Abstract
The use of hydrogels for bone regeneration has been limited due to their inherent low modulus to support cell adhesion and proliferation as well as their susceptibility to bacterial infections at the wound site. To overcome these limitations, we evaluated multifunctional polysaccharide hydrogels of varying stiffness to obtain the optimum stiffness at which the gels (1) induce proliferation of human dermal fibroblasts, human umbilical vascular endothelial cells (HUVECs), and murine preosteoblasts (MC3T3-E1), (2) induce osteoblast differentiation and mineralization, and (3) exhibit an antibacterial activity. Rheological studies demonstrated that the stiffness of hydrogels made of a polysaccharide blend of methylcellulose, chitosan, and agarose was increased by crosslinking the chitosan component to different extents with increasing amounts of genipin. The gelation time decreased (from 210 to 60 min) with increasing genipin concentrations. Proliferation of HUVECs decreased by 10.7 times with increasing gel stiffness, in contrast to fibroblasts and osteoblasts, where it increased with gel stiffness by 6.37 and 7.8 times, respectively. At day 14 up to day 24, osteoblast expression of differentiation markers—osteocalcin, osteopontin—and early mineralization marker—alkaline phosphatase, were significantly enhanced in the 0.5% (w/v) crosslinked gel, which also demonstrated enhanced mineralization by day 25. The antibacterial efficacy of the hydrogels decreased with the increasing degree of crosslinking as demonstrated by biofilm formation experiments, but gels crosslinked with 0.5% (w/v) genipin still demonstrated significant bacterial inhibition. Based on these results, gels crosslinked with 0.5% (w/v) genipin, where 33% of available groups on chitosan were crosslinked, exhibited a stiffness of 502±64.5 Pa and demonstrated the optimal characteristics to support bone regeneration.
Introduction
Hydrogels are a logical choice for nonload-bearing bone tissue-engineering applications, as they exhibit excellent biocompatibility and cause minimal inflammatory responses and tissue damage.1–4 Hydrogels have been mainly considered for soft tissue regeneration,5 but recent advances have allowed for the creation of multifunctional biomaterials with applications to hard tissue engineering.6 For bone tissue engineering, hydrogel scaffolds are able to present the seeded cells with mechanical, physical, and chemical cues similar in nature to those found in the early stages of fracture healing.7 Thus, hydrogels can recreate the complex interaction between cells and their microenvironments to regulate tissue morphogenesis and function. Despite these advantages, the use of hydrogels is associated with a number of limitations. This includes a limited ability to induce mineralization,8,9 potential for bacterial infection,2,10 dissimilarity to host tissue (together with poor cell proliferation and attachment),11 and a low mechanical modulus to support tissue regeneration.12,13 Another major impediment for using hydrogels in bone tissue engineering is poor or nonexistent growth and proliferation of endothelial cells in the newly forming tissue,14 which potentially can lead to delayed delivery of nutrients to mineralized bone and its cellular constituents.
Since bone is a highly mineralized tissue, the inability of hydrogels to induce mineralization is generally considered a major stumbling block in their application to bone tissue engineering. Osteoblast-mediated mineralization on hydrogels has been evaluated by the addition of osteoinductive growth factors, bone morphogenetic proteins, or mineral nucleation sites.5,15,16 Gaharwar et al. used chitosan to modify the material properties of PEO-silicate gels to tune cellular adhesion and control biomineralization on a cell–biomaterial interface.17 Many investigators have also evaluated cell-specific properties on gels made by different crosslinkers.18–20 The multifunctional gels created previously, as summarized by Leach et al.,21 depict the potential of current hydrogels in controlling cellular behavior, integration of engineered materials with host or transplanted tissue, and inductive factor presentation.
There have been limited studies highlighting the attachment and proliferation of osteoblast, fibroblast, and endothelial (human umbilical vascular endothelial cell [HUVEC]) cells on the same hydrogel, where stiffness has been modulated. This is important because vascularized bone regeneration at fracture sites requires all three of these cell types,22–24 and because these cell types favor substrates with different stiffness. Specifically, fibroblasts and osteoblasts exhibit better cell attachment and proliferation on substrates with higher stiffness than endothelial cells.25–27 Therefore, it becomes necessary to optimize scaffold stiffness to accommodate the substrate stiffness preference of these three cell types. Furthermore, since fracture sites and sites in the oral cavity are prone to infection,28 the incorporation of components that exhibit inherent antibacterial efficacy into these gels will enable lowering of infections at the site of application of these gels. In this study, we evaluate whether gel-based scaffolds with optimal storage modulus/stiffness can be created to fulfill a variety of functions required for the initial stages of a bone regeneration process to up to 3 weeks. These include the support for attachment of two cell types, proliferation of all three cell types, and differentiation and mineralization of osteoblasts, while preserving antibacterial efficacy.
Therefore, injectable polysaccharide hydrogel composite blends of varying stiffness were generated and evaluated for their utility as scaffolds for bone tissue engineering. Stiffness of the composite gels made of methylcellulose, agarose, and chitosan was varied by crosslinking the chitosan component with genipin. The effect of crosslinking on rheological properties and gelation times was characterized. Hydrogels with varying stiffness, induced by crosslinking, were tested to determine cell adhesion and proliferation (fibroblast, osteoblasts, and endothelial cells) as well as differentiation and mineralization (by osteoblasts). This enabled us to determine optimal gel characteristics for supporting bone tissue regeneration.
Methods and Materials
Creation of polysaccharide hydrogel blends
To make the hydrogel blends, 150 mg of low molecular weight chitosan (Sigma Aldrich) was dissolved in 4 mL of phosphate-buffered saline (Invitrogen) and 4 mL of 2.5% methacrylic acid (Sigma Aldrich). This mixture was heated at 70°C for 15 min, at which point, 225 mg of agarose (Sigma Aldrich) was added. Upon dissolution of these components, the mixture was placed on ice and 1.05 g of methylcellulose (Sigma Aldrich) was added to the mixture along with 8 mL of PBS. To obtain the 0.1%, 0.5%, and 1% by weight genipin blends, 17, 85, and 170 mg of genipin (Wako Chemicals) were added, respectively. All the gel components were sterilized using the ethylene oxide sterilization method (Anderson Anprolene Gas Sterilizer AN 74i). The preparation of the hydrogels with varying crosslinker concentrations is summarized in Table 1.
Table 1.
Components of Each of the Hydrogel Blend
| Gel component | Control (0% (w/v) Genipin) | 0.1% (w/v)Genipin | 0.5% (w/v)Genipin | 1% (w/v)Genipin |
|---|---|---|---|---|
| Methylcellulose | 1.05 g | 1.05 g | 1.05 g | 1.05 g |
| Agarose | 225 mg | 225 mg | 225 mg | 225 mg |
| Chitosan | 150 mg | 150 mg | 150 mg | 150 mg |
| Genipin | 0 mg | 17 mg | 85 mg | 170 mg |
Determination of degree of crosslinking
Ninhydrin assay as described by Solorio et al. was used to determine the degree of crosslinking,29 within 1 h after creation of the gels. The concentration of free amines in the hydrogels was determined by comparing the optical absorbance at 570 nm against the glycine standard curve using a Synergy H3 spectrophotometer (Biotek, Winooski, USA).
Rheological characterization
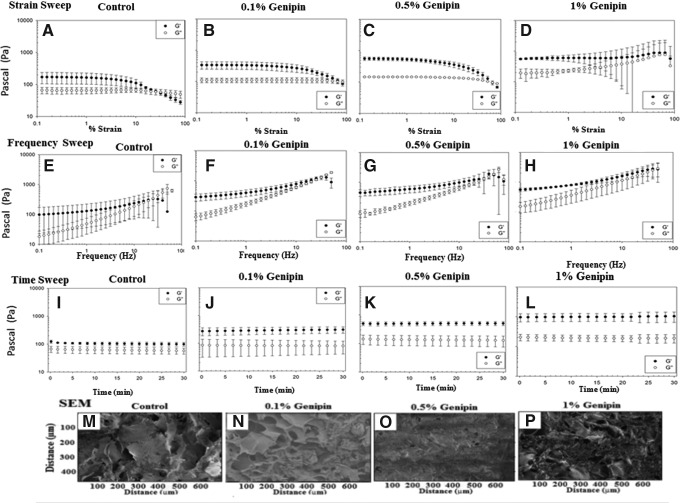
An AR-G2 rheometer (Texas Instruments) was used to perform three rheological tests on hydrogels crosslinked with varying amounts (0, 0.1, 0.5, and 1 wt.%). The test methods employed were oscillatory time, strain and frequency sweep, each performed at 37°C and a physiological pH of 7.4. For analysis, it is noted that it is important to determine the linear viscoelastic region (LVR) of the strain. Based on the data, the frequency for the hydrogel in the LVR was determined at a specific strain. Strain sweeps were conducted at a constant frequency of 1 Hz (Fig. 1A–D). The hydrogels were next subjected to a frequency sweep at a constant strain (1% strain) (Fig. 1E–H). A time sweep at constant frequency (0.5% Hz) and strain (1% strain) in the LVR was performed to monitor the in situ gelation behavior of the hydrogel dispersions (Fig. 1I–L), enabling monitoring of the elastic stored modulus G′ and viscous loss modulus G′′.
FIG. 1.
(A–L) Graphical representation of change in G′ and G′′ with increasing genipin concentrations. To determine the linear viscoelastic region of the hydrogel, strain sweep (A–D) and frequency sweep (E–H) were run on the gels. Based on the data from strain sweep and frequency sweep, a time sweep at 0.5 Hz and 1% strain was run to monitor the gel behavior. (M–P) Scanning electron micrographs of all the sample hydrogels. Due to the complex nature of the interaction of the hydrogel constituents and irregular surface morphology, the actual size of the pores could not be determined.
To obtain a gel of uniform thickness under the 15-mm parallel plate of the rheometer, the hydrogel blends were allowed to solidify and crosslink between its plates. Gels were poured and the temperature of the sample holder was maintained at 70°C for 1 h followed by an equilibration at 37°C for 10 min, before data for the time, frequency, and strain sweep were collected. To prevent drying of the sample, the annulus of the plate is filled with water and the sample holding region is sealed with a polycarbonate cover.
Dynamic rheometry is a powerful tool for monitoring microstructural changes in a material.30 This is because it allows properties to be probed in at rest conditions without microstructural disruption. The shear elastic (or storage) modulus, G′ is calculated using G′=σ0 cos ( )/γ0 and the loss modulus is calculated using G′′=σ0 sin (
)/γ0 and the loss modulus is calculated using G′′=σ0 sin ( )/γ0, where (σ0) is stress, (γ0) is the strain amplitude, and
)/γ0, where (σ0) is stress, (γ0) is the strain amplitude, and  is the phase angle between stress and strain.
is the phase angle between stress and strain.
Scanning electron microscopy
The sample hydrogels were freeze dried for 24 h in 15-mL centrifuge tubes in a dissection hood freeze dryer (Millirock Technology). All sample hydrogels were sputter coated with platinum before observation with SEM (Supra 55 Carl Zeiss).
Cell culture and tests
Osteoblasts, fibroblasts, and HUVECs were cultured on polystyrene T-75 tissue culture plastic flasks (Corning). The Dulbecco's modified Eagle's medium supplemented with 10% and 15% fetal bovine serum (Thermo Fisher Scientific) was used for osteoblasts and fibroblasts, respectively, with 1% penicillin–streptomycin (Invitrogen). HUVECs were cultured in the endothelial cell basal medium-2 supplemented with EGM-2 SingleQuots purchased from Lonza. Hydrogels were seeded with these cells 2 days after the hydrogel blend was prepared and were allowed to solidify at 37°C inside four well Lab-Tek chamber slides (Thermo Fisher Scientific) at a density of 5×105 cells. Please note that for imaging the cells, cell media were removed and the hydrogels were turned upside down. Cell attachment studies were conducted with fibroblasts and HUVECs by imaging at 24 h after cell culture. Cell proliferation assays were initiated at 7 days of postculture. Real-time PCR was performed at day 15 after initiation of cell culture. Xylenol orange was added to the cell culture media from day 15 to day 25 after initiation of cell culture. At day 25, cells were fixed and assayed for mineralization. Antimicrobial assays were conducted 24 h after culturing Streptococcus mutans on the hydrogels.
Cell attachment using lentiviral transfection of the cells with EGFP and imaging at 24 h
The fibroblasts (passage 4, ATCC PCS-201-012) and HUVECs (passage 5, ATCC CRL-1730) were transfected with EGFP (Enhanced Green Fluorescent Protein) lentivirus using the polybrene transfection agent (EMD Millipore Corporation) in accordance with the manufacturer's protocol. There was a difficulty in transfecting osteoblasts (MC3T3-E1, Subclone4, ATCC CRL-2593) with the EGFP lentivirus, and hence, the images have not been reported.
Cellular proliferation using cell titer blue florescence after 7 days of culture
The osteoblasts and fibroblasts were seeded at a density of 5×105 per well and HUVECs at 5×106 cells/well (culture area 1.9 cm2) in a 24-well plate in the wells of both the control and experimental hydrogel. After 7 days of culture, 200 μL of cell titer blue dye (Promega Biosciences) was added to each of these wells, and florescence was measured at the 24-h time point (after plating of cells on the hydrogel) using a plate reader (Synergy H4; Biotek) at excitation wavelength of 530 nm and emission wavelength of 590 nm.
Osteoblast mineralization and quantification at day 25
Mineralization-inducing media were made by adding 13.4 g of Dulbecco's modified Eagle's medium (Fisher Scientific) with 4.5 g/L glucose and L-glutamine was added to 1 L of autoclaved water and 2.2 g of sodium bicarbonate NaHCO3 (Fisher Scientific). The solution was stirred on a magnetic stirrer plate for 15 min. At the time of cell culture, 10% FBS and 1% penicillin/streptomycin were added to the media. To this solution, 3.06 g (10 mM) of β-glycerophosphate and 50 mg (50 μg/mL) of ascorbic acid were added to 1 L of media.
Xylenol orange tetrasodium salt (Sigma Aldrich) was used to monitor mineralized nodule formation in the osteoblast cell culture.31 This media was the same as the mineralizing media, but with the addition of 20 mM xylenol orange tetrasodium salt. Xylenol orange was added to the media starting at 15 days after initiation of cell culture until cells were fixed on day 25 to assay mineralization. The xylenol orange was excited at 440/570 nm and fluoresced at 610 nm, and was imaged using a Zeiss filter with excitation and emission band passes of 540–580 nm and 593–663 nm, respectively.
Quantification of mineralization
Mineralization of osteoblasts was quantified by two separate methods, first based on image thresholding of xylenol orange-labeled vital cultures using MATLAB (Mathworks), and second by atomic absorption spectroscopy (AAS). For the xylenol orange threshold, images of both phase and fluorescence (with Texas Red Filter Set) were taken in five adjacent regions of all hydrogels, and then stitched into a larger 8-bit image (4×, Nikon Ti-100). The phase channel was subtracted from the fluorescence, and a threshold taken that was set to half the level between the background and signal (−6dB). The number of pixels above the threshold were counted and used to express the percentage of mineralized area in each well. Note, the combination of phase and fluorescence allowed unbound xylenol orange (difficult to wash away without a wearing gel) to be distinguished, whereas the use of decibel levels allowed correction for the variation background levels in each image (genipin darkens the gel).
Mineralization was also quantified by atomic absorption with an atomic absorption spectrometer (AA-Perkin Elmer). Each well was prepared by adding 0.5 mL of 10% nitric acid and the resultant calcium content measured relative to a standard curve and compared between groups. Care was taken to minimize interference due to ionized calcium, precipitation of phosphate phases by adding a large excess of potassium and lanthanum ions.
Osteoblast differentiation using real-time PCR analysis on days 7, 15, and 24
Primers were designed from mRNA sequences available on the Pubmed Nucleotide database (Table 2). The specificities of primers were analyzed by BLAST (www.ncbi.nlm.nih.gov), and oligoanalyzer 3.1 (www.idtdna.com). qPCR amplifications were performed on Roche Lightcycler 480 (Roche, Indianapolis, Indiana, USA) using SYBR green master mix (Qiagen, Valencia, CA, USA). The RNA extraction and subsequent qPCR analysis were carried out on days 7, 15, and 24 postmineralization.
Table 2.
Primers for RT-PCR Analysis of MC3T3-E1 Cells
| Gene | Forward primer | Reverse primer |
|---|---|---|
| GAPDH | AACGACCCCTTCATTGAC | TCCACGACATACTCAGCAC |
| OPN | GATCAGGACAACAACGGAAAGG | CTTGTGGCTGTGAAACTTGTGG |
| OCN | AGGGAGGATCAAGTCCCG | GAACAGACTCCGGCGCTA |
| ALP | GTTGCCAAGCTGGGAAGAACAC | CCCACCCCGCTATTCCAAAC |
| BSP | TGTCTGCTGAAACCCGTTC | GGGGTCTTTAAGTACCGGC |
| OPG | CGAGGACCACAATGAACAAGTG | TTTTAGGTAGGTGCCAGGAGCA |
ALP, alkaline phosphatase; BSP, bone sialoprotein; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; OCN, osteocalcin; OPG, osteoprotegerin; OPN, osteopontin.
The osteogenic genes selected for qPCR indicate different cellular functions, but also allow the temporal differentiation of osteoblasts to be evaluated. Osteopontin (OPN) has a transient rise in expression on the onset of differentiation, but then rapidly recedes thereafter. Concurrently, osteocalcin (OCN) levels surge, which is why they are markers for differentiation of osteoblasts.32 Alkaline phosphatase (ALP) is one of the most widely used markers for early mineralization and late matrix maturation as it has a transient rise in expression after the onset of differentiation, and serves as a precursor to mineralization.33 Osteoprotegerin (OPG), which functions to decrease bone resorption,34 bone sialoprotein (BSP), and one housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were also evaluated.
Formation of S. mutans biofilms by crystal violet staining at 24 hours
An artificial saliva-like solution containing 10 mM of MgCl2 and 10 mM of CaCl2 was made in a solution of phosphate-buffered saline and was inoculated with S. mutans (ATCC 104495) culture at an optical density (OD) of 1.0 in a 12-well plate containing freeze dried composite gels. Gels were incubated with S. mutans for 24 h and lightly washed with double-distilled water to remove traces of any planktonic and loosely associated growth. After washing, gels were stained with 0.1% (w/v) crystal violet for 15 min and then rewashed. Gels were then soaked in 100% ethanol for 20 min to remove the crystal violet stain that attaches to the biofilm. The OD of the ethanol supernatant from each of the gels was measured using a plate reader at a wavelength of 575 nm and these readings were then compared to the standard curve for absorbance at 570 nm to determine the extent of biofilm formation. It is noted that crystal violet stains the extracellular matrix secreted by the bacteria35 and is an indication of the relative amounts of biofilm deposited on the different scaffolds. Note that there were no differences in crystal violet staining of the composite gels crosslinked to different extents, but not exposed to bacteria.
Hydrogel gelation time test
To determine the gelation time of the gels, an inverted tube test36 was performed (n=3/group). Each hydrogel blend (5 mL) was placed into 15-mL centrifuge tubes at 4°C and allowed to equilibrate to room temperature in 15 min. The gels were then placed into a 37°C hot plate and were assessed to determine if they solidified. The hydrogels were inverted and firmly shaken twice to determine if there was any adhesion of the gel to the sides of the tube. Once there was no movement of the hydrogels and no adhesion to the sides of the tube, the hydrogels were confirmed to be solidified.
ALP activity
The qunatative determination of ALP was carried out using the ALP detection flouresence kit (Sigma Aldrich). The cells cultured on the hydrogel scaffolds were incubated with p-nitrophenol phosphate as a substrate, and then washed with the PBS buffer and lysed with cell lytic reagents. Fluorescence was detected at 360 nm excitation and 440 nm emission. The ALP activity was normalized to the total protein measured with the Bio-Rad protein assay (BioRad, Hercules).
Statistical analysis
All experiments were performed with n=3/group and repeated thrice. Statistical analyses were performed using JMP IN software (release 8.0.1; SAS). A one-way ANOVA was run first to determine statistical differences between groups. For groups that showed differences in ANOVA, a post hoc Tukey–Kramer HSD test was used.
Results
Imaging of the hydrogel blends
SEM micrographs of the gels were taken to determine their microscopic structure (Fig. 1M–P). Due to the complex morphology and three-dimensional nature of the pores, the pore size cannot be determined with SEM micrographs. However, the addition of genipin appears to change the pore size of the hydrogels (Fig. 1M–P). There was a change in the swelling ratio observed (data not reported) with the changing genipin concentration, which corresponds to previously published results.37
Determination of degree of crosslinking and gelation time
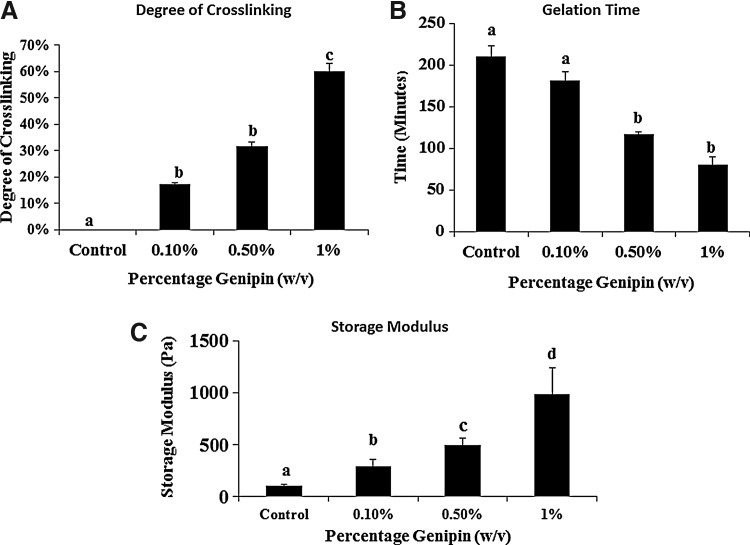
Polysaccharide hydrogel polymers crosslinked with different concentrations of genipin demonstrate different degrees of crosslinking as determined using the ninhydrin assay.29 Crosslinking with 1% (w/v) genipin (Fig. 2A) initiated ∼4 times faster gelation (Fig. 2B) as compared to the non-crosslinked control hydrogels. The degree of crosslinking increased to 60% with a 1% (w/v) genipin concentration in the hydrogel with respect to the control (0 % [w/v] genipin) hydrogel.
FIG. 2.
(A) Graphical representation of increasing degree of crosslinking with increasing genipin concentrations. The hydrogels were crosslinked to up to 60% with 1% (w/v) genipin concentrations. The hydrogel with 1% (w/v) genipin concentration was significantly different from control, 0.1% and 0.5% gel (p<0.05, n=3). (B) Gelation times increased with the control gel showing significantly higher (p<0.05, n=3) gelation time compared to crosslinked samples (C) Storage modulus was found to be significantly higher in all the groups with increasing genipin concentrations (p<0.05, n=3). Groups with different alphabetical characters are significantly different from each other at p<0.05.
Rheological characterization of hydrogel blends
The rheological analysis of the gels showed that the control gel had an average G′ value of 104.5±11.5 Pa, the 0.1% genipin gels had an average G′ of 291.4±69.3 Pa, the 0.5% genipin gels had an average G′ of 502±64.5 Pa, and the 1% genipin gel had an average G′ of 939.6±248.9 Pa, which was significantly higher (p<0.05, n=3) than all the other gels. Thus, there was a 3.5 times increase of the storage modulus in the hydrogel group with a 1% (w/v) genipin concentration (Fig. 2C). Hence, rheological characterization shows that increasing the amount of genipin increases the storage modulus of the hydrogels.
Cellular proliferation
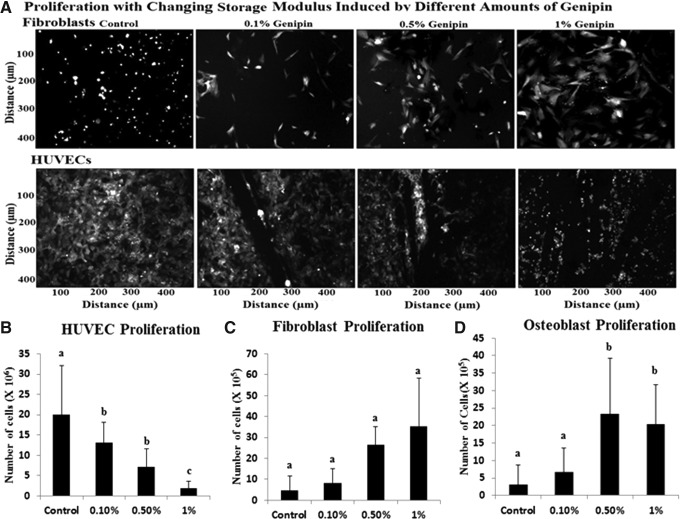
The proliferation of HUVECs, fibroblasts, and osteoblasts varied with stiffness of the gel. HUVEC proliferation (Fig. 3A Top panel and Fig. 3B) was greater on softer substrates by 10.7 times with the 0.1% and 0.5% (w/v) concentration of genipin demonstrating improved viability and adhesion. Fibroblast and osteoblast proliferation (Fig. 3A Bottom panel, Fig. 3C, D) increased with increasing stiffness gels by 7.8 and 6.4 times, respectively. It has been shown that osteoblasts and fibroblasts prefer stiffer surfaces for proliferation38–40 and according to the proliferation data obtained via the cell titer blue kit, we saw that the 0.5% (w/v) genipin crosslinked hydrogel group showed significantly higher rates of cellular proliferation (p<0.05, n=3) over 24 h for osteoblasts and fibroblasts.
FIG. 3.
(A) Cells that do not attach due to improper hydrogel stiffness do not spread out and thus appear smaller and round. A decrease in attachment and proliferation of HUVE cells is found to correspond to an increase in the storage modulus of the underlying gels. The opposite is found with fibroblasts in which, decreased attachment with increasing stiffness was demonstrated. (B–D) Proliferation of endothelial cells, human dermal fibroblasts, and MC3T3-E1 osteoblast cells on hydrogels with varying storage modulus at day 7. Groups with different alphabetical characters are significantly different from each other at p<0.05. HUVE, human umbilical vascular endothelial.
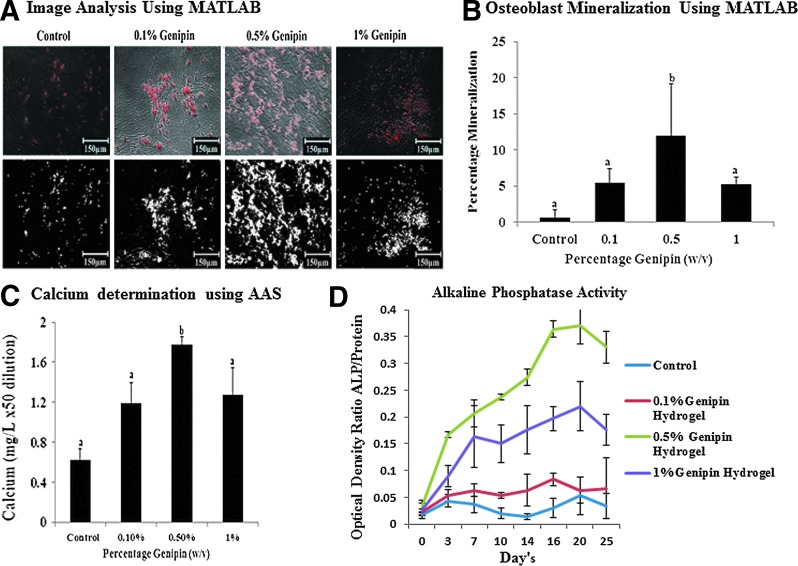
Osteoblast mineralization quantification using MATLAB and AAS
The mineralization results (Fig. 4A) showed that the hydrogel with 0.5% (w/v) genipin concentration demonstrated the maximum mineralization as compared to 0%, 0.1%, and 1% (w/v) genipin hydrogels. It was shown that the hydrogel group with a 0.5% genipin concentration and having a G′ at 502±64.5 Pa demonstrated maximum mineralization to up to 15% of the total area (2.0 cm2) of the osteoblasts growing at a confluence in a 24-well plate quantified using MATLAB image analysis (Fig. 4.B). AAS showed calcium concentrations of 1.78 mg/L dissolved in nitric acid at 50 times dilution (Fig. 4D) for osteoblasts growing on 0.5% (w/v) genipin hydrogel groups. This result is in agreement with the results for proliferation of cells on the hydrogels.
FIG. 4.
(A) Quantification of mineralization using a threshold on the fluorescence level in MATLAB (at 10×magnification). (B) Graphical representation of percentage mineralization. (C) Quantification of mineralization by atomic absorption spectroscopy groups with different alphabetical characters is significantly different from each other at p<0.05. (D) ALP detection assay for detecting the ALP activity of the cells cultured on the hydrogel scaffolds for 25 days. The ALP activity in total cell lysates were assayed and expressed as the ratio of fluorescence readings of ALP in cells growing on 0.1, 0.5, and 1 wt% hydrogels as compared to the total protein content isolated from the cells and quantified using Bio-Rad protein assay at n=3. ALP, alkaline phosphatase.
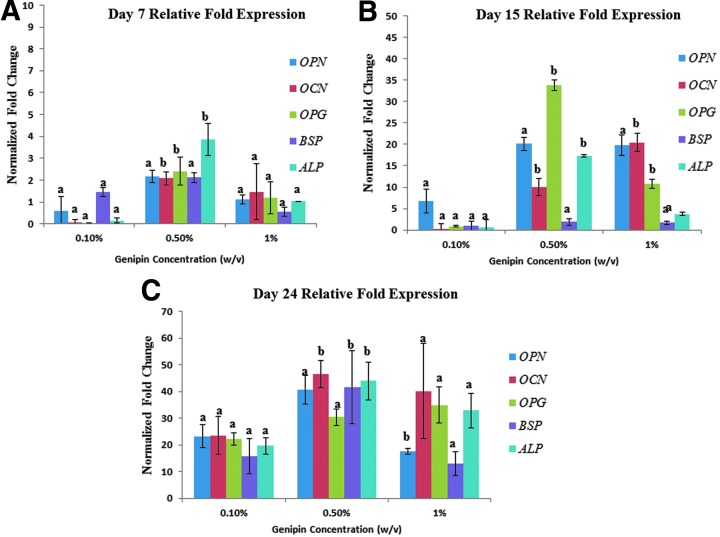
Quantitation of gene expression using qPCR
The temporal gene response indicating osteoblast differentiation was evaluated in OCN, OPN, BSP, ALP, and OPG. Of these proteins, OCN, OPN, and BSP are synthesized by osteoblasts involved in adhesion41 and, thus, are of special interest. For day 7 RT-PCR of osteogenic genes (Fig. 5A), highest upregulation was shown by cells grown on 0.5 wt% genipin hydrogel; there was no statistically significant upregulation in all the genes except for OCN, OPG, and ALP in all the genes. For day 15 RT-PCR of osteoblasts, OCN showed uniform upregulation with stiffness to up to 21 times from 0.1% to 1% (w/v) genipin concentration (p<0.05, n=3) compared to non-crosslinked control hydrogel. OPN did not upregulate significantly with increase in stiffness. OPG was found to be significantly higher in the 0.5% (w/v) hydrogel group showing up to ∼33-fold upregulation in expression relative to the control non-crosslinked hydrogels (Fig. 4C). ALP, which is an early marker for mineralization, was upregulated with increasing stiffness to up to 20-fold (p<0.05, n=3) on hydrogels with 0.5% (w/v) genipin concentration after 15 days of culture. At day 24, all the genes showed highest fold expression when compared to day 7 and day 15 cells with OCN, OPG, and ALP showing statistically significant upregulation in 0.5 % (w/v) genipin hydrogel when compared to osteoblasts grown on rest of the hydrogel groups.
FIG. 5.
As can been seen from the graphs, there is a significant upregulation of several osteogenic marker genes like OCN, OPN, and OPG (p<0.05) with increasing stiffness of the hydrogel scaffolds. (A) Relative fold expression of all the major genes is highest at 0.5% genipin concentration at day 7 with OCN and OPG showing two- to threefold increase relative to the control (p<0.05). (B) Relative fold expression of osteogenic markers for osteoblasts at day 15. ALP increased significantly with increasing stiffness to up to 20-fold (p<0.05, n=3) on hydrogel with 0.5% w/v crosslinker concentration after 15 days of culture. Osteocalcin showed a significantly uniform increase with stiffness to up to 21 times from 0.1% to 1% genipin concentration (p<0.05, n=3) and osteopontin showed highest expression in the 0.5% hydrogel group. Osteoprotegerin was found to be significantly higher in the 0.5% hydrogel group showing up to an ∼33-fold increase in expression relative to the control non-crosslinked hydrogels. (C) For day 24, all the genes were significantly upregulated compared to day 7 and day 15 expression levels of the genes. The 0.5% genipin concentration hydrogel showed significant upregulation of OCN to up to 40–50-fold, BSP to 40-fold, and ALP to 45-fold at p<0.05 compared to 0.1% and 1% genipin concentration hydrogels. Groups with different alphabetical characters are significantly different from each other at p<0.05.
ALP activity determination
The results of the ALP activity are shown in Figure 4D. There was no statistically significant difference in the ALP activity for osteoblast cells grown on hydrogel groups with genipin concentrations of 0, 0.1, and 1% (w/v). However, a significant difference in the ALP activity was seen for osteoblasts grown on hydrogels with 0.5 genipin concentration starting at day 8. The ALP activity of cells grown on 0.5 genipin concentration hydrogels was highest all throughout the 25-day period of cell culture.
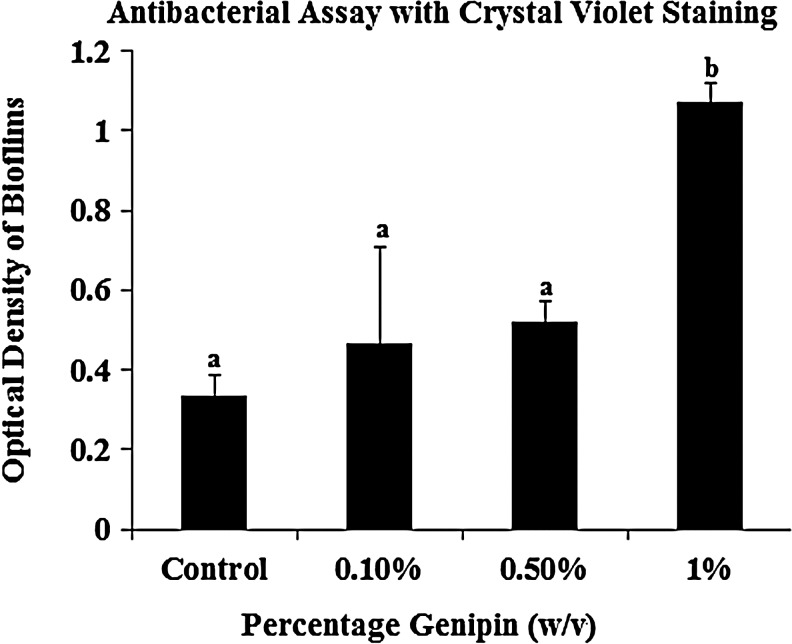
Antibacterial test results
Based on results obtained via spectrophotometry, the number of free amine groups (Fig. 2A) in the chitosan component of the hydrogel is inversely proportional to the amount of biofilm formation on the hydrogel surface. The control hydrogel, with the maximum amount of free amino groups, showed maximum antibacterial efficacy against S. mutans with spectrophotometric readings showing a significant decrease (p<0.05, n=3) in optical density from 1.5 to 0.33 of the crystal violet eluted from the gel after ethanol washing (Fig. 6). The spectrophotometric readings for 0.1%, 0.5%, and 1% (w/v) hydrogel of the eluted bioflims were 0.46, 0.522, and 1.06, respectively, indicating a lower antibacterial efficacy above a 0.5% (w/v) crosslinker concentration of the hydrogel.
FIG. 6.
Graphical representation of biofilm on gels with varying levels of crosslinked (amine) groups. Since amino groups on chitosan are responsible for the antibacterial effect of the hydrogels, crosslinking them using genipin would lead to a decrease in the antibacterial effect. It was seen that hydrogels with 1% (w/v) crosslinker concentrations had a significantly higher biofilm formation (therefore, a lesser antibacterial efficacy) compared to the non-crosslinked and crosslinked groups (p<0.05; n=3). Groups with different alphabetical characters are significantly different from each other at p<0.05.
Discussion
While hydrogel-based treatment for bone tissue engineering is promising, the hydrogel formulation that best facilitates bone regeneration in nonload-bearing areas42 can be improved. Some of the first hydrogel biomaterials employed for bone regeneration were synthetic biomaterials or some were a combination of synthetic and natural biomaterials. When applied within the bone defect, these synthetic or natural nonmodified hydrogels are generally not conducive to cellular influx. However, creating hydrogels with positively charged domains have improved the hydrogel ability to encourage cellular adhesion within in vitro models.43 Typically, synthetic hydrogels are not injectable and are not able to fill the irregular injury geometries of bone defects. Therefore, injectable strategies using polysaccharide-based hydrogels are being explored. Hydrogels fabricated from fibrin,44 fibronectin,45 or Matrigel46 have favorable characteristics of being injectable and having peptide domains that facilitate cellular attachment for bone tissue regeneration applications. However, these peptide-based hydrogels are slow to solidify and have the potential to efflux from the site of bone defect. In addition, cells are able to degrade biologically derived human protein hydrogels rapidly,47 and after degradation, there may not be a suitable matrix to facilitate osteoblast, fibroblast, and endothelial cell proliferation and adhesion.
The mechanical weakness of hydrogels is a limiting factor that restricts their use to nonload-bearing applications only.48 Mechanical properties of the hydrogels for bone tissue engineering are increased by combining the hydrogels with particles of ceramic materials, such as β-tricalcium phosphate, hydroxyapatite, demineralized bone matrix, or calcium carbonate.49 In this study, to obtain the required stiffness along with the mechanical properties, which mimic the ECM of the cell, crosslinking of positively charged hydrogels presents itself as a promising option.50 While a positive charge of the hydrogel would help the cells to adhere, the crosslinker would act as a reinforcing agent to grant sufficient mechanical stiffness to the gel to enable the cells growing on them to proliferate successfully. Rheological tests were performed on the gels to determine their mechanical properties. Strain sweeps were conducted on completely crosslinked hydrogels to determine the linear viscoelastic (LVE) region of a strain. The strain response was in the LVE region up to ∼3% strain for the control gel, 4% strain for 0.1% genipin, 2% strain for 0.5% genipin, and 2% strain for the 1% genipin gel (Fig. 1A–D). Large standard deviations are seen at strains higher than the LVE region of the 1% genipin gel; however, since this test is to determine the LVE region of each gel, these data are not used for the final time sweep. After conducting the strain sweeps, a strain of 1% was chosen for subsequent tests because it was safely in the LVE region of all hydrogels.
Frequency sweeps were also conducted on completely crosslinked hydrogels to determine the LVE region of frequency. The LVE response of all the hydrogels tested was very similar, and all the hydrogels tested had a LVE response up to 1 Hz (Fig. 1E–H). A frequency of 0.5 Hz was chosen for the subsequent time sweeps to ensure that the gels were in the LVE for every hydrogel tested. Time sweeps were conducted last to determine the storage modulus of each of the hydrogels. Since all the experiments in this study were conducted on completely crosslinked gels, time sweeps were conducted on crosslinked gels to determine the ultimate modulus and not gelation characteristics of the hydrogels. All time sweeps were conducted at 1% strain and 0.5 Hz so that the tests would be in the LVE region for both strain and frequency. Time sweeps were conducted for 30 min following complete gelation of the hydrogels. The gels show no significant changes in the modulus over this time (Fig. 1I–L), proving they have reached their ultimate storage modulus.
It is hypothesized that inclusion of genipin as the crosslinker would create stiffer hydrogels, which would effectively facilitate cellular adhesion and proliferation superior to non-crosslinked hydrogels. Within the study, the concentration of genipin, which is a naturally occurring crosslinker (while maintaining the same agarose, methylcellulose, and chitosan concentrations) was varied creating hydrogels with different storage moduli as determined using the shear rheometer. The addition of genipin to the blends leads to an increase in the opacity and darker blue color of the gels. This is observed because increasing genipin concentrations lead to increased crosslinking of primary amine groups through a nucleophilic attack of primary amine on C3 carbon of genipin in the presence of oxygen.51 The components of the gels used were previously chosen to enable better adhesion of cells to the gels. Methylcellulose, a major component of this hydrogel, is a hydrophilic polymer derived from cellulose.52 Chitosan is a biodegradable, nontoxic polysaccharide derived from chitin, a polymer of N-acetyl-D-glucose-2-amine.53,54 In this blend, agarose is used to sequester water from the methylcellulose to enable faster entanglement of methylcellulose chains.55 Crosslinking is used to enhance mechanical strength and chemical stability, to control aqueous permeability and solubility of the hydrogels, and to decrease the aqueous swelling features of chitosan-based materials.56–61 Crosslinking of the chitosan component with genipin enabled the creation of hydrogels with varying substrate stiffness, without changing the polysaccharide constituents. Results demonstrated that material properties (gel stiffness) can be leveraged to induce osteoblast differentiation in 2D culture as an alternative to biochemical cues such as soluble supplements, immobilized biomolecules and vectors, which are often expensive, labile and potentially carcinogenic.
Numerous studies have demonstrated the importance of substrate stiffness in cell function, with low stiffness gels promoting endothelial cell adhesion and growth (a requisite for neovascularization of constructs),62–64 and high stiffness gels promoting fibroblast and osteoblast attachment and differentiation (a requisite for mineralization).65 Fibroblasts migrate, proliferate, and differentiate into bone cells upon release of certain proteins (BMPs, insulin-like growth factors, transforming growth factors, and fibroblast growth factors), which enhance bone healing.66–69 Endothelial cells and osteoblasts are required for subsequent vascularization and mineralization, respectively. Endothelial cells have also been shown to enhance bone regeneration in an osseous defect.70 These processes are important because bone regeneration requires a coordinated cascade of events to enable the native cells to migrate and differentiate at the site of injury. It is widely accepted that physical properties of materials, such as topography, geometry, porosity, and stiffness, can be used to direct biological outcomes in a manner similar to traditional approaches involving chemistry or biomolecules.26,71–73 Previous studies with planar substrates (2D culture) have shown that stiffness of the underlying surface affects cellular behaviors such as proliferation, organization, migration, and differentiation.26,71,73 For previous work with osteoblasts in particular, proliferation of rat calvarial cells was enhanced on stiffer poly(N-isopropylacrylamide-co-acrylic acid) gels (2D) compared to softer gels.74 Organization, motility, and supplement-induced differentiation of MC3T3-E1 osteoblasts cultured on collagen-modified polyacrylamide gels and arginine-glycine-aspartate (RGD)-modified PEG hydrogels (2D) were influenced by matrix stiffness.75,76 Therefore, it is important to design gels of optimal stiffness that can promote growth and proliferation of these different cell types.77
Since these hydrogels are proposed for bone formation in maxillofacial and dental bone defects, there is a high chance of antimicrobial contamination in those sites. Chitosan has demonstrated a high antimicrobial activity against a wide variety of pathogenic microorganisms like S. mutans.78–82 S. mutans is widely known as an early colonizer that can lead to the formation of dental plaques83,84 and has been used for studies in antibacterial properties.85 It is an etiological agent of dental caries, colonizes the tooth surface, and forms biofilms. We quantified the antimicrobial activity of the hydrogels of different genipin concentrations against S. mutans growing under the conditions that favor biofilm formation using crystal violet staining.86,87 It is proposed that the interaction between the anionic cell surface of bacteria and the cationic amine group of chitosan weakens the cell membrane of the bacteria.88,89 As crosslinking is mediated through the amine groups, there are fewer amine groups in crosslinked chitosan, which may affect its antibacterial efficacy. Therefore, it is important to investigate the extent to which antibacterial effects of the composite gels were compromised by crosslinking. Our results suggest that the gels up to 0.5% (w/v) crosslinked gels demonstrate substantial antibacterial efficacy.
There are several limitations to this study. One is that the crosslinking of the chitosan component of the gel may affect other gel characteristics, such as its architecture, porosity, among others, which may be responsible for the observed differences in cell behavior. However, based on previous studies,68,90,91 where substrate stiffness was demonstrated to affect the attachment and proliferation of different cell types, we expect the change in substrate stiffness to be primarily responsible for cell proliferation, differentiation, and mineralization observed in our studies. Another limitation is related to complications involved in determining the calcium concentration using the MATLAB image analysis and AAS. First, in MATLAB, because of the varying colors of the gels due to different genipin concentrations, the threshold of the images, above which the pixels were counted to determine mineralization, had to be optimized. Second, in AAS, there were two important complications. First, calcium has a very low electronegativity and is thus readily oxidized. The absorbance spectrum of a calcium ion is not the same as atomic calcium. Atomic calcium absorbs strongly at 422.7 nm and calcium ions do not absorb light at 422.7 nm. Ionization of calcium therefore results in a significant decrease in sensitivity. The ionization of calcium can be suppressed by adding a large excess of a species that is more easily oxidized than calcium. Second, if phosphorus is present in the sample (some vitamins contain significant amounts of phosphorous-containing compounds), calcium can react with phosphorus in the flame to form calcium phosphate, which is a very stable compound. The formation of calcium phosphate prevents complete atomization of calcium. Phosphorus, therefore, interferes with the detection of calcium. The formation of calcium phosphate can be suppressed by introducing a species that reacts with phosphorus (or phosphate ions) more readily than does calcium. Lanthanum is a good species for this purpose. Therefore, all the hydrogel solutions in nitric acid contained large excess of potassium and lanthanum. For comparing real-time PCR data to mineralization data, the major apparent discrepancy is for OCN expression, between the 0.5% and 1% (w/v) genipin crosslinked gels. However, OCN could have peaked earlier in the 0.5% (w/v) gels. Alkaline phosphate, a marker that acts as a precursor to mineralization, tracks the observed mineralization profiles—being the highest in the 0.5% (w/v) genipin crosslinked scaffolds.
Conclusions
The work described in this article demonstrates the effect of rheological behavior of genipin crosslinked polysaccharide hydrogels on adhesion, proliferation, and gene expression in osteoblasts and proliferation and adhesion of fibroblasts and HUVEC cells, on gels formed at body temperatures and physiological pH values, to access their potential as injectable scaffolds for tissue engineering of bone. We demonstrated that polysaccharide hydrogel blends with 500–600 Pa stiffness obtained by using 0.5% (w/v) genipin resulted in optimal fibroblast, endothelial, and osteoblast proliferation and adhesion without the addition of cytokines, growth factors, or nucleation sites required for mineralization. The gel with 0.5% (w/v) genipin concentrations exhibited maximum osteoblast proliferation because of smoother topography compared to 0%, 0.1%, and 0.5% (w/v) hydrogels. The real-time PCR results demonstrated an upregulation in the osteogenic markers for proliferation (OPG) and differentiation (OPN and OCN) and an early marker for mineralization (ALP). These gains were obtained without compromising the inherent antibacterial efficacy of the hydrogel.
Disclosure Statement
No competing financial interests exist.
References
- 1.Benoit D.S.W., et al. Synthesis and characterization of a fluvastatin-releasing hydrogel delivery system to modulate hMSC differentiation and function for bone regeneration. Biomaterials. 2006;27:6102. doi: 10.1016/j.biomaterials.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 2.Lee K.Y. Alsberg E. Mooney D.J. Degradable and injectable poly (aldehyde guluronate) hydrogels for bone tissue engineering. J Biomed Mater Res. 2001;56:228. doi: 10.1002/1097-4636(200108)56:2<228::aid-jbm1089>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 3.Saito A., et al. Prolonged ectopic calcification induced by BMP‐2–derived synthetic peptide. J Biomed Mater Res Part A. 2004;70:115. doi: 10.1002/jbm.a.30071. [DOI] [PubMed] [Google Scholar]
- 4.Wang C., et al. The control of anchorage-dependent cell behavior within a hydrogel/microcarrier system in an osteogenic model. Biomaterials. 2009;30:2259. doi: 10.1016/j.biomaterials.2008.12.072. [DOI] [PubMed] [Google Scholar]
- 5.Gkioni K., et al. Mineralization of hydrogels for bone regeneration. Tissue Eng Part B Rev. 2010;16:577. doi: 10.1089/ten.TEB.2010.0462. [DOI] [PubMed] [Google Scholar]
- 6.Park J.B. The use of hydrogels in bone-tissue engineering. Med Oral. 2011;16:e115. doi: 10.4317/medoral.16.e115. [DOI] [PubMed] [Google Scholar]
- 7.Slaughter B.V., et al. Hydrogels in regenerative medicine. Adv Mater. 2009;21:3307. doi: 10.1002/adma.200802106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim J., et al. Potential of hydrogels based on poly (ethylene glycol) and sebacic acid as orthopedic tissue engineering scaffolds. Tissue Eng Part A. 2009;15:2299. doi: 10.1089/ten.tea.2008.0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nuttelman C.R., et al. The effect of ethylene glycol methacrylate phosphate in PEG hydrogels on mineralization and viability of encapsulated hMSCs. Biomaterials. 2006;27:1377. doi: 10.1016/j.biomaterials.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 10.Di Martino A. Sittinger M. Risbud M.V. Chitosan: a versatile biopolymer for orthopaedic tissue-engineering. Biomaterials. 2005;26:5983. doi: 10.1016/j.biomaterials.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 11.Molinaro G., et al. Biocompatibility of thermosensitive chitosan-based hydrogels: an in vivo experimental approach to injectable biomaterials. Biomaterials. 2002;23:2717. doi: 10.1016/s0142-9612(02)00004-2. [DOI] [PubMed] [Google Scholar]
- 12.Drury J.L. Mooney D.J. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 13.Li Z., et al. Chitosan–alginate hybrid scaffolds for bone tissue engineering. Biomaterials. 2005;26:3919. doi: 10.1016/j.biomaterials.2004.09.062. [DOI] [PubMed] [Google Scholar]
- 14.Rouwkema J. Boer J.D. Blitterswijk C.A.V. Endothelial cells assemble into a 3-dimensional prevascular network in a bone tissue engineering construct. Tissue Eng. 2006;12:2685. doi: 10.1089/ten.2006.12.2685. [DOI] [PubMed] [Google Scholar]
- 15.Burdick J.A., et al. Delivery of osteoinductive growth factors from degradable PEG hydrogels influences osteoblast differentiation and mineralization. J Controlled Release. 2002;83:53. doi: 10.1016/s0168-3659(02)00181-5. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto M. Tabata Y. Ikada Y. Ectopic bone formation induced by biodegradable hydrogels incorporating bone morphogenetic protein. J Biomater Sci Polym Ed. 1998;9:439. doi: 10.1163/156856298x00550. [DOI] [PubMed] [Google Scholar]
- 17.Gaharwar A.K., et al. Addition of chitosan to silicate cross-linked PEO for tuning osteoblast cell adhesion and mineralization. ACS Appl Mater Interfaces. 2010;21:22. doi: 10.1021/am100609t. [DOI] [PubMed] [Google Scholar]
- 18.Lee Y.M., et al. Tissue engineered bone formation using chitosan/tricalcium phosphate sponges. J Periodontol. 2000;71:410. doi: 10.1902/jop.2000.71.3.410. [DOI] [PubMed] [Google Scholar]
- 19.Shirosaki Y., et al. In vitro cytocompatibility of MG63 cells on chitosan-organosiloxane hybrid membranes. Biomaterials. 2005;26:485. doi: 10.1016/j.biomaterials.2004.02.056. [DOI] [PubMed] [Google Scholar]
- 20.Yu Y., et al. Novel injectable biodegradable glycol chitosan‐based hydrogels crosslinked by Michael‐type addition reaction with oligo (acryloyl carbonate)‐b‐poly (ethylene glycol)‐b‐oligo (acryloyl carbonate) copolymers. J Biomed Mater Res Part A. 2011;99:316. doi: 10.1002/jbm.a.33199. [DOI] [PubMed] [Google Scholar]
- 21.Leach J.K. Multifunctional cell-instructive materials for tissue regeneration. Regen Med. 2006;1:447. doi: 10.2217/17460751.1.4.447. [DOI] [PubMed] [Google Scholar]
- 22.Rutherford R.B., et al. Bone morphogenetic protein-transduced human fibroblasts convert to osteoblasts and form bone in vivo. Tissue Eng. 2002;8:441. doi: 10.1089/107632702760184709. [DOI] [PubMed] [Google Scholar]
- 23.Tabata Y., et al. Bone regeneration by basic fibroblast growth factor complexed with biodegradable hydrogels. Biomaterials. 1998;19:807. doi: 10.1016/s0142-9612(98)00233-6. [DOI] [PubMed] [Google Scholar]
- 24.Bouletreau P.J., et al. Hypoxia and VEGF up-regulate BMP-2 mRNA and protein expression in microvascular endothelial cells: implications for fracture healing. Plast Reconstr Surg. 2002;109:2384. doi: 10.1097/00006534-200206000-00033. [DOI] [PubMed] [Google Scholar]
- 25.Nemir S. West J.L. Synthetic materials in the study of cell response to substrate rigidity. Ann Biomed Eng. 2010;38:2. doi: 10.1007/s10439-009-9811-1. [DOI] [PubMed] [Google Scholar]
- 26.Engler A.J., et al. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 27.Schneider A., et al. Polyelectrolyte multilayers with a tunable Young's modulus: influence of film stiffness on cell adhesion. Langmuir. 2006;22:1193. doi: 10.1021/la0521802. [DOI] [PubMed] [Google Scholar]
- 28.Kannangara D.W. Thadepalli H. McQuirter J.L. Bacteriology and treatment of dental infections. Oral Surg Oral Med Oral Pathol. 1980;50:103. doi: 10.1016/0030-4220(80)90194-2. [DOI] [PubMed] [Google Scholar]
- 29.Solorio L., et al. Gelatin microspheres crosslinked with genipin for local delivery of growth factors. J Tissue Eng Regen Med. 2010;4:514. doi: 10.1002/term.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pai V. Srinivasarao M. Khan S.A. Evolution of microstructure and rheology in mixed polysaccharide systems. Macromolecules. 2002;35:1699. [Google Scholar]
- 31.Wang Y.H., et al. Examination of mineralized nodule formation in living osteoblastic cultures using fluorescent dyes. Biotechnol Prog. 2008;22:1697. doi: 10.1021/bp060274b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang W., et al. Signaling and transcriptional regulation in osteoblast commitment and differentiation. Front Biosci. 2007;12:92. doi: 10.2741/2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seibel M.J. Biochemical markers of bone turnover part I: biochemistry and variability. Clin Biochem Rev. 2005;26:97. [PMC free article] [PubMed] [Google Scholar]
- 34.Boyce B.F. Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 2008;473:139. doi: 10.1016/j.abb.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Westerlund B. Korhonen T. Bacterial proteins binding to the mammalian extracellular matrix. Mol Microbiol. 2006;9:687. doi: 10.1111/j.1365-2958.1993.tb01729.x. [DOI] [PubMed] [Google Scholar]
- 36.Zuidema J.M., et al. Fabrication and characterization of tunable polysaccharide hydrogel blends for neural repair. Acta Biomater. 2011;7:1634. doi: 10.1016/j.actbio.2010.11.039. [DOI] [PubMed] [Google Scholar]
- 37.Yan L.P., et al. Genipin‐cross‐linked collagen/chitosan biomimetic scaffolds for articular cartilage tissue engineering applications. J Biomed Mater Res Part A. 2010;95:465. doi: 10.1002/jbm.a.32869. [DOI] [PubMed] [Google Scholar]
- 38.Schweikl H., et al. Proliferation of osteoblasts and fibroblasts on model surfaces of varying roughness and surface chemistry. J Mater Sci Mater Med. 2007;18:1895. doi: 10.1007/s10856-007-3092-8. [DOI] [PubMed] [Google Scholar]
- 39.Baxter L., et al. Fibroblast, osteoblast adhesion, morphology on calcium phosphate surfaces. Eur Cell Mater. 2002;4:1. doi: 10.22203/ecm.v004a01. [DOI] [PubMed] [Google Scholar]
- 40.Kunzler T.P., et al. Systematic study of osteoblast and fibroblast response to roughness by means of surface-morphology gradients. Biomaterials. 2007;28:2175. doi: 10.1016/j.biomaterials.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 41.Anselme K. Osteoblast adhesion on biomaterials. Biomaterials. 2000;21:667. doi: 10.1016/s0142-9612(99)00242-2. [DOI] [PubMed] [Google Scholar]
- 42.Kong H.J. Smith M.K. Mooney D.J. Designing alginate hydrogels to maintain viability of immobilized cells. Biomaterials. 2003;24:4023. doi: 10.1016/s0142-9612(03)00295-3. [DOI] [PubMed] [Google Scholar]
- 43.Halstenberg S., et al. Biologically engineered protein-graft-poly (ethylene glycol) hydrogels: a cell adhesive and plasmin-degradable biosynthetic material for tissue repair. Biomacromolecules. 2002;3:710. doi: 10.1021/bm015629o. [DOI] [PubMed] [Google Scholar]
- 44.Yamada Y., et al. Bone regeneration following injection of mesenchymal stem cells and fibrin glue with a biodegradable scaffold. J Cranio-Maxillofac Surg. 2003;31:27. doi: 10.1016/s1010-5182(02)00143-9. [DOI] [PubMed] [Google Scholar]
- 45.Di Bella C. Farlie P. Penington A.J. Bone regeneration in a rabbit critical-sized skull defect using autologous adipose-derived cells. Tissue Eng Part A. 2008;14:483. doi: 10.1089/tea.2007.0137. [DOI] [PubMed] [Google Scholar]
- 46.Misawa H., et al. PuraMatrixTM facilitates bone regeneration in bone defects of calvaria in mice. Cell Transplantat. 2006;15:903. doi: 10.3727/000000006783981369. [DOI] [PubMed] [Google Scholar]
- 47.Sieminski A.L. Gooch K.J. Salmon fibrin supports an increased number of sprouts and decreased degradation while maintaining sprout length relative to human fibrin in an in vitro angiogenesis model. J Biomater Sci Polym Ed. 2004;15:237. doi: 10.1163/156856204322793610. [DOI] [PubMed] [Google Scholar]
- 48.Fedorovich N.E., et al. Hydrogels as extracellular matrices for skeletal tissue engineering: state-of-the-art and novel application in organ printing. Tissue Eng. 2007;13:1905. doi: 10.1089/ten.2006.0175. [DOI] [PubMed] [Google Scholar]
- 49.Tang Y., et al. A thermosensitive chitosan/poly (vinyl alcohol) hydrogel containing hydroxyapatite for protein delivery. J Biomed Mater Res Part A. 2009;91:953. doi: 10.1002/jbm.a.32240. [DOI] [PubMed] [Google Scholar]
- 50.Werner C. Pompe T. and Salchert, K. Modulating extracellular matrix at interfaces of polymeric materials. Polym Regen Med. 2006;203:63. [Google Scholar]
- 51.Butler M.F. Ng Y.F. Pudney P.D.A. Mechanism and kinetics of the crosslinking reaction between biopolymers containing primary amine groups and genipin. J Polym Science Part A Polym Chem. 2003;41:3941. [Google Scholar]
- 52.Stewart G. Wang Y. Niewiarowski S. Methylcellulose protects the ability of anchorage-dependent cells to adhere following isolation and holding in suspension. Biotechniques. 1995;19:598. [PubMed] [Google Scholar]
- 53.Ravi Kumar M.N.V. A review of chitin and chitosan applications. Reactive Funct Polym. 2000;46:1. [Google Scholar]
- 54.Zarzycki R. Modrzejewska Z. Use of chitosan in medicine and biomedical engineering. Polim Med. 2003;33:47. [PubMed] [Google Scholar]
- 55.Martin B.C. Agarose and methylcellulose hydrogel blends for nerve regeneration applications. J Neural Eng. 2008;5:221. doi: 10.1088/1741-2560/5/2/013. [DOI] [PubMed] [Google Scholar]
- 56.Francis Suh J.K. Matthew H.W.T. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: a review. Biomaterials. 2000;21:2589. doi: 10.1016/s0142-9612(00)00126-5. [DOI] [PubMed] [Google Scholar]
- 57.Mi F.L., et al. In vitro evaluation of a chitosan membrane cross-linked with genipin. J Biomater Sci Polym Ed. 2001;12:835. doi: 10.1163/156856201753113051. [DOI] [PubMed] [Google Scholar]
- 58.Jin J. Song M. Hourston D. Novel chitosan-based films cross-linked by genipin with improved physical properties. Biomacromolecules. 2004;5:162. doi: 10.1021/bm034286m. [DOI] [PubMed] [Google Scholar]
- 59.Moura M.J. Figueiredo M.M. Gil M.H. Rheological study of genipin cross-linked chitosan hydrogels. Biomacromolecules. 2007;8:3823. doi: 10.1021/bm700762w. [DOI] [PubMed] [Google Scholar]
- 60.Budtova T., et al. Chitosan modified by poly (ethylene oxide): Film and mixture properties. J Appl Polym Sci. 2002;84:1114. [Google Scholar]
- 61.Alexeev V.L., et al. Improvement of the mechanical properties of chitosan films by the addition of poly(ethylene oxide) Polym Eng Sci. 2002;40:1211. [Google Scholar]
- 62.Yamamura N., et al. Effects of the mechanical properties of collagen gel on the in vitro formation of microvessel networks by endothelial cells. Tissue Eng. 2007;13:1443. doi: 10.1089/ten.2006.0333. [DOI] [PubMed] [Google Scholar]
- 63.Thébaud N.B., et al. Human endothelial progenitor cell attachment to polysaccharide-based hydrogels: a pre-requisite for vascular tissue engineering. J Mater Sci Mater Med. 2007;18:339. doi: 10.1007/s10856-006-0698-1. [DOI] [PubMed] [Google Scholar]
- 64.Letourneur D., et al. Heparin and non‐heparin‐like dextrans differentially modulate endothelial cell proliferation: in vitro evaluation with soluble and crosslinked polysaccharide matrices. J Biomed Mater Res. 2002;60:94. doi: 10.1002/jbm.10072. [DOI] [PubMed] [Google Scholar]
- 65.Seidi A., et al. Gradient biomaterials for soft-to-hard interface tissue engineering. Acta Biomater. 2011;7:1441. doi: 10.1016/j.actbio.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 66.Saunders R.L. Hammer D.A. Assembly of human umbilical vein endothelial cells on compliant hydrogels. Cell Mol Bioeng. 2010;3:60. doi: 10.1007/s12195-010-0112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rowlands A.S. George P.A. Cooper-White J.J. Directing osteogenic and myogenic differentiation of MSCs: interplay of stiffness and adhesive ligand presentation. Am J Physiol Cell Physiol. 2008;295:C1037. doi: 10.1152/ajpcell.67.2008. [DOI] [PubMed] [Google Scholar]
- 68.Yeung T., et al. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton. 2004;60:24. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- 69.Kalfas I.H. Principles of bone healing. Neurosurg Focus. 2001;10:1. doi: 10.3171/foc.2001.10.4.2. [DOI] [PubMed] [Google Scholar]
- 70.Kaigler D., et al. Transplanted endothelial cells enhance orthotopic bone regeneration. J Dent Res. 2006;85:633. doi: 10.1177/154405910608500710. [DOI] [PubMed] [Google Scholar]
- 71.Mitragotri S. Lahann J. Physical approaches to biomaterial design. Nature Mater. 2009;8:15. doi: 10.1038/nmat2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dalby M.J., et al. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nature Mater. 2007;6:997. doi: 10.1038/nmat2013. [DOI] [PubMed] [Google Scholar]
- 73.Saha K., et al. Substrate modulus directs neural stem cell behavior. Biophys J. 2008;95:4426. doi: 10.1529/biophysj.108.132217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chung E.H., et al. Biomimetic artificial ECMs stimulate bone regeneration. J Biomed Mater Res Part A. 2006;79:815. doi: 10.1002/jbm.a.30809. [DOI] [PubMed] [Google Scholar]
- 75.Khatiwala C.B., et al. ECM compliance regulates osteogenesis by influencing MAPK signaling downstream of RhoA and ROCK. J Bone Miner Res. 2008;24:886. doi: 10.1359/JBMR.081240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hsiong S.X., et al. Differentiation stage alters matrix control of stem cells. J Biomed Mater Res Part A. 2007;85:145. doi: 10.1002/jbm.a.31521. [DOI] [PubMed] [Google Scholar]
- 77.Wells R.G. The role of matrix stiffness in regulating cell behavior. Hepatology. 2008;47:1394. doi: 10.1002/hep.22193. [DOI] [PubMed] [Google Scholar]
- 78.Wu H.D., et al. Chitosan-based polyelectrolyte complex scaffolds with antibacterial properties for treating dental bone defects. Mater Sci Eng C. 2012;32:207. [Google Scholar]
- 79.Zhao L., et al. Synthesis of antibacterial PVA/CM-chitosan blend hydrogels with electron beam irradiation. Carbohydr Polym. 2003;53:439. [Google Scholar]
- 80.Bae K., et al. Effect of water-soluble reduced chitosan on Streptococcus mutans, plaque regrowth and biofilm vitality. Clin Oral Invest. 2006;10:102. doi: 10.1007/s00784-006-0038-3. [DOI] [PubMed] [Google Scholar]
- 81.Tarsi R., et al. Inhibition of Streptococcus mutans adsorption to hydroxyapatite by low-molecular-weight chitosans. J Dent Res. 1997;76:665. doi: 10.1177/00220345970760020701. [DOI] [PubMed] [Google Scholar]
- 82.Sarasam A.R. Krishnaswamy R.K. Madihally S.V. Blending chitosan with polycaprolactone: effects on physicochemical and antibacterial properties. Biomacromolecules. 2006;7:1131. doi: 10.1021/bm050935d. [DOI] [PubMed] [Google Scholar]
- 83.Marsh P. Bradshaw D. Dental plaque as a biofilm. J Ind Microbiol Biotechnol. 1995;15:169. doi: 10.1007/BF01569822. [DOI] [PubMed] [Google Scholar]
- 84.Welin J., et al. Protein expression by Streptococcus mutans during initial stage of biofilm formation. Appl Environ Microbiol. 2004;70:3736. doi: 10.1128/AEM.70.6.3736-3741.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kreth J., et al. Competition and coexistence between Streptococcus mutans and Streptococcus sanguinis in the dental biofilm. J Bacteriol. 2005;187:7193. doi: 10.1128/JB.187.21.7193-7203.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Beckloff N., et al. Activity of an antimicrobial peptide mimetic against planktonic and biofilm cultures of oral pathogens. Antimicrob Agents Chemother. 2007;51:4125. doi: 10.1128/AAC.00208-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wen Z.T. Burne R.A. Functional genomics approach to identifying genes required for biofilm development by Streptococcus mutans. Appl Environ Microbiol. 2002;68:1196. doi: 10.1128/AEM.68.3.1196-1203.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kong M., et al. Antimicrobial properties of chitosan and mode of action: a state of the art review. Int J Food Microbiol. 2010;144:51. doi: 10.1016/j.ijfoodmicro.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 89.Roy S. Das P.K. Antibacterial hydrogels of amino acid‐based cationic amphiphiles. Biotechnol Bioeng. 2008;100:756. doi: 10.1002/bit.21803. [DOI] [PubMed] [Google Scholar]
- 90.Discher D.E. Janmey P. Wang Y.-l. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 91.Genes N.G., et al. Effect of substrate mechanics on chondrocyte adhesion to modified alginate surfaces. Arch Biochem Biophys. 2004;422:161. doi: 10.1016/j.abb.2003.11.023. [DOI] [PubMed] [Google Scholar]