Abstract
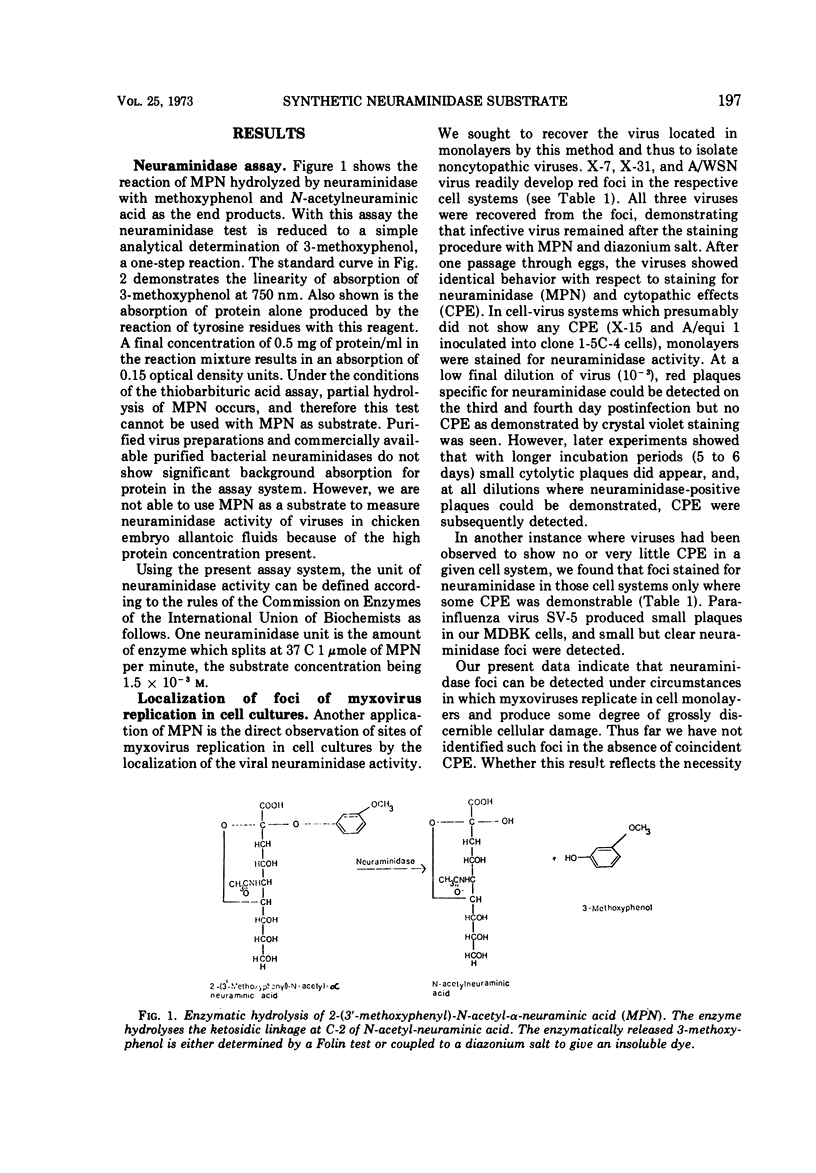
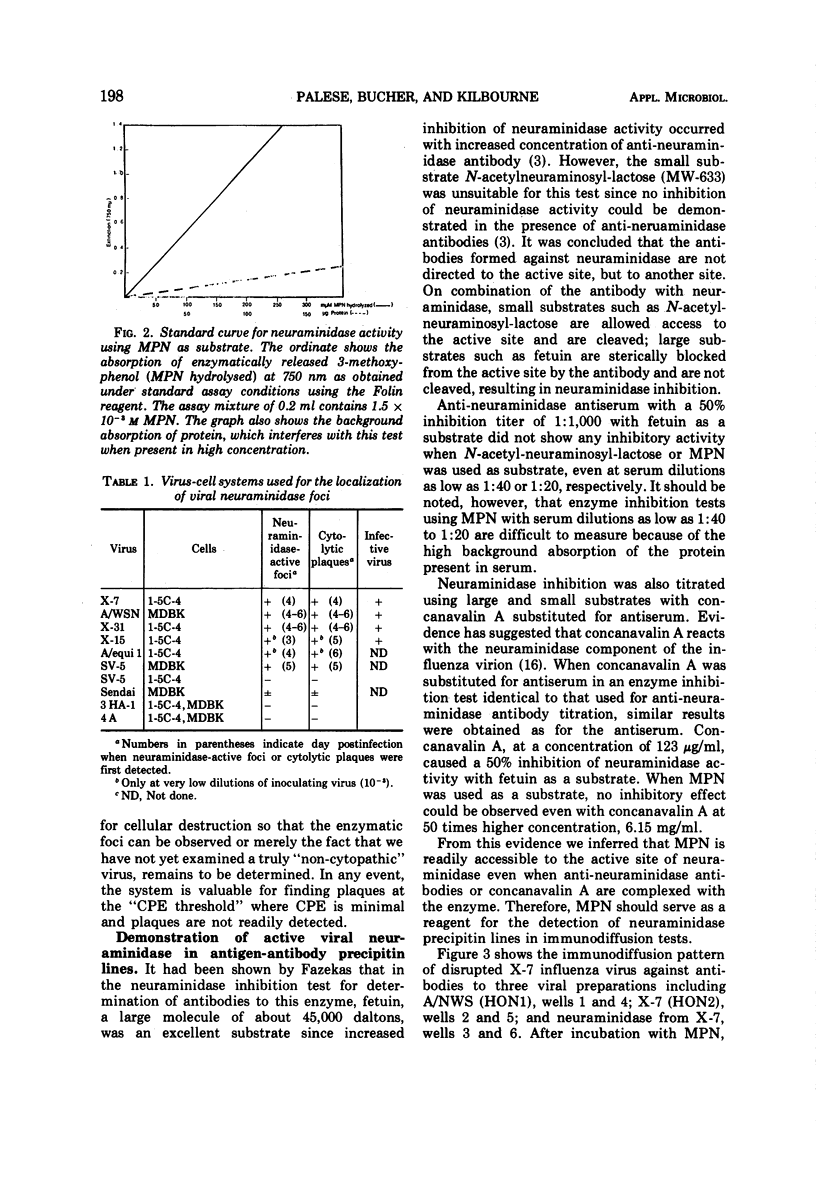
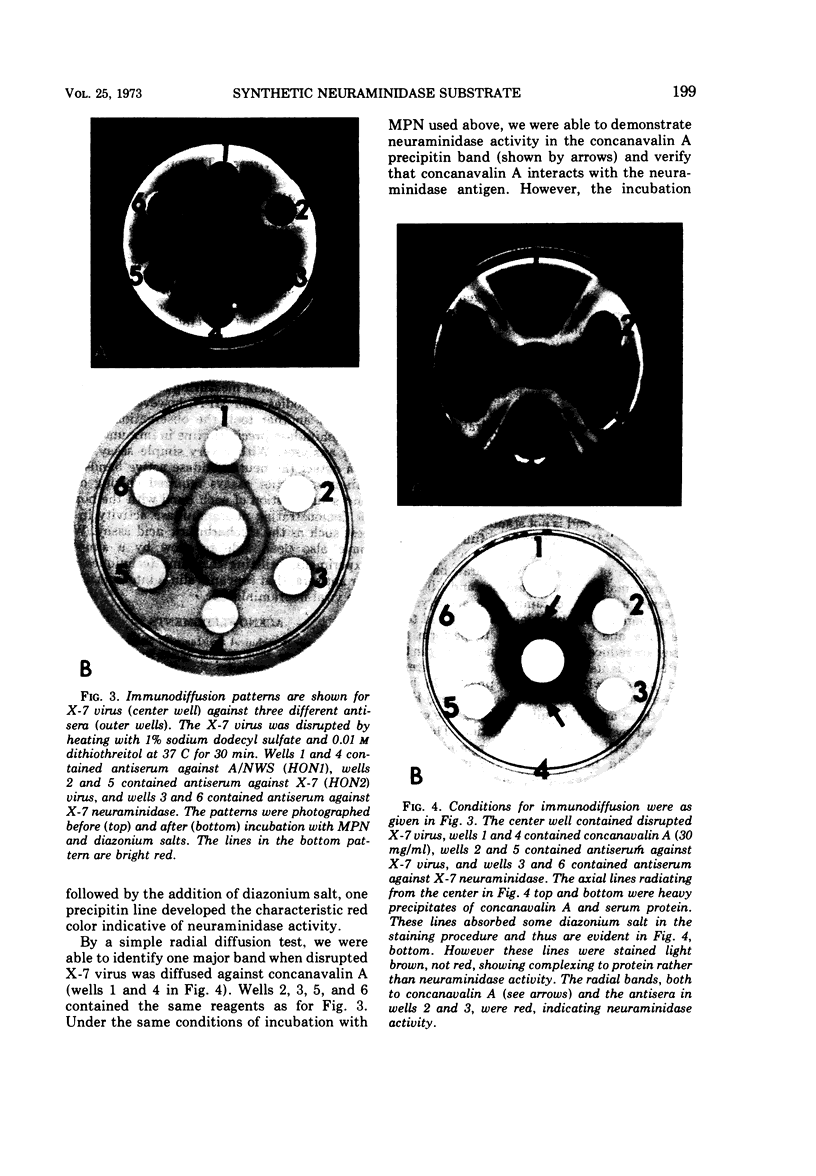
A rapid and precise assay for neuraminidase using 2-(3′-methoxyphenyl)-N-acetyl-α-neuraminic acid (MPN) is described. It is proposed that this substrate be used for the standardization of activity of neuraminidases from viral, bacterial, and mammalian sources. MPN is also used as a chromogenic substrate to localize influenza and parainfluenza virus foci in tissue culture. This technique permits the recovery of infective virus from these stained „plaques.” It has also been demonstrated that immunoprecipitin lines containing neuraminidase complexes with antibody in the Ouchterlony test can be observed by a similar staining procedure. No enzyme inhibition occurs in the presence of anti-neuraminidase antibodies or concanavalin A when MPN is used as a substrate in contrast to the results with high-molecular-weight substrates such as fetuin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bucher D. J., Kilbourne E. D. A 2 (N2) neuraminidase of the X-7 influenza virus recombinant: determination of molecular size and subunit composition of the active unit. J Virol. 1972 Jul;10(1):60–66. doi: 10.1128/jvi.10.1.60-66.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmquist L. A method for the determination of neuraminidase activity in the presence of added neuraminic acids or potential inhibitors. Acta Chem Scand. 1969;23(3):1045–1052. doi: 10.3891/acta.chem.scand.23-1045. [DOI] [PubMed] [Google Scholar]
- Kilbourne E. D., Schulman J. L., Schild G. C., Schloer G., Swanson J., Bucher D. Related studies of a recombinant influenza-virus vaccine. I. Derivation and characterization of virus and vaccine. J Infect Dis. 1971 Nov;124(5):449–462. doi: 10.1093/infdis/124.5.449. [DOI] [PubMed] [Google Scholar]
- Kolbourne E. D. Recombination of influenza A viruses of human and animal origin. Science. 1968 Apr 5;160(3823):74–76. doi: 10.1126/science.160.3823.74. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laver W. G., Kilbourne E. D. Identification in a recombinant influenza virus of structural proteins derived from both parents. Virology. 1966 Nov;30(3):493–501. doi: 10.1016/0042-6822(66)90125-5. [DOI] [PubMed] [Google Scholar]
- Palese P., Bodo G., Tuppy H. Quantitative determination of neuraminidase-active foci in cell monolayer cultures infected with influenza or newcastle disease virus. J Virol. 1970 Oct;6(4):556–558. doi: 10.1128/jvi.6.4.556-558.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rott R., Becht H., Klenk H. D., Scholtissek C. Interactions of concanavalin A with the membrane of infleunza virus infected cells and with envelope components of the virus particle. Z Naturforsch B. 1972 Mar;27(3):227–233. doi: 10.1515/znb-1972-0303. [DOI] [PubMed] [Google Scholar]
- Sugiura A., Tobita K., Kilbourne E. D. Isolation and preliminary characterization of temperature-sensitive mutants of influenza virus. J Virol. 1972 Oct;10(4):639–647. doi: 10.1128/jvi.10.4.639-647.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuppy H., Palese P. A chromogenic substrate for the investigation of neuraminidases. FEBS Lett. 1969 Apr;3(1):72–75. doi: 10.1016/0014-5793(69)80100-6. [DOI] [PubMed] [Google Scholar]
- Ziegler D. W., Hutchinson H. D. Coupled-enzyme system for measuring viral neuraminidase activity. Appl Microbiol. 1972 Jun;23(6):1060–1066. doi: 10.1128/am.23.6.1060-1066.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]