Abstract
Induced pluripotent stem cells (iPSCs) hold great potential for cell therapy and tissue engineering. Neural crest stem cells (NCSCs) are multipotent that are capable of differentiating into mesenchymal lineages. In this study, we investigated whether iPSC-derived NCSCs (iPSC-NCSCs) have potential for tendon repair. Human iPSC-NCSCs were suspended in fibrin gel and transplanted into a rat patellar tendon window defect. At 4 weeks post-transplantation, macroscopical observation showed that the repair of iPSC-NCSC-treated tendons was superior to that of non-iPSC-NCSC-treated tendons. Histological and mechanical examinations revealed that iPSC-NCSCs treatment significantly enhanced tendon healing as indicated by the improvement in matrix synthesis and mechanical properties. Furthermore, transplanted iPSC-NCSCs produced fetal tendon-related matrix proteins, stem cell recruitment factors, and tenogenic differentiation factors, and accelerated the host endogenous repair process. This study demonstrates a potential strategy of employing iPSC-derived NCSCs for tendon tissue engineering.
Introduction
Tendon injuries are a common clinical problem in either workplace or sport.1,2 At present, treatments for injured tendon include autografts, allografts, xenografts, and prosthetic devices, with unsatisfactory effects and some inevitable disadvantages such as donor site morbidity, risk of disease transmission, and limited long-term function.3,4 Clearly, better alternative therapeutic methods need to be developed.
Currently, cell therapies and tissue engineering strategies offer potential approaches toward tendon repair. Many cell types have been used with or without scaffolds for tendon repair. Tenocytes is a common cell source for tendon repair.5–7 The disadvantage of using tenocytes is the need of harvesting autologous tendon tissue, which may not be practical for clinical therapies. Mesenchymal stem cells (MSCs) are a widely used cell source.8–11 These cells have great capacity for differentiating into mesenchymal lineages, including tenocytes. However, MSCs may express alkaline phosphatase activity and form ectopic bone in repaired tendon.12,13 Therefore, how to control the specific differentiation of MSCs into tendon-forming cells remains a great challenge. Recently, the longstanding dogma that the mammalian tendon is a postmitotic organ with limited regenerative reserve had been challenged by the discovery of stem cells isolated from tendon.14–16 Though tendon stem cells show healing promotion,17–20 the application of tendon stem cells for tendon repair needs further investigation.
An exciting breakthrough in stem cell biology is that adult somatic cells can be reprogrammed into induced pluripotent stem cells (iPSCs), which bypasses immune rejection issue, ethical concerns of using embryonic stem cells (ESCs), and limited expansion of adult somatic cells. These advantages of iPSCs make it a valuable cell source for cell therapies. Our previous studies demonstrated that human iPSC-derived neural crest stem cells (iPSC-NCSCs) could differentiate into mesenchymal lineages such as osteoblasts, chondrocytes, adipocytes, and myoblasts.21–23 Here, we hypothesize that iPSC-NCSCs can also differentiate into another mesenchymal lineage, tenocytes, and, subsequently, promote tendon repair. To test this hypothesis, a rat patellar tendon model was employed, in which a window defect was created for iPSC-NCSCs transplantation. This study aimed at investigating the effect of iPSC-NCSCs on tendon repair and at exploring their roles.
Materials and Methods
Cell culture and characterization
An iPSC line derived from human bone marrow MSCs (MSC-iPS1)24 was obtained from George Q. Daley's laboratory at Children's Hospital Boston. NCSCs were derived from MSC-iPS1 as previously described.21 Briefly, detached MSC-iPS1 were cultured as embryo body-like floating cell aggregates in ESC maintenance medium without basic fibroblast growth factor (bFGF) for 5 days. The cell aggregates were then allowed to adhere to the CellStart (Invitrogen)-coated dishes in StemPro® NSC serum-free medium (StemPro® NSC SFM; Invitrogen). After 7 more days, the colonies with rosette structures were harvested mechanically, cultured in suspension in StemPro NSC SFM for 1 week, replated onto the CellStart-coated dishes, and cultured for 3 more days. Cells were dissociated into single cells by TryplE Select (Invitrogen), cultured in StemPro NSC SFM as a monolayer, and further purified by cloning, subculture, and/or flow-activated cell sorting to obtain homogeneous populations that were positive for neural crest markers (termed passage 1; P1). The iPSCs-derived NCSCs were maintained in StemPro NSC SFM for expansion without differentiation, and passages 10–15 were used in this study.
To demonstrate mesenchymal lineages differentiation potential of iPSC-NCSCs, we carried out the protocol of NCSC differentiation into osteoblasts, adipocytes, and chondrocytes as described.23
Cell labeling and graft fabrication
To trace iPSC-NCSCs within the transplant site, cells were prestained with CM-DiI (C7000; Invitrogen). Briefly, when cells had reached 80% confluency, the CM-DiI was diluted in culture medium to achieve a final concentration of 10 μg/mL. Then, cells were incubated with CM-Dil/culture medium mixture for 30 min at 37°C. Afterward, CM-Dil-stained cells were washed twice with phosphate-buffered saline (PBS) and measured by fluorescence microscopy (BX51; Olympus). About 2.5×106 iPSC-NCSCs were suspended in 50 μL of 40 mg/mL fibrinogen (F8630; Sigma-Aldrich), and mixed with 50 μL of 50 U/mL thrombin (T4648, Sigma-Aldrich); then, they were quickly pipetted into a custom-made sterile trough (2 mm in width, 2 mm in depth, and 25 mm in length) that was placed inside a 60 mm Petri dish. After incubating with culture medium for 24 h, cells-fibrin constructs (2 mm in width, 2 mm in depth, and 4 mm in length) were then cut for transplantation, ensuring each implant contained about 5×105 cells.
Cell viability detection in fibrin gel
The quality of iPSC-NCSCs within the constructs was assessed by a live/dead assay after culturing for 24 h just before transplanting. In brief, the gel was incubated in culture medium containing 5 μM calcein (C3099; Invitrogen) for 30 min to stain live cells and 10 μg/mL propidium iodide (P2667; Sigma) for 10 min to stain dead cells. After washing twice with PBS, the green or red fluorescence was visualized using fluorescence microscopy (BX51; Olympus).
Rat patellar tendon repair model
Animal experiments were carried out according to the protocols approved by Chongqing University and Third Military Medical University Animal Care and Use Committee. Thirty adult female athymic rats (nude rats) (Vital River Laboratories) weighing 200–250 g were used in all experiments. Under general anesthesia, a patellar tendon window defect was created as previously described.25 Briefly, under sterile condition, after shaving the fur of both hindlimbs, skin and the soft tissue were dissected via a ventral longitudinal incision to expose patellar tendon; then, a standardized full-thickness window defect (1×4 mm, narrower than the width of the graft to make the graft fill the defect tightly) was created in the central part of the tendon without reaching the tendon-bone interface. In the experimental limb, the window defect was filled with fibrin gel with iPSC-NCSCs, while the contralateral limb was filled with fibrin gel alone to serve as control. After transplantation, synovium and skin of both hindlimbs were sutured. Rats were not immobilized postoperatively, being permitted to move freely within cages. At scheduled time, the rats were sacrificed with an overdose of sodium pentobarbital for macroscopical observation, histology analysis, mechanical testing, and gene expression analysis. Previous studies17,26 showed that biomechanical properties in the repaired tendon reach the peak at day 30 and plateau afterward in the tendon window defect model. In this study, therefore, we determined the effect of transplanted cells on tendon repair by structural and mechanical examinations, as well as the extracellular matrix (ECM) protein expression analysis at week 4. To elucidate the mechanism of repair at an earlier time point, we examined the secretion of paracrine factors at week 1. To track the long-term fate of transplanted cells and determine whether there was teratoma and ectopic bone/cartilage formation, we examined the samples at week 8. We also monitored the expression of human and rat genes at weeks 1, 2, and 4.
Macroscopical observation, histological examination, immunostaining, macroscopical scoring, and histological scoring
Rats were sacrificed, and both iPSC-NCSC-treated and non-iPSC-NCSC-treated tendons were exposed and macroscopically evaluated at 4 weeks postsurgery. For histological examination, tendon samples were dissected and fixed in 4% paraformaldehyde at 4°C for 24 h; then, they were transferred into 15% and 30% (w/v) sucrose solutions for 24 h, respectively. Longitudinal sections (5 μm in thickness) were cryosectioned for hematoxylin and eosin (HE) staining, Masson trichrome staining, and immunostaining.
To quantify the differences between the groups, three rats were evaluated by a macroscopical scoring system (Table 1). Then, the rats were subjected to histology analysis. Three HE and Masson trichrome stained samples in each group were evaluated by a histological scoring system (Table 2). Both scoring systems were modified from a previous study,27 and scoring was performed in a blinded fashion.
Table 1.
Macroscopical Scoring Systema
| Point | |
|---|---|
| Loading/lameness | |
| Hind leg fully loaded | 1 |
| Hind leg not fully loaded | 0 |
| Connection tendon to skin/slidablity | |
| Not conjoined, slidable | 1 |
| Adhesion, not fully slidable | 0 |
| Inflammation | |
| Non existing | 1 |
| Existing (edema, swelling, and redness) | 0 |
| Tendon surface at the defect area | |
| Intact, smooth | 1 |
| Uneven, harsh | 0 |
| Neighboring tendon | |
| Unchanged | 1 |
| Changed (color, thickness, and surface) | 0 |
| Level of the defect | |
| At the niveau of tendon surface | 1 |
| Hollow, under the level of tendon | 0 |
| Defect | |
| Diminished or not delimitable | 1 |
| Augmented or size about 4×1 mm | 0 |
| Swelling/redness of tendon | |
| No swelling/no redness | 2 |
| Palpable swelling, no redness | 1 |
| Palpable swelling with redness | 0 |
| Connection tendon/paratendineum and fascia, slidability | |
| Not adnated, slidable | 1 |
| Adhesion, not slidable | 0 |
| Shape of tendon | |
| Normal | 3 |
| Slightly thickened | 2 |
| Moderately thickened | 1 |
| Intensely thickened | 0 |
| Color of tendon | |
| Bright white | 1 |
| Translucent, dull white | 0 |
Modified from previous study.27
Table 2.
Histological Scoring Systema
| Point | |
|---|---|
| ECM organization of the whole tendon | |
| Wavy, compact, and parallel-arranged collagen fibers | 2 |
| In part compact, in part loose, or not orderly | 1 |
| Loosely composed, not orderly (“granulation” tissue) | 0 |
| Cellularity/cell-matrix ratio | |
| Physiological | 2 |
| Locally increased cell density | 1 |
| Increased cell density or decreased ECM content | 0 |
| Cell alignment | |
| Uniaxial | 2 |
| Areas of irregularly arranged cells (10–50%) | 1 |
| More than 50% of cells with no uniaxial alignment | 0 |
| Cell distribution | |
| Homogeneous, physiological | 1 |
| Focal areas of elevated cell density (cell clustering) | 0 |
| Cell nucleus morphology | |
| Predominantly elongated, heterochromatic cell nuclei (tenocytes) | 2 |
| 10–30% of the cells possess large, oval, euchromatic, or polymorph heterochromatic nuclei | 1 |
| Predominantly larger, oval, euchromatic, or polymorph, heterochromatic nuclei | 0 |
| Organization of repair tissue of the tendon callus | |
| Homogeneous (whole tissue with similar composition) | 2 |
| Locally heterogeneous tissue composition | 1 |
| Whole tissue composition completely changed | 0 |
| Transition from defect to normal tissue | |
| Gel integrated, no gaps at the margin visible | 2 |
| Recognizable transition | 1 |
| Abrupt transition, splitting/gaps detectable, and callus tissue | 0 |
| Vascularization in the defect area | |
| Hypovascularized, like surrounding tendon (small capillaries) | 1 |
| Hypervascularized (increased numbers of small or larger capillaries) | 0 |
| Inflammation | |
| No inflammatory cell infiltrates | 1 |
| Infiltrating inflammatory cell types (neutrophils, macrophages, and foreign-body/giant cell) | 0 |
Modified from previous study.27
ECM, extracellular matrix.
For immunostaining, antibodies against human nuclear mitotic apparatus protein (hNuMA; Abcam), bone morphogenetic protein 12 (BMP 12) (Santa Cruz), bFGF (Santa Cruz), Collagen I (Abcam), collagen III (Abcam), Vimentin (ZSGB-Bio), and Tenascin-C (Abcam) were used. Among these antibodies, only the hNuMA antibody was specific for human cells. The sections were incubated with the primary antibodies or non-immune antiserum (negative control) at 4°C overnight, then with AlexaFluor 488-conjugated secondary antibodies (Invitrogen) for 1 h, followed by DAPI (1 μg/mL; Roche) staining for 10 min at room temperature. The sections were washed twice in PBS with 0.25% Triton (10 min each) between each incubation step. All samples were finally sealed and imaged on a fluorescence microscope (BX51; Olympus).
Quantification of hNuMA+ cells at the repair site was done by counting the hNuMA+ cells and DAPI+ nuclear cells in 6 separate slides per animal (three animals from each time point at weeks 4 and 8). The results were presented as a percentage of hNuMA+ cells/DAPI+ cells per section. Microscope acquisition parameters were kept fixed for each channel in different sections.
Biomechanical testing
Biomechanical testing was performed according to previous studies17,28,29 at 4 weeks postsurgery. After animal euthanasia, all soft tissue spanning the knee, except for the central part of the patellar tendon, was sharply transected. Then, the width and thickness of the repaired tendon were measured by a Vernier caliper, and the cross-section area was calculated. The patella-central part of patellar tendon-tibia complex (PCTC) was then rigidly fixed to custom-made clamps. In subsequent procedures, the PCTC was kept moist by saline irrigation to avoid dehydration at ambient conditions (RH ∼70%). The samples were prepared in the same manner as described earlier for normal athymic rat tendons. Mechanical properties of both normal tendons (n=4) and repaired tendons (n=8 for each group) were determined by a tabletop uniaxial testing instrument (5567; Instron) using a 50 N load cell under a crosshead speed of 10 mm/min. The stress–strain curve, failure load, and Young's modulus were obtained.
RNA isolation, reverse transcription-polymerase chain reaction, and quantitative real-time PCR
Total RNA was isolated from the central repaired tendons using RNA Extraction Kit (RP1202; Bioteke). First-strand cDNA was synthesized using PrimeScript® RT Reagent Kit with gDNA Eraser (DRR047A; TaKaRa). Specific primers (Table 3) were used to detect gene expression. To avoid interspecies cross-reactivity, all primers were designed specific to human or rat genes, and validated by PCR and gel electrophoresis. For quantitative gene expression analysis, real-time PCR experiments were performed using Platinum® SYBR® Green qPCR SuperMix-UDG (C11733; Invitrogen) with Light Cycler apparatus (C1000 Touch; Bio-Rad). Three samples at weeks 1, 2, and 4 from each group were employed for gene analysis. Each sample tested at least three experimental replicates for expression of the gene of interest. The expression level of each target gene was then relative to H β-actin or R glyceraldehyde phosphate dehydrogenase (GAPDH) using 2−ΔΔCt method. The rat gene expression levels were then normalized to non-iPSC-NCSC-treated group at week 1. All commercial kits and reagents were used according to the instructions provided by the manufacturer.
Table 3.
Sequences, GenBank Accession Number, and PCR Product Size of Specific Primer Pairs Used for Gene Expression Analysis
| Gene name | GenBank accession number | Sequences (5′-3′) | PCR product size (bp) |
|---|---|---|---|
| H β-actina | NM_001101.3 | AGCGAGCATCCCCCAAAGTT | 285 |
| GGGCACGAAGGCTCATCATT | |||
| H Collagen XIV | NM_021110.1 | AAGGATTGCCCTCCGACTAC | 319 |
| GATGCGTTCATTGCCTTCTC | |||
| H Collagen III | NM_000090.3 | CCCCGTATTATGGAGATGAACC | 109 |
| CCATCAGGACTAATGAGGCTTTCT | |||
| H Tenascin-Ca | NM_002160.2 | TCTCTGCACATAGTGAAAAACAATACC | 112 |
| TCAAGGCAGTGGTGTCTGTGA | |||
| H Smad 8 | NM_001127217.2 | TGGTGCTCGGTCGCCTACT | 294 |
| TGGGTGGAAGCCGTGTTG | |||
| H SDF-1a | NM_199168.3 | TCTCCTGGGGATGTGTAATGG | 307 |
| AGGACCTTCTGTGGATCGCAT | |||
| H Fractalkinea | NM_002996.3 | GACTTTCCTCTTTGGTCTACAC | 758 |
| GTGACTTTATGCCTACAACTTC | |||
| H BMP 12 | NM_182828.2 | GCGCGGTGCTAGTCGTCTCC | 162 |
| GTCTGCGGCCGCCAATGACT | |||
| H TGF β3 | NM_003239.2 | CACTGCCGAGTGGCTGTCCT | 724 |
| TGTTTCCCGAGGAGCGGGC | |||
| H bFGF | NM_002006.4 | ACCTGCAGACTGCTTTTTGCCCA | 91 |
| GGTGCCACGTGAGAGCAGAGC | |||
| R GAPDHa | NM_017008.3 | GCAAGTTCAACGGCACAG | 141 |
| CGCCAGTAGACTCCACGAC | |||
| R Collagen Ia | NM_053304.1 | TGGATGGCTGCACGAGT | 177 |
| TTGGGATGGAGGGAGTTTA | |||
| R Collagen IIIa | NM_032085.1 | GCCTCCCAGAACATTACATAC | 259 |
| CAATGTCATAGGGTGCGATA | |||
| R Epha 4a | NM_001162411.1 | CTTATGGATAGTGGGCTGAGACAA | 183 |
| AAAACATTCAGAAGGAACCAAGGA | |||
| R Eya 2a | NM_130427.1 | ATTTGGGAGCGTGCACCAGGAT | 89 |
| GCGGGTTGTATGATGGGCTGAA |
Primers from previous study.28
H, human; R, rat; SDF-1, stromal cell-derived factor-1; BMP 12, bone morphogenetic protein 12; GAPDH, glyceraldehyde phosphate dehydrogenase; bFGF, basic fibroblast growth factor; TGF β3, transforming growth factor beta 3; PCR, polymerase chain reaction.
Statistical analysis
Quantitative data are expressed as means±standard deviations. Statistical analysis was performed by paired t test for comparisons between two groups and by one-way ANOVA, followed by Tukey HSD test for comparisons between more than two groups (OriginLab OriginV8.0 Software). Difference was considered significant when p<0.05.
Results
In vitro characterization of iPSC-NCSCs and establishment of rat patellar tendon repair model
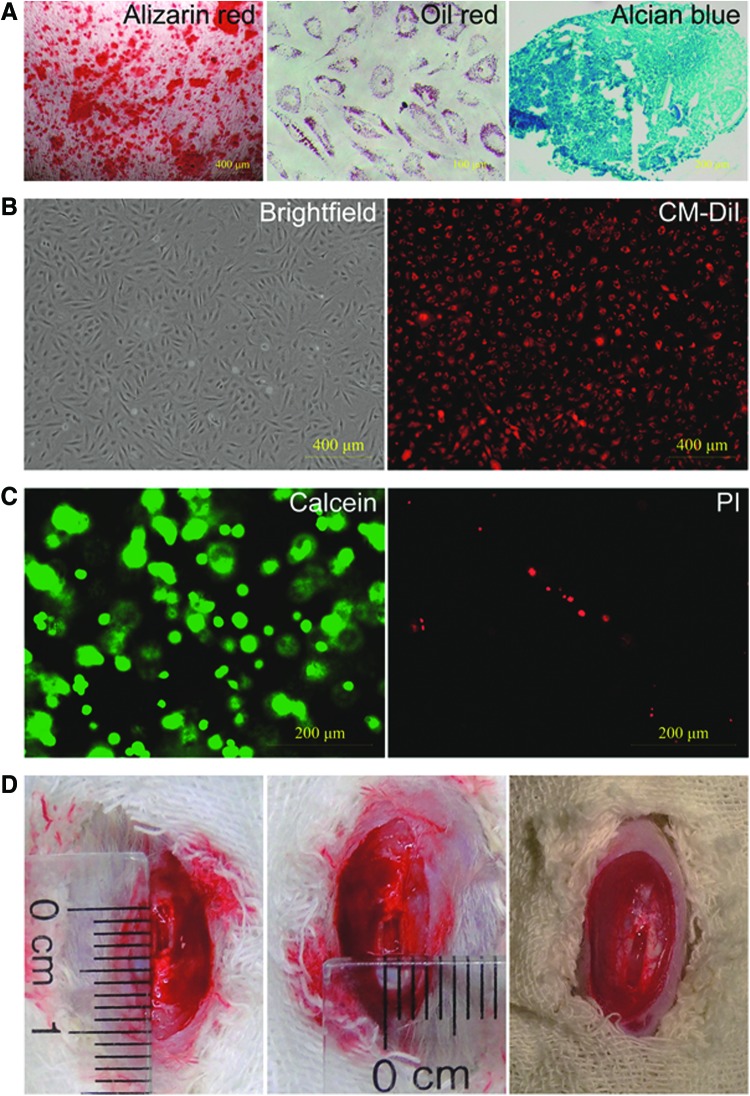
To verify the mesenchymal differentiation potential of iPSC-NCSCs, cells were cultured with specific induction medium. As shown in Figure 1A, iPSC-NCSCs differentiated into osteoblastic cells, adipogenic cells, and chondrogenic cells after being cultured with induction media for 3–4 weeks.
FIG. 1.
In vitro differentiation potential of iPSC-NCSCs and rat patellar tendon repair model. (A) Osteogenic differentiation shown by Alizarin red staining for calcified matrix; Adipogenic differentiation shown by Oil red staining for oil droplets; Chondrogenic differentiation shown by Alcian blue staining for glycosaminoglycans. (B) iPSC-NCSCs (brightfield) were prelabeled by CM-DiI (red) before transplantation. (C) Cell viability was tested by using live (calcein staining, green)/dead (PI staining, red) assay. (D) A 1×4 mm window defect was created in patellar tendon of athymic rat; the right picture shows the window defect filled with cell/fibrin gel complex after culturing in vitro by one day. PI, propidium iodide; iPSC-NCSCs, induced pluripotent stem cell-derived neural crest stem cells. Color images available online at www.liebertpub.com/tea
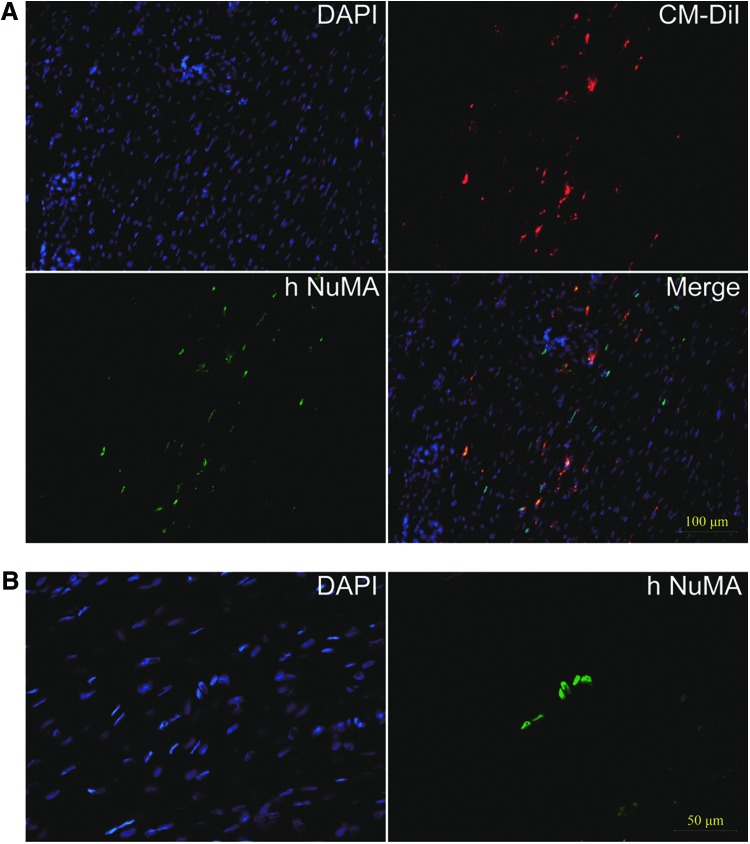
To trace the transplanted iPSC-NCSCs, cells were prelabeled by CM-DiI before being suspended in fibrin gel. Results showed that most cells can be labeled (Fig. 1B). Before surgery, a cell viability assay showed that >90% of the cells survived in the graft (Fig. 1C). Then, the cell/gel complex was transplanted into a 1 mm ×4 mm window defect within patellar tendon (Fig. 1D). In addition to tracing cells by CM-DiI, an antibody against hNuMA, which is specific to human cells, was employed to confirm the cells' survival at the transplant site. Results showed that about 9% of the repaired tendon cells were positive for hNuMA and colocalized with CM-DiI at 4 weeks after transplantation (Fig. 2A). Although the number of transplanted cells decreased greatly by time, about 2.67% of the repaired tendon cells still survived even until 8 weeks after transplantation (Fig. 2B).
FIG. 2.
Tracing of transplanted iPSC-NCSCs. (A) Tracing the transplanted iPSC-NCSCs at week 4 by CM-DiI (red) and hNuMA costaining (green). (B) hNuMA immunostaining (green) of survived iPSC-NCSCs within the window defect at 8 weeks post-transplantation. In all immunofluorescence images, nuclei were stained by DAPI (blue). hNuMA, human nuclear mitotic apparatus protein; DAPI, 4′,6-diamidino-2-phenylindole. Color images available online at www.liebertpub.com/tea
Macroscopical observation of repaired tendon
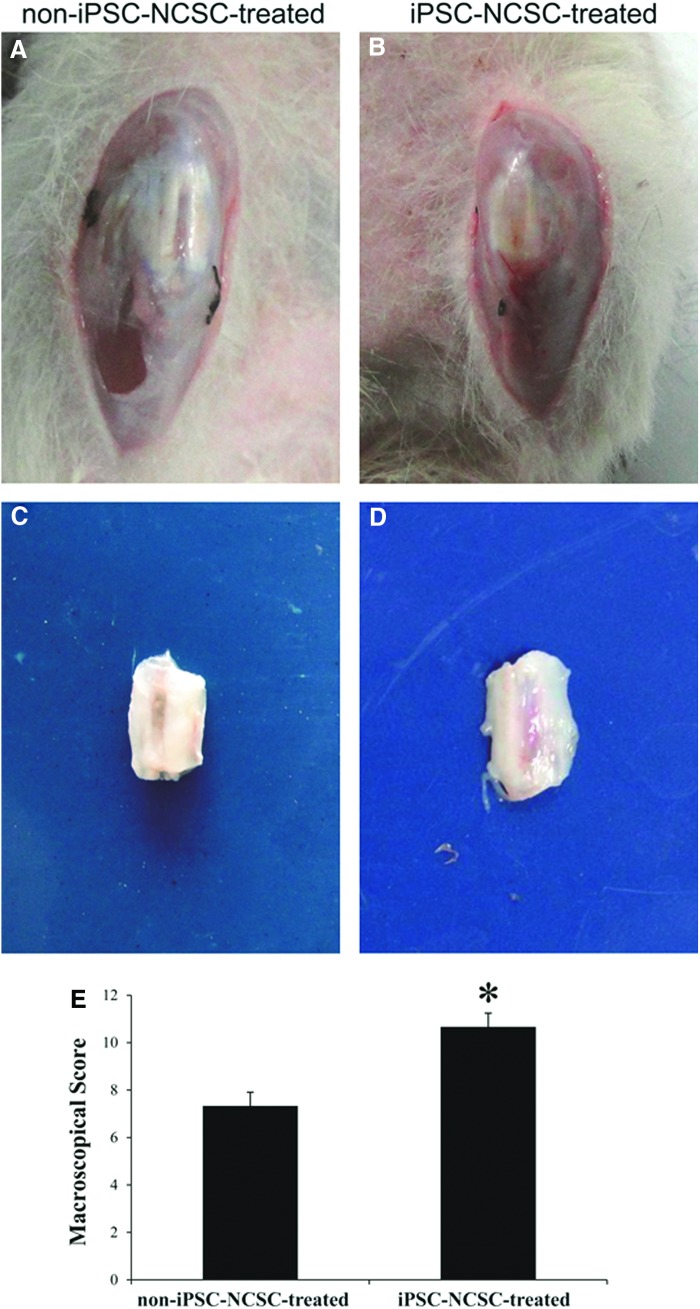
After surgery, all rats survived until scheduled euthanasia. No wound infections or any other complications were observed. A limping gait from both operative hindlimbs was observed during the first week postsurgery, and it then returned to normal since the second week.
At 4 weeks postsurgery, macroscopical morphology of the repaired tendons was observed. The window defect of repaired tendon was less visible in the iPSC-NCSC-treated group (Fig. 3B) compared with that in the non-iPSC-NCSC-treated group (Fig. 3A). After dissecting surrounding tissues, the repaired tendon looked glittering white in iPSC-NCSC-treated group (Fig. 3D), while it looked translucent, dull white in the non-iPSC-NCSC-treated group (Fig. 3C). The macroscopical score of the iPSC-NCSC-treated group was significantly higher than that of the non-iPSC-NCSC-treated group (Fig. 3E, 0.67±0.58 vs. 7.33±0.58, p<0.05).
FIG. 3.
Macroscopical morphology and macroscopical scores of repaired tendons. Macroscopical morphology of the knee (A, B) and the repaired patellar tendon (C, D) in non-iPSC-NCSC-treated group (A, C) and iPSC-NCSC-treated group (B, D) at 4 weeks postsurgery. (E) Macroscopical scores of repaired tendons. *p<0.05. n=3. Compared between the two groups. Color images available online at www.liebertpub.com/tea
Histological analysis of repaired tendon
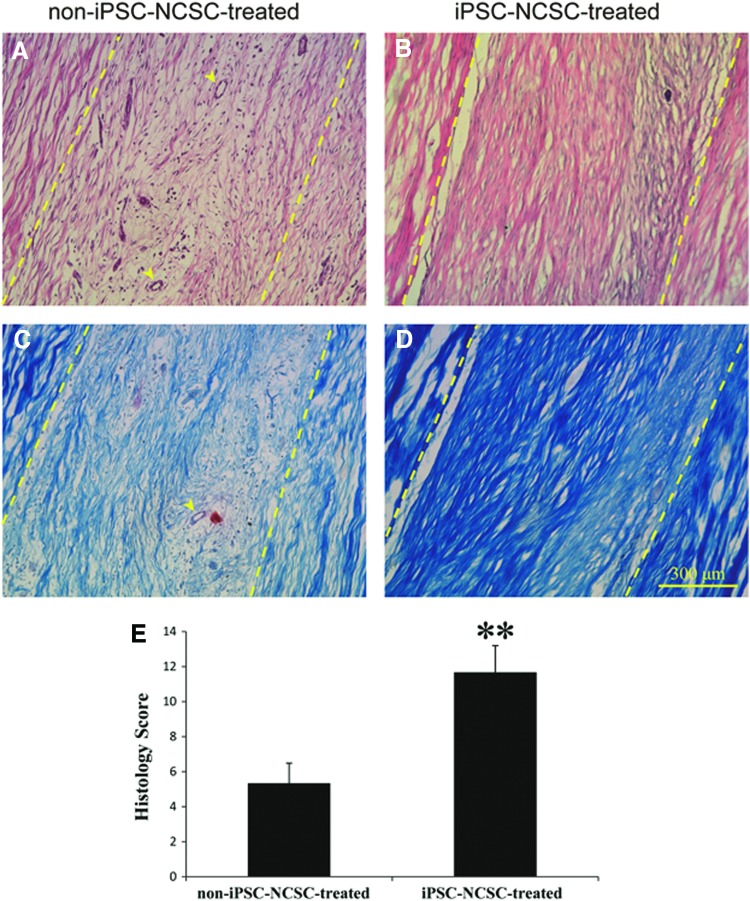
At 4 weeks postsurgery, HE staining of iPSC-NCSC-treated tendons showed denser connective tissue and more ECM deposition than that of non-iPSC-NCSC-treated tendons, with a greater number of cells exhibiting a spindle-shaped morphology aligned/organized along the longitudinal (tensile) axis of the tendon. By contrast, non-iPSC-NCSC-treated tendons exhibited minimal development of tendon-like morphology, remained poorly organized, and had much more vascularization (Fig. 4A, B). Masson trichrome staining showed that more collagen fibers were formed in iPSC-NCSC-treated tendons than that in non-iPSC-NCSC-treated tendons (Fig. 4C, D). The histology score also confirmed that the iPSC-NCSC-treated group showed better repair than the non-iPSC-NCSC-treated group (Fig. 4E, 1.67±1.53 vs. 5.33±1.15, p<0.01).
FIG. 4.
Histological analysis and histology scores of repaired tendons. Hematoxylin and eosin staining (A, B) and Masson thricome staining (C, D) of the repaired tendon in non-iPSC-NCSC-treated group (A, C) and iPSC-NCSC-treated group (B, D) at 4 weeks postsurgery. (E) Histology scores of repaired tendons. Arrowheads (A, C) show blood vessels. Dash lines (A–D) show the boundary of transplanted fibrin gel and host tendon. **p<0.01. n=3. Compared between the two groups. Color images available online at www.liebertpub.com/tea
Mechanical evaluation of repaired tendon
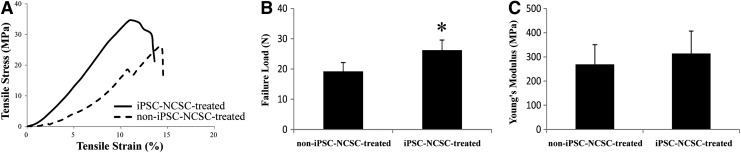
To further correlate tissue mechanical properties with their structural features, repaired tendons were harvested at 4 weeks postsurgery and subjected to mechanical testing (Fig. 5). All specimens failed at the midsubstance of the repaired tendon without avulsion from the tibial or patellar during testing. The failure load was significantly higher (p<0.05) in the iPSC-NCSC-treated group (26.21±3.34 N) compared with that in the non-iPSC-NCSC-treated group (19.20±2.92 N), which was 57.1% and 41.8% of normal rat tendon (45.90±1.41 N; p<0.05), respectively. The Young's modulus in the iPSC-NCSC-treated group (313.69±92.87 MPa) was also higher than that in the non-iPSC-NCSC-treated group (269.35±80.97 MPa); however, there was no significant difference between the two groups (p>0.05), and which was 49.7% and 42.7% of normal rat tendon (631.15±26.23 MPa; p<0.05), respectively.
FIG. 5.
The mechanical properties of repaired tendons at 4 weeks post-transplantation. (A) Representative stress-strain curves, respectively, for non-iPSC-NCSC-treated group and iPSC-NCSC-treated group. (B) Failure load. (C) Young's modulus. *p<0.05. n=8. Compared between the two groups.
The mechanism of iPSC-NCSC-promoted tendon repair
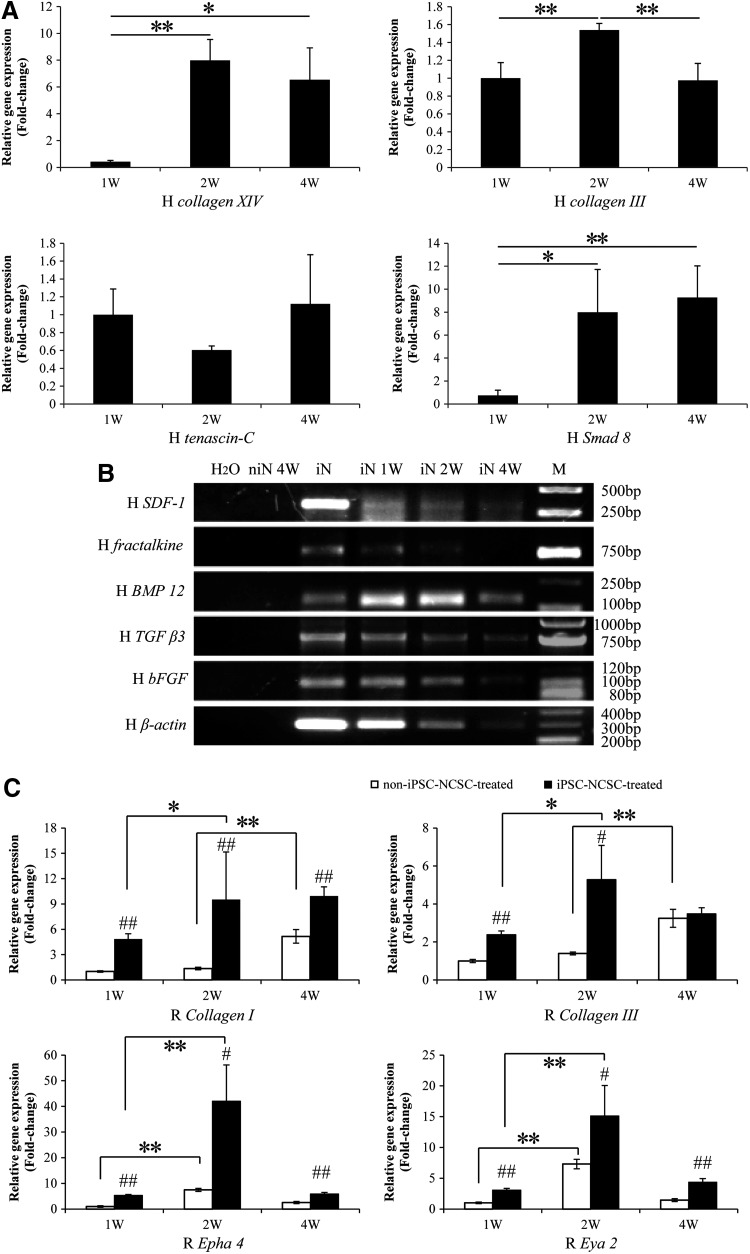
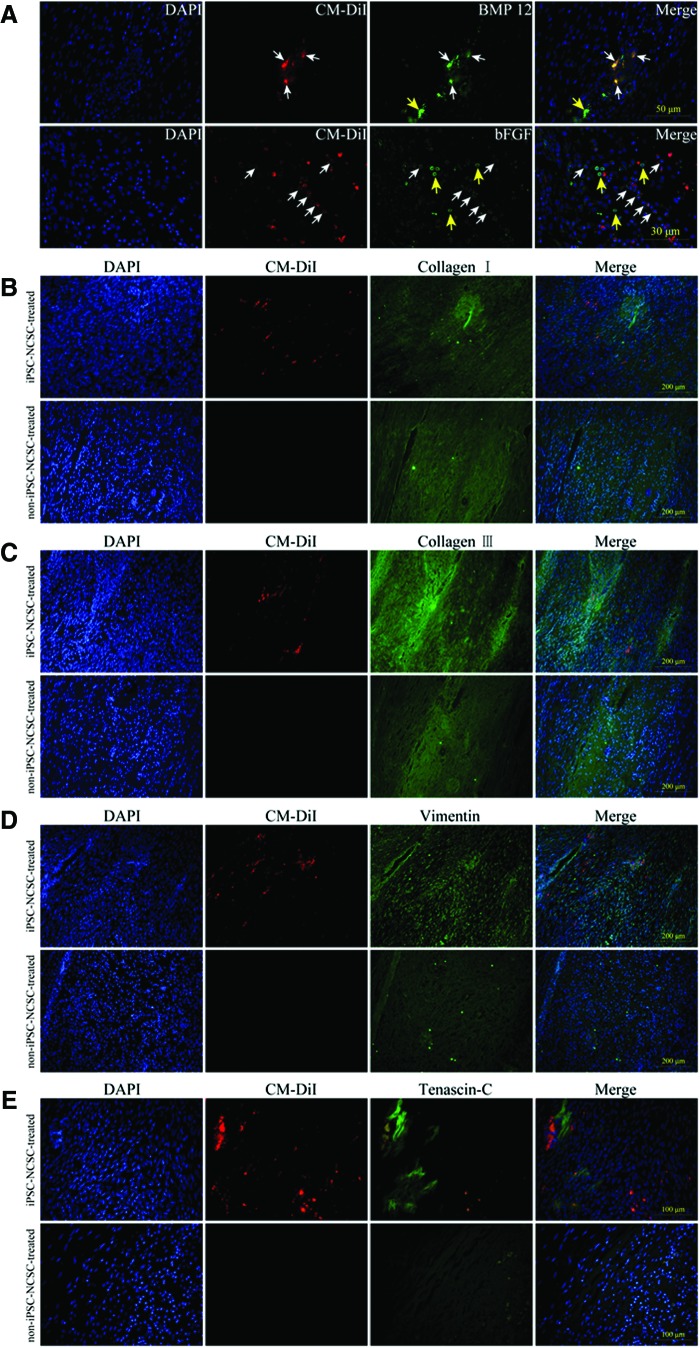
To elucidate the mechanism underlying the iPSC-NCSCs promoted tendon repair, gene transcription and protein expression were analyzed. By using human-specific primers, the transplanted cells were found expressing tendon-related genes increasingly (Fig. 6A), suggesting that the transplanted iPSC-NCSCs could differentiate into tenocyte within the tendon injury niche. PCR and gel electrophoresis showed that the transplanted cells expressed stromal cell-derived factor-1 (SDF-1), fractalkine, BMP 12, transforming growth factor beta 3 (TGF β3), and bFGF at an early time, but the amount decreased over time, along with the transplanted cell number reduction, which was reflected by the decreased human β-actin expression. (Fig. 6B). To detect host endogenous repair, qPCR was performed by using rat-specific primers. Results showed that the expression of collagen I, collagen III, epha 4, and eya 2 were higher in the iPSC-NCSC-treated group at weeks 1, 2, and 4, compared with the non-iPSC-NCSC-treated group (Fig. 6C). Moreover, the expression of epha 4 and eya 2 was increased within 2 weeks in both groups; however, the expression of collagen I and collagen III was up-regulated earlier (within 2 weeks) in the iPSC-NCSC-treated group compared with that in the non-iPSC-NCSC-treated group (within 4 weeks) (Fig. 6C), suggesting that the transplanted iPSC-NCSCs could enhance the host ECM deposit, thus accelerating the endogenous repair. Immunostaining for BMP 12 and bFGF indicated that these tenogenic factors were, indeed, secreted in the injury site at 1 week (Fig. 7A). The expression of tendon-related ECM proteins was assayed at week 4. Collagen I expression has markedly no difference between the two groups (Fig. 7B). However, a greater amount of collagen III, vimentin, and tenascin-C was deposited in the iPSC-NCSC-treated group compared with the non-iPSC-NCSC-treated group (Fig. 7C–E).
FIG. 6.
The mechanism of iPSC-NCSC-promoted tendon repair. (A) Human-specific express analysis of fetal tendon ECM genes (collagen XIV, collagen III, and tenascin-C) and tenogenic signaling molecule (smad 8) in transplanted cells. All human gene transcript levels were relative to β-actin. *p<0.05, **p<0.01. n=3. Compared between 1, 2, and 4 weeks. (B) reverse transcription polymerase chain reaction (RT-PCR) analysis of stem cell recruitment factors (SDF-1, fractalkine) and tenogenic differentiation factors (BMP 12, TGF β3, and bFGF). (C) Rat-specific express analysis of tendon-related ECM genes (collagen I, collagen III) and tenogenic signaling molecules (epha 4, eya 2). All rat gene transcript levels were relative to glyceraldehyde phosphate dehydrogenase, and normalized to non-iPSC-NCSC-treated group at week 1, respectively. #p<0.05, ##p<0.01. Compared between the two groups. *p<0.05, **p<0.01. Compared between 1, 2, and 4 weeks. n=3. niN 4W, non-iPSC-NCSC-treated group at 4 weeks; iN, iPSC-NCSCs in vitro culture at subconfluence; M, DNA ladder marker; iN 1W, iN 2W, and iN 4W, iPSC-NCSC-treated group at 1, 2, and 4 weeks; SDF-1, stromal cell-derived factor-1; BMP 12, bone morphogenetic protein 12; TGF β3, transforming growth factor beta 3; bFGF, basic fibroblast growth factor; ECM, extracellular matrix.
FIG. 7.
Immunostaining for BMP 12, bFGF in iPSC-NCSC-treated group at 1 week postsurgery (A), and collagen I (B), collagen III (C), vimentin (D), Tenasin-C (E) in both groups at 4 weeks postsurgery. White arrowheads indicate CM-DiI+/BMP 12+ or CM-DiI+/bFGF+ cells. Yellow arrowheads indicate CM-DiI−/BMP 12+ or CM-DiI−/bFGF+ cells. Color images available online at www.liebertpub.com/tea
Discussion
The present study is the first, to our knowledge, to provide evidence that iPSCs may represent a valuable cell source for tendon repair. A reproducible patellar tendon window defect model17,25,28,30 was employed to test the efficiency of iPS-NCSCs on tendon repair. At 4 weeks after transplantation, macroscopical observation, histological analysis, biomechanical evaluation of iPSC-NCSC-treated tendons showed better repair than non-iPSC-NCSC-treated tendons. These results indicate that iPSC-NCSCs accelerate ECM deposition and promote tendon repair at the injury site.
To characterize the roles that iPS-NCSCs played in tendon repair, we first investigated their survival after transplantation. We demonstrated that iPSC-NCSCs engrafted into the injury site, as indicated by CM-DiI labeling and hNuMA immunostaining, as well as RT-PCR analysis of human specific gene expression in the repaired tendons. These results showed that the cells survived in host tendon tissue after transplantation. Moreover, in the early phase of repair (week 1–4), transplanted cells participated in the repair by producing tendon-related matrix proteins and paracrine factors. However, the number of transplanted cells decreased with time, possibly due to the natural clearance of excessive cells by apoptosis at the end stage of the healing process, which is consistent with previous reports.17,28,31
Although there is evidence that transplanted cells could promote tendon/ligament repair, there is little information on the roles of these cells in the transplant site.17,28 To address this issue, we characterized the gene and protein expression in iPSC-NCSCs after their engraftment. Collagen XIV,32 collagen III,33 and tenascin-C34 are important components of fetal tendon ECM. Smad 8 is a recently reported specific tenogenic signaling molecule.35,36 By using human-specific primers, we found that the expression level of aforementioned genes, except for tenascin-C, was increased, indicating that the transplanted iPSC-NCSCs may differentiate into fetal tendon-like cells. Moreover, at an early time point, the transplanted iPSC-NCSCs expressed stem cell recruitment factors such as SDF-1 and fractalkine, which can facilitate the recruitment and homing of host stem cells to the transplant site.37,38 The transplanted iPSC-NCSCs also expressed a number of differentiation factors, which can induce tenogenic differentiation, including BMP 12,39–43 TGF β3,44 and bFGF.30,45 Subsequently, rat-specific genes expression analysis elucidated that the expression of rat tendon ECM genes and tendon-specific genes increased in iPSC-NCSC-treated tendons. Intriguingly, the iPSC-NCSCs treatment could enhance the efficacy of host endogenous repair by shortening the healing time. Moreover, protein analysis also showed that the transplanted iPSC-NCSCs secreted paracrine factors, accompanied by more ECM deposition in iPSC-NCSC-treated tendons. Taken together, these data demonstrate that iPSC-NCSCs promote rat tendon repair by not only directly differentiating into fetal tenocytes in tendon niche but also secreting stem cell recruitment factors and tenogenic differentiation factors, which may, in turn, accelerate endogenous tendon repair.
Although numerous studies have proved that iPSCs possess great potential for tissue repair, the risk for teratoma formation remains a big hurdle for iPSCs clinical applications.46 In the present study, we employed a stepwise differentiation protocol to derive multipotent NCSCs from pluripotent iPSCs, and subsequently allowed NCSCs to differentiate into tendon-like cells within tendon niche. As a result, no teratoma was found at 8 weeks after transplantation, which is consistent with our previous study using iPSC-NCSCs in a nerve regeneration model.21 Similarly, Barberi et al. also reported that teratorma can be avoided if ESCs were differentiated into neural progenitor cells before being transplanted into the nervous system.47
Developmental NCSCs can contribute to not only the peripheral nervous system, but also mesenchymal precursor cells, especially in craniofacial skeleton.48,49 In vitro cultured NCSCs also showed broad differentiation potentials, including peripheral neurons, Schwann cells, smooth muscle, and adipogenic, osteogenic, and chondrogenic cells.21,50,51 Thus, NCSCs represent a unique cell source for regenerative medicine. Based on recent progresses in safety52 and efficiency,53 iPSCs can be made at clinical-grade therapeutic cells for future applications. Therefore, multipotent NCSCs derived from iPSCs may be used as a valuable and unlimited cell source for tissue regeneration, including neural and mesenchymal tissues (tendon, ligament, etc.). However, due to the osteogenic and chondrogenic differentiation potentials, close attention should be paid to avoid ectopic bone formation in stem-cell-transplanted tendon.12 In this study, no ectopic bone or cartilage formation was observed at week 8. In spite of this, long-term studies and more broad examinations should be performed in the future. Moreover, the optimal cell sources, especially iPSC-derived cells and adult tissue-derived cells, for tendon tissue engineering need to be investigated in future studies. In addition, in this xenogeneic transplantation model, the nude rats are T-cell deficient, which cannot thoroughly address the immune rejection issues. Besides the potential for autologous cell therapies, whether iPSC-NCSCs and adult stem cells can be used for allogeneic transplantation should also be investigated.
Conclusion
Our study demonstrates that iPSC-NCSCs can survive in three-dimensional gel and engraft into injured tendon. Macroscopical morphology, histology, and biomechanical analysis indicate that iPSC-NCSCs promote tendon repair. Furthermore, mechanistic investigation demonstrates that iPSC-NCSCs differentiate into tenocyte lineages, and secrete stem cell recruitment factors and tenogenic differentiation factors, which may promote endogenous tendon repair. This study suggests that iPSC-NCSCs may be a potential cell source for tendon repair.
Acknowledgments
The authors sincerely thank Yongjin Peng for designing custom-made clamps, and Shourong Wu for carefully proof reading the article. This work was supported by grants from Innovation and Attracting Talents Program for College and University (“111” Project) (B06023), Fundamental Research Funds for the Central Universities (CDJXS11231176), National Natural Science Foundation of China (11032012, 10902130, and 30870608), Key Science and Technology Program of CQ CSTC (CSTC, 2009AA5045), Program for New Century Excellent Talents in University (NCET-10-0879), and National Institute of Health (EB12240).
Disclosure Statement
No competing financial interests exist.
References
- 1.Woo S.L. Debski R.E. Zeminski J. Abramowitch S.D. Saw S.S. Fenwick J.A. Injury and repair of ligaments and tendons. Annu Rev Biomed Eng. 2000;2:83. doi: 10.1146/annurev.bioeng.2.1.83. [DOI] [PubMed] [Google Scholar]
- 2.Longo U.G. Lamberti A. Maffulli N. Denaro V. Tissue engineered biological augmentation for tendon healing: a systematic review. Br Med Bull. 2011;98:31. doi: 10.1093/bmb/ldq030. [DOI] [PubMed] [Google Scholar]
- 3.Goh J.C. Ouyang H.W. Teoh S.H. Chan C.K. Lee E.H. Tissue-engineering approach to the repair and regeneration of tendons and ligaments. Tissue Eng. 2003;9(Suppl 1):S31. doi: 10.1089/10763270360696969. [DOI] [PubMed] [Google Scholar]
- 4.Bagnaninchi P.O. Yang Y. El Haj A.J. Maffulli N. Tissue engineering for tendon repair. Br J Sports Med. 2007;41:e10. doi: 10.1136/bjsm.2006.030643. discussion e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kryger G.S. Chong A.K. Costa M. Pham H. Bates S.J. Chang J. A comparison of tenocytes and mesenchymal stem cells for use in flexor tendon tissue engineering. J Hand Surg Am. 2007;32:597. doi: 10.1016/j.jhsa.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 6.Cao Y. Liu Y. Liu W. Shan Q. Buonocore S.D. Cui L. Bridging tendon defects using autologous tenocyte engineered tendon in a hen model. Plast Reconstr Surg. 2002;110:1280. doi: 10.1097/01.PRS.0000025290.49889.4D. [DOI] [PubMed] [Google Scholar]
- 7.Calve S. Dennis R.G. Kosnik P.E., 2nd Baar K. Grosh K. Arruda E.M. Engineering of functional tendon. Tissue Eng. 2004;10:755. doi: 10.1089/1076327041348464. [DOI] [PubMed] [Google Scholar]
- 8.Awad H.A. Butler D.L. Boivin G.P. Smith F.N. Malaviya P. Huibregtse B., et al. Autologous mesenchymal stem cell-mediated repair of tendon. Tissue Eng. 1999;5:267. doi: 10.1089/ten.1999.5.267. [DOI] [PubMed] [Google Scholar]
- 9.Young R.G. Butler D.L. Weber W. Caplan A.I. Gordon S.L. Fink D.J. Use of mesenchymal stem cells in a collagen matrix for Achilles tendon repair. J Orthop Res. 1998;16:406. doi: 10.1002/jor.1100160403. [DOI] [PubMed] [Google Scholar]
- 10.Ouyang H.W. Goh J.C. Thambyah A. Teoh S.H. Lee E.H. Knitted poly-lactide-co-glycolide scaffold loaded with bone marrow stromal cells in repair and regeneration of rabbit Achilles tendon. Tissue Eng. 2003;9:431. doi: 10.1089/107632703322066615. [DOI] [PubMed] [Google Scholar]
- 11.Hankemeier S. Hurschler C. Zeichen J. van Griensven M. Miller B. Meller R., et al. Bone marrow stromal cells in a liquid fibrin matrix improve the healing process of patellar tendon window defects. Tissue Eng. 2009;15:1019. doi: 10.1089/ten.tea.2008.0046. [DOI] [PubMed] [Google Scholar]
- 12.Awad H.A. Boivin G.P. Dressler M.R. Smith F.N. Young R.G. Butler D.L. Repair of patellar tendon injuries using a cell-collagen composite. J Orthop Res. 2003;21:420. doi: 10.1016/S0736-0266(02)00163-8. [DOI] [PubMed] [Google Scholar]
- 13.Harris M.T. Butler D.L. Boivin G.P. Florer J.B. Schantz E.J. Wenstrup R.J. Mesenchymal stem cells used for rabbit tendon repair can form ectopic bone and express alkaline phosphatase activity in constructs. J Orthop Res. 2004;22:998. doi: 10.1016/j.orthres.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Bi Y. Ehirchiou D. Kilts T.M. Inkson C.A. Embree M.C. Sonoyama W., et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 15.Rui Y.F. Lui P.P. Li G. Fu S.C. Lee Y.W. Chan K.M. Isolation and characterization of multipotent rat tendon-derived stem cells. Tissue Eng. 2010;16:1549. doi: 10.1089/ten.TEA.2009.0529. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J. Wang J. Characterization of differential properties of rabbit tendon stem cells and tenocytes. BMC Musculoskel Disord. 2010;11:10. doi: 10.1186/1471-2474-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ni M. Lui P.P. Rui Y.F. Lee Y.W. Tan Q. Wong Y.M., et al. Tendon-derived stem cells (TDSCs) promote tendon repair in a rat patellar tendon window defect model. J Orthop Res. 2012;30:613. doi: 10.1002/jor.21559. [DOI] [PubMed] [Google Scholar]
- 18.Mifune Y. Matsumoto T. Ota S. Nishimori M. Usas A. Kopf S., et al. Therapeutic potential of anterior cruciate ligament-derived stem cells for anterior cruciate ligament reconstruction. Cell Transplant. 2012;21:1651. doi: 10.3727/096368912X647234. [DOI] [PubMed] [Google Scholar]
- 19.Shen W. Chen J. Yina Z. Chen X. Liua H. Heng B.C., et al. Allogenous tendon stem/progenitor cells in silk scaffold for functional shoulder repair. Cell Transplant. 2012;21:943. doi: 10.3727/096368911X627453. [DOI] [PubMed] [Google Scholar]
- 20.Ni M. Rui Y.F. Tan Q. Liu Y. Xu L.L. Chan K.M., et al. Engineered scaffold-free tendon tissue produced by tendon-derived stem cells. Biomaterials. 2013;34:2024. doi: 10.1016/j.biomaterials.2012.11.046. [DOI] [PubMed] [Google Scholar]
- 21.Wang A. Tang Z. Park I.H. Zhu Y. Patel S. Daley G.Q., et al. Induced pluripotent stem cells for neural tissue engineering. Biomaterials. 2011;32:5023. doi: 10.1016/j.biomaterials.2011.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang A. Tang Z. Li X. Jiang Y. Tsou D.A. Li S. Derivation of smooth muscle cells with neural crest origin from human induced pluripotent stem cells. Cells Tissues Organs. 2012;195:5. doi: 10.1159/000331412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X. Chu J. Wang A. Zhu Y. Chu W.K. Yang L., et al. Uniaxial mechanical strain modulates the differentiation of neural crest stem cells into smooth muscle lineage on micropatterned surfaces. PLoS One. 2011;6:e26029. doi: 10.1371/journal.pone.0026029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park I.H. Zhao R. West J.A. Yabuuchi A. Huo H. Ince T.A., et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 25.Butler D.L. Gooch C. Kinneberg K.R. Boivin G.P. Galloway M.T. Nirmalanandhan V.S., et al. The use of mesenchymal stem cells in collagen-based scaffolds for tissue-engineered repair of tendons. Nat Protoc. 2010;5:849. doi: 10.1038/nprot.2010.14. [DOI] [PubMed] [Google Scholar]
- 26.Chan B.P. Fu S.C. Qin L. Rolf C. Chan K.M. Pyridinoline in relation to ultimate stress of the patellar tendon during healing: an animal study. J Orthop Res. 1998;16:597. doi: 10.1002/jor.1100160512. [DOI] [PubMed] [Google Scholar]
- 27.Stoll C. John T. Conrad C. Lohan A. Hondke S. Ertel W., et al. Healing parameters in a rabbit partial tendon defect following tenocyte/biomaterial implantation. Biomaterials. 2011;32:4806. doi: 10.1016/j.biomaterials.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 28.Chen X. Song X.H. Yin Z. Zou X.H. Wang L.L. Hu H., et al. Stepwise differentiation of human embryonic stem cells promotes tendon regeneration by secreting fetal tendon matrix and differentiation factors. Stem Cells. 2009;27:1276. doi: 10.1002/stem.61. [DOI] [PubMed] [Google Scholar]
- 29.Tohyama H. Yasuda K. Kitamura Y. Yamamoto E. Hayashi K. The changes in mechanical properties of regenerated and residual tissues in the patellar tendon after removal of its central portion. Clin Biomech (Bristol, Avon) 2003;18:765. doi: 10.1016/s0268-0033(03)00055-x. [DOI] [PubMed] [Google Scholar]
- 30.Chan B.P. Fu S. Qin L. Lee K. Rolf C.G. Chan K. Effects of basic fibroblast growth factor (bFGF) on early stages of tendon healing: a rat patellar tendon model. Acta Orthop Scand. 2000;71:513. doi: 10.1080/000164700317381234. [DOI] [PubMed] [Google Scholar]
- 31.Lui P.P. Cheuk Y.C. Hung L.K. Fu S.C. Chan K.M. Increased apoptosis at the late stage of tendon healing. Wound Repair Regen. 2007;15:702. doi: 10.1111/j.1524-475X.2007.00276.x. [DOI] [PubMed] [Google Scholar]
- 32.Young B.B. Gordon M.K. Birk D.E. Expression of type XIV collagen in developing chicken tendons: association with assembly and growth of collagen fibrils. Dev Dyn. 2000;217:430. doi: 10.1002/(SICI)1097-0177(200004)217:4<430::AID-DVDY10>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 33.Bland Y.S. Ashhurst D.E. Fetal and postnatal development of the patella, patellar tendon and suprapatella in the rabbit; changes in the distribution of the fibrillar collagens. J Anat. 1997;190(Pt 3):327. doi: 10.1046/j.1469-7580.1997.19030327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kardon G. Muscle and tendon morphogenesis in the avian hind limb. Development. 1998;125:4019. doi: 10.1242/dev.125.20.4019. [DOI] [PubMed] [Google Scholar]
- 35.Hoffmann A. Pelled G. Turgeman G. Eberle P. Zilberman Y. Shinar H., et al. Neotendon formation induced by manipulation of the Smad8 signalling pathway in mesenchymal stem cells. J Clin Invest. 2006;116:940. doi: 10.1172/JCI22689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shahab-Osterloh S. Witte F. Hoffmann A. Winkel A. Laggies S. Neumann B., et al. Mesenchymal stem cell-dependent formation of heterotopic tendon-bone insertions (osteotendinous junctions) Stem Cells. 2010;28:1590. doi: 10.1002/stem.487. [DOI] [PubMed] [Google Scholar]
- 37.Hattori K. Heissig B. Tashiro K. Honjo T. Tateno M. Shieh J.H., et al. Plasma elevation of stromal cell-derived factor-1 induces mobilization of mature and immature hematopoietic progenitor and stem cells. Blood. 2001;97:3354. doi: 10.1182/blood.v97.11.3354. [DOI] [PubMed] [Google Scholar]
- 38.Ji J.F. He B.P. Dheen S.T. Tay S.S. Interactions of chemokines and chemokine receptors mediate the migration of mesenchymal stem cells to the impaired site in the brain after hypoglossal nerve injury. Stem Cells. 2004;22:415. doi: 10.1634/stemcells.22-3-415. [DOI] [PubMed] [Google Scholar]
- 39.Wolfman N.M. Hattersley G. Cox K. Celeste A.J. Nelson R. Yamaji N., et al. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. J Clin Invest. 1997;100:321. doi: 10.1172/JCI119537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee J.Y. Zhou Z. Taub P.J. Ramcharan M. Li Y. Akinbiyi T., et al. BMP-12 Treatment of adult mesenchymal stem cells in vitro augments tendon-like tissue formation and defect repair in vivo. PLoS One. 2011;6:e17531. doi: 10.1371/journal.pone.0017531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Majewski M. Betz O. Ochsner P.E. Liu F. Porter R.M. Evans C.H. Ex vivo adenoviral transfer of bone morphogenetic protein 12 (BMP-12) cDNA improves Achilles tendon healing in a rat model. Gene Ther. 2008;15:1139. doi: 10.1038/gt.2008.48. [DOI] [PubMed] [Google Scholar]
- 42.Ma Y. Zhang X. Wang J. Liu P. Zhao L. Zhou C., et al. Effect of bone morphogenetic protein-12 gene transfer on posterior cruciate ligament healing in a rabbit model. Am J Sports Med. 2009;37:599. doi: 10.1177/0363546508325960. [DOI] [PubMed] [Google Scholar]
- 43.Haddad-Weber M. Prager P. Kunz M. Seefried L. Jakob F. Murray M.M., et al. BMP12 and BMP13 gene transfer induce ligamentogenic differentiation in mesenchymal progenitor and anterior cruciate ligament cells. Cytotherapy. 2010;12:505. doi: 10.3109/14653241003709652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marui T. Niyibizi C. Georgescu H.I. Cao M. Kavalkovich K.W. Levine R.E., et al. Effect of growth factors on matrix synthesis by ligament fibroblasts. J Orthop Res. 1997;15:18. doi: 10.1002/jor.1100150104. [DOI] [PubMed] [Google Scholar]
- 45.Costa M.A. Wu C. Pham B.V. Chong A.K. Pham H.M. Chang J. Tissue engineering of flexor tendons: optimization of tenocyte proliferation using growth factor supplementation. Tissue Eng. 2006;12:1937. doi: 10.1089/ten.2006.12.1937. [DOI] [PubMed] [Google Scholar]
- 46.Csete M. Translational prospects for human induced pluripotent stem cells. Regen Med. 2010;5:509. doi: 10.2217/rme.10.39. [DOI] [PubMed] [Google Scholar]
- 47.Barberi T. Klivenyi P. Calingasan N.Y. Lee H. Kawamata H. Loonam K., et al. Neural subtype specification of fertilization and nuclear transfer embryonic stem cells and application in parkinsonian mice. Nat Biotechnol. 2003;21:1200. doi: 10.1038/nbt870. [DOI] [PubMed] [Google Scholar]
- 48.Achilleos A. Trainor P.A. Neural crest stem cells: discovery, properties and potential for therapy. Cell Res. 2012;22:288. doi: 10.1038/cr.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dupin E. Sommer L. Neural crest progenitors and stem cells: from early development to adulthood. Dev Biol. 2012;366:83. doi: 10.1016/j.ydbio.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 50.Lee G. Chambers S.M. Tomishima M.J. Studer L. Derivation of neural crest cells from human pluripotent stem cells. Nat Protoc. 2010;5:688. doi: 10.1038/nprot.2010.35. [DOI] [PubMed] [Google Scholar]
- 51.Lee G. Kim H. Elkabetz Y. Al Shamy G. Panagiotakos G. Barberi T., et al. Isolation and directed differentiation of neural crest stem cells derived from human embryonic stem cells. Nat Biotechnol. 2007;25:1468. doi: 10.1038/nbt1365. [DOI] [PubMed] [Google Scholar]
- 52.Okita K. Yamakawa T. Matsumura Y. Sato Y. Amano N. Watanabe A., et al. An efficient non-viral method to generate integration-free human iPS cells from cord blood and peripheral blood cells. Stem Cells. 2012;31:458. doi: 10.1002/stem.1293. [DOI] [PubMed] [Google Scholar]
- 53.Mack A.A. Kroboth S. Rajesh D. Wang W.B. Generation of induced pluripotent stem cells from CD34+ cells across blood drawn from multiple donors with non-integrating episomal vectors. PLoS One. 2011;6:e27956. doi: 10.1371/journal.pone.0027956. [DOI] [PMC free article] [PubMed] [Google Scholar]