Abstract
Purpose
Measuring prostate cancer patient HRQOL in routine clinical practice is hindered by lack of instruments enabling efficient real-time, point-of-care scoring of multiple HRQOL domains. We sought to develop an instrument for this purpose.
Materials and Methods
The EPIC for Clinical Practice (EPIC-CP) is a one-page, 16-item questionnaire to measure urinary incontinence, urinary irritation, bowel, sexual, and hormonal HRQOL domains that we constructed by eliminating conceptually overlapping items from the 3 page EPIC-26, and revising the questionnaire format to mirror the AUA Symptom Index, thereby enabling practitioners to calculate HRQOL scores at point of care. We administered EPIC-CP to a new cohort of PCa patients in community-based and academic oncology, radiation, and urology practices to evaluate the instrument’s validity and ease of use for clinical practice.
Results
175 treated and 132 untreated PCa subjects completed EPIC-CP (N = 307). EPIC-CP domain scores correlated highly with respective domain scores from longer versions of EPIC (r ≥ 0.93 for all domains). EPIC-CP showed high internal consistency (Cronbach’s α = 0.64-0.84) and sensitivity to PCa treatment-related effects (p < 0.05 in each of 5 HRQOL domains). Patients completed EPIC-CP efficiently (96% in <10 minutes, and 11% missing items). It was deemed ‘very convenient’ by clinicians in 87% of routine clinical encounters, and clinicians accurately scored completed questionnaires 94% of the time.
Conclusions
EPIC-CP is a valid instrument that enables patient-reported HRQOL to be measured efficiently and accurately at the point of care, and can thereby facilitate improved emphasis and management of patient-reported outcomes.
Keywords: Prostatic neoplasms/therapy, Quality of Life, Outcome Assessment (Health Care), Questionnaires
INTRODUCTION
As survivorship after prostate cancer (PCa) diagnosis continues to improve with advances in detection and treatment, the effects of PCa management modalities on health-related quality of life (HRQOL) are becoming increasingly important. Patterns in HRQOL change for each PCa treatment modality are well-recognized1, but objective characterization and quantification of such changes is challenging, especially outside of the research realm in the flow of a busy clinical practice.
The 50-item Expanded Prostate Cancer Index Composite (EPIC)2, a validated domain-specific patient-reported questionnaire derived from the UCLA-Prostate Cancer Index (UCLA-PCI)3 designed to assess HRQOL after PCa treatment, was successfully reduced into a shorter 26-item form (EPIC-26)4, but can still be too cumbersome to administer in the routine clinical setting. Its three-page length is daunting to patients, it often takes longer than 10-15 minutes to administer, and it requires transformation of question answers into a 0-100 scale to calculate domain scores.
The AUA Symptom Index (AUA-SI)/International Prostate Symptom Score (IPSS) and the International Index of Erectile Function (IIEF-5)/Sexual Health Inventory for Men (SHIM) are examples of validated instruments that have become standard clinical tools in the management of lower urinary tract symptoms and erectile dysfunction, respectively.5,6 A correlate for use specifically in patients with prostate cancer has not yet been realized. In fact, no validated tool measuring HRQOL in PCa patients currently exists in a form that would be practical for use in both community and academic clinical practices. Hence, we set out to develop and validate a shorter and more accessible HRQOL instrument designed specifically for use in the routine clinical care of prostate cancer patients: the Expanded Prostate Cancer Index Composite for Clinical Practice (EPIC-CP).*
METHODS
Study populations
We performed initial psychometric analysis of EPIC-CP using the previously described EPIC-50/EPIC-26 Cohort2,4, consisting of 252 subjects who had undergone brachytherapy, external radiotherapy, and radical prostatectomy. After institutional review board exemption was obtained because no protected health information was collected, we administered EPIC-CP from June to October, 2010 in an anonymous fashion to the EPIC-CP Validation Cohort, a new cross-sectional cohort (n=307) of PCa subjects of any stage in academic and community Radiation Oncology, Medical Oncology, Urology, and multidisciplinary clinics who were either as-of-yet untreated, on active surveillance, or were within one year of receiving any PCa treatment, either localized primary or systemic (Table 1). Those unable to read English were excluded.
Table 1.
Patient and Disease Characteristics of the EPIC-CP Validation Cohort (n=307)*
| Age at enrollment – median (IQR) | 65 (59-70) |
| Race – no. (%) | |
| White/Caucasian | 245 (79.8) |
| African-American | 28 (9.1) |
| Other/Unknown | 34 (11.1) |
| PSA (ng/mL) – median (IQR) | 5.7 (4.4 – 8.2) |
| Clinical T-stage – no. (%) | |
| cT1 | 194 (63.2) |
| cT2 | 68 (22.1) |
| cT3-T4 | 20 (6.5) |
| cTX | 19 (6.2) |
| Biopsy Gleason Score – no. (%) | |
| 6 or less | 117 (38.1) |
| 7 | 125 (40.7) |
| 8-10 | 47 (15.3) |
| Clinical Setting – no. (%) | |
| Medical Oncology | 54 (17.6) |
| Radiation Oncology | 88 (28.7) |
| Urology | 74 (24.1) |
| Multidisciplinary clinic | 81 (26.4) |
| Primary Treatment – no. (%) | |
| Radical Prostatectomy | 64 (20.9) |
| External beam radiotherapy | 63 (20.5) |
| Brachytherapy | 38 (12.4) |
| Hormonal therapy only | 10 (3.3) |
| Active surveillance | 32 (10.4) |
| Untreated | 100 (32.6) |
| Hormonal therapy as part of Primary | |
| Treatment – no. (%) | |
| No | 258 (84.0) |
| Yes | 49 (16.0) |
May not sum to total patients because of occasional missing values
Abbreviations: IQR=interquartile range; PSA=prostate-specific antigen
Instrument Development
We developed EPIC-CP from item reduction and reformatting of EPIC-26.4 We gathered input and feedback regarding EPIC-CP format and content from two focused project discussion and review sessions: one project review group comprised 6 urology faculty and 9 trainees, and a separate discussion and review from a multidisciplinary group of medical oncologists (3), radiation oncologists (3), and urologists (3 academic, 2 community).
EPIC-26 reduction strategy
We performed reduction of EPIC-26 with the goal and challenge of maintaining a balance between validity, clinical relevance, sensitivity, and a manageable and consistent number of questions (three) per health domain. Items were selected for retention using an iterative process. We preserved the overall bother items from the urinary, bowel, and sexual domains for content and consistency with previous versions of EPIC2,4 and the UCLA-PCI.3 As in EPIC-26, the overall urinary bother item in EPIC-CP is evaluated separately from the urinary incontinence and irritative/obstructive scales, as it can be influenced by either or both of these domains. We examined item-score correlations between individual EPIC-26 items and their respective domain scores4, and selected questions with stronger item-score correlations. We resolved similar item-score correlations between two items in a domain by choosing the item with a higher prevalence of severity reported in a multicenter prospective trial on PCa treatment-related HRQOL effects.7 Questions whose use in routine clinical practice was particularly prevalent were favored. If multiple items within a domain addressed a single or similar construct, we retained only one of those items.
In the urinary incontinence domain, the leaking bother question and the assessment of urinary control had the highest item-score correlations. We kept the question regarding number of pads per day despite its lower relative item-score correlation because of its prevalence in clinical practice. In the urinary irritation/obstruction domain, questions regarding dysuria, weak stream, and urinary frequency had the highest item-score correlations. In the bowel domain, we retained the overall bowel bother item for its high item-score correlation as well as for content, and the frequency and urgency items for their high item-score correlations. We dropped the hematochezia item because of its low correlation with the domain score. We recognized the importance of assessing rectal pain in patients who have undergone radiotherapy; therefore, we grouped this item with the urgency item, the combination of which often characterizes tenesmus in radiation proctitis. In the sexual domain, the correlation between bother and function (r < 0.65) has been shown to be lower than in other domains.2 Therefore, we kept the overall sexual bother item for content despite its low correlation with the domain score. We preserved the question regarding the firmness of erections for its prevalence in clinical practice, and dropped the other items concerning erections for their relative redundancy. The item regarding orgasm was kept for its conceptual difference from the already-retained items. In the vitality/hormonal domain, all item-score correlations were relatively low because of the domain’s broad, systemic nature. We retained the depression and lack-of-energy items for their higher item-score correlations. We included and grouped hot flashes and breast problems because of their high prevalence, accompanying bother, and frequent need for symptomatic treatment in patients undergoing androgen deprivation therapy.8-11
The EPIC-CP scoring system
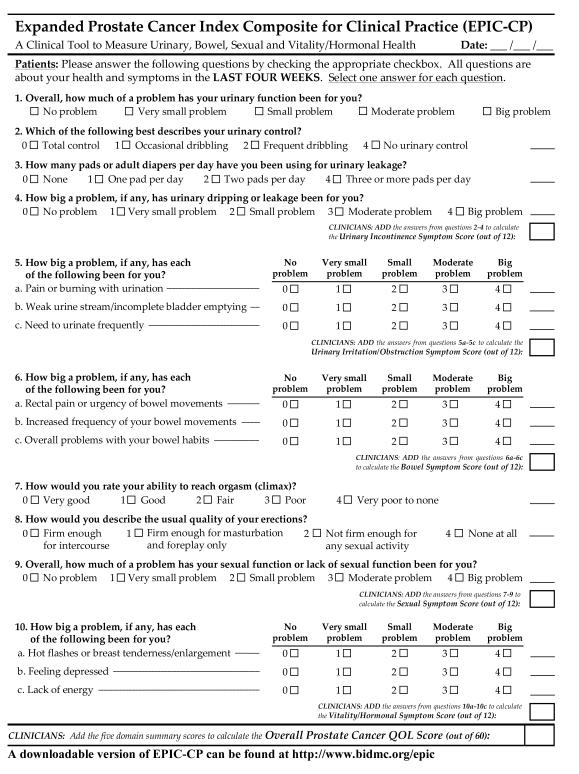
We designed EPIC-CP so that symptom scores for each domain (urinary incontinence, urinary irritative/obstructive, bowel, sexual, and vitality/hormonal) are calculated similarly as is done with the AUA-SI/IPSS,5 (i.e. higher scores reflect worse symptom/bother severity) by simply adding together the numeric values (0 to 4) that correspond to patient responses in each of 3 questions comprising each HRQOL domain (Figure 1). Consequently, the minimum symptom score (best HRQOL) = 0 and the maximum symptom score (worst HRQOL) = 12 in each domain. For consistency, the values assigned to each question range from 0 (best) to 4 (worst) regardless of whether there were 4 or 5 response options per question. This design allows calculation of domain scores at the point of care without requiring time-consuming transformations to 0-100 ranges; it also allows calculating an overall HRQOL score by summing the five domain scores (maximum of 60). The overall urinary bother item (first question) is a stand-alone item not used in scoring any of the three-item domain scores because it relates to both urinary incontinence and irritation/obstruction.
Figure 1.
Analysis and Validation
We first evaluated the EPIC-CP scoring system to previously-collected responses from the original EPIC-50/EPIC-26 Cohort (n = 252) that had been administered by mail survey2,4. Questions 2, 3, and 8 were transformed as 0, 25, 50, and 100%, as described above. The more severe answer was scored in combination questions (6a and 10a). We calculated item-score correlations between EPIC-CP items and their respective domain scores, and used the Pearson correlation coefficient to calculate the interscale correlations between the EPIC-CP, EPIC-26, and EPIC-50 domains, as well as between the EPIC-CP urinary irritative/obstructive domain and the AUA-SI. We used Cronbach’s alpha coefficient to evaluate internal consistency.
After initial psychometric analysis, we evaluated EPIC-CP in the new EPIC-CP Validation Cohort (n = 307). No study personnel assisted in the distribution or completion of EPIC-CP. Questionnaires were administered in the office setting and practitioners provided demographic and cancer-related information for each subject. Subjects were asked how long EPIC-CP took to complete, and cancer practitioners rated the convenience of the instrument’s use in the flow of clinical practice with a 4-point Likert item. We calculated mean, standard deviation, median, range, and the percentage of subjects scoring the minimum (floor) and maximum (ceiling) in each domain in all subjects who underwent any PCa therapy (n = 175). We used Cronbach’s alpha to evaluate internal consistency, and estimated its variability by using confidence intervals from 500 bootstrap samples. To determine the sensitivity of EPIC-CP to PCa treatment effects, we used ANOVA to compare mean scores of treated subjects for each HRQOL domain against that of a group untreated subjects (on active surveillance or treatment not yet started; n = 132). All statistical analyses were performed using Statistical Analysis Systems software (SAS), version 9.2.
RESULTS
A complete, ready-to-use copy of EPIC-CP can be found at http://www.bidmc.org/epic. The questionnaire is formatted to fit on one page, contains 16 items, and retains the five EPIC HRQOL domains of urinary incontinence, urinary irritation/obstruction, bowel, sexual, and vitality/hormonal. Eight questions from the UCLA-PCI (including the bother items from the urinary, bowel, and sexual domains) and 6 questions from EPIC-50/EPIC-26 are preserved. Two items combine questions from EPIC-26. Each domain contains 3 questions scored 0-4, encompassing a domain score range of 0-12, with a lower score indicating more favorable HRQOL.
After rescoring the EPIC-50/EPIC-26 Cohort using EPIC-CP, we found that EPIC-CP domain summary scores correlated strongly with corresponding scores in previous versions of EPIC (r ≥ 0.93 in all domains; Table 2). Score correlation between different domain was modest (r < 0.50 for all correlations; data not shown), indicating that the EPIC-CP domains are conceptually distinct and merit independent measure. The EPIC-CP urinary irritative/obstructive domain correlated well with the AUA-SI (r = 0.81).
Table 2.
Correlation between individual items and total scores for EPIC-26 and EPIC for Clinical Practice (EPIC-CP), and correlations of domain summary scores of EPIC-CP with previous versions of EPIC upon rescoring of the EPIC-50/EPIC-26 Cohort
| Quality-of-life domain and individual EPIC-CP questionnaire item |
Item number | Item-scale correlation | Domain score correlations of EPIC-CP with previous versions of EPIC |
|||
|---|---|---|---|---|---|---|
|
| ||||||
| EPIC-26 | EPIC-CP | EPIC-26 | EPIC-CP | EPIC-50 | EPIC-26 | |
| Urinary domains | ||||||
| Overall urinary problem (1)* | 5 | 1 | n/a | n/a | ||
| Incontinence subscale (3) | 0.96 | 0.96 | ||||
| Frequent dribbling* | 2 | 2 | 0.77 | 0.75 | ||
| Any pad use* | 3 | 3 | 0.66 | 0.66 | ||
| Leaking problem* | 4.a | 4 | 0.83 | 0.76 | ||
|
| ||||||
| Irritation/obstruction subscale (3) | 0.98 | 0.97 | ||||
| Dysuria† | 4.b | 5.a | 0.65 | 0.61 | ||
| Weak stream† | 4.d | 5.b | 0.67 | 0.69 | ||
| Frequency† | 4.e | 5.c | 0.61 | 0.62 | ||
|
| ||||||
| Bowel domain (3) | 0.97 | 0.94 | ||||
| Urgency or rectal pain‡ | 6.a/6.e | 6.a | 0.77/0.65 | 0.81 | ||
| Frequency† | 6.b | 6.b | 0.81 | 0.80 | ||
| Overall bowel problem* | 7 | 6.c | 0.83 | 0.77 | ||
|
| ||||||
| Sexual domain (3) | 0.95 | 0.93 | ||||
| Difficulty with orgasm* | 8.b | 7 | 0.68 | 0.63 | ||
| Erections not firm* | 9 | 8 | 0.79 | 0.62 | ||
| Overall sexuality problem* | 12 | 9 | 0.50 | 0.35 | ||
|
| ||||||
| Vitality or hormonal domain (3) | 0.97 | 0.94 | ||||
| Hot flashes or breast problems‡ | 13.a/13.b | 10.a | 0.38/0.31 | 0.29 | ||
| Depression† | 13.c | 10.b | 0.62 | 0.60 | ||
| Lack of energy† | 13.d | 10.c | 0.58 | 0.62 | ||
These 8 items (1, 2, 3, 4, 6c, 7, 8, 9 were original UCLA-PCI3 items retained for generalizability and clinically significant assessment; item 5 in EPIC-26 and item 1 in EPIC-CP are the UCLA urinary bother item; because this item related to both incontinence and irritative/obstructive scale in factor analyses and conceptual content, it was not included in either irritative/obstructive nor urinary incontinence scale, but was retained to enable assessment of overall urinary bother, which could be influenced by either or both of these domains.
These items were retained from both EPIC-50 and EPIC-26
Two items from EPIC-26 were combined in order to preserve conceptual content in EPIC-CP.
We then externally validated EPIC-CP by administration to a new EPIC-CP Validation Cohort (Table 3), comprised of patients before or after treatment as well as some men on active surveillance. Survey completion rate was 89%, post-treatment subjects usually completed EPIC-CP within 6 months of primary treatment (median = 174 days), and treatment effects on response rates were not evident. Floor effects were evident in the urinary incontinence, bowel, and vitality/hormonal domains, but several subjects in these domains scored a near-maximum score of 11, demonstrating the capacity of EPIC-CP to capture a large range of side-effect severity. The urinary, bowel, and sexual domains demonstrated strong internal consistency (Cronbach’s alpha = 0.72-0.84). While that of the vitality/hormonal domain was unsurprisingly lower, reflecting its broad, systemic nature (Cronbach’s alpha = 0.64; 95% CI 0.54-0.73), 0.70 was included in its 95% confidence interval.
Table 3.
Domain characteristics of EPIC for Clinical Practice (EPIC-CP) in 175 subjects treated for prostate cancer in the EPIC-CP Validation Cohort
| EPIC-CP HRQOL Domain | Mean (SD) | Median (IQR) | Range | Scoring Minimum (%) |
Scoring Maximum (%) |
Cronbach’s alpha (95% CI)* |
|---|---|---|---|---|---|---|
| Urinary | ||||||
| Incontinence | 1.76 (2.27) | 1.0 (0.0-3.0) | 0.0-11.0 | 42.2% | 0.0% | 0.81 (0.77-0.86) |
| Irritation/obstruction | 2.79 (2.62) | 2.0 (1.0-4.0) | 0.0-12.0 | 19.0% | 1.1% | 0.72 (0.63-0.77) |
| Bowel | 1.65 (2.48) | 0.0 (0.0-2.0) | 0.0-11.0 | 51.4% | 0.0% | 0.84 (0.78-0.88) |
| Sexual | 6.47 (3.92) | 7.0 (3.0-9.5) | 0.0-12.0 | 8.8% | 12.6% | 0.80 (0.74-0.84) |
| Vitality/hormonal | 2.43 (2.57) | 2.0 (0.0-4.0) | 0.0-11.0 | 29.9% | 0.0% | 0.64 (0.54-0.73) |
Domain summary scores range from 0 to 12, with 0 = best possible score and 12 = worst possible score
95% confidence intervals estimated from 500 bootstrap samples
EPIC-CP demonstrated sensitivity to detect PCa treatment-related effects when domain scores of subjects who had undergone treatment were compared to untreated subjects (Table 4). Although this study was not designed to compare differences in HRQOL between primary PCa therapies, characteristic side effect patterns were observed when comparing specific primary treatment groups to untreated subjects. For example, subjects who underwent prostatectomy had highly significant deficits in the urinary incontinence and sexual domains (both p < 0.0001), and those who had external radiation experienced deficits in the urinary irritation/obstruction (p = 0.0004), bowel (p = 0.0001), and sexual domains (p < 0.0001). Vitality/hormonal domain changes approached significance for any treatment compared to non-treatment (p = 0.08), and subjects who had received hormonal therapy had worse EPIC-CP vitality/hormonal scores than those who had not (p < 0.0001).
Table 4.
Sensitivity of EPIC for Clinical Practice (EPIC-CP) to HRQOL differences associated with prostate cancer treatment – unadjusted mean domain scores by treatment group compared to a group of untreated subjects in the EPIC-CP Validation Cohort
|
Treatment group Mean (SD) |
||||
|---|---|---|---|---|
| HRQOL Domain * | Any treatment (n=175) |
Active surveillance (n=32) |
All Untreated (n=132) |
p-value† |
| Urinary | ||||
| Overall Urinary Bother | 1.32 (1.29) | 1.25 (1.32) | 1.11 (1.17) | 0.14 |
| Incontinence | 1.76 (2.27) | 0.87 (1.41) | 1.06 (1.85) | 0.004 |
| Irritation/obstruction | 2.79 (2.62) | 2.28 (2.22) | 2.16 (1.99) | 0.02 |
| Bowel | 1.65 (2.48) | 0.94 (1.63) | 1.11 (1.88) | 0.04 |
| Sexual | 6.47 (3.92) | 4.16 (3.12) | 3.73 (3.20) | <0.0001 |
| Vitality/Hormonal | 2.43 (2.57) | 1.88 (2.14) | 1.92 (2.38) | 0.08 |
| Total score | 14.66 (9.00) | 9.77 (7.40) | 9.59 (7.75) | <0.0001 |
Domain scores range from possible 0 to 12, with 0 = best score and 12 = worst score. Overall urinary bother item is scored separately from the urinary domain scores, and is scored from a possible 0 to 4, with 0 = best score and 4 = worst score.
Mean domain scores of subjects who received any treatment (n = 175) were compared using ANOVA to a group of all untreated subjects, a combined group of untreated and active surveillance (n=132). For the hormonal/vitality domain, patients who received hormonal therapy had worse scores than men who were either untreated or had undergone primary treatment without hormonal therapy.
The ease of using EPIC-CP in the clinical setting was tested by having urologists, radiation oncologists, medical oncologists, and nurse practitioners administer the questionnaire at the point of care in routine single-specialty academic or community practices as well as in the multidisciplinary clinic setting (number of evaluated practitioners = 12) without the direct assistance of study research personnel. Clinicians found the instrument convenient to use in the flow of their routine clinical practices (Table 5). Eighty-nine percent of subjects completely filled out EPIC-CP in the patient waiting room before their appointment without missing items, and 77% completed the form in less than five minutes. When calculating patient domain scores at the point of care, clinician-calculated scores were accurate in 94% of instances.
Table 5.
Ease of using EPIC-CP in routine clinical practice (n = 307)
| Time taken for patient to fill out EPIC-CP | |
| Less than 5 minutes | 77% |
| 5 to 10 minutes | 19% |
| 10-15 minutes | 2% |
| Greater than 15 minutes | <1% |
|
Convenience of EPIC-CP administration in routine
clinical practice by clinician |
|
| Very convenient | 87% |
| Somewhat convenient | 12% |
| Somewhat inconvenient | <1% |
| Very inconvenient | 0% |
| Patient completed all questions | 89% |
DISCUSSION
Validity is a vital characteristic of any instrument, but an instrument’s clinical usefulness is determined more by the importance of what it measures and its practical clinical applicability. The AUA-SI5 exemplifies such a tool: symptomatic benign prostatic hyperplasia is highly prevalent12, and the ease of administering the AUA-SI’s questions has allowed translation of its use in groundbreaking clinical trials13-15 directly into the clinical setting.
While few would question the importance of HRQOL in PCa patients, it is not intuitively clear why objectively assessing it in a domain-specific fashion using a patient-reported questionnaire is of vital clinical interest. First, physicians rather notoriously tend to underestimate the severity of patients’ post-treatment HRQOL deficits.16-18 This discrepancy contributed to the development of validated patient-reported questionnaires specific to PCa patients, the first of which was the UCLA-PCI3, the instrument from which EPIC in its various forms2,4 was derived. Use of these questionnaires and others19,20 in multicenter prospective studies specifically examining HRQOL changes after PCa treatment have helped elucidate characteristic side effect patterns after each major primary PCa treatment modality.7, 21-24
More recent efforts have sought to bring PCa HRQOL research closer to the bedside by identifying specific pre-treatment factors that may predict post-treatment HRQOL changes. Just as a patient’s oncologic outcome is affected by cancer-related factors such as Gleason score, clinical stage, and PSA25, the side effect profile that a patient experiences with a given treatment choice is heavily impacted by his individual pre-treatment, or baseline HRQOL.26,27 This presents a unique gap between the research and clinical realms which, if bridged, has the potential to improve clinicians’ ability to more accurately represent and convey the risks of PCa treatment to an individual patient. We designed EPIC-CP to bridge this gap. Its high correlation with longer EPIC versions and indirectly with the UCLA-PCI, reflects a compatibility that can facilitate translating HRQOL research findings to routine practice. Concurrent use of a general HRQOL instrument such as the SF-8 may allow easy linkage with disease-specific HRQOL.
Our development and validation of EPIC-CP has limitations. As is inherent and expected with further item reduction, the breadth of HRQOL assessment is decreased; however, EPIC-CP retains measure of 5 prostate cancer HRQOL domains, enabling prostate cancer patient-reported outcomes to be measured in the routine outpatient practice setting and to become a more standard part of routine clinical PCa care. The cross-sectional and anonymous nature of the EPIC-CP Validation Cohort did not allow for reliability testing or the assessment of EPIC-CP’s ability to detect treatment-related changes over time. That other demographic variables (marital status, income, etc.) were not assessed, and that the cohort was nonrandomized, predominantly white, and mostly treated in the academic setting may limit the generalizability of our results. Potential treatment selection bias precludes use of this cross-sectional validation cohort for conclusive comparisons regarding treatment-specific effects on HRQOL. These limitations may be addressed through further study of EPIC-CP in the prospective setting.
CONCLUSIONS
Despite these limitations, this study demonstrates that EPIC-CP is a valid, sensitive, and practical tool that can be efficiently administered in the outpatient setting, thereby enabling patient HRQOL outcomes to be easily measured and documented at the point of care. Its ease of use provides an opportunity to incorporate HRQOL measures as a standard component of routine prostate cancer clinical care, and to facilitate the implementation and documentation of patient-centered care.
Acknowledgements
The authors would like to acknowledge Irving Kaplan, MD, Brian Hoell, NP, Courtney Collins, Research Nurse, Mary Ellen Morrissey, Nurse Coordinator, Jodi Mechaber, NP, Robert Eyre, MD, and Paul Church, MD for their help with subject accrual. We would also like to thank Catrina Crociani, MPH for project management, Neha Mehta for data management, and Meredith M. Regan, ScD for her statistical expertise and supervision.
Funding: National Institutes of Health (R01 CA95662; RC1 CA146596, M Sanda, PI)
Footnotes
The complete EPIC-CP questionnaire can be found at http://www.bidmc.org/epic.
REFERENCES/BIBLIOGRAPHY
- 1.Litwin MS, Hays RD, Fink A, et al. Quality-of-life outcomes in men treated for localized prostate cancer. JAMA. 1995;273:129–135. doi: 10.1001/jama.273.2.129. [DOI] [PubMed] [Google Scholar]
- 2.Wei JT, Dunn RL, Litwin MS, et al. Development and validation of the Expanded Prostate Cancer Index Composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56:899–905. doi: 10.1016/s0090-4295(00)00858-x. [DOI] [PubMed] [Google Scholar]
- 3.Litwin MS, Hays RD, Fink A, et al. The UCLA Prostate Cancer Index: development, reliability, and validity of a health-related quality of life measure. Med Care. 1998;36(7):1002–12. doi: 10.1097/00005650-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Szymanski KM, Wei JT, Dunn RL, et al. Development and Validation of an Abbreviated Version of the Expanded Prostate Cancer Index Composite Instrument for Measuring Health-Related Quality of Life Among Prostate Cancer Survivors. Urology. 2010 Nov;76(5):1245–50. doi: 10.1016/j.urology.2010.01.027. Epub 2010 Mar 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barry MJ, Fowler FJ, Jr, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committeee of the American Urological Association. J Urol. 1992;148(5):1549–57. doi: 10.1016/s0022-5347(17)36966-5. [DOI] [PubMed] [Google Scholar]
- 6.Rosen RC, Cappelleri JC, Smith MD, et al. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11:319–326. doi: 10.1038/sj.ijir.3900472. [DOI] [PubMed] [Google Scholar]
- 7.Sanda MG, Dunn RL, Michalski J, et al. Quality of Life and Satisfaction with Outcome among Prostate-Cancer Survivors. NEJM. 2008;358:1250–61. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 8.Spetz AC, Zetterlund EL, Varenhorst E, et al. Incidence and management of hot flashes in prostate cancer. J Support Oncol. 2003 Nov-Dec;1(4):263–6. 269–70, 272–3. discussion 267-8, 271-2. [PubMed] [Google Scholar]
- 9.Dobs A, Darkes MJ. Incidence and management of gynecomastia in men treated for prostate cancer. J Urol. 2005 Nov;174(5):1737–42. doi: 10.1097/01.ju.0000176461.75794.f8. [DOI] [PubMed] [Google Scholar]
- 10.Smith MR, Goode M, Zietman AL, et al. Bicalutamide monotherapy versus leuprolide monotherapy for prostate cancer: effects on bone mineral density and body composition. J Clin Oncol. 2004 Jul 1;22(13):2546–53. doi: 10.1200/JCO.2004.01.174. [DOI] [PubMed] [Google Scholar]
- 11.Di Lorenzo G, Perdoná S, De Placido S, et al. Gynecomastia and breast pain induced by adjuvant therapy with bicalutamide after radical prostatectomy in patients with prostate cancer: the role of tamoxifen and radiotherapy. J Urol. 2005 Dec;174(6):2197–203. doi: 10.1097/01.ju.0000181824.28382.5c. [DOI] [PubMed] [Google Scholar]
- 12.Sagnier PP, Girman CJ, Garraway M, et al. International comparison of the community prevalence of symptoms of prostatism in four countries. Eur Urol. 1996;29(1):15–20. doi: 10.1159/000473711. [DOI] [PubMed] [Google Scholar]
- 13.Lepor H, Williford WO, Barry MJ, et al. The efficacy of terazosin, finasteride, or both in benign prostatic hyperplasia. Veterans Affairs Cooperative Studies Benign Prostatic Hyperplasia Study Group. N Engl J Med. 1996 Aug 22;335(8):533–9. doi: 10.1056/NEJM199608223350801. [DOI] [PubMed] [Google Scholar]
- 14.McConnell JD, Bruskewitz R, Walsh P, et al. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. Finasteride Long-Term Efficacy and Safety Study Group. N Engl J Med. 1998 Feb 26;338(9):557–63. doi: 10.1056/NEJM199802263380901. [DOI] [PubMed] [Google Scholar]
- 15.McConnell JD, Roehrborn CG, Bautista OM, et al. the Medical Therapy of Prostatic Symptoms (MTOPS) Research Group The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003 Dec 18;349(25):2387–98. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]
- 16.Fowler FJ, Jr., Barry MJ, Luet Patient-reported complications and follow-up treatment after radical prostatectomy. The National Medicare Experience: 1988-1990 (updated June 1993) Urology. 1993;42(6):622–9. doi: 10.1016/0090-4295(93)90524-e. al. [DOI] [PubMed] [Google Scholar]
- 17.Joly F, Brune D, Couette JE, et al. Health-related quality of life and sequelae in patients treated with brachytherapy and external beam irradiation for localized prostate cancer. Ann Oncol. 1998;9(7):751–7. doi: 10.1023/a:1008276632623. [DOI] [PubMed] [Google Scholar]
- 18.Sonn GA, Sadetsky N, Presti JC, Litwin MS. Differing perceptions of quality of life in patients with prostate cancer and their doctors. J Urol. 2009 Nov;182(5):2296–302. doi: 10.1016/j.juro.2009.07.027. Epub 2009 Sep 16. [DOI] [PubMed] [Google Scholar]
- 19.Borghede G, Sullivan M. Measurement of quality of life in localized prostatic cancer patients treated with radiotherapy. Development of a prostate cancer-specific module supplementing the EORTC QLQ-C30. Qual Life Res. 1996;5(2):212–22. doi: 10.1007/BF00434743. [DOI] [PubMed] [Google Scholar]
- 20.Giesler RB, Miles BJ, Cowen ME, et al. Assessing quality of life in men with clinically localized prostate cancer: development of a new instrument for use in multiple settings. Qual Life Res. 2000;9(6):645–65. doi: 10.1023/a:1008931703884. [DOI] [PubMed] [Google Scholar]
- 21.Madalinska JB, Essink-Bot ML, de Koning HJ, et al. Health-related quality-of-life effects of radical prostatectomy and primary radiotherapy for screen-detected or clinically diagnosed localized prostate cancer. J Clin Oncol. 2001;19(6):1619–28. doi: 10.1200/JCO.2001.19.6.1619. [DOI] [PubMed] [Google Scholar]
- 22.Smith DP, King MT, Egger S, et al. Quality of life three years after diagnosis of localised prostate cancer: population based cohort study. BMJ. 2009;339:b4817. doi: 10.1136/bmj.b4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pardo Y, Guedea F, Aguiló F, et al. Quality-of-Life Impact of Primary Treatments for Localized Prostate Cancer in Patients Without Hormonal Treatment. J Clin Oncol. 2010 Oct 4; doi: 10.1200/JCO.2009.25.3245. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Potosky AL, Legler J, Albertsen PC, et al. Health outcomes after prostatectomy or radiotherapy for prostate cancer: results from the Prostate Cancer Outcomes Study. J Natl Cancer Inst. 2000 Oct 4;92(19):1582–92. doi: 10.1093/jnci/92.19.1582. [DOI] [PubMed] [Google Scholar]
- 25.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998 Sep 16;280(11):969–74. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 26.Chen RC, Clark JA, Talcott JA. Individualizing quality-of-life outcomes reporting: how localized prostate cancer treatments affect patients with different levels of baseline urinary, bowel, and sexual function. J Clin Oncol. 2009 Aug 20;27(24):3916–22. doi: 10.1200/JCO.2008.18.6486. Epub 2009 Jul 20. [DOI] [PubMed] [Google Scholar]
- 27.Stanford JL, Feng Z, Hamilton AS, et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the Prostate Cancer Outcomes Study. JAMA. 2000;283(3):354–60. doi: 10.1001/jama.283.3.354. [DOI] [PubMed] [Google Scholar]