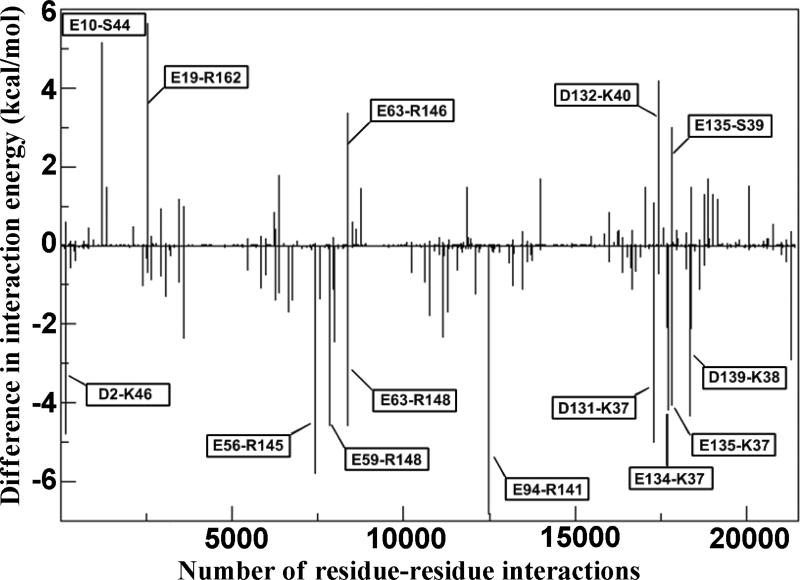
Calcium-binding changes the interactions among the three components of troponin that permits muscle contraction to occur. The effect of bepridil on the binding energies among the components was estimated using the MM-PBSA approach. Table 4 shows that with bepridil, the total interaction energy between TnC and TnI decreased by 37 kcal/mol compared to free troponin. Bepridil produced no significant changes in the TnC-TnT or TnT-TnI interactions. In order to classify the residues that were responsible for the change in the TnC-TnI interaction energy, the decomposition energy module using MM-GBSA was used to obtain pairwise residue-residue interaction energies. Having 161 residues in TnC and 133 residues in TnI, we decided to simplify the search and identify the residues by taking an energy difference of each TnC-TnI pair between the no bepridil (SYS 4A) and bepridil (SYS 4B) form, as shown below:
ΔE denotes the TnC-TnI pairwise residue-residue interaction difference, Eno-bepridil is the TnC-TnI pairwise residue-residue interaction in SYS4A, and Ewith-bepridil is the TnC-TnI pairwise residue-residue interaction in SYS4B. A negative value for ΔE indicates that bepridil makes the pairwise interaction less favorable; while a positive value for ΔE indicates that bepridil makes the pairwise interaction more favorable. As shown in Figure 5, bepridil increased the number of residue-residue interaction energy differences (ΔE) below 0 demonstrating that bepridil made more residue-residue interactions less favorable. This result was expected as the total interaction between TnC-TnI subunits was shown to be decreased in the MM-PBSA calculation in Table 4.
Table 4.
The interaction among three components of troponin by MMPBSA in SYS 4.
| Interaction (kcal/mol) | Ligand | Eele | EvdW | Esol | ΔG | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | STD | Mean | STD | Mean | STD | Mean | STD | ||
| TnC-TnI | None | −6151.4 | 181.15 | −210.7 | 10.98 | 6106.96 | 166.21 | −255.13 | 20.71 |
| Bepridil | −5730.3 | 153.77 | −179.1 | 12.02 | 5690.81 | 153.29 | −218.57 | 15.18 | |
| TnC-TnT | None | −935.82 | 83.36 | −36.41 | 4.09 | 947.48 | 85.6 | −24.75 | 9.09 |
| Bepridil | −1089.8 | 92.79 | −41.34 | 5.31 | 1102.27 | 88.39 | −28.89 | 11.34 | |
| TnT-TnI | None | −170.77 | 67.66 | −203.3 | 7.62 | 211.07 | 66.76 | −162.99 | 11.99 |
| Bepridil | −269.79 | 57.24 | −212.4 | 7.71 | 317.54 | 57.6 | −164.6 | 11.06 | |
Figure 5.
The TnC-TnI pairwise residue-residue interaction difference between SYS 4A (no bepridil) and SYS 4B (with bepridil). The prominent differences are labeled with the TnC residue-TnI residue in the box.
Figure 5 shows that the destabilized interactions include the N-domain of TnC with the switch segment of TnI and the C-domain of TnC with N-terminal region of TnI. Bepridil weakened the interaction of the N-domain of TnC, including residues E56, E59 and E63, with the N-terminal tail of the switch segment of TnI including residues R145 and R148. Our data are in concord with NMR studies that show a negative cooperativity of binding of bepridil to TnC in the presence of TnI owing to steric and repulsive effects of TnI R148 with the pyrollidine ring of bepridil.12 These effects were suggested to cause a reorientation of TnI to fit in the hydrophobic pocket. This reorientation of the TnI switch segment with bepridil may explain the decrease in the interactions with the N-domain of TnC as shown in our simulations.
Bepridil binding to TnC also decreased the interaction energies of residues D131, E134, E135 and D139 of TnC with the residues N-terminal to the H1 helix of TnI (residues K37 and K38). This drop in interaction energies may be a result of bepridil decreasing the Ca2+ sensitivity of the C-domain sites as seen in our simulation (Table 3). The C-domain calcium sites are also EF-hands that change structurally with the binding of calcium and allow binding of the other segments of troponin including TnI and TnT. With the desensitization of these sites, TnI may be losing some of its interactions with TnC C-domain.
Correlated Motion
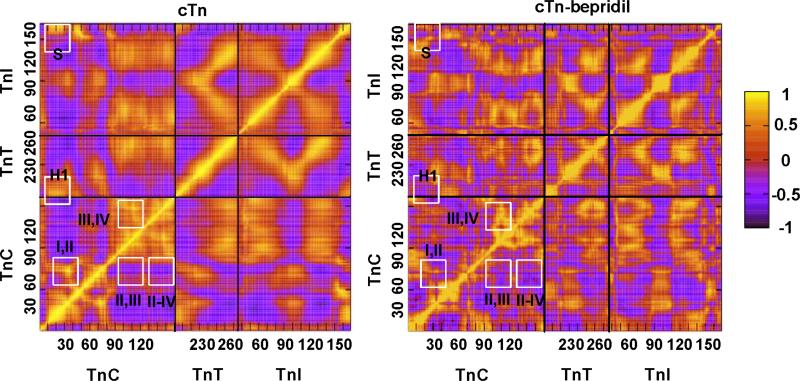
A Cross-correlation analysis38,39 was applied to investigate the effect of bepridil on the correlated motions within troponin. Strong positive correlations have correlation coefficients or values close to 1 and strong negative correlations have coefficients close to -1. The correlated motions of the large complex (SYS 4B) with bepridil were analyzed and compared with that of SYS 4A15 without bepridil to explore any differences with the addition of bepridil and shown in Figure 6. While the correlation patterns are similar for both cases, the pattern with bepridil is more fragmented. That is, the areas of correlation do not appear to run in long contiguous stretches as well when bepridil is present.
Figure 6.
Cross Correlation map of cardiac troponin without bepridil (SYS 4A15, left) and with bepridil (SYS 4B, right). A strong positive correlation is denoted by 1 and a strong negative correlation is denoted by −1.
In the wild type system (without bepridil, SYS 4A) the loop residues in the N-domain of calcium-binding sites I and site II are highly positively correlated as indicated by the bright yellow spot inside the white square designated as I,II in the left of the Figure 6. This correlation is maintained even though site I is dysfunctional in cardiac troponin. These sites are also correlated in skeletal troponin where both sites are functional and cooperative.40,41 A similar positive correlation is seen in the C-domain calcium ions and loop residues of site III and site IV as indicated in Figure 6 as III, IV. However there appears to be minimal correlation between the N-domain sites and C-domain sites. The cross correlation coefficient values are as follows: sites III and IV with 0.735 showing strong positive correlation, sites II and III with -0.320 showing minimal negative correlation and sites II and IV with -0.496 showing minimal negative correlation.
With the addition of bepridil in the N-domain of TnC in SYS 4B, the calcium correlation tended to change. The C-domain sites maintained high positive correlations with a correlation coefficient of 0.699, however site II of the N-domain and site IV of C-domain showed a stronger negative correlation than in the no-bepridil system. The calcium correlation for site II, IV increased from -0.496 to -0.774 with bepridil while site II, III correlation remained minimal with a value of -0.420. Moreover the loop residues of site I and II showed lower correlation in the bepridil form than in the no bepridil form as shown in Figure 6.
In SYS 4A, the N-domain of TnC (cNTnC1-89) showed a strong positive correlation with both the N-terminus helix of TnT (H1, TnT200-222) and the switch segment of TnI (S, TnI151-159). At the same time the N-domain of TnC showed a negative correlation with the C-domain of TnC, the C-terminus helix of TnT (H2, TnT226-270) and particularly with TnI except for the switch segment. The close association of C-domain of TnC (cCTnC94-161) with the other domains of troponin can be seen by its strong positive correlations with the large helix of TnT (H2, TnT226-270) and the two major helices of TnI (H1, TnI43-79 and H2, TnI91-135). Bepridil caused two main changes in SYS 4. The N-domain of TnC lost its correlations with the switch segment of TnI (S in Figure 6) and H1 of TnT as shown in Figure 6. Previous experiments on structures including the cNTnC and TnI switch segment suggested a negative cooperativity in the tertiary form (cNTnC·TnI·bepridil) compared to the binary form (cNTnC·TnI) which results in the reorientation of the TnI switch segment that allows the bepridil to bind in the hydrophobic pocket of TnC.12 The TnI switch segment may lose correlation with the N-domain as it is less associated with TnC in the presence of bepridil.
IV. Experimental Verification
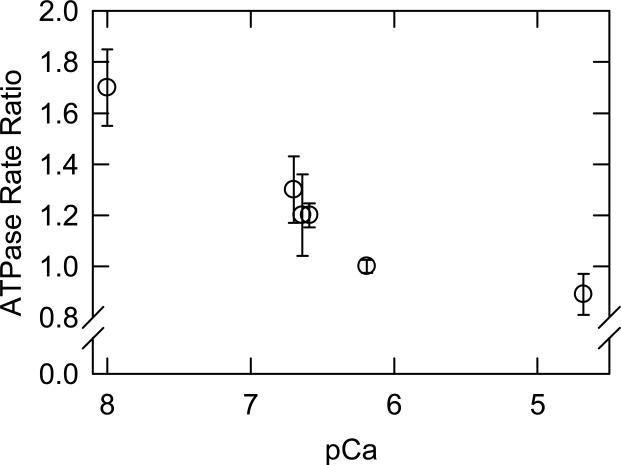
Several experiments were performed to test the computational predictions of bepridil's effects on troponin. We first observed that bepridil had effects on the ATPase activity of myosin S1 even in the absence of troponin. Bepridil reduced the ATPase activity of S1 alone (0.072/sec to 0.059/sec), of actin and S1 (1.9/sec to 1.6/sec) and of tropomyosin-actin and S1 (0.64 to 0.52.sec). To correct for these effects when studying the complete system (troponintropomyosin-actin and S1), we increased all rates in the presence of bepridil by a factor of 1.23. Figure 7 shows the effect of bepridil on troponin-tropomyosin-actin-activated ATPase activity of myosin S1 in solution. Bepridil had a stimulatory effect at low free calcium concentrations, but as the calcium concentration was increased this became a mild inhibitory effect. The lack of a stimulatory effect at high calcium saturation has been observed in muscle fibers.10 Our observation that bepridil effects the troponin complex in the absence of calcium is in contrast to the general view that calcium is required for bepridil binding to TnC.3 For example, bepridil did not alter isolated TnC-felodipine fluorescence except in the presence of calcium.10 Furthermore, bepridil produced only small effects on the NMR spectrum of isolated TnC in the absence of calcium.11 The authors of that study concluded that bepridil binding to isolated TnC is weaker in the absence of calcium. Our experimental system differs from those earlier studies in that we used the complete S1-actin-tropomyosin-troponin complex. Furthermore we used high concentrations of bepridil that would permit low affinity interactions. The ability of bepridil to stimulate activity at low calcium concentrations and to inhibit activity at high calcium concentrations is reminiscent of the effects of some mutations of troponin I that activate the intermediate state of the regulatory complex.6 It is possible that bepridil stabilizes the intermediate state more than the active state of regulated actin. Any drug designed for treating disorders of the troponin complex should be screened for effects at both high and low free concentrations as changes in the distribution of regulated states can be deleterious to cardiac function.
Figure 7.
Bepridil enhances ATPase activity at low free calcium concentrations. The ratio of rate of ATP hydrolysis (with bepridil/without bepridil) is shown with standard deviations as a function of the pCa. The bepridil concentration is 125 μM with 1% ethanol as a carrier. Conditions: 25° C with 0.1 μM S1, 20 μM actin, 5.7 μM tropomyosin and 5.7 μM troponin. The reaction buffer contained 1 mM ATP, 3 mM MgCl2, 20 mM MOPS pH 7, 1mM dithiothreitol and sufficient CaCl2 and EGTA to reach the target pCa. The ionic strength was adjusted to 62 mM by the addition of KCl. Values in the presence of bepridil were multiplied by 1.23 to correct for inhibition of actin-tropomyosin stimulated ATPase activity by bepridil.
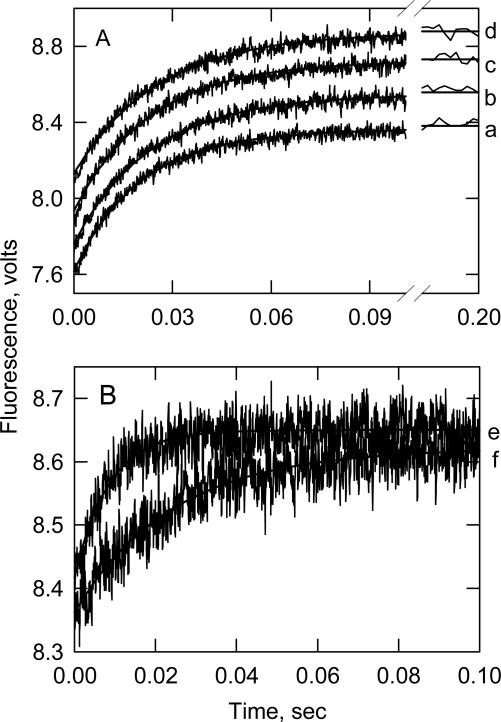
We tested the prediction that bepridil has little effect on calcium binding to the intact troponin (SYS 4) by measuring the rate of calcium detachment at different bepridil concentrations. Detachment rates should decrease with increasing bepridil concentrations if bepridil strengthens calcium binding. That is, the calcium release rate is inversely related to the calcium affinity in TnC.11 Figure 8 shows time courses of calcium detachment from the calcium specific sites of human cardiac troponin. The transients for intact troponin were not affected to a great extent by bepridil while a large difference was seen in the case of the TnI-TnC complex (Figure 8B).
Figure 8.
Time courses of calcium release from intact cardiac troponin (A) and from the TnI-TnC complex (B). Ca2+ dissociation was followed by the increase in Quin 2 fluorescence.A. Calcium release from whole troponin was measured in the absence of bepridil (a) or in the presence of 62.5 (b), 125 (c) and 250 (d) μM bepridil. B. Calcium release from the TnI-TnC complex was measured in the absence (e) or presence of 250 μM bepridil (f). Conditions 3.5 μM troponin or 2.5 μM TnI-TnC, 15 μM CaCl2, 100 mM KCl, 20 mM MOPS pH 7, 1 mM dithiothreitol, 60 μM Quin 2, 20° C. The time scale for data collection was chosen to observe Ca2+ release from the low affinity regulatory site.
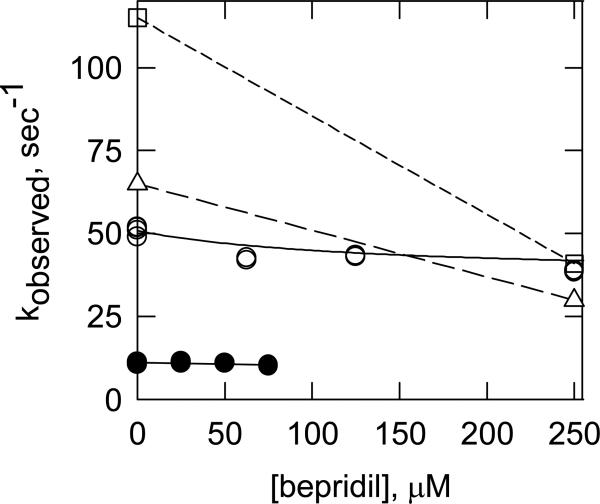
The bepridil concentration dependencies of the calcium dissociation rates for different troponin complexes are shown in Figure 9. The largest effect of bepridil was seen on the human cardiac TnI-TnC complex at 20 °C (open squares). Bepridil had a 2-fold effect on the TnC complex alone (bovine shown here) in agreement with published reports.9,42 The human troponin complex was only slightly affected by bepridil at 20 °C while no effect was seen with bovine cardiac troponin at 4° C. These experimental results are consistent with the predictions of the MD simulations of Table 3.
Figure 9.
Bepridil has little effect on the rate of Ca2+ dissociation from whole troponin. Calcium dissociation was measured by the increase in Quin 2 fluorescence as shown in Fig. 8. Dissociation was measured from the TnI-TnC complex at 20 °C (squares), TnC alone at 20 °C (triangles), whole troponin at 20° C (open circles) and whole troponin at 4° C (solid circles).
V. Conclusions
The computational and experimental approaches in this work suggest that bepridil is not a simple calcium sensitizer. Bepridil enhances the calcium binding affinity for the isolated TnC but not for the intact troponin complex. Bepridil increases the calcium sensitivity in the isolated TnC by changing the calcium coordination; but in the whole troponin complex, bepridil changes the correlation of the calcium-binding sites by weakening the positive correlation of the calcium-binding sites in the same domain and enhancing the negative correlation of calcium-binding sites at different domains. Bepridil binding decreases the TnC-TnI interaction at both N- and C-domain of TnC. Predicted calcium binding affinities were verified by the measured Ca2+-detachment constants. The Ca2+-detachment constants decrease with the increasing of bepridil concentration in the isolated TnC, but was little affected in the whole complex at various bepridil concentrations. Bepridil has a mild inhibitory effect on ATPase activity at high calcium concentration. The results show that molecular dynamics simulations are useful for examining bepridil induced changes in troponin. As changes in the structure of troponin in different states of activity become defined the utility of molecular dynamics simulations for predicting drug efficacy will increase.
Acknowledgment
This work is supported by grants from the National Institution of Health R01AR044504 to J.M.C. and a Shared Resources Grant from the Brody School of Medicine to J.M.C. and Y.L.
Abbreviations
- MOPS
3-(N-morpholino)-propane sulfonic acid
- S1
myosin subfragment 1
- EGTA
ethylene glycos-bis(2-aminoethyl ether)-N,N,N’N’-tetraacetic acid
- cTn
cardiac Troponin
- cTnC
cardiac Troponin C
- TnI
Troponin I
- TnT
Troponin T
References
- 1.Kobayashi T, Solaro RJ. Annu. Rev. Physiol. 2005;67:39–67. doi: 10.1146/annurev.physiol.67.040403.114025. [DOI] [PubMed] [Google Scholar]
- 2.Haselgro JC. Cold Spring Harbor Symposia on Quantitative Biology. 1973;37:341–352. [Google Scholar]
- 3.Li MX, Wang X, Sykes BD. Journal of Muscle Res. and Cell Mot. 2004;25:559–579. doi: 10.1007/s10974-004-5879-2. [DOI] [PubMed] [Google Scholar]
- 4.Chalovich JM, Eisenberg E. Journal of Biol. Chem. 1982;257:2432–2437. [PMC free article] [PubMed] [Google Scholar]
- 5.Gomes A, Potter J. Ann. N.Y. Acad. Sci. 2004;1015:214–224. doi: 10.1196/annals.1302.018. [DOI] [PubMed] [Google Scholar]
- 6.Mathur MC, Kobayashi T, Chalovich JM. Biophys J. 2009;96:2237–2244. doi: 10.1016/j.bpj.2008.12.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gafurov B, Fredricksen S, Cai A, Brenner B, Chase PB, Chalovich JM. Biochemistry. 2004;43:15276–15285. doi: 10.1021/bi048646h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burhop J, Rosol M, Craig R, Tobacman LS, Lehman WJ. Biol. Chem. 2001;276:20788–20794. doi: 10.1074/jbc.M101110200. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Love ML, Putkey JA, Cohen C. PNAS. 2000;97:5140–5145. doi: 10.1073/pnas.090098997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solaro RJ, Bousquet P, Johnson JD. J. Pharmocol. Experim. Therap. 1986;238:502–507. [PubMed] [Google Scholar]
- 11.MacLachlan LK, Reid DG, Mitchell RC, Salter CJ, Smith SJ. J. Biol. Chem. 1990;265:9764–9770. [PubMed] [Google Scholar]
- 12.Wang X, Li MX, Sykes BD. J. of Biol. Chem. 2002;277:31124–31133. doi: 10.1074/jbc.M203896200. [DOI] [PubMed] [Google Scholar]
- 13.Takeda S, Yamashita A, Maeda Y. Nature. 2003;424:35–41. doi: 10.1038/nature01780. [DOI] [PubMed] [Google Scholar]
- 14.Baker D, Sali A. Science. 2001;294:93–96. doi: 10.1126/science.1065659. [DOI] [PubMed] [Google Scholar]
- 15.Varughese JF, Chalovich JM, Li Y. J. Biomol. Str. Dyn. 2010;28:159–174. doi: 10.1080/07391102.2010.10507350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Case DA, Cheatham TE, Darden T, Gohlke H, Luo R, Merz KM, et al. J Comp. Chem. 2005;26:1668–1688. doi: 10.1002/jcc.20290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim K, Jordan KD. J. Phys. Chem. 1994;98:10089–10094. [Google Scholar]
- 18.Toukmaji A, Sagui C, Board J, Darden T. J. Chem. Phys. 2000;113:10913–10927. [Google Scholar]
- 19.Essmann U, Perera L, Berkowitz ML, Darden T, Lee H, Pedersen LG. J. Chem. Phys. 1995;103:8577–8593. [Google Scholar]
- 20.Crowley MF, Darden TA, Cheatham III TE, Deerfield II DW. J. Supercomputing. 1997;11:255–278. [Google Scholar]
- 21.Ryckaert JP, Ciccotti G, Berendsen HJC. J. Comp. Phys. 1977;23:327–341. [Google Scholar]
- 22.Wang J, Morin P, Wang W, Kollman PA. J. Amer. Chem. Soc. 2001;123:5221–5230. doi: 10.1021/ja003834q. [DOI] [PubMed] [Google Scholar]
- 23.Wang W, Kollman PA. J. Mol. Biol. 2000;303:567–582. doi: 10.1006/jmbi.2000.4057. [DOI] [PubMed] [Google Scholar]
- 24.Kollman PA, Massova I, Reyes C, Kuhn B, Huo S, Chong L, et al. Acc. Chem. Res. 2000;33:889–897. doi: 10.1021/ar000033j. [DOI] [PubMed] [Google Scholar]
- 25.Kielley WW, Harrington WF. Biochim. Biophys. Acta. 1960;41:401–21. doi: 10.1016/0006-3002(60)90037-8. [DOI] [PubMed] [Google Scholar]
- 26.Eisenberg E, Kielley WW. Cold Spring Harbor Symposia on Quantitative Biology. 1972;37:145–52. [Google Scholar]
- 27.Potter JD. Methods in Enzymology. 1982;85:241–63. doi: 10.1016/0076-6879(82)85024-6. [DOI] [PubMed] [Google Scholar]
- 28.Eisenberg E, Kielley WW. J. Biol. Chem. 1974;249:4742–48. [PubMed] [Google Scholar]
- 29.Gafurov B, Fredricksen S, Brenner B, Chase PB, Chalovich JM. Biochemistry. 2004;43(48):15276–85. doi: 10.1021/bi048646h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobayashi T, Solaro RJ. J. Biol. Chem. 2006;281(19):13471–77. doi: 10.1074/jbc.M509561200. [DOI] [PubMed] [Google Scholar]
- 31.Chalovich JM, Eisenberg E. J. Biol. Chem. 1982;257:2432–37. [PMC free article] [PubMed] [Google Scholar]
- 32.Kleerekooper Q, Putkey JA. J. Biol. Chem. 1999;274:23932–23939. doi: 10.1074/jbc.274.34.23932. [DOI] [PubMed] [Google Scholar]
- 33.Abusamhadneh E, Abott MB, Dvoretsky A, Finley N, Sasi S, Rosevear PR. FEBS Lett. 2001;506:51–54. doi: 10.1016/s0014-5793(01)02790-9. [DOI] [PubMed] [Google Scholar]
- 34.Potter JD, Gergely J. J. Biol. Chem. 1975;250(12):4628–4633. [PubMed] [Google Scholar]
- 35.Pirani A, Xu C, Hatch V, Craig R, Tobacman L, Lehman WJ. Mol. Bio. 2005;346:761–72. doi: 10.1016/j.jmb.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 36.Eisenberg E, Weihing RR. Nature. 1970;228(276):1092–93. doi: 10.1038/2281092a0. [DOI] [PubMed] [Google Scholar]
- 37.Bremel RD, Murray JM, Weber A. Cold Spring Harbor Symposia on Quantitative Biology. 1972;37:267–75. [Google Scholar]
- 38.Ichiye T, Karplus M. Prot Struct Funct Genet. 1991;11:205–217. doi: 10.1002/prot.340110305. [DOI] [PubMed] [Google Scholar]
- 39.Karplus M, Ichiye T. J Mol Biol. 1996;263:120–122. doi: 10.1006/jmbi.1996.0562. [DOI] [PubMed] [Google Scholar]
- 40.Teleman O, Drakenberg T, Forsén S, Thulin E. Eur. J. Biochem. 1983;134:453–457. doi: 10.1111/j.1432-1033.1983.tb07588.x. [DOI] [PubMed] [Google Scholar]
- 41.Iida S. J. Biochem. 1987;103:482–486. doi: 10.1093/oxfordjournals.jbchem.a122296. [DOI] [PubMed] [Google Scholar]
- 42.Smith SJ, England PJ. Br. J. Pharmacol. 1990;100(4):779–785. doi: 10.1111/j.1476-5381.1990.tb14092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]