Abstract
Fibrin glue has been widely investigated as a cell delivery vehicle for improving the therapeutic effects of mesenchymal stem cells (MSCs). Implanted MSCs produce their therapeutic effects by secreting paracrine factors and by replacing damaged tissues after differentiation. While the influence of fibrin glue on the differentiation potential of MSCs has been well documented, its effect on paracrine function of MSCs is largely unknown. Herein we investigated the influence of fibrin glue on the paracrine effects of MSCs. MSCs were isolated from human adipose tissue. The effects of fibrin glue on survival, migration, secretion of growth factors, and immune suppression of MSCs were investigated in vitro. MSCs in fibrin glue survived and secreted growth factors such as the vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF) over 14 days. VEGF and immune modulators, including the transforming growth factor (TGF)-β1 and prostaglandin E2, secreted from MSCs in fibrin glue significantly increased under inflammatory conditions. Thus, MSCs in fibrin glue effectively suppressed immune reactions. In addition, fibrin glue protected the MSCs from oxidative stress and prevented human dermal fibroblast death induced by exposure to extreme stress. In contrast, MSCs within fibrin glue hardly migrated. These results suggest that fibrin glue may sustain survival of implanted MSCs and their paracrine function. Our results provide a mechanistic data to allow further development of MSCs with fibrin glue as a clinical treatment.
Introduction
Mesenchymal stem cells (MSCs) are multipotent stem cells, which were first isolated from bone marrow and later from other tissues, including muscle, cartilage, dental pulp, adipose tissue, placenta, and umbilical cord blood.1,2 In recent years, many clinical as well as preclinical studies using MSCs have been conducted because of their great potential to repair tissue, including their capacity to differentiate into various cell lineages, their ability to release soluble factors that are crucial to cell survival, proliferation, neovascularization, and their ability to modulate the immune response.3–8
The MSC implantation route is associated with their efficiency of travel to the target organs and tissues. The most commonly used routes for MSC transplantation are systemic (intravenous) and local injections. Systemically implanted MSCs migrate into damaged tissue in response to biological signals.9–11 This route of implantation is less invasive, and a large number of cells can be administered; however, most of the implanted MSCs become trapped in the lung, spleen, and liver capillaries; thus, only some of the implanted cells reach the damaged tissue.12,13 In contrast, the local MSC implantation method is more invasive, but offers improved cell engraftment. Nevertheless, directly implanted cells have limited cell retention and survival at the target site. Therefore, numerous studies are being carried out to develop further optimized implantation methods to prevent cell loss.
The fields of tissue engineering and drug delivery systems using biomaterials such as hyaluronic acid, collagen, and fibrin provide a proper solution to this problem. Specifically, fibrin glue is the most commonly used biomaterial because of its high biocompatibility, biodegradability, injectability, and ease of handling. Furthermore, fibrin glue is nontoxic, nonallergenic, and nonimmunogenic. Therefore, clinical applications of MSCs with fibrin glue have been employed to treat several conditions, including Crohn's disease, diabetic ulcers, and cartilage defects.14–18 As a cell-delivery vehicle, fibrin glue facilitates cell attachment, proliferation, differentiation, and ultimately, tissue formation and organization due to the three-dimensional cell structure.19,20 Therefore, implanting MSCs with fibrin glue could induce a synergistic effect to repair damaged tissue.
Despite many animal and clinical studies, previous studies19,21,22 have focused on the effect of fibrin glue on proliferation and differentiation potential of MSCs, and consequential replacement of damaged tissue. However, the contribution of fibrin glue to the paracrine effects of MSCs has not been examined. Considering the emerging concept of a therapeutic effect for the paracrine mechanism of MSCs,23,24 we systemically investigated the effect of fibrin glue on the paracrine mechanism of MSCs, including production of growth factors and anti-inflammatory cytokines, the antiapoptotic effect, and migration in vitro.
Materials and Methods
MSC isolation and culture
MSCs were isolated from lipoaspirates of human subcutaneous fat tissue obtained from healthy donors who provided informed consent. Lipoaspirates were washed at least three times with phosphate-buffered saline (PBS) and digested in an equal volume of PBS containing 1% bovine serum albumin and 0.025% collagenase type I (Invitrogen, Gaithersburg, MD) for 80 min at 37°C with intermittent shaking. Isolated cells were cultured in the Dulbecco's modified Eagle's medium (DMEM; Invitrogen) supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, UT) and 1 ng/mL human basic fibroblast growth factor (bFGF) and used at passage 3.
Fibrin glue preparation
The fibrin glue was from the commercially available Greenplast kit (Greencross, Seoul, Korea), which consisted of the aprotinin solution (1100 KIU/1.1 mL), calcium chloride solution (13.9–15.6 mg/2.5 mL), lyophilized human plasma fibrinogen (126.5–256.3 mg), and thrombin (4.9–11.1 mg). The fibrinogen and thrombin were dissolved with 1 mL of the aprotinin and calcium chloride solution, respectively. A cell suspension was mixed with the thrombin solution at a 4:1 ratio. Then, the cell-thrombin suspension was mixed with the fibrinogen solution at 1:1 using a Duploject applicator and simultaneously added to each well.
MSC viability and proliferation test in fibrin glue
To determine whether MSCs were capable of surviving in fibrin glue, MSCs incorporated into fibrin glue were prepared at a final concentration of 5×105 cells/200 μL/well and incubated in a 37°C CO2 incubator. MSCs without fibrin glue were cultured on tissue culture plates in the DMEM as a control. On days 0 (2 h after mixing), 3, 7, and 14, they were sectioned and stained with 10 μg/mL acridine orange/ethidium bromide (AO/EtBr). Then, the sections were analyzed under a fluorescence microscope at 200× magnification. Cell proliferation was assessed by using 5-bromo-2′-deoxy-uridine (Roche, Indianapolis, IN) according to the manufacturer's instructions.
Growth factor secretion assay
The MSC-fibrin glue mixture was prepared at final concentrations of 1×105, 1×106, and 1×107 cells/well. One milliliter of DMEM was added to each well, and the cells were incubated in a CO2 incubator. The MSC-conditioned medium was collected every 3 days for 15 days. To assess the change in cytokines released from MSCs with or without fibrin glue under an inflammatory condition, 1×105 MSCs in fibrin glue were cocultured with 5×105 peripheral blood mononuclear cells (PBMCs) from an unrelated MSC donor in the presence or absence of the mitogen phytohemagglutinin (PHA, 5 μg/mL). PBMCs were isolated by Ficoll–Paque (1.077 g/mL; Amersham Biosciences, Uppsala, Sweden) density gradient centrifugation from the peripheral blood of healthy donors who provided informed consent. The concentrations of factors released from MSCs, including the vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), transforming growth factor (TGF)-β1, and prostaglandin E2 (PGE2) were assessed using an enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions.
Immune inhibition assay
MSCs in fibrin glue were prepared at a final concentration of 2.5×105 cells/well of a 48-well plate. PBMCs (5×105cells/well) were added to each well with MSCs and fibrin glue in the presence of PHA (5 μg/mL). After 48 h of incubation, supernatants were collected and analyzed for the inflammatory cytokine tumor necrosis factor (TNF)-α by ELISA (R&D Systems). To assess the proliferation rate of lymphocytes, the remaining cells were treated with 5-ethynyl-2′-deoxyuridine (EdU; Invitrogen, Eugene, OR) for 20 h, harvested, stained with the anti-EdU antibody, and then analyzed by flow cytometry.
Cell-death protection of MSCs in fibrin glue
Human dermal fibroblasts (HDFs; CCD-986) were suspended in the DMEM containing 1% FBS and seeded at a concentration of 3×104 cells/well into the lower chamber of a 24-Transwell plate (Corning, Corning, NY). After 24 h of incubation, the cells were washed with the DMEM. Then, the upper chambers, including fibrin glue alone (control), 1×105 and 1×106 MSCs with or without fibrin glue were inserted into the 24-Transwell plate. At the same time, cells were exposed to 100, 200, and 400 μM of tert-butyl hydroperoxide (tbOOH) in the DMEM. After 12 h of incubation, the upper chambers containing MSCs alone or MSCs incorporated into fibrin glue were removed and stained with AO/EtBr. A 20-μL aliquot of WST-1 (Roche) was added into the lower chamber and incubated for 3 h to measure HDF viability. A 100-μL aliquot of the supernatants was transferred to a 96-well microplate, and absorbance was measured at 450 nm.
Migration of MSCs in fibrin glue
The migration capacity of MSCs in fibrin glue was evaluated using 8-μm pore size Transwell membrane filters. MSCs (2.5×104 cells/well) with or without fibrin glue were added to the upper chamber, and the lower chambers were filled with PBMCs (5×105 cells/well) and stimulated with PHA. After 2–5 days of incubation, the filters were stained with DAPI or hematoxylin and eosin, and the cells on the upper side of the filters were removed with a cotton swab. Transwell filters were cut out and mounted onto a glass slide. A randomly selected field was photographed, and migrated cells were counted.
Statistical analysis
Data are expressed as mean±SD from three or four different donor-derived MSCs. The statistical analysis was performed using ANOVA followed by the Tukey test; a p-value <0.05 was considered to indicate statistical significance.
Results
MSC morphology and viability inside the fibrin glue
The morphology and viability of MSCs in fibrin glue were assessed on days 0, 3, 7, and 14. MSCs without fibrin glue were analyzed as a control. As shown in Figure 1A, MSCs were evenly distributed in the fibrin glue and the cells maintained a rounded shape throughout the experimental period. More than 95% of the MSCs in the fibrin glue survived until day 14 (Fig. 1A, C). In contrast, the viability of MSCs without fibrin glue significantly declined to 80.4%±10% on day 7 (p<0.05) and 76.7%±3.6% on day 14 (p<0.01) (Fig. 1B, C). The BrdU incorporation assay did not indicate any proliferation of the MSCs within the fibrin glue (Fig. 1D) when they were cultured under the DMEM medium without FBS.
FIG. 1.
Survival and proliferation of mesenchymal stem cells (MSCs) in fibrin glue. (A, B) MSCs with or without fibrin were stained with acridine orange/ethidium bromide (AO/EtBr) on days 0, 3, 7, and 14 of incubation and visualized by fluorescence microscopy (Green: live cells [AO], red: dead cells [EtBr]). (C) Percentage of surviving MSCs with or without fibrin glue. At day 0, no differences were observed in the survival rate of MSCs with or without fibrin glue. Survival of MSCs in fibrin glue was sustained for 14 days, while the survival rate of MSCs without fibrin glue significantly decreased. Data are expressed as mean±SD of three different donor-derived MSCs. *p<0.05, **p<0.01 versus on day 0. (D) MSCs with or without fibrin glue were treated with BrdU for 20 h in a CO2 incubator, and then stained with the anti-BrdU antibody. MSCs with or without fibrin glue did not stain with anti-BrdU. Inset picture shows a positive control of BrdU staining in MSCs cultured under the Dulbecco's modified Eagle's medium (DMEM) containing 10% of fetal bovine serum. FG, fibrin glue. Color images available online at www.liebertpub.com/tea
MSCs in fibrin glue do not migrate to the inflammatory site
We next performed an in vitro Transwell cell migration assay to examine whether the therapeutic effect of MSCs in fibrin glue would be result from migrating to the injured site. As shown in Figure 2, migration of MSCs without fibrin glue, but not with fibrin glue, increased significantly under the inflammatory condition compared with that of the control on day 2 (3.6%±0.3% versus 88.7%±8.1%). However, only 1.8%±0.7% of MSCs in fibrin glue migrated from the fibrin glue despite the inflammatory stimulation. Migration of MSCs in fibrin glue did not increase further on day 5 (data not shown).
FIG. 2.
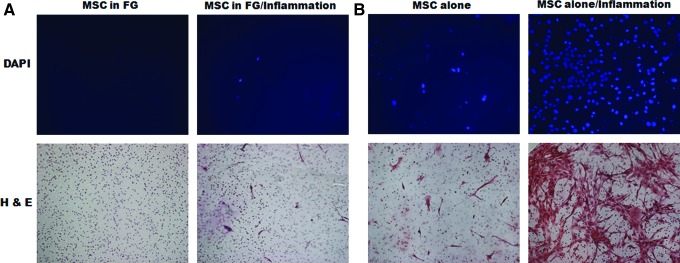
Migration of MSCs in fibrin glue. A Transwell migration assay was performed. (A) MSCs with or (B) without fibrin glue were cultured in the upper Transwell chamber in the presence or absence of inflammation in the lower chamber. After 2 days of incubation, migrated cells that remained on the lower face of the filters were stained with DAPI or hematoxylin and eosin (H&E) stain and counted. (A) MSCs in fibrin glue barely migrated when they were exposed to the inflammatory condition, whereas (B) most of the MSCs incubated without fibrin glue migrated. Color images available online at www.liebertpub.com/tea
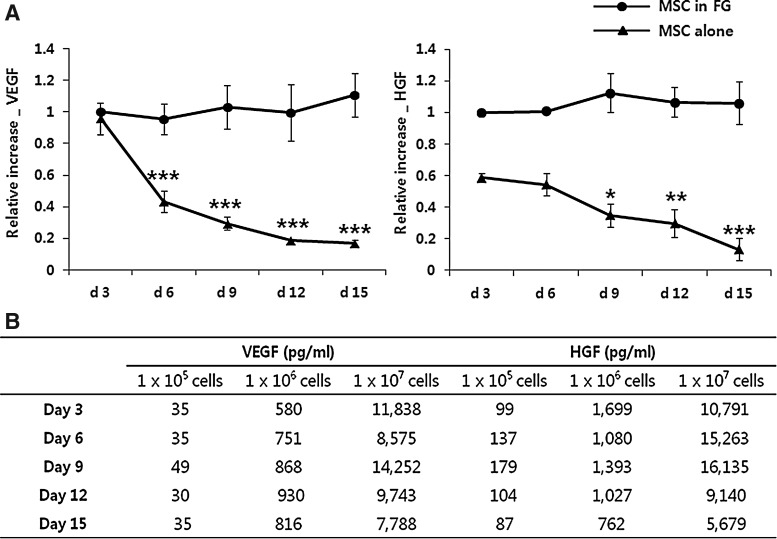
MSCs in fibrin glue continually secreted growth factors
We then examined whether MSCs in fibrin glue function for long-term culture. We incorporated 1×105/well of MSCs into fibrin glue and incubated them in the DMEM. Supernatants were collected every 3 days for 15 days and assessed for secretion of VEGF and HGF from the MSCs. All of the studies were performed using three or four different donor-derived MSCs. The baseline levels of secreted growth factors differed for each donor cell, and the data were expressed as the mean relative increase. As shown in Figure 3A, both VEGF and HGF were secreted continuously from the MSCs in fibrin glue for 15 days, while the levels of these factors secreted from MSCs without fibrin glue significantly decreased. Different numbers of MSCs in fibrin glue, 1×105, 1×106, and 1×107 MSCs/well were incubated in the same culture conditions to determine that the growth factor secreted from the MSCs in fibrin glue was correlated with the cell number. The MSCs in fibrin glue secreted VEGF and HGF in a cell number-dependent manner for 15 days (Fig. 3B).
FIG. 3.
Growth factors secreted from MSCs in fibrin glue. (A) MSCs with or without fibrin glue were incubated for 15 days in the DMEM. Supernatants were collected every 3 days and assessed for the vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF). The levels of VEGF and HGF secreted from MSCs in fibrin glue were sustained throughout the experimental period. In contrast, levels of these factors were significantly lower at all times for MSCs cultured without fibrin glue. Data are expressed as the relative changes from levels of MSCs in fibrin glue during the first 3 days. *p<0.05, **p<0.01, ***p<0.001 versus on day 3. (B) MSCs in fibrin glue were plated at 1×105–1×107 cells/well and incubated for 15 days. MSCs in fibrin glue secreted VEGF and HGF in a cell number-dependent manner. Results of one representative experiment are shown.
The effect of inflammation on secretion of soluble factors from MSCs in fibrin glue
As damaged tissues are often accompanied by inflammation, implanted MSCs may also be exposed to an inflammatory condition. Therefore, we assessed the influence of an inflammatory condition on the release of soluble factors, including VEGF, HGF, TGF-β1, and PGE2 from MSCs in fibrin glue. The baseline levels of VEGF, HGF, TGF-β1, and PGE2 secreted from MSCs in fibrin glue were 120.9±54.1, 614.5±295.4, 19.8±8.8, and 367.7±158.2 pg/105 cells, respectively, and those secreted from MSCs without fibrin glue were 114.8±30.9, 264.1±57.9, 15.0±8.8, and 91.5±13.0 pg/105 cells, respectively. These factors were not detected in fibrin glue alone, PBMC alone, and PBMC+PHA. When MSCs with or without fibrin glue were exposed to an inflammatory environment, the levels of each factor increased markedly (Fig. 4). Specifically, TGF-β1 and PGE2, which are major immune modulators secreted from MSCs,25,26 increased at least 3- and 10-fold, respectively.
FIG. 4.
The influence of inflammatory condition on soluble factors secreted from MSCs in fibrin glue. MSCs with or without fibrin glue were cultured in the presence or absence of an inflammatory stimulus such as human peripheral blood mononuclear cells (hPBMCs) and phytohemagglutinin (PHA) (5 μg/mL). Soluble factors secreted from MSCs in fibrin glue were measured after 3 days of incubation. The levels of VEGF, HGF, transforming growth factor (TGF-β1), and prostaglandin E2 (PGE2) secreted from MSCs in fibrin glue or MSCs alone increased when they were exposed to an inflammatory condition. Data are expressed as mean±SD of three or four donor-derived MSCs. *p<0.05, **p<0.01, ***p<0.001 for significant differences between normal and inflammatory test conditions. N, normal culture conditon; I, inflammatory culture condition.
MSCs in fibrin glue suppress the immune reaction
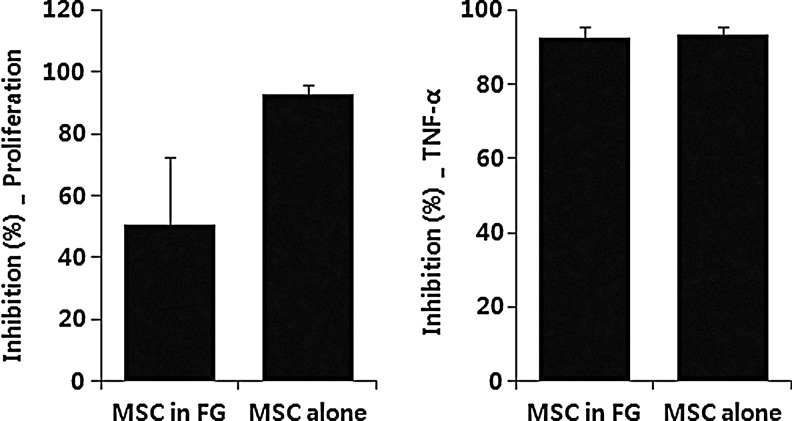
Increased release of HGF, TGF-β1, and PGE2 from MSCs supports an immunosuppressive effect of MSCs; therefore, we investigated whether the immunosuppressive function of MSCs in fibrin glue was exerted on lymphocyte proliferation and secretion of TNF-α. As shown in Figure 5, PHA-mediated proliferation of lymphocytes was significantly inhibited by MSCs in fibrin glue (50.4%±21.8%) and by MSCs alone (92.8%±3.2%). The TNF-α levels also decreased >90% in MSCs with or without fibrin glue.
FIG. 5.
Immunosuppressive effect of MSCs in fibrin glue. hPBMCs were cocultured with MSCs with or without fibrin glue in the presence or absence of the inflammatory stimulus, PHA (5 μg/mL). The PHA-mediated proliferation of lymphocytes was significantly inhibited by MSCs in fibrin glue (50.4%±21.8%) and by MSCs without fibrin glue (92.8%±3.2%). Levels of tumor necrosis factor (TNF)-α secreted from PBMCs were decreased >90% by addition of MSCs with or without fibrin glue. Data are expressed as mean±SD of four different donor-derived MSCs.
The influence of fibrin glue on preventing MSC death
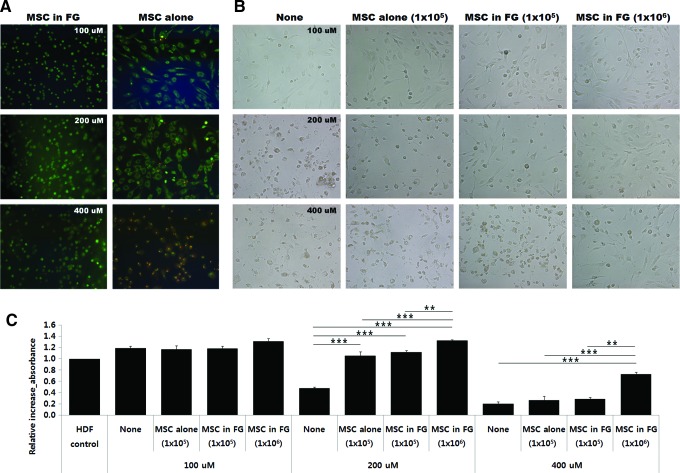
We finally examined whether incorporated MSCs in fibrin glue could prevent cell death from microenvironmental stress. No significant difference was observed between MSC viability with or without fibrin glue (94.8%±3.9% vs. 91.2%±2.3%) when MSCs were exposed to low (100 μM) concentrations of tbOOH. MSC viability decreased to 82.6%±2.3% and 0%, respectively, when MSCs alone were exposed to 200 or 400 μM tbOOH (Fig. 6A). However, MSCs in fibrin glue showed good viability of 94.9%±0.8% under severe oxidative stress (400 μM of tbOOH). These results indicate that fibrin glue protected MSCs from severe oxidative stress.
FIG. 6.
The influence of MSCs in fibrin glue on oxidative stress-induced cell death. Human dermal fibroblasts (HDFs) were plated into the lower chamber of a Transwell and incubated for 24 h. Following adhesion of the HDFs, the upper chambers, including fibrin glue alone (control), 1×105, or 1×106 MSCs with or without fibrin glue were inserted into the Transwell, and then incubated with tert-butyl hydroperoxide (tbOOH) for another 12 h. (A) Inserts were removed and stained with AO/EtBr, and (B) the remaining HDFs in the lower chamber were observed by microscopy and (C) HDFs were quantified by the WST-1 assay. (A) MSCs in fibrin glue maintained a high survival rate under severe oxidative stress and (B, C) protected the HDFs from severe oxidative stress. Data are expressed as mean±SD of three different donor-derived MSCs. **p<0.01, ***p<0.001 versus control culture at each concentration of tbOOH. Color images available online at www.liebertpub.com/tea
We further investigated whether MSCs in fibrin glue could protect other surrounding cells from a noxious environment such as oxidative stress. HDFs were exposed to tbOOH in the presence of 1×105 or 1×106 MSCs with or without fibrin glue, cell morphology was observed under a microscope, and viability was assessed by the WST-1 assay. HDFs detached from the culture plate and became rounded and shrunken after tbOOH treatment. Cell injury became more severe as the tbOOH concentration was increased (Fig. 6B). In contrast, HDFs treated with MSCs alone or MSCs in fibrin glue were relatively less seriously injured, and they maintained their spindle-like shape in the presence of 200 μM tbOOH. The HDFs maintained their normal shape only in the presence of fibrin glue containing 1×106 MSCs when a higher (400 μM) tbOOH concentration was tested (Fig. 6B).
When HDF survival was assessed by the WST-1 assay, no significant differences were observed among the nontreated (control), 1×105 MSCs alone, and the 1×105 and 1×106 MSCs in fibrin glue-treated cells exposed to 100 μM tbOOH. At 200 μM tbOOH, the HDF survival rate increased 1.8±0.6, 1.9±0.6, and 2.3±0.7-fold, respectively (p<0.001), compared to that in the control when they were treated with 1×105 MSCs alone, 1×105 or 1×106 MSCs in fibrin glue. When cells were exposed to a 400 μM tbOOH, the survival rate of HDFs treated with 1×106 MSCs in fibrin glue increased 3.7±0.7-fold compared to that in the control (p<0.001). In contrast, treatment with 1×105 MSCs alone or MSCs in fibrin glue did not protect HDFs from severe oxidative stress. We could not assess the effect of 1×106 MSCs without fibrin glue because it was impossible to load the MSCs into the upper chamber.
Discussion
Our results suggest that fibrin glue may prolong the therapeutic function of MSCs by sustaining the microenvironment through secretion of growth factors, cytokines, and immunomodulatory factors as a result of their increased survival rate.
In the present study, we carried out using MSCs isolated from adipose tissue because they are easy to harvest and yield a large number of cells, and we have confirmed their therapeutic effects through preclinical and clinical studies.13,15,16 Implanted MSCs should stably and functionally survive for a sufficient time to have a therapeutic effect at the damaged or wound site. We showed that MSCs within fibrin glue survived >14 days at 37°C without additional nutrients such as FBS or growth factors, whereas the viability of MSCs without fibrin glue decreased gradually. This result is consistent with previous reports.18,27 The reason for the high survival rate of MSCs in fibrin glue may be the suitable matrix environment, such as temporary retention space, cytoprotective effects, and cell–matrix interactions provided by the fibrin glue. However, we could not observe evidence of MSC proliferation within fibrin glue in a BrdU incorporation assay, which was different from a previous study.20 This discrepancy could be explained by the fact that Ho et al.20 used growth factors or serum in their experiments. Considering that implanted cells to a damaged site practically do not get sufficient nutrients due to obstructed blood vessels, our study design is considered to be more representative of in vivo conditions. In addition, the fibrinogen concentration is important for cell morphology, proliferation, and migration. An undiluted high concentration of fibrin glue has a longer degradation time compared to that of diluted fibrin glue, which forms a denser mass with smaller pore sizes and thicker fibrin fibers compared with diluted fibrin glue.28 Thus, a lower fibrin glue concentration (10 mg/mL) promotes cell proliferation relatively more than a higher fibrin glue concentration.19,29 In the present study, we used an undiluted high concentration of fibrin glue (126–256 mg/mL) to keep the MSCs incorporated into fibrin glue at the implanted site. For the same reason, MSCs in fibrin glue hardly migrated during the 5 days of incubation, whereas most of the MSCs without fibrin glue migrated to the inflammatory site in vitro. During the experimental period, fibrin glue did not degrade in vitro. However, as stated above, it is biodegraded by plasmin in vivo within 1–2 weeks depending on its formulation and is then eliminated by the kidneys. These results suggest that MSCs implanted into damaged tissue with fibrin glue could effectively survive without cell migration for at least 1–2 weeks during fibrin glue degradation.
We also analyzed the amounts of VEGF and HGF, these well recognized angiogenic and antiapoptotic factors secreted from MSCs.30 We found that MSCs in fibrin continuously secreted VEGF and HGF in a cell number-dependent manner for 15 days without sufficient nutrients or stimulation. On the other hand, as time passed, those levels significantly decreased in MSCs alone, suggesting that MSCs implanted into damaged tissue with fibrin glue could produce a long-lasting therapeutic action through longer survival. In addition to these factors, we assessed TGF-β1, insulin-like growth factor (IGF), and bFGF. TGF-β1 was detected less than 50 pg/mL per 105 cells, but bFGF and IGF were not detected regardless of fibrin glue under our experimental condition (data not shown). These factors have been reported to be expressed in MSCs,31 but some studies have shown that bFGF and EGF were not expressed in MSCs.32 This discrepancy is due to the use of different cell culture conditions, such as the culture medium, serum, and growth factors.33
Inflammation is a common finding in most wound sites and various diseases. Therefore, MSCs implanted into a wound or disease site are exposed to an inflammatory environment and are activated by interferon (IFN)-γ, TNF-α, interleukin (IL)-1α, or IL-1β. Thus, we examined the influence of these conditions on MSCs in fibrin glue by mimicking the inflammatory condition in vitro. The baseline levels of each soluble factor secreted from MSCs depending on the presence of fibrin glue were different. HGF and PGE2 secretion increased in the presence of fibrin glue, but no differences were observed for VEGF and TGF-β1 secretion in the presence of fibrin glue. However, each factor dramatically increased when MSCs were exposed to the inflammatory condition regardless of the presence of fibrin glue, suggesting that MSCs in fibrin glue could effectively activate at a wound or disease site. Moreover, TGF-β1 and PGE2 levels increased at least 3- and 10-fold, respectively, from the normal condition.
HGF, TGF-β1, and PGE2 are anti-inflammatory mediators that prevent proliferation of T cells, inhibit production of TNF-α and IL-12, and downregulate major histocompatibility complex-II on the macrophage surface.34–38 Consistent with these results, MSCs in fibrin glue effectively inhibited excess immune reactions (Fig. 4). Fibrin glue contains countless pores that may allow the passage of soluble factors secreted from MSCs (e.g., HGF, TGF-β1, or PGE2) or PBMCs (e.g., TNF-α or IFN-γ). Inhibition of lymphocyte proliferation by MSCs in fibrin glue was less than MSCs without fibrin glue, although the exact reason for this decrease is not clear, but a direct inhibition between MSCs and PBMCs may require full inhibitory activity.39,40 TNF-α released by PHA-stimulated PBMCs was effectively inhibited by MSCs in fibrin glue.
Cells undergo apoptosis and/or necrosis resulting from injuries or infections. MSCs also suffer from an adverse environment after they are implanted to disease sites. We showed that fibrin glue effectively protected MSCs from severe oxidative stress by tbOOH, resulting in the prevention of fibroblast death during exposure to extreme stress. These results are consistent with the study of Guo et al.19 who showed that MSCs cultured in fibrin glue resist apoptosis and necrosis by inducing oxygen and glucose deprivation.
In conclusion, the present in vitro data demonstrated that MSCs in fibrin glue sustain functional survival and paracrine function and suggest further development of MSCs with fibrin glue as a clinical treatment. The influence of fibrin glue on MSC paracrine function in vivo requires further investigation.
Acknowledgments
This work was supported by a research grant from the Korea Health 21 R&D Project, Ministry of Health Welfare and Family Affairs, Republic of Korea (No. A100041), and the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MEST) (No. 2012-0000952).
Disclosure Statement
No competing financial interests exist.
References
- 1.Pittenger M.F. Mackay A.M. Beck S.C. Jaiswal R.K. Douglas R. Mosca J.D. Moorman M.A. Simonetti D.W. Craig S. Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 2.da Silva Meirelles L. Chagastelles P.C. Nardi N.B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 3.Kuzmina L.A. Petinati N.A. Parovichnikova E.N. Lubimova L.S. Gribanova E.O. Gaponova T.V. Shipounova I.N. Zhironkina O.A. Bigildeev A.E. Svinareva D.A. Drize N.J. Savchenko V.G. Multipotent mesenchymal stromal cells for the prophylaxis of acute graft-versus-host disease: a phase II study. Stem Cells Int. 2012;2012:8. doi: 10.1155/2012/968213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Connick P. Kolappan M. Crawley C. Webber D.J. Patani R. Michell A.W. Du M.Q. Luan S.L. Altmann D.R. Thompson A.J. Compston A. Scott M.A. Miller D.H. Chandran S. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an open-label phase 2a proof-of-concept study. Lancet Neurol. 2012;11:150. doi: 10.1016/S1474-4422(11)70305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orozco L. Soler R. Morera C. Alberca M. Sánchez A. García-Sancho J. Intervertebral disc repair by autologous mesenchymal bone marrow cells: a pilot study. Transplantation. 2011;92:822. doi: 10.1097/TP.0b013e3182298a15. [DOI] [PubMed] [Google Scholar]
- 6.Jiang R. Han Z. Zhuo G. Qu X. Li X. Wang X. Shao Y. Yang S. Han Z.C. Transplantation of placenta-derived mesenchymal stem cells in type 2 diabetes: a pilot study. Front Med. 2011;5:94. doi: 10.1007/s11684-011-0116-z. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y. Wang F. Chen J. Ning Z. Yang L. Bone marrow-derived mesenchymal stem cells versus bone marrow nucleated cells in the treatment of chondral defects. Int Orthop. 2012;36:1079. doi: 10.1007/s00264-011-1362-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kharaziha P. Hellström P.M. Noorinayer B. Farzaneh F. Aghajani K. Jafari F. Telkabadi M. Atashi A. Honardoost M. Zali M.R. Soleimani M. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: a phase I-II clinical trial. Eur J Gastroenterol Hepatol. 2009;21:1199. doi: 10.1097/MEG.0b013e32832a1f6c. [DOI] [PubMed] [Google Scholar]
- 9.Baek S.J. Kang S.K. Ra J.C. In vitro migration capacity of human adipose tissue-derived mesenchymal stem cells reflects their expression of receptors for chemokines and growth factors. Exp Mol Med. 2011;43:596. doi: 10.3858/emm.2011.43.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ponte A.L. Marais E. Gallay N. Langonné A. Delorme B. Hérault O. Charbord P. Domenech J. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25:1737. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- 11.da Silva Meirelles L. Caplan A.I. Nardi N.B. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26:2287. doi: 10.1634/stemcells.2007-1122. [DOI] [PubMed] [Google Scholar]
- 12.Schrepfer S. Deuse T. Reichenspurner H. Fischbein M.P. Robbins R.C. Pelletier M.P. Stem cell transplantation: the lung barrier. Transplant Proc. 2007;39:573. doi: 10.1016/j.transproceed.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 13.Hyun Y.Y. Kim I.O. Kim M.H. Nam D.H. Lee M.H. Kim J.E. Song H.K. Cha J.J. Kang Y.S. Lee J.E. Kim H.W. Han J.Y. Cha D.R. Adipose-derived stem cells improve renal function in a mouse model of IgA nephropathy. Cell Transplant. 2012;21:2425. doi: 10.3727/096368912X639008. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Olmo D. Herreros D. Pascual I. Pascual J.A. Del-Valle E. Zorrilla J. De-La-Quintana P. Garcia-Arranz M. Pascual M. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Dis Colon Rectum. 2009;52:79. doi: 10.1007/DCR.0b013e3181973487. [DOI] [PubMed] [Google Scholar]
- 15.Cho Y.B. Lee W.Y. Park K.J. Kim M. Yoo H.W. Yu C.S. Autologous adipose tissue-derived stem cells for the treatment of Crohn's fistula: a phase I clinical study. Cell Transplant. 2013;22:279. doi: 10.3727/096368912X656045. [DOI] [PubMed] [Google Scholar]
- 16.Lee Y.W. Park K.J. Cho Y.B. Yoon S.N. Song K.H. Kim D.S. Jung S.H. Kim M. Yoo H.W. Kim I. Ha H. Yu C.S. Autologous adipose tissue-derived stem cells treatment demonstrated favorable and sustainable therapeutic effect for crohn's fistula. Stem Cells. 2013 doi: 10.1002/stem.1357. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Han S.K. Kim H.R. Kim W.K. The treatment of diabetic foot ulcers with uncultured, processed lipoaspirate cells: a pilot study. Wound Repair Regen. 2010;18:342. doi: 10.1111/j.1524-475X.2010.00593.x. [DOI] [PubMed] [Google Scholar]
- 18.Haleem A.M. Singergy A.A. Sabry D. Atta H.M. Rashed L.A. Chu C.R. El Shewy M.T. Azzam A. Abdel Aziz M.T. The clinical use of human culture-expanded autologous bone marrow mesenchymal stem cells transplanted on platelet-rich fibrin glue in the treatment of articular cartilage defects: a pilot study and preliminary results. Cartilage. 2010;1:253. doi: 10.1177/1947603510366027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo H.D. Wang H.J. Tan Y.Z. Wu J.H. Transplantation of marrow-derived cardiac stem cells carried in fibrin improves cardiac function after myocardial infarction. Tissue Eng Part A. 2011;17:45. doi: 10.1089/ten.TEA.2010.0124. [DOI] [PubMed] [Google Scholar]
- 20.Ho W. Tawil B. Dunn J.C. Wu B.M. The behavior of human mesenchymal stem cells in 3D fibrin clots: dependence on fibrinogen concentration and clot structure. Tissue Eng. 2006;12:1587. doi: 10.1089/ten.2006.12.1587. [DOI] [PubMed] [Google Scholar]
- 21.Ahmed T.A. Giulivi A. Griffith M. Hincke M. Fibrin glues in combination with mesenchymal stem cells to develop a tissue-engineered cartilage substitute. Tissue Eng Part A. 2011;17:323. doi: 10.1089/ten.TEA.2009.0773. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X. Wang H. Ma X. Adila A. Wang B. Liu F. Chen B. Wang C. Ma Y. Preservation of the cardiac function in infarcted rat hearts by the transplantation of adipose-derived stem cells with injectable fibrin scaffolds. Exp Biol Med (Maywood) 2010;235:1505. doi: 10.1258/ebm.2010.010175. [DOI] [PubMed] [Google Scholar]
- 23.Wang J. Wang J. Liao L. Tan J. Mesenchymal-stem-cell-based experimental and clinical trials: current status and open questions. Expert Opin Biol Ther. 2011;11:893. doi: 10.1517/14712598.2011.574119. [DOI] [PubMed] [Google Scholar]
- 24.Asanuma H. Meldrum D.R. Meldrum K.K. Therapeutic applications of mesenchymal stem cells to repair kidney injury. J Urol. 2010;184:26. doi: 10.1016/j.juro.2010.03.050. [DOI] [PubMed] [Google Scholar]
- 25.Uccelli A. Moretta L. Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 26.Cui L. Yin S. Liu W. Li N. Zhang W. Cao Y. Expanded adipose-derived stem cells suppress mixed lymphocyte reaction by secretion of prostaglandin E2. Tissue Eng. 2007;13:1185. doi: 10.1089/ten.2006.0315. [DOI] [PubMed] [Google Scholar]
- 27.Girandon L. Kregar-Velikonja N. Božikov K. Barlič A. In vitro models for adipose tissue engineering with adipose-derived stem cells using different scaffolds of natural origin. Folia Biol (Praha) 2011;57:47. [PubMed] [Google Scholar]
- 28.Lee H.H. Haleem A.M. Yao V. Li J. Xiao X. Chu C.R. Release of bioactive adeno-associated virus from fibrin scaffolds: effects of fibrin glue concentrations. Tissue Eng Part A. 2011;17:1969. doi: 10.1089/ten.tea.2010.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cox S. Cole M. Tawil B. Behavior of human dermal fibroblasts in three-dimensional fibrin clots: dependence on fibrinogen and thrombin concentration. Tissue Eng. 2004;10:942. doi: 10.1089/1076327041348392. [DOI] [PubMed] [Google Scholar]
- 30.Rehman J. Traktuev D. Li J. Merfeld-Clauss S. Temm-Grove C.J. Bovenkerk J.E. Pell C.L. Johnstone B.H. Considine R.V. March K.L. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 31.Williams A.R. Hare J.M. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011;109:923. doi: 10.1161/CIRCRESAHA.111.243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoo K.H. Jang I.K. Lee M.W. Kim H.E. Yang M.S. Eom Y. Lee J.E. Kim Y.J. Yang S.K. Jung H.L. Sung K.W. Kim C.W. Koo H.H. Comparison of immunomodulatory properties of mesenchymal stem cells derived from adult human tissues. Cell Immunol. 2009;259:150. doi: 10.1016/j.cellimm.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 33.Sotiropoulou P.A. Perez S.A. Salagianni M. Baxevanis C.N. Papamichail M. Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells. 2006;24:462. doi: 10.1634/stemcells.2004-0331. [DOI] [PubMed] [Google Scholar]
- 34.Yañez R. Oviedo A. Aldea M. Bueren J.A. Lamana M.L. Prostaglandin E2 plays a key role in the immunosuppressive properties of adipose and bone marrow tissue-derived mesenchymal stromal cells. Exp Cell Res. 2010;316:3109. doi: 10.1016/j.yexcr.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 35.Goodwin J.S. Bankhurst A.D. Messner R.P. Suppression of human T-cell mitogenesis by prostaglandin. Existence of a prostaglandin-producing suppressor cell. J Exp Med. 1977;146:1719. doi: 10.1084/jem.146.6.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scales W.E. Chensue S.W. Otterness I. Kunkel S.L. Regulation of monokine gene expression: prostaglandin E2 suppresses tumor necrosis factor but not interleukin-1 alpha or beta-mRNA and cell-associated bioactivity. J Leukoc Biol. 1989;45:416. [PubMed] [Google Scholar]
- 37.van der Pouw Kraan T.C. Boeije L.C. Smeenk R.J. Wijdenes J. Aarden L.A. Prostaglandin-E2 is a potent inhibitor of human interleukin 12 production. J Exp Med. 1995;181:775. doi: 10.1084/jem.181.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Snyder D.S. Beller D.I. Unanue E.R. Prostaglandins modulate macrophage Ia expression. Nature. 1982;299:163. doi: 10.1038/299163a0. [DOI] [PubMed] [Google Scholar]
- 39.Sotiropoulou P.A. Perez S.A. Gritzapis A.D. Baxevanis C.N. Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24:74. doi: 10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
- 40.Jiang X.X. Zhang Y. Liu B. Zhang S.X. Wu Y. Yu X.D. Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]