Abstract
Voltage-gated sodium (Nav) channels are required for impulse conductance in excitable tissues. Navs have been linked to human cancers, including prostate. The expression and distribution of Nav isoforms (Nav1.1-Nav1.9) in human prostate cancer are not well established. Here, we evaluated the expression of these isoforms and investigated the expression of Nav1.8 in human prostate cancer tissues. Nav1.8 was highly expressed in all examined cells. Expression of Nav1.1, Nav1.2, and Nav1.9 were high in DU-145, PC-3 and PC-3M cells compared to LNCaP (hormone-dependent), C4-2, C4-2B, and CWR22Rv-1 cells. Nav1.5 and Nav1.6 were expressed in all cells examined. Nav1.7 expression was absent in PC-3M and CWR22Rv-1, but expressed in the other cells examined. Immunohistochemistry revealed intensive Nav1.8 staining correlated with more advanced pathologic stage of disease. Increased intensity of nuclear Nav1.8 correlated with increased Gleason grade. Our results revealed that Nav1.8 is universally expressed in human prostate cancer cells. Nav1.8 expression statistically correlated with pathologic stage (P=0.04) and Gleason score (P=0.01) of human prostate tissue specimens. The aberrant nuclear localization of Nav1.8 with advanced prostate cancer tissues warrant further investigation into use of Nav1.8 as a potential biomarker to differentiate between early and advanced disease.
Keywords: Voltage-gated sodium channel, Prostate cancer, Prostate biomarker, Gleason score
Introduction
Prostate cancer is the most common cancer diagnosed in men and the second leading cause of cancer death among men in the United States [1]. A major challenge encountered in the treatment of prostate cancer is that a significant portion of the tumors will recur. The genomic/proteomic alterations underlying prostate cancer progression and therapy resistance are poorly understood. Hence, the discovery of novel biomarkers remains central to earlier and improved accuracy of detection and diagnosis of human prostate cancer disease.
Recently, Nav channels have been linked to human prostate cancer cell proliferation [2], invasion [3] and metastasis [4]. Navs are members of a single gene family with at least nine isotypes (Nav1.1-1.9) which are commonly found in excitable cells including heart (Nav1.5), skeletal muscles (Nav1.4) and neurons (CNS, Nav1.1–Nav1.2, Nav1.6; PNS, Nav1.7–Nav1.9) [5]. Navs mediate sodium influx to generation action potentials which are critical for normal cellular functions [5].
Nav is a pore forming trans-membrane protein composed of a large α subunit (>200 kDa) and at least two associated β subunits (∼30-40 kDa) [5]. The large pore forming alpha subunit consists of four internal homologous transmembrane domains (I-IV) linked by three interdomain cytoplasmic loops which contain a number of phosphorylation sites that participate in intracellular signaling [6]. Protein kinases (PKA, PKC, receptor tyrosine kinase (RTK), Ca2+/calmodulin-dependent protein kinase (CaMK), p38MAPK, and p42/44 MAPK) have been shown to modulate Nav current by promoting protein-protein interactions, stimulating channel trafficking and insertion to the membrane, and rapid translation of Nav channels [6,7]. Expression of the α subunit alone is sufficient for cellular function [5].
Navs are classified into two groups based on their sensitivity to a sodium channel specific toxin, tetrodotoxin, (TTX). Nav1.5, 1.8, and 1.9 are resistant to TTX (IC50, μM) while the other six isotypes are highly sensitive to TTX (IC50, nM) [5]. Various Nav mRNA transcripts have been detected in a variety of human cancers [4,8-11]. Abdul and Hoosein have provided evidence that Navs were highly expressed in human prostate cancer tissues using a pan-Nav antibody, which recognizes the conserved DIII-DIV linker of all Nav isotypes [12]. However, the expression of Nav specific protein isotypes and its subcellular localization in human prostate cancer remain elusive. In this present study, we have examined the protein expression of Nav specific α subunits (Nav1.1-1.9) in hormone-dependent and hormone-independent human prostate cancer cell lines and explored the clinical relevance of a specific voltage gated sodium channel Nav1.8 in human prostate cancer disease progression.
Materials and Methods
Cell culture
LNCaP, C4-2, and C4-2B (gif from Dr. Robert Sikes, University of Delaware, Department of Biological Sciences, Newark, DE) and CWR22Rv-1, DU-145, PC-3, and PC-3M cell lines (ATCC, Manassas, VA) were cultured in RPMI-1640 with L-glutamine (Mediatech, Inc., Herdon, VA) containing 5% fetal bovine serum (FBS), 2.5 mM L-glutamine at 37°C with 5% CO2. LNCaP cells were cultured in the presence of 0.5 nM dihydrotestosterone (5α-androstan-17β-ol-3-one) (Sigma-Aldrich, St. Louis, MO).
Western blot analysis
Western protocols were adapted from Collins et al. [13]. Briefly, prostate cancer cells were lysed in the radioimmunoprecipitation (RIPA) buffer (50 mM Tris-HCl, pH 7.6, 5 mM EDTA, 150 mM NaCl, 30 mM sodium pyrophosphate, 50 mM sodium fluoride, 1 mM sodium orthovanadate, 1% Triton X-100, 0.01% SDS, 0.5% sodium deoxycholate). Protease inhibitor cocktails (Sigma-Aldrich) were added to RIPA buffer prior to use. The protein samples were separated by 4% Tris-Glycine SDS-PAGE or 4-12% Bis-Tris SDS-PAGE (Invitrogen, Carlsbad, CA) and transferred into immuno-Blot PVDF membranes (Biorad Laboratories, Hercules, CA). The membranes were blocked with blocking buffer (50 mM Tris-Cl, 150 mM NaCl, 10 g/L BSA) and probed with the following antibodies: anti-Nav1.1, anti-Nav1.2, anti-Nav1.5, anti-Nav1.6, anti-Nav1.7, anti-Nav1.8, anti-Nav1.9 (Upstate/Millipore, Billerica, MA); anti-EGFR, anti-PARP (Cell Signaling Technology, Danvers, MA); and anti-alpha tubulin (Sigma-Aldrich). Chemiluminescent detection was performed using ECL reagents according to the vendor's instructions (Pierce, Rockford, IL).
Subcellular fractionation
C4-2 and PC-3 cells were fractionated using the Fraction PREP Cell Fractionation system (Biovision, Mountain View, CA) according to manufacturer's instructions. Fractionation was accomplished on cells (107) treated with a series of extraction buffers, followed by sequential centrifugation to separate the cytoplasmic, the plasma membrane, and nuclear enriched fractions. Western blot was performed on the fractions using antibodies to Nav1.1, 1.7 and 1.8. The purity of the enriched fraction was determined using antibodies to PARP (nucleus), EGFR (plasma membrane) and α-tubulin (cytosol).
Immunohistochemical detection of Nav1.8 in human prostate specimens
Paraffin-embedded cells or arrayed prostate cancer specimens (US Biomax, Inc, Rockville, VA) containing normal (17) and malignant (160) prostate tissues were deparaffinized, rehydrated, boiled with citrate buffer (pH 6), treated with 0.3% H2O2, and preincubated in blocking solution (10% normal goat serum). The primary antibody, anti-Nav1.8, was incubated with the specimens at a concentration of 1:50 for one hour at room temperature. Antigen-antibody complexes were detected using a horseradish-peroxidase complexed anti-rabbit secondary antibody (Dako Envision-Plus) (Dako North America, Inc., Carpinteria, CA). 3,3'-diaminobenzidine (Dako) was used as chromogen and hematoxylin as counterstain. A subtype-specific IgG was used as a negative control. Sprague-Dawley rat sciatic nerve dorsal root ganglion (DRG) was used as a positive control tissue. Samples were imaged with Olympus BX61 camera/DP-70 inverted microscope (Center Valley, PA) using provided DP Controller software. Individual prostate samples were scored (TH and SS) using semi-quantitative steps of increasing staining intensity, where 0 was undetectable, low immunostaining, 1+; intermediate immunostaining, 2+; and high immunostaining, 3+ as previously described [14]. Fisher's exact test was used to compare presence of staining (negative stain vs. positive stain) and localization in each site among the clinical features. The exact Jonckheere-Terpstra test was used to determine if staining intensity was associated with the clinical features. The association between two sites of localization controlling for clinical factors were compared using the exact Cochran-Mantel-Haenszel test. All statistical analyses were performed using SAS software (SAS Institute Inc., Cary, NC).
Results
Voltage-gated sodium channel isoforms are differentially expressed in human prostate cancer cell lines
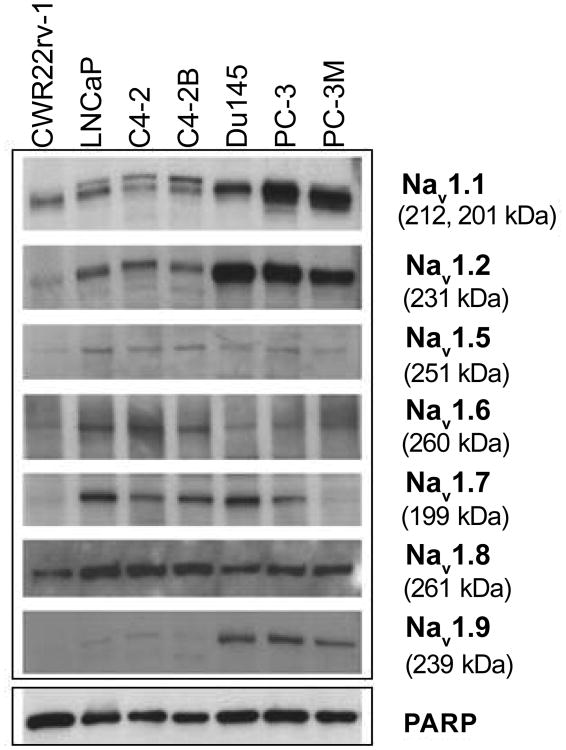
We examined the expression of specific Nav isotypes in seven human prostate cancer cells, including hormone-dependent (LNCaP) and hormone-independent (C4-2, C4-2B, CWR22Rv-1, DU145, PC-3, and PC-3M) cells. Antibodies against unique epitopes of the α-subunit of sodium channel isoforms were used to detect the neuronal (Nav1.1–Nav1.2, Nav1.6), cardiac (Nav1.5), and peripheral neurons (Nav1.7–Nav1.9) isoforms. The specificity of the antibodies was verified by peptide competition studies (data not shown). The isotype-specific antibodies detected band of appropriate molecular weight by Western blot analysis (Figure 1).
Figure 1. Expression of Nav isoforms in human prostate cancer cells.
Western blot analyses of extracts from human prostate cancer cell lines. Cell lysates were analyzed by immunoblotting with anti-Nav1.1, Nav1.2, Nav1.5, Nav1.6, Nav1.7, Nav1.8, and Nav1.9. Blots were probed with anti-PARP-1 antibody to normalize for protein loading.
As shown in Figure 1, Nav1.8 demonstrated ubiquitous expression in both hormone-dependent and independent human prostate cancer cells. In contrast, the expression of Nav isoforms, 1.1, 1.2 and 1.9, were higher in DU-145, PC-3, and PC-3M cells as compared to LNCaP and its two lineage cell lines, C4-2 and C4-2B, and CWR22Rv-1. Nav1.5 and Nav1.6 were expressed in all prostate cancer cells examined. The expression of Nav1.7 was absent in PC-3M and CWR22Rv-1, but expressed in the other prostate cancer cells examined. The levels of Nav1.3 and Nav 1.4 were undetectable (data not shown). This could be due to the quality of the antibodies and/or the lack of expression of these isoforms.
Nav1.8 expression in human prostate cancer tissues
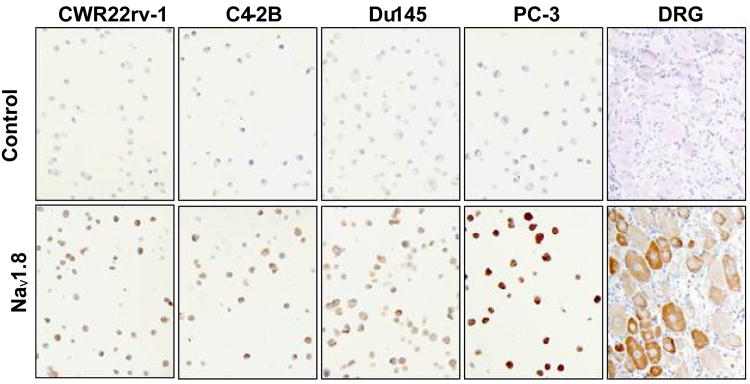
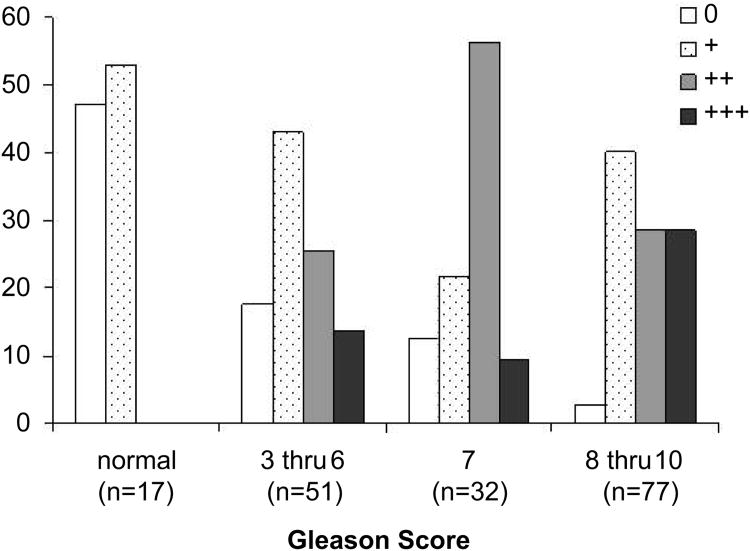
Because Nav1.8 was highly expressed in all the human prostate cancer cell lines examined, we asked whether the expression of Nav1.8 increased in prostate cancer tissues with advancing Gleason score. For optimization of Nav1.8 tissue staining, we initially performed an immunocytochemical analysis of Nav1.8 in both hormone-dependent and independent cells. The specificity of Nav1.8 antibody was verified by peptide inhibition (negative control) and rat DRG staining (positive control) (Figure 2). Human prostate tissue specimens were then immunostained and sections were scored with a semi-quantitative scoring method (0-3+) based on the intensity of Nav1.8 staining. Normal prostate epithelium was either absent or weakly stained (intensity score of 0 to 1+) for Nav1.8 (A representative sample in Figure 3A). The malignant prostate tissues with GS 4 or less were either absent or weak Nav1.8 stained (A representative sample in Figure 3B, GS 4). A moderate (A representative sample in Figures 3C and 3D; GS 6, GS 7) to strong (A representative sample in Figures 3E and 3F; GS 8, GS 10) Nav1.8 staining was observed in more advanced prostate tissue specimens. Figure 4 shows the distribution of Nav1.8 staining intensity (0-3) and Gleason score. The percentage of prostate cancer specimens with high Nav1.8 immunoreactivity (2+ and 3+) increased as GS increased. In contrast, the percentage of prostate cancer specimens with little or low Nav1.8 immunoreactivity decreased as GS increased.
Figure 2. Nav1.8 immunohistochemical analysis of human prostate cancer cell lines.
DRG from rat tissue was used as a positive control for Nav1.8. Negative control (top panels) represents neutralization of Nav1.8 antibody with Nav1.8 peptide.
Figure 3. Expression of Nav 1.8 in human prostate cancer tissues.
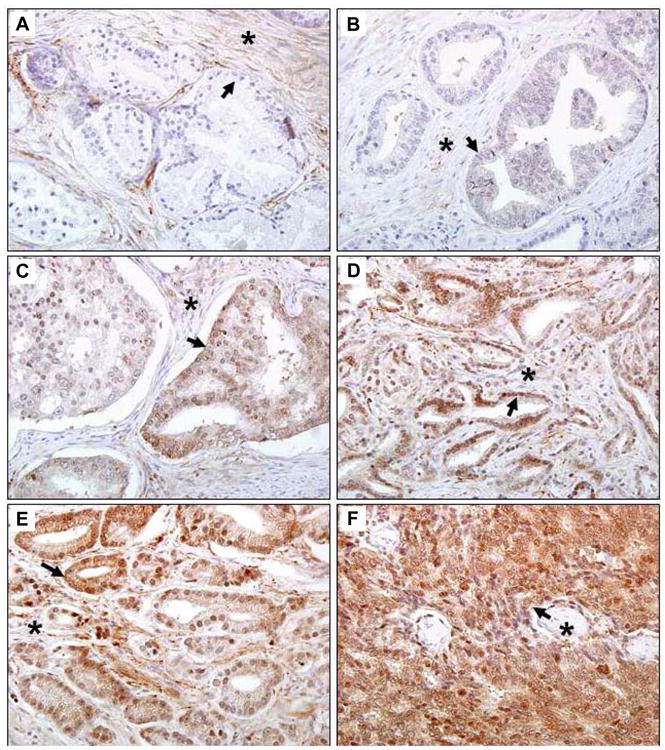
Human prostate tissue specimens consist of normal (n=17) and malignant (n=160) were analyzed by immunohistochemical staining using a polyclonal anti- Nav 1.8 antibody. Representative samples are shown. A. Normal human prostate specimen with very low Nav1.8 staining. B. Prostatic adenocarcinoma specimen GS 4 with very low Nav1.8 staining. Prostatic adenocarcinoma specimens with moderate Nav1.8 staining, GS 6 (C) and GS 7(D). Prostatic adenocarcinoma specimens with strong Nav1.8 staining, GS 8 (E) and GS10 (F). Images were taken at 40× (Olympus BX61 Camera/DP70 inverted microscope) using DP Controller Software. Arrows indicate prostate epithelium. Asterisks indicate stroma. GS, Gleason score.
Figure 4. Relationship between Nav 1.8 staining intensity and Gleason score.
Human prostate tissue specimens consist of normal (n=17) and malignant (n=160) were analyzed by immunohistochemical staining using a polyclonal anti- Nav 1.8 antibody. Prostate tissue specimens were divided into four groups (normal, GS 3-6, GS 7, and GS 8-10) and individually scored for Nav 1.8 staining: 0 = no detectable, 1+ = low Nav 1.8 staining, 2+ = moderate Nav 1.8 staining, 3+ = strong Nav 1.8 staining.
Table 1 provides a history of the patient specimens and statistical correlations. The observed difference in staining intensity between normal and malignant tissues was statistically significant (P<0.0001). When Nav1.8 expression was compared with PSA secretion, pathologic stage, Gleason score, and lymph node involvement in malignant prostate cancer tissues, statistically significant correlations were observed in all clinical features (p-values for pathological stage, p=0.04; Gleason Score, p= 0.01; pathologic lymph node stage, p<0.001). These findings were not observed with PSA secretion and Nav1.8 staining (p=0.15).
Table 1.
Summary of Patient History and Correlations to Nav1.8 Epithelial Staining.
| P-values | |||
|---|---|---|---|
| Characteristic | |||
| n | Negative vs. Positive1 | Intensity2 | |
| All healthy tissues | 17 | <0.0001 | <0.0001 |
| All tumors | 160 | ||
| PSA (ng/ml) | --- | 0.15 | |
| < 4 | 12 | ||
| 4–10 | 10 | ||
| 10–20 | 17 | ||
| > 20 | 24 | ||
| Pathologic Stage | 0.05 | 0.04 | |
| pT2 | 27 | ||
| pT3 | 47 | ||
| pT4 | 27 | ||
| Gleason Score | 0.01 | 0.01 | |
| 3–6 | 51 | ||
| 7 | 32 | ||
| 8–10 | 77 | ||
| Pathologic lymph node stage | 0.0006 | <0.0001 | |
| pN0 | 61 | ||
| pN+ | 31 | ||
P-values computed using the fisher's exact test
P-values computed using the exact Jonckheere-Terpstra test
Localization of Nav1.8 in human prostate cancer tissues
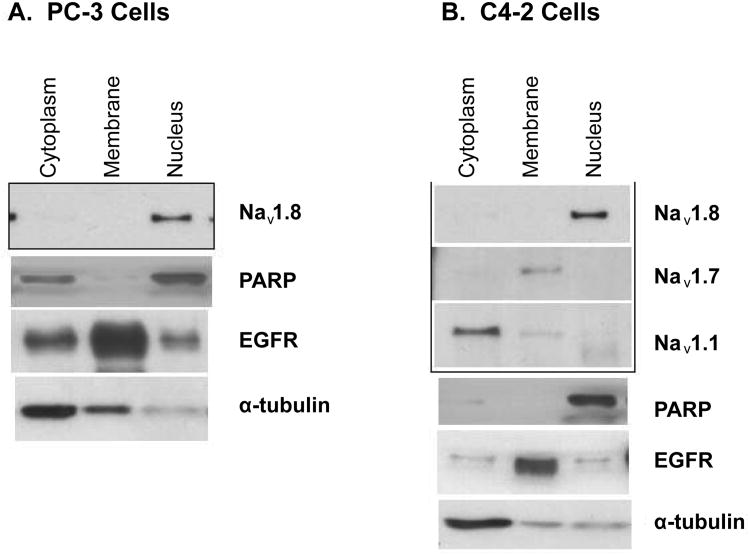
While previous studies show Navs localize to the plasma membrane in excitable cells (such as neurons), the cellular localization of Navs in prostate cancer is not well established. Cellular localization of Nav1.8 in human prostate tissue is shown in Figure 5. In normal human prostate tissue, the nuclei of the prostatic acinar basal cells showed either absent or weak Nav1.8 staining (Figure 5A). In a case of GS 6 tumor, Nav1.8 staining was observed in both the plasma membrane and cytosol of the prostate epithelial cells (Representative sample in Figure 5B). We noticed a disappearance of Nav1.8 staining in the plasma membrane of many prostate cancer tissue specimens with higher Gleason grade. In epithelial cells of a case of GS7 tumor, moderate Nav1.8 staining was seen in the nucleus (Representative sample in Figure 5C). Strong staining was observed in the cytosol (Figure 5D, GS10), or strong mixed cytosol and nucleus staining (Figure 5E) in advanced prostate cancer tissues. The observation that Nav1.8 localized to the nucleus was unexpected. To verify that Nav1.8 localized to the nucleus, subcellular enriched fractions of C4-2 and PC-3 cells were immunoblotted with antibody to Nav1.8. Western blot analysis revealed detection of Nav1.8 in the nuclear enriched fractions of PC-3 cells (Figure 6A) and C4-2 cells (Figure 6B). We also probed the subcellular enriched fractions of C4-2 for the presence of Nav1.1 and Nav1.7. As illustrated in Figure 6B, Nav1.1 was detected in the cytosolic enriched fractions while Nav1.7 was present in the membrane enriched fractions. These results further validate our observation of nuclear Nav1.8 in human prostate cancer. The purity of the subcellular enriched fractions was probed using EGFR as a plasma membrane marker, cytosolic marker alpha-tubulin, and nuclear marker PARP (Figures 6A and 6B).
Figure 5. Localization of Nav 1.8 in human prostate cancer tissues.
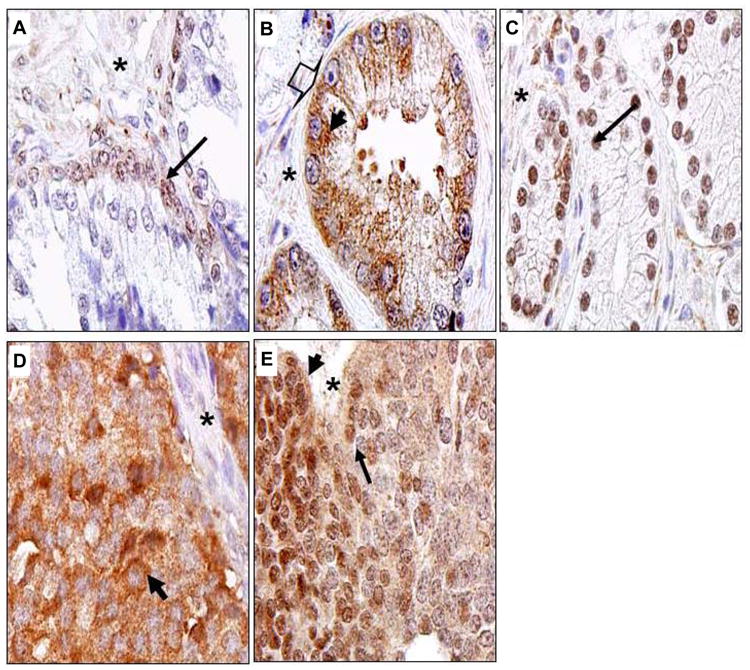
Human prostate tissue specimens consist of normal (n=17) and malignant (n=160) were analyzed by immunohistochemical staining using a polyclonal anti- Nav 1.8 antibody. Representative samples are shown. A. Normal human prostate specimen, nuclei of prostatic acinar basal cells with very low Nav1.8 staining. B. Prostatic adenocarcinoma specimen GS 6 with moderate Nav1.8 staining in the plasma membrane (arrow head) and cytosol (bold thick arrow). C. Nuclear staining of a case of GS 7. D. Cytosolic staining of a case of GS10. E. A case of GS10 with mixed cytosolic and nuclear staining. Images were taken at 100× (Olympus BX61 Camera/DP70 inverted microscope) using DP Controller Software. Asterick represents stroma. Long thin arrows denote nuclei.
Figure 6. Nav 1.8 is localized to the nucleus.
Human prostate cancer cell lines were fractionated into plasma membrane, cytosolic and nuclear enriched fractions. Proteins were separated on a 4-12% SDS-PAGE gradient gel. A. Subcellular fractions of PC-3 cells immunoblotted with Nav 1.8. B. Subcellular fractions of C4-2 cells immunoblotting with Nav 1.1 and 1.7 and 1.8. Purity of the enriched fractions were shown using anti-α-tubulin for the cytoplasm, EGFR for the plasma membrane, and PARP for the nucleus.
Discussion
Although a number of reports have linked voltage-gated sodium channels to human prostate cancer, little information is known regarding the expression of Nav specific proteins and its cellular distribution [4,9,12,15-17]. In this study, we demonstrate for the first time that Nav1.8, normally found in excitable cells like dorsal root ganglia, is highly expressed in human prostate cancer cells. Additionally, Nav1.8 expression is associated with advanced histopathological grade of human prostate cancer disease. Our current observations are consistent with Abdul and Hoosein [12] studies in which the authors used a pan-Navs antibody and demonstrated that Nav s immunoreactivity is greater in human prostate cancer tissues as compared to normal human prostate.
The molecular mechanisms for the upregulation of Nav1.8 are unknown. It has been suggested that that increased Nav1.8 expression by p38MAPK is regulated at the post-transcriptional level [18]. Our laboratory is currently exploring a possible connection between Nav1.8 and p38 MAPK activation in human prostate cancer. Our Western blot data demonstrated that both LNCaP and PC3 cells have comparable of Nav1.7 protein. This result did not agree with previous reports identifying Nav1.7 as the major isoform found in PC-3 prostate cancer cells [9]. These discrepancies suggest that increased Nav1.7 mRNA in prostate cancer may not be a reflective of functional expression of Nav1.7.
Localization of Nav1.8 in the nucleus is another intriguing finding from our current study. Recently the voltage-independent potassium ion channel Trek-1 was found to be localized to the nucleus of human prostate cancer cells [19]. Furthermore, the nuclear localization of a plasma membrane protein ErbB3 has been linked to poor prognosis in prostate cancer survival [20]. These studies strongly support that the translocation of membrane proteins to the nucleus of human prostate cancer cells.
The functional and mechanistic roles of Nav1.8 in prostate cancer cells are not known. However, the altered cytoplasmic to nuclear ratio of Nav1.8 in prostate cancer tissues may be useful in differentiating early and advanced stages of prostate cancer. Currently, the role of this isoform and other Nav isoforms in human prostate cancer cells and tissues are under investigation by our laboratory. Finally, our study identified Nav1.8, a voltage regulated ion channel protein, in human prostate cancer cells and tissues and strongly supports the further study of Nav1.8 as a potential biomarker and a therapeutic target in this disease.
Acknowledgments
This work was supported by NIH-R01 grant CA105435-04, The Georgetown Drug Discovery Program, and the Lombardi Comprehensive Cancer Center at Georgetown Medical Center.
Footnotes
Conflict of Interest: The authors do not have conflict of interest for this study.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Anderson JD, Hansen TP, Lenkowski PW, Walls AM, Choudhury IM, et al. Voltage-gated sodium channel blockers as cytostatic inhibitors of the androgen-independent prostate cancer cell line PC-3. Mol Cancer Ther. 2003;2:1149–1154. [PubMed] [Google Scholar]
- 3.Bennett ES, Smith BA, Harper JM. Voltage-gated Na+ channels confer invasive properties on human prostate cancer cells. Pflugers Arch. 2004;447:908–914. doi: 10.1007/s00424-003-1205-x. [DOI] [PubMed] [Google Scholar]
- 4.Smith P, Rhodes NP, Shortland AP, Fraser SP, Djamgoz MB, et al. Sodium channel protein expression enhances the invasiveness of rat and human prostate cancer cells. FEBS Lett. 1998;423:19–24. doi: 10.1016/s0014-5793(98)00050-7. [DOI] [PubMed] [Google Scholar]
- 5.Yu FH, Yarov-Yarovoy V, Gutman GA, Catterall WA. Overview of molecular relationships in the voltage-gated ion channel superfamily. Pharmacol Rev. 2005;57:387–395. doi: 10.1124/pr.57.4.13. [DOI] [PubMed] [Google Scholar]
- 6.Costa MR, Catterall WA. Phosphorylation of the alpha subunit of the sodium channel by protein kinase C. Cell Mol Neurobiol. 1984;4:291–297. doi: 10.1007/BF00733592. [DOI] [PubMed] [Google Scholar]
- 7.Catterall WA, Hulme JT, Jiang X, Few WP. Regulation of sodium and calcium channels by signaling complexes. J Recept Signal Transduct Res. 2006;26:577–598. doi: 10.1080/10799890600915100. [DOI] [PubMed] [Google Scholar]
- 8.Diaz D, Delgadillo DM, Hernandez-Gallegos E, Ramirez-Dominguez ME, Hinojosa LM, et al. Functional expression of voltage-gated sodium channels in primary cultures of human cervical cancer. J Cell Physiol. 2007;210:469–478. doi: 10.1002/jcp.20871. [DOI] [PubMed] [Google Scholar]
- 9.Diss JK, Archer SN, Hirano J, Fraser SP, Djamgoz MB. Expression profiles of voltage-gated Na(+) channel alpha-subunit genes in rat and human prostate cancer cell lines. Prostate. 2001;48:165–178. doi: 10.1002/pros.1095. [DOI] [PubMed] [Google Scholar]
- 10.Fraser SP, Diss JK, Chioni AM, Mycielska ME, Pan H, et al. Voltage-gated sodium channel expression and potentiation of human breast cancer metastasis. Clin Cancer Res. 2005;11:5381–5389. doi: 10.1158/1078-0432.CCR-05-0327. [DOI] [PubMed] [Google Scholar]
- 11.Roger S, Rollin J, Barascu A, Besson P, Raynal PI, et al. Voltage-gated sodium channels potentiate the invasive capacities of human non-small-cell lung cancer cell lines. Int J Biochem Cell Biol. 2007;39:774–786. doi: 10.1016/j.biocel.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Abdul M, Hoosein N. Voltage-gated sodium ion channels in prostate cancer: expression and activity. Anticancer Res. 2002;22:1727–1730. [PubMed] [Google Scholar]
- 13.Collins SP, Reoma JL, Gamm DM, Uhler MD. LKB1, a novel serine/threonine protein kinase and potential tumour suppressor, is phosphorylated by cAMP-dependent protein kinase (PKA) and prenylated in vivo. Biochem J. 2000;3:673–680. [PMC free article] [PubMed] [Google Scholar]
- 14.Li H, Ahonen TJ, Alanen K, Xie J, LeBaron MJ, et al. Activation of signal transducer and activator of transcription 5 in human prostate cancer is associated with high histological grade. Cancer Res. 2004;64:4774–4782. doi: 10.1158/0008-5472.CAN-03-3499. [DOI] [PubMed] [Google Scholar]
- 15.Fiske JL, Fomin VP, Brown ML, Duncan RL, Sikes RA. Voltage-sensitive ion channels and cancer. Cancer Metastasis Rev. 2006;25:493–500. doi: 10.1007/s10555-006-9017-z. [DOI] [PubMed] [Google Scholar]
- 16.Laniado ME, Lalani EN, Fraser SP, Grimes JA, Bhangal G, et al. Expression and functional analysis of voltage-activated Na+ channels in human prostate cancer cell lines and their contribution to invasion in vitro. Am J Pathol. 1997;150:1213–1221. [PMC free article] [PubMed] [Google Scholar]
- 17.Prevarskaya N, Skryma R, Bidaux G, Flourakis M, Shuba Y. Ion channels in death and differentiation of prostate cancer cells. Cell Death Differ. 2007;14:1295–1304. doi: 10.1038/sj.cdd.4402162. [DOI] [PubMed] [Google Scholar]
- 18.Hudmon A, Choi JS, Tyrrell L, Black JA, Rush AM, et al. Phosphorylation of sodium channel Na(v)1.8 by p38 mitogen-activated protein kinase increases current density in dorsal root ganglion neurons. J Neurosci. 2008;28:3190–3201. doi: 10.1523/JNEUROSCI.4403-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voloshyna I, Besana A, Castillo M, Matos T, Weinstein IB, et al. TREK-1 is a novel molecular target in prostate cancer. Cancer Res. 2008;68:1197–1203. doi: 10.1158/0008-5472.CAN-07-5163. [DOI] [PubMed] [Google Scholar]
- 20.Koumakpayi IH, Diallo JS, Le Page C, Lessard L, Gleave M, et al. Expression and nuclear localization of ErbB3 in prostate cancer. Clin Cancer Res. 2006;12:2730–2737. doi: 10.1158/1078-0432.CCR-05-2242. [DOI] [PubMed] [Google Scholar]